Abstract
A laboratory activity was developed to teach freezing point depression and colligative properties to introductory-level chemistry students. The laboratory uses food-grade reagents and is delivered in two units that can be taught in a single 2 hour session or two separate sessions. The total cost of the consumables is 1 USD. In the first part of this two-part activity, students perform measurements on the properties of five salt solutions to better know and understand freezing point depression. In the second part of the activity, students apply their knowledge and understanding of freezing point depression to make ice cream. The ice-cream-making experiment is delivered as a group activity to encourage reflection. Centering this experiment on ice cream allows students to connect properties described in chemistry to everyday life. The solutions used in the experiment are reusable and nonhazardous. The experiment can be implemented in a classroom, in a teaching laboratory, or at home.
Keywords: First-Year Undergraduate/General, Laboratory Instruction, Hands-On Learning/Manipulatives, Green Chemistry
Introduction
Colligative properties and freezing point depression are taught in both the introductory and upper-level chemistry curriculum; however, various misconceptions about these concepts have been documented for preservice teachers, some of whom are chemistry majors.1 New strategies to deliver chemistry content at the introductory level are needed to address this challenge. While several laboratory exercises have been documented for upper-level laboratories,2−5 an introductory-level laboratory experiment may be more suitable to mitigate the misconceptions surrounding freezing point depression and colligative properties. Moreover, experiences that provide opportunities for students to transition from lower learning levels6 (e.g., comprehension) to the application of chemistry concepts are more likely to address content misconceptions.7 Introductory laboratories that relate scientific principles to the real world have been demonstrated to enhance learning.8 Integrating food chemistry into laboratory experiments is one strategy to increase the relevance of science to daily life as well as to increase science participation and diversity.9 In addition, food-based activities are often utilized to connect chemistry to the real world because of the knowledge commonly held about food.9−13 This is convenient for demonstrating colligative properties and freezing point depression, which are easily connected to the process of making ice cream.
The cost of materials and the difficulty in acquiring supplies are major factors that motivate the implementation of laboratory experiments.14 Additionally, the value in integrating teaching laboratories based on green chemistry has recently been emphasized.15 Different experiments reported to teach freezing point depression involve a variety of approaches. While a few involve food,3,16 chemicals that require specific handling or disposal are also used, such as organic solvents,5 aromatics,5 fatty acids,2 ammonium sulfate,4 or p-dichlorobenzene.17 Of the published experiments, these measurements involve a freezing point instrument,5 a differential scanning calorimeter,4 or a lab-built calorimeter.2,3 These are costly and generally restricted to advanced students. To address the need for a low-cost, simple, and environmentally friendly laboratory experience, an experiment was designed to use common materials, namely, a thermometer, a freezer, and four consumable reagents: table salt, Epsom salt, calcium-based road salt, and commercially available ready-to-churn ice cream mix. The cost of the consumables, which is $1 per student, is further reduced if the salt solutions are recycled, setting the framework to conduct chemistry in a greener manner. Another benefit of using home-grade materials is the reduced risk relative to experiments that rely on traditional chemicals.5 Since the experiment is performed using common equipment, it follows that the skillset required is minimal, further increasing the accessibility.
The design of the experiment outlined in this report was driven by cost and simplicity, reducing barriers to implementing it in a wide variety of learning environments. To ensure that the activity is accessible, inexpensive Styrofoam cup calorimeters are used to measure the freezing points of nontoxic aqueous salt solutions. Frozen ice cubes of various salt types and concentrations are used to make ice cream. The consistency of the ice cream made with different salt cubes, and consequently at different freezing points, is measured, and the results obtained by each individual are shared to allow students to reflect on the outcome. These experiments can be completed in a single 2 hour period or split into two separate sessions, making them amenable to a classical laboratory setting as well as a high school or university classroom. The experiment is easy to complete using the written protocol and the video recording demonstrating the procedure, both of which are included in this report. The experiment has been successfully tested by students in an introductory chemistry laboratory for non-chemistry majors as well as a second-semester general chemistry laboratory for chemistry majors. The students in the non-chemistry-major laboratory increased in both knowledge and understanding of the concepts. Although the students in the major-honors laboratory had sufficient knowledge and understanding of these concepts, they demonstrated an improvement in their ability to apply the concepts.
