Abstract
Background
That foot infections are predominately polymicrobial has long been recognized, but it is not clear if the various species co-occur randomly or in patterns. We sought nonrandom species co-occurrence patterns that might help better predict prognosis or guide antimicrobial selection.
Methods
We analyzed tissue (bone, skin, and other soft tissue), fluid, and swab specimens collected from initial foot infection episodes during a 10-year period using a hospital registry. Nonrandom co-occurrence of microbial species was identified using simple pairwise co-occurrence rates adjusted for multiple comparisons, Markov and conditional random fields, and factor analysis. A historical cohort was used to validate pattern occurrence and identify clinical significance.
Results
In total, 156 unique species were identified among the 727 specimens obtained from initial foot infection episodes in 694 patients. Multiple analyses suggested that Staphylococcus aureus is negatively associated with other staphylococci. Another pattern noted was the co-occurrence of alpha-hemolytic Streptococcus, Enterococcus fecalis, Klebsiella, Proteus, Enterobacter, or Escherichia coli, and absence of both Bacteroides and Corynebacterium. Patients in a historical cohort with this latter pattern had significantly higher risk-adjusted rates of treatment failure.
Conclusions
Several nonrandom microbial co-occurrence patterns are frequently seen in foot infection specimens. One particular pattern with many Proteobacteria species may denote a higher risk for treatment failure. Staphylococcus aureus rarely co-occurs with other staphylococci.
Keywords: species co-occurrence, diabetic foot ulcer, ecological, foot infection, osteomyelitis, polymicrobial
In selecting antimicrobial therapy, the most recent management guidelines from the Infectious Disease Society of America have recommended that “just targeting aerobic gram-positive cocci…is sufficient” because of a high prevalence of gram-positive organisms [1]. Two important observations have emerged since the publication of these guidelines. The first is that certain gram-negative bacteria—including Pseudomonas aeruginosa, Escherichia coli, and other extended-spectrum beta-lactamase (ESBL)–producing bacteria—are associated with worse treatment outcomes [2–5]. Methicillin-resistant Staphylococcus aureus, on the other hand, is common but not associated with treatment failure or leg amputation [5–7].
The second observation is that organisms found in foot infections may occur in certain nonrandom polymicrobial patterns. That foot infections are predominately polymicrobial has long been recognized in conventional culture results [8–10] and more recently in 16s RNA analyses [11–13]. Some of these 16s studies report patterning of microbial species that correlates with clinical characteristics [14] or foot wound chronicity [15]. The identification of co-occurrence patterns could have clinical relevance in both optimizing antimicrobial selection (covering organisms both identified and anticipated while leaving uncovered organisms neither seen nor anticipated) and understanding the prognosis of polymicrobial foot infections (especially the interaction between that can have a synergistic or ameliorating effect on quantity, gene expression, or pathogenicity) [16, 17].
This study was therefore designed to identify such nonrandom species co-occurrences or other patterns in a large series of foot infections. Our hope was to find patterns or other observations that could have clinical relevance in determining the prognosis or guiding management of foot infections.
METHODS
We studied microbiology specimens collected from initial infection episodes at the Michael E. DeBakey Veterans Affairs Medical Center in Houston, Texas, during the 10-year period ranging from April 1, 2010, through April 30, 2020. The study was done with institutional review board approval at the Baylor College of Medicine (protocol H-34858). Culture results were reviewed using our hospital's Theradoc (Premiere Inc., Charlotte, NC, USA) registry of clinical microbiology specimens. The full analysis included all tissue (bone, skin, and other soft tissue), fluid, and swab specimens. We excluded blood specimens, liver biopsy specimens, cerebrospinal fluid, specimens obtained from unspecified body locations, and specimens that included only gram stain results. The clinical management of foot infections during this time period was guideline-directed and multidisciplinary, involving multiple surgical and medical specialties. We obtain fluid, tissue, and/or bone specimens at surgery in most patients with foot infections at our hospital [3].
