Abstract
Objective
To examine whether feeding patterns from birth to age 6 months modify the association between birth weight and weight at 7–12 months of age.
Study design
Longitudinal mixed models were used to examine feeding trajectories across categories of birth weight and weight at 7–12 months of age in 1799 mother-infant dyads enrolled in the Infant Feeding Practices Study II. The percentage of breast milk received and the average daily formula consumption were calculated from birth to 6 months of age. Birth weights were classified as high (≥4000 g) and normal (≥2500 g and <4000 g). Weights at 7–12 months of age were categorized as high (z score >1) or normal (z score ≤1). A secondary analysis was performed using categories defined by birth weight adjusted for gestational age percentiles (>90% and 10th-90th percentile).
Results
High birth weight (HBW) infants with high weights at 7–12 months of age demonstrated a rapid decline in the percentage of breast milk feedings compared with HBW infants with normal weights at 7–12 months of age. Normal birth weight infants with high weights at 7–12 months of age received a lower percentage of breast milk and had greater absolute intakes of formula than those with normal weights at 7–12 months of age; these associations did not vary over time. Results were similar when infants were categorized by birth weight percentiles.
Conclusions
A lower proportion of breast milk feedings was associated with excess weight at 7–12 months of age in HBW infants. These findings suggest an initial target for obesity prevention programs focusing on the first 6 months after birth.
Many factors that are present in the first 1000 days of life (ie, from conception to age 2 years) possess a strong relationship with later weight,1 but a high birth weight (HBW, ≥4000 g) is a consistent predictor of later overweight or obesity in childhood, adolescence, and adulthood.2–10 In a large prospective cohort study, the prevalence of obesity was nearly double among HBW infants from early childhood to adolescence compared with normal birth weight (NBW, 2500–3999 g) infants.11 Moreover, 36% of youth who were obese at 14 years of age had a birth weight ≥4000 g.11 In addition to obesity, HBW infants also have increased risk for comorbid medical problems including type 2 diabetes12,13 and cardiovascular concerns.14–16
Given these concerns, a better understanding of the mechanisms that shape the association between HBW and later obesity is needed.9 Prenatal (eg, prepregnancy overweight/obesity and diabetes mellitus)17 and postnatal influences (eg, breast milk feeding, introduction to cereal, feeding practices, and eating behaviors)2,18,19 appear to be important in determining later weight trajectories.19 An understanding of potentially modifiable practices may provide insight as to how HBW infants become obese children and inform targeted prevention efforts to improve growth patterns and reduce the risk for overweight/obesity.
Exclusive breastfeeding is protective against high weight20 and is recommended by the American Academy of Pediatrics for the first 6 months.21 Because breastfeeding rates can be improved with education and support,22–25 we examined associations between feeding practices from birth to 6 months of age and weight-for-age in months 7–12 of infancy using observational data from the Infant Feeding Practices Study II. We hypothesized that HBW infants would receive a lower percentage of breast milk and consume more formula than NBW infants. We further hypothesized that HBW infants with high weight z scores at 7–12 months of age would consume a lower percentage of breast milk and higher volume of formula compared with HBW infants with a normal weight z score at 7–12 months of age.
Methods
The Infant Feeding Practices Study II (IFPS II) was a longitudinal survey of 4902 women in the US conducted by the Food and Drug Administration and Centers for Disease Control and Prevention from May 2005 to June 2007. Mother-infant dyads were eligible if the mother was ≥18 years of age and the infant was a singleton born at >35 weeks of gestation with a birth weight ≥2.25 kg. Infants were ineligible if they had an illness or condition that would impair feeding in the first year. Following the completion of an initial prenatal questionnaire, mothers completed postnatal questionnaires monthly (neonatal, months 2, 3, 4, 5, 6, 7) and then every 7 weeks (months 9, 10.5, 12) until the infant was 12 months of age. Additional information about the IFPS II is presented elsewhere.26 Given that this was a secondary analysis of deidentified data, the current study was not considered human subjects research by the Cincinnati Children’s Hospital Medical Center Institutional Review Board and did not require Institutional Review Board approval.
Maternal information collected prenatally included age, race and ethnicity, parity, smoking, prepregnancy body mass index (BMI), and poverty-to-income ratio. Gestational weight gain was assessed via self-report at the first postnatal survey. Delivery and neonatal information included gestational age (in weeks), method of delivery, infant sex, and birth weight (in grams). Following the definition used in 2 recent meta-analyses, absolute birth weight was categorized as high (≥4000 g) or normal (≥2500 and <4000 g).9,10 Based on sex-specific fetal growth reference tables,27 birth weight percentiles were classified as large for gestational age (LGA, birth weight > 90th percentile) or appropriate for gestational age (AGA, 10th-90th percentile).
At each postnatal questionnaire from birth to 6 months, mothers completed a food frequency measure reporting the average number of infant feedings of breast milk, formula, and other milk (eg, soy, rice milk) per day during the previous 7 days. The percentage breast milk variable was calculated at each postnatal survey as follows28: [(number of breast milk feedings/(number of breast milk + formula + cow’s milk + other milk feedings)] × 100%. The percentage of breast milk was calculated for each completed postnatal survey through 6 months; mother-infant dyads could have up to 6 measurements.
Mothers who offered formula to their infants during the first 6 months were asked to indicate the approximate number of ounces consumed per feeding from birth to 6 months. Response options included 1–2, 3–4, 5–6, 7–8, and 8 or more ounces. Response options were converted to the midpoint (ie, 1.5, 3.5, 5.5, 7.5, and 8.5).29 The midpoint formula consumption and the average number of formula feedings per day were used to estimate the average daily formula consumption in ounces per month from birth to 6 months. Because not all mothers offered formula in the first 6 months, only those reporting formula feedings during at least 1 measured time point were included in analyses using this variable. Once an infant had been given formula on a postnatal survey, the infant remained in the formula group for all subsequent time points even if reverting back to exclusive breastmilk.
Infant weight and length were obtained by maternal report on postnatal questionnaires at the month 3, 5, 7, and 12 surveys. The appointment date and measurements were obtained from the infant’s most recent doctor’s appointment. Because of concerns about parent-reported infant length,28 we used weight z scores rather than weight-for-length or length measurements. Infants with at least 2 weight measurements were included, and at least 1 measurement must have been obtained at the month 7 (n = 414) or 12 (n = 1385) survey. Weight-for-age z scores were calculated using the sex-specific Centers for Disease Control and Prevention national growth reference data.30 Biologically implausible z scores were excluded. Weight was dichotomized as a z score >1 (high) or ≤1 (normal) at 7–12 months of age.28
Descriptive statistics are reported as means (with SDs) or medians (with IQRs) and frequencies unless otherwise indicated. Longitudinal mixed effects models were fit using restricted maximum likelihood with an unstructured covariance pattern to allow for an unbalanced data structure (ie, unequal number of measurements and measurement intervals) and correlations between infants. Several models were estimated to determine the most robust statistical model for the associations between group and feeding practices from birth to 6 months. Full group analyses examined feeding practices across categories of absolute birth weight (high vs normal) and birth weight percentile (LGA vs AGA).
Three models were estimated. Model 1 was an unadjusted linear model examining the relationship between group and feeding trajectories. Model 2 was composed of model 1 with adjustment for covariates that could potentially alter the association between early feeding or birth weight: mother’s age, prepregnancy BMI, race/ethnicity (white used as reference), poverty-to-income ratio, gestational age (not used for LGA or AGA comparisons), and infant sex (male as reference). Model 3 was composed of model 2 with a quadratic component to determine if trajectories were nonlinear. The respective terms age and age2 were used to capture the linear and non-linear effects of time (chronological infant age) on the outcome variable, and whether the outcome varied as a function of group (ie, group × infant age interaction). Random intercepts and random slopes were included to allow for infant-specific variation in the intercepts and rates of change. For all models, goodness-of-fit was estimated using deviance (−2 × log-likelihood), Akaike’s information criteria, and Bayesian information criteria for which lower values represent better model fit. All analyses were conducted in Stata 15 (StataCorp LP, College Station, Texas) and using the function “mixed,” the procedure for estimating multilevel mixed-effects regression models.
Results
Birth weight was available for 3389 infants. Of these, 1521 mother-infant pairs were excluded for missing weight information at 7–12 months of age, and 68 were excluded for missing covariate information. The final sample size was 1799 (HBW, n = 209) for the analysis of percentage breast milk and 1265 (HBW, n = 133) for the average daily formula consumption. Because not all infants received formula and the timing of introduction varied, the sample size and mean number of observations is lower for formula consumption than analyses examining percentage breast milk. Information at all 6 postnatal surveys was available for 1267 (70.4%) respondents for the percentage breast milk outcome and 521 (41.2%) for average daily formula consumption. Mean observations across all full group and subgroup analyses ranged from 5.5–5.6 for percentage breast milk and 4.5–4.6 on average daily formula consumption.