Hazards and Safety Precautions
The health effects resulting from contact with temperatures as low as −18 °C are reduced by using gloves. The safety data sheets for food-grade table salt, pharmaceutical-grade Epsom salt, and commercial-grade road salt composed of calcium chloride should be consulted. If the activity is delivered in a laboratory in which eating is prohibited, the students should be reminded to follow the safety guidelines. If the activity is performed in a sanitary setting where eating is not prohibited and the ice cream is prepared according to the manufacturer’s instructions, the ice cream can be considered to be food-grade.
Freezing Point Depression Experiments
Two experiments to teach the concepts of colligative properties and freezing point depression are described. The prelaboratory instructions and experimental protocols shared with the students are included as Supporting Information. These experiments involve measuring the freezing points of five aqueous salt solutions and comparing them to the values predicted using the kf value for water. These activities reinforce fundamental learning at the levels of knowledge and comprehension, as categorized by Bloom’s taxonomy. A second experiment expands on this learning by guiding students to apply the concepts by using a frozen salt solution to make ice cream as part of a classroom competition. These experiments are delivered sequentially.
The first part of the experiment (i.e., part A) involves measurements of salt solutions to investigate the freezing point depression. Upon completing part A of the experiment, students will be able to describe the characteristics of colligative properties and predict how concentration and salt type affect the freezing point of a solvent. To achieve these goals, students are given ice cubes and the apparatus shown in Figure 1, which allows them to measure the freezing points of five aqueous salt solutions, including 1, 3, and 5 molal sodium chloride (NaCl), 1 molal magnesium sulfate (MgSO4), and 1 molal calcium chloride (CaCl2) solutions. Overall, this process can be completed at a cost of $0.29 per student for consumable materials (i.e., salts). Common labware items are also used (i.e., thermometer, ice cube trays, Styrofoam cup, plastic bottles). If these items are not available, they can be purchased at a cost of $6.87 per student (see the Supporting Information for an itemized list). If purchased, they should be stored and used repeatedly for future experiments. By measuring the temperature of the three NaCl solutions with the Styrofoam calorimeter, students observe the change in freezing point as a function of solute concentration. The measurements collected for the 1, 3, and 5 molal NaCl solutions should approach the predicted values of −3.7, −11.2, and −18.6 °C, respectively. The students compare these measured values to the freezing point depression values predicted using the freezing point depression constant of water (kf = −1.86 °C kg/mol). Next, by comparing the freezing point depression values for the 1 molal NaCl and 1 molal MgSO4 solutions, which are the same (i.e., predicted to be −3.7 °C), the students appreciate that colligative properties do not depend on the identity of the solute. Finally, by comparing the freezing point depression values for the 1 molal NaCl and 1 molal CaCl2 solutions (i.e., predicted to be −3.7 and −5.6 °C, respectively), the students better appreciate the role of the van’t Hoff factor (i) in calculating the freezing point depression. If the students’ experience in the lab is successful, these observations help students to achieve knowledge and comprehension, which are the basic levels of learning in Bloom’s taxonomy.6 Some questions have been included in the laboratory handout to help the instructor assess learning. Additional assessments of learning may be used as well.
Figure 1.

Photograph of thermal calorimetry apparatus used in the first experiment (part A) of this activity. The image also includes the frozen salt cubes, shown in a food-grade ice-cube tray, as well as bottles of chilled aqueous salt solutions.
