Abstract
Background
Robotic surgery has been widely used in the radical treatment of colonic cancer. However, it is unclear what advantages the robotic approach offers over other approaches in left colectomy. This study aims to explore the advantage of robotic surgery in left colectomy by comparing open, laparoscopic, and robotic surgery.
Methods
A retrospective analysis was performed on the clinical data of patients with radical left colectomy for colon cancer who were admitted to the Department of General Surgery, The First Affiliated Hospital of Nanchang University, from November 2012 to November 2017. Two hundred eleven patients included were divided into the open surgery group (OS, n=49), laparoscopic surgery group (LS, n=92), and robotic surgery group (RS, n=70) according to surgical techniques. The clinicopathologic data were collected for clinical outcome assessment. Finally, the clinical value of RS in radical left colectomy was further evaluated by propensity score matching (PSM) analysis.
Results
Three groups were similar in demographics and clinical characteristics. Compared with OS, LS and RS groups had better intraoperative and perioperative clinical outcomes. Moreover, the RS group exhibited the minimum operative times, length of stay (LOS), and evaluated blood loss. LS and RS also exhibited less perioperative and postoperative long-term complications. Three groups showed similar postoperative pathological outcomes. The overall survival and disease-free survival were also similar among the three groups (all P > 0.05). Cox regression analysis showed surgical approach was not a prognostic factor for overall survival (P = 0.671) and disease-free survival (P = 0.776). PSM analysis of RS and LS by clinical characteristics showed RS showed shorter operation time (P < 0.001) and LOS for patients without complications (P = 0.005). However, no significant differences were found in perioperative and long-term postoperative complications, pathological outcomes, overall survival, and disease-free survival.
Conclusions
Among three techniques for radical left colectomy, LS and RS had significant advantages over OS in short-term clinical outcomes, and no significant differences were found in overall, disease-free survival, local recurrence, and distant metastasis incidence. Moreover, RS shows better perioperative clinical outcomes but without compromising survival compared with LS.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12957-022-02796-8.
Keywords: Robotic surgery, Laparoscopic surgery, Open surgery, Left colectomy
Background
Colorectal cancer (CRC) is the second most deadly cancer and accounts for approximately 10% of all annually diagnosed cancers and cancer-related deaths worldwide [1]. In particular, left-sided colon cancer showed a worrying rise in young patients [2]. Surgical resection is still the only method to achieve radical treatment for resectable local left colonic cancer in the middle and advanced stages under elective conditions [3, 4]. Historically, OS has long been the most classic and useful surgical approach to treat left colonic cancer [5]. However, OS brings great trauma to the patients, such as long abdominal incision and severe postoperative pain, which is not conducive to patients’ postoperative recovery. Over the last several decades, minimally invasive surgery (MIS) including LS and RS has become more popular and has been adopted in colorectal surgery [6]. The studies showed MIS can decrease perioperative morbidity, facilitate accurate anatomical dissection, and improve the quality of the resection specimens [7, 8], but without compromising oncologic principles [9]. Results [10, 11] showed that LS has better cosmesis, shorter postoperative hospitalization, and faster recovery than OS. Recently, RS using the da Vinci surgical system shows several technical advantages [12] such as 3D visualization, elimination of fulcrum effect, multi-manipulator operation, and better ergonomic positioning and achieves similar or even better outcomes than LS in colorectal cancer [13, 14]. However, few investigations on surgery for radical left colectomy for colonic cancer are available according to current literature.
Our gastrointestinal center has evolved from open, laparoscopic, and robotic surgery for radical resection of CRC. In rectal cancer, our study revealed short-term advantages of RS, but without a beneficial effect on survival [15]. In this study, a comprehensive comparison of perioperative outcomes and survival in consecutive series of 211 patients who were treated by three different surgical techniques (open, laparoscopic, and robotic) for radical left colectomy, and the safety and efficacy were also analyzed. Moreover, the value of MIS, special for RS, in radical left colectomy was verified.
Methods
Study population and data collection
This is a retrospective study of patients undergoing radical left colectomy for colonic cancer under elective conditions from November 2012 to November 2017 at the largest gastrointestinal center in Jiangxi Province, The First Affiliated Hospital of Nanchang University. Detailed eligibility criteria are shown as follows:
The inclusion criteria are (1) tumor in the left side including the distal 1/3 of the transverse colon (TC), splenic flexure (SF), the upper segment of descending colon (UDC), or the lower segment of descending colon (LDC); (2) age older than 18 and younger than 80 years; (3) primary colonic adenocarcinoma confirmed pathologically by endoscopic biopsy; (4) pathologic T1-4aN0-2M0 at postoperative evaluation according to 8th AJCC Cancer Staging Manual; (5) no history of malignancy in other organs; (6) ASA scores I, II, or III; and (7) written informed consent.
