Abstract
We herein report the use of phenyl trimethylammonium iodide (PhMe3NI) as a safe, nontoxic, and easy-to-handle reagent for an absolutely monoselective N-methylation of amides and related compounds as well as for the N-methylation of indoles. In addition, we expanded the method to N-ethylation using PhEt3NI. The ease of operational setup, high yields of ≤99%, high functional group tolerance, and especially the excellent monoselectivity for amides make this method attractive for late-stage methylation of bioactive compounds.
Nitrogen-containing compounds are privileged structures in organic chemistry. For example, among thousands of FDA-approved, small molecule drugs, more than 80% contain at least one nitrogen atom with an average of 2.3 nitrogens per drug.1 These impressive numbers outline the importance of nitrogen-containing motifs in medicinal chemistry and drug discovery. When checking the top 200 small molecule drugs by retail sales in 20212 (see the bottom of Figure 1 for a selection), one notices the nitrogen atom is found in a majority of pharmaceuticals and herein appears in different structural modifications. Repeatedly occurring nitrogen-containing functionalities include amines, amides, sulfonamides, and N-heterocycles. Simple structural modifications, e.g., alkylation, of such groups often drastically change the physiological and biological properties of pharmaceutically active molecules.3,4 Considering alkylation as a late-stage modification in bioactive compounds in general, the simplest and smallest of all alkyl groups, the methyl group, seems to have the most profound impact on altering the biological properties of a molecule.5−7 This phenomenon is well-known as the “magic-methyl effect”.4,8,9
Figure 1.

Strategies for the methylation of amides (top) and selected N-methylated small molecule pharmaceuticals and their retail sales in 2021 (bottom).2
Hence, new strategies for efficient and selective N-methylation of amides and related structures are of great interest.10 However, major challenges with these specific transformations need to be considered (see Figure 1). First, traditionally applied methylating agents, such as iodomethane11 or dimethyl sulfate,12 often suffer from undesired properties, such as high toxicity, carcinogenicity, and volatility. Some strategies require transition metal catalysts, e.g., when using peroxides13 or MeOH14−16 as the single-carbon source.
Still others have a relatively narrow substrate scope, which limits the broad application of the respective methylating agent, e.g., when using formaldehyde17,18 under reductive conditions or PO(OMe)3.19 The Schoenebeck group recently reported a safe and metal-free methylation protocol using tetramethylammonium fluoride.20 This method is characterized by a relatively broad substrate scope, including amides, N-heterocycles, and alcohols. However, this strategy lacks monoselectivity when methylating primary amides. In general, the tendency of primary amides to undergo overalkylation features the second serious challenge when searching for new N-methylation strategies.
We describe herein a novel, safe, and monoselective protocol for efficient methylation of amides using phenyl trimethylammonium iodide (PhMe3NI) as the CH3 source under mildly basic conditions, which is characterized by the ease of operational setup. In addition, we demonstrate the broad applicability of this new method by expanding the scope toward N-heterocycles, e.g., indoles, and prove its potential use in the late-stage functionalization of bioactive compounds. Furthermore, the monoselective introduction of an ethyl group can be realized using the related quaternary ammonium salt PhEt3NI.
For all optimization screenings, we used 4-fluoro benzyl amide (1a) as the substrate. The fluoro substituent enables facile quantification directly from the reaction solution without preceding solvent removal or workup via 19F NMR using trifluoro toluene as the internal standard.