Mothers of HBW infants were more likely to have a greater prepregnancy BMI and gestational weight gain and were more likely to be white, multiparous, and less likely to smoke relative to mothers of NBW infants (Table I). HBW infants were more likely to be male, born by cesarean delivery, and had a longer gestation. Thirty-three percent of HBW infants (n = 69) and 17% of NBW infants (n = 277) had high weight z scores (>1) at 7–12 months of age.
Table I.
Baseline demographics and dyadic characteristics as a function of infant absolute birth weight
| Characteristics | NBW (n = 1590) | HBW (n = 209) | P value |
|---|---|---|---|
| Mother | |||
| Prepregnancy BMI, kg/m2 | 26.44 ± 6.51 | 28.83 ± 7.46 | <.001 |
| Gestational weight gain, kg | 13.70 ± 6.09 | 15.69 ± 6.94 | <.001 |
| Age, y | 29.70 ± 5.36 | 30.11 ± 4.91 | .29 |
| Primiparous, % | 29.8 | 20.9 | <.01 |
| Prenatal smoking, % | 7.4 | 2.4 | <.01 |
| <185% PIR, % | 36.7 | 35.4 | .93 |
| Caucasian, % | 86.6 | 91.4 | <.001 |
| Cesarean delivery, % | 27.8 | 37.3 | <.01 |
| Gestational diabetes, % | 6.5 | 7.8 | .77 |
| Infant | |||
| Birth weight, g | 3379.15 ± 357.10 | 4267.91 ± 222.73 | <.001 |
| Infant sex, % male | 47.8 | 62.2 | <.001 |
| Gestational age, wk | 39.26 ± 1.23 | 39.99 ± 1.02 | <.001 |
PIR, poverty-to-income ratio.
Of the 209 HBW infants, 85% (n = 177) were also LGA using birth weight percentiles and the remaining 15% (n = 32) were AGA. Sixty-four infants who were previously coded as NBW were classified as small-for-gestational age (<10th percentile)27 and subsequently removed from the LGA vs AGA analyses. Of the 1526 remaining NBW infants, 4% (n = 61) were classified as LGA and the remainder were AGA (n = 1465). For these groups, the final sample sizes for percentage breast milk and daily formula consumption were 1735 (LGA, n = 238) and 1217 (LGA, n = 156), respectively. Thirty-six percent of LGA infants (n = 87) and 17% of AGA infants (n = 254) had high weight z scores at 7–12 months of age.
For the whole sample, breast milk comprised 67.08% (SD = 42.68) of total milk feeds at the first postnatal questionnaire and 47.31% (SD = 47.03) in month 6. In the first postnatal survey, 24% of mothers fed 0% breast milk, rising to 47% at 6 months. Feeding 100% breast milk was reported by 48% of mothers in the first postnatal questionnaire and 34% of mothers at 6 months. Among the subset of mothers who fed formula, the mean volumes were 18.81 (SD = 14.56) and 26.22 (SD = 14.44) ounces per day initially and at month 6. The median number of formula feedings per day in the first postnatal survey was 6 (IQR, 2–8) and 5 (IQR, 3–6) at month 6.
A drop in the percentage of breast milk feedings occurred in all participants in the first few months; this decline leveled off over time (Table II and Figure 1; available at www.jpeds.com). Daily formula consumption increased in early infancy and leveled off in later months. No significant effects of group or group × infant age were found for percentage of breast milk feedings or formula consumption. Results were similar when analyzed using LGA and AGA birth weight percentiles (Table III and Figure 2; available at www.jpeds.com).
Table II.
Unadjusted and adjusted* longitudinal mixed models for the association between absolute birth weight group and percentage breast milk (n = 1799) and daily formula consumption (n = 1265) between birth to 6 months
| Model 1 ß (95% CI) |
Model 2* ß (95% CI) |
Model 3* ß (95% CI) |
||||
|---|---|---|---|---|---|---|
| Variable | Percentage breast milk | Daily formula consumption | Percentage breast milk | Daily formula consumption | Percentage breast milk | Daily formula consumption |
| HBW | 3.27 (−3.11, 9.64) | −1.36 (−4.26, 1.54) | 4.57 (−1.89, 11.04) | −2.06 (−4.99, 0.86) | 5.03 (−1.46, 11.53) | −1.99 (−5.03, 1.04) |
| Age | −0.94 (−1.02, −0.86)† | 0.42 (0.37, 0.47)† | −0.94 (−1.02, −0.86)† | 0.42 (0.37, 0.47)† | −1.14 (−1.27, −1.01)† | 0.90 (0.79, 1.01)† |
| HBW × age | 0.28 (.04, 0.52)† | .05 (−0.10, 0.19) | 0.28 (.04, 0.51)‡ | .04 (−0.10, 0.19) | .07 (−0.30, 0.44) | 0.11 (−0.22, 0.43) |
| Age2 | .01 (.00, .01)† | −.02 (−.03, −.02)† | ||||
| HBW × age2 | .01 (−.00, .02) | −.00 (−.02, .01) | ||||
| Model Fit Statistics | ||||||
| Deviance | 87 930 | 44 286 | 87 870 | 44 230 | 87 844 | 44 130 |
| AIC | 87 947 | 44 302 | 87 902 | 44 262 | 87 881 | 44 167 |
| BIC | 88 005 | 44 355 | 88 017 | 44 369 | 88 011 | 44 287 |
AIC, Akaike’s information criterion; BIC, Bayesian information criterion.
Models are adjusted for mother’s age, prepregnancy BMI, gestational age, infant sex, race/ethnicity, and PIR.
P < .001.
P <.05.
Figure 1.
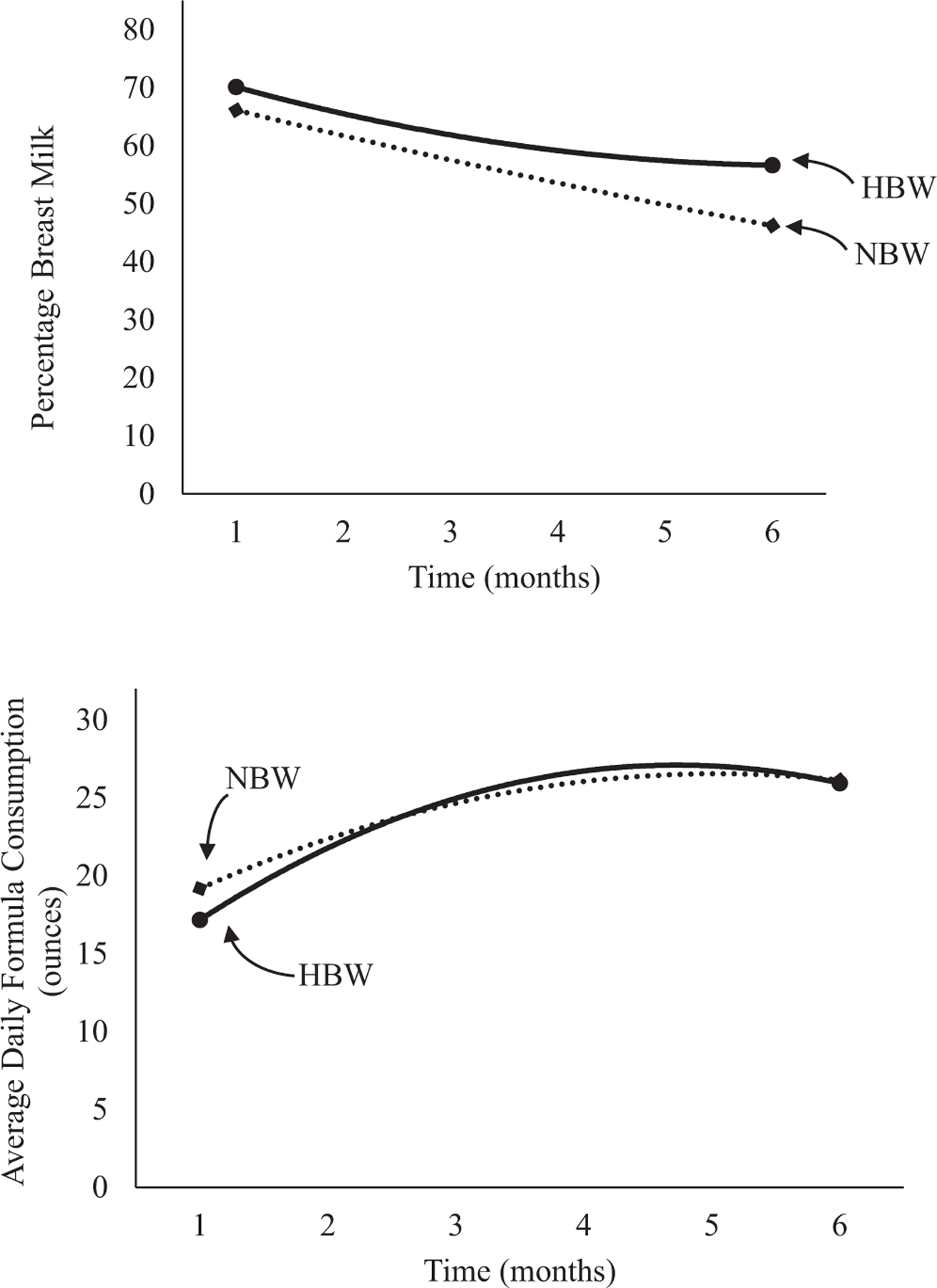
Unadjusted percentage breast milk and average daily formula consumption stratified by absolute birth weight.