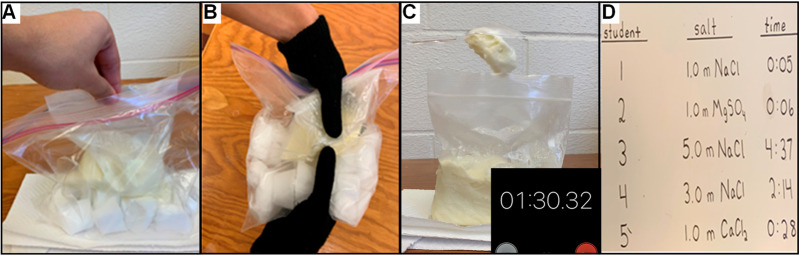
The second part of this experiment (i.e., part B) is designed to guide students to apply their knowledge and comprehension of freezing point depression and colligative properties to a real-world application. This activity engages each student to consider which salt cubes will most effectively set the ice cream. The experiment can be completed in under 30 min. The cost of consumables (i.e., salt, ice cream, plastic bags) is $0.73 per student. Common items are also used (i.e., ice cube trays, winter gloves). If these items are not available, they can be purchased at a cost of $3.54 per student (see the Supporting Information for an itemized list) and reused in the future. To begin the activity, students are given different salt ice cubes along with the unset ice cream and gloves. All students are instructed to place the unset ice cream in the bag of salt cubes (Figure 2a). The students begin to hand mix the ice cream (Figure 2b) at the same time. When the first student has made fully set ice cream, an announcement is made to everyone in the laboratory to stop mixing in order to measure the thickness of the ice cream. The consistency of the ice cream is measured using a flipped spoon test (Figure 2c). As outlined in the handout, which is available in the Supporting Information, this is done by scooping out ice cream with a spoon and holding the spoon upside down with a timer on. Well-set ice cream will stick to the spoon for several minutes, while thinly set ice cream will drop from the spoon quickly. Upon completing this activity and reporting all of the measurements (Figure 2d), the students will observe that the consistency of the ice cream follows the temperature of the frozen salt cubes. The salt cubes that produce the thickest to thinnest set ice cream are as follows: 5 molal NaCl, 3 molal NaCl, 1 molal CaCl2, and 1 molal NaCl/1 molal MgSO4. When the second experiment is delivered as outlined here, the frozen cubes are essentially at the eutectic point of −21 °C and can be considered as a mixture of solid ice and sodium chloride dihydrate until the cubes undergo a phase change. When the students knead the bags with gloves, the heat is transferred between the ice cream mix and the cubes. The eutectic halt is longer for higher-molality preparations.18 Phase diagrams of the water–sodium chloride system are available,10,19 including a discussion of the phase transitions relevant to the process of ice cream.10 An advantage to delivering the activity as a contest is that it stimulates group discussion as well as reflection. A student’s mastery of applying the concept of freezing point depression to a real-world topic may occur during the activity, following group discussion once the results are posted, or following completion of the reflective questions included in the student handout.
Figure 2.
Photographs illustrating the application of freezing point depression to the portion of the experiment based on making ice cream. (A) A bag of the unset ice cream mix is placed inside of a bag containing the frozen salt cubes. (B) The unset ice cream is mixed by the student wearing gloves. (C) Once the ice cream has set, the student quantifies the consistency of the ice cream by recording the amount of time it takes for the ice cream to drop from a spoon. (D) The data from all of the students are posted to demonstrate the correlation between the type of salt used to set the ice cream and the ice cream consistency. Posting the data facilitates reflection on the application of freezing point depression to cost-effective and rapid preparation of ice cream.
Implementation and Evaluation
This activity is designed for a general chemistry audience in a high school or college setting. The lab can also be delivered remotely (i.e., as a take-home or lab-in-a-box experiment), as most students have access to a freezer. To establish the feasibility of this experiment, it was tested in a general chemistry lab for chemistry majors as well as in a general chemistry class for nonmajors. While our experience is favorable, some considerations are helpful. When preparing the reagents, the temperature of the freezer must be checked. If necessary, the thermal control must be adjusted so that the freezer temperature is below the freezing point of the salt solution. It should also be noted that the higher-concentration solutions (i.e., solutions with the lowest freezing point depression) require 2–4 days to freeze, depending on the number of trays placed in the freezer.
