Abstract
Secretory leukocyte protease inhibitor (SLPI) has been found to possess activity against the human immunodeficiency virus type 1 (HIV-1) in vitro at physiological concentrations. A study was undertaken to evaluate SLPI levels in human saliva and plasma among HIV-positive (HIV+) patients with various HIV-1 viral loads in comparison to uninfected controls. Whole blood in EDTA and unstimulated saliva samples were collected from 37 HIV+ patients, of whom 20 had a history of intravenous drug abuse (IVDA). Control samples were collected from 20 appropriate age- and sex-matched HIV-1-negative individuals. SLPI was estimated from both saliva and serum samples by an enzyme-linked immunosorbent assay. HIV viral load was determined using a quantitative reverse transcription-PCR. SLPI levels were increased 16.7% in plasma and 10.3% in saliva among HIV+ patients in comparison to uninfected controls. SLPI levels were increased 5.9% in saliva and 3.9% in plasma among HIV+ patients with a high viral load (>10,000 copies/ml) as compared to patients with a low viral load (<400 copies/ml). Only 23% of patients with a high viral load used combination therapy with protease inhibitor drugs, whereas 92.9% of HIV+ patients with a low viral load used protease inhibitors. SLPI levels did not differ significantly among the IVDA patients, patients with different viral loads, or patients using protease inhibitor drugs. There was a statistically significant increase in SLPI levels in saliva among HIV patients in comparison to non-HIV-infected controls. An increase in SLPI levels among HIV+ patients may be a natural consequence of HIV pathogenesis and an important factor in preventing oral transmission of HIV, but this increase may not be evident during plasma viremia in patients with a high viral load.
Transmission of the human immunodeficiency virus (HIV) by the oral route (6, 8, 9, 16) is virtually nonexistent, although infectious virions have been isolated from saliva and gingival crevicular fluid (5). The mechanism of this oral immunity to HIV transmission is poorly understood. Reports of antiviral activity in the saliva of both healthy individuals (1, 3–5, 7) and HIV type 1 (HIV-1)-infected individuals (5) suggest the presence of a factor or factors in saliva that can inhibit HIV-1 infection. Aggregation of HIV-1 by high-molecular-weight molecules (mucins, etc.) in the saliva has been implicated in this regard (1, 17, 19) and is consistent with the HIV-1 particle aggregation and entrapment by saliva as observed ultrastructurally on filters (2, 11). Secretory leukocyte protease inhibitor (SLPI), a major human elastase inhibitor present in upper-airway secretions (10) and in human neutrophils (18), has been reported to show activity against HIV-1 (13, 14). SLPI inhibits HIV-1 infection of human monocytes at physiological concentrations (1 to 10 μg/ml), and its depletion from saliva results in a significant loss of antiviral activity (13). SLPI is a potent serine protease inhibitor with biologic activity against a broad range of proteases, such as neutrophil elastase and cathepsin G, and its antiviral activity could be due to inhibition of a protease-mediated event that is required for virus entry and infectivity of susceptible cells (14).
In vitro studies have determined the molecular mass of SLPI from epithelial cells to be between 10 and 14 kDa (18). SLPI is synthesized and secreted in vitro by epithelial cells and has been located in bronchial glands and bronchiolar epithelial cells (18). SLPI is also found in abundance in parotid secretions, in cervical secretions, and in sputum from patients with chronic obstructive pulmonary diseases (18). SLPI accounts for 80 to 90% of the human neutrophil elastase-inhibitory activity present in sputum (10). Also of interest is the finding that pulmonary sputum samples from patients with cystic fibrosis (characterized by pronounced neutrophil inflammation) contained higher-than-normal amounts of SLPI, although lower-than-normal amounts of SLPI were reported in the sputum of smokers (18).
