Abstract
The immunoglobulin subclass responses to homologous lipopolysaccharide (LPS) and to cholera toxin (CT) in adult patients infected with Vibrio cholerae O1 and V. cholerae O139 were studied. LPS-specific antibody-secreting cells (ASC) of both the immunoglobulin A1 (IgA1) and IgA2 subclasses were seen, with the IgA1 ASC response predominating in both V. cholerae O1- and O139-infected patients. For antibodies in plasma, by day 11 after onset of disease, all V. cholerae O1- infected patients responded to homologous LPS with the IgA1 subclass (P = 0.001), whereas fewer (68%) responded with the IgA2 subclass (P = 0.007). About 89% of V. cholerae O139-infected patients responded with the IgA1 subclass (P = 0.003), and only 21% responded with the IgA2 subclass (not significant [NS]). Both groups of cholera patients showed significant increases in LPS-specific IgG1, IgG2, and IgG3 antibodies in plasma. In feces, the response to homologous LPS occurred in both groups of patients with the IgA1 and IgA2 subclasses, with 55 to 67% of patients showing a positive response. V. cholerae O1- and O139-infected patients showed CT-specific ASC responses of the different IgG and IgA subclasses in the circulation, and the pattern followed the order IgG1 > IgA1 > IgG2 > IgA2, with low levels of IgG3 and IgG4 ASC. Plasma anti-CT antibody responses in all subclasses were seen by day 11 after onset of disease. Although there were no increases in CT-specific ASC of the IgG3 (NS) and IgG4 (NS) subtypes, there were significant increases of these two subclasses in plasma (P ≤ 0.001). The response to CT in the fecal extracts was contributed to by both IgA1 and IgA2 isotypes, with 67 to 75% of the patients responding. Thus, the mucosa-derived ASC and fecal antibodies to LPS and CT were of both the IgA1 and IgA2 subclasses; in plasma, the contribution from IgA2 was lower. Very little difference in the B-cell responses to LPS and CT in the different subclasses was seen in the two groups of cholera patients. Vaccines against O1 and O139 cholera ideally should stimulate antibody subclasses that are likely to offer protection.
The subclasses of immunoglobulin A (IgA) and IgG antibodies are known to have different functions. Several factors may influence the subclass pattern of the antibody response in infections, important among which are the type of antigen (whether proteins or polysaccharides) (6, 29), the age of the individual (13), and exposure to the antigens and the site of induction of the immune response (7). Different cytokines are also involved in the regulation of the B-cell isotype and subclass switch process (3). The different isotypes and subclasses have preferential capacities to generate immunity and protection against diseases (31). Knowledge about the subclass distribution of specific antibodies in acute watery diarrhea caused by Vibrio cholerae O1 is limited, and no information is available on the disease caused by V. cholerae O139, which has emerged as the second causative agent of cholera (2, 41). The main difference between the two serogroups is the presence of different lipopolysaccharide (LPS) antigens (14) and a capsular polysaccharide in V. cholerae O139 (37).
An earlier study of cholera vaccinees and patients (17) has shown that cholera toxin (CT) induces responses of the four IgG subclasses (IgG1, IgG2, IgG3, and IgG4) and the IgA1 subclass in serum. A study on North American volunteers has shown that secondary challenge with V. cholerae O1 results in LPS-specific responses of the IgG1 and IgG3 subclasses (21), whereas after primary exposure, the major response to LPS was of IgG4 antibodies. In this study, we have focused on understanding the subclass distributions of the LPS- and CT-specific antibody responses in patients with cholera due to V. cholerae O1 and to the relatively new serogroup O139 in order to understand the contributions of the different subclasses as well as the differences in the subclass distributions of the responses in the two groups of cholera patients.
Patients infected with V. cholerae O1 and O139 respond to homologous LPS with antibody-secreting cells (ASC) of the IgA and IgM isotypes (28). For CT, responses of the IgG and IgA isotypes are mainly seen. Antibodies of the IgG and IgA isotypes in the circulation increase in response to CT and homologous LPS (28, 35). In this study we have attempted to analyze the mucosal and systemic immune responses of the different IgG and IgA subclasses. The local antibody response in the gut has been studied by assessing the ASC in the circulation, which serve as a proxy indicator of the mucosal immune response (10, 23), as well as antibodies in feces. For the systemic response, we have analyzed antibodies in plasma.
MATERIALS AND METHODS
Study group.
Eighteen adult male patients with cholera due to V. cholerae O139 and 19 patients with cholera due to V. cholerae O1 El Tor were studied. The degree of dehydration (mild to severe) was assessed by a physician according to the Denver system (39).
Confirmation of bacterial strains.
The stool specimens of patients suffering from acute watery diarrhea were studied by dark-field microscopy (4) to detect vibrio-like bacterial movement and were cultured on taurocholate-tellurite-gelatin agar (24), and serological confirmation of suspected vibrio colonies was carried out by slide agglutination with specific antisera (28). Stool cultures were also carried out to exclude the presence of copathogens, such as Salmonella, Shigella, and Campylobacter spp. (40) and enterotoxigenic Escherichia coli (28), and in addition, direct microscopy of stool was performed to detect parasites and helminths.
Sample collection.
After microbiological confirmation of cholera, venous blood was collected from patients on the second day of hospitalization, which generally corresponded to 2 days after the onset of diarrhea (day 2). Blood was also collected 5 and 9 days later, during convalescence (that is, around 7 and 11 days after onset of disease, respectively). All patients were treated with either erythromycin or tetracycline.
