Abstract
Introduction:
Medullary breast carcinomas (MBCs) are distinguished by circumscribed, high-grade morphology with dense chronic inflammation; they are associated with the basal phenotype but have a relatively good prognosis.
Methods:
This study aimed to review the clinicopathological features of MBCs diagnosed at the Department of Pathology, Singapore General Hospital and correlate them with immunohistochemical expression of hormonal markers and c-erbB-2, the basal markers p53, cytokeratin (CK) 14, epidermal growth factor receptor (EGFR) and 34BE12, and the follow-up outcome.
Results:
Using Ridolfi's criteria for histologic reviews, 62 patients previously diagnosed as having ‘typical MBC’ (n = 26), ‘atypical MBC’ (n = 32) and ‘invasive carcinoma with focal medullary-like features’ (n = 4) were re-classified as follows: ‘typical MBC’ (n = 6; 9.7%), ‘atypical MBC’ (n = 46; 74.2%), and ‘non-medullary infiltrating carcinoma’ (n = 10; 16.1%). Clinicopathological parameters, including ethnicity, age, tumour size and concurrent ductal carcinoma in situ (DCIS), showed no statistically significant correlation with review diagnoses and immunohistochemical findings. Presence of lymphovascular invasion and nodal stage were significantly correlated with recurrence and breast cancer-related deaths, respectively. ER negativity was significantly correlated with triple positivity for basal markers CK14, EGFR and 34BE12, which comprised patients who showed a significantly decreased disease-free survival rate within a 10–15-year follow-up period.
Conclusions:
Lymphovascular invasion and high nodal stage as well as triple negativity among typical and atypical MBCs that have basal-like phenotype represent a portion of invasive carcinomas with medullary features that may have poor outcomes in this otherwise relatively good prognostic group.
Keywords: Clinical outcomes, immunohistochemistry, medullary breast carcinoma, survival, tissue microarray
INTRODUCTION
Medullary breast carcinomas (MBCs) have been recognised since 1949, when Moore first described a 'medullary-like carcinoma' with lymphoid infiltrate, syncytial growth and low rate of metastasis associated with a favourable prognosis.[1] Owing to the lack of universally accepted criteria for diagnosing MBC in the 1950s, the survival data may have been skewed by the inclusion of high-grade invasive ductal carcinomas (IDCs). In 1977, Ridolfi refined the criteria for diagnosing MBC,[2] subdividing it into typical medullary carcinoma and atypical medullary carcinoma, and subsequently found significant prognostic differences between the two subtypes, with the typical cases harbouring a better prognosis. The World Health Organization classified MBC as a special subtype of invasive breast carcinoma with specific histologic characteristics, refined from Ridolfi's criteria, as follows:[2,3] complete microscopic circumscription; predominantly syncytial growth pattern; moderate to marked diffuse mononuclear stromal infiltrate; absence of microglandular features and in situ component; moderate or marked nuclear pleomorphism; and numerous mitoses. MBC has been reported to have a better prognosis than that of conventional breast carcinoma,[1,2,3,4,5,6] with the five-year survival rates ranging from 82% to 84% and 50% to 63%, respectively,[1,2] compared with IDC, not otherwise specified (NOS). Hence, it is important to distinguish MBC from high-grade infiltrative ductal cancers that have poorer prognosis.
Since the classification of medullary carcinoma as a distinct entity, there have been controversies with regard to its morphologic criteria, immunological profile, and genetic and prognostic implications. MBCs have a relatively good prognosis despite their poorly differentiated histomorphology and their diagnostic criteria show the lowest inter-observer reproducibility among all breast tumour subtypes.[7] They are frequently associated with BRCA1 germline mutations, with a large overlap between medullary features and the phenotype of BRCA1 germline-associated tumours,[8,9] and a high rate of TP53 mutations, with higher TP53 accumulation than that observed in common IDCs.[10] Their typical lack of oestrogen receptors and low incidence of c-erbB-2 amplification classify them into a distinct subgroup of triple negative basal-like cancers, which has been confirmed by several gene expression profiling studies.[11,12,13,14] Although triple negativity and basal phenotype generally imply a poor prognosis, MBCs have long been associated with a 'relatively good prognosis', augmenting the paradox of the prognostic implications of MBCs.
Several studies have been conducted in Western countries in order to better understand the clinicomorphologic presentation and biologic behaviour of MBCs.[15,16,17] Our study aimed to correlate the clinicopathologic features and immunohistochemical findings of hormonal markers and c-erbB-2, common basal markers and follow-up outcomes of Asian women diagnosed as having breast carcinomas harbouring medullary features.
METHODS
The files of the Singapore General Hospital's Department of Pathology were searched for mastectomies and wide excision specimens diagnosed as medullary or atypical medullary carcinoma from 1991 to 2009. Demographic and clinicopathological parameters such as patient ethnicity, age, tumour size, concurrent ductal carcinoma in situ (DCIS), presence of lymphovascular invasion and number of axillary lymph node metastases were studied. Light microscopic review was performed concurrently by two pathologists including an experienced subspecialist in breast pathology, and the cases were reclassified using the criteria established by Ridolfi et al. The criteria proposed by Ridolfi for typical MBC in his original paper are shown in Box 1.
Box 1.