The exclusion criteria are (1) age>80 and <18 years; (2) with other malignant tumors; (3) TNM stage at 0, IV; (4) multi-visceral resection (tumors invading adjacent organs or tumors located in multiple bowel tubes); (5) palliative surgery; (6) history of CRC surgery; and (7) emergency surgery due to complications (bleeding, obstruction, or perforation) caused by left-sided colon cancer.
Eligible patients received routine preoperative chest X-ray or CT scan, abdominal CT scan, tumor markers, colonoscopy, and other liver ultrasound or MRI. All patients were assigned to robotic, laparoscopic, and open surgical procedure groups according to local medical insurance and the intention-to-treat principle. Some cases with LS and RS were converted to the OS due to intraperitoneal adhesions and were included in the OS group if met with inclusion criteria. Moreover, to reduce potential bias caused by the limitations of a retrospective cohort study, the propensity score matching (PSM) method was used to conduct a 1-to-1 matching analysis between the LS group and the RS group.
Surgical treatment and follow-up
All surgical operations were performed by surgeons of similar experience and seniority. Surgical indication followed the guide treatment of colorectal cancer [16]. The length of bowel resection and scope of the lymph node dissection for radical left colectomy that was treated, the distal 1/3 of the transverse colon (TC), splenic flexure (SF), the upper segment of descending colon (UDC), or the lower segment of descending colon (LDC) are shown in the Supplementary Fig 1. The surgery was to remove the tumor-bearing colon segment and its corresponding mesocolon and ligate the origin of the inferior mesenteric artery (IMA) to maximize lymph node dissection (LND) without damaging the visceral fascia layer. Our surgical team attempted to secure 10 cm or more for the proximal and distal resection margin and followed D3 lymphadenectomy [17] and complete mesocolic excision (CME) principles [18]. The procedures for radical left colectomy were as follows: firstly, dissociated inferior mesenteric artery (IMA) and then ligated the origin of the left colon artery (LCA) while preserving the superior rectal artery and part of the sigmoid artery. Secondly, the mesocolon was incised along the inferior pancreatic border, transecting the left colic vein or inferior mesenteric vein (IMV), and the left branch of the middle colic artery. Thirdly, the division of the splenocolic and gastrocolic ligament and the lateral peritoneal fold completed the mobilization of the splenic flexure and accomplished the CME; Forthly, the mobilized left colon was removed to accomplish side-to-end or side-to-side stapled extracorporeal or intracorporeal anastomosis; Lastly, final operations were to flush the abdominal cavity, place a drainage tube after inspection, and suture the auxiliary incision.
All patients were regularly followed-up after surgery. A follow-up visit involved standard clinical examination, serum tumor marker test, and imaging by computer tomography (CT) or/and magnetic resonance or/and ultrasounds. Patients were treated with adjuvant chemotherapy (CHT) when indicated.
Definitions
Comorbidity was measured using the Charlson Comorbidity Index (CCI) and selected elements of Elixhauser comorbidity score (cardiovascular, pulmonary, endocrine, gastrointestinal, renal, inflammatory, and neuropsychiatric disorders) [19].
The conversion was defined as the use of a midline or periumbilical long incision for any reason at any time during the procedure. Cases, converted to OS, who met the inclusion criteria, were to the OS group.
Postoperative complications were stratified by the Clavien–Dindo classification of surgical complications [20]. Adverse events were defined as any deviation from the normal postoperative course, and major adverse events were defined as any event that is life-threatening, requires inpatient hospitalization, results in a single organ or multiorgan failure, or requires operative, endoscopic, or radiological intervention [21]. Major adverse events correspond to Grade III/IV/V of the Clavien–Dindo classification [20, 22]. Long-term complications were defined as postoperative complications that occurred after the 30th postoperative day. The short-term clinical outcomes included intraoperative and perioperative outcomes, perioperative postoperative complications, and postoperative pathologic outcomes. Then, long-term postoperative complications and survival outcomes were incorporated into long-term clinical outcomes.
Overall survival was calculated in months from the time of surgery to the last follow-up or death. Recurrence was defined as the presence of locoregional recurrence, the presence of distant metastases, or death from colorectal cancer. Locoregional recurrence was defined as the relapse of the tumor at the primary site confirmed by radiological or histological evidence. Distant metastasis was considered as metastatic lesions that were diagnosed in other organs beyond the primary site.
