Abstract
Objective
To examine the dose-dependent influence of oral alpha-lipoic acid (ALA) supplementation on cardiometabolic risk factors in patients with type 2 diabetes (T2D).
Design
We followed the instructions outlined in the Cochrane Handbook for Systematic Reviews of Interventions and the Grading of Recommendations, Assessment, Development, and Evaluation Handbook to conduct our systematic review. The protocol of the study was registered in PROSPERO (CRD42021260587).
Method
We searched PubMed, Scopus, and Web of Science to May 2021 for trials of oral ALA supplementation in adults with T2D. The primary outcomes were HbA1c, weight loss, and LDL cholesterol (LDL-C). Secondary outcomes included fasting plasma glucose (FPG), triglyceride (TG), C-reactive protein (CRP), and blood pressure. We conducted a random-effects dose–response meta-analysis to calculate the mean difference (MD) and 95% CI for each 500 mg/day oral ALA supplementation. We performed a nonlinear dose–response meta-analysis using a restricted cubic spline.
Results
We included 16 trials with 1035 patients. Each 500 mg/day increase in oral ALA supplementation significantly reduced HbA1c, body weight, CRP, FPG, and TG. Dose–response meta-analyses indicated a linear decrement in body weight at ALA supplementation of more than 600 mg/day (MD600 mg/day: −0.30 kg, 95% CI: −0.04, −0.57). A relatively J-shaped effect was seen for HbA1c (MD: −0.32%, 95% CI: −0.45, −0.18). Levels of FPG and LDL-C decreased up to 600 mg/day ALA intake. The point estimates were below minimal clinically important difference thresholds for all outcomes.
Conclusion
Despite significant improvements, the effects of oral ALA supplementation on cardiometabolic risk factors in patients with T2D were not clinically important.
Keywords: alpha-lipoic acid, cardiometabolic, type 2 diabetes, diabetes, systematic review, meta-analysis
Introduction
Diabetes mellitus epidemics and complications resulted in major health problems and have contributed tremendously to the global burden of mortality and disability. The Global Burden of Disease Study 2013 identified diabetes mellitus as the ninth major cause of reduced life expectancy (1).
Many studies have shown that type 2 diabetes is associated with increased formation of free radicals and decreased antioxidant potential, leading to oxidative damage of cell components (2, 3, 4). Previous research has shown that most patients with type 2 diabetes suffer from dyslipoproteinemia (5), as defined by increased levels of total cholesterol (TC), triglycerides (TG), and LDL cholesterol and reduced levels of HDL cholesterol (5, 6). Increased formation of free radicals and decreased antioxidant potential, coupled with lipid abnormalities, are substantial risk factors for developing complications, especially microvascular ones.
Even though many synthetic medications have been created, none of the molecules is a complete treatment, and prolonged use of some of these synthetic drugs can have dangerous side effects (7). Using plants was reported to be a beneficial alternative therapy to manage and treat diabetes mellitus with little to no side effects compared to oral synthetic oral antidiabetic medications (7, 8). Because they are more effective at treating diabetes than Western pharmaceutical drugs, many plants have garnered a lot of attention (9).
Alpha-lipoic acid (ALA), also known as thioctic acid, is a naturally occurring compound that is synthesized enzymatically in plant and animal mitochondria from octanoic acid and cysteine (10). ALA is considered as a strong antioxidant that elicits its antioxidant effects by clearing free radicals and chelating metal ions, as well as acting on other antioxidants such as ascorbic acid and vitamin E, and increasing the intracellular glutathione levels (11, 12). ALA has gained considerable attention for managing diabetic complications due to its antioxidant properties. However, studies evaluating the effect of ALA supplementation on cardiometabolic risk factors remain controversial. While some studies found a significant effect of supplementation of ALA on inflammatory markers (13, 14), serum lipids (11), and weight (15), others failed to find such favourable effects (16, 17, 18).
Although previous reviews have investigated the effect of ALA supplementation on cardiometabolic risk factors, many of these reviews were conducted in non-type 2 diabetes patients with conflicting results (11, 13, 14). There is only one meta-analysis of intervention studies conducted among patients with type 2 diabetes (16) that included both oral and i.v. ALA supplementations. In addition, the estimates of the effect of ALA supplementation, including the certainty of the evidence for each estimate and the magnitude of the observed impact based on the minimal clinically important difference (MCID), have not been evaluated. Furthermore, potential dose-dependent effects of ALA supplementation on cardiometabolic risk factors in patients with type 2 diabetes have not been evaluated.
Dose–response meta-analysis of differences in means is a new statistical approach that can help evaluate the dose-dependent effect of interventions on continuous outcomes and thus can help present useful information needed for decision-making (19). Due to lack of observational studies addressing the association between ALA intake and cardiometabolic risk factors, we did not include observational studies in the present review. Therefore, we performed a systematic review and dose–response meta-analysis of randomized controlled trials (RCT) on the effect of oral supplementation of ALA on cardiometabolic risk factors in patients with type 2 diabetes.
Research design and methods
We followed instructions outlined in the Cochrane Handbook for Systematic Reviews of Interventions (20) and the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) Handbook (21) to conduct our systematic review. The present dose–response meta-analysis has been reported following the Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement (22). The protocol of the systematic review was registered in PROSPERO (CRD42021260587).
Systematic search
To find potential eligible RCTs for inclusion in the present review, we searched PubMed, Scopus, and Web of Science from inception up to May 2021. We supplemented the database search by manually reviewing the reference lists of all existing related reviews. The search in the databases and reference lists was not language restricted. We combined keywords related to intervention, outcome, and study design to find potential eligible RCTs. The complete search strategy is described in Supplementary Table 1 (see section on supplementary materials given at the end of this article). A team of two reviewers (ATJ and AJ) independently screened titles and abstracts according to the predefined inclusion and exclusion criteria to identify potential eligible trials. Differences between the two reviewers were resolved by a third reviewer (SS-B). The between-reviewer agreement at the full-text screening stage was assessed and reported as Cohen’s kappa coefficient (κ) (23).
Study selection
We defined inclusion and exclusion criteria according to the PICOS (population, intervention/exposure, comparator, outcome, and study design) framework (Table 1). Published human intervention studies were considered eligible for inclusion in the present dose–response meta-analysis if they had the following criteria: (1) human RCTs with a minimum intervention period of 4 weeks, conducted in adults with existing type 2 diabetes aged 18 years or older, with or without cardiovascular conditions and regardless of medication use or glucose concentration and HbA1c level; (2) evaluated the effects of ALA supplementation orally, with or without calorie restriction, physical activity, and behavioral support, against a control group; (3) considered change in body weight (weight and BMI), HbA1c, fasting plasma glucose (FPG), systolic (SBP) and diastolic blood pressure (DBP), LDL cholesterol, HDL cholesterol, TC, TG, and C-reactive protein (CRP) as the outcome of interest; (4) provided mean and s.d. of change in aforementioned outcomes or reported sufficient information to estimate those values; and (5) provided dose of ALA supplementation in the intervention group.
Table 1.
Criteria used for inclusion of randomized controlled trials.
| Participants | Type 2 diabetes patients (men and women), 18 years and above, with any sample size |
| Intervention | Oral supplementation of ALA ≥4 weeks |
| Comparator | Placebo |
| Outcome | HbA1c, LDL-C, weight and BMI, FPG, SBP, DBP, HDL-C, TC, TG, and CRP |
| Study design | Randomized controlled trials |
| Time/date | Studies published up to 21 May 2021 |
| Other | English only Full text only |
Trials with nonrandomized design, quasi-experimental studies, trials conducted in adolescents (under 18 years of age), pregnant and lactating women, trials that did not specify the amount of ALA consumption, trials that used i.v. ALA, and trials that compared the combined effects of ALA and other supplements (e.g. ALA + vitamin E) were excluded. We excluded trials that compared the combined effects of ALA plus other supplements due to their potential synergistic effects.
Outcomes
For the present review, we considered the change in HbA1c, LDL cholesterol, SBP, and body weight as primary outcomes. Secondary outcomes included change in TC, HDL cholesterol, TG, DBP, and CRP. We also considered a reduction in hypoglycemic medications and adverse events as our secondary outcomes. Adverse events included any undesirable events reported in primary trials such as hypoglycemic events and gastrointestinal discomfort.