Ridolfi’s criteria for typical medullary breast carcinoma:(2)
| 1. Syncytial growth pattern Syncytial sheets of pleomorphic cells arranged in anastomosing trabeculae should comprise >75% of the tumour [Figure 1]. |
| 2. Circumscription The tumour was considered ‘well-circumscribed’ when all margins were microscopically circumscribed and convex [Figure 2]. |
| 3. Absence of DCIS component DCIS peripheral to or distinct from the main tumour mass was categorised as ‘absent’ if not visualised at all outside the tumour and ‘present’ if DCIS was detected in at least one microscopic field peripheral to or distinct from the tumour. The nuclear grade of the DCIS was also classified as low, intermediate or high. |
| 4. Absence of microglandular formation Any presence of gland formation within the tumour was regarded as microglandular formation. |
| 5. Moderate to marked diffuse mononuclear infiltrate Ridolfi had used a four-point scale (0 to 1+, 2+, 3+ and 4+) to quantify the intensity of mononuclear inflammation and further characterised distribution (diffuse vs. limited to borders only). |
DCIS: ductal carcinoma in situ
Figure 1.
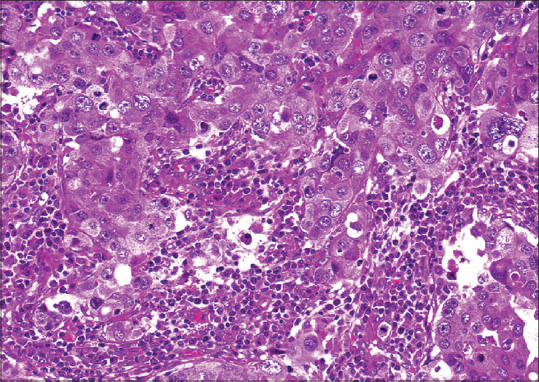
Photomicrograph shows syncytial growth pattern exhibited by a medullary breast carcinoma, with distinct intercellular borders (H&E, ×40).
Figure 2.
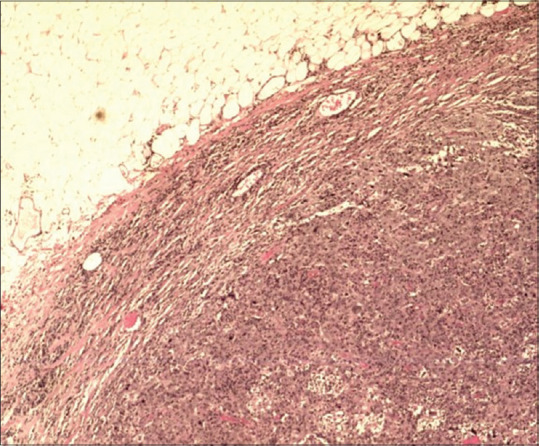
Photomicrograph shows a well-circumscribed border in a medullary breast carcinoma (H&E, ×20).
For our study, we used three descriptive categories, namely 'mild' [Figure 3], 'moderate' and 'marked' [Figure 4], with the latter two corresponding closely to diffuse mononuclear inflammation comparable to the photomicrographic examples of 'moderate' to 'marked' intensities characterised by Ridolfi. Ridolfi had used a four-point scale (0 to 1+, 2+, 3+ and 4+) to quantify the intensity of mononuclear inflammation and further characterised distribution (diffuse vs. limited to borders only). For our study, we used three descriptive categories, namely 'mild' [Figure 3], 'moderate' and 'marked' [Figure 4], with the latter two corresponding closely to diffuse mononuclear inflammation comparable to the photomicrographic examples of 'moderate' to 'marked' intensities characterised by Ridolfi.
Figure 3.
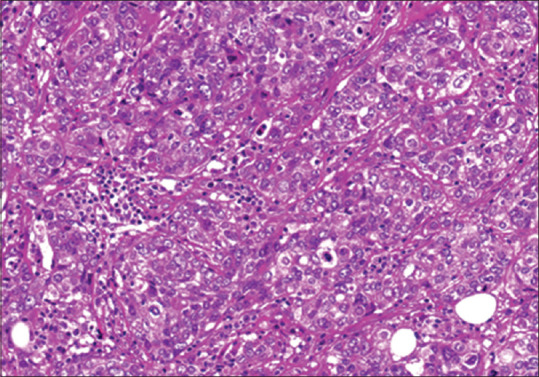
Example photomicrograph shows mild lymphocytic infiltrate within a medullary breast carcinoma (H&E, ×40).
Figure 4.
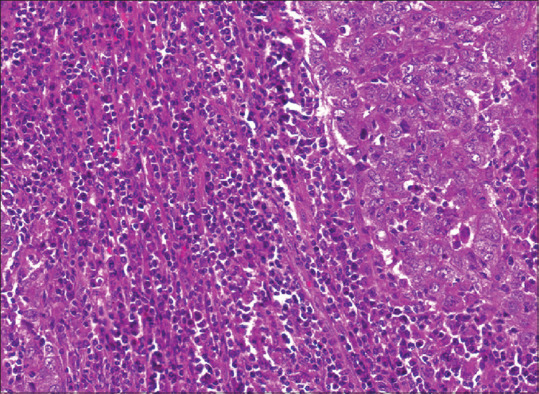
Photomicrograph shows a marked lymphocytic infiltrate within and around the tumour, in contrast to [Figure 3] (H&E, ×40).