Viral load measurement has revolutionized the medical management of HIV-1-infected patients. There are several clinical applications of viral load measurements, including predicting prognosis, determining when to initiate antiretroviral therapy, and assessing response to antiretroviral therapy. Plasma HIV-1 RNA levels have been shown to be a strong predictor of a rapid progression to AIDS after seroconversion that is independent of CD4 cell count (15). In a study of 62 homosexual men with documented HIV-1 seroconversion, an initial plasma HIV-1 RNA level greater than 100,000 equivalents/ml soon after seroconversion was associated with a >10-fold increase in the risk of developing AIDS (15). Patients who maintained viral load levels of <10,000 particles/ml did not progress to AIDS over the next five years (15).
HIV-1 RNA has been detected in saliva and gingival crevicular fluid of HIV-positive (HIV+) patients by our laboratory (data not shown) and also by other researchers (5). SLPI inhibits HIV-1 infection of human monocytes at physiological concentrations (1 to 10 μg/ml), and its depletion from saliva results in a significant loss of anti-viral activity (13). SLPI distribution in both saliva and plasma samples among HIV+ patients with a history of intravenous drug abuse (IVDA) has not been well studied, and there have not been enough studies regarding SLPI concentrations in saliva or plasma among HIV-1-infected patients in comparison to uninfected populations. Furthermore, SLPI distribution among HIV+ patients with different viral loads has never been evaluated. Hence, in this descriptive study we have investigated SLPI levels among HIV+ patients with various viral loads and also among IVDA HIV+ populations in comparison to uninfected patients.
MATERIALS AND METHODS
Selection of patients.
A total of 37 subjects (20 males and 17 females, from 29 to 59 years old) were randomly selected from patients seeking evaluation and treatment at the University of Maryland Dental School. The only criterion for inclusion in the study was the determination that the patients were HIV seropositive. Twenty age- and sex-matched HIV seronegative patients were also selected as negative controls. Medical histories were recorded and each patient was clinically evaluated. Each of the participants in this study granted informed consent.
Collection of saliva.
Whole human saliva (∼5 ml, unstimulated) was collected from each of the HIV+ patients and from age- and sex-matched negative controls by tilting the head forward and dribbling saliva from the lower lip into a 50-ml graduated centrifuge tube. After 5 min, the subject was asked to expectorate any remaining saliva. The saliva sample was divided into aliquots and stored immediately in a freezer (−20°C) until used for SLPI estimation.
Hematologic sampling.
Approximately 10 ml of venous blood was collected by a phlebotomist directly into a Vacutainer tube containing EDTA as an anticoagulant. The blood was placed in double-containment transport tubes and immediately carried to the biosafety level III laboratory. The blood was then centrifuged at 300 × g for 15 min at 25°C. The supernatant plasma was collected, divided into aliquots, and stored at −80°C until assayed. The whole process, from the collection of blood to the storage of plasma, was performed within 30 min to avoid degeneration of HIV RNA.
SLPI assay.
Saliva and plasma from HIV+ patients and uninfected controls (samples stored at −20 and −80°C, respectively) were used to determine SLPI levels with a commercial enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, Minn.). The ELISA procedure employed a quantitative solid-phase sandwich enzyme immunoassay technique wherein a monoclonal antibody specific for SLPI was used to coat the microtiter plate provided in the kit. The different concentrations of standard samples (62.5 to 4,000 pg/ml) were prepared from a 4,000-pg/ml stock solution by using a serial twofold dilution. The plasma and saliva samples were prediluted (plasma, 20-fold, and saliva, 40-fold) with solutions provided in the kit. Also, SLPI assay experiments were repeated with further dilution of the samples when the values of the unknown samples were above the highest of the standard value. Duplicate optical density readings for each standard and for each unknown sample were taken with an ELISA reader and averaged. A curve was prepared, plotting the optical density versus the concentration of SLPI in the standard wells. By comparing the optical density of the unknown samples to this standard curve, the concentration of the SLPI in the unknown samples was determined. Results were expressed as nanograms of SLPI per milliliter of the saliva or plasma.