Data extraction
Two reviewers (ATJ and AJ) independently and in duplicate screened the full texts of eligible trials and extracted the following data: author and year, population location, study design and duration, characteristics of the population (% female, mean age ± s.d., baseline BMI and body weight, health status), total sample size, intervention characteristics (dose of ALA supplementation in the intervention group), comparison group, calorie restriction, physical activity, behavioral support, outcome measures, and main results for the outcomes included. Disagreements between the two reviewers were resolved by a third reviewer (SS-B).
Risk of bias (quality) assessment
Two reviewers (ATJ and AJ) independently and in duplicate performed the risk of bias assessments using the Cochrane risk of bias tool (20). An overall quality score was given to the trials based on bias domains: low risk of bias (≤1/5 items were unknown and none were high), some concerns (≤2/5 items were unclear or at least one high), and high risk of bias (≥2/5 items were high). Disagreements regarding the risk of bias assessment were resolved by a third reviewer (SS-B).
Statistical analysis
We considered weighted mean difference (MD) and 95% CI of change in HbA1c, LDL cholesterol, body weight, FPG, SBP, DBP, HDL cholesterol, TC, TG, and CRP as the effect size for reporting the results of the present study.
First, we calculated changes from baseline values in each study. If the mean values and s.d.s of changes were not available, we calculated these values by using data from measures before and after the intervention, according to the guidelines of the Cochrane Handbook (20). When standard errors instead of s.d.s were presented, the former was converted to s.d. If either s.d. or standard error was not reported in the trials, we used the average s.d.s obtained from other trials included in the meta-analysis (24). For trials that reported median data instead of mean data, we converted the former to mean data using standard methods (25, 26).
Second, we used the method introduced by Crippa and Orsini (19) to calculate MD and its corresponding standard error of change in HbA1c, LDL cholesterol, weight, BMI, FPG, SBP, DBP, HDL cholesterol, TC, TG, and CRP for each 500 mg/day oral ALA supplementation in the intervention group relative to the control group in each trial. This method requires the dose of ALA supplementation in each study arm, the mean, and its corresponding s.d. of change in primary and secondary outcomes in each study arm, and the number of participants in each arm. Trial-specific results were pooled using DerSimonian and Laird random-effects model (27).
Based on our a priori protocol, we then performed a series of predefined subgroup analyses based on baseline weight (normal weight or overweight/obese), presence of calorie restriction and physical activity in the intervention program, and risk of bias assessment. We considered subgroup differences credible based on eight criteria introduced by the Instrument to assess the Credibility of Effect Modification Analyses (ICEMAN) (28). P for subgroup difference was generated using meta-regression analysis. Influence analysis was carried out to test the potential impact of each trial on the pooled effect size. The potential for publication bias was tested using Egger’s test (29), Begg’s test (30), and by inspection of the funnel plot when ≥10 trials were available. We assessed heterogeneity quantitatively using the I2 statistic and performed a χ2 test for homogeneity (P heterogeneity > 0.10) (31).
Finally, we performed a dose–response meta-analysis to clarify the shape of the effect of different doses of ALA on primary and secondary outcomes. Nonlinear dose–response associations were assessed with restricted cubic splines with three knots at Harrell’s recommended centiles (10%, 50%, 90%) (32). Statistical analyses were conducted using STATA software version 16.1. A two-tailed P value of less than 0.05 was considered significant.
Grading the evidence
We assessed the certainty of the evidence using the GRADE approach (33). Pairs of authors (AJ and SS-B) independently performed GRADE assessment. GRADE rates the certainty of evidence as high, moderate, low, and very low. Detailed information about the domains of the GRADE tool and how to judge each domain are provided in Supplementary Text 1. With regards to imprecision domain, we set the MCID values as 0.5% for HbA1c; 1.6 mmol/L for FPG; 4.4 kg for body weight; 1.5 kg/m2 for BMI; 0.26 mmol/L for TC; 0.10 mmol/L for LDL and HDL cholesterol; 0.09 mmol/L for TG; 2 mmHg for SBP and DBP; and 0.5 mg/dL for CRP (34).
Results
Selection of studies for inclusion in the meta-analysis
The primary search yielded 1137 studies from 3 databases (PubMed, Scopus, and Web of Sciences). After removing duplicates, 842 identified articles remained, of which, 756 were excluded during the review of title and abstract (Supplementary Fig. 1). Full texts of the remaining 86 studies were obtained and assessed. Of those, 16 studies met our criteria for inclusion in the meta-analysis. The between-reviewer agreement for including studies was near perfect (Cohen’s kappa = 0.87) at the full-text screening step. Supplementary Table 2 contains a summary of the excluded articles based on full-text assessment with reasons for exclusion.
Characteristics of primary trials included in the dose–response meta-analysis
Table 2 summarizes the general characteristics of 16 studies (15, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49) with 1035 participants included in this dose–response meta-analysis. In brief, the included RCTs had parallel designs and were published from March 1997 to October 2020. Of the 16 included studies, 8 studies were conducted in Asia (36, 40, 41, 42, 43, 45, 48, 50), 5 were conducted in Europe (15, 35, 39, 47, 49), and 3 were conducted in the United States (37, 44, 46). All trials included adult patients with type 2 diabetes. The intervention duration ranged from 4 weeks (47) to 52 weeks (35). The median sample size was 65 participants (range 14–135). In all, 11 studies reported on baseline glycemic control of patients (35, 36, 37, 39, 42, 44, 45, 46, 47, 49, 50) while only 5 studies (35, 41, 46, 49, 50) reported the use of insulin by the subjects. Only one study implemented calorie restriction (43) and programmed physical activity (36) alongside ALA supplementation. The daily doses of oral ALA ranged from 200 mg/day (40) to 1200 mg/day (36, 43). The duration of diabetes ranged from 2 (37, 42) to over 20 (46) years. No study reported on the change of medication during the intervention period. Meanwhile, six studies (36, 40, 42, 43, 48, 50) reported minor adverse events, including anorexia, diarrhea, heart burn, and other gastrointestinal problems.
Table 2.
Characteristics of trials included in dose–response meta-analysis of alpha-lipoic acid supplementation in patients with type 2 diabetes.