Using criteria 1 to 5, under light microscopic review, tumours fulfilling all five criteria were diagnosed as 'typical medullary carcinoma'. Tumours that fulfilled Criterion 1 and at least three of the criteria from 2 to 5 were described as 'atypical'. Those that failed to display more than 75% syncytial growth (i.e. Criterion 1) or satisfied less than three criteria from Criteria 2 to 5 were designated as 'non-medullary infiltrating carcinoma'.
Archived 10% formalin-fixed paraffin-embedded material for the cases studied was retrieved where available. Suitable representative areas of the tumour were selected and tissue microarray (TMA) construction was performed using methods described previously in the literature.[18] Briefly, 1-mm diameter cores were bored from the preselected areas of the donor paraffin blocks and arrayed into a recipient paraffin block using a manual microarrayer (Beecher Instruments, Sun Prairie, WI, USA). Two cores were bored for each case; 4-micron thick TMA paraffin sections were then cut and subjected to immunohistochemistry for oestrogen and progesterone receptors (ER and PR), c-erbB-2 and p53. A tri-panel of basal cell markers was used to evaluate basal-like characteristics of the tumours. Cytokeratin 14 (CK14), epidermal growth factor receptor (EGFR) and 34BE12 were used as basal markers. Immunohistochemistry was performed using commercially available antibodies. Briefly, the TMA slides were deparaffinised with xylene and then rehydrated through serial alcohol washes. Antigens were retrieved using Tris-buffered EDTA before incubation with the primary antibody, followed by automated immunohistochemical staining through a Ventana Benchmark XT instrument (Ventana Medical Systems, Tucson, AZ, USA). The chromogen used was 3,3′-diaminobenzidine tetrahydrochloride (Dako Liquid DAB+; Dako, Glostrup, Denmark). All sections were counterstained with Mayer's haematoxylin. Signal detection was performed using the ultraView Universal DAB Detection Kit (Ventana Medical Systems, Tucson, AZ, USA). Positive and negative external controls were included as per the manufacturers' datasheets [Table 1].
Table 1.
Immunohistochemical markers and their cut-off positivity.
| Marker | Clone | Host | Catalogue number | Dilution | Antigen retrieval | Antibody incubation time | Positivity cut-off |
|---|---|---|---|---|---|---|---|
| ER | SP1 | Rabbit Monoclonal | #RM-9101-R7 | 1:50 | 0.01M Tris-EDTA pH 8.7 at 98°C for 15 min | 30 min, RT | 1+ (1%) |
|
| |||||||
| PR | PgR 636 | Mouse Monoclonal | Dako M3569 | 1:200 | 0.01M Tris-EDTA pH 8.7 at 98°C for 15 min | 30 min, RT | 1+ (1%) |
|
| |||||||
| c-erb-B2 | SP3 | Rabbit Monoclonal | #RM-9103-R7 | 1:200 | 0.01M Tris-EDTA pH 8.7 at 98°C for 15 min | 30 min, RT | 3+ (30%) |
|
| |||||||
| CK14 | LL002 | Mouse Monoclonal | Novocastra NCL-LL002 | 1:20 | 0.01M Tris-EDTA pH 8.7 at 98°C for 15 min | 30 min, RT | 1+ (1%) |
|
| |||||||
| EGFR | E30 | Mouse Monoclonal | Dako M7239 | 1:50 | Protease at 40°C for 10 min | 30 min, RT | 1+ (1%) |
|
| |||||||
| 34BE12 | 34BE12 | Mouse Monoclonal | Dako M0630 | 1:200 | 0.01M Tris-EDTA pH 8.7 at 98°C for 15 min | 30 min, RT | 1+ (1%) |
|
| |||||||
| P53 | D07 | Mouse Monoclonal | Dako M7001 | 1:70 | Standard CC1* at 99°C for 60 min | 32 min, 42°C | 1+ (1%) |
EDTA: ethylenediaminetetraacetic acid; RT: room temperature
The results were reported as positive if nuclear staining of at least 1+ staining intensity was observed in 1% of tumour cells for ER and PR, and membranous staining of at least 3+ was observed in 30% tumour cells for c-erbB-2, as per the manufacturers' recommendations. Cytoplasmic staining of at least 1+ staining intensity in 1% of tumour cells for CK14, 34BE12 and p53, and cytoplasmic membrane staining of at least 1+ intensity in 1% of tumour cells for EGFR were interpreted as positive results [Table 1]. Cases that showed positive results for at least one out of the markers CK14, EGFR or 34BE12 were designated as 'basal-like'.
Data was analysed using statistical software SPSS for Windows version 16.0 (SPSS Inc, Chicago, IL, USA). Clinicopathologic features were correlated with each other, with immunohistochemical findings, and with the follow-up outcomes using Chi-square and Fisher's exact tests. Survival analysis was based on deaths due to breast cancer, and the time from initial surgery to death due to breast cancer was considered for analysis. Disease-free survival was defined as the time from diagnosis to recurrence or breast cancer-specific death. Overall survival was defined as the time from diagnosis to the date of the last follow-up. Survival curves were estimated using the Kaplan-Meier method and compared between groups using the log-rank statistics. A P value <0.05 was considered statistically significant. The median follow-up period for all patients was 7.5 (range 1.5–19) years, with nine cases of recurrences/metastases to distant sites and seven cancer-related deaths.