Initial episodes were defined as the first episode for which bone or soft tissue specimens otherwise meeting inclusion and exclusion criteria were collected. Only 1 tissue specimen type per patient was included from the index episode in this analysis. In the event of 2 or more specimens from 1 patient or tissue type, we included the specimen with the highest number of organisms. Analyses that examined co-occurrence patterns used only 1 specimen per patient.
Nonrandom pairwise associations were identified in several ways. Observed-to-expected ratios were used with a threshold P value of .001 to adjust for multiple comparisons. The presence and strength of additional pairwise interactions were also identified and quantified using Markov and conditional random fields [18–20]. These analyses differ from a simple analysis of pairwise associations in that they consider both observed (direct) and unobserved (indirect) associations to assess whether 2 species have a nonrandom co-occurrence pattern. Lastly, patterning involving >2 organisms was examined with factor analysis [21].
A previously characterized cohort of patients with foot osteomyelitis from this same hospital [3] was used to determine whether species co-occurrence patterns were associated with treatment outcomes. For this, unadjusted time-to-treatment failure was assessed with Kaplan-Meier plotting and log-rank comparisons. As with the previous analysis, treatment failure in this cohort was defined as either an unplanned resection of additional bone in an area contiguous with or adjacent to the initial site of osteomyelitis or as a leg (“major,” or above-ankle) amputation [3]. A multivariate model of treatment failure was used to determine if these co-occurrence patterns had an impact on treatment failure rates after adjusting for demographics, clinical characteristics, and other risk factors. All analyses were done in R, version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria), using RStudio build 351 (RStudio, Boston, MA, USA).
RESULTS
Basic Characterization of Study Specimens Suggested That Microbial Species Occurrences of Foot Bone and Soft Tissue or Fluid Are Similar but Distinct From Other Bodily Locations
A total of 3733 specimens from 3573 patients with index infections met study inclusion and exclusion criteria. The number of specimens obtained from various source locations is provided in Supplementary Table 1.
We first confirmed the validity of analyzing microbial isolates from foot soft tissue or fluid together with isolates from foot bone specimens. This was confirmed by seeing no significant difference in the proportion with polymicrobial results, the number of isolates per specimen, the proportion that were gram-positive vs -negative and within various metabolic categories, species diversity as measured by the Shannon index, or prevalence rates of specific organisms of interest (Supplementary Tables 2–5, Supplementary Figures 1–3). Foot specimens were, as expected, significantly different from specimens from other locations in each of these characteristics (Supplementary Tables 6–9, Supplementary Figures 4–6). In light of these findings, all subsequent analyses focused on foot specimens only and included 156 unique species identified among the foot specimens obtained from index infection episodes in 694 patients. Species with a 1% or higher prevalence in foot specimens are listed in Table 1.
Table 1.
The Prevalence and Incidence of the Most Frequent Microbial Isolates in Foot Specimens
| Genus ± Species Name | Prevalence,a % | Incidence,b % |
|---|---|---|
| Staphylococcus aureus | 40.17 | 17.39 |
| Corynebacterium, species not specified | 18.29 | 7.92 |
| Staphylococcus epidermidis | 18.16 | 7.86 |
| Enterococcus faecalis | 16.78 | 7.27 |
| Streptococcus, group B, species not specified | 13.76 | 5.96 |
| Proteus mirabilis | 11.55 | 5.00 |
| Escherichia coli | 11.42 | 4.94 |
| Enterococcus, species not specified | 10.18 | 4.41 |
| Pseudomonas aeruginosa | 9.77 | 4.23 |
| Klebsiella pneumoniae | 4.95 | 2.14 |
| Enterobacter cloacae | 4.95 | 2.14 |
| Morganella morganii | 4.54 | 1.97 |
| Streptococcus, species not specified | 4.13 | 1.79 |
| Stenotrophomonas maltophilia | 3.30 | 1.43 |
| Citrobacter freundii | 3.03 | 1.31 |
| Serratia marcescens | 2.20 | 0.95 |
| Streptococcus anginosus | 2.20 | 0.95 |
| Staphylococcus lugdunensis | 2.06 | 0.89 |
| Candida, species not specified | 1.38 | 0.60 |
| Enterococcus faecium | 1.24 | 0.54 |
The percentage of specimens with a specific isolate.