Statistical analysis
Categorical variables were presented as counts and percentages. Normally distributed continuous variables were expressed as mean ± standard deviations (SD). Comparisons of categorical variables were performed using Fisher’s exact test or χ2 test, whereas comparisons of quantitative variables were carried out using Kruskal-Wallis tests or Wilcoxon rank sum tests. A matched comparative analysis between the LS and RS groups was conducted based on propensity score matching (PSM, a logistic regression model with a match tolerance value of 0.01) by one-to-one nearest-neighbor matching with covariates as follows: age, sex, BMI, ASA score, previous abdominal surgery, smoking and drinking history, family history of CRC, CEA, lymph node metastasis, CCI score, with comorbidities, postoperative chemotherapy, tumor location, tumor differentiation, and pTNM stage. The overall survival (OS), disease-free survival (DFS) rates, and cumulative incidence of local recurrence (LR) and distant metastasis were calculated by using the Kaplan–Meier method and compared by log-rank test. All statistically significant factors determined by univariate analysis were then analyzed by multivariate analysis using the Cox proportional hazards regression model. PSM and all statistical analyses were conducted using SPSS 26.0 (SPSS Inc., Chicago IL, USA). P<0.05 was set as the criterion for statistical significance.
Results
Demographics and clinical characteristics
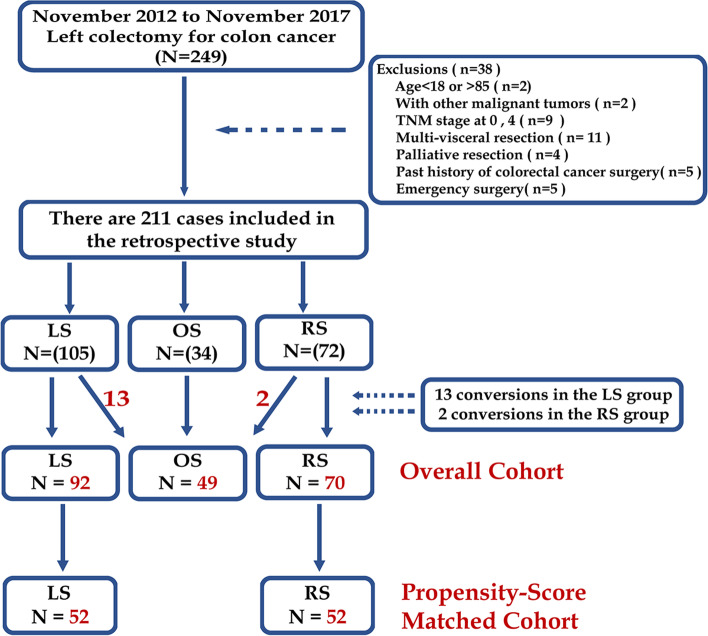
A total of 211 patients met the inclusion criteria and were evaluated in three groups (Fig. 1). Due to tumor with advanced T stage, severe peripheral adhesion and anatomical ambiguity, 13 of 105 patients originally scheduled for LS and 2 of 72 in the RS group were converted to OS. A statistical difference was found in conversion rates between the LS and RS groups (12.4 vs. 2.8%; P = 0.028). The demographics and clinical characteristics of the three groups were shown no significant differences among groups in age, sex, BMI, ASA score, smoking and drinking history, family history of CRC, lymph node metastasis, and preoperative comorbidities among three groups (all P > 0.05; Supplementary Table 1). Though no significance was revealed, cases with previous abdominal surgery in OS (32.7%) were more than that in LS (18.5%) and RS (17.1%). The average preoperative number of red blood cells in OS was significantly highest (P = 0.022; Supplementary table 1), but no significance was shown between RS and LS groups (P = 0.798; Supplementary table 1). Preoperative SEMS insertion and postoperative adjuvant chemotherapy were similar among the three groups (P > 0.05; Supplementary table 1).
Fig. 1.
Flow diagram of case selection
Intraoperative and perioperative clinical outcomes
Operative time in the RS group was 141.4±40.0 min and was the shortest among the three groups (P < 0.001; Table 1), but no difference between the LS group (186.1±43.7 min) and the OS group (175.9±43.6 min). MIS including LS and RS exhibited less blood loss and shorter length of hospital stay than OS (P < 0.05; Table 1), while RS had the least blood loss (127.6±70.0ml) and shortest length of hospital stay (7.7±3.1 days). The time to first bowel movement, first flatus, and first liquid diet was the significantly longest in the OS group (P < 0.001; Table 1), but no difference was observed between LS and RS (P > 0.05; Table 1). Except for WBC, hematology examination, including RBC, TP, and ALB, showed significant differences between pre- and post-operation in three groups (all P < 0.001; Supplementary Fig. 2A1&B1&C1&D1). However, the changes from pre-operation to post-operation in RBC (P = 0.373, Supplementary Fig. 2A2), WBC (P = 0.747, Supplementary Fig. 2B2), TP (P = 0.210, Supplementary Fig. 2C2), and ALB (P = 0.112, Supplementary Fig. 2D2) were similar among three groups (Supplementary Fig. 2).
Table 1.
