Abstract
Background
Volatile organic compounds (VOCs) are produced systemically due to varied physiological states such as oxidative stress and are excreted through the lungs. Benchtop and preliminary clinical data suggest that breath testing may be a useful diagnostic modality for viral respiratory tract infections.
Methods
Patients with influenza-like illness (ILI) presenting to a single clinic in San Antonio, Texas, from 3/2017 to 3/2019 submitted a 2-minute breath sample in addition to a nasopharyngeal swab collected for polymerase chain reaction (PCR) assay for respiratory pathogens. VOCs were assayed with gas chromatography–mass spectrometry (GC-MS), and data were analyzed to identify breath VOC biomarkers that discriminated between ILI patients with and without a polymerase chain reaction (PCR) assay that was positive for influenza.
Results
Demographic, clinical, PCR, and breath data were available for 237 episodes of ILI, among which 32 episodes (13.5%) were PCR positive for influenza. Twenty candidate VOCs identified patients with influenza with greater than random accuracy. A predictive algorithm using 4 candidate biomarkers identified this group with 78% accuracy (74% sensitivity, 70% specificity). Based on their mass spectra, most of these biomarkers were n-alkane derivatives, consistent with products of oxidative stress.
Conclusions
A breath test for VOC biomarkers accurately identified ILI patients with PCR-proven influenza. These findings bolster those of others that a rapid, accurate, universal point-of-care influenza diagnostic test based on assay of exhaled-breath VOCs may be feasible. The next step will be a study of patients with ILI using a simplified method of breath collection that would facilitate translation for use in clinical practice.
Keywords: breath test, diagnosis, influenza, polymerase chain reaction PCR, respiratory tract infection, volatile organic compound VOC
A breath test utilizing volatile organic compound (VOC) biomarkers accurately identified patients with PCR-proven influenza. These findings bolster those of others that a rapid, accurate, universal point-of-care influenza diagnostic test based on assay of exhaled breath VOCs may be feasible.
Seasonal influenza is a common respiratory illness resulting in up to 41 million cases, 700 000 hospitalizations, and 50 000 deaths per year in the United States, with direct and indirect costs of about $87 billion [1, 2]. The economic and clinical burdens of influenza will worsen in the future as novel strains of the virus emerge: it is estimated that, in the United States, a “medium-level” pandemic would infect from 15% to 35% of the population, kill up to 207 000 people, and generate associated costs of up to $167 billion [3].
The ongoing coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) further illustrates the threat posed by novel respiratory viruses. COVID-19 resulted in >800 000 deaths in the United States in 2020–2021, with an estimated total cost of >$16 trillion [4]. Globally, by 2024, COVID-19 is projected to result in a gross domestic product (GDP) 3% below a no-COVID scenario [5]. These costs far exceed those associated with conventional recessions—they are akin to those associated with global climate change.
The severity and duration of influenza can be reduced by early detection and treatment of individual patients. Diagnostic approaches that can rapidly and accurately detect newly emerging variants are required to facilitate prompt implementation of containment and mitigation strategies [6, 7]. Unfortunately, laboratory testing for influenza has historically been of questionable value.
Presently, there are 57 US Food and Drug Administration (FDA)–cleared molecular assays (rapid molecular and nucleic acid amplification tests [NATs]) and antigen detection tests (rapid influenza diagnostic tests [RIDTs] and immunofluorescence assays) available for influenza diagnosis [8]. NATs, polymerase chain reaction (PCR) in particular, are often considered the gold standard for influenza diagnosis. While many of the limitations of these tests (primarily logistical concerns including transportation and batching of samples resulting in long turnaround times) have been overcome by current generation platforms, their relatively high cost and the need to develop new reagents for novel strains are persistent concerns [9].
RIDTs are antigen-based tests developed for rapid diagnosis of influenza at the point of care (POC) in the community. While the simplicity (many are Clinical Laboratory Improvement Amendments waived) and speed (completed in <30 minutes) of RIDTs are attractive, their low sensitivity for detecting seasonal influenza (62.3% in 1 large meta-analysis) significantly limits their usefulness [6]. RIDT performance during the influenza A(H1N1)pdm09 pandemic of 2009–2010 was even poorer (10%–40% in 1 large study), prompting the US Centers for Disease Control and Prevention (CDC) to advise physicians not to discontinue antiviral therapy despite a negative RIDT result [10].
The COVID-19 pandemic has underscored the urgent need for rapid, accurate tests for established and novel respiratory pathogens [4, 5]. Of note, by one estimate, a single additional COVID-19 screening test per 100 people was associated with an 8% reduction in mortality risk [11]. Delays in the development, distribution, and availability of accurate NATs and RIDTs led to a significant increase in the morbidity, mortality, and cost associated with the last 2 novel respiratory virus outbreaks in the United States [12, 13]. Assay of exhaled breath for volatile organic compounds (VOCs) via gas chromatography–mass spectrometry (GC-MS) is an emerging diagnostic modality with characteristics that might allow it to fill the respiratory virus diagnostics gap.
Many VOCs have a high vapor pressure at body temperature, and they are readily excreted through the lungs and may be detected in the breath. VOCs are produced systemically in humans as a result of varied physiological states and are excreted in the lungs [14]. These biomarkers of oxidative stress correlate with systemic inflammation. Current technology allows for detection of >2000 different VOCs in low concentrations, mostly in parts per billion or parts per trillion, in exhaled breath via laboratory-based GC-MS [14]. Portable, tabletop gas chromatographs employing surface acoustic wave detection (GC-SAW) can detect up to 60 different VOCs in exhaled breath in as little as 10 minutes. These instruments have been studied as point-of-care breath tests to identify VOC biomarkers in pulmonary tuberculosis and breast cancer [15, 16].