We started our investigations by building on our previous results for the selective α-C(sp3)-methylation21 using PhMe3NI as the methylating agent and KOH as the base in toluene at 120 °C (Table 1, entry 1). The mono-N-methylated product was obtained with a moderate yield of 56%. Other hydroxy bases showed significantly lower conversion (entries 2 and 3). Gratifyingly, we found Cs2CO3 as a mild base giving the mono-N-methylated product (2a) in 85% yield (entry 4). All other bases tested turned out to be inefficient (see the Supporting Information for details). Next, we tested different quaternary ammonium salts as the methylating agents. The tetramethylammonium halides gave lower overall yields for 2a, with a rapid decrease in conversion from the respective fluoride to iodide salts (entries 6–9). Tetramethylammonium fluoride, which was applied for methylating secondary amides toward tertiary ones, gave a 1:1 mixture of mono- and bis-methylated products 2a and 3a, respectively (entry 6), and obviously lacks monoselectivity. As anticipated, the phenyl trimethylammonium halides performed best, with the phenyl trimethylammonium iodide outperforming the respective chloride and bromide (entries 4, 10, and 11). We also tested a variety of solvents that are considered to be more environmentally benign such as t-BuOH, cyclopentyl methyl ether (CPME),22 and anisole.23 Indeed, they turned out to be suitable solvents for this specific reaction; however, ∼10–20% lower yields of 2a were obtained (entries 12–14) compared to those with toluene, which consequently remained the solvent of choice (entry 4).
Table 1. Optimization of the Reaction Conditionsa.

| yield
(%)b |
||||||
|---|---|---|---|---|---|---|
| entry | solvent | ammonium salt | base | conversion (%) | 2a | 3a |
| 1 | toluene | PhMe3NI | KOH | 81 | 56 | 19 |
| 2 | toluene | PhMe3NI | NaOH | 43 | 11 | 7 |
| 3 | toluene | PhMe3NI | LiOH·H2O | 28 | 6 | 0 |
| 4 | toluene | PhMe3NI | Cs2CO3 | 91 | 85 | 5 |
| 5 | toluene | PhMe3NI | no base | 7 | 0 | 0 |
| 6 | toluene | Me4NF | Cs2CO3 | 97 | 26 | 24 |
| 7 | toluene | Me4NCl | Cs2CO3 | 73 | 67 | 3 |
| 8 | toluene | Me4NBr | Cs2CO3 | 31 | 23 | 0 |
| 9 | toluene | Me4NI | Cs2CO3 | 8 | 4 | 0 |
| 10 | toluene | PhMe3NCl | Cs2CO3 | 96 | 78 | 7 |
| 11 | toluene | PhMe3NBr | Cs2CO3 | 99 | 78 | 11 |
| 12 | t-BuOHc | PhMe3NI | Cs2CO3 | 79 | 65 | 7 |
| 13 | CPME | PhMe3NI | Cs2CO3 | 94 | 74 | 7 |
| 14 | anisole | PhMe3NI | Cs2CO3 | 89 | 73 | 5 |
Reactions were performed on a 0.35 mmol scale, with 2 equiv of the base and 2 equiv of the ammonium salt under an Ar atmosphere at 120 °C with a reaction time of 18 h.
Yields were determined by quantitative 19F NMR using trifluoro toluene as the internal standard.
At 100 °C.
In our previous publication on selective α-methylation of aryl ketones,21 we could prove that a reaction pathway via thermal decomposition of the methylammonium salt to its respective methyl halide, which in turn could act as the actual methylating agent, can be excluded. Additionally, when the N-methylation of benzyl amide is performed with MeI under basic conditions, the N-bis-methylated product is obtained exclusively, and no monoselectivity is observed.24 The latter results corroborate the hypothesis of a direct nucleophilic substitution mechanism rather than a pathway via thermal decomposition to MeI and even more emphasize the importance of finding novel monoselective protocols employing alternative reagents.
We performed additional experiments to demonstrate the remarkable selectivity of this new protocol. When monomethylated amides 2a and 2b were subjected again to the best performing reaction conditions (Table 1, entry 4), only 27% and 35% of bis-methylated products 3a and 3b were obtained (Scheme 1). For both reactions, mainly unreacted monomethylated starting material was recovered. This underlines the applicability of the developed reaction conditions for selective monomethylation because even when trying to enforce a second methylation, this works only poorly. These findings corroborate the hypothesis that an attachment of a sterically demanding CH3 group makes the nitrogen less prone to further deprotonation by a weak base and alters its nucleophilicity. Therefore, a second alkylation via the bulky ammonium salt is slowed significantly.
Scheme 1. Reaction Using Monomethylated Benzamides as Starting Materials.

Yield determined by quantitative 19F NMR using trifluoro toluene as the internal standard.