The percentage of total isolates comprised of a specific isolate.
Several Nonrandom Species Co-occurrences Were Identified Using Pairwise Co-occurrence Analyses
Nonrandom pairwise species co-occurrences in the foot were first assessed by examining observed vs expected co-occurrences. With a random co-occurrence threshold probability adjusted to <.001% to adjust for multiple comparisons, analysis of the pairwise co-occurrences of species representing at least 0.1% of total isolates in foot specimens suggested that the Bacteroides, Enterobacter, and non-aureus staphylococcal species occurred much less frequently than expected when Staphylococcus aureus was present (Table 2).
Table 2.
Observed vs Expected Co-occurrences and Ratios for Pairs of Microbial Isolates With Nonrandom Co-occurrences
| Species 1 | Species 2 | Observed Co-occurrences | Expected Co-occurrences | Observed:Expected Ratio | Probability of Random Occurrence |
|---|---|---|---|---|---|
| Bacteroides | Staphylococcus aureus | 0 | 8.5 | 0.0000000 | 0.00002 |
| Staphylococcus aureus | Staphylococcus, species not specified | 0 | 5.6 | 0.0000000 | 0.00067 |
| Staphylococcus aureus | Staphylococcus epidermidis | 3 | 12.5 | 0.2400000 | 0.00014 |
| Staphylococcus aureus | Staphylococcus, coagulase-negative species | 18 | 51.6 | 0.3488372 | 0.00000 |
| Enterobacter | Staphylococcus aureus | 7 | 18.1 | 0.3867403 | 0.00022 |
Examining pairwise species co-occurrences in foot infection specimens using Markov and conditional random fields yielded more detailed results (Supplementary Materials). In brief, these analyses showed many positive associations between E. coli, Proteus, Klebsiella, Enterobacter (genus Proteobacteria), and Enterococcus species. These species generally also had negative associations with staphylococcal species, and a negative association was also seen between Enterococcus faecalis and other nonspeciated Enterococcus.
The Markov and conditional random field analyses confirmed findings of the simple pair-wise co-occurrence analysis in also indicating negative associations between Staphylococcus aureus, Staphylococcus epidermidis, and other coagulase-negative staphylococcal species (CONS). Additionally, the Markov and random field analyses found additional negative associations between Staphylococcus aureus and Candida species and between Staphylococcus epidermidis nonspeciated group B Streptococcus.
Factor Analysis Identified 3 Patterns
A factor analysis was done to identify clustering of species in polymicrobial infections beyond pairwise co-occurrences. Scree plotting yielded 2 as the optimal number of factors or patterns (Supplementary Figure 7). This analysis suggested the following 3 species co-occurrence patterns:
A Staphylococcus aureus–dominant pattern: The presence of Staphylococcus aureus and absence of Staphylococcus epidermidis, other coagulase-negative staphylococci, and nonspeciated staphylococci. This pattern was present in 37.9% of all foot specimens and 38 of the 284 (92.6%) total specimens with Staphylococcus aureus.
A CONS-dominant pattern (the inverse of the Staphylococcus aureus–dominant pattern): Staphylococcus epidermidis, coagulase-negative staphylococci, or other non-aureus staphylococci present with absence of Staphylococcus aureus. This pattern was present in 21.6% of all foot specimens.
“Pattern C”: the presence of 2 or more of the following: alpha-hemolytic Streptococcus, Enterococcus fecalis, Klebsiella, Proteus, Enterobacter, or Escherichia coli; and absence of both Bacteroides and Corynebacterium. This pattern was present in 11.4% of all foot specimens.