A group at the University of California at Davis undertook a series of elegant in vitro experiments examining the VOCs produced in cell culture by 3 influenza virus subtypes [17] and by human rhinovirus (HRV) [18]. The patterns of VOCs elaborated by infected cells were unique for each influenza virus subtype. Despite these differences, a conserved group of VOCs was produced following infection with all 3 influenza strains, raising the possibility that both a universal test and an etiological test may be feasible. The use of 2 control arms in the HRV study (uninfected control cells and cells treated with heat-killed HRV and polyinosinic-polycytidylic acid double-stranded RNA [poly(I:C)], a Toll-like receptor 3 [TLR3] agonist) allowed them to demonstrate that the production of VOCs observed early in HRV infection is not due to inflammation, but to active viral replication.
While cancer detection has been the predominant focus of breath testing clinical research, there have been a number encouraging studies of its potential application to infectious diseases. Before the arrival of SARS-CoV-2, tuberculosis (TB) was the most extensively studied infectious disease with respect to breath analysis [15, 19–21]. Mycobacterium tuberculosis (MTB) produces VOCs as byproducts of its metabolic processes. Analysis of the exhaled breath of patients with active pulmonary TB reveals the presence of both such MTB-specific VOCs and VOCs reflecting the increased oxidative stress caused by serious systemic infection in the human host. At least 9 clinical studies and 1 meta-analysis have suggested that breath VOCs can accurately distinguish between those with and without SARS-CoV-2 infection [22–31].
Two clinical studies have examined the effect of receipt of live attenuated influenza vaccine (LAIV) on the VOCs in exhaled breath in humans by collecting breath samples before and after vaccination [32, 33]. In both, LAIV caused measurable changes in the abundances of breath VOCs, suggesting that breath testing may be a plausible means for diagnosing influenza infection. In the second study, breath VOC biomarkers of oxidative stress identified postvaccination subjects with 82% accuracy on day 2, rising to 95% accuracy on day 14.
Given in vitro cell culture data and the observation that subclinical infection with LAIV caused measurable changes in the abundances of breath VOCs, symptomatic, naturally occurring influenza infection would be expected to cause similar or more pronounced changes. This study was undertaken to test the hypothesis that breath VOC biomarkers could identify influenza infection among those presenting with ILI.
METHODS
Human Subjects
Patients with ILI presenting to the Troop Medical Clinic (TMC) on Joint Base San Antonio (JBSA)–Fort Sam Houston, Texas, between 3/2017 and 3/2019 were asked to participate in the Acute Respiratory Infection Consortium (ARIC) Natural History study [34]. ARIC is a multisite study designed to explore the epidemiology, etiology, and clinical manifestations of ILI among Military Health System (MHS) beneficiaries. The TMC serves military trainees who have completed basic training and who are undergoing technical training for future service in the MHS. These personnel tend to be young (median age, 25 years) and healthy. ILI was defined as fever (temperature ≥100.4°F) and at least 1 respiratory symptom (sore throat, cough, sputum production, shortness of breath, or chest pain).
Study Procedures
After obtaining informed consent, patient data were recorded via a standard questionnaire including the influenza patient-reported outcome (FLU-PRO) instrument [35], and samples were collected: nasopharyngeal (NP) swab (Nylon-flocked; Copan Diagnostics, Corona, CA, USA) and breath sample. The method of breath collection has been previously described [36]. Briefly, the participant wore a nose clip and breathed normally through a disposable valved mouthpiece and bacterial filter into the breath collection apparatus (BCA) for 2 minutes [37]. VOCs in 1 L of alveolar breath were collected in graphitized carbon black in stainless steel thermal desorption tubes (Carbotrap; Supelco, Inc., Bellefonte, PA, USA). Samples were sealed and transported in TDS3 storage containers (Supelco, Inc., Bellefonte, PA, USA). Samples were stored at room temperature and shipped approximately monthly in batches to Menssana Research, Inc. (Fort Lee, NJ, USA). At Menssana, samples were stored at −20°C before analysis.
Molecular Methods
NP swabs were placed immediately into viral transport media, stored at −80°C, and shipped on dry ice to the Naval Health Research Center (San Diego, CA, USA). All specimens were tested for influenza by the CDC human influenza virus real-time reverse transcription polymerase chain reaction (rtRT-PCR) assay [38]. Assay for influenza B lineage was not performed. An aliquot was also tested by multiplex assay for presence of other viral respiratory pathogens using the Respiratory Viral Panel Target-Enriched Multiplex PCR (TEM-PCR; Diatherix Laboratories, LLC, Huntsville, AL, USA) [39] for samples collected between 3/2017 and 12/2018 and the BioFire FilmArray Respiratory Panel (FilmArray; BioFire Diagnostics, Salt Lake City, UT, USA) for samples collected between 1/2019 and 3/2019. Both panels have 8 viral pathogens in common: adenovirus, coronavirus, human metapneumovirus, HRV/enterovirus, influenza A, influenza B, parainfluenza, and respiratory syncytial virus.
Breath Sample Analysis
Using automated instrumentation, VOCs were thermally desorbed from the sorbent trap, cryogenically concentrated, and assayed by GC-MS, as has been described previously [36]. A known quantity of an internal standard (bromofluorobenzene) was automatically loaded onto all samples to normalize the abundance of VOCs and to facilitate alignment of chromatograms.
Statistical Analysis
The methods have been described previously [40]. In summary, chromatograms were processed with metabolomic analysis software (XCMS in R) to normalize peak retention times to the bromofluorobenzene internal standard in each chromatogram [41]. The aligned data were binned into a series of 5-second retention time segments (RTS). Multiple Monte Carlo simulations were used to select the RTS that identified participants in whom influenza was detected with greater than random accuracy while minimizing the risk of including random identifiers of disease. The average random behavior of each RTS was determined by randomly assigning participants to the “disease” or “disease-free” group and performing 40 estimates of the concordance statistic (C-statistic) or area under the curve (AUC) of the receiver operating characteristics (ROC) curve. The RTS that exhibited greater diagnostic accuracy with correct assignment than with multiple random assignments identified the apparent biomarkers of influenza infection. These apparent biomarkers of influenza infection were entered into a multivariate predictive algorithm using multivariate weighted digital analysis (WDA) to determine the sensitivity and specificity of the breath test and displayed in an ROC curve [42]. The accuracy of the breath test was defined as the C-statistic, or AUC of the ROC. The major VOC in each RTS was tentatively identified by matching its mass spectral signature to a library of mass spectra (NIST 2.0, Gaithersburg, MD, USA).