Viral load assay.
Viral load was determined in plasma samples by the Amplicor HIV-1 monitor (version 1.0) test kit (Roche Diagnostics, Raritan, N.J.). In this quantitative reverse transcription-PCR based method, after PCR amplification, denaturation, and hybridization reactions, the bound HIV-1 mRNA was estimated by a sandwich ELISA technique. The Amplicor assay has a lower limit of sensitivity of 400 copies/ml and is linear to 750,000 copies/ml. Due to the smaller number of patients in our study population, the viral load measurements have been divided into high (>10,000 copies/ml) and low (<400 copies/ml).
Statistical analysis.
Student’s t test or Mann-Whitney rank-sum tests were used to determine significant differences between the means of the two groups. All experiments were run in duplicate and repeated twice.
RESULTS
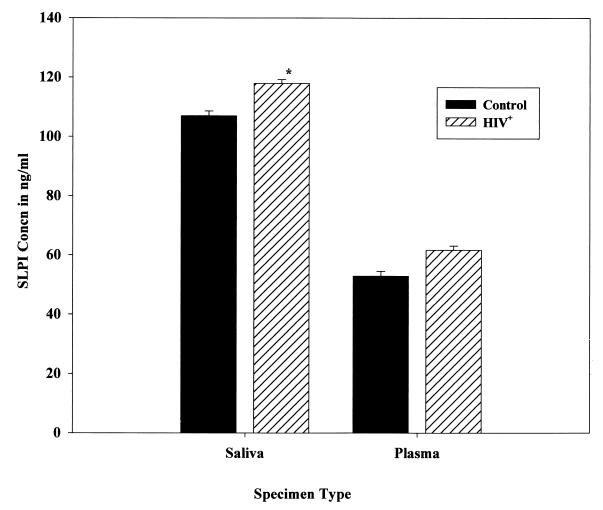
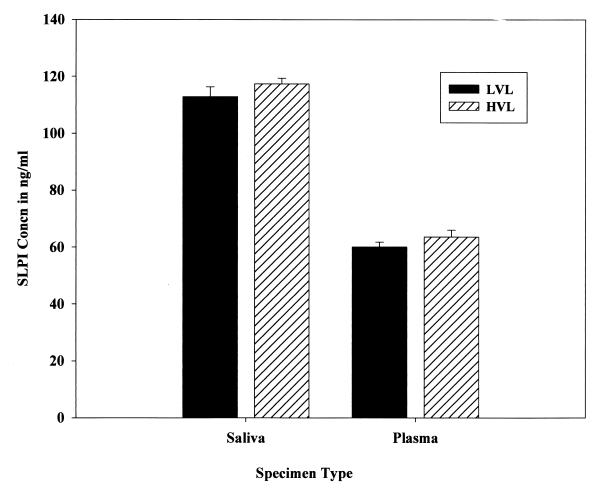
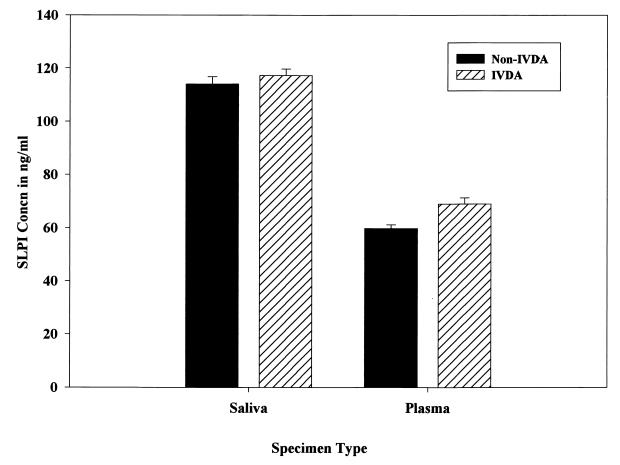
SLPI was present on average at 117.9 ng/ml in saliva and at 61.7 ng/ml in plasma among HIV+ patients in comparison to 106.9 and 52.9 ng/ml, respectively, among non-HIV-infected controls (Fig. 1). The increase in SLPI levels in saliva samples from HIV+ patients in comparison to uninfected controls was statistically significant (P < 0.05), but the increase in SLPI levels in plasma samples from the same group in comparison to uninfected controls was not significant (P > 0.05). The SLPI levels in both saliva and plasma samples were also analyzed in relation to the viral load status of the HIV+ patients (Fig. 2). Among the HIV+ patients examined, 35.2% (n = 13) were in the high (>10,000 copies/ml)-viral-load group (ranging from 12,000 to 170,000 HIV-1 RNA copies/ml), and 32.4% (n = 12) were in the low (<400 copies/ml)-viral-load group. There was definitely an increasing trend in SLPI levels in both saliva (3.9%) and plasma (5.9%) among HIV+ patients with a high viral load (>10,000 copies/ml) in comparison to levels in those with a low viral load. But the increased trend of SLPI levels in both saliva and plasma samples among the high-viral-load population in comparison to levels in low-viral-load patients did not reach statistical significance (P > 0.05). The viral load was significantly (χ2 = 14.377, df = 2, P < 0.001) related to use of protease inhibitor drugs, and hence SLPI levels were not analyzed and presented in relation to use of protease inhibitor drugs. Whether a history of IVDA among HIV+ patients had an effect on SLPI was also analyzed (Fig. 3). There was no significant difference in saliva and plasma SLPI levels among HIV+ patients with a history of IVDA in comparison to non-IVDA HIV+ patients. SLPI was also analyzed among HIV+ patients using protease inhibitor drugs in their treatment regimen. Though there was an increase in SLPI levels among HIV+ patients using protease inhibitor drugs, the difference did not reach statistical significance in either saliva or plasma samples (data not shown).