Table III.
Unadjusted and adjusted* longitudinal mixed models for the association between categories of birth weight-for-gestational age group and percentage breast milk (n = 1735) and daily formula consumption (n = 1217) between birth to 6 months
| Model 1 ß (95% CI) |
Model 2* ß (95% CI) |
Model 3* ß (95% CI) |
||||
|---|---|---|---|---|---|---|
| Variable | Percentage breast milk | Daily formula consumption | Percentage breast milk | Daily formula consumption | Percentage breast milk | Daily formula consumption |
| LGA | 2.67 (−3.38, 8.72) | −2.19 (−4.92, 0.54) | 4.43 (−1.62, 10.48) | −2.44 (−5.17, 0.29) | 4.24 (−1.85, 10.33) | −2.18 (−5.05, 0.69) |
| Age | −0.94 (−1.03, −0.86)† | 0.42 (0.37, 0.47)† | −0.95 (−1.02, −0.86)† | 0.42 (0.37, 0.47)† | −1.20 (−1.33, −1.06)† | 0.92 (0.80, 1.03)† |
| LGA × age | 0.28 (.05, 0.51)‡ | .01 (−0.13, 0.15) | 0.28 (.05, 0.51)‡ | .01 (−0.12, 0.15) | 0.34 (−.01, 0.70) | −.04 (−0.36, 0.27) |
| Age2 | .01 (.01, .02)† | −.02 (−.03, −.02)† | ||||
| LGA × age2 | −.00 (−.02, .01) | .00 (−.01, .02) | ||||
| Model fit statistics | ||||||
| Deviance | 84 842 | 42 558 | 84 786 | 42 506 | 84 760 | 42 408 |
| AIC | 84 858 | 42 575 | 84 817 | 42 536 | 84 795 | 42 443 |
| BIC | 84 915 | 42 628 | 84 924 | 42 636 | 84 917 | 42 556 |
Models are adjusted for mother’s age, prepregnancy BMI, infant sex, race/ethnicity, and PIR.
P < .001.
P < .05.
Figure 2.
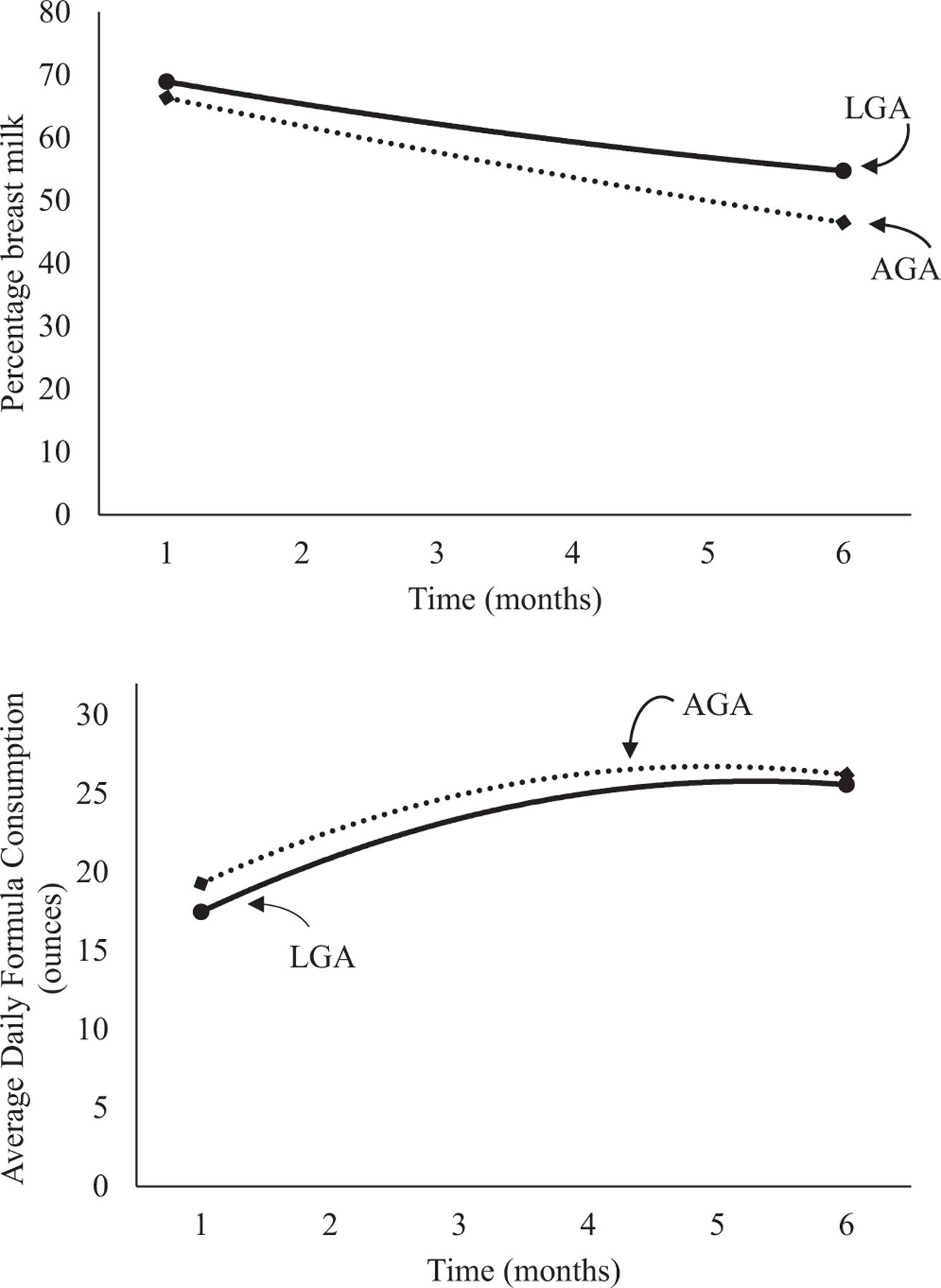
Unadjusted percentage breast milk and average daily formula consumption for infants by birth weight percentiles.
The percentage of breast milk feedings gradually declined across the first 6 months of infancy for HBW infants (Table IV). HBW infants with high weights at 7–12 months of age demonstrated an early nonlinear decline in the percentage of breast milk feedings (a significant interaction of HBW-high weight group and infant age2) from birth to 6 months compared with HBW infants with normal weights at 7–12 months of age (Figure 3). Although formula intake increased over time, there was no significant group or group × infant age interaction. In the LGA infants, the percentage of breast milk feedings decreased and formula intake increased over time (Table V; available at www.jpeds.com). LGA infants with high weights at 7–12 months of age demonstrated a greater decrease in the percentage of breast milk compared with LGA infants with normal weights at 7–12 months of age though the nonlinear interaction failed to reach significance (Figure 4; available at www.jpeds.com).
Table IV.
