Abstract
Objectives
To assess the cardiovascular and renal efficacy and safety of sodium-glucose cotransporter-2 (SGLT2) inhibitors in patients without diabetes.
Methods
We searched PubMed, MEDLINE, Embase and Cochrane Library for publications up to 17 August 2022. Certainty of evidence was assessed using the Grading of Recommendations, Assessment, Development and Evaluation approach. Random-effects meta-analyses were performed to pool effect measures across studies. Risk ratios (RRs) with 95% CIs are expressed for composite cardiovascular outcome of cardiovascular death or hospitalisation for heart failure, cardiovascular death, hospitalisation for heart failure, all-cause mortality and composite renal outcome of ≥50% reduction in estimated glomerular filtration rate (eGFR), end-stage kidney disease or renal death. Annual rate of change in eGFR is expressed as the mean difference with 95% CI.
Results
We identified four trials with 8927 patients with heart failure or chronic kidney disease (CKD). Compared with placebo, SGLT2 inhibitors showed favourable effects on the composite cardiovascular outcome (RR: 0.79, 95% CI: 0.71 to 0.87; moderate certainty), cardiovascular death (0.85, 0.74 to 0.99; moderate certainty), hospitalisation for heart failure (0.72, 0.62 to 0.82; moderate certainty), the composite renal outcome (0.64, 0.48 to 0.85; low certainty) and the annual rate of change in eGFR (mean difference: 0.99, 0.59 to 1.39 mL/min/1.73 m2/year; moderate certainty), while there was no significant difference in all-cause mortality (0.88, 0.77 to 1.01; very low certainty). Moderate certainty evidence indicated that SGLT2 inhibitors reduced the risk of serious adverse events and acute renal failure. Low certainty evidence suggested that SGLT2 inhibitors increased the risk of urinary tract infection and genital infection, while there were no differences in discontinuation due to adverse events, amputation, fracture, hypoglycaemia, ketoacidosis or volume depletion.
Conclusions
Evidence of low to moderate certainty suggests that SGLT2 inhibitors provide cardiorenal benefits but have increased risk for urinary tract infection and genital infection in patients without diabetes and with heart failure or CKD.
PROSPERO registration number
CRD42021239807.
Keywords: heart failure, diabetic nephropathy & vascular disease, cardiology, nephrology, chronic renal failure
Strengths and limitations of this study.
Extraction of non-diabetic data from currently available randomised clinical trials (RCTs), this systematic review and meta-analysis enrolled 8927 patients with heart failure or chronic kidney disease, and over 3500 events of cardiovascular and renal outcomes.
Six different types of efficacy outcomes and 10 safety outcomes were analysed to evaluate the cardiorenal protective effects and drug safety of sodium-glucose cotransporter-2 inhibitors.
The Grading of Recommendations, Assessment, Development and Evaluation approach was used to appraise the body of the evidence.
Only four RCTs were included, and most of the trials had a relatively short study duration, which limited the power of the analyses of endpoints such as all-cause mortality.
Focusing on long-term clinical outcomes of chronic conditions, studies with acute conditions or follow-up duration less than 1 year were not included.
Introduction
Sodium-glucose cotransporter-2 (SGLT2) inhibitors were initially developed and approved as glucose-lowering drugs with the unique mechanism of inducing glycosuria in patients with type 2 diabetes.1 Recent large randomised clinical trials (RCTs) have reported that SGLT2 inhibitors improved cardiovascular (CV) and renal outcomes, most notably reducing the risks of heart failure and kidney failure among patients with diabetes with high CV risk.2 3 Post hoc analyses of these trials suggested that the favourable CV and renal effects of SGLT2 inhibitors could not be completely explained by the modest improvement in metabolic profiles.4–6 These beneficial effects appeared to be maintained at decreased levels of renal function with attenuated glycosuric effects and seemed to be independent of their glucose-lowering effects.7 8 Therefore, SGLT2 inhibitors were proposed to provide additional cardioprotective and renoprotective effects beyond the mechanisms of promoting glycosuria.9–11
RCTs comparing SGLT2 inhibitors with placebo, in which one-third to half of the participants did not have pre-existing diabetes, reported that SGLT2 inhibitors reduced the risk of CV and renal events, and the CV and renal benefits were similar among participants with and without diabetes.12–14 These encouraging effects in reducing CV and renal risks may not be directly linked to glucose-lowering effects, suggesting that the benefits of SGLT2 inhibitors might also be extended to individuals without diabetes. Following the results from the Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trial,14 the US Food and Drug Administration recently approved the use of dapagliflozin to reduce the risk of kidney function decline, kidney failure, CV death and heart failure in adults with chronic kidney disease (CKD) regardless of their diabetes status.15
To date, effective interventions to improve cardiorenal outcomes in patients without diabetes mellitus have been scarce, and there is an urgent need to identify therapeutic agents that may provide organ-protective effects.16 17 It is not known whether the routine use of SGLT2 inhibitors would provide additional cardiorenal benefits in patients without diabetes. Given the great promise in providing remarkable cardiorenal benefits that are independent of glycaemic control, we hypothesised that SGLT2 inhibitors could have cardiorenal protective effects in patients without diabetes mellitus in addition to the background standard of care for heart failure or CKD. In this systematic review and meta-analysis, we synthesised results from RCTs to evaluate the effects of SGLT2 inhibitors versus placebo on CV and renal outcomes in patients without diabetes with heart failure or CKD. We also assessed the safety outcomes of treatment with SGLT2 inhibitors compared with placebo.
Methods
Data sources and search strategies
We conducted electronic literature searches in PubMed, MEDLINE, Embase and Cochrane Library from inception until 17 August 2022. The search terms included Medical Subject Headings and text words that were relevant to SGLT2 inhibitors, CV outcomes, renal outcomes and RCTs. We hand-searched the reference lists of all identified publications to identify additional studies. There was no restriction on the language of publication. The searches were rerun prior to the final analyses, and any further studies identified were retrieved for inclusion. Additional details of study protocol and search strategies are provided in online supplemental appendices 1 and 2. The study protocol for this review was registered in the International Prospective Register of Systematic Reviews (PROSPERO, registration number CRD42021239807). This study was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines.18
bmjopen-2021-060655supp001.pdf (724.3KB, pdf)
Study selection
We included randomised, parallel-group designed clinical trials comparing SGLT2 inhibitors with placebo that enrolled adult participants older than 18 years without pre-existing diabetes. The included studies reported at least one prespecified CV or renal outcome. We excluded review articles, articles with irrelevant study designs, study protocols and RCTs assessing active comparisons or with a study duration of less than 1 year. We also excluded articles that enrolled solely patients with diabetes. Studies reporting outcomes from subgroups without diabetes were also included.