MNC and plasma.
Peripheral blood mononuclear cells (MNC) were isolated from heparinized venous blood by gradient centrifugation on Ficoll-Isopaque (Pharmacia, Uppsala, Sweden). Plasma collected from the top of the Ficoll gradient was stored in aliquots at −20°C.
Detection of ASC.
The MNC were assayed for specific and total ASC numbers by the enzyme-linked immunospot technique (9) with an amplified biotin-streptavidin procedure (25). Since earlier studies with cholera patients have shown that specific ASC peak at around 7 days after the onset of cholera (26, 28), we studied the ASC response at this time point as well as at the acute stage at day 2. Assays were carried out with nitrocellulose-bottomed 96-well plates (Millititer HA; Millipore Corp., Bedford, Mass.). Plates were coated with purified LPS obtained from V. cholerae O1 El Tor Ogawa (strain 25049) or V. cholerae O139 (strain 4260B) for detection of LPS-specific responses (16, 28). For the detection of CT-specific ASC responses, the GM1-CTB enzyme-linked immunospot procedure was used (38), and purified recombinant CT subunit B (rCTB) was used to coat the plates (30). Suspensions of 0.1 ml of MNC (105 to 106 cells) were added to wells, and the plates were incubated at 37°C for 3 to 4 h in the presence of 5% CO2. Assays for specific ASC responses were carried out with murine monoclonal antibodies to human IgG1 (clone HP6070A), IgG2 (clone HP 6014A), and IgG3 (clone HP6047) (Calbiochem, La Jolla, Calif.) and IgG4 (clone HP6022) (Centers for Disease Control and Prevention, Atlanta, Ga.). All antibodies were used at a 1:250 dilution in 1% fetal bovine serum (Gibco, Gaithersburg, Md.)–phosphate-buffered saline (10 mM, pH 7.2) containing 0.05% Tween 20. After overnight incubation, biotin-labeled goat IgG anti-mouse F(ab′)2 antibodies (Jackson Immunoresearch Laboratories, Inc., West Grove, Pa.) were used (25). For the IgA subclass responses, biotin-labeled mouse anti-human IgA1 (clone B3506B4) and IgA2 (clone A9604D2) antibodies (Southern Biotechnology Associates [SBA], Birmingham, Ala.) were used. The enzyme conjugate used was streptavidin conjugated to horseradish peroxidase (Amersham, Buckinghamshire, United Kingdom), and ASC were detected by using aminoethyl carbazole (Sigma, St. Louis, Mo.) and 0.01% H2O2. The number of ASC per total number of MNC were counted. For total ASC responses, plates were coated with goat IgG anti-human kappa- and lambda-chain antisera (SBA). For CT-specific responses, ASC responses of the IgA and IgG subclasses were studied. For LPS-specific responses, only the IgA subclasses were studied. For these purposes, plates were coated with purified LPS from V. cholerae O1 or O139 (28). A fourfold or greater increase in specific ASC per 106 MNC on day 7 from that seen at the acute stage of onset of diarrhea (day 2) was indicative of a positive response.
Fecal extracts for antibody assays.
Feces collected from patients at the acute stage (day 2) and convalescence (day 11) were frozen immediately at −70°C. Fecal extracts were prepared as previously described (1, 26). Total IgA contents were determined by enzyme-linked immunosorbent assay (1) with pooled Swedish milk with a known IgA concentration of 1 mg/ml as a standard (35). The specific antibody response in fecal extracts was expressed by dividing the antigen-specific IgA1 or IgA2 antibody titers (units per milliliter) by the total IgA concentration (micrograms per milliliter) in the sample and multiplying by 10. Specimens with total IgA contents of <20 μg/ml were excluded from analyses. Acute- and convalescent-phase samples in which the total IgA concentration varied more than 10-fold were also excluded. On the basis of these exclusion criteria, only fecal samples from 12 O1 cholera patients and from 13 O139 patients could be used for the analysis of IgA subclass-specific antibody responses.
Detection of CT- and LPS-specific antibodies in plasma and fecal extracts.
Plasma and fecal extracts from patients (at the acute stage and at day 11 after onset of illness) were tested for CT and LPS subclass-specific antibodies. Plasma samples were tested for IgG and IgA subclasses (22), and fecal extracts were tested for IgA subclass antibodies only. For detecting CT-specific responses, the GM1-CT procedure (34) was used, with rCTB as the coating antigen in microtiter plates (Nunc, Roskilde, Denmark). For LPS-specific responses, microtiter plates were coated with purified V. cholerae O1 or O139 LPS (16, 28). Twentyfold-diluted serum samples and twofold diluted samples of fecal extracts were added in duplicate wells, and serial threefold dilutions were carried out. Biotinylated mouse monoclonal anti-human subclass-specific IgG1 (clone HP6019), IgG2 (clone HP6014), IgG3 (clone HP6050), and IgG4 (clone HP6025) conjugates (Sigma) and IgA1 and IgA2 conjugates (SBA) were added in appropriate wells at a 1:2,000 dilution in PBS-Tween and incubated overnight at 4°C. The detection enzyme consisted of a 1:4,000 dilution of streptavidin-conjugated horseradish peroxidase (Amersham). Plates were developed by using the substrate 3,3′,5,5′-tetramethylbenzidine (Sigma) and 0.01% H2O2. After 10 min, the reaction was stopped by the addition of 1.0 M H2SO4 (50 μl/well), and the absorbance was measured at 450 nm. The end point titer was determined as the reciprocal of the highest dilution giving an optical density of 0.2 unit above background. Titer calculations were carried out with a computer program, Multi (DataTree Inc., Watthams, Mass.). A positive response in plasma at convalescence was defined as greater than 2 standard errors of the mean (SEM) above the geometric mean titer (GMT) for CT or LPS at the acute stage (day 2). A twofold or greater increase in the specific antibody response in fecal extracts between acute (day 2)- and convalescent (day 11)-phase samples was considered a positive response.