Intraoperative and perioperative clinical outcomes (overall cohort)
| Variables | OS N=49 |
LS N=92 |
RS N=70 |
P |
|---|---|---|---|---|
| Operation time (min) | 175.9±43.6 | 186.1±43.7 | 141.4±40.0 | <0.001§ |
|
RS vs OS; P<0.001 RS vs LS; P<0.001 LS vs OS; P=0.646 |
||||
| Blood loss (ml) | 223.6±143.2 | 156.4±79.8 | 127.6±70.0 | <0.001§ |
|
RS vs OS; P<0.001 RS vs LS; P=0.028 LS vs OS; P=0.012 |
||||
| Time to first bowel movement (h) | 60.0±12.2 | 39.6±16.8 | 33.6±16.3 | <0.001§ |
|
RS vs OS; P<0.001 RS vs LS; P=0.126 LS vs OS; P<0.001 |
||||
| Time to first flatus (h) | 78.5±19.4 | 65.5±16.8 | 63.6±9.6 | <0.001§ |
|
RS vs OS; P<0.001 RS vs LS; P=0.674 LS vs OS; P=0.001 |
||||
| Time to first liquid diet (h) | 105.5±15.8 | 88.4±21.3 | 81.6±14.4 | <0.001§ |
|
RS vs OS; P<0.001 RS vs LS; P=0.119 LS vs OS; P<0.001 |
||||
| LOS for all patients (d) | 9.8±3.8 | 8.7±4.8 | 7.7±3.1 | <0.001§ |
|
RS vs OS; P<0.001 RS vs LS; P=0.002 LS vs OS; P=0.002 |
||||
| LOS for patients without complications (d) | 8.1±1.3 | 7.2±0.7 | 6.7±0.8 | <0.001§ |
|
RS vs OS; P<0.001 RS vs LS; P=0.018 LS vs OS; P=0.004 |
||||
| LOS for patients with complications (d) | 13.2±4.8 | 14.5±8.3 | 14.5±5.4 | 0.754§ |
Values were expressed as mean (SD = standard deviation) or n (%)
Abbreviation: RS Robotic surgery, LS Laparoscopic surgery, OS Open surgery, LOS Length of stay
§Kruskal-Wallis test
Perioperative and long-term postoperative complications
No deaths, reoperations, and readmissions happened in the LS and RS groups (all P > 0.05; Table 2), but one death was for septic shock in the OS group. Overall perioperative morbidity was the highest in the OS group (38.8%; Table 2), but no significant difference was found between RS and LS (19.6 vs. 11.4%; P > 0.05; Table 2). According to Clavien–Dindo grade, the OS group still had the highest incidence in grade III/IV complications (P = 0.004; Table 2). No significant differences were observed in the occurrence of overall and major long-term complications (all P > 0.05; Table 2). However, the rate of incisional hernia in OS was the highest (P = 0.036; Table 2).
Table 2.
Perioperative and long-term postoperative complications (overall cohort)
| Variables | Open N=49 |
Laparoscopic N=92 |
Robotic N=70 | P |
|---|---|---|---|---|
| Perioperative complicationsa | ||||
| Mortality, n (%) | 1 (2.0%) | 0 (0.0%) | 0 (0.0%) | 0.232‡ |
| Reoperation, n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | / |
| Readmission, n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | / |
| Overall morbidity, n (%) | 19 (38.8%) z | 18 (19.6%) y | 8 (11.4%) y | 0.001† |
| Grade I/II complications, n (%) | 13(26.5%) | 17 (18.5%) | 8 (11.4%) | 0.107† |
| Wound infection | 4 (8.2%) | 6 (6.5%) | 5(7.1%) | 0.942‡ |
| Intra-abdominal infection | 0 (0.0%) | 2 (2.2%) | 0 (0.0%) | 0.506‡ |
| Ileus | 0 (0.0%) | 2 (2.2%) | 1 (1.4%) | 0.796‡ |
| Acute pneumonia | 1 (2.0%) | 1 (1.1%) | 1 (1.4%) | 1.000‡ |
