Abstract
Objectives
This study aimed to evaluate and compare the cell viability, differentiation potential and anti-inflammatory potential of propolis and Biodentine™ on stem cells isolated from human exfoliated deciduous teeth (SHED).
Materials and methods
SHED were segregated and cultured from the dental pulp of children after therapeutic extraction. Microculture Tetrazolium Assay (MTT) assay was carried out for assessing cell proliferation potential of propolis and Biodentine at different concentrations. As per the results from cell proliferation assay, cell differentiation potential of SHED was evaluated at concentration of 12.5 μg/ml using Alizarin Red staining. The anti-inflammatory potential of test materials was evaluated using gelatin zymography by detecting MMP-2 and MMP-9.
Results
The maximum cell proliferation percentage of SHED treated with propolis and Biodentine was observed at a concentration of 12.5 μg/ml, on day 7, 14 and 21 with Biodentine having maximum cell proliferation potential followed by propolis. SHED treated with Biodentine showed maximum cell differentiation on day 7 (107.16), 14 (106.29) and 21 (107.72). However, anti-inflammatory activity against MMP-2 was 95 % with propolis and 85 % with Biodentine and whereas, against MMP-9 it was 65 % for propolis and 47 % for Biodentine.
Conclusion
Propolis shows comparable cell viability, cell proliferation and differentiation potential on SHED when compared to Biodentine. It also exhibits better invitro anti-inflammatory activity on SHED compared to Biodentine. Further studies are warranted to validate the application of propolis as an effective and economical alternative biocompatible agent to Biodentine for vital pulp therapies.
Keywords: Propolis, Biodentine, Stem-cells, Immunofluorescence Assay, MTT Assay, Gelatin Zymography
1. Introduction
Stem cells from human exfoliated deciduous teeth (SHED) have a high potential to differentiate into numerous specialised cells (Abdullah, 2013) compared to other dental stem cells such as dental pulp stem cells (DPSC) and Bone marrow mesenchymal stem cells (BMMSC) (Nakamura et al., 2009). Therapeutic approaches involving such cells have enormous potential for reconstructing tissues, including structured native pulp with the highly organised pattern of normal dental pulp tissues. Such therapies are required when normal pulpal integrity is compromised due to dental caries, trauma, or iatrogenic pulp damages (Chen et al., 2020, Sui et al., 2019).
Owing to their bio-inductive properties calcium silicate-based materials (CSBM) such as Mineral Trioxide Aggregate (MTA) and Biodentine can maintain the vitality of exposed pulp via deposition of hard tissues (Petta et al., 2020). Biodentine has demonstrated superior physical and biological properties (Rajasekharan et al., 2017). However, it has also shown intense cytotoxicity and negative influence on the pulp tissues at the area of contact, compromising the viability of the native cells due to apoptosis and necrosis (Küçükkaya et al., 2016). Such unfavourable fallouts led to an attempt to identify natural, biocompatible materials with similar bio-inductive properties.
Propolis, a derivative from honey bees (Apis mellifera), is one such natural biomaterial. Several studies documented its applications in dentistry (Al-Shaher et al., 2004, Bruschi et al., 2006, Esmeraldo et al., 2013, Gjertsen et al., 2011, Hayacibara et al., 2005), including anti-microbial and cariostatic properties. Propolis is also used as an intracanal irrigant and wound healing material. It has also demonstrated promising results as a vital pulp therapy agent in animal teeth (Parolia et al., 2010a, Parolia et al., 2010b). Although numerous biofunctional properties of propolis have been identified, limited data are available regarding the responses of SHED after direct contact with propolis and Biodentine. Therefore, the present study evaluate cell viability, cell differentiation, and anti-inflammatory potential of propolis and Biodentine on SHED through a direct contact method using various assays.
2. Materials and methods
After obtaining clearance from the institutional ethics committee, this study was conducted at the Department of Pediatric and Preventive Dentistry, MIDSR Dental College, Latur, Maharashtra, India and the Department of Molecular Biology and Immunology, Maratha Mandal Dental College and Research Centre, Belgaum, Karnataka, India.
2.1. Collection, isolation, and culture of SHED
Under strict aseptic conditions, SHED were obtained from freshly extracted, clinically healthy primary teeth collected from children (aged 8 to 12 years) after obtaining written and informed consent from the parents or guardians, following the institutional ethics committee protocols. The pulp tissue was extirpated through the access opening at the cemento-enamel junction or the open sub-pulpal wall (Athanasiadou et al., 2018), followed by suspension into screw-capped test tubes containing Dulbecco’s Modified Eagle’s medium (DMEM). The pulp tissue was then digested in phosphate-buffered saline (PBS) containing 3 mg/mL collagenase type I and 4 mg/mL diphase II and incubated for 60 min at 37 °C till the release of individual cells from the tissue. The cell cultures were re-suspended in 5–10 mL PBS followed by 5 mL of DMEM to obtain single-cell suspensions and again incubated at 37 °C in 5 % CO2 in 24-well microtiter plates (Goorha and Reiter, 2017). The outgrown cells were subcultured in the DMEM growth medium supplemented with FSB, l-glutamine, penicillin, streptomycin, and amphotericin (PSA) antibiotic solution, followed by incubation at 37 °C in a humidified atmosphere. The cells derived after the fifth passage were subcultured at a ratio of 1:4.