Data extraction and certainty/quality of evidence assessment
Two reviewers (W-CT and H-YW) independently extracted the following data: details of the study design, year of publication, study duration, generic name and dose of SGLT2 inhibitors, patient characteristics (age, sex and ethnicity), systolic blood pressure, estimated glomerular filtration rate (eGFR), glycated haemoglobin (HbA1c), underlying diseases, outcome events and adverse events. Two investigators (W-CT and H-YW) independently evaluated the methodological quality of the eligible trials by using the Cochrane Collaboration’s tool for assessing the risk of bias.19 The certainty of evidence was assessed independently using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach.20 Disagreements between the two authors were resolved by discussion or consultation.
Outcomes
Our outcomes of interest were (1) the composite CV outcome of CV death or hospitalisation for heart failure; (2) CV death; (3) hospitalisation for heart failure; (4) all-cause mortality; (5) the composite renal outcome of 50% or greater reduction in eGFR, end-stage kidney disease (ESKD) or renal death; and (6) the annual rate of change in eGFR (mL/min/1.73 m2/year). The prespecified outcome major adverse cardiovascular events (defined as a composite of CV death, non-fatal myocardial infarction and non-fatal stroke), individually or in combination, were not available and were not included in this study even though multiple attempts through various modes of communication (email, industry and social media) were made to achieve relevant data. For safety outcomes, we assessed adverse events, including any serious adverse event, discontinuation of the study drug due to adverse events, hypoglycaemia, ketoacidosis, amputation, fracture, volume depletion, acute renal failure, urinary tract infection and genital infection.
Data synthesis and analysis
Analyses were conducted with R software (V.4.0.5, R Foundation for Statistical Computing, Vienna, Austria).21 Tables of the GRADE summary of findings were developed with GRADEpro GDT (Guideline Development Tool), showing the certainty of the evidence for each outcome across studies.22 The pooled estimates of effect measures and 95% CIs of comparisons between the use of SGLT2 inhibitors and placebo were calculated using both the fixed-effect model and the DerSimonian and Laird random-effects model.23 The effect size of binary outcomes, including the composite CV outcome, CV death, hospitalisation for heart failure, all-cause mortality and the composite renal outcome, is expressed as risk ratios (RRs) with 95% CIs. Therapy with SGLT2 inhibitors would provide a better protective effect if the RR was significantly less than 1, and vice versa. The continuous outcome, the annual rate of change in eGFR, is expressed as the mean difference (MD) with 95% CI. Therapy with SGLT2 inhibitors would provide a better renoprotective effect if the MD was significantly greater than zero (ie, a lower rate of decline in eGFR), and vice versa. For the data needed to pool the annual rate of change in eGFR, we used imputation methods to reconstruct the missing values as recommended in the Cochrane Handbook (online supplemental appendix 3).23 Since the included studies in our systematic review enrolled populations with different types of chronic diseases, the between-study variance could be substantial, and the use of a fixed-effect model might not properly summarise the effect measures.24 Therefore, the random-effects model was used as the primary analytical model to calculate the pooled estimates for the effect measures of the included studies. The between-study heterogeneity was assessed by the I2 statistic and the Cochrane Q-test.23 There were no study-level covariates available to explore the potential sources of heterogeneity, and we did not perform subgroup analyses or meta-regression in this study. To assess publication bias, we performed the funnel plot and Egger’s test.25 For study outcomes with fewer than three included studies, Egger’s test could not be performed. Two-sided p values <0.05 were considered statistically significant.
Patient and public involvement
Patients or the public were not involved in this study.
Results
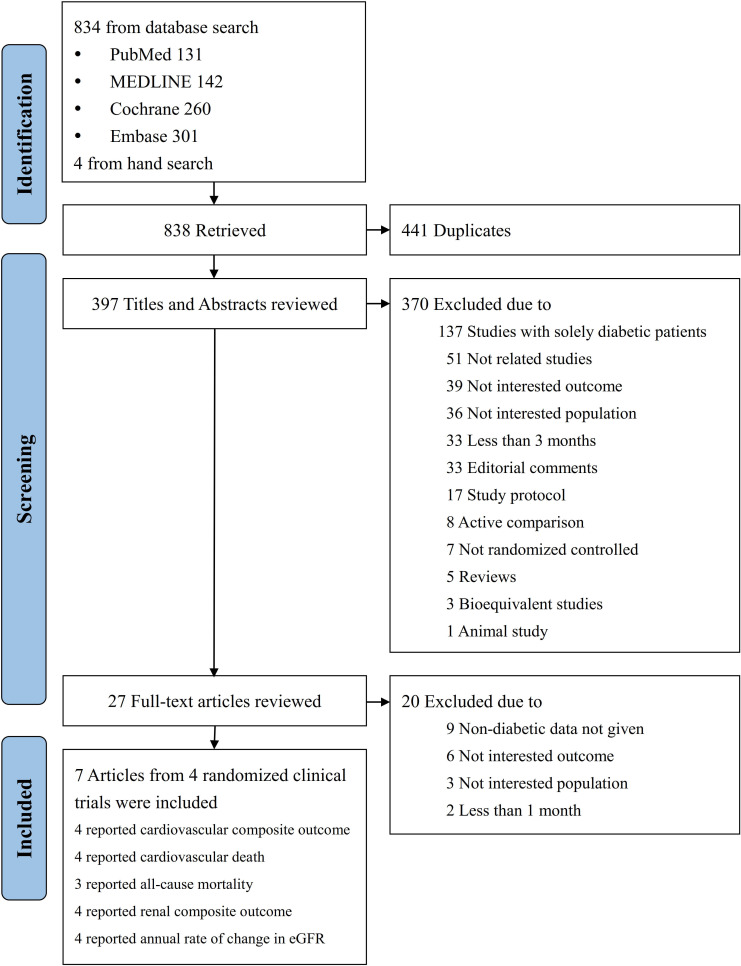
As shown in figure 1, a total of 838 articles were identified by the literature search. Of these, 27 articles were reviewed in full text, and 7 articles from four trials were included.
Figure 1.
Summary of study identification and selection. eGFR, estimated glomerular filtration rate.
Study characteristics
There were four RCTs from seven eligible articles26–29 that enrolled a total of 8927 participants without diabetes. All studies were multicentre, double-blind, placebo-controlled, randomised trials. The clinical and methodological characteristics of each study are summarised in table 1.
Table 1.