Analyses.
The Wilcoxon signed rank test and the Mann-Whitney U test were used for statistical analysis. The Fisher exact test was used where applicable to evaluate the statistical significance of differences between groups. A P value of <0.05 was considered the criterion for a significant difference. Analyses were carried out with the statistical software SigmaStat (Jandel Scientific, San Rafael, Calif.). The geometric means and SEMs of the values were calculated for all samples.
RESULTS
Clinical history of patients.
The patients with V. cholerae O1 disease were 18 to 45 years old (median age, 30 years), and 79% experienced severe dehydration, while 21% had moderate dehydration. They were admitted with a history of 2 to 18 h (median, 6.5 h) of watery diarrhea prior to hospitalization. The patients with O139 cholera were between 20 and 50 years (median age, 30 years), and 94% had severe dehydration, while 6% had moderate dehydration; they had a history of 4 to 16 h of diarrhea (median duration, 4 h) before hospitalization.
Examination of stools from cholera patients revealed no other bacterial copathogens. However, stool microscopy showed the presence of Ascaris lumbricoides in three V. cholerae O1- and two V. cholerae O139-infected patients. In addition, in two V. cholerae O1-infected patients and one V. cholerae O139-infected patients, vegetative forms of Giardia lamblia were present.
LPS-specific ASC responses.
ASC responses to homologous LPS, i.e., LPS of the infecting serogroup, were studied only for the IgA isotype. In both groups of patients peak ASC responses, at around day 7 after onset of illness, were of the IgA1 (GMT of 341 and 116 ASC/106 MNC for V. cholerae O1- and V. cholerae O139-infected patients, respectively) and IgA2 (GMT of 40 and 24 ASC/106 MNC for V. cholerae O1- and for V. cholerae O139-infected patients, respectively) subtypes. The IgA1 response, however, predominated with all patients responding; for the IgA2 subtype, 88% of the O1-infected patients and 78% of the O139-infected patients also showed a positive response.
CT antigen-specific ASC response.
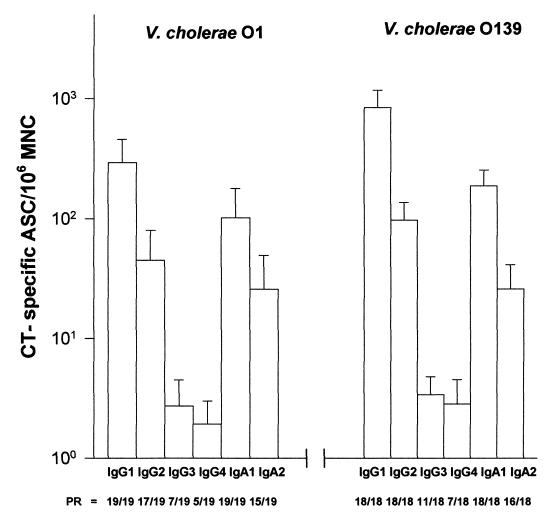
Figure 1 illustrates the CT-specific ASC responses detected in the blood of patients 7 days after onset of illness. A majority of both V. cholerae O1- and O139-infected cholera patients showed a positive CT-specific ASC response of the IgG1, IgG2, IgA1, and IgA2 subclasses. The responses in both groups were of similar magnitude and followed the order IgG1 > IgA1 > IgG2 > IgA2.
FIG. 1.
CT-specific ASC response of the IgG and IgA subclasses in V. cholerae O1 and V. cholerae O139-infected patients. ASC responses (GM ± SEM) recorded on day 7 after onset of illness are shown. PR, number of patients showing a positive ASC response to CT/total number studied.
The CT-specific ASC responses of the IgG3 and IgG4 subclasses were poor, with lower numbers of patients with positive responses.
Total ASC response.
In V. cholerae O1-infected patients, levels of total ASC of the four IgG subclasses remained unchanged from the acute stage to convalescence at day 7 (GMT of 1,295, 610, 105, and 80 ASC/106 MNC for IgG1, IgG2, IgG3, and IgG4, respectively; P = not significant [NS]). In V. cholerae O139-infected patients, there were significant increases in ASC of all the IgG subclasses from the acute stage to convalescence at day 7 (GMT of 1,106, 634, 136, and 95 ASC/106 MNC for IgG1, IgG2, IgG3, and IgG4, respectively; P = 0.001 to <0.001). In both V. cholerae O1- and O139-infected patients, the total IgA1 ASC numbers remained unchanged from the acute stage to convalescence at day 7 (GMT of 1,615 and 1,742 ASC/106 MNC for O1- and O139-infected patients, respectively; P = NS). The levels of IgA2 ASC remained unchanged in both groups of cholera patients over the course of the illness (GMT of 741 and 627 ASC/106 MNC at day 7 after onset of illness for O1 and O139 patients, respectively).
LPS- and CT-specific antibody responses of the IgG and IgA subclasses in plasma.