| Fever of unknown origin | 4 (8.2%) z | 3 (3.3%) zy | 0 (0.0%) y | 0.037‡ |
| Anastomotic hemorrhage | 0 (0.0%) | 1 (1.1%) | 0 (0.0%) | 1.000‡ |
| Anastomotic leak | 0 (0.0%) | 1 (1.1%) | 0 (0.0%) | 1.000‡ |
| Blood transfusion due to anemia | 4 (8.2%) z | 1 (1.1%) y | 1 (1.4%) y | 0.049‡ |
| Grade III/IV complications, n (%) | 5 (10.2%) z | 1 (1.1%) y | 0 (0.0%) y | 0.004‡ |
| Wound dehiscence (fascia) | 1 (2.0%) | 0 (0.0%) | 0 (0.0%) | 0.232‡ |
| Intra-abdominal infection and effusion | 4 (8.2%) z | 0 (0.0%) y | 0 (0.0%) y | 0.003‡ |
| Ileus | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1.000‡ |
| Acute liver failure | 0 (0.0%) | 1 (1.1%) | 0 (0.0%) | 1.000‡ |
| Long-term postoperative complicationsb | ||||
| Overall morbidity, n (%) | 5 (10.2%) | 3 (3.3%) | 2 (2.9%) | 0.133‡ |
| Grade I/II complications, n (%) | 1 (2.0%) | 1 (1.1%) | 1 (1.4%) | 1.000‡ |
| Incisional hernia | 0 (0.0%) | 1 (1.1%) | 0 (0.0%) | 1.000‡ |
| Ileus | 1 (2.0%) | 0 (0.0%) | 1 (1.4%) | 0.317‡ |
| Grade III/IV complications, n (%) | 4 (8.2%) | 2 (2.2%) | 1 (1.4%) | 0.160‡ |
| Incisional hernia | 2 (4.1%) z | 0 (0.0%) y | 0 (0.0%) y | 0.036‡ |
| Adhesion | 2 (4.1%) | 1 (1.1%) | 0 (0.0%) | 0.171‡ |
| Anastomotic stricture | 0 (0.0%) | 1 (1.1%) | 1 (1.4%) | 1.000‡ |
| Major perioperative complications, n (%) | 6 (12.2%) z | 1 (1.1%) y | 0 (0.0%) y | 0.001‡ |
| Major long-term complications, n (%) | 4 (8.2%) | 2 (2.2%) | 1 (1.4%) | 0.160‡ |
Values are expressed as n (%)
Major complications were defined as complications with a Grade III and higher of the Clavien–Dindo classification. Different letters (z and y) marked on the right side of the cell frequency indicated that there is a statistically different between the two groups that is the probability of the significance test for the comparison between the two groups is less than 0.05 (P < 0.05)
†Pearson’s chi-squared test
‡Fisher’s exact test
aComplications within 30 days from operation date
bNew complications 30 days after operation date
Postoperative pathological outcomes
There were no significant differences among the three groups in tumor location, with adenomatous polyps, neoplasm diameter, tumor differentiation, pTNM stage, lymph node metastasis, lympho-vascular invasion, and perineural invasion (all P > 0.05; Table 3). In addition, the number of retrieved lymph nodes and distal resection margin showed no statistical difference (all P > 0.05; Table 3). No positive margin was detected in the three groups (Table 3).
Table 3.
Postoperative pathologic outcomes (overall cohort)
| Variables | Open N=49 |
Laparoscopic N=92 |
Robotic N=70 |
P |
|---|---|---|---|---|
| Tumor location | 0.067† | |||
| TC&SF, n (%) | 14 (28.6%) | 26 (28.3%) | 16 (22.9%) | |
| UDC, n (%) | 24 (49.0%) | 29 (31.5%) | 21 (30.0%) | |
| LDC, n (%) | 11 (22.4%) | 37 (40.2%) | 33 (47.1%) | |