FIG. 1.
SLPI concentrations in saliva and plasma of HIV+ patients and HIV-1-negative controls. Error bars represent standard errors of the means. ∗, P < 0.05 by Student’s t test.
FIG. 2.
SLPI concentrations in saliva and plasma of HIV+ patients with high viral load (HVL) and low viral load (LVL). Error bars represents standard errors of the means.
FIG. 3.
SLPI concentrations in saliva and plasma of HIV+ patients with a history of IVDA. Error bars represent standard errors of the means.
DISCUSSION
SLPI is produced by serous cells of tracheal and bronchial submucosal glands and by nonciliated bronchial epithelial cells (12). Although nonspecific antiviral activity may be found in other body fluids (14), antiviral activity specific for HIV-1 has thus far been ascribed, at least in part, to the SLPI found in saliva (13). The findings of significantly (P < 0.05) increased SLPI levels in saliva samples from the HIV+ patients in comparison to uninfected controls in our study may support the view that SLPI has a role in inhibition of HIV transmission via the oral route. Smaller increases in SLPI in plasma samples from HIV+ patients in comparison to uninfected controls, as noted in our study, may contribute to some extent to the increased transudation of SLPI through the gingival sulcus and hence to increased saliva SLPI concentration in this population. Among the HIV+ patients, the significant (P < 0.05) elevations of SLPI levels in saliva compared to SLPI levels in the plasma also support the view that local cells within the oral cavity produce SLPI.
Direct quantitation of plasma HIV-1 RNA is now being performed as a sensitive and specific measurement of HIV virus load among patients at all HIV disease stages. There is no reported study observing the difference in SLPI levels according to the HIV-1 viral load status. We used the Amplicor HIV-1 monitor test for viral load assay in our study, because it is sensitive and requires only a small volume of plasma (200 μl). The levels of SLPI also demonstrated that nonresponders to therapy, i.e., patients whose viral loads remained elevated (>10,000 copies/ml), may also exhibit high plasma and saliva SLPI concentrations. Neither the biological nor the clinical significance of the association of SLPI with viral load status is well understood at this time. In high-viral-load HIV+ patients, higher SLPI levels could be a factor that effectively reduces the risk of oral transmission of HIV-1 virus.