| Reference, country | Participants (n) | Age (mean ± s.d. or range) | Study design (duration, week) | Diabetes duration (years) | Intervention (dose) | Control |
|---|---|---|---|---|---|---|
| Didangelos, 2020, Greece (35) | Type 2 diabetes mellitus patients with diabetes duration and metformin treatment for at least 4 years and HbA1C between 6.5 and 7.5% (85) | >50 | Parallel (52) | 15 | ALA (570) | Placebo |
| Baziar, 2020, Iran (36) | Non-insulin-dependent diabetes mellitus patients with a BMI between 18.5 and 29.9 kg/m2, aged between 40 and 60 years old, diagnosis of type 2 diabetes mellitus for at least two years, and HbA1c < 7% (70) | 40–60 | Parallel (8) | 3.6 | ALA (1200 mg/day) | Placebo |
| Mendoza-Núñez, 2019, Mexico (37) | Type 2 diabetes mellitus patients with a body mass indexBMI between <35 kg/m2, mean age of 64 ± 1 years, diagnosis of type 2 diabetes mellitus for at least 2 years, and HbA1C < 7% (135) | 64 ± 1 | Parallel (26) | 1–3 | ALA (600 mg/day) | Placebo |
| Lee, 2017, Korea (38) | Type 2 diabetes patients between 20 and 80 years, with HbA1c < 11% (75) | >50 | Parallel (12) | 12.6 | ALA (600 mg/day) | Placebo |
| Derosa, 2016, Italy (39) | 18–75 years of age, with type 2 diabetes, HbA1c > 7%, and BMI ≥ 25 and < 30 kg/m2 (105) | 18–75 | Parallel (12) | Not reported (NR) | ALA (600 mg/day) | Placebo |
| Al-Saber, 2016, Bahrain (40) | Type 2 diabetes mellitus with HbA1c ≥ 6.5 and ≤ 10%, between 20 and 75 years old, BMI ≤ 44 kg/m2 (53) | >40 | Parallel (12) | NR | ALA (200 mg/day) | Placebo |
| Okanović, 2015, Bosnia and Herzegovina (15) | Obese patients with type 2 diabetes mellitus, BMI between 18.50 and 24.99 kg/m2, concentration of glucose between 4.4 and 6.1 mmol/L (60) | ≥60 | Parallel (20) | NR | ALA (600 mg/day) | Placebo |
| Udupa, 2013, India (41) | Type 2 diabetes mellitus patients between 21 and 65 years, with fasting blood sugar between 110 and 250 mg/dL (50) | 21–65 | Parallel (12) | NR | ALA (300 mg/day) | Placebo |
| Porasuphatana, 2012, Thailand (42) | Type 2 diabetes mellitus patients. Inclusion criteria included glycemic status and microalbuminuria (20–200 mg/dL) (38) | 44 ± 0.88 | Parallel (26) | 2.1 | ALA (600 mg/day) | Placebo |
| Koh, 2011, Korea (43) | Type 2 diabetes mellitus obese subjects aged 18–65 years with a BMI ≥ 30 kg/m2, fasting plasma glucose concentration ≥ 126 mg/dL (98) | >35 | Parallel (20) | NR | ALA (1200 mg/day) | Placebo |
| de Oliveira, 2011, Brazil (44) | Type 2 diabetes mellitus patients ranging in age from 38 to 75 years, at least 2 years since the diagnosis of diabetes, HbA1C > 7%, (52) | Parallel (16) | NR | ALA (600 mg/day) | Placebo | |
| Ansar, 2011, Iran (45) | Type 2 diabetes mellitus adults with a mean age of 53 years, diabetes’ duration > 1 year, and a fasting blood glucose > 126 mg/dL (57) | 53 | Parallel (8) | 7 | ALA (300 mg/day) | Placebo |
| Lukaszuk, 2009, USA (46) | Type 2 diabetics, 21–65 years, nonpregnant or lactating (20) | 21–65 | Parallel (12) | ≥1 | ALA (600 mg/day) | Placebo |
| Gianturco, 2009, Italy (47) | Type 2 diabetes mellitus patients, >50 years, diabetes diagnosis at least 5 years, HbA1C < 7% (14) | >50 | Parallel (4) | 5 | ALA (400 mg/day) | Placebo |
| Chang, 2007, Korea (48) | Hemodialysis type 2 diabetes patients, mean age of 64 years (50) | >55 | Parallel (12) | NR | ALA (600 mg/day) | Placebo |
| Ziegler, 1997, Germany (49) | Type 2 diabetes mellitus patients, aged >18 and ≤70 years, (73) | >55 | Parallel (16) | 15.2 | ALA (800 mg/day) | Placebo |
Risk of bias
All included studies were at low risk of bias in terms of incomplete outcome data, selective reporting, and other sources of bias. Ten out of 16 studies were at low risk of bias in terms of random sequence generation, 13 out 16 were at low risk in terms of allocation concealment and blinding of participants and personnel, and 9 out of 16 were at low risk in terms of blinding of the outcome assessment. A summary of the result is shown in Supplementary Table 3.
Findings from this meta-analysis
Primary outcomes
Effect of ALA supplementation on HbA1c
ALA supplementation resulted in a significant reduction in HbA1c for each 500 mg/day increase in the intervention group compared with the control group based on the analysis of 11 studies with 782 participants (35, 37, 39, 40, 41, 42, 43, 46, 48, 49, 50) (MD: –0.17%; 95% CI: −0.30 to −0.05, P = 0.008) (Table 3 and Supplementary Fig. 2). A substantial between-study heterogeneity was noted (I2 = 91.5%, P < 0.001). In the sensitivity analysis, we found that this association was influenced by the results of three studies (39, 42, 43). When each of these studies were excluded at a time from the analysis, the respective pooled effect sizes became statistically nonsignificant (Supplementary Table 4). There was a credible subgroup difference, where trials of low risk of bias showed a significant reduction (MD: −0.28%; 95% CI: −0.38 to −0.18, P = 0.009), but trials with a high risk of bias or some concerns did not (P for subgroup difference < 0.009, Supplementary Table 5).
Table 3.
Summary of the effect of supplementation with alpha-lipoic acid (each 500 mg/day) on levels of cardiometabolic risk factors in patients with type 2 diabetes.
| Outcome | Participants (studies) | Mean difference (95% CI) | MCID value | Clinically important? (≥MCID) | GRADE certainty |
|---|---|---|---|---|---|
| Primary outcomes | |||||
| HbA1c(%) | 782 (11) | −0.17 (−0.30, −0.05) | 0.5% | No | Moderate |
| BMI (kg/m2) | 563 (7) | −1.07 (−1.90, −0.25) | 1.5 kg/m2 | No | Low |
| Body weight (kg) | 349 (5) | −0.68 (−0.71, −0.64) | 4.4 kg | No | Moderate |
| LDL cholesterol (mg/dL) | 524 (9) | −5.05 (−12.93, 2.82) | 3.87 mg/dL | No | Very low |
| SBP (mmHg) | 388 (5) | −1.71 (−5.48, 2.07) | 2 mmHg | No | Very low |
| Secondary outcomes | |||||
| C-reactive protein (mg/dL) | 254 (3) | −0.03 (−0.04, −0.01) | 0.5 mg/dL | No | Low |
| DBP (mmHg) | 388 (5) | 1.03 (0.05, 2.02) | 2 mmHg | No | Low |
| FPG (mg/dL) | 620 (9) | −6.08 (−9.74, −2.42) | 29 mg/dL | No | Low |
| Triglyceride (mg/dL) | 669 (10) | −19.18 (−38.19, −0.17) | 8 mg/dL | No | Moderate |
| Total cholesterol (mg/dL) | 659 (10) | −3.57 (−24.36, 17.23) | 10 mg/dL | No | Very low |
| HDL cholesterol (mg/dL) | 609 (9) | 0.71 (−0.41, 1.83) | 3.87 mg/dL | No | Very low |
DBP, diastolic blood pressure; FPG, fasting plasma glucose; MCID, minimal clinically important difference; SBP, systolic blood pressure.
Effect of ALA supplementation on weight loss
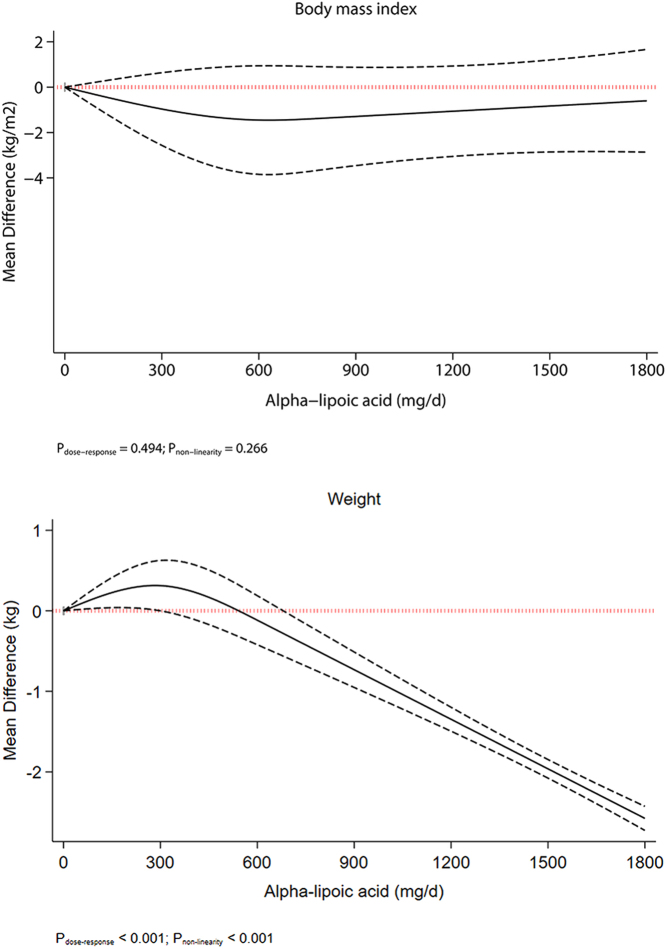
Pooled results from the random-effects model on 7 RCTs with 563 participants (15, 36, 37, 39, 43, 46, 50) showed that each 500 mg/day increase in ALA supplementation can result in a significant reduction in BMI (MD: −1.07 kg/m2; 95% CI: −1.90 to −0.25, P = 0.01) with substantial heterogeneity between the eligible studies (I2 = 94.7%, P heterogeneity < 0.0001) (Table 3 and Supplementary Fig. 3). In the sensitivity analysis, we found that this association was influenced by the results of the study by Okanovic and colleagues (15). When this study was excluded from the analysis, the pooled effect size was not statistically significant (MD: −1.08, 95% CI: −2.22, 0.05) (Supplementary Table 6). The subgroup analysis showed caloric restriction, physical activity, and the risk of bias as potential sources of heterogeneity (Supplementary Table 7).