| With adenomatous polyps, n (%) | 11 (22.4%) | 31 (33.7%) | 14 (20.0%) | 0.112† |
| Neoplasm longest diameter (cm) | 5.6±2.4 | 5.1±1.8 | 5.0±1.8 | 0.243§ |
| Tumor differentiation | 0.834‡ | |||
| Well, n (%) | 3 (6.1%) | 10 (10.9%) | 8 (11.4%) | |
| Moderate, n (%) | 36 (73.5%) | 69 (75.0%) | 54 (77.1%) | |
| Poor, n (%) | 7 (14.3%) | 8 (8.7%) | 5 (7.1%) | |
| Mucinous, n (%) | 3 (6.1%) | 5 (5.4%) | 3 (4.3%) | |
| pTNM stage | 0.261† | |||
| I, n (%) | 5 (10.2%) | 14 (15.2%) | 15 (21.4%) | |
| II, n (%) | 22 (44.9%) | 45 (48.9%) | 24 (34.3%) | |
| III, n (%) | 22 (44.9%) | 33 (35.9%) | 31 (44.3%) | |
| pT stage | 0. 412‡ | |||
| T1, n (%) | 2 (4.1%) | 10 (10.9%) | 10 (14.3%) | |
| T2, n (%) | 4 (8.2%) | 4 (4.3%) | 5 (7.1%) | |
| T3, n (%) | 6 (12.2%) | 11 (12.0%) | 4 (5.7%) | |
| T4a, n (%) | 35 (75.5%) | 62 (72.8%) | 51 (72.9%) | |
| pN stage | 0.399† | |||
| N0, n (%) | 27 (55.1%) | 59 (64.1%) | 39 (55.7%) | |
| N1, n (%) | 13 (26.5%) | 21 (22.8%) | 24 (34.3%) | |
| N2, n (%) | 9 (18.4%) | 12 (13.0%) | 7 (10.0%) | |
| Number of lymph node harvest | 14.7±6.2 | 14.0±6.0 | 14.0±4.8 | 0.725§ |
| Positive resection margin, n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | / |
| With lymph node metastasis, n (%) | 22 (44.9%) | 32 (34.8%) | 31 (44.3%) | 0.358† |
| With lymphovascular invasion, n (%) | 11 (22.4%) | 14 (15.2%) | 10 (14.3%) | 0.447† |
| With extranodal tumor deposits, n (%) | 6 (12.2%) | 14 (15.2%) | 13 (18.6%) | 0.639† |
| With perineural invasion, n (%) | 17 (34.7%) | 34 (37.0%) | 24 (34.3%) | 0.931† |
Values are expressed as mean (SD = standard deviation) or n (%)
Abbreviation: TC The distal 1/3 of the transverse colon, SF Splenic flexure, UDC Upper segment of descending colon, LDC Lower segment of descending colon, pTNM Pathological tumor-node-metastasis
§Kruskal-Wallis test
†Pearson’s chi-squared test
‡Fisher’s exact test
Survival analyses and prognostic factors
The median follow-up period was 40 months for the overall population. No statistical differences were observed in overall survival among the three groups (P = 0.671; Fig. 2A1). Also, no statistical difference was found in the overall survival by subtype analysis according to the TNM stage among three groups (all P > 0.05; Fig. 2A2, A3, and A4). Similarly, the disease-free survival rate showed no significant differences among the three groups (P = 0.671; Fig. 2B1). Subtype analysis of the disease-free survival still showed no significant difference according to the TNM stage among the three groups (all P > 0.05; Fig. 2B1, B2, and B3). Cumulative local recurrence rate (P = 0.797; Fig. 2C) and cumulative distant metastatic incidence (P = 0.790; Fig. 2D) was similar among three groups (Fig. 2).
Fig. 2.
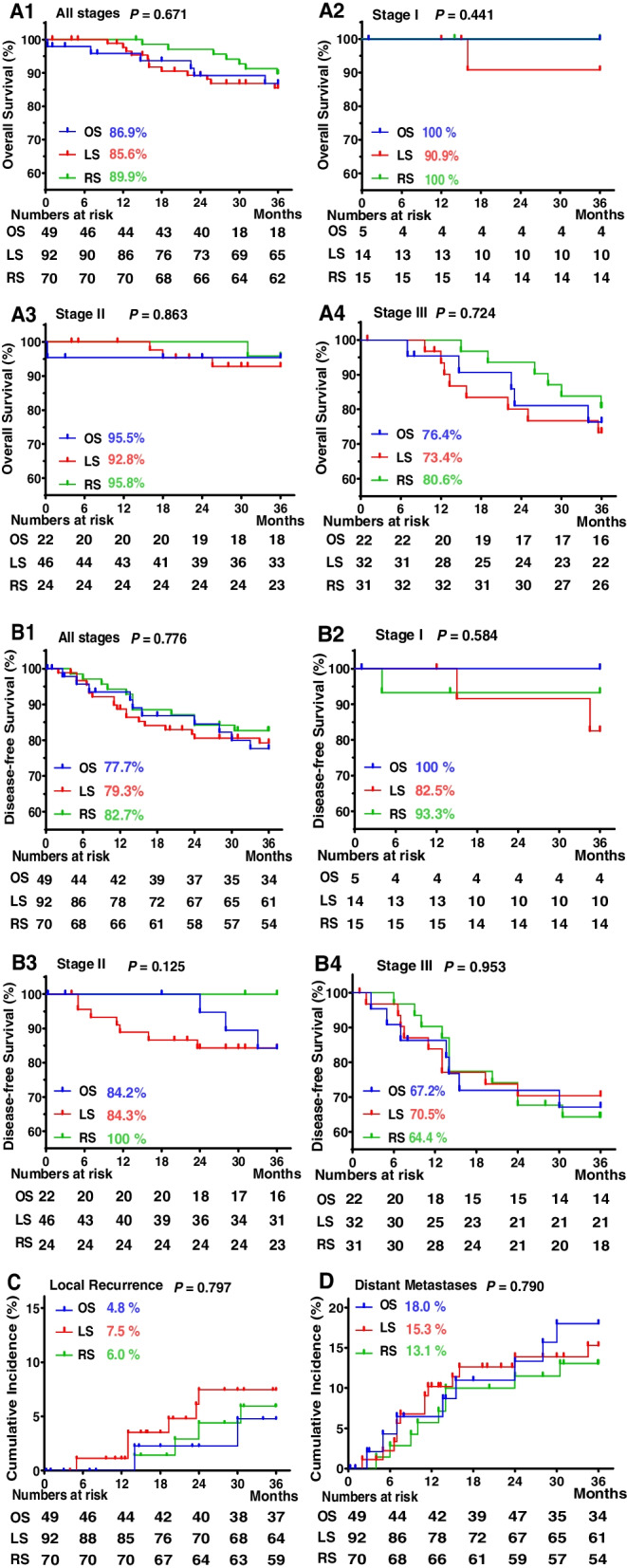