The same approach was used to select RTS that might allow for discrimination between other subsets of participants: influenza detected vs no virus detected, influenza detected vs any other virus detected, and any virus detected vs no virus detected. If candidate RTS were identified via Monte Carlo simulations, these candidate biomarkers were entered into a multivariate predictive algorithm using multivariate WDA.
RESULTS
Demographic, clinical, PCR, and breath data were available for 237 episodes of ILI among 235 unique patients (2 patients presented twice with ILI during the study period). Influenza was detected alone or in combination with another virus in 32 episodes and other respiratory viruses in 62 (Table 1). The median age of participants (interquartile range) was 21 (19–23) years, and 69% were male. There were no differences in age, gender, education level, race, smoking status, or military affiliation (branch of service) between the influenza-positive and -negative groups (Table 2). Likewise, there was no difference in days of limited activity, days of missed work, or symptoms at presentation between the groups (Table 3).
Table 1.
Viral Detection by PCR During 237 Episodes of ILI
| Any | 94 |
| Human rhinovirus/enterovirus | 38 |
| Influenza A (no subtype = 5, H3 = 19, H1 = 3) | 27 |
| Coronavirus (NL63 = 4, OC43 = 2, 229E = 1, HKU1 = 1) | 8 |
| Human metapneumovirus | 5 |
| Parainfluenza (Type 1/3 = 3, Type 4 = 1) | 4 |
| Influenza A/H3 + human rhinovirus/enterovirus | 2 |
| Human rhinovirus/enterovirus + human metapneumovirus | 2 |
| Human rhinovirus/enterovirus + parainfluenza (Type 1/3 = 1, Type 4 = 1) | 2 |
| Influenza B | 2 |
| Respiratory syncytial virus | 2 |
| Influenza A/no subtype + human rhinovirus/enterovirus | 1 |
| Human metapneumovirus + respiratory syncytial virus | 1 |
| None | 143 |
| Total | 237 |
Abbreviations: H1, hemagglutinin 1 subtype; H3, hemagglutinin 3 subtype; ILI, influenza-like illness; PCR, polymerase chain reaction.
Table 2.
Characteristics of Participants With and Without Influenza During 237 Episodes of ILI
| … | Influenza (n = 32), Median (IQR) or No. (%) | No Influenza (n = 205), Median (IQR) or No. (%) | P Value |
|---|---|---|---|
| Age, y | 21 (19–23.5) | 21 (19–24) | .71 |
| Gender | … | … | .68 |
| Male | 24 (75) | 139 (67.8) | |
| Female | 8 (25) | 65 (31.7) | |
| Missing | 0 (0) | 1 (0.5) | |
| Race | … | … | .72 |
| Black | 2 (6.2) | 28 (13.7) | |
| Hispanic | 6 (18.8) | 40 (19.5) | |
| Unknown/other | 4 (12.5) | 22 (10.7) | |
| White | 20 (62.5) | 115 (56.1) | |
| Military affiliation | … | … | .62 |
| Army | 23 (71.8) | 128 (62.4) | |
| Navy | 6 (18.8) | 56 (27.3) | |
| Air Force | 3 (9.4) | 21 (10.3) | |
| Smoking status | … | … | .2 |
| Current | 2 (6.2) | 4 (1.9) | |
| Former | 4 (12.5) | 19 (9.3) | |
| Never | 26 (81.3) | 182 (88.8) | |
| Vaccinated | 32 (100) | 203 (99) | .57 |
| Education level | … | … | .39 |
| High school | 21 (65.6) | 154 (75.1) | |
| Associate's degree | 7 (21.9) | 33 (16.1) | |
| Bachelor's degree | 4 (12.5) | 13 (6.4) | |
| Higher degree | 0 (0) | 5 (2.4) |
“Vaccinated” refers to individuals who received influenza vaccine during the current influenza season.
Abbreviations: ILI, influenza-like illness; IQR, interquartile range.
Table 3.
Clinical Presentation of Participants With and Without Influenza During 237 Episodes of ILI
| … | Influenza (n = 32), Median (IQR) or No. (%) | No Influenza (n = 205), Median (IQR) or No. (%) | P Value |
|---|---|---|---|
| Days of limited activity | 4 (4–5.5) | 4 (3–6) | .73 |
| Days of missed work | 2 (2–3) | 3 (2–4) | .2 |
| Hospitalization | 0 (0) | 2 (1) | 1 |
| FLU-PRO symptom score | … | … | |
| Lower respiratory tract | 3 (2–6.5) | 4 (2–5) | .92 |
| Upper respiratory tract | 3 (2–6) | 3 (1–5) | .51 |
| Gastrointestinal | 2 (0–4) | 2 (1–5) | .35 |
| Systemic | 6 (4–10) | 6 (4–8) | .49 |
| Total | 14 (10–27) | 16 (11–21) | .91 |
Abbreviations: FLU-PRO = influenza patient-reported outcome instrument; ILI, influenza-like illness; IQR, interquartile range.