2.2. Preparation of test materials
In this study, SHED cultured and maintained in DMEM were used as the negative control. Whereas, cells in the osteoinduction medium were used as the positive control. Biodentine and the ethanolic extract of propolis were used as the test materials. Biodentine was mixed as per the manufacturer’s instructions, and the ethanolic extract of propolis was prepared by refluxing with 70 % ethanol for 1 h (Park and Ikegaki, 1998). Predetermined concentrations of premixed Biodentine and ethanolic extract of propolis were added to the culture medium.
2.3. Stem cell identification and characterisation by immunofluorescence assay
Cultured SHED were identified and characterised using antibody markers CD73, CD90, and CD105 conjugated to fluorophores using flow cytometry (Tomás-Catalá et al., 2018, Yu et al., 2016). Cells were seeded in a 24-well flat-bottom microplate and incubated overnight. Further, SHED were fixed in 4 % paraformaldehyde for 30 min before PBS wash. Thereafter, the cells were treated with 100 µl of blocking solution for 30 min, followed by PBS wash. Finally, a single drop of primary antibodies CD73, CD90, and CD105 was added to these cells, followed by incubation for 1 h. After the last PBS wash, the cells were examined under the fluorescence microscope at 20X magnification (Kumbar et al., 2020).
2.4. Cell proliferation ability using MTT assay
The proliferation rate of negative control and experimental group cells (treated with bio-inductive materials) was assessed using MTT assay on the 7th, 14th, and 21st days. All the cells were seeded in a 96-well flat-bottom microplate and incubated at 37 °C and 5 % CO2 overnight. The experimental group cells were treated with different concentrations of test materials (viz. 200, 100, 50, 25, 12.5, 6.125 µg/mL). After a double PBS wash, the cells were added with 20 µl of MTT staining solution to each well, followed by incubation at 37 °C for 4 h. A hundred microliters of Dimethyl sulfoxide (DMSO) were added to these cells to dissolve the formazan crystals. The absorbance of the viable cells was measured at 570 nm (Abs570) with an automatic microplate reader (Dahake et al., 2021).
Cell viability was calculated using the following formula:
2.5. Cell differentiation potential using Alizarin red staining assay
Cell differentiation potential of the test materials on SHED was determined using Alizarin red staining assay on 7th, 14th, and 21st days. SHED treated with the control and test groups were maintained at 37 °C in 95 % humidity and 5 % CO2 overnight. After incubation, the cells were washed and fixed with 95 % ethanol for 15 min at 4 °C. Further, the cells were stained with 2 % Alizarin red S (pH 4.1 – 4.3) for 15 min. After staining, the absorbance of the stain was measured by a spectrophotometer at 415 nm (Kukreja et al., 2021).
2.6. Anti-inflammatory activity using gelatin zymography
Preparation of Matrix metalloproteinases (MMP) samples: 5 mL of tris buffer was added to the chopped tissue samples and centrifuged at 6000 rpm for 30 min at 4 °C to obtain the supernatant.
Preparation of extract: 50 µl of test materials were added separately to 50 µl of MMP sample and incubated for 1 h. 50 µl of MMP and 50 µl of tetracycline HCL were taken as the positive control, whereas 50 µl of MMP sample was taken as the negative control.
Gel Electrophoresis: Aliquots of 20 µl of the prepared sample of test materials was mixed with sample buffer in equal proportion and subjected to electrophoresis in the gel containing 10 % sodium dodecyl sulfate–polyacrylamide and 0.1 % gelatin. Further, the gel was removed and washed with 2.5 % Triton x-100 (Kudalkar et al., 2014). The zymogen renaturing buffer was removed carefully, and the gel was incubated at 37 °C overnight. The gels were then stained with Coomassie blue R-250 and destained with Acoomassie R-250 to visualise gelatinolytic activity in the zymogram. After staining, the clear zones were scanned to measure the relative MMP-2 and MMP-9 expression levels. The lower bands were gelatinases-A (MMP-2), and the upper bands were gelatinases-B (MMP-9) (Tajhya et al., 2017).
2.7. Statistical analysis
All assays were performed in triplicate. Statistical analysis of the results of the experiments was performed using descriptive statistics, and the data were expressed as mean and standard deviation. One-way ANOVA test was performed to compare measurements of mean among the groups. All the results were considered significant with a probability p-value < 0.05. A Post hoc test was applied to analyse multiple pairwise individual comparisons among the groups.