Characteristics of the non-diabetic study participants from the included studies
| Source | Petrie et al 2020 (DAPA-HF)26 |
Anker et al 2021 (EMPEROR-Reduced)28 |
Wheeler et al 2021 (DAPA-CKD)29 |
Filippatos et al 2022 (EMPEROR-Preserved)30* |
| Inclusion criteria | Chronic HFrEF, NYHA class II–IV with LVEF ≤40% and elevated NT-proBNP | Chronic HFrEF, NYHA class II–IV with LVEF ≤40% and elevated NT-proBNP | CKD, eGFR 25–75 mL/min/1.73 m2 and UACR 200–5000 mg/g | Chronic HFpEF, NYHA class II–IV with LVEF >40% and elevated NT-proBNP |
| Received standard of care | Yes | Yes | Yes | Yes |
| Diabetes as a stratification variable in the trial | Yes | Yes | Yes | Yes |
| Follow-up (years) | 1.5 | 1.3 | 2.4 | 2.2 |
| Total number of participants without diabetes | 2605 | 1874 | 1398 | 3050 |
| Type of intervention | Dapagliflozin 10 mg once daily | Placebo | Empagliflozin 10 mg once daily | Placebo | Dapagliflozin 10 mg once daily | Placebo | Empagliflozin 10 mg once daily | Placebo |
| Number of participants in each group | 1298 | 1307 | 936 | 938 | 697 | 701 | 1531 | 1519 |
| Age (years) | 66±12 | 66±12 | 68±12 | 66±12 | 57±15 | 56±15 | 73±10 | |
| Women, n (%) | 324 (25) | 308 (24) | 227 (24) | 238 (25) | 215 (31) | 245 (35) | 1420 (47) | |
| Race, n (%) | ||||||||
| White | 918 (71) | 926 (71) | 679 (73) | 665 (71) | 373 (54) | 376 (54) | 2334 (77) | |
| Black | 50 (4) | 48 (4) | 66 (7) | 71 (8) | 28 (4) | 26 (4) | 117 (4) | |
| Asian | 311 (24) | 314 (24) | 154 (17) | 160 (17) | 268 (38) | 267 (38) | 431 (14) | |
| Other | 19 (2) | 19 (2) | 37 (4) | 42 (5) | 28 (4) | 32 (5) | 167 (6) | |
| History of heart failure, n (%) | 1295 (100) | 1305 (100) | 936 (100) | 937 (100) | 58 (8) | 49 (7) | 3050 (100) | |
| Systolic blood pressure (mm Hg) | 121±16 | 120±16 | 122±16 | 120±15 | 132±16 | 133±17 | 130±15 | |
| Haemoglobin A1c (%) | 5.7±0.4 | 5.8±0.4 | 5.8±0.4 | 5.7±0.4 | 5.6±0.4 | 5.6±0.4 | 5.7±0.4 | |
| eGFR (mL/min/1.73 m2) | 68±19 | 68±19 | 63±21 | 63±21 | 42±12 | 42±12 | 62±19 | |
| eGFR <60 mL/min/1.73 m2, n (%) | 480 (37) | 464 (36) | 434 (46) | 426 (45) | 642 (92) | 650 (93) | 1478 (49) | |
| ACE inhibitor, n (%) | 737 (57) | 752 (58) | 451 (48) | 425 (45) | 222 (32) | 238 (34) | 1229 (40) | |
| ARB, n (%) | 357 (28) | 335 (26) | 213 (23) | 227 (24) | 460 (66) | 452 (64) | 1093 (36) | |
| Diuretic, n (%) | 1191 (92) | 1214 (93) | 779 (83) | 809 (86) | 210 (30) | 207 (30) | 2358 (77) | |
Data are the mean±SD or n (%).
*Characteristics of overall non-diabetic group data are presented for the EMPEROR-Preserved trial as no data for each intervention group can be extracted.
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CKD, chronic kidney disease; DAPA-CKD, Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease; DAPA-HF, Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure; eGFR, estimated glomerular filtration rate; EMPEROR-Preserved, Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction; EMPEROR-Reduced, Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; SGLT2, sodium-glucose cotransporter-2; UACR, urinary albumin-to-creatinine ratio (with albumin measured in milligrams and creatinine measured in grams).
Three studies enrolled patients with chronic heart failure, and one study focused on those with CKD. All studies were designed to compare SGLT2 inhibitors with placebo as an adjunct to the standard of care. The status of diabetes at baseline was one of the stratification variables in all four trials. The length of follow-up ranged from 1.3 to 2.4 years. In terms of the SGLT2 inhibitors, dapagliflozin was prescribed in two studies, and empagliflozin was prescribed in another two studies. All regimens were administered at a dosage of 10 mg once daily. The mean age of the participants in the studies ranged from 56 to 73 years, with females accounting for 33%. Regarding ethnicity, 70% of the participants were white, one-fifth were Asian and 5% were black. Overall, the majority of the participants (85%) had a history of chronic heart failure. The mean HbA1C of the participants in the studies ranged from 5.6% to 5.8%. Half of the participants (51%) had eGFR levels less than 60 mL/min/1.73 m2. As a standard of care, 45% of the participants received angiotensin-converting enzyme inhibitors, 35% received angiotensin receptor blockers and 76% received diuretics.
Assessment of risk of bias and body of evidence
The risk of bias of the included studies is summarised in online supplemental figures S1 and S2. All four studies were deemed to be at low risk of bias in all domains.
For efficacy outcomes, certainty of evidence was rated ‘moderate’ for composite CV outcome, CV death, hospitalisation for heart failure and annual rate of change in eGFR, ‘low’ for composite renal outcome and ‘very low’ for all-cause mortality (online supplemental table S1). For safety outcomes, certainty of evidence was rated ‘moderate’ for any serious adverse event and acute renal failure, and ‘low’ for amputation, fracture, volume depletion, urinary tract infection and genital infection (online supplemental table S2).