Increases in LPS-specific responses of the IgG1, IgG2, IgG3, and IgG4 subclasses were seen by day 11 after the onset of illness compared to those at the acute stage in V. cholerae O1-infected patients (Table 1). A similar type of pattern was seen in O139-infected patients, with responses of the different IgG subclasses (Table 1). Although 60% of the O1- and O139-infected patients responded with IgG4 antibodies by day 11, this difference from the levels seen at the acute stage was not statistically significant. The responses to LPS of the different IgG1 to IgG3 subclasses were similar in the two groups of patients over the course of the illness. The only difference was that the LPS-specific IgG4 antibody response was significantly higher in V. cholerae O139-infected patients than in V. cholerae O1-infected patients at the acute stage at day 2 (P = 0.026). There were no differences in the frequency of response or in the magnitude of response between the two groups of patients at convalescence.
TABLE 1.
IgG subclass responses to homologous LPS in plasma of cholera patients
| V. cholerae serotype | IgG subclass | GMTa (rangeb) | Fold increasec | Responder frequency (%)d | Pe |
|---|---|---|---|---|---|
| O1 | IgG1 | 393 (251–604) | 12.4 | 90 | <0.007 |
| IgG2 | 101 (42–240) | 20 | 90 | 0.009 | |
| IgG3 | 322 (214–467) | 6.6 | 80 | 0.009 | |
| IgG4 | 49 (32–75) | 6 | 80 | NS | |
| O139 | IgG1 | 676 (485–942) | 5 | 80 | 0.009 |
| IgG2 | 37 (28–50) | 9.4 | 70 | 0.03 | |
| IgG3 | 764 (586–916) | 5 | 90 | 0.002 | |
| IgG4 | 146 (92–231) | 2.5 | 60 | NS |
At 11 days after onset of disease.
GMT ± 1 SEM.
Increase of GMT at day 11 compared to that at the acute stage at day 2 after onset of disease.
Percentage showing a twofold increase in titer.
Statistical analyses (difference between titers at the acute stage and convalescence) were carried out by using the Wilcoxon signed rank test; P < 0.05 was considered significant.
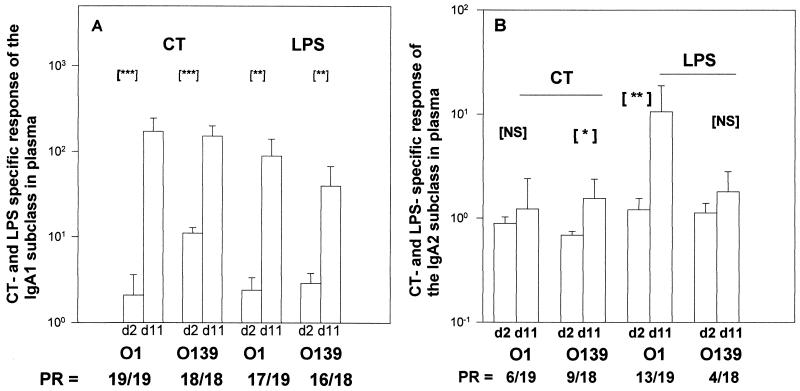
Over 89% of the patients in both groups responded with LPS-specific IgA1 antibodies in plasma by day 11 after onset of illness (Fig. 2A). However, 68% of the V. cholerae O1-infected patients responded to LPS with the IgA2 subclass (Fig. 2B), whereas only 22% of the V. cholerae O139-infected patients showed a positive response. However, there were no differences in the magnitude of the LPS-specific IgA1 or IgA2 responses between O1- and O139-infected patients over the course of the illness.
FIG. 2.
CT- and LPS-specific antibody responses, in plasma, of the IgA1 (A) and the IgA2 (B) subclasses in V. cholerae O1- and V. cholerae O139-infected patients. The GMT and SEM are shown. Asterisks indicate statistically significant differences in the responses between the acute stage (day [d] 2) and at convalescence (day 11): ∗, P < 0.05 to 0.01; ∗∗, P < 0.01 to 0.001; ∗∗∗, P < 0.001. PR, number of patients showing positive response/total number of patients studied.
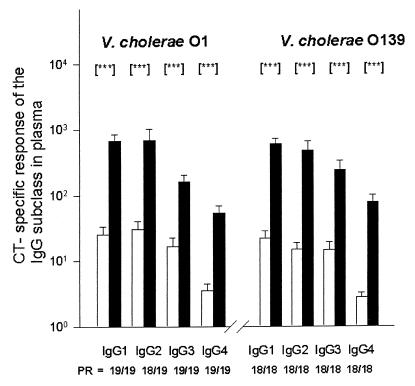
Over 95% of the cholera patients showed a positive response in CT-specific IgG antibody titers of the four IgG subclasses by day 11 after onset of illness (Fig. 3). Although the CT-specific IgG3 and IgG4 levels were low, they were significantly higher than the levels at the acute stage. The two groups of patients showed similar magnitudes of responses of the different IgG subclasses from the acute phase to convalescence.
FIG. 3.
CT-specific IgG subclass responses in the plasma of patients with cholera due to V. cholerae O1 or V. cholerae O139. The GMT and SEM are shown. Asterisks denote statistically significant differences (P < 0.001) in responses between the acute stage at day 2 (□) and at convalescence at day 11 after onset of illness (█). PR, number of patients showing a positive antibody response to CT/total number studied.