3. Results
3.1. Stem cell identification and characterisation by immunofluorescence assay
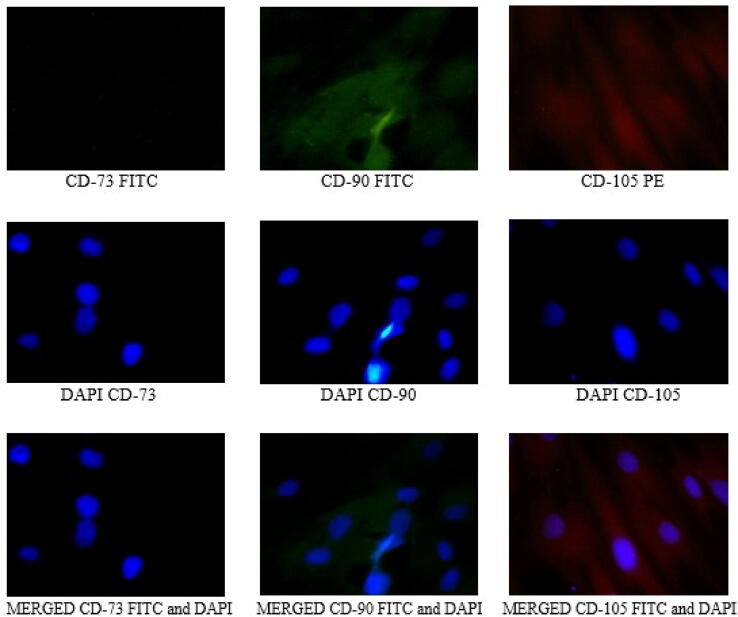
Immunofluorescence assay was performed using the fluorescein isothiocyanate (FITC)-conjugated antibody markers CD73, CD90, and phycoerythrin (PE)-conjugated CD105. The nuclei were stained with the counter-stain DAPI, shows the distribution pattern of fluorescent immunostaining that confirmed the presence of CD73+, CD90+, and CD105 + cells (see Fig. 1).
Fig. 1.
Stem cells from human exfoliated deciduous teeth (SHED) characterization using Immunofluorescence assay.
3.2. Cell proliferation ability using MTT assay
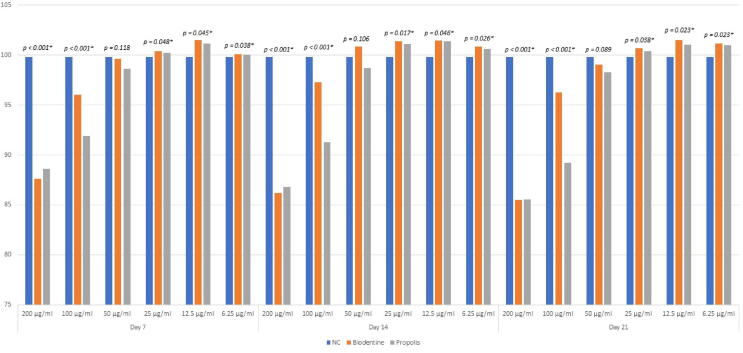
One way ANOVA was used to compare test cell viability (%) among three groups, and a statistically significant difference (p < 0.05) was found between three groups at a concentration of 200, 100, 25,12.5, and 6.25 μg/mL on the 7th, 14th, and 21st days (Fig. 3). However, a statistically nonsignificant difference was found among three groups at a concentration of 50 μg/mL (p > 0.05) on the 7th, 14th, and 21st days. The highest cell proliferation for Biodentine and propolis was observed at a concentration of 12.5 μg/mL on the 7th, 14th, and 21st days, which was statistically significant (p < 0.05) (see Fig. 2, Fig. 3).
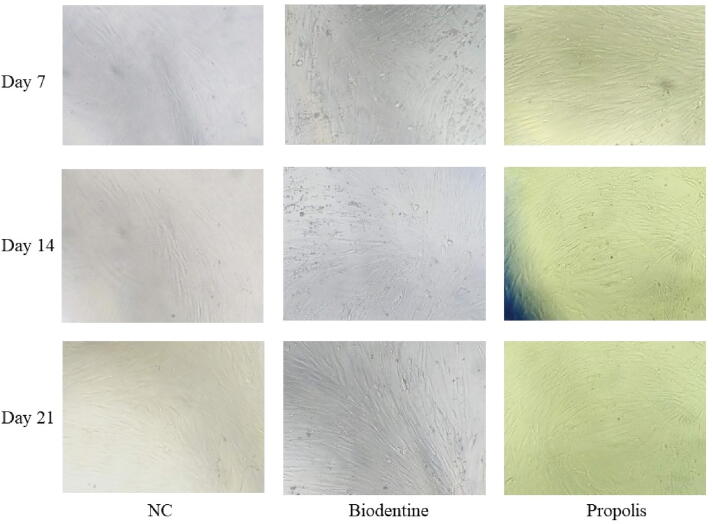
Fig. 3.
MTT assay of SHED treated with (a) Negative control, (b) Biodentine, (c) Propolis on day 7, 14 and 21.
Fig. 2.
Cell proliferation potential of SHED treated with test materials at different concentrations using MTT assay on day 7, 14 and 21.