Effects of SGLT2 inhibitors on CV and renal outcomes
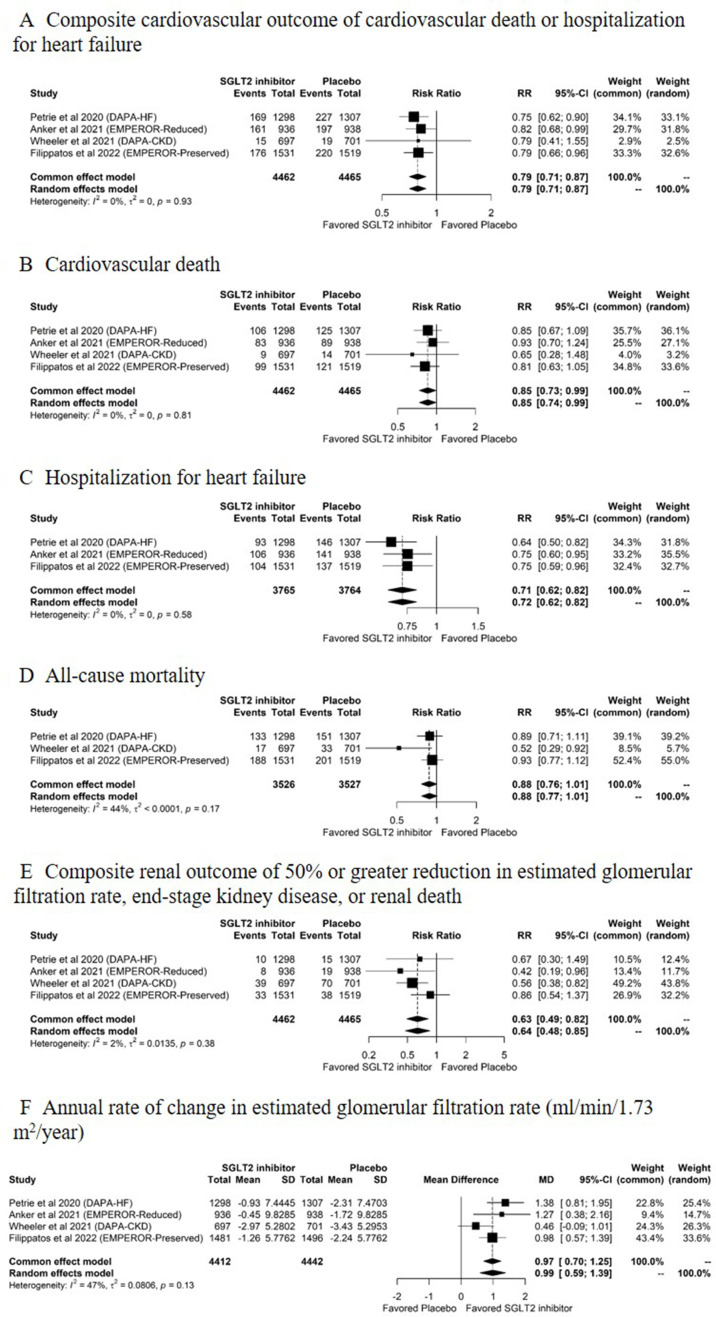
There were a total of 3512 CV and renal events in the four RCTs, including 1184 composite CV outcomes, 646 CV deaths, 727 hospitalisations for heart failure, 723 deaths and 232 composite renal outcomes. Figure 2 shows the pooled estimates of CV and renal outcomes. The composite renal outcome generally included renal death, ESKD and a sustained reduction in eGFR of 50% or greater in the DAPA-HF trial26 and DAPA-CKD trial29 and 40% or greater in the EMPEROR-Reduced trial28 and EMPEROR-Preserved trial30; the composite renal outcome did not include renal-related death in the EMPEROR-Reduced trial28 and EMPEROR-Preserved trial30 (online supplemental table S3). Between-study heterogeneity was not present in the CV and renal outcomes (figure 2A–F). The funnel plots and Egger’s test indicated no significant publication bias for the study outcomes except for all-cause mortality that had funnel plot asymmetry (Egger’s test, p=0.01) (online supplemental figure S3).
Figure 2.
Pooled estimates of the efficacy outcomes comparing SGLT2 inhibitors with placebo. (A) Composite cardiovascular outcome of cardiovascular death or hospitalisation for heart failure, (B) cardiovascular death, (C) hospitalisation for heart failure, (D) all-cause mortality, (E) composite renal outcome of 50% or greater reduction in eGFR, end-stage kidney disease or renal death and (F) annual rate of change in eGFR (mL/min/1.73 m2/year) for comparisons between SGLT2 inhibitors and placebo. DAPA-CKD, Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease; DAPA-HF, Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure; eGFR, estimated glomerular filtration rate; EMPEROR-Preserved, Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction; EMPEROR-Reduced, Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction; MD, mean difference; RR, risk ratio; SGLT2, sodium-glucose cotransporter-2.
Compared with placebo, SGLT2 inhibitors significantly reduced the risk of the composite CV outcome (RR: 0.79, 95% CI: 0.71 to 0.87, p<0.001; figure 2A; moderate certainty evidence, online supplemental table S1), CV death (RR: 0.85, 95% CI: 0.74 to 0.99, p=0.04; figure 2B; moderate certainty evidence, online supplemental table S1), hospitalisation for heart failure (RR: 0.72, 95% CI: 0.62 to 0.82, p<0.001; figure 2C; moderate certainty evidence, online supplemental table S1), the composite renal outcome (RR: 0.64, 95% CI: 0.48 to 0.85, p=0.002; figure 2E; low certainty evidence, online supplemental table S1) and the annual rate of change in eGFR (MD: 0.99, 95% CI: 0.59 to 1.39 mL/min/1.73 m2/year, p<0.001; figure 2F; moderate certainty evidence, online supplemental table S1). SGLT2 inhibitors did not reduce the risk of all-cause mortality (RR: 0.88, 95% CI: 0.77 to 1.01, p=0.07; figure 2D; very low certainty evidence, online supplemental table S1) compared with placebo.
Safety profile of therapy with SGLT2 inhibitors
Table 2 summarises the adverse events reported in the included studies.
Table 2.
Adverse events reported in the included studies
| Source | Overall | Petrie et al 2020 (DAPA-HF)26 |
Anker et al 2021 (EMPEROR-Reduced)28 |
Wheeler et al 2021 (DAPA-CKD)29 |
Filippatos et al 2022 (EMPEROR-Preserved)30 |
|||||
| Type of intervention | SGLT2 inhibitor (n=4458) | Placebo (n=4459) | Dapagliflozin (n=1295) | Placebo (n=1305) | Empagliflozin (n=936) | Placebo (n=937) | Dapagliflozin (n=696) | Placebo (n=699) | Empagliflozin (n=1531) | Placebo (n=1518) |
| Any serious adverse event* | 1677 (38) | 1832 (41) | 448 (35) | 481 (36) | 375 (40) | 439 (47) | 150 (22) | 167 (24) | 704 (46) | 745 (49) |
| Discontinuation of the study drug due to adverse events | 532 (12) | 503 (11) | 68 (5) | 59 (5) | 147 (16) | 152 (16) | 36 (5) | 29 (4) | 281 (18) | 263 (17) |
| Hypoglycaemia | 2 (<1) | 2 (<1) | 0 | 0 | 0 | 0 | 0 | 0 | 2 (<1) | 2 (<1) |
| Ketoacidosis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Amputation | 3 (<1) | 7 (<1) | 1 (<1) | 3 (<1) | 1 (<1) | 1 (<1) | 0 | 1 (<1) | 1 (<1) | 2 (<1) |
| Fracture | 72 (2) | 59 (2) | 27 (2) | 25 (2) | 25 (3) | 16 (2) | 20 (3) | 18 (3) | NA | NA |
| Volume depletion | 418 (9) | 347 (8) | 94 (7) | 79 (6) | 94 (10) | 100 (11) | 35 (5) | 19 (3) | 195 (13) | 149 (10) |
| Acute renal failure | 306 (7) | 374 (8) | 34 (5) | 40 (6) | 77 (8) | 94 (10) | 62 (5) | 78 (6) | 133 (9) | 162 (11) |
| Urinary tract infection | 194 (4) | 150 (3) | NA | NA | 39 (4) | 34 (4) | 6 (<1) | 4 (<1) | 149 (10) | 112 (7) |
| Genital infection | 43 (1) | 16 (<1) | NA | NA | 13 (1) | 8 (<1) | 0 | 0 | 30 (2) | 8 (<1) |
Data are n (%).