RESULTS
In total, 62 women with a mean age of 51.1 (range 32–71, median 52) years who were originally diagnosed with medullary or atypical medullary carcinoma were recruited in this study; of these, 46 (75.2%) were Chinese, 6 (9.7%) were Malay, 4 (6.5%) were Indian and 6 (9.7%) were of other Asian ethnicities. In total, 42 patients underwent simple mastectomies and 20 patients underwent wide excision biopsies. The mean tumour size was 2 (range 0.55–4.50) cm.
The original pathology reports showed 26 (41.9%) 'medullary carcinomas', 32 (51.6%) 'atypical medullary carcinomas' and 4 (6.4%) 'invasive ductal carcinomas with focal medullary features', whereas review diagnoses showed typical MBC in 6 (9.7%) cases, five of which were concordant with the original diagnosis and one case was originally diagnosed as 'atypical medullary carcinoma'. Atypical MBCs being reviewed (46 of 62; 74.2%) were originally diagnosed as 'medullary carcinoma', 18 of which were specified as 'typical medullary carcinoma' in the original report; 10 (16.1%) cases reviewed as non-medullary infiltrating carcinoma were originally diagnosed as 'typical medullary carcinoma' (n = 2), 'atypical medullary carcinoma' (n = 4) and 'invasive ductal carcinoma with focal medullary features' (n = 4).
DCIS was present in 15 (24.2%) cases, 12 of which were reviewed as atypical MBC and three as non-medullary infiltrating carcinoma. Six of these cases were of high-grade, solid subtype, three of which had comedonecrosis. The remaining nine cases were of low to intermediate grade with either solid or cribriform architecture, uniformly lacking necrosis. The presence of DCIS did not correlate significantly with the review diagnoses, immunohistochemical findings or follow-up outcome.
Only 4 (6.5%) cases had lymphovascular invasion, three of which were atypical MBCs and one was a non-medullary infiltrating carcinoma. Interestingly, three of these four cases developed contralateral breast cancer two to six years after diagnosis, presenting as triple negative invasive ductal carcinoma, Grade 3. Presence of lymphovascular invasion was significantly correlated to decreased disease-free survival (P = 0.001) but had no statistical correlation with age, ethnicity, tumour size, presence of DCIS or immunohistochemical markers.
Of the 59 cases with axillary clearance, 6 (10.2%) were associated with nodal metastases; four cases were atypical MBCs (three cases staged as N1, one case as N3) and two cases of typical MBC had N1 and N2 nodal stages [Table 2]. The case of typical MBC with N2 nodal stage showed contralateral axillary nodal recurrence and metastasis to bone marrow within five years after diagnosis, and that of atypical MBC with N3 nodal stage showed metastasis to supraclavicular node within two years after diagnosis. Both patients died within two years of recurrence/metastases. High nodal stage (N2 or higher) was significantly correlated to poor overall survival (P = 0.03), but had no statistical correlation with age, ethnicity, tumour size, presence of DCIS or immunohistochemical markers.
Table 2.
Details of node-positive cases.
| Tumour subtype | Involved axillary nodes/total nodes | Nodal stage | Size of main tumour (cm) | Follow-up outcome (years after surgery) |
|---|---|---|---|---|
| Atypical | 1/22 | N1 | 2 | No recurrence (7) |
|
| ||||
| Atypical | 16/23 | N3 | 3.5 | Metastasis to supraclavicular node (1.8); died 1 year after recurrence |
|
| ||||
| Atypical | 1/9 | N1 | 1.9 | No recurrence (19) |
|
| ||||
| Atypical | 1/(not specified) | N1 | Not specified | No recurrence (13) |
|
| ||||
| Typical | 3/20 | N1 | 1.7 | No recurrence (7) |
|
| ||||
| Typical | 4/20 | N2 | 2.4 | Metastases to contralateral axilla and bone marrow (4.8); died 1.5 years after recurrence |
The majority (45/62; 72.6%) of cases showed negative results for ER, PR and c-erbB-2 immunohistochemical expression, with typical MBCs showing triple negative findings in all six cases. Atypical MBCs and non-medullary infiltrating carcinomas were ER+ (2 of 62; 3.2%), PR+ (1 of 62; 1.6%) or positive for both (10 of 62, 6.2%) and negative for c-erbB-2. Five cases were positive for c-erbB-2 on immunohistochemistry, and were negative for ER and PR [Table 3]. Correlation with disease-free survival showed significantly poor prognosis for the triple-negative cancers compared with the non-triple-negative cases (P = 0.021; Figure 5). The overall survival of patients with triple-negative cancer showed a downward trend compared with that of patients with non-triple-negative cancer, with a statistically significant difference (P = 0.056).
Table 3.
Immunohistochemistry results for each subtype.
| Tumour subtype | No. (%) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| ER/PR | c-erbB-2 | |||||
|
|
|
|||||
| ER+/PR+ | ER+/PR− | ER−/PR+ | ER−/PR− | Positive | Negative | |
| Typical (n=6) | 0 | 0 | 0 | 6 (100.0) | 0 | 6 (100.0) |
|
| ||||||
| Atypical (n=46) | 9 (19.6) | 2 (4.3) | 1 (2) | 34 (73.9) | 4 (8.7) | 42 (91.3) |
|
| ||||||
| Non-medullary (n=10) | 1 (10.0) | 0 | 0 | 9 (90.0) | 1 (10.0) | 9 (90.0) |
|
| ||||||
| Total (n=62) | 10 (16.1) | 2 (3.2) | 1 (1.6) | 49 (79.0) | 5 (8.1 | 57 (91.9) |
ER: oestrogen receptor; PR: progesterone receptor
Figure 5.