Similarly, we observed a significant effect for each 500 mg/day increase in ALA supplementation on body weight after combining 5 trials with 349 participants (39, 43, 45, 46, 49) (MD: -0.68 kg; 95% CI −0.71 to −0.64, P < 0.001) with low heterogeneity (I2 = 0.0%, P = 0.94) (Supplementary Fig. 4). Sensitivity analysis showed that this association was influenced by the results of one study (43) which, when excluded from the analysis, rendered the pooled effect size statistically nonsignificant (MD: −0.53, 95% CI: −1.46, 0.41) (Supplementary Table 8). Subgroup analysis can be found in Supplementary Table 9, with no evidence of significant and credible effect modification.
Effect of ALA supplementation on LDL cholesterol
A nonsignificant effect for each 500 mg/day increase in ALA supplementation was observed on LDL cholesterol concentration after combining and analyzing 9 studies with 524 participants (35, 36, 37, 39, 40, 44, 46, 47, 50) (MD: −5.05 mg/dL; 95% CI: −12.93 to 2.82, P = 0.21) (Supplementary Fig. 5). The lack of association of ALA with LDL was robust as it persisted in sensitivity analyses excluding one study at a time (Supplementary Table 10). There was no credible difference across subgroups (Supplementary Table 11).
Effect of ALA supplementation on SBP
Again, a nonsignificant effect for each 500 mg/day increase in ALA supplementation was observed on SBP from the analysis of 5 trials with 388 participants (35, 37, 46, 49, 50) (MD: −1.71 mmHg; 95% CI: −5.48 to 2.07, P = 0.38) (Supplementary Fig. 6). The lack of association persisted in sensitivity analyses excluding one study at a time (Supplementary Table 12).
Secondary outcomes
Effect of ALA supplementation on CRP
Pooled results of 3 trials with 254 patients with type 2 diabetes (37, 39, 47) showed that each 500 mg/day increase in ALA supplementation resulted in a significant reduction in CRP concentration (MD: −0.03 mg/dL; 95% CI: −0.04 to −0.01, P < 0.001) (Supplementary Fig. 7) without heterogeneity between the included studies (I2 = 42.3%, Pheterogeneity = 0.18). Findings from the sensitivity analysis showed that the significant inverse association was robust, such that exclusion of each study at a time did not change the pooled effect size (Supplementary Table 13).
Effect of ALA supplementation on DBP
By combining findings from 5 trials with 388 participants (35, 37, 46, 49, 50), we found a small increase in DBP with each 500 mg/day increase in ALA supplementation (MD: 1.03 mmHg; 95% CI: 0.05–2.02, P = 0.04) (Supplementary Fig. 8) without any heterogeneity (I2 = 0.0%, P = 0.46). The association with ALA was driven by two studies and did not persist when these studies were excluded (Supplementary Table 14). Results of the subgroup analyses can be found in Supplementary Table 15, with no evidence of credible subgroup differences.
Effect of ALA supplementation on FPG
A pooled analysis of 9 trials with 620 participants (15, 36, 37, 39, 40, 41, 42, 44, 45) reported a significant effect of ALA supplementation on reduction of FPG for each 500 mg/day increase (MD: −6.08 mg/dL; 95% CI: −9.74 to −2.42, P = 0.001), with substantial between-study heterogeneity (I2 = 94.2%, P < 0.001) (Supplementary Fig. 9). The significant inverse association was robust, such that exclusion of each study at a time did not change the pooled effect size based from the findings from the sensitivity analysis (Supplementary Table 16). The subgroup analysis showed the risk of bias partly explained the heterogeneity, where a greater decrease was observed in the studies with a low risk of bias (Supplementary Table 17).
Effect of ALA supplementation on TG
Pooling the results of 10 trials with 669 participants (15, 35, 36, 37, 39, 40, 44, 46, 47, 50) showed a significant effect for each 500 mg/day increase in ALA supplementation in reducing TG levels (MD: −19.18 mg/dL; 95% CI: −38.19 to −0.17, P = 0.05) (Supplementary Fig. 10). The association with ALA was driven by six studies (36, 37, 39, 40, 44, 46) and did not persist when these studies were excluded (Supplementary Table 18). Subgroup analysis did not show credible differences between groups (Supplementary Table 19).
Effect of ALA supplementation on TC and HDL
We did not observe any statistical significant effect on serum TC concentration (MD: −3.57 mg/dL; 95% CI: −24.36 to 17.23, P = 0.74) for each 500 mg/day increase in ALA supplementation (Supplementary Fig. 11) based on the analysis of 10 studies (35, 36, 37, 39, 40, 44, 46, 47, 48, 50) and HDL (MD: 0.71 mg/dL; 95% CI: −0.41 to 1.83 mg/dL, P = 217) (Supplementary Fig. 12) based on the analysis of 9 studies with 524 participants (35, 36, 37, 39, 40, 44, 46, 47, 50). The lack of association of ALA with TC (Supplementary Table 20) and HDL (Supplementary Table 22) persisted in sensitivity analyses excluding one study at a time. The credibility of subgroup difference was rated low and the number of trials was very small.
Publication bias
Funnel plots and Egger’s regression tests indicated no evidence of substantial publication bias for HbA1c (P = 0.70), BMI (P = 0.54), weight (P = 95), LDL cholesterol (P = 0.52), CRP (P = 86), DBP (P = 0.54), TG (P = 0.96), TC (P = 0.37), HDL cholesterol (P = 0.39), and SBP (P = 0.06). However, there was evidence of a significant small-study effect for FPG (P = 0.04). The results for funnel plots are indicated in Supplementary Figures 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, and 23.
Nonlinear dose–response meta-analyses
We performed a nonlinear dose–response meta-analysis using a restricted cubic spline. Dose–response meta-analysis indicated a linear reduction in body weight up to ALA supplementation of 1800 mg/day (Fig. 1). A J-shaped effect was seen for HbA1c, with the greatest reduction at 300 mg/day (MD: −0.32%, 95% CI: −0.45, −0.18; Fig. 2). We also found the greatest reductions for FPG (MD600 mg/day : −9.2 mg/dL, 95% CI: −2.3, −15.42) and LDL cholesterol (MD600 mg/day : −7.8 mg/dL, 95% CI: −0.17, −13.28) at 600 mg/day, with the flattening of the curve at higher intake (Fig. 2).
Figure 1.
Nonlinear dose–response meta-analysis of the effect of alpha-lipoic acid supplementation on body weight.
Figure 2.