All V. cholerae O1- and O139-infected patients responded with CT-specific plasma antibodies of the IgA1 subtype by day 11 after the onset of illness (Fig. 2A). Only 32 to 50% of O1 and O139 cholera patients showed CT-specific responses of the IgA2 subclass in plasma (Fig. 2B). A significant increase in the magnitude of the CT IgA2 response from the acute stage was seen only in the case of V. cholerae O139-infected patients. However, the magnitudes of the responses observed by day 11 were similar in V. cholerae O1- and O139-infected patients for both the IgA1 and IgA2 subclasses.
CT- and LPS-specific IgA1 and IgA2 responses in fecal extracts.
Antibodies of the IgA1 and IgA2 subclasses to homologous LPS were seen in feces from 55 to 67% of the V. cholerae O1- and O139-infected patients at convalescence (Table 2). The response to CT in feces was of both the IgA1 and IgA2 subclasses (Table 2), and between 67 and 100% of the cholera patients showed a positive response. There were no significant differences in the proportions of patients showing responses of the IgA1 versus the IgA2 subclass to CT or to LPS. Similarly, there were no differences between the proportions of O1- and O139-infected patients responding to the two CT antigens.
TABLE 2.
IgA subclass responses to rCTB and homologous LPS in fecal extracts from cholera patients
| IgA subclass | Antigen | V. cholerae serotype | GMTa (rangeb) | Fold increasec | Responder frequencyd (no. of responders/total) | Pe |
|---|---|---|---|---|---|---|
| IgA1 | rCTB | O1 | 12 (8.4–16.8) | 7.7 | 75 (9/12) | NS |
| O139 | 5.4 (3.4–9.0) | 3.3 | 67 (9/13) | NS | ||
| LPS | O1 | 7.0 (4–12) | 2.7 | 67 (8/12) | NS | |
| O139 | 4.2 (3.2–5.6) | 2.3 | 67 (9/13) | 0.34 | ||
| IgA2 | rCTB | O1 | 22.0 (16–30) | 5.2 | 100 (12/12) | NS |
| O139 | 16.0 (10.4–23) | 5.2 | 75 (10/13) | 0.03 | ||
| LPS | O1 | 7.5 (6–10) | 2.5 | 67 (8/12) | NS | |
| O139 | 7.5 (4–15) | 2.0 | 55 (7/13) | NS |
GMT (specific titer/total IgA) at 11 days after onset of disease.
GMT ± 1 SEM.
Increase of GMT at day 11 compared to that at the acute stage at day 2 after onset of disease.
Percentage showing a twofold increase in titer.
Statistical analyses (difference between titers at the acute stage and convalescence) were carried out by using the Wilcoxon signed rank test; P < 0.05 was considered significant.
Comparison of responses of the IgA subclass between ASC and plasma and fecal antibodies.
More V. cholerae O1- and O139-infected patients responded with CT-specific ASC of the IgA1 subtype than with fecal antibodies (P = 0.023 to 0.049). Similarly, more responders to CT with the IgA1 isotype were seen in plasma than in feces (P = 0.023 to 0.049). Higher proportions of O1 (P = 0.008) and O139 (P = 0.027) patients responded with CT-specific IgA2 ASC than with IgA2 plasma antibodies. Again, a higher proportion of O1 (P < 0.001), but not O139 (P = NS), patients responded with IgA2 antibodies in feces than in plasma. When the response to LPS was analyzed, relatively more V. cholerae O1- and O139-infected patients responded with IgA1 ASC than with IgA1 fecal antibodies (P = 0.02). Relatively more V. cholerae O139-infected patients showed a positive LPS-specific IgA2 ASC response than showed a plasma antibody response (P = 0.002). No other difference was seen in the IgA2 response to LPS in the different compartments in either V. cholerae O1- or O139-infected patients.
DISCUSSION
The main objective of this study was to characterize the subclass distribution of the mucosal and systemic antibody responses in patients to the two important V. cholerae antigens, LPS and CT (15, 33, 34), and to assess if there were differences in the response to these antigens between patients infected with V. cholerae O1 and O139. Knowledge of the specific antibody responses in the different subclasses in natural cholera infection is limited (17), but since the disease gives long-lasting protection, more information is needed to understand the induction of the different subclasses after infection. This is particularly important since the different IgG and IgA subclasses may have distinct functional roles in the protection against disease (31).
We observed IgA ASC responses to LPS and CT of both the IgA1 and IgA2 (in about 4:1 ratio) subclasses in both groups of patients. However, in plasma the LPS- and CT-specific responses of the IgA1 subclass were high, which is similar to results obtained earlier (17). This difference between the ASC and plasma antibodies can be explained by the relative distributions of the IgA1 and IgA2 subtypes in the ileum (19) and plasma. In the small intestine, IgA1 and IgA2 immunocytes are present in ratios of about 60 and 40%, respectively, whereas in plasma about 86 and 14%, respectively, contribute to the concentration of the IgA1 and IgA2 subtypes. In the fecal extracts, however, about 60% of the IgA responses to both of the cholera-specific antigens were of the IgA2 subclass. This is understandable, since IgA1 antibodies are more prone to proteolytic degradation than IgA2 antibodies (12). Although the feces collected from the patients were treated with protease inhibitors and stored at −70°C, some denaturation may have taken place, leading to loss of IgA1 antibodies and a decreased proportion of IgA1 responses.