Isolated yields given.
With the optimized reaction conditions in hand, we applied the N-methylation reaction to various substrates, including amides, indoles, and a variety of structurally related bioactive compounds, to demonstrate the broad applicability of our developed protocol (Scheme 2).
Scheme 2. Scope of N-Methylation and N-Ethylation.
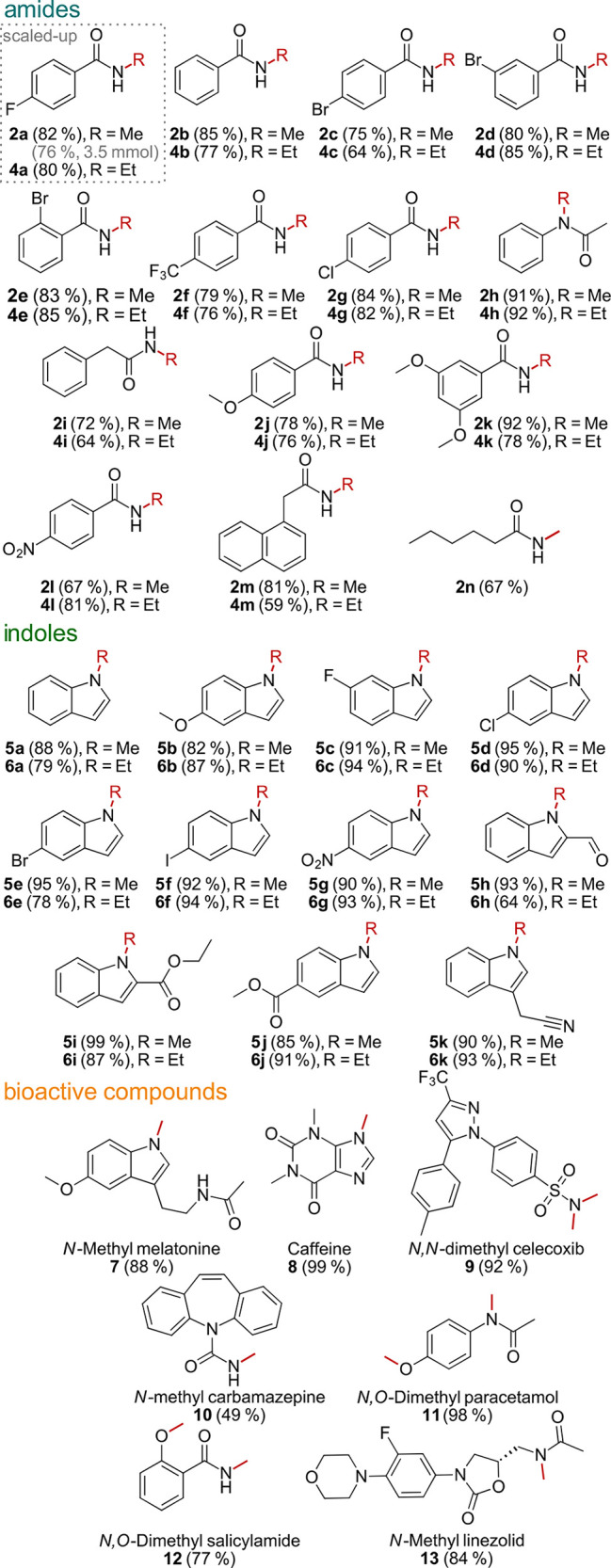
Reactions performed on a 100 mg scale, Cs2CO3 (2 equiv), ammonium salt (2.5 equiv): PhMe3NI (for products 2a–2m, 5a–5k, and 9–15), PhEt3NI (for products 4a–4m and 6a–6k), toluene (0.23 M), 120 °C for 16–24 h.
In all reactions, N,N-dimethylamine is formed as a stoichiometric byproduct from PhMe3NI after its methyl group transfer. This byproduct can be either quenched in situ by conversion to its water-soluble HCl salt and subsequently removed in a mild acidic workup or, for acid-sensitive compounds, easily removed via column chromatography. The obtained results are compiled in Scheme 2.