Unadjusted and adjusted* longitudinal mixed models for the association between categories of HBW group/weight-for-age status and percentage breast milk (n = 209) and daily formula consumption (n = 133) between birth to 6 months
| Model 1 ß (95% CI) |
Model 2* ß (95% CI) |
Model 3* ß (95% CI) |
||
|---|---|---|---|---|
| Variable | Percentage breast milk | Daily formula consumption | Percentage breast milk | Percentage breast milk |
| HBW-high | −10.57 (−22.84, 1.70) | 4.22 (−1.28, 9.72) | −8.29 (−20.72, 4.14) | −7.26 (−19.72, 5.20) |
| Age | −0.52 (−0.74, −0.30)† | 0.48 (0.29, 0.68)† | −0.52 (−0.74, −0.30)† | −0.75 (−1.10, −0.39)† |
| HBW-high × age | −0.43 (−0.81, −.05) | −.09 (−0.39, 0.22) | −0.43 (−0.82, −.05) | −0.94 (−1.55, −0.34)‡ |
| Age2 | .01 (−.00, .02) | |||
| HBW-high × age2 | .03 (.00, .05)‡ | |||
| Model fit statistics | ||||
| Deviance | 10 036 | 4650 | 10 018 | 10 002 |
| AIC | 10 052 | 4666 | 10 051 | 10 038 |
| BIC | 10 092 | 4701 | 10 133 | 10 130 |
HBW-high, HBW infants with high weight-for-age.
Models are adjusted for mother’s age, prepregnancy BMI, gestational age, infant sex, race/ethnicity, and PIR.
P < .001.
P < .05.
Figure 3.
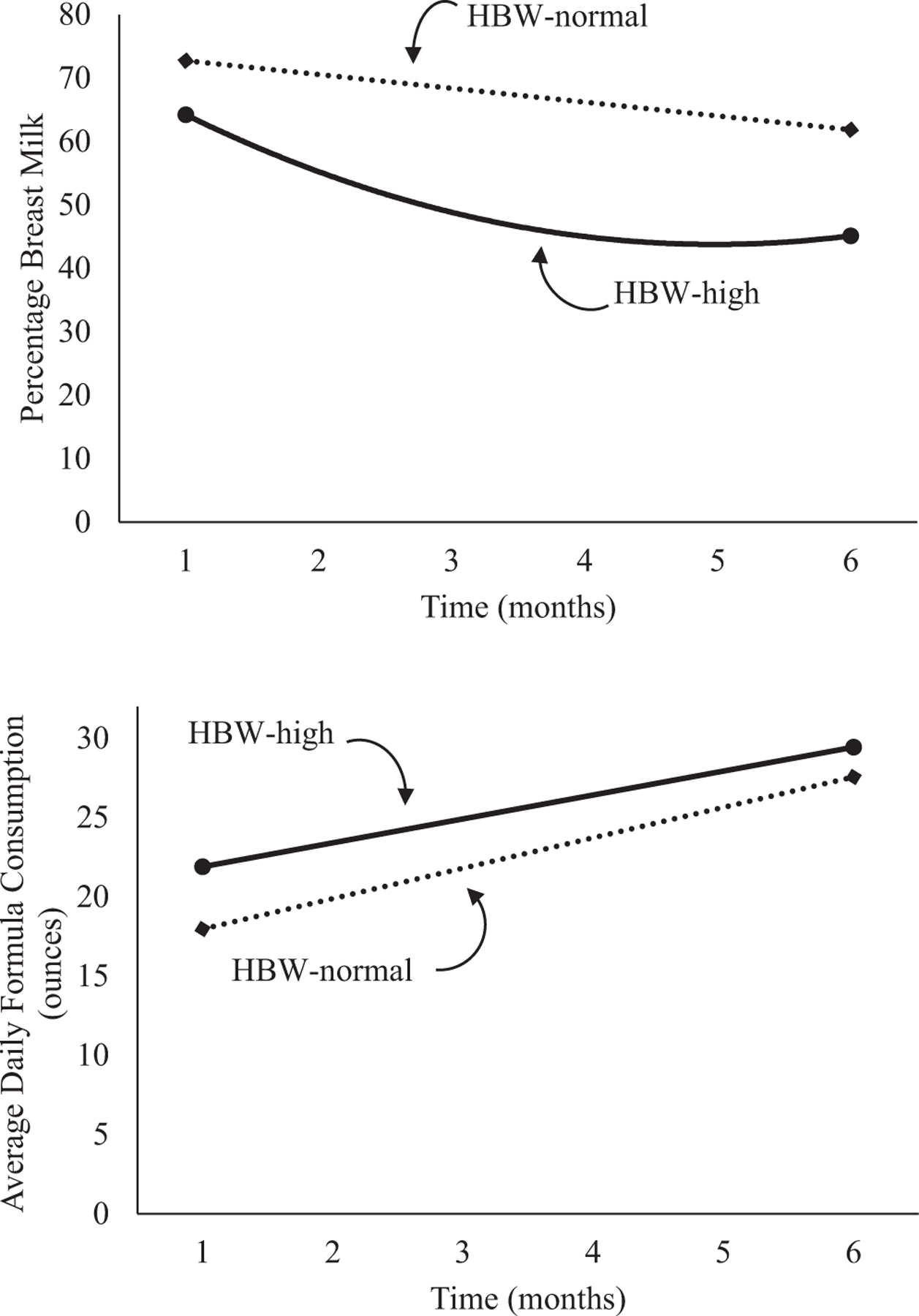
Unadjusted percentage breast milk and average daily formula consumption stratified by categories of HBW and weight z score at 7–12 months of age.
Table V.
Unadjusted and adjusted* longitudinal mixed models for the association between categories of large-for-gestational age/weight-for-age status and percentage breast milk (n = 238) and daily formula consumption (n = 156) between birth to 6 months
| Empty Cell | Model 1 ß (95% CI) |
Model 2* ß (95% CI) |
Model 3* ß (95% CI) |
|
|---|---|---|---|---|
| Variable | Percentage breast milk | Daily formula consumption | Percentage breast milk | Percentage breast milk |
| LGA-high | −6.28 (−18.18, 5.62) | 5.31 (−0.67, 11.29) | −3.54 (−15.53, 8.45) | −0.57 (−13.12, 12.18) |
| Age | −0.51 (−0.73, −0.30)† | 0.49 (0.32, 0.66)† | −0.51 (−0.73, −0.30)† | −0.58 (−0.93, −0.23)† |
| LGA-high × age | −0.40 (−0.76, −.04)‡ | −0.13 (−0.39, 0.13) | −0.40 (−0.76, −.04)‡ | −0.91 (−1.67, −0.14)‡ |
| Age2 | .00 (−.01, .02) | |||
| LGA-high × age2 | .02 (−.01, .04) | |||
| Model fit statistics | ||||
| Deviance | 11 370 | 6076 | 11 350 | 11 346 |
| AIC | 11 387 | 5291 | 11 381 | 11 380 |
| BIC | 11 429 | 5327 | 11 459 | 11 469 |
LGA-high, LGA infants with high weight-for-age.
Models are adjusted for mother’s age, prepregnancy BMI, infant sex, race/ethnicity, and PIR.
P < .001.
P <.05.
Figure 4.