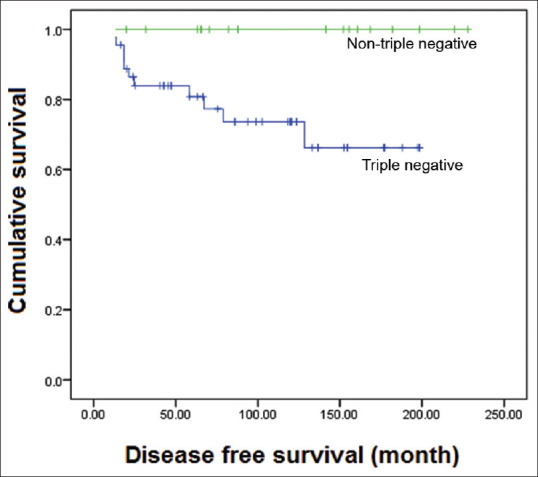
Graph shows the disease-free survival curve of cases negative for oestrogen receptor, progesterone receptor and c-erbB-2, compared with non-triple-negative cases (P = 0.021).
Regardless of the histologic subtype, the majority of cases were positive for p53 and at least one of the basal markers CK14, EGFR and 34BE12 [Table 4]. All typical MBCs were positive for 34BE12, which significantly correlated to their being negative for ER (p <0.001), and five of the six typical MBCs were positive for all three basal markers and three were positive for p53. Three atypical MBCs were positive for all the three basal markers. EGFR showed significant inverse correlation with PR staining (P = 0.021). Cases that were positive for all three basal markers (8 of 62; 12.9%) were also significantly correlated to ER negativity (P = 0.003). 4 (6.4%) cases were negative for the three basal markers (i.e. non-basal like). Three of these non-basal-like breast cancers were atypical MBCs and one was a non-medullary infiltrating carcinoma, all of which were positive for ER and PR. Basal-like carcinomas showed a distinct downward trend towards poor disease-free survival and overall survival compared with the non-basal-like phenotype, although their correlation was not statistically significant (P = 0.312) [Figure 6].
Table 4.
Basal-myoepithelial marker expression for each subtype.
| Tumour subtype | No. (%) | ||||
|---|---|---|---|---|---|
|
| |||||
| p53 | CK14 | EGFR | 34BE12 | CK14/EGFR/34BE12 positive | |
| Typical (n=6) | 3 (50.0) | 4 (66.6) | 5 (83.3) | 6 (100.0) | 5 (83.3) |
|
| |||||
| Atypical (n=46) | 37 (80.4) | 32 (69.6) | 31 (67.3) | 41 (89.1) | 3 (6.5) |
|
| |||||
| Non-medullary (n=10) | 9 (90.0) | 6 (60.0) | 6 (60.0) | 7 (70.0) | 0 |
|
| |||||
| Total (n=62) | 49 (79.0) | 42 (67.7) | 42 (67.7) | 54 (87.0) | 8 (12.9) |
CK14: cytokeratin 14; EGFR: epidermal growth factor receptorTable 5. Breast-cancer specific deaths and sites of recurrences per tumour subtype within a ten-year follow-up period.
Figure 6.
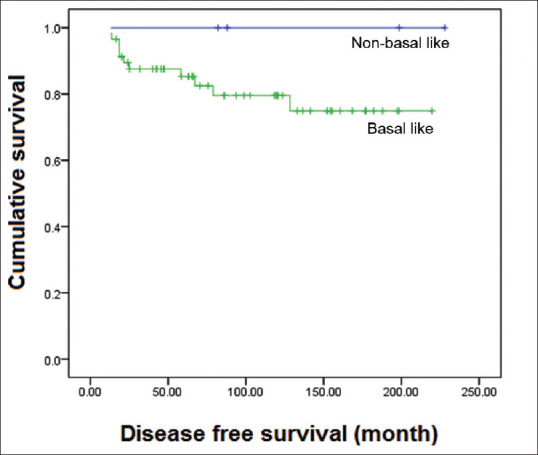
Graph shows the disease-free survival curve for basal-like carcinomas (positive for at least one basal marker CK14, EGFR, or 34BE12) compared with those that were negative for any basal marker. The downward trend of basal-like cancers is evident, although not statistically significant (P = 0.312).
The majority of cases experienced neither recurrence nor metastases after excision or mastectomy within a follow-up period of 1.5–19.0 years. 9 (14.5%) cases developed recurrence/metastases within a follow-up period of 0.5–9.6 years. Seven of these cases were of atypical MBC, and one each of typical MBC and non-medullary infiltrating carcinoma. Six of these cases lead to breast cancer-specific deaths within the same day up to 18 months (range 0–1.5 years) after recurrence (four atypical MBCs, and one each of typical MBC and non-medullary infiltrating carcinoma). The sites of recurrence were the other breast (n = 4), chest wall (n = 1), supraclavicular node (n = 1 ipsilateral and n = 1 contralateral), lung and brain (n = 1), and contralateral axilla and bone marrow (n = 1). The single case of typical MBC with recurrence in ipsilateral axillary nodes 72 months after diagnosis developed metastasis to the bone marrow, with subsequent death 18 months after the recurrence [Table 5].
Table 5.