The monoalkylated amides were obtained in yields of ≤91% for the methylation (products 2a–2n) and 92% for the ethylation (products 4a–4m). In all cases, a variety of functional groups on the benzamide, e.g., halides (products 2a, 2c–2g, 4a, and 4c–4e), a nitro group (products 2l and 4l), ether (products 2j, 2k, 4j, and 4k), and fused aromatic rings (products 2m and 4m), were used. Interestingly, an amide functionality at a benzylic position reacted chemoselectively without substitution at the α-position (products 2i, 4i, 2m, and 4m). This method, however, is not restricted to para-substituted amides but can be used to methylate ortho- and meta-substituted benzamides with comparable yields (cf. 2c and 4c to 2d, 2e, 4d, and 4e; cf. 2j and 4j to 2k and 4k).
The aliphatic amide hexanamide could be selectively monomethylated in a moderate yield of 67% (product 2n). No bis-methylated product could be detected in the crude reaction mixture by NMR and LC-MS analysis, but unreacted starting material could be. This was also true for all other products with moderate yields. Only for products 2i and 2m could trace amounts (<8%) of bis-methylated species be detected via crude NMR. The reaction of N-acetylaniline also yielded the desired methylated and ethylated products 2h and 4h, showing that depending on the specific structure some secondary amides can be alkylated in excellent yields.
Because the experimental pKa values in DMSO for 1h (pKa = 21.525) and 2a (pKa = 21.526) are in the same range, we hypothesize that the facile methylation of secondary amide 1h, in comparison to the methylation of 2a (see Scheme 1), might be caused by the lower steric demand of planar phenyl groups compared to a bulky methyl substituent directly attached to the nitrogen. Therefore, the nitrogen would be more readily approached by PhMe3NI for substrate 1h than for 2a. However, as the monomethylation toward secondary amides is much more demanding, we mainly focused on primary amides as starting materials. To further prove this method’s applicability and ease of operational setup, we performed the methylation of 1a on a 3.52 mmol scale, giving 2a in a 76% isolated yield.
In addition to amides, the indole motif is considered a privileged heterocyclic structure in biologically active compounds, as well.27−29 Hence, we tested whether indoles could be N-methylated and N-ethylated as well with our new protocol. Overall, indole-derived substances performed slightly better in this specific N-alkylation reaction than primary amides. A great range of functional groups was well tolerated, including halides (products 5c–5f and 6c–6f), ether (products 5b and 6b), nitro (products 5g and 6g), aldehyde (products 5h and 6h), esters (products 5i, 5j, 6i, and 6j), and nitrile (products 5k and 6k). The described methylation of selected indole derivatives with PhMe3NI under mild basic conditions gave yields as high as those of the methylation with Me4NF performed by the group of Schönebeck.20 In contrast to Me4NF, however, the use of anhydrous PhMe3NI and storage of the reagent in a glovebox are not required, which makes this presented method even more convenient.
To outline the potential of this method for late-stage functionalization of bioactive molecules, we performed methylation on a selection of established pharmaceuticals. Tryptamine-derived compounds, like melatonin, are methylated exclusively at the indole nitrogen atom in 88% yield (product 7). Upon subjecting the N-monomethylated melatonin (product 7) again to the reaction conditions mentioned above, we could observe no further methylation at the nitrogen of the secondary amide. Theophylline can be fully methylated to give caffeine (product 8) in quantitative yield. The sulfonamide moiety in celecoxib is fully bis-methylated in an excellent yield of 92% (product 9). Sulfonamides exhibit significantly lower pKa values compared to those of benzamides; hence, a monomethylated sulfonamide readily undergoes a second substitution at the nitrogen. As in carbamazepine, a urea-derived functionality is monomethylated in moderate yield (product 10). From previous results,21 we found hydroxy groups being readily methylated. Thus, as expected, paracetamol and salicylamide were methylated at the phenolic position and the amide moiety (products 11 and 12), and the antibiotic linezolid can be N-methylated at the acetamide moiety with an 84% yield (product 13).