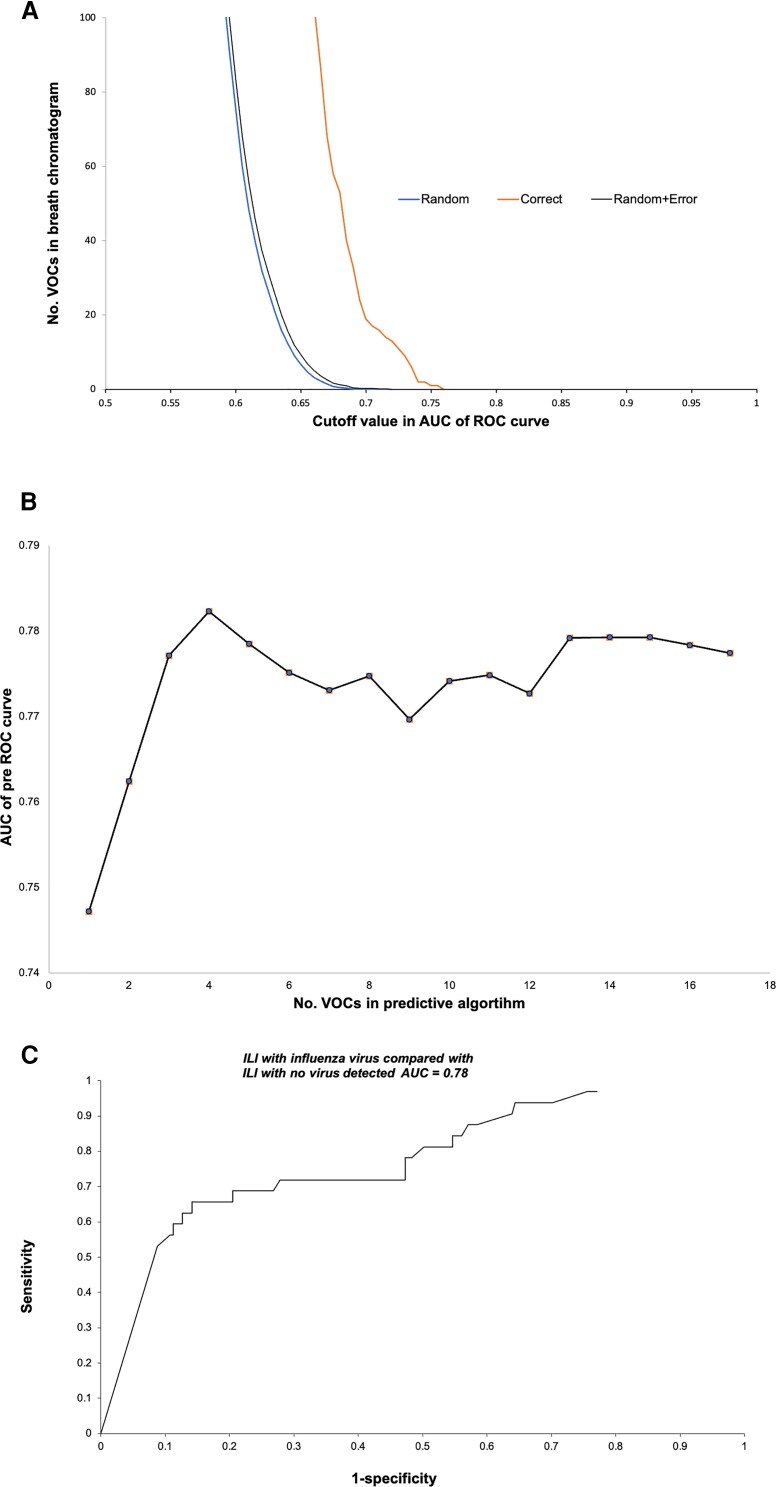
Monte Carlo analysis of breath biomarkers (Figure 1A) identified 20 breath VOC biomarkers that distinguished between influenza-positive (n = 32) and -negative (n = 205) cases with greater than random accuracy. The chemical structures of the top 5 biomarker VOCs were tentatively identified from their mass spectra (Table 4). The majority of these were either straight-chain n-alkane hydrocarbons (eg, heptane) or their methylated derivatives (eg, hexane-3-methyl), which are consistent with products of oxidative stress. When these VOCs were entered into a WDA multivariate algorithm, the accuracy of the predictive model reached a plateau with 4 (Figure 1B). The accuracy of the 4-VOC predictive model was 78%. With a cutoff point at the “shoulder” of the ROC curve, the test had 74% sensitivity and 70% specificity (Figure 1C).
Figure 1.
ILI with influenza infection vs ILI without influenza infection. A, Monte Carlo analysis of breath VOC biomarkers. Multiple Monte Carlo simulations were employed to identify chromatogram segments that distinguished participants with influenza from those without with greater than random accuracy. The average random behavior of each chromatogram segment was determined by randomly assigning subjects to the “influenza” or “no influenza” group and performing 40 estimates of the C-statistic (AUC of the ROC curve). For any given value of the C-statistic, it was then possible to identify the chromatogram segments that exhibited greater diagnostic accuracy with correct assignment than with multiple random assignments. This figure shows that the random assignment curve fell to 0 where the C-statistic ∼0.7. However, about 20 chromatogram segments exhibited greater than random diagnostic accuracy, thereby fulfilling the requirements of true biomarkers. These segments were entered into the multivariate predictive algorithm. B, Effect of number of VOCs in the algorithm on predictive accuracy. This graph displays variation in AUC of the ROC curve as a function of the number of biomarkers in the algorithm. The algorithm achieved maximal predictive accuracy (∼78%) with 4 biomarkers (chromatogram segments) in the model, and addition of more biomarkers to the algorithm did not significantly improve its performance. C, ROC curve. This curve displays the sensitivity (true-positives) vs 1-specificity (true-negatives) of the breath test utilizing the algorithm with 4 biomarkers. Test accuracy was 78% (ie, the C-statistic, or AUC of the ROC curve). With a cutoff point at the “shoulder” of the ROC curve, the test had ∼74% sensitivity and ∼70% specificity. Abbreviations: AUC, area under the curve; ILI, influenza-like illness; ROC, receiver operating characteristic; VOCs, volatile organic compounds.
Table 4.