3.3. Cell differentiation potential using Alizarin red staining assay
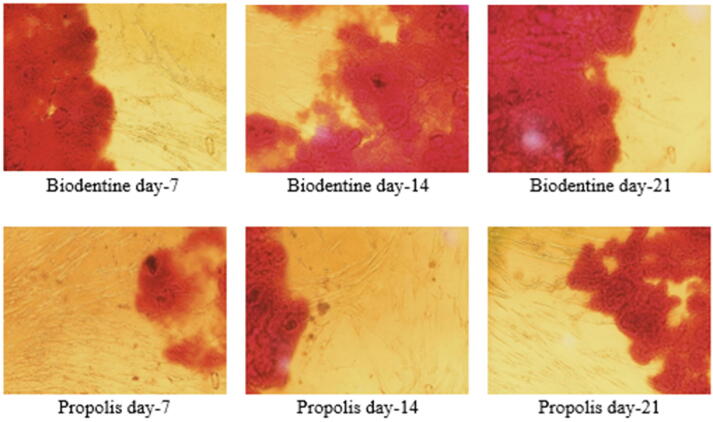
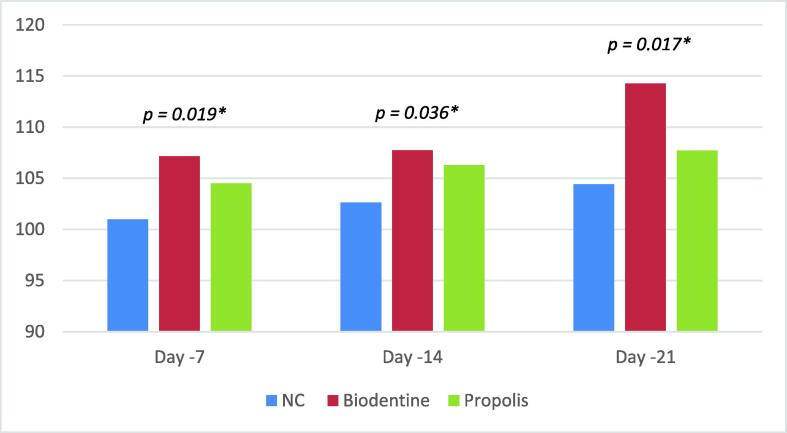
Significant formation of the mineralized nodule was verified using Alizarin red staining. Cell cultured with Biodentine showed the highest osteoinduction/odonto-induction capacity, closely followed by propolis. Fig. 5 shows cell differentiation results of all test groups by staining cells with Alizarin red stain to identify Ca2+ deposits. At 12.5 μg/mL a highly significant difference was noted among all the groups on the 7th (p = 0.019), 14th (p = 0.036), and 21st (p = 0.017) days (see Fig. 4).
Fig. 5.
Cell differentiation potential of SHED treated with test materials using Alizarin red staining assay.
Fig. 4.
Cell differentiation potential of SHED treated with test materials using Alizarin red staining assay.
3.4. Anti-inflammatory activity using gelatin zymography
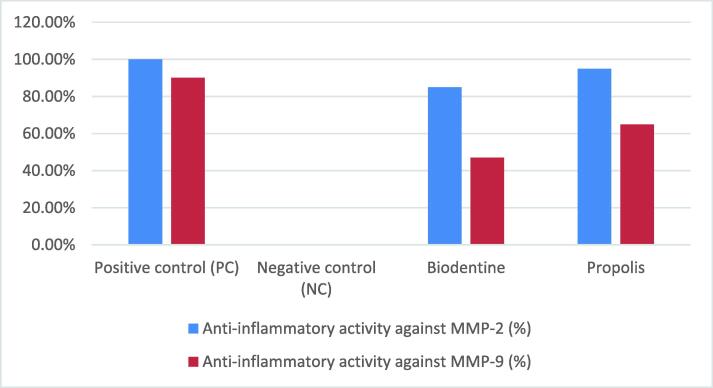
Gelatin zymography was used to characterize MMPs and found that both materials enhanced MMP-2 and MMP-9. Propolis, Biodentine, positive control, and negative control showed appearance of 5 %, 15 %, 0 %, and 100 % MMP-2 bands respectively, which corresponds to 95 %, 85 %, 100 %, and 0 % of anti-inflammatory activity against MMP-2. Similarly, propolis, Biodentine, positive control, and negative control showed the appearance of 35 %, 35 %, 10 %, and 100 % MMP-9 bands, respectively, which corresponds to 65 %, 65 %, 90 %, and 0 % of anti-inflammatory activity against MMP-9. (Fig. 6).
Fig. 6.
Anti-inflammatory activity against MMP-2 and MMP-9.
4. Discussion
Different biomaterials are used in vital pulp therapies to preserve injured dental pulp to promote pulp form, function, and health. These biomaterials create a microenvironment that promotes dentin formation. However, the outcomes may vary and can be attributed to pulp state, host immune response (Leprince et al., 2012), and associated bacterial virulence (Coil et al., 2004). Therefore, these materials must be practically easy to use, non-hazardous and effective in stimulating dentin repair or regeneration.
In the present study, cell viability, bioinductive and anti-inflammatory properties of readily available, low-cost biomaterial propolis were compared with Biodentine using MTT assay, Alizarin red staining, and gelatin zymography. As SHED shows a higher proliferation rate and possesses a higher ability to differentiate than DPSC, and BMMSCs, they were used in this study. In the present study stem cell were cultivated by suspending the cells in culture media following standard protocols. The confirmation of SHED was done by identification of positive markers for antibodies against human mesenchymal stem cells (MSC) with specific surface markers CD73, CD90, and CD105 (Charan and Kantharia, 2013).