*The original definition in each trial included any adverse event that required hospitalisation, resulted in death, and so on.
DAPA-CKD, Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease; DAPA-HF, Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure; EMPEROR-Preserved, Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction; EMPEROR-Reduced, Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction; NA, not available; SGLT2, sodium-glucose cotransporter-2.
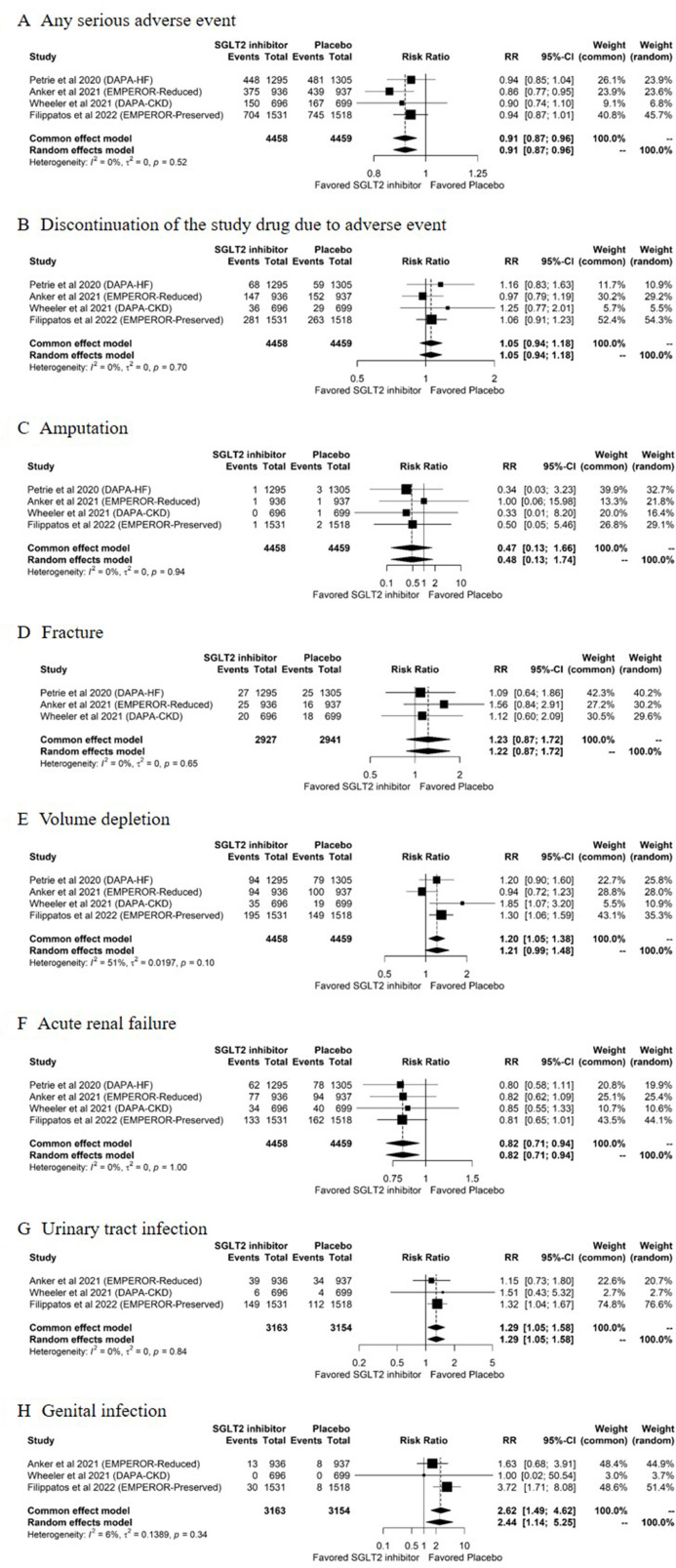
Figure 3 displays the pooled estimates for the safety outcomes. All four trials26 28–30 reported data on adverse events, including any serious adverse event, discontinuation of the study drug due to adverse events, hypoglycaemia, ketoacidosis, amputation, volume depletion and acute renal failure. Three trials26 28 29 reported the risk of fracture. Three trials28–30 reported the risk of urinary tract infection and genital infection. Three trials26 28 29 reported that there was no event of hypoglycaemia in either group and one trial30 reported two hypoglycaemic events in each group. All four trials reported that there was no event of ketoacidosis in either group. Heterogeneity between studies was not present in any of the safety outcomes (figure 3A–H). No evidence of publication bias was detected in the funnel plots and Egger’s test for the safety outcomes (online supplemental figure S4).
Figure 3.
Pooled estimates of the safety outcomes comparing SGLT2 inhibitors with placebo. (A) Any serious adverse event, (B) discontinuation of the study drug due to adverse events, (C) amputation, (D) fracture, (E) volume depletion, (F) acute renal failure, (G) urinary tract infection and (H) genital infection, for comparisons between SGLT2 inhibitors and placebo. DAPA-CKD, Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease; DAPA-HF, Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure; EMPEROR-Preserved, Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction; EMPEROR-Reduced, Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction; RR, risk ratio; SGLT2, sodium-glucose cotransporter-2.
Of the 8917 participants, 3509 (39%) experienced serious adverse events: 38% in the SGLT2 inhibitor group and 41% in the placebo group. Compared with participants in the placebo group, those in the SGLT2 inhibitor group had a lower risk of any serious adverse event (RR: 0.91, 95% CI: 0.87 to 0.96, p<0.001; figure 3A; moderate certainty evidence, online supplemental table S2), and acute renal failure (RR: 0.82, 95% CI: 0.71 to 0.94, p=0.006; figure 3F; moderate certainty evidence, online supplemental table S2). Compared with placebo, SGLT2 inhibitors significantly increased the risk of urinary tract infection (RR: 1.29, 95% CI: 1.05 to 1.58, p=0.02; figure 3G; low certainty evidence, online supplemental table S2) and genital infection (RR: 2.44, 95% CI: 1.14 to 5.25, p=0.02; figure 3H; low certainty evidence, online supplemental table S2). There were no between-group differences in discontinuation of the study drug due to adverse events (RR: 1.05, 95% CI: 0.94 to 1.18, p=0.38; figure 3B; low certainty evidence, online supplemental table S2), amputation (RR: 0.48, 95% CI: 0.13 to 1.74, p=0.26; figure 3C; low certainty evidence, online supplemental table S2), fracture (RR: 1.22, 95% CI: 0.87 to 1.72, p=0.25; figure 3D; low certainty evidence, online supplemental table S2) or volume depletion (RR: 1.21, 95% CI: 0.99 to 1.48, p=0.07; figure 3E; low certainty evidence, online supplemental table S2).