Tentative Identification of VOC Biomarkers of Influenza Infection
| VOC Number | AUC | Fit | VOC Identification |
|---|---|---|---|
| 1 | 0.75 | 879 | (R)-(-)-2-Amino-1-Propanol |
| … | … | 859 | Hydroxyurea |
| … | … | 848 | Topotecan |
| 2 | 0.74 | 725 | 2-Butanamine, N-Nitro- |
| … | … | 701 | 1-Propanamine, N, 2-Dimethyl-N-Nitro- |
| … | … | 650 | Isothiourea, 2-(2-Octylsulfonyl)Ethyl- |
| 3 | 0.73 | 747 | Hexanal, 3-Methyl- |
| … | … | 669 | Butane, 1-(Ethenyloxy)-3-Methyl |
| … | … | 643 | Pentane, 1-(Ethenyloxy)- |
| 4 | 0.73 | 973 | Heptane |
| … | … | 948 | Hexane, 3-Methyl- |
| … | … | 906 | Pentane, 2,3-Dimethyl- |
| 5 | 0.73 | 917 | (1R)-26,6-Trimethylbicyclo[3.1.1]Hept-2 |
| … | … | 907 | Alpha-Pinene |
| … | … | 901 | Trans-Beta-Ocimene |
The list displays the 5 major breath biomarkers of influenza infection, each with its top 3 tentative identifications. AUC indicates a biomarker's accuracy when it was employed alone. “Fit” is a numerical value assigned by the NIST computer-based library as an indicator of “goodness of fit” of the mass spectrum of a breath VOC compared with similar mass spectra in the library. Fit values range from 0 (no fit) to 1000 (perfect fit). Values >700 or >800 are generally considered “good” to “very good” fits. However, these identifications may only be considered tentative. A number of the tentative VOC identifications comprised either straight-chain n-alkane hydrocarbons (eg, heptane) or their methylated derivatives (eg, hexane-3-methyl). These VOCs are all consistent with products of oxidative stress. Abbreviations: AUC, area under the curve; NIST, National Institute of Standards and Technology; VOC, volatile organic compound.
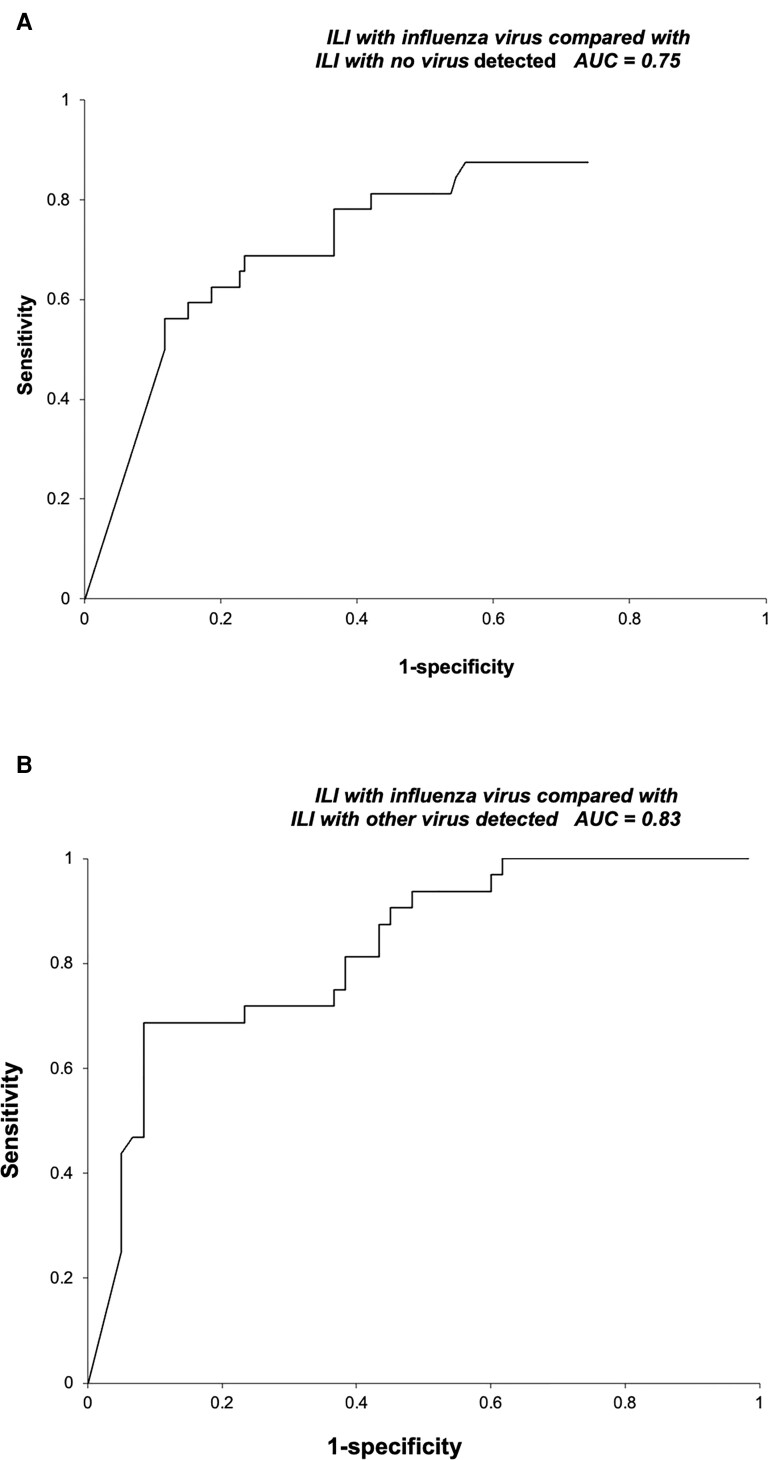
Analyses comparing the influenza-positive group with other groups yielded similar results (Table 5). The accuracy of a 5-VOC model comparing the influenza-positive group with the group with any other virus detected was 75% (Figure 2A), while the accuracy of a 3-VOC model comparing the influenza-positive group with the group with no virus detected was 83% (Figure 2B). By contrast, Monte Carlo analysis did not identify any VOCs, which significantly distinguished between the group with any virus detected and that with no virus detected.