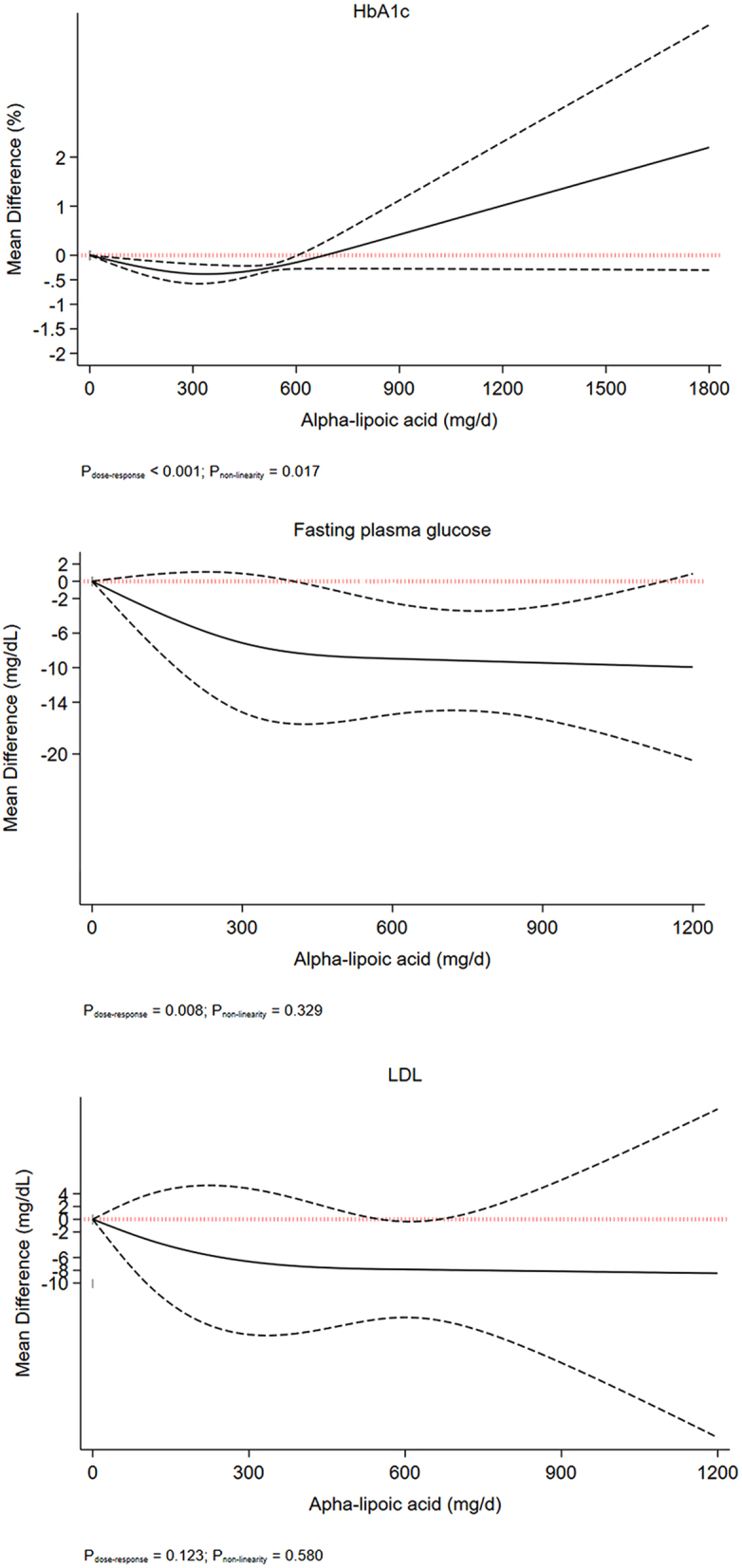
Nonlinear dose–response meta-analysis of the effect of alpha-lipoic acid supplementation on glycated hemoglobin, fasting plasma glucose, and LDL cholesterol.
Adverse events
Overall, 6 out of 16 primary trials reported adverse events after ALA supplementation in individuals with type 2 diabetes. One trial indicated that ALA was well tolerated with only 1 person had complained of heartburn after 5 weeks of ALA supplementation (36). Furthermore, another trial reported three minor adverse reactions including gastrointestinal problems, urological problems, and nervous problems (50). In the study earlier, in terms of serious adverse events, three including one gastric cancer in the ALA group and two duodenitis in the placebo group were observed. Al-Saber and colleagues (40) reported diverse adverse events with the most frequently reported events being mild to moderate gastrointestinal. Again, one patient in each group self-reported a mild event of hypoglycemia, judged by the investigator to be not related to ALA intake. Meanwhile, one serious adverse event of a nasal abscess requiring hospitalization was reported by a subject receiving ALA, which was reported not to be related to the study product. Furthermore, another study reported anorexia in one patient who voluntary dropped out of the study, and skin rash among two subjects (42). Again, in the same study, some patients reported a bitter taste in the throat after swallowing ALA capsules. Moreover, some adverse events including fever, headache, dizziness, abdominal pain, palpitation, epigastric soreness, itching, and urticarial were reported in another study, with itching sensation being the most common adverse event in subjects treated with ALA which led to the withdrawal of four subjects (43).
Grading the evidence
We applied the GRADE rating tool to rate the certainty of evidence (Table 3 and Supplementary Table 24). For primary outcomes, the certainty of the evidence was rated moderate for HbA1c and body weight, low for BMI, and very low for SBP and LDL cholesterol. For secondary outcomes, the certainty of the evidence was rated moderate for serum TG and low and very low for other outcomes. The effects of ALA supplementation on cardiometabolic outcomes were smaller than thresholds settled as MCID for all outcomes, suggesting small and unimportant effects.
Discussion
We included 16 RCTs with 1035 patients with type 2 diabetes in this study. Pooled results from the random-effects models showed that each 500 mg/day oral ALA supplementation reduced HbA1c and body weight but did not affect LDL cholesterol concentration. ALA supplementation reduced CRP, FPG, serum TG, and DBP but had no effects on serum TC and HDL cholesterol concentrations and SBP. Dose–response meta-analyses indicated a strong linear decrement in body weight at ALA supplementation of more than 600 mg/day, while a relatively J-shaped effect was seen for HbA1c, with the greatest reduction at 300 mg/day. Levels of FPG and LDL concentration decreased up to 600 mg/day ALA intake, with flattening of the curve thereafter. The certainty of the evidence was rated moderate to very low, with the point estimates well below MCID thresholds for all outcomes. No serious adverse events were reported in the original trials. To the best of our knowledge, our study is the first dose–response meta-analysis of RCTs that investigated the effect of oral ALA supplementation on cardiometabolic risk factors in patients with type 2 diabetes.
In line with our study, a previous meta-analysis reported that ALA supplementation was effective for short-term weight loss and reduction in BMI compared with placebo in various populations including patients with diabetes mellitus, metabolic syndrome, rheumatoid arthritis, and nonalcoholic fatty liver disease (51). In line with our findings, Rahimlou and colleagues also reported a significant decrease in HbA1c, CRP, and FPG after ALA supplementation in their study (52). Moreover, other studies found that ALA supplementation had beneficial effects on CRP levels (13, 14) and FPG (53). We did not observe any effect of ALA supplementation on lipid profile, including TC, LDL cholesterol, and HDL cholesterol concentrations, which was in line with observations of previous studies (16, 53). In contrast, serum TC and LDL cholesterol levels significantly decreased following ALA supplementation (11, 54).
Our study has its advantages over the previous studies in terms of methodology. While in this study we included only patients with type 2 diabetes, the previous meta-analyses included patients with different health outcomes (13, 53, 54) and with smaller sample sizes (51, 53, 54). Furthermore, others included both oral and i.v. interventions in their analyses (13, 52). Also, this study is the first dose–response meta-analysis on the effect of ALA among patients with type 2 diabetes.
Although we found statistically significant effects of ALA supplementation on cardiometabolic risk factors, these effects were smaller than MCID thresholds for all our primary outcomes, suggesting small and unimportant effects. For example, for HbA1c, the effect was −0.17% (−0.30, −0.05), which was lower than the MCID threshold for HbA1c (0.50%). Similar to our results, previous meta-analyses did not observe any clinical effectiveness of ALA supplementation on HbA1c (52), BMI and body weight (51), despite statistical significance.
We performed prespecified subgroup analyses based on the risk of bias and the presence or absence of calorie restriction or physical activity in the intervention program, and then identified potential credible differences in the subgroups based on eight criteria introduced by the ICEMAN tool (28). Although there were some significant subgroup differences, the credibility of subgroup differences was rated low due to very small number of trials in the subgroups. The subgroup analyses indicated that the size and the direction of the effects were generally the same in the subgroup of the trials with a low risk of bias that confirmed the robustness of the main findings. We also found a significant subgroup difference for BMI, where one trial that implemented calorie restriction indicated a large reduction in body weight (MD: −4.79 kg/m2; 95% CI: −5.64 to −3.49); however, the credibility of subgroup difference was rated low due to very small number of trials (n = 1) in that subgroup. Nevertheless, the combined effects of ALA supplementation and calorie restriction on body weight in patients with type 2 diabetes could be assessed in future research.
We also found a large and important improvement in LDL (MD: −15.39 mg/dL, 95% CI: −26.99, −3.78; n = 1) and HDL (MD: 19.49 mg/dL 95% CI: 10.69, 28.28; n = 1) in trials that were conducted on those without overweight/obesity. But there was no credible subgroup difference and the number of trials was very small, suggesting for further research.