In both groups of cholera patients, plasma antibody responses of all four IgG subclasses to LPS were seen, although the contribution from IgG1 and IgG3 antibodies predominated. North American volunteers have also shown increases in O1 LPS-specific IgG3 antibodies after secondary exposure to V. cholerae O1 (21). For V. cholerae O139, which has recently emerged, the IgG3 response probably reflects a primary exposure to the O139 LPS. The response of the IgG1 and IgG3 subclasses to LPS may be thought of as one that contributes to the antibacterial antibody response measured by the vibriocidal antibodies, since both of these antibodies have the capacity to bind complement and hence play a role in the antibacterial antibody assay. The LPS-specific IgG1 and IgG3 responses in the study carried out with North American volunteers were highly associated with vibriocidal activity in the IgG fraction, suggesting that these subclasses may also contribute to vibriocidal antibodies (21). Although vibriocidal antibodies have been utilized as a useful proxy measure for assessing the protective immune response in patients and vaccinees (18, 27), there are limitations in the usefulness of this assay under conditions of secondary V. cholerae O1 exposure (8, 20, 36). Further studies will have to be carried out to see whether the LPS-specific response in the different subclasses can be used as an alternative marker of immunity. It is not surprising that patients responded with antibodies of the IgG4 subclass also. It is believed that IgG4 antibodies are elicited by chronic antigenic stimulation (32). The patients studied here live in an area where cholera, and related diarrheal diseases are endemic, and they are constantly being exposed to these antigens. Even Swedish vaccinees on secondary immunization with the oral cholera vaccine show a higher IgG4 response to CT than those given a primary dose (17). We have found that the IgG ASC response to CT was dominated by IgG1 and IgG2, whereas plasma antibodies of all four IgG subclasses to CT were detected. In cholera, bacteria colonize the enterocytes of the small intestine and produce disease. In this location of the gut, only about 5% of the immunoglobulin-producing immunocytes are of the IgG isotype (5), of which 55% consist of IgG1 cells, followed by about 20 to 35% IgG2 and less IgG3 or IgG4. It is therefore not surprising that the ASC response, which represents the mucosal response to CT, was seen mainly in the IgG1 and IgG2 subclasses of the IgG isotype. In plasma, on the other hand, although CT-specific IgG1 and IgG2 ASC were mainly detected, responses of the IgG3 and IgG4 isotypes were also seen. In a study carried out earlier (17), cholera patients were found to respond with systemic antibodies of the IgG1 to IgG4 isotypes. The isotype and subclass pattern of the immune response are determined by the immunogenicity and adjuvanticity, as well as the isotype-switching property, of the antigen. The IgG subclass switch, especially that of the IgG1 and IgG4 subtypes, and the mucosal IgA responses are believed to be modulated by CT as well as by the Th2-regulated synthesis of interleukin-4 or -5 (11, 21, 22). Further studies are needed to determine the role of these cytokines in patients with cholera and the modulation of the subclass-specific responses.
We have demonstrated that both groups of cholera patients showed local and systemic responses of the different antibody subclasses, but with no pattern of restriction of any IgG or IgA subclass for CT or LPS. In cholera, which is a noninvasive disease, the antitoxic and antibacterial antibodies present in the intestinal lumen are believed to offer protection against disease by neutralizing the effects of CT and inhibiting colonization (35). The relative abundances of LPS- and CT-specific IgA2 antibodies in intestinally derived ASC in blood as well as in feces suggest that protection in the gut in cholera may largely be due to this component of the IgA isotype by blocking of the activity of the virulence antigens. Studies on the protective role of the IgA2 antibodies in cholera are needed.
The present study showed that despite possessing a capsule and an LPS structurally different from that of V. cholerae O1, V. cholerae O139 induced antibody subclasses similar to those seen in O1 cholera. This information should be kept in mind when designing vaccines against both O1 and O139 cholera, and antigens that induce antibodies of a particular subclass(s) that afford protection should be better represented in the vaccine.
ACKNOWLEDGMENTS
This research was supported by the Swedish Agency for Research Cooperation with Developing Countries (Sida-SAREC) and the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B). The Centre is supported by agencies and countries which share its concern for the health problems of developing countries. Current donors providing unrestricted support include the goverments of Australia, Bangladesh, Belgium, Canada, Saudi Arabia, Sri Lanka, Sweden, Switzerland, the United Kingdom, and the United States; international organizations include United Nations Children’s Fund (UNICEF).