Kaplan–Meier survival curves and cumulative incidence curves (overall cohort). A Kaplan–Meier survival curves for overall survival rates according to TNM stage. A1 All stages, A2 Stage I, A3 Stage II, and A4 Stage III. B Kaplan–Meier survival curves for disease-free survival rates according to TNM stage. B1 All stages, B2 Stage I, B3 Stage II, and B4 Stage III. C Cumulative incidence curves of local recurrence rates. D Cumulative incidence curves of distant metastasis rates
In univariate analysis, factors that affected both overall and disease-free survival were postoperative adjuvant chemotherapy, tumor differentiation, pTNM stage, pN stage, number of lymph node harvest, lymphovascular invasion, extranodal tumor deposits, and perineural invasion (all P < 0.05; Table 4). The local recurrence rate was affected by three risk factors such as the level of serum CEA> 6.5, pN stage, and perineural invasion (all P < 0.05; Table 4). However, surgical approaches were not prognostic factors for overall and disease-free survival and local recurrence rates in this study (all P < 0.05; Table 4). In multivariate analysis, the prognostic factors for the overall survival were postoperative adjuvant chemotherapy (HR [CI] 2.407 (1.056–5.483); P = 0.037; Table 5), pN stage (HR [CI] 3.251 (1.856–5.695); P < 0.001; Table 5), and prognostic factors for disease-free survival were CEA (HR [CI] 2.036 (1.069–3.877); P=0.030; Table 5), and pN stage (HR [CI] 2.516 (1.689–3.747); P < 0.001; Table 5). Perineural invasion was the only prognostic risk factors for local recurrence (HR [CI] 9.275 (2.014–42.721); P=0.004; Table 5).
Table 4.
Prognostic factors of a 3-year survival and local recurrence by univariate analysis (overall cohort)
| Variables | N=211 | Overall survival (%) | P* | Disease-free survival (%) | P* | Cumulative local recurrence (%) | P* |
|---|---|---|---|---|---|---|---|
| Age (years) | 0.707 | 0.809 | 0.410 | ||||
| ≤65 | 132 | 88.1 | 79.9 | 7.4 | |||
| > 65 | 79 | 86.0 | 80.2 | 4.4 | |||
| Sex | 0.090 | 0.898 | 0.312 | ||||
| Male | 124 | 90.6 | 79.9 | 7.8 | |||
| Female | 87 | 82.5 | 80.4 | 3.9 | |||
| BMI (kg/m2) | 0.364 | 0.255 | 0.822 | ||||
| ≤25 | 169 | 86.2 | 78.4 | 6.6 | |||
| >25 | 42 | 92.1 | 86.9 | 5.4 | |||
| ASA score | 0.385 | 0.144 | 0.373 | ||||
| 1 | 1 | 100.0 | 100.0 | 0.0 | |||
| 2 | 85 | 83.9 | 75.8 | 9.7 | |||
| 3 | 125 | 89.5 | 85.5 | 4.3 | |||
| CEA (ng/ml) | 0.056 | 0.001 | 0.010 | ||||
| ≤6.5 | 164 | 89.7 | 84.7 | 4.0 | |||
| > 6.5 | 47 | 78.6 | 63.5 | 15.5 | |||
| CA199 (ng/ml) | <0.001 | 0.015 | 0.124 | ||||
| ≤27 | 182 | 90.7 | 82.4 | 5.4 | |||
| > 27 | 29 | 64.5 | 63.0 | 13.5 | |||
| Surgical approach | 0.671 | 0.776 | 0.797 | ||||
| Open | 49 | 86.9 | 77.7 | 4.8 | |||
| Laparoscopy | 92 | 85.6 | 79.3 | 7.5 | |||
| Robot | 70 | 89.9 | 82.7 | 6.0 | |||
| Previous abdominal surgery | 0.528 | 0.307 | 0.285 | ||||
| Yes | 45 | 90.2 | 85.5 | 9.6 | |||
| No | 166 | 86.6 | 78.6 | 5.4 | |||
| Smoking and drinking history | 0.433 | 0.222 | 0.155 | ||||
| Yes | 66 | 90.1 | 74.5 | 4.6 | |||
| No | 145 | 86.1 | 82.6 | 10.1 | |||