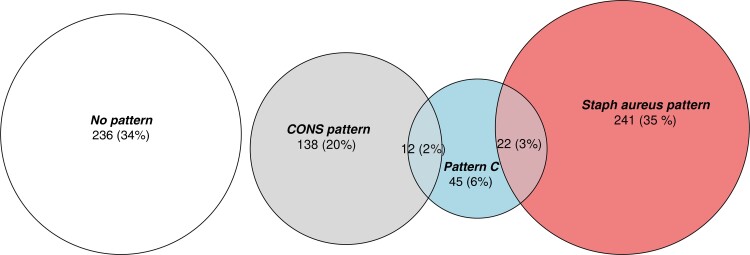
The factor loadings for various isolates in each of these 4 patterns are provided in Supplementary Table 10. The relative occurrences and overlap of these 3 patterns are shown in Figure 1.
Figure 1.
The absolute and relative occurrences and overlap of the 3 described microbial co-occurrence patterns. Abbreviation: CONS, coagulase-negative staphylococcal species.
Pattern C in Foot Osteomyelitis Is Associated With Treatment Failure
Finally, we used these patterns to re-analyze data from a cohort of patients with osteomyelitis for whom we had long-term treatment failure rates [3]. Of 130 initial foot osteomyelitis episodes in unique patients with both microbiological data and outcome data, 30 (24%) were categorized as having a Staphylococcus aureus pattern, 20 (16%) were categorized as the CONS pattern, 9 (7%) were categorized as having pattern C, and 62 (50%) were categorized as having no pattern.
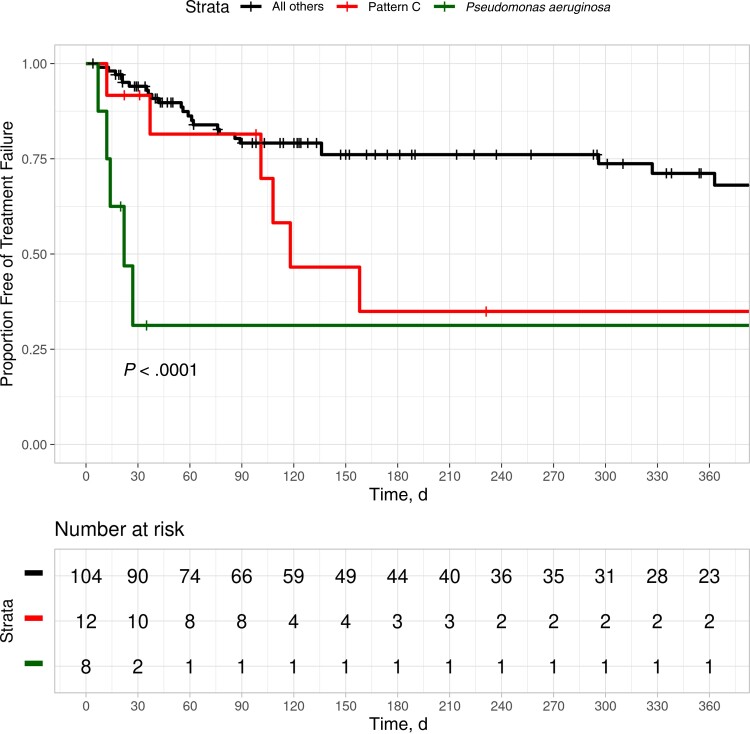
Log-rank analysis demonstrated that osteomyelitis treatment failure was significantly more frequent in osteomyelitis episodes as having pattern C or Pseudomonas aeruginosa (P < .0001) (Figure 2). Rates of freedom from treatment failure at 1 year were 34.9%, 31.2%, and 68.1% for patients with pattern C, patients with Pseudomonas aeruginosa, and all other patients, respectively (Figure 2). The presence of ESBL-producing organisms reached marginal significance on log-rank analysis but was not significant in multivariate modeling (Supplementary Figure 8 and Supplementary Table 11, respectively).
Figure 2.
Freedom from treatment failure among 130 patients with foot osteomyelitis.
A multivariate Cox proportional hazards model was then used to estimate the influence of various factors on treatment failure for osteomyelitis in this cohort. This analysis showed that, even with adjustment for other characteristics previously found significant, the presence of pattern C was strongly associated with treatment failure (Table 3). Including the microbiological patterns resulted in an overall model R2 value of 0.802, slightly better than the R2 value of 0.786 in the best performing model that included individual organisms instead of the patterns.
Table 3.