Discussion
In this systematic review and meta-analysis comparing SGLT2 inhibitors with placebo in patients without diabetes with chronic heart failure or CKD, we found that SGLT2 inhibitors provided cardiorenal protective effects with additional adverse effects. A total of 8927 participants were analysed, and all received medical standards of care. The majority of the participants had pre-existing chronic heart failure and half of them had CKD. Compared with placebo, the pooled treatment effects showed that SGLT2 inhibitors reduced the risk of the composite CV outcome of CV death or hospitalisation for heart failure by 21%, CV death by 15%, hospitalisation for heart failure by 28% and decreased the risk of the composite renal outcome of ≥50% reduction in eGFR, ESKD or renal death by 36%. SGLT2 inhibitors also postponed the decline in eGFR by 0.99 mL/min/1.73 m2 per year. Compared with those who received placebo, patients treated with SGLT2 inhibitors had a lower risk of serious adverse events and acute renal failure but did show an increased risk of urinary tract infection and genital infection. Adopting the GRADE approach, low to moderate certainty evidence demonstrated that SGLT2 inhibitors should be considered in individuals without diabetes and with chronic heart failure or CKD to prevent the deleterious effects of CV and renal diseases. However, evidence of low certainty suggested that SGLT2 inhibitors might cause clinically important adverse events such as urinary tract infection and genital infection, which could jeopardise tolerability of long-term treatment with SGLT2 inhibitors.
Strengths of this study
To the best of our knowledge, this is one of the largest systematic review and meta-analysis comparing SGLT2 inhibitors with placebo in terms of cardiorenal protective effects and safety among patients without diabetes. Following a standard study protocol and using a comprehensive search strategy, this systematic review enrolled four large-scale RCTs with more than 8900 patients and over 3500 events of CV and renal outcomes. Six different types of efficacy outcomes were analysed to evaluate the cardiorenal protective effects of SGLT2 inhibitors, and most of the pooled treatment effects showed significant protective effects. The safety of SGLT2 inhibitors was also demonstrated after evaluating ten different types of safety outcomes. Quality appraisals used the GRADE approach. Accordingly, our data favourably provide comprehensive evidence of the cardiorenal protective effects and drug safety of SGLT2 inhibitors in patients without diabetes and with heart failure or CKD.
Results in relation to other studies and reviews
Despite substantial evidence of the beneficial effects of SGLT2 inhibitors on important clinical outcomes in patients with type 2 diabetes,31 32 few studies have attempted to focus on populations without diabetes. Recently, several large-scale RCTs have enrolled both patients with and without diabetes to evaluate the clinical benefits of SGLT2 inhibitors.12–14 The Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) trial, which included individuals without diabetes accounting for 55% of the enrollees, evaluated the effects of dapagliflozin in 4744 participants with chronic heart failure.12 After a median follow-up of 18.2 months, dapagliflozin reduced the risk of the primary outcome of CV death or worsening heart failure by 26% (HR: 0.74, 95% CI: 0.65 to 0.85), with similar benefits in patients with and without diabetes (p value for interaction=0.83).12 The DAPA-CKD trial enrolled a total of 4304 participants with CKD, among which one-third were individuals without diabetes.14 After a median follow-up of 2.4 years, dapagliflozin reduced the risk of the primary outcome of ≥50% sustained eGFR decline, ESKD or death from renal or CV causes by 39% (HR: 0.61, 95% CI: 0.51 to 0.72), with similar benefits in patients with and without diabetes (p value for interaction=0.24).14 Evidence of the clinical benefits of SGLT2 inhibitors in the population without diabetes was obtained from subgroup analyses of these trials, which were generally underpowered. In a systematic review, Teo et al reported better cardiac outcomes in patients without diabetes who received SGLT2 inhibitors than in those who received placebo,33 but the landmark DAPA-CKD trial was not included in this review. In a meta-analysis of the DAPA-HF and the Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction (EMPEROR-Reduced) trials, Zannad et al reported that treatment with SGLT2 inhibitors reduced the risk of the composite outcome of hospitalisation for heart failure or CV death by 25% (HR: 0.75, 95% CI: 0.65 to 0.87) in patients without diabetes.34 In a recent systematic review and meta-analysis reported by Salah et al, initiation of SGLT2 inhibitors in patients hospitalised for acute heart failure reduced the risk of rehospitalisation for heart failure by 48%, while the effect on adverse events remained uncertain as the findings from included studies were limited due to few events.35 After extracting information on participants without diabetes from the four latest large-scale trials, our findings were consistent with those of individual trials and previous systematic reviews. Our data not only supported the CV and renal efficacy but also uncovered the adverse effect of SGLT2 inhibitors in patients without diabetes with heart failure or CKD.
The mechanisms underlying the organ-protective effects of SGLT2 inhibitors in these populations are not yet completely understood but may be beyond their metabolic effects of enhancing glycosuria. Within a few years, there has been an increasing number of proposed pathways for the systemic organ-protective effects of SGLT2 inhibitors, which are related to preventing sodium and water retention,36 favourable metabolic adaptations for energy production,37 38 restored myocardial sodium and calcium balance by the inhibition of sodium-hydrogen exchanger 1,39 reduced tissue sodium content,40 attenuations of tubuloglomerular feedback and subsequent intraglomerular hypertension leading to renoprotection,41 42 activation of the depressor arm of the renal-angiotensin-aldosterone system evoking vasodilatory, antioxidant, anti-inflammatory and sympathoinhibitory effects,11 suppression of inflammation and fibrosis,43 induction of erythropoiesis44 and adaptive reprogramming of stressed cells via the activation of sirtuin 1, which promotes homeostasis and survival.9
By evaluating a total of 10 safety outcomes, the results of our study showed that treatment with SGLT2 inhibitors elicited some adverse events though had lower risk for any serious adverse events and acute renal failure in the population without diabetes. It is also important to note that there were no events of hypoglycaemia or ketoacidosis in the included trials, except for EMPEROR-Preserved trial reported by Filippatos et al30 that showed a similar hypoglycaemic event in both groups. Compared with those in the placebo group, participants in the SGLT2 inhibitors group experienced an increased risk of urinary tract infection by 29% (RR: 1.29, 95% CI: 1.05 to 1.58, p=0.02; figure 3G; low certainty evidence, online supplemental table S2) and a 2.44-fold higher risk of genital infection (RR: 2.44, 95% CI: 1.14 to 5.25, p=0.02; figure 3H; low certainty evidence, online supplemental table S2). However, there was a lower risk of any serious adverse events by 9% (RR: 0.91, 95% CI: 0.87 to 0.96, p<0.001; figure 3A; moderate certainty evidence, online supplemental table S2), and acute renal failure by 18% (RR: 0.82, 95% CI: 0.71 to 0.94, p=0.006; figure 3F; moderate certainty evidence, online supplemental table S2) among participants who received SGLT2 inhibitors than among those who received placebo, while the risks of other adverse events including discontinuation of the study drug due to adverse events, amputation, fracture and volume depletion were similar among participants in the SGLT2 inhibitor and placebo groups (figure 3B–E). The increased risk of clinically important adverse events such as urinary tract infection and genital infection observed in our study must be balanced with the cardiorenal benefits of SGLT2 inhibitors, especially in the context of long-term use.