Table 5.
Summary of Breath Biomarker Findings
| Index Group | Comparator Group | AUC |
|---|---|---|
| ILI with influenza (n = 32) | ILI without influenza (n = 205) | 0.78 |
| ILI with influenza (n = 32) | ILI with no virus detected (n = 143) | 0.75 |
| ILI with influenza (n = 32) | ILI with any other virus detected (n = 62) | 0.83 |
| ILI with any virus detected (n = 94) | ILI with no virus detected (n = 143) | NS |
Abbreviations: AUC, area under the curve; ILI, influenza-like illness; NS, not significant.
Figure 2.
ROC curves for ILI with influenza infection vs ILI with no virus detected and ILI with influenza infection vs ILI with any other virus detected. Abbreviations: AUC, area under the curve; ILI, influenza-like illness; ROC, receiver operating characteristic.
DISCUSSION
In this clinical study of patients presenting with ILI, breath VOC biomarkers accurately identified those with PCR-proven influenza infection and distinguished them from those who were infected with other viruses or in whom no virus was detected. These data corroborate and expand upon those from other recent studies of patients with influenza and with SARS-CoV-2.
A prospective observational study performed at a single tertiary care medical center in Bayreuth, Germany, before the COVID-19 pandemic, enrolled 24 participants: 20 admitted for ILI (14 positive for influenza by PCR) and 4 asymptomatic health care workers [43]. In a procedure that took <5 minutes for each participant, nasal breath aspirated for 10 seconds during normal respiration was directly analyzed by multicapillary column coupled ion mobility spectrometry (MCC-IMS). Cluster analysis of chromatographic data followed by multivariate analysis utilizing proprietary software allowed development of an algorithm comprised of 4 VOCs that distinguished influenza-positive from -negative participants with 100% sensitivity and specificity. The identified VOCs were not reported.
At least 9 clinical studies (6 of which were reviewed in a recent meta-analysis [31]) have suggested that breath VOCs can accurately distinguish between those with and without SARS-CoV-2 infection. In 3 of the studies, participants breathed directly into the aperture of an instrument containing a nanomaterial-based hybrid sensor “electronic nose” (e-nose) for varying amounts of time [22–24]. E-noses base the analysis on an array of sensors, which are not fully selective and thus provide a certain multiplicity of detection, as each is sensitive to a different substance but responds to other compounds as well. The information on the sample is obtained by comparing the signals of several sensors. Because of this cross-specificity and cross-selectivity, sample analysis using an e-nose is not based on recognizing the individual VOCs, but rather on detecting a particular chemical signature. Each of the 3 studies utilized a different e-nose.
Four of these studies examined exhaled breath collected into bags [25–27] or a syringe [28]. One study examined exhaled breath transferred directly from the end of the endotracheal tube to the analytical equipment [29]. The final study examined exhaled breath condensate (EBC) [30]. Varied techniques were used to analyze these samples: GC-MS [26], GC time-of-flight mass spectrometry (GC-ToFMS) [25, 30], GC ion mobility spectrometry (GC-IMS) [28], proton transfer reaction ToFMS (PTR-ToFMS) [29], and Fourier transform infrared (FTIR) spectroscopy [27]. Pooled sensitivity and specificity for VOC vs PCR for the 6 studies included in the meta-analysis were 98.2% (97.5% CI, 93.1%–99.6%) and 74.3% (97.5% CI, 66.4%–80.9%), respectively [31].
The influenza and SARS-CoV-2 studies to date are best considered preliminary or exploratory. In general, they have been limited by small sample size, lack of a comparator group (healthy controls), and widely varied methodologies. Translation of breath testing research results into clinical practice has perhaps been most limited by this lack of standardization: It is hard to directly compare or replicate results [44–46]. Studies have utilized a variety of samples: exhaled oral breath, exhaled nasal breath, EBC, air from the ventilator circuit, and headspace air above culture media. Likewise, the method of breath sample collection has differed considerably: sorbent trap, polyvinyl fluoride (Tedlar) bag, polyethylene terephthalate (Mylar) bag, and direct interface to lab equipment, among others. Furthermore, the equipment used to analyze samples has included a wide array of spectrometers and e-noses. Lastly, candidate VOC biomarkers are not reported in a uniform fashion (eg, some are reported by product ion mass only, and some are not reported at all).
These observations suggest several useful next steps for the field of breath testing: first, development of standardized protocols for uniform and repeatable breath sampling; second, studies of large groups of healthy volunteers to explore the consistency of breath composition seen under different conditions and to more fully characterize the human volatilome; third, reporting of candidate VOC biomarkers with as much detail as is possible—the ability of research groups in different geographic locations and using different equipment to identify the same biomarkers in the exhaled breath of diverse patient populations with the same infection would significantly further the case for breath testing as a diagnostic modality. Indeed, such reporting would largely remove variability introduced by use of different laboratory equipment.
The finding that no VOC biomarkers discriminated between ILI patients in whom any virus was detected by PCR vs those in whom no virus was detected may suggest that the latter were either infected by a virus not included in the multiplex PCR or that the PCR was falsely negative. Specifically, the absence of a difference in VOCs in the exhaled breath of these 2 groups suggests that all patients had increased levels of oxidative stress. The study design (no baseline sample when participants were asymptomatic and no control group) does not, however, allow for a definite conclusion. Another limitation of the study is the relatively small and homogeneous population, which limits its generalizability.
Overall, these findings bolster those of others that assay of breath VOCs might allow for both a universal test (presence of any viral respiratory tract infection) and an etiological test (identification of the causative virus and its subtype). The next step will be a study of patients with ILI using a simplified method of breath collection (single breath exhaled into a polyethylene terephthalate bag). This method would facilitate translation into clinical practice because samples could be collected outside of medical settings to include self-collection at home.