Comparison of 2 Multivariate Models of Time to Treatment Failure Among Patients With Foot Osteomyelitis
| Characteristic | Original 2016 Model | Revised Model With Microbial Patterns | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Severe or unaddressed peripheral artery disease | 9.90 | 3.72–26.3 | <.001 | 6.91 | 2.48–19.3 | <.001 |
| Insulin-dependent diabetes | 4.09 | 1.55–10.8 | .004 | 6.59 | 2.22–19.6 | <.001 |
| Serum albumin <2.8 gm/dL | 4.59 | 1.93–10.9 | <.001 | 4.44 | 1.93–10.2 | <.001 |
| Homeless | 5.72 | 1.68–19.5 | .005 | 6.03 | 1.72–21.2 | .005 |
| First toe (hallux) location | 1.59 | 0.64–3.93 | .3 | 2.27 | 0.91–5.67 | .079 |
| First metatarsal location | 0.59 | 0.17–2.06 | .4 | 0.56 | 0.16–1.95 | .4 |
| Fifth toe location | 0.38 | 0.10–1.48 | .2 | 0.38 | 0.10–1.52 | .2 |
| Fifth metatarsal location | 0.98 | 0.34–2.83 | >.9 | … | … | |
| Species-appropriate antibiotics for <7 d | 3.09 | 1.25–7.64 | .014 | 3.56 | 1.39–9.13 | .008 |
| Staphylococcus aureus | 0.47 | 0.16–1.33 | .2 | … | … | |
| Pseudomonas aeruginosa | 8.42 | 2.58–27.5 | <.001 | 7.97 | 2.50–25.4 | <.001 |
| Escherichia coli | 2.12 | 0.72–6.21 | .2 | … | … | |
| CONS-dominant microbial co-occurrence pattern | … | … | 2.39 | 0.81–7.04 | .11 | |
| Staphylococcus aureus–dominant microbial co-occurrence pattern | … | … | 0.55 | 0.19–1.61 | .3 | |
| Microbial co-occurrence pattern C | … | … | 2.74 | 0.92–8.17 | .071 | |
Abbreviations: CONS, coagulase-negative staphylococcal species; HR, hazard ratio.
DISCUSSION
Microbial co-occurrence associations in foot infection episodes are not so strong that they have been apparent in smaller series with basic descriptive statistics. The need to expand beyond the original and molecular Koch's postulates [22] to a more ecological view of clinical infections may also represent a newer concept underlying the idea that nonrandom co-occurrence patterns may occur and may have clinical relevance.
Nonetheless, nonrandom co-occurrence patterns are indeed present in foot infection specimens. Summarized simply, Staphylococcus aureus only rarely occurs with Staphylococcus epidermidis, coagulase-negative staphylococcus, or other nonspeciated staphylococcal species. Several Proteobacteria—namely Klebsiella, Proteus, Enterobacter, or Escherichia coli—appear to co-occur together with each other and alpha-hemolytic Streptococcus and Enterococcus fecalis but not with Corynebacterium or Bacteroides species (“pattern C”). Patients with polymicrobial foot infections including 2 or more organisms from this latter group (“pattern C”) have significantly higher rates of osteomyelitis treatment failure.
We note some similarity between the patterns identified in our study and the patterns described in a genus-level 16s RNA analysis of 52 diabetic foot ulcers by Gardner, Grice, and colleagues [14]. Their study reported a high-diversity pattern with more Proteobacteria, a Staphylococcus-predominant pattern, and a Streptococci-dominated pattern. These first 2 patterns have obvious overlap with those of our study, with the Staphylococcus-predominant pattern of their genus-level study comprising the Staphylococcus aureus– and CONS-predominant patterns seen in our species-level study. We did not see a Streptococcus-dominated pattern, but this may be because ours was a largely species-level analysis rather than a genus-level analysis. We would also point out that pattern C was identified through a bottom-up approach. The organisms in pattern C do not fall neatly into the Proteobacteria genus or any other previously described groupings such as gram stain appearance, extended-spectrum beta-lactamase–producing, or ESKAPE pathogens [23].