Breast-cancer specific deaths and sites of recurrences per tumour subtype within a ten-year follow-up period.
| Tumour subtype with recurrence/death (n=9) | Site of recurrence | No. of mth (no. of yr) at recurrence after diagnosis | No. of mth (no. of yr) at death after recurrence | Triple negative for ER, PR, c-erbB-2 | Basal phenotype |
|---|---|---|---|---|---|
| Typical (n=1) | Contralateral axillary nodes and bone marrow | 58 (4.80) | 18 (1.50) | Yes | Yes |
|
| |||||
| Atypical (n=7) | Opposite breast (n=3) | 56, 66, 115 (4.60, 5.50, 9.60) | Alive at last follow-up | Yes | Yes |
|
| |||||
| Chest wall (n=1) | 13 (1.10) | 10 (0.83) | Yes | Yes | |
|
| |||||
| Supraclavicular node (ipsilateral, n=1; contralateral, n=1) | 6, 22 (0.50, 1.83) | 12 (1.00) for both cases | Yes | Yes | |
|
| |||||
| Lung and brain (n=1) | 15 (1.25) | 9 (0.75) | Yes | Yes | |
|
| |||||
| Non-medullary infiltrating carcinoma (n=1) | Contralateral breast, axillary and neck nodes | 18 (1.50) | 18 (1.50) | Yes | Yes |
ER: oestrogen receptor; PR: progesterone receptor
DISCUSSION
Upon histologic review with strict adherence to Ridolfi's criteria, only six cases fulfilled the five criteria for typical MBC, and the majority of those previously diagnosed as 'typical MBC' were more consistent with the criteria for atypical MBC, with one case reviewed as non-medullary infiltrating carcinoma. Previously diagnosed 'atypical MBCs' remained predominantly atypical MBC upon review. The four cases diagnosed as 'invasive ductal carcinoma with focal medullary features' were concordant with the review diagnoses of non-medullary infiltrating carcinoma. While all the cases presented with syncytial sheets of pleomorphic cells and mononuclear inflammation in variable amounts, adherence to the combination of the quantum >75% syncytial sheets, anastomosing pattern of the syncytial pleomorphic cells, diffuse rather than peritumoral distribution of at least moderate to marked mononuclear inflammation, complete rather than partial tumour circumscription, and absence of microglandular patterns and DCIS were the criteria to be fulfilled for a tumour to be classified as 'typical medullary', which was restricted to only a few cases. Although diagnostic concordance was not strictly measured in this study, the difference in diagnoses between the original reports and the reviewers' consensus diagnoses reflects the high inter-observer variability in the morphologic interpretation of medullary carcinoma, as frequently reported.[5,6,7,15,19]
Ridolfi's criteria have since been tested for not only their reproducibility but also prognostic significance, and have since been modified by several authors,[4,5,6,15,16,17,19,20,21] with additional features such as sparse necrosis, absence of fibrosis and nuclear pleomorphism proving to be prognostically significant. In this study, we applied Ridolfi's criteria with strict observance of the quantum and pattern of the histologic features to distinguish between typical and atypical medullary carcinomas and non-medullary carcinomas, and to compare their immunologic findings and outcome, with the premise that morphologically typical MBCs are those with significantly better prognosis, regardless of triple negativity and/or basal phenotype.
One of the six typical MBCs that were reviewed recurred as contralateral axillary metastases within five years after mastectomy, with involvement of the bone marrow, leading to the death of the patient after one and a half years. This spread of breast cancer was similar to that observed in the unusual and distant metastatic sites of the nine other non-typical MBCs, which also lead to poor disease-free survival. Among the histologic parameters evaluated, N2 nodal stage (four of 20 axillary nodes) of this typical MBC, compared with N0 or N1 nodal stages of the other typical MBCs, resulted in statistically significantly poor overall survival. This result concurs with Ridolfi's finding that none of the typical MBCs in his study with N0 or N1 disease experienced recurrence or death,[2] and the general notion that MBCs with more than three metastatic axillary lymph nodes are associated with poor survival.[3] In our study, the atypical MBC with N3 nodal stage metastasised to the lung and the brain, leading to death within two years after mastectomy.
Lymphovascular invasion was present in only 4 (6.4%) of the 62 cases and was correlated to the few recurrences/metastases, manifesting as contralateral breast carcinomas that were similarly negative for ER, PR and c-erbB-2 as the original tumours, in three of the four cases with lymphovascular emboli. Compared with a larger previous study on triple-negative breast cancers in our institution,[22] where lymphovascular invasion was observed in 29% of the cases, such low percentage of lymphovascular invasion among the medullary group is relatively uncommon. Manifestation of the recurrence episode as contralateral breast cancer suggests that the subsequent cancer on the other breast might be a new primary tumour rather than spread from the original tumour, especially since the 'recurrences' were reported as high-grade IDCs. However, the fact that the primary tumours were triple negative and basal-like suggests that these are manifestations of unusual aggressive behaviour associated with triple negativity and basal phenotype, and that the presence of lymphovascular emboli may be a predictor of such unusual spread.