REFERENCES
- 1.Ahren C, Wenneras C, Holmgren J, Svennerholm A-M. Intestinal antibody response after oral immunization with a prototype cholera B subunit-colonization factor antigen enterotoxigenic Escherichia coli vaccine. Vaccine. 1993;11:929–939. doi: 10.1016/0264-410x(93)90380-g. [DOI] [PubMed] [Google Scholar]
- 2.Albert M J, Siddique A K, Islam M S, Faruque A S G, Ansaruzzaman M, Faruque S M, Sack R B. A large outbreak of clinical cholera due to V. cholerae non-O1 in Bangladesh. Lancet. 1993;341:704. doi: 10.1016/0140-6736(93)90481-u. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 3.Barington T, Juul L, Gyhrs A, Heilmann C. Heavy-chain isotype patterns of human antibody-secreting cells induced by Haemophilus influenzae type b conjugate vaccines in relation to age and preimmunity. Infect Immun. 1994;62:3066–3074. doi: 10.1128/iai.62.8.3066-3074.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benenson A S, Islam M R, Greenough W B., III Rapid identification of Vibrio cholerae by dark field microscopy. Bull W H O. 1964;30:827–831. [PMC free article] [PubMed] [Google Scholar]
- 5.Bjerke K, Brandtzaeg P. Terminally differentiated human intestinal B cells. IgA and IgG subclass-producing immunocytes in the distal ileum, including Peyer’s patches, compared with lymph nodes and palatine tonsils. Scand J Immunol. 1990;32:61–67. doi: 10.1111/j.1365-3083.1990.tb02894.x. [DOI] [PubMed] [Google Scholar]
- 6.Bradwell A R. IgG subclasses in diseases. 2nd ed. England: Kidderminster Worcester; 1993. pp. 4–5. [Google Scholar]
- 7.Brandtzaeg P. Distribution and characteristics of mucosal immunoglobulin producing cells. In: Ogra P L, Mestecky J, Lamm M E, Strober W, McGhee J R, Bienenstock J, editors. Handbook of mucosal immunology. New York, N.Y: Academic Press Inc.; 1994. pp. 251–262. [Google Scholar]
- 8.Clements M L, Levine M M, Young C R, Black R E, Lim Y L, Robins-Browne R M, Craig J P. Magnitude, kinetics and duration of vibriocidal antibody responses in North Americans after ingestion of Vibrio cholerae. J Infect Dis. 1982;143:818–820. doi: 10.1093/infdis/145.4.465. [DOI] [PubMed] [Google Scholar]
- 9.Czerkinsky C, Moldoveanu Z, Mestecky J, Nilsson L A, Ouchterlony O. A novel two colour ELISPOT assay. I. Simultaneous detection of distinct types of antibody-secreting cells. J Immunol Methods. 1988;115:31–37. doi: 10.1016/0022-1759(88)90306-7. [DOI] [PubMed] [Google Scholar]
- 10.Czerkinsky C, Svennerholm A-M, Holmgren J. Induction and assessment of immunity at enteromucosal surfaces in humans: implications for vaccine development. Clin Infect Dis. 1993;16:S106–S116. doi: 10.1093/clinids/16.supplement_2.s106. [DOI] [PubMed] [Google Scholar]
- 11.Elson C O, Walding W, Woogen S, Gaspari M. Some new perspectives on IgA immunization and oral tolerance derived from the unusual properties of cholera toxin as a mucosal immunogen. In: Strober W, Lamm M E, McGhee J R, James S P, editors. Mucosal immunity and infections at mucosal surfaces. New York, N.Y: Oxford University Press; 1988. pp. 392–400. [Google Scholar]
- 12.Feinstein D, Franklin E C. Two antigenically distinguishable subclasses of human A myeloma proteins differing in their heavy chains. Nature. 1966;212:1496–1498. doi: 10.1038/2121496a0. [DOI] [PubMed] [Google Scholar]
- 13.Freijd A, Hammarstrom L, Persson M A A, Smith C I E. Specific antibody levels in otitiis prone children. I. An analysis of plasma anti-pneumococcal antibody activity of the IgG class and subclasses. Clin Exp Immunol. 1984;56:233–238. [PMC free article] [PubMed] [Google Scholar]
- 14.Hisatunse K, Kondo R S, Isshiki Y, Iguchi T, Kawamata U, Shimada T. O-antigenic lipopolysaccharide of Vibrio cholerae O139 Bengal, a new epidemic strain for recent cholera in the Indian subcontinent. Biochem Biophys Res Commun. 1993;196:1309–1315. doi: 10.1006/bbrc.1993.2395. [DOI] [PubMed] [Google Scholar]
- 15.Holmgren J, Svennerholm A-M. Cholera and the immune response. Prog Allergy. 1983;33:106–119. [PubMed] [Google Scholar]
- 16.Jertborn M, Svennerholm A-M, Holmgren J. Intestinal and systemic immune responses in humans after oral immunization with a bivalent B subunit O1/O139 whole cell cholera vaccine. Vaccine. 1996;14:1459–1465. doi: 10.1016/s0264-410x(96)00071-0. [DOI] [PubMed] [Google Scholar]
- 17.Jertborn M, Svennerholm A-M, Holmgren J. IgG and IgA subclass distribution of antitoxin antibody responses after oral cholera vaccination or cholera disease. Int Arch Allergy Appl Immunol. 1988;85:358–363. doi: 10.1159/000234532. [DOI] [PubMed] [Google Scholar]
- 18.Jertborn M, Svennerholm A-M, Holmgren J. Saliva, breast milk, and serum antibody responses as indirect measures of intestinal immunity after oral cholera vaccination or natural disease. J Clin Microbiol. 1986;24:203–209. doi: 10.1128/jcm.24.2.203-209.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kett K, Brandtzaeg P, Radl J, Haaijman J F. Different subclass distribution of IgA-producing cells in human lymphoid organs and various secretory tissues. J Immunol. 1986;136:3631–3635. [PubMed] [Google Scholar]
- 20.Levine M M, Black R E, Clements M L, Cisneros L, Nalin D R, Young C R. Duration of infection derived immunity to cholera. J Infect Dis. 1981;143:818–820. doi: 10.1093/infdis/143.6.818. [DOI] [PubMed] [Google Scholar]