MTT assay was performed to analyse the effects of propolis and Biodentine at different concentrations on cell viability and cell proliferation potential of SHED. In the present study, a significant difference in cell viability was noted between Biodentine and propolis among the test materials. The highest cell viability was observed at 12.5 µg for both, Biodentine. However, a subsequent reduction in cell viability as the concentration of test materials increased. This was following the study conducted by Chew Shi Fung (2015), where it was found that SHED were only viable when treated with the lowest concentration of propolis used in the study (0.005 mg/mL, 0.125 mg/mL, 0.25 mg/mL and 0.5 mg/mL) (Fung et al., 2015). In previous studies, Biodentine has shown good cell viability in DPSC (Hasweh et al., 2018, Zanini et al., 2012). Similarly, propolis has also been reported to enhance the proliferation of periodontal ligament fibroblast (Gjertsen et al., 2011) and SHED (Fung et al., 2015). The mechanism underlying the cell viability potential of propolis could be linked to the active compounds present in propolis like flavonoids (Huang et al., 2012) and caffeic acid phenethyl ester (CAPE), which have cytoprotective and DNA-protective effects in inflammatory pathologies (Duque et al., 2022, Wang et al., 2008).
Alizarin red staining was used to detect the differentiation potential of test materials on SHED. Biodentine promoted higher calcified nodule formation, closely followed by propolis. Propolis showed comparable results to Biodentine in the amount of calcified nodule formation throughout the 7th, 14th, and 21st days. This might be because of CAPE in propolis which attenuates osteoclastogenesis while protecting osteoblasts via suppression of RANKL/OPG signalling (Tolba et al., 2017). Propolis flavonoids have potent inhibitory activity against protein kinases in activated immune cells, promoting health promotion (Middleton and Kandaswami, 1992). In a study by Kuramoto et al., CAPE was capable of significantly inducing mRNA expression and production of VEGF in rat clonal odontoblast-like KN-3 cells. Simultaneously, VEGF increased the mineralisation activity in KN-3 cells. These findings suggested that CAPE might be useful as a novel biological material for dental pulp therapy (Kuramoto et al., 2019). Therefore, propolis and its active components are most likely responsible for osteogenic/odontogenic differentiation of SHED. The main components of Biodentine are ions such as Si, Ca, P, Zn, Mg, Cu, and Sr. All of them could be components of minerals produced either biologically or controlled induced mineralisation process (Weiner, 2003). Furthermore, they might have a significant role in biomineralisation, as recently demonstrated for Sr, Cu, Zn, and Mg (Huang et al., 2016, Jin et al., 2014, Qu et al., 2014, Rodríguez et al., 2002). A study by Lou et al. revealed that Biodentine significantly increased alkaline phosphate activity, OCN, DSPP, DMP1, BSP gene expression, and mineralizsed nodule formation (Luo et al., 2014). In another study, Araújo et al. selected DMP-1 as a differentiation marker for detecting the odontogenic potential of SHED with Biodentine and demonstrated a progressive increase in DMP-1 gene expression (Araújo et al., 2018). Several investigators studied the production of mineralised nodules in human DPSC cultured with Biodentine (Jung et al., 2015) and propolis (Kim et al., 2019). However, in the current study, SHED were used, and the results are similar to those reported in the previous one, indicating that Biodentine and propolis promote odontogenic differentiation of SHED. (Araújo et al., 2018).
MMP-2 and MMP-9 are released from cells in the form of zymogen, a proteolytically inactive preform that can be detected using zymography in cell-conditioned media. (Toth and Fridman, 2001). MMP-2 and MMP-9 are two MMPs that we are particularly interested in because they are produced by fibroblasts and pulp cells and have been associated with the pathogenesis of pulpal inflammation (Shin et al., 2002). In this study, Biodentine and propolis treatment reduced MMP-2, and MMP-9 production, and propolis, among the two compounds, has demonstrated excellent anti-inflammatory activity against MMP-2 (95 %) and MMP-9 (47 %). The inherent properties and micro-ingredients of test materials might be the reason behind the observed results. Biodentine possesses immunomodulatory properties by suppressing pro-inflammatory and augmenting anti-inflammatory cytokines (Eraković et al., 2020), whereas CAPE, different flavonoids including artepillin C in propolis, plays a key role in its anti-inflammatory action (Szliszka et al., 2013). Similarly, the anti-inflammatory properties of flavonoids are in accordance with the results obtained from Alipour M. et al., where plant flavonoids were shown their antioxidative and anti-inflammatory properties (Alipour et al., 2021).