Limitations
Our study has several limitations. First, subgroup analysis and meta-regression of the study outcomes were not performed in this study because there were no study-level covariates available. Although there was no significant between-study heterogeneity for all efficacy and safety outcomes, whether the cardiorenal benefits differ among different stages of heart failure or CKD deserves further study. Second, our study included patients without diabetes with chronic heart failure or CKD. Therefore, the organ-protective effects of SGLT2 inhibitors that we observed are restricted to these populations. Ongoing trials such as EMPA-Kidney45 should contribute to expanding the population that benefits from SGLT2 inhibition if they meet their primary endpoints. Third, the number of included RCTs was small, and most of the trials had a relatively short study duration, which limited the power of the analyses of endpoints such as all-cause mortality. However, the power to detect a true benefit might be increased by the inclusion of over 8900 patients with heart or kidney disease and by the collection of more than 3500 events of cardiorenal outcomes in this study. Fourth, the included studies were not designed to enrol solely patients without diabetes. Because participants were stratified by status of diabetes at randomisation in all included trials, the baseline characteristics of the participants were similar among the SGLT2 inhibitor and placebo groups. As a result, it was reasonable to extract data from participants without diabetes in these trials. Finally, the SGLT2 inhibitors prescribed in the included trials were dapagliflozin or empagliflozin. Whether other SGLT2 inhibitors provide similar cardioprotective or renoprotective effects in patients without diabetes deserves further study.
Conclusions
Our analyses showed that treatment with SGLT2 inhibitors provided additional cardiorenal benefits in patients without diabetes who had received standard of care for heart failure or CKD. However, there were safety concerns, such as urinary tract infection and genital infection, regarding the use of SGLT2 inhibitors. With the evidence of low to moderate certainty, our study confers substantial evidence supporting the routine use of SGLT2 inhibitors in individuals without diabetes and with chronic heart failure or CKD to reduce CV and renal morbidities and mortalities, but the integrity of such strategy might be compromised due to an increased risk of adverse events.
Supplementary Material
Footnotes
Contributors: W-CT, S-PH, Y-LC, Y-KT, Y-SP and H-YW conceived and designed the study. W-CT and H-YW independently collected, screened and extracted the data. W-CT and H-YW independently evaluated risk of bias and quality of evidence. W-CT, S-PH, Y-SP and H-YW resolved disagreement through discussion. W-CT, J-YY, M-FP, M-JK, Y-KT and W-CT performed the analyses or interpretation of data. W-CT, Y-LC, Y-SP and H-YW conducted the drafting of the work. All authors critically revised the manuscript for important intellectual content and final approval of the version to be published. W-CT and H-YW had grants for the study. Y-KT, K-YH and K-LC supervised the study. H-YW and Y-SP had full access to all of the data in the study, took responsibility for the conduct of the study, the integrity of the data and the accuracy of the data analysis, and controlled the decision to publish. H-YW and Y-SP contributed equally as corresponding authors to this work.
Funding: This study was supported by research grants to Dr Hon-Yen Wu from the National Health Research Institutes, Taiwan (NHRI-EX110-11026PI, NHRI-EX111-11026PI) and the Far Eastern Memorial Hospital, New Taipei City, Taiwan (FEMH-EX110-11026PI, FEMH-EX111-11026PI) and to Dr Wan-Chuan Tsai from the Far Eastern Memorial Hospital, New Taipei City, Taiwan (FEMH-2021-C-044 and FEMH-2022-C-007).
Disclaimer: The funders had no role in the design and conduct of the study; the collection, management, analysis and interpretation of the data; the preparation, review and approval of the manuscript; or the decision to submit the manuscript for publication.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Choi C-I. Sodium-glucose cotransporter 2 (SGLT2) inhibitors from natural products: discovery of next-generation antihyperglycemic agents. Molecules 2016;21. 10.3390/molecules21091136. [Epub ahead of print: 27 Aug 2016]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–28. 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 3.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–57. 10.1056/NEJMoa1812389 [DOI] [PubMed] [Google Scholar]
- 4.Inzucchi SE, Kosiborod M, Fitchett D, et al. Improvement in cardiovascular outcomes with Empagliflozin is independent of glycemic control. Circulation 2018;138:1904–7. 10.1161/CIRCULATIONAHA.118.035759 [DOI] [PubMed] [Google Scholar]
- 5.Cahn A, Wiviott SD, Mosenzon O, et al. Cardiorenal outcomes with dapagliflozin by baseline glucose-lowering agents: post hoc analyses from DECLARE-TIMI 58. Diabetes Obes Metab 2021;23:29–38. 10.1111/dom.14179 [DOI] [PubMed] [Google Scholar]
- 6.Inzucchi SE, Zinman B, Fitchett D, et al. How does Empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG outcome trial. Diabetes Care 2018;41:356–63. 10.2337/dc17-1096 [DOI] [PubMed] [Google Scholar]
- 7.Heerspink HJL, Desai M, Jardine M, et al. Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol 2017;28:368–75. 10.1681/ASN.2016030278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrykiv S, Sjöström CD, Greasley PJ, et al. Differential effects of dapagliflozin on cardiovascular risk factors at varying degrees of renal function. Clin J Am Soc Nephrol 2017;12:751–9. 10.2215/CJN.10180916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Packer M. SGLT2 inhibitors produce cardiorenal benefits by promoting adaptive cellular reprogramming to induce a state of fasting mimicry: a paradigm shift in understanding their mechanism of action. Diabetes Care 2020;43:508–11. 10.2337/dci19-0074 [DOI] [PubMed] [Google Scholar]
- 10.Rajasekeran H, Cherney DZ, Lovshin JA. Do effects of sodium-glucose cotransporter-2 inhibitors in patients with diabetes give insight into potential use in non-diabetic kidney disease? Curr Opin Nephrol Hypertens 2017;26:358–67. 10.1097/MNH.0000000000000343 [DOI] [PubMed] [Google Scholar]
- 11.Tsimihodimos V, Filippatos TD, Elisaf MS. SGLT2 inhibitors and the kidney: effects and mechanisms. Diabetes Metab Syndr 2018;12:1117–23. 10.1016/j.dsx.2018.06.003 [DOI] [PubMed] [Google Scholar]
- 12.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 13.Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with Empagliflozin in heart failure. N Engl J Med 2020;383:1413–24. 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 14.Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–46. 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- 15.FDA Approves treatment for chronic kidney disease, 2021. Available: https://www.fda.gov/news-events/press-announcements/fda-approves-treatment-chronic-kidney-disease [Accessed 27 May 2021].