Acknowledgments
Disclaimers. This study, IDCRP-045, was conducted by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense (DoD) program executed by the Uniformed Services University of the Health Sciences (USUHS) through a federal assistance agreement with The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. (HJF).
Financial support. This work was supported by federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), under Inter-Agency Agreement (12012-001-07000); the Defense Health Program; and the Department of the Air Force, 59th Medical Wing/Science and Technology Branch.
The contents of this publication are the sole responsibility of the authors and do not necessarily reflect the views, opinions, or policies of the Department of Veterans Affairs, the Uniformed Services University of the Health Sciences (USUHS), the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., the Department of Defense (DoD), the Departments of the Army, Navy, or Air Force, Brooke Army Medical Center, or the US Government. Mention of trade names, commercial products, or organizations does not imply endorsement by the US Government.
Patient consent. All participants provided written informed consent. Procedures followed were in accordance with the ethical standards of the Helsinki Declaration (1964, amended most recently in 2008) of the World Medical Association. The investigators adhered to policies for the protection of human subjects as prescribed in 45CFR46. The study was approved by the Infectious Disease Institutional Review Board (IRB) at the USUHS, Bethesda, Maryland, and at the Brooke Army Medical Center, San Antonio, Texas.
ClinicalTrials.gov identifier. NCT01021098.
Contributor Information
Patrick J Danaher, Medicine Service, Infectious Diseases Section, South Texas Veterans Health Care System, San Antonio, Texas, USA.
Michael Phillips, Menssana Research Inc., Fort Lee, New Jersey, USA.
Peter Schmitt, Schmitt & Associates, Newark, New Jersey, USA.
Stephanie A Richard, Infectious Disease Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA; The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda, Maryland, USA.
Eugene V Millar, Infectious Disease Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA; The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda, Maryland, USA.
Brian K White, Air Force Medical Readiness Agency, Defense Health Headquarters, Falls Church, Virginia, USA.
Jason F Okulicz, Department of Medicine, Infectious Diseases Service, Brooke Army Medical Center, San Antonio, Texas, USA.
Christian L Coles, Infectious Disease Clinical Research Program, The Henry M. Jackson Foundation for the Advancement of Military Medicine, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA.
Timothy H Burgess, Infectious Disease Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA.
References
- 1. Centers for Disease Control and Prevention . Disease burden of flu. Available at:https://www.cdc.gov/flu/about/burden/index.html. Accessed January 1, 2022.
- 2. Molinari NM. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 2007; 25:5086–96. [DOI] [PubMed] [Google Scholar]
- 3. Cross M. Influenza pandemic: challenges in preparedness and response. Government Accountability Office (GAO) 2005. Available at:https://www.hsdl.org/?view&did=454016. Accessed January 16, 2022.
- 4. Cutler DM. The COVID-19 pandemic and the $16 trillion virus. JAMA 2020; 324:1495–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yeyati EL, Filippini F. Social and economic impact of COVID-19. Brookings Global Working Paper #158, June 2021. Available at:https://www.brookings.edu/research/social-and-economic-impact-of-covid-19. Accessed January 16, 2022.
- 6. Vemula SV, Zhao J, Liu J, Wang X, Biswas S, Hewlett I. Current approaches for diagnosis of influenza infections in humans. Viruses 2016; 8:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention . National strategy for pandemic influenza. Homeland Security Council 2005. Available at:https://www.cdc.gov/flu/pandemic-resources/pdf/pandemic-influenza-implementation.pdf. Accessed January 16, 2022.
- 8. Centers for Disease Control and Prevention . Overview of influenza testing methods. Available at:https://www.cdc.gov/flu/professionals/diagnosis/overview-testing-methods.htm. Accessed April 11, 2022.
- 9. Wang R, Sheng ZM, Taubenberger JK. Detection of novel (swine origin) H1N1 influenza A virus by quantitative real-time reverse transcriptase-PCR. J Clin Microbiol 2009; 47:2675–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention . Evaluation of rapid influenza diagnostic tests for detection of novel influenza A (H1N1) virus—United States, 2009. MMWR Morb Mortal Wkly Rep 2009; 58:826–9. [PubMed] [Google Scholar]
- 11. Liang LL, Tseng CH, Ho HJ, Wu CY. COVID-19 mortality is negatively associated with test number and government effectiveness. Sci Rep 2020; 10:12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Department of Health and Human Services . An HHS retrospective on the 2009 H1N1 influenza pandemic to advance all hazards preparedness. Revised June 15, 2012. Available at:https://www.phe.gov/Preparedness/mcm/h1n1-retrospective/Documents/h1n1-retrospective.pdf. Accessed August 7, 2022.