5. Conclusion
Propolis has demonstrated comparable cell proliferation, differentiation potential as well as anti-inflammatory properties on SHED in-vitro when compared to Biodentine. However, the authors would like to recommend further in-vitro and in-vivo studies to evaluate other properties of propolis before concluding it as an effective, economical alternative biocompatible agent to Biodentine for vital pulp therapies.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
References
- Abdullah M.F. DPSCs and SHED in Tissue Engineering and Regenerative Medicine. TOSCJ. 2013;4:1–6. doi: 10.2174/1876893801304010001. [DOI] [Google Scholar]
- Alipour M., Pouya B., Aghazadeh Z., SamadiKafil H., Ghorbani M., Alizadeh S., Aghazadeh M., Dalir Abdolahinia E. The Antimicrobial, Antioxidative, and Anti-Inflammatory Effects of Polycaprolactone/Gelatin Scaffolds Containing Chrysin for Regenerative Endodontic Purposes. Stem Cells Int. 2021;2021 doi: 10.1155/2021/3828777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shaher A., Wallace J., Agarwal S., Bretz W., Baugh D. Effect of propolis on human fibroblasts from the pulp and periodontal ligament. J. Endod. 2004;30:359–361. doi: 10.1097/00004770-200405000-00012. [DOI] [PubMed] [Google Scholar]
- Araújo L.B., Cosme-Silva L., Fernandes A.P., de Oliveira T.M., Cavalcanti B.das N., Gomes Filho J.E., Sakai V.T. Effects of mineral trioxide aggregate, BiodentineTM and calcium hydroxide on viability, proliferation, migration and differentiation of stem cells from human exfoliated deciduous teeth. J. Appl. Oral Sci. 2018;26 doi: 10.1590/1678-7757-2016-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasiadou E., Paschalidou M., Theocharidou A., Kontoudakis N., Arapostathis K., Bakopoulou A. Biological interactions of a calcium silicate based cement (BiodentineTM) with Stem Cells from Human Exfoliated Deciduous teeth. Dent. Mater. 2018;34:1797–1813. doi: 10.1016/j.dental.2018.09.014. [DOI] [PubMed] [Google Scholar]
- Bruschi M.L., Lara E.H.G., Martins C.H.G., Vinholis A.H.C., Casemiro L.A., Panzeri H., Gremião M.P.D. Preparation and Antimicrobial Activityof Gelatin Microparticles Containing Propolis Against Oral Pathogens. Drug Dev. Ind. Pharm. 2006;32:229–238. doi: 10.1080/03639040500466312. [DOI] [PubMed] [Google Scholar]
- Charan J., Kantharia N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013;4:303–306. doi: 10.4103/0976-500X.119726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Fu H., Wu X., Duan Y., Zhang S., Hu H., Liao Y., Wang T., Yang Y., Chen G., Li Z., Tian W. Regeneration of pulpo-dentinal–like complex by a group of unique multipotent CD24a + stem cells. Sci. Adv. 2020;6:eaay1514. doi: 10.1126/sciadv.aay1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coil J., Tam E., Waterfield J. Proinflammatory Cytokine Profiles in Pulp Fibroblasts Stimulated with Lipopolysaccharide and Methyl Mercaptan. J. Endodont. 2004;30:88–91. doi: 10.1097/00004770-200402000-00006. [DOI] [PubMed] [Google Scholar]
- Dahake P.T., Baliga S.M., Kumbar V.M., Bhat K.G. Cytotoxicity of Novel Polymeric Gel Matrix Triple Antibiotic Paste—an In Vitro Study. Regen. Eng. Transl. Med. 2021;7:21–29. doi: 10.1007/s40883-020-00191-x. [DOI] [Google Scholar]
- Duque C., Hussein H., Bortolatto J., Prakki A., Kishen A. Effect of taxifolin and epigallocatechin-3-gallate on biomineralization potential of stem cells from dental apical papilla. Arch. Oral Biol. 2022;105413 doi: 10.1016/j.archoralbio.2022.105413. [DOI] [PubMed] [Google Scholar]
- Eraković M., Duka M., Bekić M., Tomić S., Ismaili B., Vučević D., Čolić M. Anti-inflammatory and immunomodulatory effects of Biodentine on human periapical lesion cells in culture. Int. Endod. J. 2020;53:1398–1412. doi: 10.1111/iej.13351. [DOI] [PubMed] [Google Scholar]
- Esmeraldo M.R.A., de Carvalho M.G.F., de Carvalho R.A., Lima R. de F., Costa E.M.M. de B. Inflammatory effect of green propolis on dental pulp in rats. Braz. Oral Res. 2013;27:417–422. doi: 10.1590/S1806-83242013005000022. [DOI] [PubMed] [Google Scholar]
- Fung C., Mohamad H., Hashim S., Htun A., Ahmad A. Proliferative Effect of Malaysian Propolis on Stem Cells from Human Exfoliated Deciduous Teeth: An In vitro Study. BJPR. 2015;8:1–8. doi: 10.9734/BJPR/2015/19918. [DOI] [Google Scholar]