Regarding the delivery of the reagents, there are several advantages to controlling the circulation of the solutions. Orderly distribution and collection of materials (e.g., by row or group) of a single salt at a time is highly encouraged. This is especially helpful if the activity is delivered in a typical higher-education laboratory setting (e.g., 20 to 50 students per lab section). If the freezer is not colocated in the laboratory, each type of salt should be moved from the freezer to a cooler when transported to the students. This is necessary because the ice cubes will begin to equilibrate to ambient temperature when removed from the freezer. If swift transportation (i.e., 15 min) is not feasible, then freezer packs also brought to temperature in the freezer should be included in the cooler. It should be noted that all of the materials from part A of the activity are recyclable. Providing wide-mouth waste containers allows the liquids and cubes to be collected and reused in subsequent experiments by refreezing them.
Several points regarding part B of the activity are important to note. First, overzealous students can rupture the inner ice cream bag during mixing. Therefore, the students should be advised to avoid doing this. Second, students must wear gloves to reduce exposure of their hands to the frozen cubes. When measuring the ice cream consistency, the students should scoop from the center of the ice cream in the bag for an accurate measure of thickness.
The laboratory handout contains some questions to guide the students in order to assess the learning outcomes. The activity was evaluated with a group of 30 students enrolled in a first-semester general chemistry course for nonmajors and a group of nine students enrolled in a second-semester general chemistry course for chemistry majors. Additional assessments for this activity were designed to address the first three levels of Bloom’s taxonomy: knowledge, comprehension, and application. When the students enrolled in the nonmajor chemistry course were evaluated, the students had a statistically significant improvement in all categories as calculated using Student’s t test (ρ = 0.05). The gains observed between the assessments were 23% to 52%, 35% to 52%, and 26% to 41% for knowledge, comprehension, and application, respectively. When the same assessments were given to the students enrolled in the second-semester course for chemistry majors, a statistically significant improvement (Student’s t test, ρ = 0.05) was observed in application of the principles of freezing point depression, with scores increasing from 33% to 63%.
Limitations
Errors in the temperature measurements can be introduced through bias in the thermometer or if the ice–brine phase is not equilibrated or mixed properly. Relative standard deviations in temperature measurements ranged from 0.5% to 0.9%. Additionally, the measurements of freezing point depression in the first experiment are performed using salt concentrations that are relevant to setting ice cream in the follow-on activity. Although simple calculations of the predicted freezing point depression will be close to the observed values, the calculation is accurate for lower concentrations of salt. More complex computational models for predicting the freezing point depression at higher concentrations are available.19,20 For the first experiment, the amount of time needed for equilibration depends upon the relative amounts of the chilled brine (4 °C) cubes (−21 °C). When the temperature is measured at the onset of mixing, the readings may appear to be unstable for several minutes. Additional cubes may need to be added if the calorimeter transitions to brine lacking solid ice. Regarding the ice cream competition, the amount of ice cream mix must be constant for all students, and smaller quantities, such as the recommended 200 g, will set quickly. As per safe food handling, the ice cream must be refrigerated (2–4 °C).
Some considerations warrant additional discussion. Although the salts are available to the public, students should be advised not to consume food-grade table salt, pharmaceutical-grade Epsom salt, or commercial-grade road salt. Additionally, the size of the freezer compartment must be considered. In our experience, a maximum of 60 standard ice cube trays fit in the freezer of a typical combination refrigerator/freezer. We estimate that each student requires 2.75 ice cube trays, making a single freezer sufficient for 21 student experiments. Additionally, while the calculations for each salt solution are provided in the Supporting Information, the salt purity and reported hydration must be checked. For example, table salt is exceptionally pure, whereas the purity of calcium chloride in road salt depends upon the formulation that is used. Pharmaceutical-grade Epsom salt with no additives (e.g., lavender, menthol) should be used and is generally sold as a heptahydrate. As mentioned previously, the achievable temperature of the freezer must be measured before beginning. Finally, once frozen, the salt cubes should be used shortly after being removed from the freezer, as they will equilibrate to ambient temperature.