The SLPI levels in IVDA HIV+ patients did not differ significantly from those in their non-IVDA counterparts. Among the current population of UMB, 70 to 80% had a previous history of IVDA. Our data may be reflective of our available HIV+ population, and they should eventually be compared to treatment trends in larger populations. The variation in SLPI levels may be caused by the drug-abuse lifestyle, wherein patients may not seek the latest treatment options, or they may not fully comply with their treatment regimens. It is likely that IVDA may affect the distribution of patients into the high-viral-load group due to the response to treatment issues. HIV+ patients using the protease inhibitor drug regimens showed expected low viral loads and a trend of lower SLPI levels in both saliva and plasma samples.
In conclusion, there was a significantly increased level of SLPI from whole, unstimulated saliva samples in HIV+ patients in comparison to uninfected controls. Although there were other trends noted in the data regarding a potential relationship between HIV+ patients with a high viral load (>10,000 copies/ml) and SLPI levels, the differences were not statistically significant compared to data for low-viral-load patients. The clinical significance of elevated SLPI levels in HIV+ patients with different viral load statuses should be investigated further with a larger sample of patients.
REFERENCES
- 1.Archibald D W, Cole G A. In vitro inhibition of HIV-1 infectivity by human salivas. AIDS Res Hum Retroviruses. 1990;6:1425–1432. doi: 10.1089/aid.1990.6.1425. [DOI] [PubMed] [Google Scholar]
- 2.Bergey E J, Cho M-I, Hammarskjold M-L, Rekosh D, Levine M J, Blumberg B M, Epstein L G. Aggregation of human immunodeficiency virus type 1 by human salivary secretions. Crit Rev Oral Biol Med. 1993;4:467–473. doi: 10.1177/10454411930040033001. [DOI] [PubMed] [Google Scholar]
- 3.Coppenhaver D H, Sriyuktasuth-Woo P, Baron S, Barr C E, Qureshi M N. Correlation of nonspecific antiviral activity with the ability to isolate infectious HIV-1 from saliva. N Engl J Med. 1994;330:1314–1315. doi: 10.1056/NEJM199405053301815. [DOI] [PubMed] [Google Scholar]
- 4.Fox P C, Wolff A, Yeh C-K, Atkinson J C, Baum B J. Saliva inhibits HIV-1 infectivity. J Am Dent Assoc. 1988;116:635–637. doi: 10.14219/jada.archive.1988.0002. [DOI] [PubMed] [Google Scholar]
- 5.Fox P C, Wolff A, Yeh C-K, Atkinson J C, Baum B J. Salivary inhibition of HIV-1 infectivity: functional properties and distribution in men, women and children. J Am Dent Assoc. 1989;118:709–711. doi: 10.14219/jada.archive.1989.0165. [DOI] [PubMed] [Google Scholar]
- 6.Friedland G H, Saltzman B R, Rogers M F, Kahl P A, Lesser M L, Meyers M M, Klein R S. Lack of household transmission of HTLV-III infection. N Engl J Med. 1986;314:344–349. doi: 10.1056/NEJM198602063140604. [DOI] [PubMed] [Google Scholar]
- 7.Fultz P N. Components of saliva inactivate human immunodeficiency virus. Lancet. 1986;ii:1215. doi: 10.1016/s0140-6736(86)92218-x. [DOI] [PubMed] [Google Scholar]