The de facto approach to polymicrobial findings in foot infections has been to simply report incidence rates of the individual species, an approach that implicitly assumes a random co-occurrence of organisms. Foot infections clearly involve a diverse array of microbes and are more frequently polymicrobial than soft tissue and bone infections in other locations. As previously suggested [24], results from this study also confirm that the microbiological spectrum of clinical specimens from foot bone and foot soft tissue are comparable to each other but distinct from the spectra of other bodily locations. Still, foot infections may serve as a prototype for a more ecological understanding of polymicrobial infections in general.
These polymicrobial co-occurrence patterns have practical applications nonetheless. First, patients with foot infection categorized as pattern C—or, as previously reported, Pseudomonas aeruginosa—should be recognized as being at risk for treatment failure. Follow-up should be more frequent than typical. Vigilant monitoring for signs of failure should lead to resampling of the area of infection, as microbes present in foot infections appear to change over time [25, 26]. Second, identifying patterns may help clinicians interested in antimicrobial stewardship consider avoiding empirical Staphylococcus aureus coverage not only if Staphylococcus aureus is not seen on culture but also if Staphylococcus epidermidis, other CONS species, or other non-aureus Staphylococcus species are seen on conventional culture.
Finally, identifying microbial co-occurrence patterns may help identify important synergistic, facilitative, or competitive relationships between organisms. For example, a high prevalence of Corynebacterium and other organisms typically categorized as simple commensals or as contaminants has been reported in many 16s RNS studies [11, 27, 28] and also in some studies of conventional cultures [3, 29]. In vitro studies have demonstrated that Corynebacterium striatum has a moderating effect on the pathogenicity of Staphylococcus aureus [16]. In addition to improving prognostic accuracy, understanding nuanced species interactions such as this might suggest that eliminating or even fostering the presence of specific bacteria (ie, probiotics) may provide clinical benefit [30, 31].
More research work is needed to bridge the gap between conventional culture findings and 16s RNA results. This study is among the first to search for co-occurrence patterns in clinical microbiology results rather than 16s RNA results. Seeing similar patterns in the current study and in 16s RNA studies should make the seeming disconnect between these different methodologies seem not quite as disparate. The statistical approaches to identifying co-occurrence patterns are recent and will likely continue to evolve. Our findings also need to be corroborated with similar analyses done using data from other centers, especially non-US centers, to determine whether previously described local or regional variations in the relative prevalence rates of various organisms—including a higher prevalence rate of gram-negative organisms in low-income countries [32]—impact co-occurrence patterns.
In summary, microbial co-occurrence patterns are common in foot infections and have a risk-adjusted impact on treatment failure. Identification and further characterization of co-occurrence patterns may have practical clinical relevance.
Supplementary Material
Acknowledgments
Financial support. This research was funded internally and no external funding sources were obtained.
Potential conflicts of interest. The authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. Conceptualization: N.R.B., C.M. Methodology: N.R.B., D.B., J.H.D., N.J.C. Validation: verification, whether as a part of the activity or separate, of the overall replication/reproducibility of results/experiments and other research outputs; formal analysis: N.R.B., D.B., J.H.D., N.J.C. Resources: provision of study materials, reagents, materials, patients, laboratory samples, animals, instrumentation, computing resources, or other analysis tools; data curation management activities; writing—original draft: N.R.B., N.J.C., C.M. Writing—review & editing: C.M., M.C.R.B. Supervision: N.R.B.
Availability of data and material. Data will not be made publicly available.
Code availability. Code will not be made publicly available.
Ethics approval. Approved by Baylor College of Medicine Institutional Review Board (protocol H-34858).
Consent to participate. Retrospective research, individual patient consent not obtained. Consent for publication has been given by all authors.
Contributor Information
Neal R Barshes, Division of Vascular Surgery and Endovascular Therapy, Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, Texas, USA; Michael E. DeBakey Veterans Affairs Medical Center, Houston, Texas, USA; Infectious Disease Section, Department of Medicine, One Baylor Plaza, Houston, Texas, USA.