Conclusions and Future Directions
The experiment described in this report demonstrates freezing point depression to students in a safe and straightforward manner. The activity is appropriate to teach freezing point depression and colligative properties to a wide range of students enrolled in general chemistry. The materials required for the experiment are readily available to the public and can be used by a typical adult with minimal risk to human health, provided that the salts are not consumed. The experiment can be performed in a traditional laboratory setting or nontraditional environment, such as in a large lecture hall, a home, or a high school classroom. By making ice cream after measuring the freezing points of aqueous salt solutions, students are able to connect the concept of freezing point depression to a real-world application.
Beyond the protocol described in this report, the activity can be expanded. For example, with an additional lab period, the students can be empowered to prepare their own solutions used in the experiment. This provides an opportunity for each student to practice basic laboratory skills, such as calculating and weighing masses required to prepare solutions. Additionally, a solution containing a nonionic analyte, such as glucose, could be introduced to provide an example of a solid that dissolves but does not dissociate in solvent. For the experiments involving churning the ice cream, flavor can be included as an evaluation criterion in addition to the cost of the salt and the consistency of the ice cream. In this scenario, the students could be encouraged to personalize the ice cream by adding dry ingredients prior to churning. A range of ice cream chemistry experiments are possible, such as correlating the brine molality and measured temperature16 as well as studies of the ice cream temperature equilibration10 or change in ice cream viscosity.10
Acknowledgments
The experiment was evaluated at West Virginia University following the approval of IRB protocol number 2108389463R001. This material is based upon work supported by the National Science Foundation (Grant CHE2004021).
Supporting Information Available
The Supporting Information is available at https://pubs.acs.org/doi/10.1021/acs.jchemed.2c00626.
The authors declare no competing financial interest.
Supplementary Material
References
- Pinarbasi T.; Sozbilir M.; Canpolat N. Prospective Chemistry Teachers’ Misconceptions About Colligative Properties: Boiling Point Elevation and Freezing Point Depression. Chem. Educ. Res. Pract. 2009, 10, 273–280. 10.1039/B920832C. [DOI] [Google Scholar]
- Hunnicutt S. S.; Grushow A.; Whitnell R. How Is the Freezing Point of a Binary Mixture of Liquids Related to the Composition? A Guided Inquiry Experiment. J. Chem. Educ. 2017, 94, 1983–1988. 10.1021/acs.jchemed.7b00409. [DOI] [Google Scholar]
- Novo M.; Reija B.; Al-Soufi W. Freezing Point of Milk: A Natural Way to Understand Colligative Properties. J. Chem. Educ. 2007, 84, 1673. 10.1021/ed084p1673. [DOI] [Google Scholar]
- Bodzewski K. Y.; Caylor R. L.; Comstock A. M.; Hadley A. T.; Imholt F. M.; Kirwan K. D.; Oyama K. S.; Wise M. E. Investigating Freezing Point Depression and Cirrus Cloud Nucleation Mechanisms Using a Differential Scanning Calorimeter. J. Chem. Educ. 2016, 93, 729–732. 10.1021/acs.jchemed.5b00663. [DOI] [Google Scholar]
- Li S.; Guo J.; Wang K.; Chen L.; Hu D.; Bai Y. Adapting and Modifying the Apparatus for Students to Accurately Determine the Freezing Point of a Solvent and Solution. J. Chem. Educ. 2017, 94, 1590–1593. 10.1021/acs.jchemed.7b00253. [DOI] [Google Scholar]