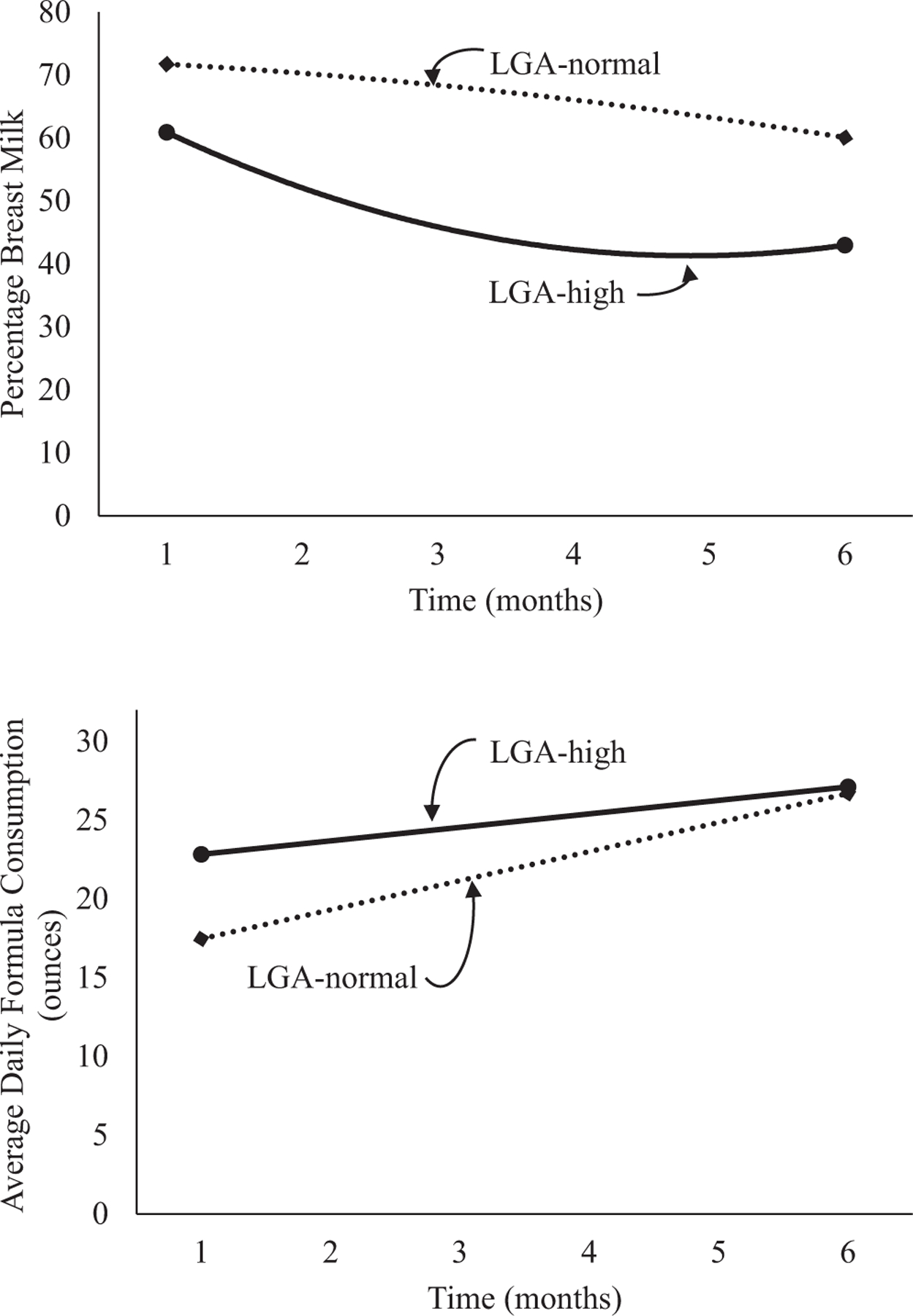
Unadjusted percentage breast milk and average daily formula consumption stratified by categories of LGA and weight z score at 7–12 months of age.
The percentage of breast milk feeding rapidly decreased in the early months for all NBW infants (Table VI; available at www.jpeds.com). A main effect of group indicated that NBW infants with high weights at 7–12 months of age received a lower percentage of breast milk compared with those with normal weights at 7–12 months of age and this relationship did not vary over time. Across all NBW infants, formula consumption rapidly increased in the early months and then leveled off. Compared with NBW infants with normal weights at 7–12 months of age, those with high weights at 7–12 months of age received more ounces of formula per day and this relationship did not vary over time (Figure 5; available at www.jpeds.com). Findings were similar for AGA infants with normal or high weights at 7–12 months of age (Table VII and Figure 6; available at www.jpeds.com).
Table VI.
Unadjusted and adjusted* longitudinal mixed models for the association between categories of NBW group/weight-for-age status and percentage breast milk (n = 1590) and daily formula consumption (n = 1132) between birth to 6 months
| Empty Cell | Model 1 ß (95% CI) |
Model 2* ß (95% CI) |
Model 3* ß (95% CI) |
|||
|---|---|---|---|---|---|---|
| Variable | Percentage breast milk | Daily formula consumption | Percentage breast milk | Daily formula consumption | Percentage breast milk | Daily formula consumption |
| NBW-high | −13.87 (−19.60, −8.14)† | 6.29 (3.93, 8.64)† | −12.22 (−17.90, −6.54) | 5.68 (3.35, 8.01)† | −11.67 (−17.41, −5.93)† | 5.34 (2.86, 7.82)† |
| Age | −0.92 (−1.01, −0.83)† | 0.43 (0.38, 0.49)† | −0.92 (−1.01, −0.83)† | 0.44 (0.38, 0.49)† | −1.09 (−1.24, −0.95)† | 0.90 (0.77, 1.02)† |
| NBW-high × age | −0.10 (−0.32, 0.12) | −.09 (−0.20, .04) | −0.10 (−0.32, 0.12) | −.08 (−0.20, .04) | −0.27 (−0.62, .08) | −.01 (−0.29, 0.27) |
| Age2 | .01 (.00, .01)‡ | −.02 (−.03, −.02)† | ||||
| NBW-high × age2 | .01 (−.01, .02) | −.00 (−.01, .01) | ||||
| Model fit statistics | ||||||
| Deviance | 77 802 | 39 592 | 77 758 | 39 538 | 77 740 | 39 456 |
| AIC | 77 818 | 39 608 | 77 790 | 39 571 | 77 777 | 39 492 |
| BIC | 77 875 | 39 660 | 77 903 | 39 675 | 77 905 | 39 610 |
NBW-high, NBW infants with high weight-for-age.
Models are adjusted for mother’s age, prepregnancy BMI, gestational age, infant sex, race/ethnicity, and PIR.
P < .001.
P < .05.
Figure 5.
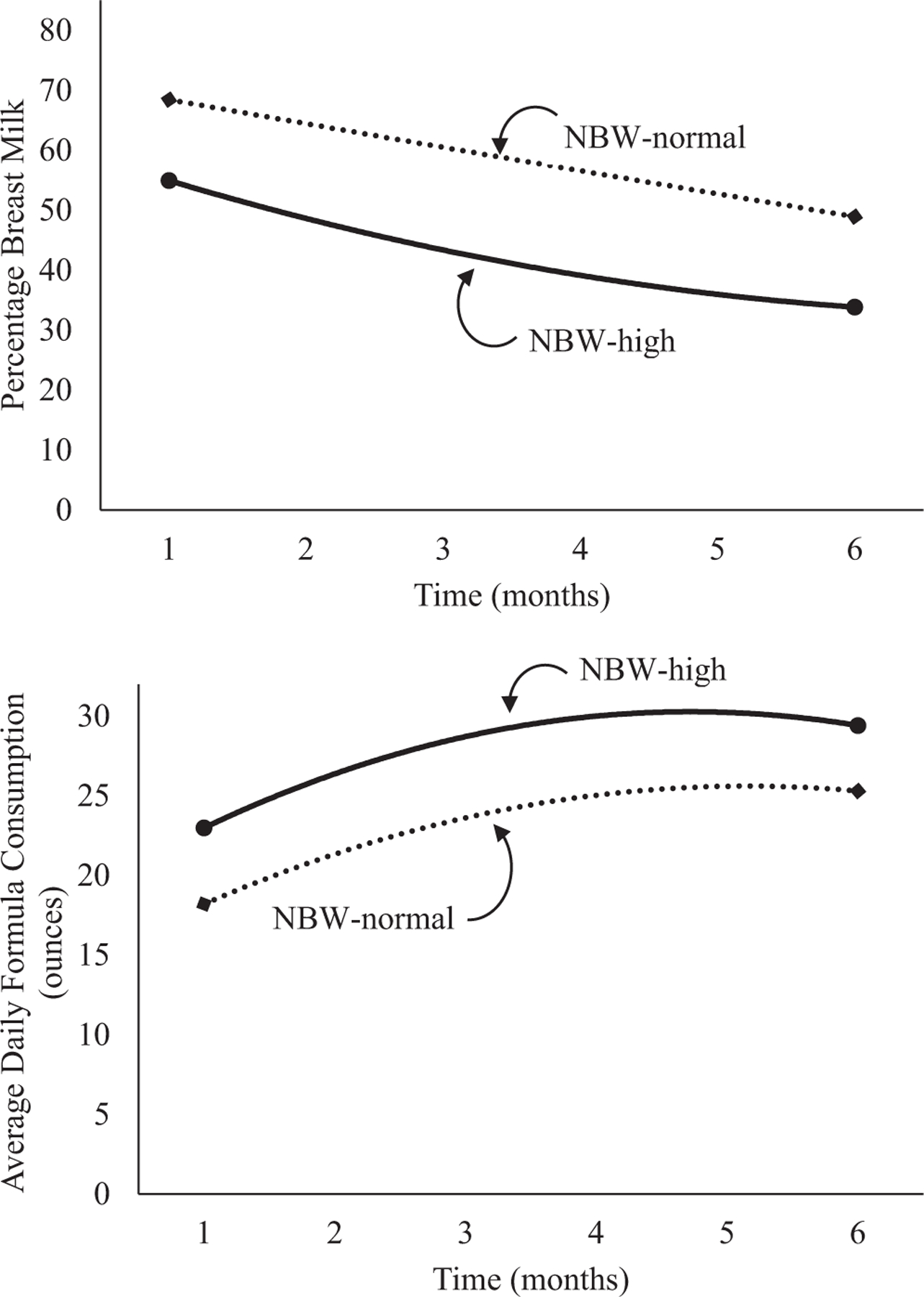
Unadjusted percentage breast milk and average daily formula consumption stratified by categories of NBW and weight z score at 7–12 months of age.