- Gjertsen A.W., Stothz K.A., Neiva K.G., Pileggi R. Effect of propolis on proliferation and apoptosis of periodontal ligament fibroblasts. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011;112:843–848. doi: 10.1016/j.tripleo.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Goorha S., Reiter L.T. Culturing and Neuronal Differentiation of Human Dental Pulp Stem Cells. Curr. Protoc. Hum. Genet. 2017;92 doi: 10.1002/cphg.28. 21.6.1-21.6.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasweh N., Awidi A., Rajab L., Hiyasat A., Jafar H., Islam N., Hasan M., Abuarqoub D. Characterization of the biological effect of Biodentine TM on primary dental pulp stem cells. Indian J. Dent. Res. 2018;29:787. doi: 10.4103/ijdr.IJDR_28_18. [DOI] [PubMed] [Google Scholar]
- Hayacibara M.F., Koo H., Rosalen P.L., Duarte S., Franco E.M., Bowen W.H., Ikegaki M., Cury J.A. In vitro and in vivo effects of isolated fractions of Brazilian propolis on caries development. J. Ethnopharmacol. 2005;101:110–115. doi: 10.1016/j.jep.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Huang M., Hill R.G., Rawlinson S.C.F. Strontium (Sr) elicits odontogenic differentiation of human dental pulp stem cells (hDPSCs): A therapeutic role for Sr in dentine repair? Acta Biomater. 2016;38:201–211. doi: 10.1016/j.actbio.2016.04.037. [DOI] [PubMed] [Google Scholar]
- Huang X.-F., Yuan S.-J., Yang C. Effects of total flavonoids from Drynaria fortunei on the proliferation and osteogenic differentiation of rat dental pulp stem cells. Mol. Med. Rep. 2012;6:547–552. doi: 10.3892/mmr.2012.974. [DOI] [PubMed] [Google Scholar]
- Jin G., Cao H., Qiao Y., Meng F., Zhu H., Liu X. Osteogenic activity and antibacterial effect of zinc ion implanted titanium. Colloids Surf., B. 2014;117:158–165. doi: 10.1016/j.colsurfb.2014.02.025. [DOI] [PubMed] [Google Scholar]
- Jung J.-Y., Woo S.-M., Lee B.-N., Koh J.-T., Nör J.E., Hwang Y.-C. Effect of Biodentine and Bioaggregate on odontoblastic differentiation via mitogen-activated protein kinase pathway in human dental pulp cells. Int. Endod. J. 2015;48:177–184. doi: 10.1111/iej.12298. [DOI] [PubMed] [Google Scholar]
- Kim J.-H., Kim S.-Y., Woo S.-M., Jeong H.-N., Jung J.-Y., Kim S.-M., Lim H.-S. Combination of mineral trioxide aggregate and propolis promotes odontoblastic differentiation of human dental pulp stem cells through ERK signaling pathway. Food Sci. Biotechnol. 2019;28:1801–1809. doi: 10.1007/s10068-019-00609-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küçükkaya S., Görduysus M.Ö., Zeybek N.D., Müftüoğlu S.F. In Vitro Cytotoxicity of Calcium Silicate-Based Endodontic Cement as Root-End Filling Materials. Scientifica (Cairo) 2016;2016:9203932. doi: 10.1155/2016/9203932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudalkar M., Nayak A., Bhat K., Nayak R. Effect of Azadirachta indica (Neem) and Aloe vera as compared to subantimicrobial dose doxycycline on matrix metalloproteinases (MMP)-2 and MMP-9: An in-vitro study. Ayu. 2014;35:85. doi: 10.4103/0974-8520.141947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukreja B., Bhat K., Kukreja P., Kumber V., Balakrishnan R., Govila V. Isolation and immunohistochemical characterization of periodontal ligament stem cells: A preliminary study. J. Indian Soc. Periodontol. 2021;25:295. doi: 10.4103/jisp.jisp_442_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumbar V.M., Muddapur U.M., Bhat K.G., Shwetha H.R., Kugaji M.S., Peram M.R. Indirect Immunofluorescence and Tumorspheres Enrichment Technique for Identifying Cancer Stem Cell Markers in Cancer Cell Lines From Primary Oral Cancer Tissues: An In Vitro Study. J. Adv. Oral Res. 2020;11:224–230. doi: 10.1177/2320206820941379. [DOI] [Google Scholar]
- Kuramoto H., Hirao K., Yumoto H., Hosokawa Y., Nakanishi T., Takegawa D., Washio A., Kitamura C., Matsuo T. Caffeic Acid Phenethyl Ester (CAPE) Induces VEGF Expression and Production in Rat Odontoblastic Cells. Biomed Res. Int. 2019;2019:5390720. doi: 10.1155/2019/5390720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leprince J.G., Zeitlin B.D., Tolar M., Peters O.A. Interactions between immune system and mesenchymal stem cells in dental pulp and periapical tissues: Immune system and MSCs interactions. Int. Endod. J. 2012;45:689–701. doi: 10.1111/j.1365-2591.2012.02028.x. [DOI] [PubMed] [Google Scholar]
- Luo Z., Kohli M.R., Yu Q., Kim S., Qu T., He W. Biodentine Induces Human Dental Pulp Stem Cell Differentiation through Mitogen-activated Protein Kinase and Calcium-/Calmodulin-dependent Protein Kinase II Pathways. J. Endodont. 2014;40:937–942. doi: 10.1016/j.joen.2013.11.022. [DOI] [PubMed] [Google Scholar]
- Middleton E., Kandaswami C. Effects of flavonoids on immune and inflammatory cell functions. Biochem. Pharmacol. 1992;43:1167–1179. doi: 10.1016/0006-2952(92)90489-6. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Yamada Y., Katagiri W., Sugito T., Ito K., Ueda M. Stem cell proliferation pathways comparison between human exfoliated deciduous teeth and dental pulp stem cells by gene expression profile from promising dental pulp. J. Endod. 2009;35:1536–1542. doi: 10.1016/j.joen.2009.07.024. [DOI] [PubMed] [Google Scholar]