- 21.Losonsky G A, Yunyongying J, Lim V, Reymann M, Lim Y L, Wasserman S S, Levine M M. Factors influencing secondary vibriocidal immune responses: relevance for understanding immunity to cholera. Infect Immun. 1996;64:10–15. doi: 10.1128/iai.64.1.10-15.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marinaro M, Staats H F, Hiroi T, Jackson R J, Costo M, Boyaka P N, Okahashi N, Yamamoto M, Kiyono H, Bluethmann H, Fujihashi K, McGhee J R. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J Immunol. 1995;155:4621–4629. [PubMed] [Google Scholar]
- 23.McDermott M R, Bienestock J. Evidence for a common mucosal immune system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J Immunol. 1979;122:1892–1898. [PubMed] [Google Scholar]
- 24.Monsur K A. A highly selective gelatin-taurocholate tellurite medium for the isolation of Vibrio cholerae. Trans R Soc Trop Med Hyg. 1961;5:440–442. doi: 10.1016/0035-9203(61)90090-6. [DOI] [PubMed] [Google Scholar]
- 25.Ogawa T, Tarkowski A, McGhee M L, Moldoveanu Z, Mestecky J, Hirsch H Z, Koopman W J, Hamada S, McGhee J R, Kiyono H. Analysis of human IgG and IgA subclass antibody-secreting cells from localized chronic inflammatory tissue. J Immunol. 1989;142:1150–1158. [PubMed] [Google Scholar]
- 26.Qadri F, Jonson G, Begum Y A, Wennerås C, Albert M J, Salam M A, Svennerholm A-M. Immune response to the mannose-sensitive hemagglutinin in patients with cholera due to Vibrio cholerae O1 and O139. Clin Diagn Lab Immunol. 1997;4:429–434. doi: 10.1128/cdli.4.4.429-434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qadri F, Mohi G, Hossain J, Azim T, Khan A M, Salam M A, Sack R B, Albert M J, Svennerholm A-M. Comparison of the vibriocidal antibody response in cholera due to Vibrio cholerae O139 Bengal with the response in cholera due to Vibrio cholerae O1. Clin Diagn Lab Immunol. 1995;2:685–688. doi: 10.1128/cdli.2.6.685-688.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qadri F, Wennerås C, Albert M J, Hossain J, Mannoor K, Begum Y A, Mohi G, Salam M A, Sack R B, Svennerholm A-M. Comparison of immune responses in patients infected with Vibrio cholerae O139 and O1. Infect Immun. 1997;65:3571–3576. doi: 10.1128/iai.65.9.3571-3576.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russel M W, Kilian M, Lamm M E. Biological activities of IgA. In: Ogra P L, Mestecky J, Lamm M E, Strober W, Bienenstock J, McGhee J R, editors. Handbook of mucosal immunology. 2nd ed. New York, N.Y: Academic Press Inc.; 1999. pp. 225–240. [Google Scholar]
- 30.Sanchez J, Holmgren J. Recombinant system for over-expression of cholera toxin B subunit in Vibrio cholerae as a basis for vaccine development. Proc Natl Acad Sci USA. 1989;86:481–485. doi: 10.1073/pnas.86.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schreiber J R, Barrus V, Cates K L, Siber G R. Functional characterization of human IgG, IgM and IgA antibody directed to the capsule of Haemophilus influenzae type b. J Infect Dis. 1986;153:8–16. doi: 10.1093/infdis/153.1.8. [DOI] [PubMed] [Google Scholar]
- 32.Shakib F. The IgG4 subclass. Monogr Allergy. 1986;19:223–226. [PubMed] [Google Scholar]
- 33.Svennerholm A-M, Holmgren J. Synergistic protective effect in rabbits of immunization with Vibrio cholerae. Infect Immun. 1976;13:735–740. doi: 10.1128/iai.13.3.735-740.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Svennerholm A-M, Holmgren J, Black R, Levine M, Merson M. Serologic differentiation between antitoxic response to infection with Vibrio cholerae and enterotoxin-producing Escherichia coli. J Infect Dis. 1983;147:514–521. doi: 10.1093/infdis/147.3.514. [DOI] [PubMed] [Google Scholar]
- 35.Svennerholm A-M, Jertborn M, Gothefors L, Karim M M, Sack D A, Holmgren J. Mucosal antibodies and antibacterial immunity after cholera disease and after immunization with a combined B subunit-whole cell vaccine. J Infect Dis. 1984;149:884–893. doi: 10.1093/infdis/149.6.884. [DOI] [PubMed] [Google Scholar]
- 36.Tacket C O G A, Losonsky, Nataro J P, Cryz S J, Edelman R, Kaper J B, Levine M M. Onset and duration of protective immunity in challenged volunteers after vaccination with live and cholera vaccine CVD 103-HgR. J Infect Dis. 1992;166:837–841. doi: 10.1093/infdis/166.4.837. [DOI] [PubMed] [Google Scholar]
- 37.Waldor M K, Colwell R, Mekalanos J J. The Vibrio cholerae O139 serogroup antigen includes capsular and lipopolysaccharide virulence determinants. Proc Natl Acad Sci USA. 1994;91:11388–11392. doi: 10.1073/pnas.91.24.11388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wennerås C, Svennerholm A-M, Ahren A-C, Czerkinsky C. Antibody-secreting cells in human peripheral blood after oral immunization with an inactivated enterotoxigenic Escherichia coli vaccine. Infect Immun. 1992;60:2605–2611. doi: 10.1128/iai.60.7.2605-2611.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization. Manual for laboratory investigations of acute enteric infections. Geneva, Switzerland: World Health Organization; 1987. Programme for control of diarrhoeal diseases (CDD/83.3 Rev 1) pp. 9–20. [Google Scholar]
- 40.World Health Organization. Manual for the treatment of diarrhoea: for use by physicians and other senior health workers (WHO/CDD/SER/80-2, Rev. 2). Geneva, Switzerland: World Health Organization; 1990. [Google Scholar]
- 41.World Health Organization. Epidemic diarrhoea due to Vibrio cholerae non-O1. Weekly Epidemiol Rec. 1993;68:141–142. [PubMed] [Google Scholar]