Table VII.
Unadjusted and adjusted* longitudinal mixed models for the association between categories of appropriate-for-gestational age/weight-for-age status and percentage breast milk (n = 1497) and daily formula consumption (n = 1061) between birth to 6 months†
| Empty Cell | Model 1 ß (95% CI) |
Model 2* ß (95% CI) |
Model 3* ß (95% CI) |
|||
|---|---|---|---|---|---|---|
| Variable | Percentage breast milk | Daily formula consumption | Percentage breast milk | Daily formula consumption | Percentage breast milk | Daily formula consumption |
| AGA-high | −14.66 (−21.02, −8.30)† | 7.14 (4.29, 9.99)† | −13.42 (−19.94, −7.10)† | 6.50 (3.68, 9.33)† | −11.22 (−18.06, −4.39)† | 6.90 (3.14, 10.65)† |
| Age | −0.92 (−1.02, −0.83)† | 0.43 (0.37, 0.49)† | −0.92 (−1.02, −0.83)† | 0.43 (0.38, 0.49)† | −1.14 (−1.29, −0.99)† | 0.94 (0.81, 1.07)† |
| AGA-high × age | −0.13 (−0.36, 0.10) | −.08 (−0.20, .05) | −0.12 (−0.35, 0.11) | −.08 (−0.20, .05) | −0.47 (−0.95, .01) | −0.15 (−0.55, 0.25) |
| Age2 | .01 (.00, .02)† | −.02 (−.03, −.02)† | ||||
| AGA-high × age2 | .01 (−.00, .02) | .00 (−.01, .01) | ||||
| Model fit statistics | ||||||
| Deviance | 73 368 | 37 222 | 73 332 | 37 174 | 73 306 | 37 090 |
| AIC | 73 385 | 37 238 | 73 363 | 37 204 | 73 341 | 37 125 |
| BIC | 73 441 | 37 290 | 73 468 | 37 301 | 73 461 | 37 235 |
AGA-high, AGA infants with high weight-for-age.
Models are adjusted for mother’s age, prepregnancy BMI, infant sex, race/ethnicity, and PIR.
P < .001.
Figure 6.
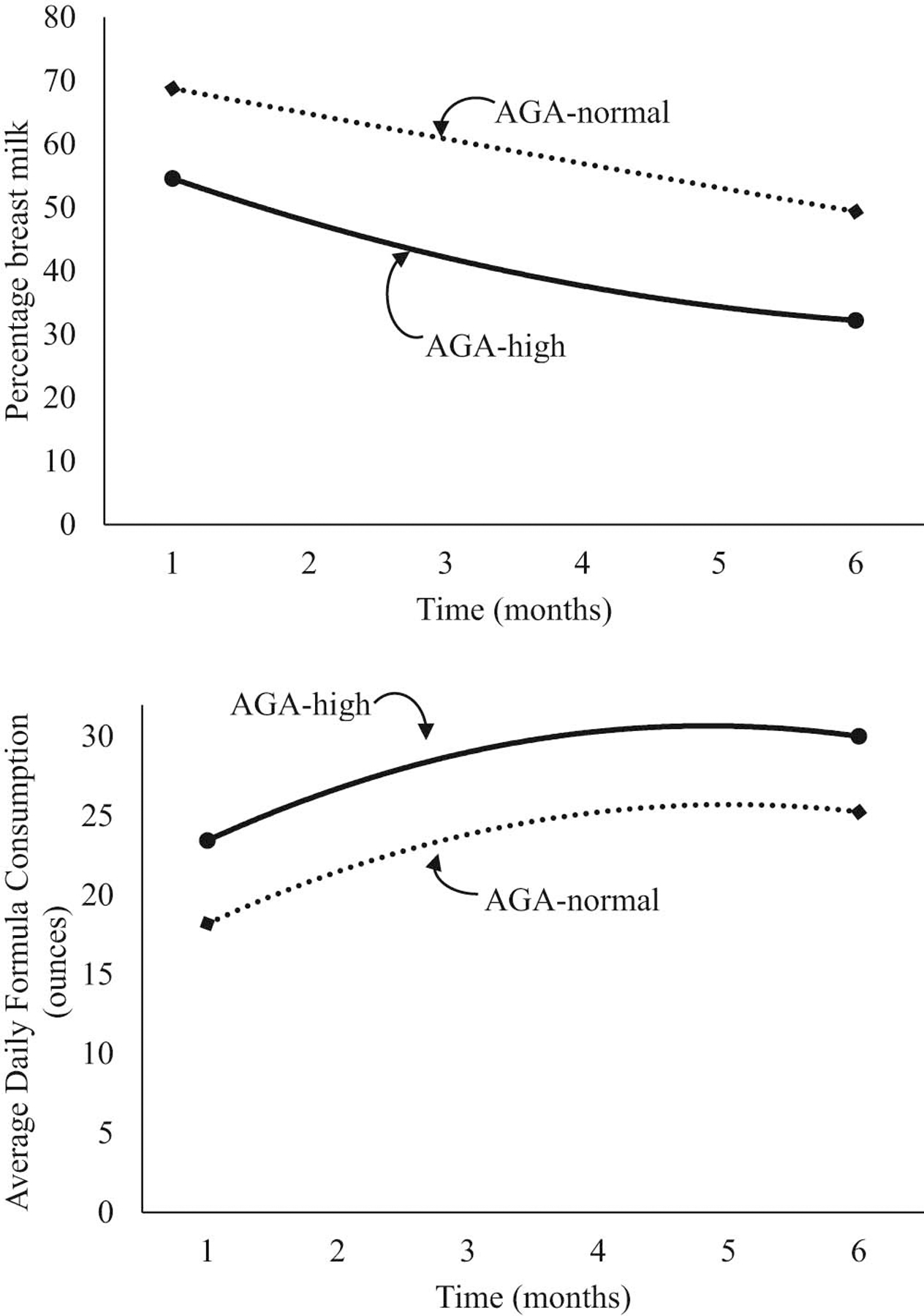
Unadjusted percentage breast milk and average daily formula consumption stratified by categories of AGA and weight z score at 7–12 months of age.
Discussion
A robust association exists among infants born at a HBW and later risk for overweight/obesity2–4,7–10,15; however, not all HBW infants follow this trajectory. To better understand the association between modifiable factors and risk for excess weight among HBW infants, we examined breast and formula feeding from birth to age 6 months. It was hypothesized that, irrespective of weight status at 7–12 months of age, infants born heavy (HBW and LGA) would receive a lower ratio of breast to nonbreast milk and consume more formula compared with infants born at a normal weight (NBW and AGA). However, these differences were not found, regardless of how infant birth weight was defined (using absolute birth weight or birth weight percentiles). These results are consistent with one other retrospective study that found no differences between NBW and HBW infants on infant eating behaviors or maternal feeding practices.19
We also hypothesized that infants born heavy (HBW or LGA) with a high weight at 7–12 months of age would consume a lower percentage of breast milk and higher volume of formula compared with infants born heavy and with a normal weight at 7–12 months of age. The results showed that among HBW infants with high weight at 7–12 months of age (based on a previously used criterion),28 breast milk feeding rapidly declined in the earliest months, compared with HBW infants with a normal weight at 7–12 months of age. In other words, among infants who were heavy at birth, other milks/formula were introduced earlier in infants with high weights at 7–12 months of age than in those with normal weights at 7–12 months of age. Findings were similar regardless of whether infants born heavy was defined as HBW or LGA.
Infants born at a normal weight who proceeded on a high weight trajectory at 7–12 months of age consumed a lower percentage of breast milk and had greater formula intake compared with those with normal weight at 7–12 months of age. Our results are consistent with findings that have shown that HBW infants who achieved a normal weight-for-length at 7–8 months of age appeared to respond better to internal cues of satiety compared with HBW infants with a high weight-for-length.19 Moreover, others have shown that heavy offspring born to mothers with overweight/obesity and diabetes have rapidly rising BMIs in the first several years of life.17 Taken together, both pre- and postnatal factors may elevate risk for continued weight gain among infants born heavy.
