Abstract
Introduction and importance
Thrombotic thrombocytopenic purpura (TTP) is a rare disease due to deficiency of ADAMTS13 which can present with anemia and thrombocytopenia. The study highlights the role of PLASMIC score in diagnosis and rituximab in the treatment of this condition.
Case presentation
Herein, we report a case of 38 years old female who had presented with fever, irritability, and altered sensorium. On investigations, she had hemolytic anemia, and thrombocytopenia with peripheral blood smear showing occasional schistocytes and managed with steroids and plasma exchange. As her platelet, LDH, and a few other lab parameters failed to normalize and met the criteria of refractory TTP, hence she was started on 5 cycles of rituximab and her condition improved.
Clinical discussion
Thrombotic thrombocytopenic purpura can be presumed based upon PLASMIC score where if the score is 5 or more while ADAMTS13 assay is required for confirmation. It is a life-threatening condition where treatment options include therapeutic plasma exchange (PEX), glucocorticoids, Rituximab, and caplacizumab. Rituximab is considered particularly in refractory cases.
Conclusion
Thrombotic thrombocytopenic purpura can lead to complications due to low platelet counts. Hence, early diagnosis and intervention are crucial to prevent such complications.
Keywords: Anemia, PLASMIC score, Rituximab, Thrombocytopenia, Thrombotic thrombocytopenic purpura
Highlights
-
•
Thrombotic thrombocytopenic purpura is a rare disease due to deficiency of ADAMTS13 where patients can present with anemia and thrombocytopenia.
-
•
PLASMIC score can be used for presumptive diagnosis of this condition while ADAMTS13 assay is required for confirmation.
-
•
Steroids and plasma exchange are the preferred treatment.
-
•
In case of refractory cases, Rituximab can play a role to treat as well as prevent recurrence.
1. Introduction
Thrombotic Thrombocytopenic Purpura (TTP) is rare thrombotic microangiopathy caused by deficiency of ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) resulting in the formation of microvascular platelet-rich thrombi [1,2]. It can be inherited (genetic mutation) or acquired (anti-ADAMTS13 autoantibodies). The latter is further classified into primary with no apparent cause identified and secondary when it is associated with infections, drugs, and autoimmune conditions [[1], [2], [3]].
Thrombocytopenia and microangiopathic hemolytic anemia are consistent features of TTP during presentation whereas clinical manifestations of organ ischemia/injury can be variable. Earlier on, pentad of anemia, thrombocytopenia, fever, and neurological and renal dysfunction was required for the definition of TTP [4]. However, < 10% has all the five features simultaneously and the presence of only anemia and thrombocytopenia should raise high suspicion for TTP(1) [5] [6].
Herein, we have presented a case of TTP in a 38-year-old female, who had a clinical improvement following 8 days of daily therapeutic plasma exchange (PEX) and steroids. However, her platelet, LDH, and a few other lab parameters failed to normalize. Thereafter 5 cycles of Rituximab were initiated which later normalized her lab parameters. The study also highlights the role of PLASMIC scores in making a presumptive diagnosis of TTP where ADAMTS13 assay is not available as well as starting Rituximab therapy to treat this life-threatening condition. The case has been reported as per SCARE guidelines [7].
2. Case presentation
38 years female, a known case of diabetes mellites, presented to the emergency department via ambulance with complaints of altered level of consciousness, abdominal pain, fever, vomiting, and irritability for 3 days.
On examination, she looked ill and icteric and had mucosal pallor. Her blood pressure was elevated (150/80 mm Hg) with a temperature of 99 F. At presentation, her findings were Hb 9.3, platelets 25,000 with normal leucocytes count. Random blood sugar was 263 mg/dl and renal function showed urea and creatinine to be 95/1.33 respectively. Routine urine examination showed plenty of RBCs most likely due to menstruation. Arterial blood gas analysis was non-revealing. D-dimer level was normal. She was started on empiric therapy with Inj piperacillin/tazobactam, paracetamol, and iv fluids. Therein, her condition deteriorated soon with GCS 9 (E2, V1, M6) with SpO2 in the range of 80–90% in room air. So, with oxygen therapy, she was transferred to ICU. Medical and hematology consultations were done and her cause for bicytopenia was evaluated.