- Adams N. E. Bloom’s Taxonomy of Cognitive Learning Objectives. J. Med. Libr. Assoc. 2015, 103, 152–153. 10.3163/1536-5050.103.3.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King D.; Ritchie S. M., Learning Science through Real-World Contexts. In Second International Handbook of Science Education; Fraser B. J., Tobin K., McRobbie C. J., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp 69–79. [Google Scholar]
- Broman K.; Parchmann I. Students’ Application of Chemical Concepts When Solving Chemistry Problems in Different Contexts. Chem. Educ. Res. Pract. 2014, 15, 516–529. 10.1039/C4RP00051J. [DOI] [Google Scholar]
- Levine M.; DiScenza D. J. Sweet, Sweet Science: Addressing the Gender Gap in Stem Disciplines through a One-Day High School Program in Sugar Chemistry. J. Chem. Educ. 2018, 95, 1316–1322. 10.1021/acs.jchemed.7b00900. [DOI] [Google Scholar]
- Gibbon D. L.; Kennedy K.; Reading N.; Quieroz M. The Thermodynamics of Home-Made Ice Cream. J. Chem. Educ. 1992, 69, 658. 10.1021/ed069p658. [DOI] [Google Scholar]
- Casanova R. S.; Civelli J. L.; Kimbrough D. R.; Heath B. P.; Reeves J. H. Distance Learning: A Viable Alternative to the Conventional Lecture-Lab Format in General Chemistry. J. Chem. Educ. 2006, 83, 501. 10.1021/ed083p501. [DOI] [Google Scholar]
- Bayline J. L.; Tucci H. M.; Miller D. W.; Roderick K. D.; Brletic P. A. Chemistry of Candy: A Sweet Approach to Teaching Nonscience Majors. J. Chem. Educ. 2018, 95, 1307–1315. 10.1021/acs.jchemed.7b00739. [DOI] [Google Scholar]
- Miles D. T.; Borchardt A. C. Laboratory Development and Lecture Renovation for a Science of Food and Cooking Course. J. Chem. Educ. 2014, 91, 1637–1642. 10.1021/ed5003256. [DOI] [Google Scholar]
- Boesdorfer S. B.; Livermore R. A. Secondary School Chemistry Teacher’s Current Use of Laboratory Activities and the Impact of Expense on Their Laboratory Choices. Chem. Educ. Res. Pract. 2018, 19, 135–148. 10.1039/C7RP00159B. [DOI] [Google Scholar]
- O’Neil N. J.; Scott S.; Relph R.; Ponnusamy E. Approaches to Incorporating Green Chemistry and Safety into Laboratory Culture. J. Chem. Educ. 2021, 98, 84–91. 10.1021/acs.jchemed.0c00134. [DOI] [Google Scholar]
- Sund R. Quantifying a Colligative Property Associated with Making Ice Cream. J. Chem. Educ. 1989, 66, 669. 10.1021/ed066p669. [DOI] [Google Scholar]
- College S. M.10: Determination of the Molar Mass by Freezing Point Depression (Experiment). Santa Monica College, 2021. https://chem.libretexts.org/Ancillary_Materials/Laboratory_Experiments/Wet_Lab_Experiments/General_Chemistry_Labs/Online_Chemistry_Lab_Manual/Chem_12_Experiments/10%3A_Determination_of_the_Molar_Mass_by_Freezing_Point_Depression_(Experiment) (accessed 2022-06-06).
- Atkins P.; De Paula J.. 5C.4 Liquid-Solid Phase Diagrams. In Physical Chemistry: Thermodynamics, Structure, and Change, 10th ed.; W.H. Freeman and Company: New York, 2014; pp 212–214. [Google Scholar]
- Li D.; Zeng D.; Yin X.; Han H.; Guo L.; Yao Y. Phase Diagrams and Thermochemical Modeling of Salt Lake Brine Systems. II. NaCl+H2O, KCl+H2O, MgCl2+H2O and CaCl2+H2O Systems. Calphad 2016, 53, 78–89. 10.1016/j.calphad.2016.03.007. [DOI] [Google Scholar]
- Lamas C. P.; Vega C.; Noya E. G. Freezing Point Depression of Salt Aqueous Solutions Using the Madrid-2019 Model. J. Chem. Phys. 2022, 156, 134503. 10.1063/5.0085051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.