In the current study, infants who were born heavy and showed a gradual reduction in breast milk feedings also showed slower weight gain than those with a more rapid reduction in breast milk. These results suggest that a greater proportion of breast milk feedings may buffer against high weight at 7–12 months of age. Prolongation of breastfeeding has received much attention20,31,32 as a method to reduce the risk of overweight.20 In a retrospective cohort study, breastfeeding ≥12 months was associated with a similar BMI at 4 years among AGA and LGA infants,33 suggesting that a longer duration of breastfeeding may protect against excessive weight gain for infants born heavy. Further, formula is considered more energy-dense and has a higher protein content than breast milk and the use of formula has been associated with greater weight gain from 3–6 and 6–9 months, compared with the intakes and growth patterns of breastfed infants.34
Although the current study examined potentially modifiable feeding practices, it is important to reflect on other predictors of breastfeeding difficulty such as maternal weight, delivery mode, and maternal gestational diabetes mellitus (GDM) that could be confounding the association between breastfeeding and later weight status in infants born heavy. Maternal overweight/obesity has been previously associated with reduced intention to breastfeed, shortened duration of breastfeeding, and delayed onset of lactogenesis II,35 although the mechanisms that drive these relationships (eg, anatomic, psychosocial) are not well understood.36 Although our study showed a larger proportion of prepregnancy overweight/obesity among mothers of infants with HBW and high weight at 7–12 months of age (72.5%) compared with those with normal weights at 7–12 months of age (63.6%), this difference was not statistically significant. Delivery mode may also contribute to poor breastfeeding37–39 as emergency cesarean delivery is associated with shortened breastfeeding duration compared with spontaneous vaginal delivery.40 However, our study showed no differences between HBW subgroups on method of delivery.
Maternal GDM is a risk factor increasing the likelihood of delivering a heavy infant and reduced duration of breastfeeding. Although BMI at age 1 year has been shown to differ between LGA infants born to mothers with and without GDM,41 and breastfeeding at least 3 months appears protective against overweight in children born to mothers with GDM,42 the current study did not find differences in maternal GDM between the HBW subgroups. Overall our study did not find relationships between breast milk feedings and other prenatal and intrauterine indicators that are traditionally associated with reduced breastfeeding.
Encouragingly, breastfeeding practices can be increased following brief education.23–25,43 For example, telephone-based support increased the duration of any and exclusive breastfeeding among obese women.22 The current findings convey the importance of addressing early feeding and providing support to mothers of infants born heavy to curtail a high weight trajectory and potentially impact later obesity. Furthermore, the critical period of infancy which encompasses multiple well-child visits in the first year of life may be an appropriate front line for tailoring anticipatory guidance and education strategies around sustained breastfeeding specifically for mothers of infants born heavy.
Although HBW infants have almost double the prevalence of overweight/obesity at later ages compared with NBW infants, it is still the minority of either population that become obese.11 It may be that this relatively low incidence resulted in failure to find group differences between our HBW and NBW groups. Breastfeeding was not different between these groups but did distinguish between HBW infants with normal or high weights at 7–12 months of age. These findings suggest that following a sample of HBW infants over time and examining mechanisms that may be associated with excess weight gain would be a productive avenue for developing prevention programs to mitigate obesity risk among infants at high risk for obesity (ie, birth weight ≥4000 g).44
The current investigation is not without limitations. Because these data come from an observational study, lack of causality and possible confounding are important methodologic concerns. Although the current study controlled for prenatal and sociodemographic characteristics, there are likely other factors that could not be adjusted for that may impact these results. In addition, data were collected via maternal report which may contain inaccuracies, specifically with report of absolute birth weight, gestational age, and weight at 7–12 months of age. Generalization of these findings is also a concern. Compared with women who participated in other nationally representative surveys,26 mothers in the IFPS II were more likely to be older, employed, and more highly educated. They were more likely to breastfeed for longer durations, to have fewer children, and were less likely to be low income or smoke. Finally, these data are more than 10 years old and although breastfeeding has been largely stable over recent years (eg, 2005–2008 vs 2009–2012),45 additional contemporary analysis and HBW specific cohort studies are needed to enhance understanding of the HBW-obesity pathway. A strength of the study was the longitudinal mixed modeling approach that allowed for an examination of feeding practices over birth to age 6 months.
In conclusion, although there is a strong association between HBW and later overweight/obesity, not all HBW infants will follow this trajectory. The current study examined relationships between feeding practices among categories of birth weight and weight at 7–12 months of age. HBW infants with later high weight-for-age showed an early decline in breast milk intake over the first 6 months of life compared with HBW infants with normal weights at 7–12 months of age. These associations held regardless of whether HBW was defined by absolute birth weight or birth weight percentiles. Given the complexity of accurately calculating gestational age,46–48 absolute birth weight appears to be an equally appropriate as well as established risk marker1 for future excess weight. Future studies should seek to elucidate other variables (eg, complementary feeding, sleep) that may be implicated in the HBW-obesity association. ■
Acknowledgments
Supported the National Institute of Diabetes and Digestive and Kidney Diseases (T32 DK063929).
Glossary
- HBW
High birth weight
- NBW
Normal birth weight
- IFPS II
Infant Feeding Practices Study II
- BMI
Body mass index
- LGA
Large for gestational age
- AGA
Appropriate for gestational age
- GDM
Gestational diabetes mellitus
Footnotes
The authors declare no conflicts of interest.
References
- 1.Woo Baidal JA, Locks LM, Cheng ER, Blake-Lamb TL, Perkins ME, Taveras EM. Risk factors for childhood obesity in the first 1,000 days: a systematic review. Am J Prev Med 2016;50:761–79. [DOI] [PubMed] [Google Scholar]
- 2.Yu Z, Sun JQ, Haas JD, Gu Y, Li Z, Lin X. Macrosomia is associated with high weight-for-height in children aged 1–3 years in Shanghai, China. Int J Obes (Lond) 2008;32:55–60. [DOI] [PubMed] [Google Scholar]
- 3.Sparano S, Ahrens W, De Henauw S, Marild S, Molnar D, Moreno LA, et al. Being macrosomic at birth is an independent predictor of overweight in children: results from the IDEFICS study. Matern Child Health J 2013;17:1373–81. [DOI] [PubMed] [Google Scholar]
- 4.Li N, Liu E, Sun S, Guo J, Pan L, Wang P, et al. Birth weight and overweight or obesity risk in children under 3 years in China. Am J Hum Biol 2014;26:331–6. [DOI] [PubMed] [Google Scholar]
- 5.Taveras EM, Rifas-Shiman SL, Belfort MB, Kleinman KP, Oken E, Gillman MW. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics 2009;123:1177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phillips D, Young JB. Birth weight, climate at birth, and the risk of obesity in adult life. Int J Obes (Lond) 2000;24:281–7. [DOI] [PubMed] [Google Scholar]
- 7.Rugholm S, Baker JL, Olsen LW, Schack-Nielsen L, Bua J, Sorensen TIA. Stability of the association between birth weight and childhood overweight during the development of the obesity epidemic. Obes Res 2005;13:2187–94. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Gao E, Wu J, Zhou J, Yang Q, Walker MC, et al. Fetal macrosomia and adolescence obesity: results from a longitudinal cohort study. Int J Obes (Lond) 2009;33:923–8. [DOI] [PubMed] [Google Scholar]