Nicholas J Clark, School of Veterinary Science, School of Veterinary Science, The University of Queensland, Gatton, Queensland, Australia.
Deeksha Bidare, Baylor College of Medicine, One Baylor Plaza, Houston, Texas, USA.
J H Dudenhoeffer, Department of Biology and Biochemistry, University of Houston, Houston, Texas, USA.
Cezarina Mindru, Michael E. DeBakey Veterans Affairs Medical Center, Houston, Texas, USA; Infectious Disease Section, Department of Medicine, One Baylor Plaza, Houston, Texas, USA.
Maria C Rodriguez-Barradas, Michael E. DeBakey Veterans Affairs Medical Center, Houston, Texas, USA; Infectious Disease Section, Department of Medicine, One Baylor Plaza, Houston, Texas, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Lipsky BA, Berendt AR, Cornia PB, et al. Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2012; 2012:e132–73. [DOI] [PubMed] [Google Scholar]
- 2. Saltoglu N, Yemisen M, Ergonul O, et al. Predictors for limb loss among patient with diabetic foot infections: an observational retrospective multicentric study in Turkey. Clin Microbiol Infect 2015; 21:659–64. [DOI] [PubMed] [Google Scholar]
- 3. Barshes NR, Mindru C, Ashong C, Rodriguez-Barradas M, Trautner BW. Treatment failure and leg amputation among patients with foot osteomyelitis. Int J Low Extrem Wounds 2016; 15:303–12. [DOI] [PubMed] [Google Scholar]
- 4. Hinojosa CA, Boyer-Duck E, Anaya-Ayala JE, et al. Impact of the bacteriology of diabetic foot ulcers in limb loss. Wound Repair Regen 2016; 24:923–7. [DOI] [PubMed] [Google Scholar]
- 5. Leibovitch M, Cahn A, Gellman YN, et al. Predictors and outcomes of diabetic foot ulcer infection with ESBL-producing bacteria in a large tertiary center. Int J Infect Dis 2021; 113:318–24. [DOI] [PubMed] [Google Scholar]
- 6. Ashong CN, Raheem SA, Hunter AS, Mindru C, Barshes NR. Methicillin-resistant Staphylococcus aureus in foot osteomyelitis. Surg Infect 2017; 18:143–8. [DOI] [PubMed] [Google Scholar]
- 7. Aragon-Sanchez J, Lazaro-Martinez J, Quintana-Marrero Y, et al. Are diabetic foot ulcers complicated by MRSA osteomyelitis associated with worse prognosis? Outcomes of a surgical series. Diabetic Med 2009; 26:552–5. [DOI] [PubMed] [Google Scholar]
- 8. Lavery L, Harkless L, Felder-Johnson K, Mundine S. Bacterial pathogens in infected puncture wounds in adults with diabetes. J Foot Ankle Surg 1994; 33:91–7. [PubMed] [Google Scholar]
- 9. Gerding DN. Foot infections in diabetic patients: the role of anaerobes. Clin Infect Dis 1995; 20:S283–8. [DOI] [PubMed] [Google Scholar]
- 10. Citron DM, Goldstein EJ, Merriam CV, Lipsky BA, Abramson MA. Bacteriology of moderate-to-severe diabetic foot infections and in vitro activity of antimicrobial agents. J Clin Microbiol 2007; 45:2819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van Asten S, La Fontaine J, Peters E, Bhavan K, Kim P, Lavery L. The microbiome of diabetic foot osteomyelitis. Eur J Clin Microbiol Infect Dis 2016; 35:293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zou M, Cai Y, Hu P, et al. Analysis of the composition and functions of the microbiome in diabetic foot osteomyelitis based on 16S rRNA and metagenome sequencing technology. Diabetes 2020; 69:2423–39. [DOI] [PubMed] [Google Scholar]
- 13. Moon J, Kim N, Lee HS, et al. Nanopore 16S amplicon sequencing enhances the understanding of pathogens in medically intractable diabetic foot infections. Diabetes 2021; 70:1357–71. [DOI] [PubMed] [Google Scholar]