- Park Y.K., Ikegaki M. Preparation of Water and Ethanolic Extracts of Propolis and Evaluation of the Preparations. Biosci. Biotechnol. Biochem. 1998;62:2230–2232. doi: 10.1271/bbb.62.2230. [DOI] [PubMed] [Google Scholar]
- Parolia A., Thomas M.S., Kundabala M., Mohan M. Propolis and its potential uses in oral health. Int. J. Med. Medical Sci. 2010;2:210–215. [Google Scholar]
- Parolia A., Thomas M., Mala K., Mohan M. Propolis and its potential uses in oral health. Int. J. Med. Medical Sci. 2010;2:210–215. [Google Scholar]
- Petta T.de M., Pedroni A.C.F., Saavedra D.F., Faial K.do C.F., Marques M.M., Couto R.S.D. The effect of three different pulp capping cements on mineralization of dental pulp stem cells. Dent. Mater. J. 2020;39:222–228. doi: 10.4012/dmj.2018-349. [DOI] [PubMed] [Google Scholar]
- Qu T., Jing J., Jiang Y., Taylor R.J., Feng J.Q., Geiger B., Liu X. Magnesium-Containing Nanostructured Hybrid Scaffolds for Enhanced Dentin Regeneration. Tissue Eng. Part A. 2014;20:2422–2433. doi: 10.1089/ten.tea.2013.0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekharan S., Martens L.C., Vandenbulcke J., Jacquet W., Bottenberg P., Cauwels R.G.E.C. Efficacy of three different pulpotomy agents in primary molars: a randomized control trial. Int. Endod. J. 2017;50:215–228. doi: 10.1111/iej.12619. [DOI] [PubMed] [Google Scholar]
- Rodríguez J.P., Ríos S., González M. Modulation of the proliferation and differentiation of human mesenchymal stem cells by copper. J. Cell. Biochem. 2002;85:92–100. [PubMed] [Google Scholar]
- Shin S., Lee J., Baek S., Lim S. Tissue Levels of Matrix Metalloproteinases in Pulps and Periapical Lesions. J. Endodont. 2002;28:313–315. doi: 10.1097/00004770-200204000-00013. [DOI] [PubMed] [Google Scholar]
- Sui B., Chen C., Kou X., Li B., Xuan K., Shi S., Jin Y. Pulp Stem Cell-Mediated Functional Pulp Regeneration. J. Dent. Res. 2019;98:27–35. doi: 10.1177/0022034518808754. [DOI] [PubMed] [Google Scholar]
- Szliszka E., Kucharska A.Z., Sokół-Łętowska A., Mertas A., Czuba Z.P., Król W. Chemical Composition and Anti-Inflammatory Effect of Ethanolic Extract of Brazilian Green Propolis on Activated J774A.1 Macrophages. Evidence-Based Complement. Alternative Med. 2013;2013:1–13. doi: 10.1155/2013/976415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajhya R.B., Patel R.S., Beeton C. In: Matrix Metalloproteases, Methods in Molecular Biology. Galea C.A., editor. Springer; New York, New York, NY: 2017. Detection of Matrix Metalloproteinases by Zymography; pp. 231–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolba M.F., El-Serafi A.T., Omar H.A. Caffeic acid phenethyl ester protects against glucocorticoid-induced osteoporosis in vivo : Impact on oxidative stress and RANKL/OPG signals. Toxicol. Appl. Pharmacol. 2017;324:26–35. doi: 10.1016/j.taap.2017.03.021. [DOI] [PubMed] [Google Scholar]
- Tomás-Catalá C.J., Collado-González M., García-Bernal D., Oñate-Sánchez R.E., Forner L., Llena C., Lozano A., Moraleda J.M., Rodríguez-Lozano F.J. Biocompatibility of New Pulp-capping Materials NeoMTA Plus, MTA Repair HP, and Biodentine on Human Dental Pulp Stem Cells. J. Endod. 2018;44:126–132. doi: 10.1016/j.joen.2017.07.017. [DOI] [PubMed] [Google Scholar]
- Toth M., Fridman R. Metastasis Research Protocols. Humana Press; New Jersey: 2001. Assessment of Gelatinases (MMP-2 and MMP-9) by Gelatin Zymography; pp. 163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Chen L., Wu W., Long Y., Wang R. Potential cytoprotection: antioxidant defence by caffeic acid phenethyl ester against free radical-induced damage of lipids, DNA, and proteins. Can. J. Physiol. Pharmacol. 2008;86:279–287. doi: 10.1139/Y08-029. [DOI] [PubMed] [Google Scholar]
- Weiner S. An Overview of Biomineralization Processes and the Problem of the Vital Effect. Rev. Mineral. Geochem. 2003;54:1–29. doi: 10.2113/0540001. [DOI] [Google Scholar]
- Yu F., Dong Y., Yang Y., Lin P., Yu H., Sun X., Sun X.-F., Zhou H., Huang L., Chen J. Effect of an Experimental Direct Pulp-capping Material on the Properties and Osteogenic Differentiation of Human Dental Pulp Stem Cells. Sci. Rep. 2016;6:34713. doi: 10.1038/srep34713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanini M., Sautier J.M., Berdal A., Simon S. Biodentine Induces Immortalized Murine Pulp Cell Differentiation into Odontoblast-like Cells and Stimulates Biomineralization. J. Endodont. 2012;38:1220–1226. doi: 10.1016/j.joen.2012.04.018. [DOI] [PubMed] [Google Scholar]