- 16.Tsai W-C, Wu H-Y, Peng Y-S, et al. Association of intensive blood pressure control and kidney disease progression in nondiabetic patients with chronic kidney disease: a systematic review and meta-analysis. JAMA Intern Med 2017;177:792–9. 10.1001/jamainternmed.2017.0197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakagawa Y, Kuwahara K. Sodium-glucose cotransporter-2 inhibitors are potential therapeutic agents for treatment of non-diabetic heart failure patients. J Cardiol 2020;76:123–31. 10.1016/j.jjcc.2020.03.009 [DOI] [PubMed] [Google Scholar]
- 18.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JPT, Savović J, Page MJ, et al. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, et al., eds. Cochrane Handbook for systematic reviews of interventions version 6.3 (updated February 2022. Cochrane, 2022. www.training.cochrane.org/handbook [Google Scholar]
- 20.Schünemann HJ, Higgins JPT, Vist GE, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, et al., eds. Cochrane Handbook for systematic reviews of interventions version 6.3 (updated February 2022). Cochrane, 2022. www.training.cochrane.org/handbook [Google Scholar]
- 21.R: A Language and Environment for Statistical Computing [program]. 4.0.5. version, 2021
- 22.GRADEpro GDT . GRADEpro Guideline Development Tool [Software] McMaster University and Evidence Prime; 2022. gradepro.org [Google Scholar]
- 23.Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for systematic reviews of interventions version 6.3 (updated February 2022. Cochrane, 2022. www.training.cochrane.org/handbook [Google Scholar]
- 24.Borenstein M, Hedges LV, Higgins JPT, et al. Introduction to meta-analysis. John Wiley & Sons, Ltd, 2009. [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrie MC, Verma S, Docherty KF, et al. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA 2020;323:1353–68. 10.1001/jama.2020.1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jhund PS, Solomon SD, Docherty KF, et al. Efficacy of dapagliflozin on renal function and outcomes in patients with heart failure with reduced ejection fraction: results of DAPA-HF. Circulation 2021;143:298–309. 10.1161/CIRCULATIONAHA.120.050391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anker SD, Butler J, Filippatos G, et al. Effect of Empagliflozin on cardiovascular and renal outcomes in patients with heart failure by baseline diabetes status: results from the EMPEROR-Reduced trial. Circulation 2021;143:337–49. 10.1161/CIRCULATIONAHA.120.051824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wheeler DC, Stefánsson BV, Jongs N, et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol 2021;9:22–31. 10.1016/S2213-8587(20)30369-7 [DOI] [PubMed] [Google Scholar]
- 30.Filippatos G, Butler J, Farmakis D, et al. Empagliflozin for heart failure with preserved left ventricular ejection fraction with and without diabetes. Circulation 2022;146:101161CIRCULATIONAHA122059785. 10.1161/CIRCULATIONAHA.122.059785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmer SC, Tendal B, Mustafa RA, et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ 2021;372:m4573. 10.1136/bmj.m4573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu J, Yu X, Zheng Y, et al. Association of glucose-lowering medications with cardiovascular outcomes: an umbrella review and evidence MAP. Lancet Diabetes Endocrinol 2020;8:192–205. 10.1016/S2213-8587(19)30422-X [DOI] [PubMed] [Google Scholar]
- 33.Teo YH, Teo YN, Syn NL, et al. Effects of sodium/glucose cotransporter 2 (SGLT2) inhibitors on cardiovascular and metabolic outcomes in patients without diabetes mellitus: a systematic review and meta-analysis of randomized-controlled trials. J Am Heart Assoc 2021;10:e019463. 10.1161/JAHA.120.019463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-reduced and DAPA-HF trials. Lancet 2020;396:819–29. 10.1016/S0140-6736(20)31824-9 [DOI] [PubMed] [Google Scholar]
- 35.Salah HM, Al'Aref SJ, Khan MS, et al. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors initiation in patients with acute heart failure, with and without type 2 diabetes: a systematic review and meta-analysis. Cardiovasc Diabetol 2022;21. 10.1186/s12933-022-01455-2. [Epub ahead of print: [published Online First: 20220205]]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMurray J. EMPA-REG - the "diuretic hypothesis". J Diabetes Complications 2016;30:3–4. 10.1016/j.jdiacomp.2015.10.012 [DOI] [PubMed] [Google Scholar]
- 37.Ferrannini E, Baldi S, Frascerra S, et al. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes 2016;65:1190–5. 10.2337/db15-1356 [DOI] [PubMed] [Google Scholar]
- 38.Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG outcome study? A unifying hypothesis. Diabetes Care 2016;39:1115. 10.2337/dc16-0542 [DOI] [PubMed] [Google Scholar]
- 39.Uthman L, Baartscheer A, Bleijlevens B, et al. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na+/H+ exchanger, lowering of cytosolic Na+ and vasodilation. Diabetologia 2018;61:722–6. 10.1007/s00125-017-4509-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karg MV, Bosch A, Kannenkeril D, et al. SGLT-2-inhibition with dapagliflozin reduces tissue sodium content: a randomised controlled trial. Cardiovasc Diabetol 2018;17:5. 10.1186/s12933-017-0654-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cherney DZI, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014;129:587–97. 10.1161/CIRCULATIONAHA.113.005081 [DOI] [PubMed] [Google Scholar]
- 42.Cherney DZI, Dekkers CCJ, Barbour SJ, et al. Effects of the SGLT2 inhibitor dapagliflozin on proteinuria in non-diabetic patients with chronic kidney disease (diamond): a randomised, double-blind, crossover trial. Lancet Diabetes Endocrinol 2020;8:582–93. 10.1016/S2213-8587(20)30162-5 [DOI] [PubMed] [Google Scholar]
- 43.Heerspink HJL, Perco P, Mulder S, et al. Canagliflozin reduces inflammation and fibrosis biomarkers: a potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia 2019;62:1154–66. 10.1007/s00125-019-4859-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mazer CD, Hare GMT, Connelly PW, et al. Effect of Empagliflozin on erythropoietin levels, iron stores, and red blood cell morphology in patients with type 2 diabetes mellitus and coronary artery disease. Circulation 2020;141:704–7. 10.1161/CIRCULATIONAHA.119.044235 [DOI] [PubMed] [Google Scholar]
- 45.Herrington WG, Preiss D, Haynes R, et al. The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: a rationale for the EMPA-KIDNEY study. Clin Kidney J 2018;11:749–61. 10.1093/ckj/sfy090 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-060655supp001.pdf (724.3KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.