- 14. Gardner SE, Hillis SL, Heilmann K, Segre JA, Grice EA. The neuropathic diabetic foot ulcer microbiome is associated with clinical factors. Diabetes 2013; 62:923–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Malone M, Johani K, Jensen SO, et al. Next generation DNA sequencing of tissues from infected diabetic foot ulcers. EBioMedicine 2017; 21:142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ramsey MM, Freire MO, Gabrilska RA, Rumbaugh KP, Lemon KP. Staphylococcus aureus shifts toward commensalism in response to Corynebacterium species. Front Microbiol 2016; 7:1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hardy BL, Dickey SW, Plaut RD, et al. Corynebacterium pseudodiphtheriticum exploits Staphylococcus aureus virulence components in a novel polymicrobial defense strategy. MBio 2019; 10:e02491-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harris DJ. Inferring species interactions from co-occurrence data with Markov networks. Ecology 2016; 97:3308–14. [DOI] [PubMed] [Google Scholar]
- 19. Cheng J, Levina E, Wang P, Zhu J. A sparse ising model with covariates. Biometrics 2014; 70:943–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clark NJ, Wells K, Lindberg O. Unravelling changing interspecific interactions across environmental gradients using Markov random fields. Ecology 2018; 99:1277–83. [DOI] [PubMed] [Google Scholar]
- 21. Muhlebach MS, Hatch JE, Einarsson GG, et al. Anaerobic bacteria cultured from cystic fibrosis airways correlate to milder disease: a multisite study. Eur Respir J 2018; 52:1800242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Falkow S. Molecular Koch's postulates applied to microbial pathogenicity. Rev Infect Dis 1988; 2:S274–6. [DOI] [PubMed] [Google Scholar]
- 23. Sandiumenge A, Lisboa T, Gomez F, Hernandez P, Canadell L, Rello J. Effect of antibiotic diversity on ventilator-associated pneumonia caused by ESKAPE organisms. Chest 2011; 140:643–51. [DOI] [PubMed] [Google Scholar]
- 24. Lipsky BA, Armstrong DG, Citron DM, Tice AD, Morgenstern DE, Abramson MA. Ertapenem versus piperacillin/tazobactam for diabetic foot infections (SIDESTEP): prospective, randomised, controlled, double-blinded, multicentre trial. Lancet 2005; 366:1695–703. [DOI] [PubMed] [Google Scholar]
- 25. Barshes NR, Mindru C, Trautner BW, Rodriguez-Barradas MC. Discordant isolates in bone specimens from patients with recurrent foot osteomyelitis. Eur J Clin Microbiol Infect Dis 2019; 38:767–9. [DOI] [PubMed] [Google Scholar]
- 26. Sloan TJ, Turton JC, Tyson J, et al. Examining diabetic heel ulcers through an ecological lens: microbial community dynamics associated with healing and infection. J Med Microbiol 2019; 68:230–40. [DOI] [PubMed] [Google Scholar]
- 27. Suryaletha K, John J, Radhakrishnan MP, George S, Thomas S. Metataxonomic approach to decipher the polymicrobial burden in diabetic foot ulcer and its biofilm mode of infection. Int Wound J 2018; 15:473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smith K, Collier A, Townsend EM, et al. One step closer to understanding the role of bacteria in diabetic foot ulcers: characterising the microbiome of ulcers. BMC Microbiol 2016; 16:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bessman AN, Geiger PJ, Canawati H. Prevalence of Corynebacteria in diabetic foot infections. Diabetes Care 1992; 15:1531–3. [DOI] [PubMed] [Google Scholar]
- 30. Xu Z, Hsia HC. The impact of microbial communities on wound healing: a review. Ann Plast Surg 2018; 81:113–23. [DOI] [PubMed] [Google Scholar]
- 31. Knackstedt R, Knackstedt T, Gatherwright J. The role of topical probiotics on wound healing: a review of animal and human studies. Int Wound J 2020; 17:1687–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Macdonald KE, Boeckh S, Stacey HJ, Jones JD. The microbiology of diabetic foot infections: a meta-analysis. BMC Infect Dis 2021; 21:770. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.