Research from clinical studies suggests that ALA mimics the action of insulin via the insulin cascade. Some potential mechanisms have been shown to be associated with the effect of ALA in decreasing blood glucose including the upregulation of glucose transporters (GLUT 1 and GLUT4) (55) and via the phosphoinositide 3-kinase pathway (56, 57). Evidence supports the recruitment of GLUT4 by ALA from the Golgi body into the sarcolemma, thereby stimulating glucose uptake in the skeletal muscle. A cascade of substrate phosphorylation is known to be triggered by the acute activation of insulin receptors following ALA ingestion, which triggers the translocation of glucose transporters (GLUT) from the cytoplasm to the cell surface (58, 59). Moreover, ALA has been reported to suppress gluconeogenesis in the liver (60) while increasing glucose transport within the skeletal muscle (61).
The exact pathway of action of ALA in decreasing weight and BMI has not been identified; however, some potential mechanisms have been postulated. In animal studies, ALA was suggested to lead to decreased food consumption and enhanced energy usage via inhibiting hypothalamus AMP-activated protein kinase activity (62). In peripheral tissues, ALA leads to weight reduction by increasing fat breakdown while inhibiting its synthesis (63, 64). Furthermore, a prior investigation on people with type 2 diabetes mellitus found that taking ALA supplements increased muscular ATP production via improving mitochondrial activity (65). Therefore, increased peripheral glucose use and muscle ATP production may help with weight management.
Chronic hyperglycemia is well known to favor the production of reactive oxygen species (ROS), which in turn leads to the deterioration of beta-cell function, leading to increasing either insulin insensitivity or resistance, thus worsening type 2 diabetes. Both ROS and diabetes are associated with a wide range of inflammatory markers such as CRP, TNF-a, and IL-6, and in chronic states, these inflammatory markers may be elevated (66), particularly via the nuclear factor-κB (NF-κB) activation pathway (67). ALA and its reduced form (dihydrolipoic acid (DHLA)) have been shown to have a potent scavenging effect on neutralizing a variety of ROS (55) via suppressing NF-κB activation (68). Studies on cell lines have revealed that ALA in physiologic quantities inhibits NF-kB nuclear translocation, preventing its impact on target gene expression later on (69). Also, the decreasing effect may be associated directly with the antioxidant effect of ALA or the effect of other antioxidants (vitamin E and ascorbic acid) activated by its reduced form (DHLA) (70). Due to its synergistic effects on other antioxidants, it has been proposed that ALA may still have antioxidant benefits long after being eliminated from the body (71).
High heterogeneity across trials might be explained by different reasons such as the study sample, aims, and durations diversity. Previous studies of ALA treatment found improvements in LDL and TC (11, 54). We did not find significant improvements in these metabolic indices in the current study. One possible reason for the discordant results may be the sample sizes or differences in the study subjects. Participants in the previous studies were fewer and suffering from different health conditions compared to only diabetes mellitus in our study. Trial duration, diabetes duration, and baseline glycemic control status might be other effective factors that influenced the lipid-modulating effect of ALA supplementation. While our study averaged 16 weeks in duration, a report from a previous study (11) indicates supplementation duration of fewer than 12 weeks results in a significant reduction in serum LDL.
Clinical implications
Several dietary intervention strategies have been proposed to perform in patients with type 2 diabetes for improving glycemic control and reducing levels of cardiometabolic risk factors. People with type 2 diabetes may consume several supplements, including ALA, without robust evidence supporting their clinically important effects in patients with type 2 diabetes. In this dose–response meta-analysis of randomized trials, we indicated that the use of ALA supplementation may be an effective intervention in people with type 2 diabetes; however, its effects were small and unimportant. Therefore, current evidence does not support supplementation with ALA in patients with type 2 diabetes.
Limitations of the research
There are limitations in our meta-analysis that deserve consideration. First, we had limited evidence for the effect of ALA supplementation on some outcomes such as CRP; thus, more research is needed on the effects of oral ALA on inflammation. Second, we found that ALA resulted in a more improvement of BMI in studies with some concerns in terms of quality; thus, weight-reducing effects of ALA supplementation should be interpreted with caution. Third, we did not do trials of i.v. ALA on patients with type 2 diabetes; thus, our findings cannot be generalized to those trials. Fourth, most studies included in the present review used a dose of 600 mg/day; thus, we had limited evidence for the efficacy of ALA supplementation at a dose larger than 600 mg/day.
Conclusions
Despite significant improvements, the effects of oral ALA supplementation on cardiometabolic risk factors in patients with type 2 diabetes were not clinically important. Thus, our findings do not support its use in clinical practice for patients with type 2 diabetes. The lack of clinical importance, however, does not disprove its benefits, as we observed statistically significant effects of ALA supplementation on levels of cardiometabolic risk factors.
Supplementary Material
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author contribution statement
A J and S S-B contributed to conception/design of the research; A T J and A J contributed to acquisition, analysis, and interpretation of the data; A T J and A J drafted the manuscript; S S-B critically revised the manuscript; and S S-B agrees to be fully accountable for ensuring the integrity and accuracy of the work. All authors read and approved the final manuscript.
References
- 1.Abubakar I, Tillmann T, Banerjee A. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015385117–171. ( 10.1016/S0140-6736(1461682-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bashan N, Kovsan J, Kachko I, Ovadia H, Rudich A. Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiological Reviews 20098927–71. ( 10.1152/physrev.00014.2008) [DOI] [PubMed] [Google Scholar]
- 3.Mohsen IH, Jawad MA, Kadhim AJ, Al-Terehi MN. The oxidative stress state in diabetes mellitus type 2 patients with different medications types. Journal of Chemical Health Risks 202212 523–525. ( 10.22034/JCHR.2022.690774) [DOI] [Google Scholar]
- 4.Adnan Khalaf M, Ghassan Zainal I. Investigation of antioxidant markers in diabetic patients. Archives of Razi Institute 202176 1453–1460. ( 10.22092/ari.2021.355755.1717) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin JA, Lee JH, Lim SY, Ha HS, Kwon HS, Park YM, Lee WC, Kang MI, Yim HW, Yoon KH, Son H-Y.Metabolic syndrome as a predictor of type 2 diabetes, and its clinical interpretations and usefulness. Journal of Diabetes Investigation 20134334–343. ( 10.1111/jdi.12075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi SH, Ginsberg HN. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends in Endocrinology and Metabolism 201122353–363. ( 10.1016/j.tem.2011.04.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar S, Mittal A, Babu D, Mittal A. Herbal medicines for diabetes management and its secondary complications. Current Diabetes Reviews 202117437–456. ( 10.2174/1573399816666201103143225) [DOI] [PubMed] [Google Scholar]
- 8.Patle D, Vyas M, Khatik GL. A review on natural products and herbs used in the management of diabetes. Current Diabetes Reviews 202117186–197. ( 10.2174/1573399816666200408090058) [DOI] [PubMed] [Google Scholar]
- 9.Le Q-U, Lay H-L, Wu M-C. Herbs for the management of diabetes mellitus in traditional Vietnamese medicine. Journal of Applied Biopharmaceutics and Pharmacokinetics 201971–7. [Google Scholar]