Immunohistochemical positivity for p53 and basal markers, and triple negativity for ER, PR and c-erbB-2 among the majority of our cases theoretically imply aggressive tumour behaviour and poor prognosis.[20,21,22] It is noteworthy that among the 42 (67.7%) cases that were p53 positive and the 45 (72.6%) cases that were ER, PR and c-erbB-2 negative in our study, only 4 (9.5%) had axillary metastases upon mastectomy and axillary clearance. In the previous study on triple-negative breast cancers of various subtypes that were predominantly ductal carcinomas,[21] lymph node metastases were observed in 40% of the cases, a proportion that was significantly greater than the 1% proportion among non-basal like cancers in that cohort. In a subsequent study on triple-negative breast cancers with basal-like phenotype,[22] 20% recurrence and 24% deaths were reported after surgery for basal-like cancers, including double, triple and quadruple recurrences. As a subgroup of triple-negative breast cancers with basal-like phenotype, therefore, the low percentage of axillary metastases in our medullary group and the relatively low percentage (14.5%) of recurrences/metastases suggest that medullary features may indeed be predictors of better prognosis, compared with their triple-negative basal-like predominantly ductal counterparts. Rakha et al. attributed this relatively good prognosis to the prominent inflammation that accompanies the otherwise high-grade features of medullary carcinomas;[17] in addition, among the studies modifying Ridolfi's criteria, prominent inflammation has also remained a consistently reproducible histologic criterion.
Our use of the triple panel CK14, EGFR and 34BE12 as surrogate markers to depict basal-like phenotype was based on the 78% sensitivity and 100% specificity of this tri-panel in detecting basal-like cancer among the cohort of expression-profiled triple-negative breast cancers in our institution.[22,23] Using these markers for this medullary group, tri-panel positivity was observed in three cases of atypical and five cases of typical MBC, with inverse correlation to ER positivity. Positivity for at least one of these basal markers was observed in all but four cases (three atypical, one non-medullary infiltrating carcinoma). Cases with basal phenotype regardless of the histologic subtype showed a downward trend in disease-free survival and overall survival when compared with the few non-basal-like cancers or those that did not express any of these basal markers. Although this follow-up outcome was not statistically significant, it supports the finding that even among carcinomas with medullary features, those with basal-like phenotype have poorer prognosis than that of non-basal like carcinomas. The EGFR positivity in the majority of the cases not only categorises them as basal-like but also renders them potential candidates for future targeted treatment, similar to cetuximab or EGFR tyrosine kinase inhibitors, as investigated by many clinical trials intended to develop treatment for triple-negative breast cancers.[24]
Although we distinguished the typical MBCs from the atypical and non-medullary infiltrating carcinomas, this histologic subtyping did not correlate significantly with patient age and ethnicity; tumour size; presence of DCIS; lymphovascular or nodal stage; ER, PR and c-erbB-2 status; as well as basal markers. This lends credence to the preferred term of 'medullary-like carcinoma' for any tumour that may otherwise be typical or atypical MBC.[7] Designating a tumour with at least syncytial patterns of high-nuclear-grade carcinoma cells with dense lymphoid inflammation and almost complete circumscription as 'medullary-like' may help distinguish it from the high-grade ductal carcinomas that may also be triple negative and/or basal like, in view of the relatively lower proportion of recurrence, metastasis or death attributed to the subtype 'medullary'. Triple negativity for ER, PR and c-erbB-2 and basal-like phenotype may take precedence over whether the carcinoma with medullary features is typical or atypical, separating the relatively more aggressive medullary-like carcinomas from those that are non-basal like. The findings of this study suggest that the presence of lymphovascular emboli and at least N2 nodal stage in a carcinoma with medullary-like features are pathologic parameters that may predict its possible aggressive and fatal behaviour.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We wish to thank Prof Tan Puay Hoon, our Head of Department, for her support and valuable advice on this project.
REFERENCES
- 1.Moore OS, Jr, Foote FW., Jr The relatively favorable prognosis of medullary carcinoma of the breast. Cancer. 1949;2:635–42. doi: 10.1002/1097-0142(194907)2:4<635::aid-cncr2820020411>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 2.Ridolfi RL, Rosen PP, Port A, Kinne D, Miké V. Medullary carcinoma of the breast: a clinicopathologic study with 10 year follow-up. Cancer. 1977;40:1365–85. doi: 10.1002/1097-0142(197710)40:4<1365::aid-cncr2820400402>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 3.Tavassoli FA, Devilee P. Pathology and Genetics: World Health Organization Classification of Tumours. Tumours of the Breast and Female Genital Organs. Lyon: IARC Press; 2003. pp. 28–9. [Google Scholar]
- 4.Wargotz ES, Silverberg SG. Medullary carcinoma of the breast: a clinicopathologic study with appraisal of current diagnostic criteria. Hum Pathol. 1998;19:1340–6. doi: 10.1016/s0046-8177(88)80290-9. [DOI] [PubMed] [Google Scholar]
- 5.Pedersen L, Holck S, Schiødt T, Zedeler K, Mouridsen HT. Inter- and intraobserver variability in the histopathological diagnosis of medullary carcinoma of the breast, and its prognostic implications. Breast Cancer Res Treat. 1989;14:91–9. doi: 10.1007/BF01805979. [DOI] [PubMed] [Google Scholar]
- 6.Reinfuss M, Stelmach A, Mitus J, Rys J, Duda K. Typical medullary carcinoma of the breast: a clinical and pathological analysis of 52 cases. J Surg Oncol. 1995;60:89–94. doi: 10.1002/jso.2930600205. [DOI] [PubMed] [Google Scholar]
- 7.Pathology reporting of breast disease. A joint document incorporating the third edition of the NHS Breast Screening Programme's Guidelines for Pathology Reporting in Breast Cancer Screening and the second edition of the Royal College of Pathologists' Minimum Dataset for Breast Cancer Histopathology. NHSBSP Publication No. 58. 2005. pp. 41–87. Available at: https://www.cmcanceralliance.nhs.uk/application/files/3615/4815/5660/Guidelines_for_NHSBSP58_January_2005_Reviewed_CNG_June_2010.pdf .