- 9.Yu ZB, Han SP, Zhu GZ, Zhu C, Wang XJ, Cao XG, et al. Birth weight and subsequent risk of obesity: a systematic review and meta-analysis. Obes Rev 2011;12:525–42. [DOI] [PubMed] [Google Scholar]
- 10.Schellong K, Schulz S, Harder T, Plagemann A. Birth weight and longterm overweight risk: systematic review and a meta-analysis including 643,902 persons from 66 studies and 26 countries globally. PLoS ONE 2012;7:e47776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunningham SA, Kramer MR, Narayan KM. Incidence of childhood obesity in the United States. N Engl J Med 2014;370:403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harder T, Rodekamp E, Schellong K, Dudenhausen JW, Plagemann A. Birth weight and subsequent risk of type 2 diabetes: a meta-analysis. Am J Epidemiol 2007;165:849–57. [DOI] [PubMed] [Google Scholar]
- 13.Whincup PH, Kaye SJ, Owen CG, Huxley R, Cook DG, Anazawa S, et al. Birth weight and risk of type 2 diabetes: a systematic review. JAMA 2008;300:2886–97. [DOI] [PubMed] [Google Scholar]
- 14.Johnsson IW, Haglund B, Ahlsson F, Gustafsson J. A high birth weight is associated with increased risk of type 2 diabetes and obesity. Pediatr Obes 2015;10:77–83. [DOI] [PubMed] [Google Scholar]
- 15.Skilton MR, Siitonen N, Wurtz P, Viikari JS, Juonala M, Seppala I, et al. High birth weight is associated with obesity and increased carotid wall thickness in young adults: the cardiovascular risk in young Finns study. Arterioscler Thromb Vasc Biol 2014;34:1064–8. [DOI] [PubMed] [Google Scholar]
- 16.Bowers K, Liu G, Wang P, Ye T, Tian Z, Liu E, et al. Birth weight, postnatal weight change, and risk for high blood pressure among chinese children. Pediatrics 2011;127:e1272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie C, Wang Y, Li X, Wen X. Childhood growth trajectories of etiological subgroups of large for gestational age newborns. J Pediatr 2016;170:60–6, e5. [DOI] [PubMed] [Google Scholar]
- 18.Leonard SA, Rasmussen KM. Larger infant size at birth reduces the negative association between maternal prepregnancy body mass index and breastfeeding duration. J Nutr 2011;141:645–53. [DOI] [PubMed] [Google Scholar]
- 19.Stough CO, Bolling C, Zion C, Stark LJ Comparison of High and Normal Birth Weight Infants on Eating, Feeding Practices, and Subsequent Weight (Under Review). [DOI] [PMC free article] [PubMed]
- 20.Harder T, Bergmann R, Kallischnigg G, Plagemann A. Duration of breastfeeding and risk of overweight: a meta-analysis. Am J Epidemiol 2005;162:397–403. [DOI] [PubMed] [Google Scholar]
- 21.Gartner LM, Morton J, Lawrence RA, Naylor AJ, O’Hare D, Schanler RJ, et al. Breastfeeding and the use of human milk. Pediatrics 2005;115:496–506. [DOI] [PubMed] [Google Scholar]
- 22.Carlsen EM, Kyhnaeb A, Renault KM, Cortes D, Michaelsen KF, Pryds O. Telephone-based support prolongs breastfeeding duration in obese women: a randomized trial. Am J Clin Nutr 2013;98:1226–32. [DOI] [PubMed] [Google Scholar]
- 23.Gross RS, Mendelsohn AL, Gross MB, Scheinmann R, Messito MJ. Randomized controlled trial of a primary care-based child obesity prevention intervention on infant feeding practices. J Pediatr 2016;174:171–7, e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kramer MS, Chalmers B, Hodnett ED, Sevkovskaya Z, Dzikovich I, Shapiro S, et al. Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA 2001;285:413–20. [DOI] [PubMed] [Google Scholar]
- 25.Pugh LC, Milligan RA, Frick KD, Spatz D, Bronner Y. Breastfeeding duration, costs, and benefits of a support program for low-income breastfeeding women. Birth 2002;29:95–100. [DOI] [PubMed] [Google Scholar]
- 26.Fein SB, Labiner-Wolfe J, Shealy KR, Li R, Chen J, Grummer-Strawn LM. Infant Feeding Practices Study II: study methods. Pediatrics 2008;122(Suppl 2):S28–35. [DOI] [PubMed] [Google Scholar]
- 27.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li R, Fein SB, Grummer-Strawn LM. Association of breastfeeding intensity and bottle-emptying behaviors at early infancy with infants’ risk for excess weight at late infancy. Pediatrics 2008;122(Suppl 2):S77–84. [DOI] [PubMed] [Google Scholar]
- 29.Perrine CG, Sharma AJ, Jefferds MED, Serdula MK, Scanlon KS. Adherence to vitamin D recommendations among US infants. Pediatrics 2010;125:627–32. [DOI] [PubMed] [Google Scholar]
- 30.Kuczmarski RJ, Ogden CL, Grummer-Strawn LS, Flegal KM, Guo SS, Wei R, et al. CDC Growth Charts: United States. Advance Data: Center for Disease Control and Prevention/National Center for Health Statistics; 2000. [PubMed]
- 31.Arenz S, Ruckerl R, Koletzko B, von Kries R. Breast-feeding and childhood obesity—a systematic review. Int J Obes Relat Metab Disord 2004;28:1247–56. [DOI] [PubMed] [Google Scholar]
- 32.Hediger ML, Overpeck MD, Kuczmarski RJ, Ruan W. Association between infant breastfeeding and overweight in young children. JAMA 2001;285:2453–60. [DOI] [PubMed] [Google Scholar]
- 33.Çamurdan MO, Çamurdan AD, Polat S, Beyazova U. Growth patterns of large, small, and appropriate for gestational age infants: impacts of longterm breastfeeding: a retrospective cohort study. J Pediatr Endocrinol Metab 2011;24:463–8. [DOI] [PubMed] [Google Scholar]
- 34.Heinig M, Nommsen L, Peerson J, Lonnderdal B, Dewey K. Energy and protein intakes of breast-fed and formula-fed infants during the first year of life and their association with growth velocity: the DARLING Study. Am J Clin Nutr 1993;58:152–61. [DOI] [PubMed] [Google Scholar]
- 35.Turcksin R, Bel S, Galjaard S, Devlieger R. Maternal obesity and breastfeeding intention, initiation, intensity and duration: a systematic review. Matern Child Nutr 2014;10:166–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amir LH, Donath S. A systematic review of maternal obesity and breastfeeding intention, initiation and duration. BMC Pregnancy Childbirth 2007;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dewey KG, Nommsen-Rivers LA, Heinig MJ, Cohen RJ. Risk factors for suboptimal infant breastfeeding behavior, delayed onset of lactation, and excess neonatal weight loss. Pediatrics 2003;112:607–19. [DOI] [PubMed] [Google Scholar]
- 38.Pérez-Escamilla R, Maulén-Radovan I, Dewey KG. The association between cesarean delivery and breast-feeding outcomes among Mexican women. Am J Public Health 1996;86:832–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rowe-Murray HJ, Fisher JR. Baby friendly hospital practices: cesarean section is a persistent barrier to early initiation of breastfeeding. Birth 2002;29:124–31. [DOI] [PubMed] [Google Scholar]
- 40.Ahluwalia IB, Li R, Morrow B. Breastfeeding practices: does method of delivery matter? Matern Child Health J 2012;16:231–7. [DOI] [PubMed] [Google Scholar]
- 41.Vohr BR, McGarvey ST. Growth patterns of large-for-gestational-age and appropriate-for-gestational-age infants of gestational diabetes mothers and control mothers at 1 year. Diabetes Care 1997;20:1066–72. [DOI] [PubMed] [Google Scholar]
- 42.Schaefer-Graf UM, Hartmann R, Pawliczak J, Passow D, Abou-Dakn M, Vetter K, et al. Association of breast-feeding and early childhood overweight in children from mothers with gestational diabetes mellitus. Diabetes Care 2006;29:1105–7. [DOI] [PubMed] [Google Scholar]
- 43.Ibanez G, de Reynal de Saint Michel C, Denantes M, Saurel-Cubizolles M-J, Ringa V, Magnier A-M. Systematic review and meta-analysis of randomized controlled trials evaluating primary care-based interventions to promote breastfeeding in low-income women. Fam Pract 2011;29:245–54. [DOI] [PubMed] [Google Scholar]
- 44.Blake-Lamb TL, Locks LM, Perkins ME, Baidal JAW, Cheng ER, Taveras EM. Interventions for childhood obesity in the first 1,000 days a systematic review. Am J Prev Med 2016;50:780–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miles G, Siega-Riz AM. Trends in food and beverage consumption among infants and toddlers: 2005–2012. Pediatrics 2017;139:pii: e20163290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalish RB, Thaler HT, Chasen ST, Gupta M, Berman SJ, Rosenwaks Z, et al. First-and second-trimester ultrasound assessment of gestational age. Am J Obstet Gynecol 2004;191:975–8. [DOI] [PubMed] [Google Scholar]
- 47.Dietz PM, England LJ, Callaghan WM, Pearl M, Wier ML, Kharrazi M. A comparison of LMP-based and ultrasound-based estimates of gestational age using linked California livebirth and prenatal screening records. Paediatr Perinat Epidemiol 2007;21:62–71. [DOI] [PubMed] [Google Scholar]
- 48.Lynch CD, Zhang J. The research implications of the selection of a gestational age estimation method. Paediatr Perinat Epidemiol 2007;21:86–96. [DOI] [PubMed] [Google Scholar]