- 10.Golbidi S, Badran M, Laher I. Diabetes and alpha lipoic acid. Frontiers in Pharmacology 20112 69. ( 10.3389/fphar.2011.00069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mousavi SM, Shab-Bidar S, Kord-Varkaneh H, Khorshidi M, Djafarian K. Effect of alpha-lipoic acid supplementation on lipid profile: a systematic review and meta-analysis of controlled clinical trials. Nutrition 201959121–130. ( 10.1016/j.nut.2018.08.004) [DOI] [PubMed] [Google Scholar]
- 12.Packer L.α-Lipoic acid: a metabolic antioxidant which regulates NF-κB signal transduction and protects against oxidative injury. Drug Metabolism Reviews 199830245–275. ( 10.3109/03602539808996311) [DOI] [PubMed] [Google Scholar]
- 13.Haghighatdoost F, Hariri M. The effect of alpha-lipoic acid on inflammatory mediators: a systematic review and meta-analysis on randomized clinical trials. European Journal of Pharmacology 2019849115–123. ( 10.1016/j.ejphar.2019.01.065) [DOI] [PubMed] [Google Scholar]
- 14.Akbari M, Ostadmohammadi V, Tabrizi R, Mobini M, Lankarani KB, Moosazadeh M, Heydari ST, Chamani M, Kolahdooz F, Asemi Z. The effects of alpha-lipoic acid supplementation on inflammatory markers among patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials. Nutrition and Metabolism 2018151–10. ( 10.1186/s12986-018-0274-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okanović A, Prnjavorac B, Jusufović E, Sejdinović RJ. Alpha-lipoic acid reduces body weight and regulates triglycerides in obese patients with diabetes mellitus. Medicinski Glasnik 201512122–127. ( 10.17392/798-15) [DOI] [PubMed] [Google Scholar]
- 16.Ebada MA, Fayed N, Fayed L, Alkanj S, Abdelkarim A, Farwati H, Hanafy A, Negida A, Ebada M, Noser Y. Efficacy of alpha-lipoic acid in the management of diabetes mellitus: a systematic review and meta-analysis. Iranian Journal of Pharmaceutical Research 2019182144–2156. ( 10.22037/ijpr.2019.1100842) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun Y-D, Dong Y-D, Fan R, Zhai L-L, Bai Y-L, Jia L-H. Effect of (R)-α-lipoic acid supplementation on serum lipids and antioxidative ability in patients with age-related macular degeneration. Annals of Nutrition and Metabolism 201260293–297. ( 10.1159/000338444) [DOI] [PubMed] [Google Scholar]
- 18.Li N, Yan W, Hu X, Huang Y, Wang F, Zhang W, Wang Q, Wang X, Sun K. Effects of oral α‐lipoic acid administration on body weight in overweight or obese subjects: a crossover randomized, double‐blind, placebo‐controlled trial. Clinical Endocrinology 201786680–687. ( 10.1111/cen.13303) [DOI] [PubMed] [Google Scholar]
- 19.Crippa A, Orsini N. Dose-response meta-analysis of differences in means. BMC Medical Research Methodology 201616 91. ( 10.1186/s12874-016-0189-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schünemann H, Brozek J, Oxman A. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendation, Version 3.2. The GRADE Working Group, 2008. [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 20096 e1000097. ( 10.1136/bmj.b2535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen J.A coefficient of agreement for nominal scales. Educational and Psychological Measurement 19602037–46. ( 10.1177/001316446002000104) [DOI] [Google Scholar]
- 24.Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. Journal of Clinical Epidemiology 2006597–10. ( 10.1016/j.jclinepi.2005.06.006) [DOI] [PubMed] [Google Scholar]
- 25.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 2014141–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Statistical Methods in Medical Research 2018271785–1805. ( 10.1177/0962280216669183) [DOI] [PubMed] [Google Scholar]
- 27.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 19867177–188. ( 10.1016/0197-2456(8690046-2) [DOI] [PubMed] [Google Scholar]
- 28.Schandelmaier S, Briel M, Varadhan R, Schmid CH, Devasenapathy N, Hayward RA, Gagnier J, Borenstein M, van der Heijden GJMG, Dahabreh IJet al. Development of the Instrument to assess the credibility of effect modification analyses (ICEMAN) in randomized controlled trials and meta-analyses. Canadian Medical Association Journal 2020192E901–E906. ( 10.1503/cmaj.200077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997315629–634. ( 10.1136/bmj.315.7109.629) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Begg CB.Publication bias. In The Handbook of Research Synthesis, pp. 299–409. Eds Cooper H, Hedges LV. New York: Russell Sage Foundation, 1994. [Google Scholar]
- 31.Higgins JP, Thompson SG, Deeks JJ, Altman DGJB. Measuring inconsistency in meta-analyses. BMJ 2003327557–560. ( 10.1136/bmj.327.7414.557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frank EH.Regression Modeling Strategies with Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. Spinger, 2015. [Google Scholar]
- 33.Brożek JL, Akl EA, Alonso‐Coello P, Lang D, Jaeschke R, Williams JW, Phillips B, Lelgemann M, Lethaby A, Bousquet JJAet al. Grading quality of evidence and strength of recommendations in clinical practice guidelines: part 1 of 3. Allergy 200964669–677. ( 10.1111/j.1398-9995.2009.01973.x) [DOI] [PubMed] [Google Scholar]
- 34.Goldenberg JZ, Day A, Brinkworth GD, Sato J, Yamada S, Jönsson T, Beardsley J, Johnson JA, Thabane L, Johnston BC. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: systematic review and meta-analysis of published and unpublished randomized trial data. BMJ 2021372m4743. ( 10.1136/bmj.m4743) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Didangelos T, Karlafti E, Kotzakioulafi E, Kontoninas Z, Margaritidis C, Giannoulaki P, Kantartzis K. Efficacy and safety of the combination of superoxide dismutase, alpha lipoic acid, vitamin B12, and carnitine for 12 months in patients with diabetic neuropathy. Nutrients 2020123254. ( 10.3390/nu12113254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baziar N, Nasli-Esfahani E, Djafarian K, Qorbani M, Hedayati M, Mishani MA, Faghfoori Z, Ahmaripour N, Hosseini S. The beneficial effects of alpha lipoic acid supplementation on Lp-PLA2 mass and its distribution between HDL and apoB-containing lipoproteins in type 2 diabetic patients: a randomized, double-blind, placebo-controlled trial. Oxidative Medicine and Cellular Longevity 20202020 5850865. ( 10.1155/2020/5850865) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mendoza-Núñez VM, García-Martínez BI, Rosado-Pérez J, Santiago-Osorio E, Pedraza-Chaverri J, Hernández-Abad VJ. The effect of 600 mg alpha-lipoic acid supplementation on oxidative stress, inflammation, and RAGE in older adults with type 2 diabetes mellitus. Oxidative Medicine and Cellular Longevity 20192019 3276958. ( 10.1155/2019/3276958) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee J, Cho JH. Letter: Effects of high-dose α-lipoic acid on heart rate variability of type 2 diabetes mellitus patients with cardiac autonomic neuropathy in Korea (Diabetes Metab J 2017;41:275-283). Diabetes and Metabolism Journal 201741417–419. ( 10.4093/dmj.2017.41.5.417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Derosa G, D'Angelo A, Romano D, Maffioli P. A clinical trial about a food supplement containing alpha-lipoic acid on oxidative stress markers in type 2 diabetic patients. International Journal of Molecular Sciences 201617 1802. ( 10.3390/ijms17111802) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Saber F, Aldosari W, Alselaiti M, Khalfan H, Kaladari A, Khan G, Harb G, Rehani R, Kudo S, Koda Aet al. The safety and tolerability of 5-aminolevulinic acid phosphate with sodium ferrous citrate in patients with type 2 diabetes mellitus in Bahrain. Journal of Diabetes Research 20162016 8294805. ( 10.1155/2016/8294805) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Udupa A, Nahar P, Shah S, Kshirsagar M, Ghongane B. A comparative study of effects of omega-3 fatty acids, alpha lipoic acid and vitamin E in type 2 diabetes mellitus. Annals of Medical and Health Sciences Research 20133442–446. ( 10.4103/2141-9248.117954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Porasuphatana S, Suddee S, Nartnampong A, Konsil J, Harnwong B, Santaweesuk A. Glycemic and oxidative status of patients with type 2 diabetes mellitus following oral administration of alpha-lipoic acid: a randomized double-blinded placebo-controlled study. Asia Pacific Journal of Clinical Nutrition 20122112–21. [PubMed] [Google Scholar]