- 8.Gerberding J L, Bryant-LeBlanc C E, Nelson K, Moss A R, Osmond D, Chambers H F, Carlson J R, Drew W L, Levy J A, Sand M A. Risk of transmitting the human immunodeficiency virus, cytomegalovirus, and hepatitis B virus to health care workers exposed to patients with AIDS and AIDS related conditions. J Infect Dis. 1987;156:1–8. doi: 10.1093/infdis/156.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Klein R S, Phelan J A, Freeman P H, Schable M S, Friedland G H, Trieger N, Steigbigel N H. Low occupational risk of human immunodeficiency virus infection among dental professionals. N Engl J Med. 1988;318:86–90. doi: 10.1056/NEJM198801143180205. [DOI] [PubMed] [Google Scholar]
- 10.Kramps J A, Franken C, Dijkman J H. Quantity of antileucoprotease relative to alpha-1 proteinase inhibitor in peripheral air spaces of the human lung. Clin Sci. 1988;75:351–353. doi: 10.1042/cs0750351. [DOI] [PubMed] [Google Scholar]
- 11.Malamud D, Davis C, Berthold P, Roth E, Friedman H. Human submandibular saliva aggregates HIV. AIDS Res Hum Retroviruses. 1993;9:633–637. doi: 10.1089/aid.1993.9.633. [DOI] [PubMed] [Google Scholar]
- 12.Masuda K-I, Suga T, Takeuchi A, Kanesaki M, Imaizumi A, Suzuki Y. Specific cleavage of secretory leukocyte protease inhibitor by neutrophil elastase and saliva. Biochem Pharmacol. 1994;48:651–657. doi: 10.1016/0006-2952(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 13.McNeely T B, Dealy M, Dripps D J, Orenstein J M, Eisenberg S P, Wahl S M. Secretory leukocyte protease inhibitor: a human saliva protein exhibiting anti-human immunodeficiency virus 1 activity in vitro. J Clin Investig. 1995;96:456–464. doi: 10.1172/JCI118056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNeely T B, Shugars D C, Rosendahl M, Tucker C, Eisenberg S P, Wahl S M. Inhibition of human immunodeficiency virus type 1 infectivity by secretory leukocyte protease inhibitor occurs prior to viral reverse transcription. Blood. 1997;90:1141–1149. [PubMed] [Google Scholar]
- 15.Mellors J W, Kingsley L A, Rinaldo C R, Todd J A, Hoo B S, Kokka R P, Gupta P. Quantitation of HIV-1 RNA in plasma predicts outcome after seroconversion. Ann Intern Med. 1995;122:573–579. doi: 10.7326/0003-4819-122-8-199504150-00003. [DOI] [PubMed] [Google Scholar]
- 16.Moore B E, Flaitz C M, Coppenhaver D H, Nichols C M, Kalmaz G D, Bessman J D, Cloyd M W, Lynch D P, Prabhakar B S, Baron S. HIV recovery from saliva before and after dental treatment: inhibitors may have critical role in viral inactivation. J Am Dent Assoc. 1993;124:67–74. doi: 10.14219/jada.archive.1993.0197. [DOI] [PubMed] [Google Scholar]
- 17.Rovinovitch M R, Iversen J M, Resnick L. Anti-infectivity activity of human salivary secretions toward human immunodeficiency virus. Crit Rev Oral Biol Med. 1993;4:455–459. doi: 10.1177/10454411930040032801. [DOI] [PubMed] [Google Scholar]
- 18.Sallenave J-M, Har M S-T, Cox G, Chignard M, Gauldie J. Secretory leukocyte proteinase inhibitor is a major leukocyte elastase inhibitor in human neutrophils. J Leukoc Biol. 1997;61:695–702. doi: 10.1002/jlb.61.6.695. [DOI] [PubMed] [Google Scholar]
- 19.Yeh C-K, Handleman B, Fox P C, Baum B. Further studies of salivary inhibition of HIV-1 infectivity. J Acquir Immune Defic Syndr. 1992;9:898–903. [PubMed] [Google Scholar]