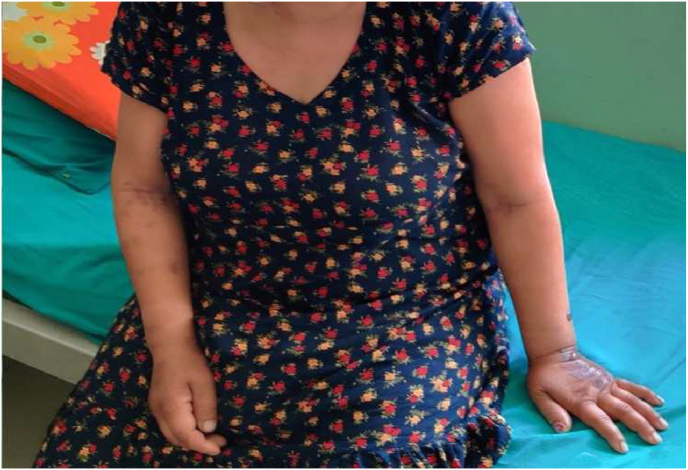
The following day, her temperature reached 104F and she developed one episode of generalized tonic-clonic seizures. Lots of petechiae were scattered and noticeable over her bilateral upper limbs as shown in Fig. 1. Her GCS was still 9/15 while platelet counts decreased to 7000. ANA level to look for autoimmune disease was >500, liver function test revealed increased bilirubin levels (Total bilirubin-6.47, direct bilirubin- 1.24), and peripheral blood smear revealed occasional schistocytes with mild polychromasia. Her LDH level was 294 IU/l and her INR was 1.4. Based on clinical and laboratory parameters a diagnosis of thrombotic thrombocytopenic purpura (TTP) was made. Differentials included immune thrombocytopenia and disseminated intravascular coagulation, however her D-dimers and bicytopenia ruled them out.
Fig. 1.
Multiple petechiae and ecchymosis noted over bilateral upper limbs.
On the 3rd day of admission, her GCS deteriorated to 3/15 with episodes of nasal and oral bleeding. She was transfused with III pints of packed RBCs and II pints of platelet-rich platelet for her decreasing hemoglobin and platelets levels respectively. In addition, PEX along with methylprednisolone was started and continued daily for 8 days.
Gradually her GCS started to improve and she was started on oral feed. At that time CT scan of the head revealed calcified granuloma in the left frontal lobe. Her platelet level began to rise (75,0000 and her hemoglobin level gradually improved however, they never reached a satisfactory level. Owing to sub-optimal response to therapies during her hospital stay, she was labeled as refractory TTP as well as to prevent recurrence of the condition, Rituximab was planned for five cycles (total calculated dose of 660mg IV).
After her 5 cycles, there was a significant improvement in her blood parameters. Currently, her hemoglobin level is 9, and her platelet count is 152,000. Renal function and ANA levels reached baseline values and petechiae disappeared. Thus, Rituximab helped her in achieving remission, and through her follow-up a month later or in between, she will be observed whether she develops recurrence in terms of clinical or laboratory parameters or not.
3. Clinical discussion
Rituximab is a monoclonal antibody that binds CD20 on B-cells [8]. Although initially developed for the treatment of lymphoma, it is now being more widely used for the treatment of autoimmune diseases like systemic lupus erythematosus, antiphospholipid syndrome, rheumatoid arthritis, granulomatosis with polyangiitis and other antineutrophil cytoplasmic antibody-associated vasculitides [8]. Further, it has also been used in the treatment of TTP [2,8,9]. About TTP, it is used in the treatment of acute and severe cases of TTP [10], along with refractory cases of TTP [11] and TTP that have shown a suboptimal response [5,12]. It has also been shown to hasten the recovery process and decrease the frequency of relapses.
TTP is a life-threatening disease and timely intervention even with PEX can improve survival from about 10 to 80–90%. Therapies available for TTP include therapeutic plasma exchange(PEX), glucocorticoids, Rituximab, and caplacizumab [2]. For a suspected TTP, ADAMTS13 is the recommended next step in the diagnosis [1]. However, due to the unavailability of the ADAMTS 13 activity assay in our region, a presumptive diagnosis of TTP with a PLASMIC score was made in our TTP case.
PLASMIC Score incorporates clinical and laboratory testing results and is calculated by summating 1 point each for the following criteria: thrombocytopenia, anemia, lack of cancer, organ or hematopoietic stem cell transplant, macrocytosis, coagulopathy, and renal failure [13]. PLASMIC Score also indirectly measures ADAMTS13 activity with a higher score associated with a higher probability of TTP (ADAMTS 13 activity, 10%). A PLASMIC score of ≥5 has a sensitivity of 99% and a specificity of 57% whereas a score of ≥6 has a sensitivity of 85% and specificity of 89% [13]. In our case, the patient had anemia with raised indirect bilirubin, thrombocytopenia, INR of 1.4, creatinine of 1.33, and no history of active cancer or organ transplant. Thus, the diagnosis of TTP was made in our patient based on the PLASMIC score.
Plasma exchange therapy with steroids was soon initiated and continued for a month. Although the patient made a gradual clinical improvement, her platelet count and LDH failed to reach a normal level. A decision was then made to start a rituximab therapy since a complete clinical response was not seen. A clinical response is achieved when platelet count normalizes to greater than 1,50,000 per microliter and LDH level falls to <1.5 × the upper limit of normal. In contrast, refractory TTP is defined when there is persistent thrombocytopenia (<50 × 109/L) and elevated LDH level even after 5 cycles of PEX and glucocorticoid treatment [1,3].
4. Conclusions
Thrombotic thrombocytopenic purpura can present with various complications and hence early diagnosis and treatment are crucial. Rituximab can be considered in cases when they are refractory to plasma exchange therapy and steroids.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Please state any conflicts of interest
None.