- 43.Koh EH, Lee WJ, Lee SA, Kim EH, Cho EH, Jeong E, Kim DW, Kim M-S, Park J-Y, Park K-G. Effects of alpha-lipoic acid on body weight in obese subjects. American Journal of Medicine 201112485.e, 1–85.e8. [DOI] [PubMed] [Google Scholar]
- 44.de Oliveira AM, Rondo PHC, Luzia LA, D’Abronzo FH, Illison VK. The effects of lipoic acid and alpha-tocopherol supplementation on the lipid profile and insulin sensitivity of patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled trial. Diabetes Research and Clinical Practice 201192253–260. ( 10.1016/j.diabres.2011.02.010) [DOI] [PubMed] [Google Scholar]
- 45.Ansar H, Mazloom Z, Kazemi F, Hejazi N. Effect of alpha-lipoic acid on blood glucose, insulin resistance and glutathione peroxidase of type 2 diabetic patients. Saudi Medical Journal 201132584–588. [PubMed] [Google Scholar]
- 46.Lukaszuk JM, Schultz TM, Prawitz AD, Hofmann E. Effects of R-alpha lipoic acid on HbA1C, lipids and blood pressure in type-2 diabetics: a preliminary study. Journal of Complementary and Integrative Medicine 20096 [epub]. ( 10.2202/1553-3840.1297) [DOI] [Google Scholar]
- 47.Gianturco V, Bellomo A, D’ottavio E, Formosa V, Iori A, Mancinella M, Troisi G, Marigliano V. Impact of therapy with α-lipoic acid (ala) on the oxidative stress in the controlled niddm: a possible preventive way against the organ dysfunction? Archives of Gerontology and Geriatrics 200949 (Supplement 1) 129–133. [DOI] [PubMed] [Google Scholar]
- 48.Chang JW, Lee EK, Kim TH, Min WK, Chun S, Lee KU, Kim SB, Park JS. Effects of alpha-lipoic acid on the plasma levels of asymmetric dimethylarginine in diabetic end-stage renal disease patients on hemodialysis: a pilot study. American Journal of Nephrology 20072770–74. ( 10.1159/000099035) [DOI] [PubMed] [Google Scholar]
- 49.Ziegler D, Schatz H, Conrad F, Gries FA, Ulrich H, Reichel G. Effects of treatment with the antioxidant alpha-lipoic acid on cardiac autonomic neuropathy in NIDDM patients – a 4-month randomized controlled multicenter trial (DEKAN study ). Diabetes Care 199720369–373. ( 10.2337/diacare.20.3.369) [DOI] [PubMed] [Google Scholar]
- 50.Lee SJ, Jeong SJ, Lee YC, Lee YH, Lee JE, Kim CH, Min KW, Cha BY. Effects of high-dose alpha-lipoic acid on heart rate variability of type 2 diabetes mellitus patients with cardiac autonomic neuropathy in Korea. Diabetes and Metabolism Journal 201741275–283. ( 10.4093/dmj.2017.41.4.275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kucukgoncu S, Zhou E, Lucas KB, Tek C. Alpha-lipoic acid (ALA) as a supplementation for weight loss: results from a meta-analysis of randomized controlled trials. Obesity Reviews 201718594–601. ( 10.1111/obr.12528) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rahimlou M, Asadi M, Jahromi NB, Mansoori A. alpha-Lipoic acid (ALA) supplementation effect on glycemic and inflammatory biomarkers: a systematic review and meta-analysis. Clinical Nutrition ESPEN 20193216–28. [DOI] [PubMed] [Google Scholar]
- 53.Tabrizi R, Borhani-Haghighi A, Mirhosseini N, Lankarani KB, Naghibzadeh-Tahami A, Akbari M, Heydari ST, Sangari M, Kolahdooz F, Raygan Fet al. The effects of alpha-lipoic acid supplementation on fasting glucose and lipid profiles among patients with stroke: a systematic review and meta-analysis of randomized controlled trials. Journal of Diabetes and Metabolic Disorders 201918585–595. ( 10.1007/s40200-019-00423-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haghighatdoost F, Hariri M. Does alpha-lipoic acid affect lipid profile? A meta-analysis and systematic review on randomized controlled trials. European Journal of Pharmacology 20198471–10. [DOI] [PubMed] [Google Scholar]
- 55.Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM. Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochimica et Biophysica Acta 200917901149–1160. ( 10.1016/j.bbagen.2009.07.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Estrada DE, Ewart HS, Tsakiridis T, Volchuk A, Ramlal T, Tritschler H, Klip A. Stimulation of glucose uptake by the natural coenzyme α-lipoic acid/thioctic acid: participation of elements of the insulin signaling pathway. Diabetes 1996451798–1804. ( 10.2337/diab.45.12.1798) [DOI] [PubMed] [Google Scholar]
- 57.Moini H, Tirosh O, Park YC, Cho K-J, Packer L. R-α-lipoic acid action on cell redox status, the insulin receptor, and glucose uptake in 3T3-L1 adipocytes. Archives of Biochemistry and Biophysics 2002397384–391. [DOI] [PubMed] [Google Scholar]
- 58.Konrad D, Somwar R, Sweeney G, Yaworsky K, Hayashi M, Ramlal T, Klip A. The antihyperglycemic drug α-lipoic acid stimulates glucose uptake via both GLUT4 translocation and GLUT4 activation: potential role of p38 mitogen-activated protein kinase in GLUT4 activation. Diabetes 2001501464–1471. ( 10.2337/diabetes.50.6.1464) [DOI] [PubMed] [Google Scholar]
- 59.Konrad D.Utilization of the insulin-signaling network in the metabolic actions of α-lipoic acid – reduction or oxidation? Antioxidants and Redox Signaling 200571032–1039. ( 10.1089/ars.2005.7.1032) [DOI] [PubMed] [Google Scholar]
- 60.Lee WJ, Song KH, Koh EH, Won JC, Kim HS, Park HS, Kim MS, Kim SW, Lee KU, Park JY. α-Lipoic acid increases insulin sensitivity by activating AMPK in skeletal muscle. Biochemical and Biophysical Research Communications 2005332885–891. ( 10.1016/j.bbrc.2005.05.035) [DOI] [PubMed] [Google Scholar]
- 61.Saengsirisuwan V, Kinnick TR, Schmit MB, Henriksen EJ. Interactions of exercise training and lipoic acid on skeletal muscle glucose transport in obese Zucker rats. Journal of Applied Physiology 200191145–153. [DOI] [PubMed] [Google Scholar]
- 62.Kim M-S, Park J-Y, Namkoong C, Jang P-G, Ryu J-W, Song H-S, Yun J-Y, Namgoong I-S, Ha J, Park I-Set al. Anti-obesity effects of α-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nature Medicine 200410727–733. [DOI] [PubMed] [Google Scholar]
- 63.Fernández‐Galilea M, Pérez‐Matute P, Prieto‐Hontoria PL, Sáinz N, López‐Yoldi M, Houssier M, Martínez JA, Langin D, Moreno‐Aliaga MJ. α‐Lipoic acid reduces fatty acid esterification and lipogenesis in adipocytes from overweight/obese subjects. Obesity 2014222210–2215. ( 10.1002/oby.20846) [DOI] [PubMed] [Google Scholar]
- 64.Fernández-Galilea M, Pérez-Matute P, Prieto-Hontoria PL, Martinez JA, Moreno-Aliaga MJ. Effects of lipoic acid on lipolysis in 3T3-L1 adipocytes. Journal of Lipid Research 2012532296–2306. ( 10.1194/jlr.M027086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barbiroli B, Medori R, Tritschler H, Iotti S, Lodi R, Zaniol P. Thioctic acid stimulates muscle ATP production in patients with type-2-diabetes and diabetic polyneuropathy. Diabetes und Stoffwechsel 1996571–76. [Google Scholar]
- 66.Kaneto H, Katakami N, Matsuhisa M, Matsuoka T-a. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediators of Inflammation 20102010453892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marseglia L, Manti S, D’Angelo G, Nicotera A, Parisi E, Di Rosa G, Gitto E, Arrigo T. Oxidative stress in obesity: a critical component in human diseases. International Journal of Molecular Sciences 201516378–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hofmann MA, Schiekofer S, Isermann B, Kanitz M, Henkels M, Joswig M, Treusch A, Morcos M, Weiss T, Borcea Vet al. Peripheral blood mononuclear cells isolated from patients with diabetic nephropathy show increased activation of the oxidative-stress sensitive transcription factor NF-kappaB. Diabetologia 199942222–232. ( 10.1007/s001250051142) [DOI] [PubMed] [Google Scholar]
- 69.Zhang WJ, Frei B. α‐Lipoic acid inhibits TNF‐a‐induced NF‐κB activation and adhesion molecule expression in human aortic endothelial cells. FASEB Journal 2001152423–2432. ( 10.1096/fj.01-0260com) [DOI] [PubMed] [Google Scholar]
- 70.Tibullo D, Volti GL, Giallongo C, Grasso S, Tomassoni D, Anfuso CD, Lupo G, Amenta F, Avola R, Bramanti V. Biochemical and clinical relevance of alpha lipoic acid: antioxidant and anti-inflammatory activity, molecular pathways and therapeutic potential. Inflammation Research 201766947–959. ( 10.1007/s00011-017-1079-6) [DOI] [PubMed] [Google Scholar]
- 71.Packer L, Witt EH, Tritschler HJ. Alpha-lipoic acid as a biological antioxidant. Free Radical Biology and Medicine 199519227–250. ( 10.1016/0891-5849(9500017-r) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a