- 8.Lakhani SR, Jacquemier J, Sloane JP, et al. Multifactorial analysis of differences between sporadic breast cancers and cancers involving BRCA1 and BRCA2 mutations. J Natl Cancer Inst. 1998;20:1138–45. doi: 10.1093/jnci/90.15.1138. [DOI] [PubMed] [Google Scholar]
- 9.Eisinger F, Jacquemier J, Charpin C, et al. Mutations at BRCA1: the medullary breast carcinoma revisited. Cancer Res. 1998;58:1588–92. [PubMed] [Google Scholar]
- 10.de Cremoux P, Salomon AV, Liva S, et al. p53 mutation as a genetic trait of typical medullary breast carcinoma. J Natl Cancer Inst. 1999;91:641–3. doi: 10.1093/jnci/91.7.641. [DOI] [PubMed] [Google Scholar]
- 11.Jacquemier J, Padovani L, Rabayrol L, et al. Typical medullary breast carcinomas have a basal/myoepithelial phenotype. J Pathol. 2005;207:260–8. doi: 10.1002/path.1845. [DOI] [PubMed] [Google Scholar]
- 12.Bertucci F, Finetti P, Cervera N, et al. Gene expression profiling shows medullary breast cancer is a subgroup of basal breast cancers. Cancer Res. 2006;66:4636–44. doi: 10.1158/0008-5472.CAN-06-0031. [DOI] [PubMed] [Google Scholar]
- 13.Vincent-Salomon A, Gruel N, Lucchesi C, et al. Identification of typical medullary breast carcinoma as a genomic sub-group of basal-like carcinomas, a heterogeneous new molecular entity. Breast Cancer Res. 2007;9:R24. doi: 10.1186/bcr1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodríguez-Pinilla SM, Rodríguez-Gil Y, Moreno-Bueno G, et al. Sporadic invasive breast carcinomas with medullary features display a basal-like phenotype: an immunohistochemical and gene amplification study. Am J Surg Pathol. 2007;31:501–8. doi: 10.1097/01.pas.0000213427.84245.92. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen L, Holck S, Schiødt T, Zedeler K, Mouridsen HT. Medullary carcinoma of the breast, prognostic importance of characteristic histopathological features evaluated in a multivariate Cox analysis. Eur J Cancer. 1994;30A:1792–7. doi: 10.1016/0959-8049(94)00251-y. [DOI] [PubMed] [Google Scholar]
- 16.Jensen ML, Kiaer H, Andersen J, Jensen V, Melsen F. Prognostic comparison of three classifications for medullary carcinomas of the breast. Histopathology. 1997;30:523–32. doi: 10.1046/j.1365-2559.1997.5720795.x. [DOI] [PubMed] [Google Scholar]
- 17.Rakha EA, Aleskandarany M, El-Sayed ME, et al. The prognostic significance of inflammation and medullary histological type in invasive carcinoma of the breast. Eur J Cancer. 2009;45:1780–7. doi: 10.1016/j.ejca.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Tan Y, Hilmy MH, Hung H, Tan PH. Initial experience with tissue microarray (TMA) in a surgical pathology laboratory: technical considerations. J Histotechnol. 2004;27:113–7. [Google Scholar]
- 19.Marginean F, Rakha EA, Ho BC, Ellis IO, Lee AH. Histological features of medullary carcinoma and prognosis in triple-negative basal-like carcinomas of the breast. Mod Pathol. 2010;23:1357–63. doi: 10.1038/modpathol.2010.123. [DOI] [PubMed] [Google Scholar]
- 20.Rigaud C, Theobald S, Noël P, et al. Medullary carcinoma of the breast.A multicenter study of its diagnostic consistency. Arch Pathol Lab Med. 1993;117:1005–8. [PubMed] [Google Scholar]
- 21.Lakhani SR, Van De Vijver MJ, Jacquemier J, et al. The pathology of familial breast cancer: predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. J Clin Oncol. 2002;20:2310–8. doi: 10.1200/JCO.2002.09.023. [DOI] [PubMed] [Google Scholar]
- 22.Thike AA, Cheok PY, Jara-Lazaro AR, et al. Triple negative breast cancer: clinicopathological characteristics and relationship with basal-like breast cancer. Mod Pathol. 2010;23:123–33. doi: 10.1038/modpathol.2009.145. [DOI] [PubMed] [Google Scholar]
- 23.Thike AA, Iqbal J, Cheok PY, et al. Triple negative breast cancer: outcome correlation with immunohistochemical detection of basal markers. Am J Surg Pathol. 2010;34:956–64. doi: 10.1097/PAS.0b013e3181e02f45. [DOI] [PubMed] [Google Scholar]
- 24.Teng YH, Tan WJ, Thike AA, et al. Mutations in the epidermal growth factor receptor (EGFR) gene in triple negative breast cancer: possible implications for targeted therapy. Breast Cancer Res. 2011;13:R35. doi: 10.1186/bcr2857. [DOI] [PMC free article] [PubMed] [Google Scholar]