Please state any sources of funding for your research
None.
Ethical approval
N/A.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Author 1: Led data collection, concept of study, and literature review.
Author 2: Literature review, revising and editing the manuscript.
Author 3: Literature review, data collection, revising and editing the manuscript.
Author 4: Literature review, revising, and editing the manuscript into final version.
Author 5: Literature review, writing rough manuscript draft.
Author 6: Literature review, revising and editing the manuscript.
All authors were involved in manuscript drafting and revising, and approved the final version.
Registration of research studies
-
1.
Name of the registry: N/A.
-
2.
Unique Identifying number or registration ID: N/A.
-
3.
Hyperlink to your specific registration (must be publicly accessible and will be checked): N/A.
Guarantor
Saurab Karki, Military Hospital, Itahari-4 Sunsari, Nepal. Email: saurabkarki1010@gmail.com, Phone: +977–9841098336.
Acknowledgement
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2022.104789.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sukumar S., Lämmle B., Cataland S.R. Thrombotic thrombocytopenic purpura: pathophysiology, diagnosis, and management. J. Clin. Med. 2021 Feb 2;10(3):536. doi: 10.3390/jcm10030536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knöbl P. Thrombotic thrombocytopenic purpura. Memo. 2018;11(3):220–226. doi: 10.1007/s12254-018-0429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scully M., Cataland S., Coppo P., de la Rubia J., Friedman K.D., Kremer Hovinga J., et al. Consensus on the standardization of terminology in thrombotic thrombocytopenic purpura and related thrombotic microangiopathies. J Thromb Haemost JTH. 2017 Feb;15(2):312–322. doi: 10.1111/jth.13571. [DOI] [PubMed] [Google Scholar]
- 4.Amorosi E.L., Ultmann J.E. Thrombotic thrombocytopenic purpura: report of 16 cases and review of the literature. Medicine (Baltim.) 1966 Mar;45(2):139–160. [Google Scholar]
- 5.Joly B.S., Coppo P., Veyradier A. Thrombotic thrombocytopenic purpura. Blood. 2017 May 25;129(21):2836–2846. doi: 10.1182/blood-2016-10-709857. [DOI] [PubMed] [Google Scholar]
- 6.Mariotte E., Azoulay E., Galicier L., Rondeau E., Zouiti F., Boisseau P., et al. Epidemiology and pathophysiology of adulthood-onset thrombotic microangiopathy with severe ADAMTS13 deficiency (thrombotic thrombocytopenic purpura): a cross-sectional analysis of the French national registry for thrombotic microangiopathy. Lancet Haematol. 2016 May 1;3(5):e237–e245. doi: 10.1016/S2352-3026(16)30018-7. [DOI] [PubMed] [Google Scholar]
- 7.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A., Thoma A., et al. The SCARE 2020 guideline: updating Consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020 Dec 1;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 8.Hanif N., Anwer F. StatPearls [Internet]. Treasure Island (FL) StatPearls Publishing; 2022. Rituximab.http://www.ncbi.nlm.nih.gov/books/NBK564374/ [cited 2022 Jul 30]. Available from: [Google Scholar]
- 9.Jasti S., Coyle T., Gentile T., Rosales L., Poiesz B. Rituximab as an adjunct to plasma exchange in TTP: a report of 12 cases and review of literature. J. Clin. Apher. 2008;23(5):151–156. doi: 10.1002/jca.20172. [DOI] [PubMed] [Google Scholar]
- 10.Chemnitz J., Draube A., Scheid C., Staib P., Schulz A., Diehl V., et al. Successful treatment of severe thrombotic thrombocytopenic purpura with the monoclonal antibody rituximab. Am. J. Hematol. 2002 Oct;71(2):105–108. doi: 10.1002/ajh.10204. [DOI] [PubMed] [Google Scholar]
- 11.Efficacy and safety of rituximab in Japanese patients with acquired thrombotic thrombocytopenic purpura refractory to conventional therapy. Int. J. Hematol. 2016 Aug;104(2):228–235. doi: 10.1007/s12185-016-2019-x. [DOI] [PubMed] [Google Scholar]
- 12.Froissart A., Buffet M., Veyradier A., Poullin P., Provôt F., Malot S., et al. Efficacy and safety of first-line rituximab in severe, acquired thrombotic thrombocytopenic purpura with a suboptimal response to plasma exchange. Experience of the French Thrombotic Microangiopathies Reference Center. Crit. Care Med. 2012 Jan;40(1):104–111. doi: 10.1097/CCM.0b013e31822e9d66. [DOI] [PubMed] [Google Scholar]
- 13.Paydary K., Banwell E., Tong J., Chen Y., Cuker A. Diagnostic accuracy of the PLASMIC score in patients with suspected thrombotic thrombocytopenic purpura: a systematic review and meta-analysis. Transfusion (Paris) 2020 Sep;60(9):2047–2057. doi: 10.1111/trf.15954. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.