In conclusion, we described a novel protocol for monoselective methylation and ethylation of amides, indoles, and related structures using solid, nontoxic, and easy-to-handle quaternary ammonium salts under mildly basic conditions. The method can also be applied to complex bioactive compounds and hence for late-stage modification of active pharmaceutical ingredients in drug discovery programs.
Acknowledgments
This work was funded by the Austrian Science Fund (FWF, Project P33064-N).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.2c02766.
Detailed procedure for quantitative 19F NMR measurements, complete optimization screening data, experimental procedures, and characterization data for all compounds isolated (PDF)
Open Access is funded by the Austrian Science Fund (FWF).
The authors declare no competing financial interest.
Supplementary Material
References
- Vitaku E.; Smith D. T.; Njardarson J. T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- Njardarson J.Top 200 Small Molecule Pharmaceuticals by Retail Sales in 2021; 2021. [Google Scholar]
- Chatterjee J.; Gilon C.; Hoffman A.; Kessler H. N-Methylation of Peptides: A New Perspective in Medicinal Chemistry. Acc. Chem. Res. 2008, 41, 1331–1342. 10.1021/ar8000603. [DOI] [PubMed] [Google Scholar]
- Barreiro E. J.; Kümmerle A. E.; Fraga C. A. M. The Methylation Effect in Medicinal Chemistry. Chem. Rev. 2011, 111, 5215–5246. 10.1021/cr200060g. [DOI] [PubMed] [Google Scholar]
- Feng K.; Quevedo R. E.; Kohrt J. T.; Oderinde M. S.; Reilly U.; White M. C. Late-stage oxidative C(sp3)-H methylation. Nature 2020, 580, 621–627. 10.1038/s41586-020-2137-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernak T.; Dykstra K. D.; Tyagarajan S.; Vachal P.; Krska S. W. The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev. 2016, 45, 546–576. 10.1039/C5CS00628G. [DOI] [PubMed] [Google Scholar]
- Börgel J.; Ritter T. Late-Stage Functionalization. Chem. 2020, 6, 1877–1887. 10.1016/j.chempr.2020.07.007. [DOI] [Google Scholar]
- Aynetdinova D.; Callens M. C.; Hicks H. B.; Poh C. Y. X.; Shennan B. D. A.; Boyd A. M.; Lim Z. H.; Leitch J. A.; Dixon D. J. Installing the “magic methyl” - C-H methylation in synthesis. Chem. Soc. Rev. 2021, 50, 5517–5563. 10.1039/D0CS00973C. [DOI] [PubMed] [Google Scholar]
- Schönherr H.; Cernak T. Profound Methyl Effects in Drug Discovery and a Call for New C–H Methylation Reactions. Angew. Chem., Int. Ed. 2013, 52, 12256–12267. 10.1002/anie.201303207. [DOI] [PubMed] [Google Scholar]
- Greenberg A.; Breneman C. M.; Liebman J. F.. The amide linkage: Structural significance in chemistry, biochemistry, and materials science; John Wiley & Sons, 2000. [Google Scholar]
- Sulikowski G. A.; Sulikowski M. M.; Haukaas M. H.; Moon B.. Iodomethane. In e-EROS Encyclopedia of Reagents for Organic Synthesis, 2005. https://doi.org/10.1002/047084289X.ri029m.pub2. [Google Scholar]
- Merriman G.Dimethyl Sulfate. In e-EROS Encyclopedia of Reagents for Organic Synthesis, 2001, https://doi.org/10.1002/047084289X.rd369. [Google Scholar]
- Xia Q.; Liu X.; Zhang Y.; Chen C.; Chen W. Copper-catalyzed N-methylation of amides and O-methylation of carboxylic acids by using peroxides as the methylating reagents. Org. Lett. 2013, 15, 3326–3329. 10.1021/ol401362k. [DOI] [PubMed] [Google Scholar]