- 13. Schneider EC. Failing the test—the tragic data gap undermining the US pandemic response. N Engl J Med 2020; 383:299–302. [DOI] [PubMed] [Google Scholar]
- 14. Phillips M, Cataneo RN, Chaturvedi A, et al. . Detection of an extended human volatome with comprehensive two-dimensional gas chromatography time of flight mass spectrometry. PLoS One 2013; 8:e75274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Phillips M, Basa-Dalay V, Blais J, et al. . Point-of-care breath test for biomarkers of active pulmonary tuberculosis. Tuberculosis 2012; 92:314–20. [DOI] [PubMed] [Google Scholar]
- 16. Phillips M, Cataneo RN, Cruz-Ramos JA, et al. . Prediction of breast cancer risk with volatile biomarkers in breath. Breast Cancer Res Treat 2018; 170:343–50. [DOI] [PubMed] [Google Scholar]
- 17. Aksenov AA, Sandrock CE, Zhao W, et al. . Cellular scent of influenza virus infection. Chembiochem 2014; 15:1040–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schivo M, Aksenov AA, Linderholm AL, et al. . Volatile emanations from in vitro airway cells infected with human rhinovirus. J Breath Res 2014; 8:037110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schito M, Migliori GB, Fletcher HA, et al. . Perspectives on advances in tuberculosis diagnostics, drugs and vaccines. Clin Infect Dis 2015; 61(Suppl 3):S102–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Phillips M, Cataneo RN, Condos R, et al. . Volatile biomarkers of pulmonary tuberculosis in the breath. Tuberculosis 2007; 87:44–52. [DOI] [PubMed] [Google Scholar]
- 21. Phillips M, Basa-Dalay V, Bothamley G, et al. . Breath biomarkers of active pulmonary tuberculosis. Tuberculosis 2010; 90:145–51. [DOI] [PubMed] [Google Scholar]
- 22. Wintjens AGWE, Hintzen KFH, Engelen SME, et al. . Applying the electronic nose for pre-operative SARS-CoV-2 screening. Surg Endosc 2020; 35:6671–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. De Vries R, Vigeveno RM, Mulder S, et al. . Ruling out SARS-CoV-2 infection using exhaled breath analysis by electronic nose in a public health setting. medRxiv 2021.02.14.21251712 [Preprint]. February 16, 2021. Available at: 10.1101/2021.02.14.21251712. Accessed October 7, 2022. [DOI]
- 24. Shan B, Broza YY, Li W, et al. . Multiplexed nanomaterial-based sensor array for detection of COVID-19 in exhaled breath. ACS Nano 2020; 14:12125–32. [DOI] [PubMed] [Google Scholar]
- 25. Berna AZ, Akaho EH, Harris RM, Congdon M, Korn E. Reproducible breath metabolite changes in children with SARS-CoV-2 infection. ACS Infect Dis 2021; 7:2596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ibrahim W, Cordell RL, Wilde MJ, et al. . Diagnosis of COVID-19 by exhaled breath analysis using gas chromatography-mass spectrometry. ERJ Open Res 2021; 7:00139-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shlomo IB, Frankenthal H, Laor A, Greenhut AK. Detection of SARS-CoV-2 infection by exhaled breath spectral analysis: introducing a ready-to-use point-of-care mass screening method. EClinicalMedicine 2022; 45:101308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ruszkiewicz DM, Sanders D, O’Brien R, et al. . Diagnosis of COVID-19 by analysis of breath with gas chromatography-ion mobility spectrometry—a feasibility study. EClinicalMedicine 2020; 29:100609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grassin-Delyle S, Roquencourt C, Moine P, et al. . Metabolomics of exhaled breath in critically ill COVID-19 patients: a pilot study. EBioMedicine 2021; 63:103154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barberis E, Amede E, Khoso S, et al. . Metabolomics diagnosis of COVID-19 from exhaled breath condensate. Metabolites 2021; 11:847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Subali AD, Wiyono L, Yusuf M, Zaky MFA. The potential of volatile organic compounds-based breath analysis for COVID-19 screening: a systematic review & meta-analysis. Diagn Microbiol Infect Dis 2022; 102:115589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mashir A, Paschke KM, van Duin D, et al. . Effect of the influenza A (H1N1) live attenuated intranasal vaccine on nitric oxide (FENO) and other volatiles in exhaled breath. J Breath Res 2011; 5:037107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Phillips M, Cataneo RN, Chaturvedi A, et al. . Effect of influenza vaccination on oxidative stress products in breath. J Breath Res 2010; 4:026001. [DOI] [PubMed] [Google Scholar]
- 34. Coles C, Millar EV, Ottolini MG. The Acute Respiratory Infection Consortium: a multi-site, multi-disciplinary clinical research network in the Department of Defense. Mil Med 2019; 184:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Powers JH, Bacci ED, Leidy NK, et al. . Performance of the inFLUenza patient-reported outcome (FLU-PRO) diary in patients with influenza-like illness (ILI). PLoS One 2018; 13:e0194180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Phillips M, Cataneo RN, Greenberg J, Gunawardena R, Naidu A, Rahbari-Oskoui F. Effect of age on the breath methylated alkane contour, a display of apparent new markers of oxidative stress. J Lab Clin Med 2000; 136:243–9. [DOI] [PubMed] [Google Scholar]
- 37. Menssana Research . Menssana systems: breath collection apparatus. Available at:http://menssanaresearch.com/systems_BCA_Menssana.html. Accessed August 12, 2022.
- 38. Shu B, Wu KH, Emery S, et al. . Design and performance of the CDC real-time reverse transcriptase PCR swine flu panel for detection of 2009 a (H1N1) pandemic influenza virus. J Clin Microbiol 2011; 49:2614–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bouvier M, Chen WJ, Arnold JC, et al. . Species-specific clinical characteristics of human coronavirus infection among otherwise healthy adolescents and adults. Influenza Other Respir Viruses 2018; 12:299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Phillips M, Bauer TL, Cataneo RN, et al. . Blinded validation of breath biomarkers of lung cancer, a potential ancillary to chest CT screening. PLoS One 2015; 10:e0142484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gowda H, Ivanisevic J, Johnson CH, et al. . Interactive XCMS online: simplifying advanced metabolomic data processing and subsequent statistical analyses. Anal Chem 2014; 86:6931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Phillips M, Altorki N, Austin JH, et al. . Detection of lung cancer using weighted digital analysis of breath biomarkers. Clin Chim Acta 2008; 393:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Steppert C, Steppert I, Bollinger T, Sterlacci W. Rapid non-invasive detection of influenza-A-infection by multicapillary column coupled ion mobility spectrometry. J Breath Res 2020; 15:011001. [DOI] [PubMed] [Google Scholar]
- 44. Jia Z, Patra A, Kutty VK, Venkatesan T. Critical review of volatile organic compound analysis in breath and in vitro cell culture for detection of lung cancer. Metabolites 2019; 9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Risby TH. Critical issues for breath analysis. J Breath Res 2008; 2:030302. [Google Scholar]
- 46. Beauchamp JD, Pleil JD. Simply breath-taking? Developing a strategy for consistent breath sampling. J Breath Res 2013; 7:042001. [DOI] [PubMed] [Google Scholar]