- Dai X.; Shi F. N-Alkyl amide synthesis via N-alkylation of amides with alcohols. Organic & Biomolecular Chemistry 2019, 17, 2044–2054. 10.1039/C8OB03091J. [DOI] [PubMed] [Google Scholar]
- Jenner G. Homogeneous ruthenium catalysis of N-alkylation of amides and lactams. Journal of molecular catalysis 1989, 55, 241–246. 10.1016/0304-5102(89)80257-3. [DOI] [Google Scholar]
- Paul B.; Panja D.; Kundu S. Ruthenium-Catalyzed Synthesis of N-Methylated Amides using Methanol. Org. Lett. 2019, 21, 5843–5847. 10.1021/acs.orglett.9b01925. [DOI] [PubMed] [Google Scholar]
- Auerbach J.; Zamore M.; Weinreb S. M. N-Methylation of amides, lactams, and ureas. Journal of organic chemistry 1976, 41, 725–726. 10.1021/jo00866a038. [DOI] [Google Scholar]
- Basha A.; Orlando J.; Weinreb S. M. N-Methylation of Amides and Related Compounds by Reduction of Methylols. Synth. Commun. 1977, 7, 549–552. 10.1080/00397917709409275. [DOI] [Google Scholar]
- Asai S.; Ban K.; Monguchi Y.; Sajiki H.; Sawama Y. Selective N-Monoalkylation of Amide Derivatives with Trialkyl Phosphates. Synlett 2018, 29, 322–325. 10.1055/s-0036-1591494. [DOI] [Google Scholar]
- Cheng H.-G.; Pu M.; Kundu G.; Schoenebeck F. Selective Methylation of Amides, N-Heterocycles, Thiols, and Alcohols with Tetramethylammonium Fluoride. Org. Lett. 2020, 22, 331–334. 10.1021/acs.orglett.9b04400. [DOI] [PubMed] [Google Scholar]
- Templ J.; Schnürch M. Selective α-Methylation of Aryl Ketones Using Quaternary Ammonium Salts as Solid Methylating Agents. Journal of Organic Chemistry 2022, 87, 4305–4315. 10.1021/acs.joc.1c03158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gonzalo G.; Alcántara A. R.; Domínguez de María P. Cyclopentyl Methyl Ether (CPME): A Versatile Eco-Friendly Solvent for Applications in Biotechnology and Biorefineries. ChemSusChem 2019, 12, 2083–2097. 10.1002/cssc.201900079. [DOI] [PubMed] [Google Scholar]
- Prat D.; Hayler J.; Wells A. A survey of solvent selection guides. Green Chem. 2014, 16, 4546–4551. 10.1039/C4GC01149J. [DOI] [Google Scholar]
- Johnstone R. A. W.; Rose M. E. A rapid, simple, and mild procedure for alkylation of phenols, alcohols, amides and acids. Tetrahedron 1979, 35, 2169–2173. 10.1016/0040-4020(79)87035-0. [DOI] [Google Scholar]
- Alcade E.Anion recognition in supramolecular chemistry; Springer Science & Business Media, 2010; Vol. 24. [Google Scholar]
- Bordwell F. G.; Ji G. Z. Effects of structural changes on acidities and homolytic bond dissociation energies of the hydrogen-nitrogen bonds in amidines, carboxamides, and thiocarboxamides. J. Am. Chem. Soc. 1991, 113, 8398–8401. 10.1021/ja00022a029. [DOI] [Google Scholar]
- Norwood V. M. IV; Huigens R. W. III Harnessing the Chemistry of the Indole Heterocycle to Drive Discoveries in Biology and Medicine. ChemBioChem. 2019, 20, 2273–2297. 10.1002/cbic.201800768. [DOI] [PubMed] [Google Scholar]
- Rosales P. F.; Bordin G. S.; Gower A. E.; Moura S. Indole alkaloids: 2012 until now, highlighting the new chemical structures and biological activities. Fitoterapia 2020, 143, 104558. 10.1016/j.fitote.2020.104558. [DOI] [PubMed] [Google Scholar]
- Thanikachalam P. V.; Maurya R. K.; Garg V.; Monga V. An insight into the medicinal perspective of synthetic analogs of indole: A review. Eur. J. Med. Chem. 2019, 180, 562–612. 10.1016/j.ejmech.2019.07.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