| Family history of CRC | 0.285 | 0.637 | 0.233 | ||||
| Yes | 21 | 95.0 | 85.2 | 0.0 | |||
| No | 190 | 86.4 | 79.5 | 7.1 | |||
| With comorbidities | 0.264 | 0.906 | 0.775 | ||||
| Yes | 80 | 83.4 | 80.0 | 5.6 | |||
| No | 131 | 89.7 | 80.1 | 6.8 | |||
| Postoperative adjuvant chemotherapy | <0.001 | <0.001 | 0.220 | ||||
| Yes | 47 | 70.6 | 85.0 | 10.2 | |||
| No | 164 | 92.1 | 62.9 | 5.4 | |||
| Perioperative morbidity | 0.963 | 0.758 | 0.740 | ||||
| Yes | 45 | 87.8 | 77.8 | 5.4 | |||
| No | 166 | 87.2 | 80.6 | 6.6 | |||
| Tumor location | 0.821 | 0.542 | 0.511 | ||||
| TC&SF | 50 | 89.2 | 85.6 | 4.4 | |||
| UDC | 74 | 85.6 | 76.8 | 4.8 | |||
| LDC | 87 | 87.7 | 79.6 | 8.7 | |||
| With adenomatous polyps | 0.009 | 0.067 | 0.137 | ||||
| Yes | 56 | 98.0 | 88.6 | 1.8 | |||
| No | 155 | 83.6 | 77.0 | 8.1 | |||
| Neoplasm diameter (cm) | 0.395 | 0.816 | 0.089 | ||||
| ≤5 | 136 | 85.8 | 80.7 | 4.2 | |||
| >5 | 75 | 89.9 | 79.0 | 10.2 | |||
| Tumor differentiation | 0.022 | 0.002 | 0.089 | ||||
| Well | 21 | 100.0 | 89.7 | 0.0 | |||
| Moderate | 159 | 88.5 | 83.4 | 5.5 | |||
| Poor | 20 | 74.4 | 59.2 | 13.8 | |||
| Mucinous | 11 | 70.0 | 50.5 | 22.2 | |||
| pTNM stage | <0.001 | <0.001 | 0.030 | ||||
| I | 34 | 96.6 | 90.1 | 0.0 | |||
| II | 91 | 95.3 | 89.5 | 3.6 | |||
| III | 86 | 75.9 | 66.4 | 11.9 | |||
| pT stage | 0.067 | 0.148 | 0.434 | ||||
| T1 | 22 | 100.0 | 95.0 | 0.0 | |||
| T2 | 13 | 100.0 | 91.7 | 0.0 | |||
| T3 | 21 | 95.2 | 85.4 | 5.0 | |||
| T4a | 155 | 83.5 | 76.4 | 7.9 | |||
| pN stage | <0.001 | <0.001 | 0.007 | ||||
| N0 | 125 | 95.7 | 89.6 | 2.7 | |||
| N1 | 58 | 87.5 | 74.9 | 9.1 | |||
| N2 | 28 | 52.0 | 48.9 | 19.4 | |||
| Number of lymph nodes detected | 0.395 | 0.856 | 0.306 | ||||
| ≤15 | 137 | 85.8 | 79.8 | 5.0 | |||
| >15 | 74 | 89.9 | 80.4 | 8.8 | |||
| With lymphovascular invasion | <0.001 | 0.001 | 0.059 | ||||
| Yes | 35 | 69.9 | 59.8 | 14.8 | |||
| No | 176 | 90.8 | 83.9 | 4.9 | |||
| With extranodal tumor deposits | 0.004 | <0.001 | 0.331 | ||||
| Yes | 33 | 72.5 | 57.3 | 10.9 | |||
| No | 178 | 90.3 | 84.5 | 5.6 | |||
| With perineural invasion | 0.001 | <0.001 | <0.001 | ||||
| Yes | 75 | 77.1 | 65.9 | 15.9 | |||
| No | 136 | 92.8 | 87.7 | 1.6 |
Abbreviation: BMI Body mass index, ASA American Society of Anesthesiology, CRC Colorectal cancer, TC The distal 1/3 of the transverse colon, SF Splenic flexure, UDC Upper segment of descending colon, LDC Lower segment of the descending colon, pTNM Pathological tumor-node-metastasis
*The 3-year overall and disease-free survival rates and cumulative local recurrence were calculated by using the Kaplan–Meier method
Table 5.
Prognostic factors of a 3-year survival and local recurrence by multivariate analysis (overall cohort)
| Variables | Overall survival HR (95% CI) |
P* | Disease-free survival HR (95% CI) |
P* | Cumulative local recurrence HR (95% CI) |
P* |
|---|---|---|---|---|---|---|
| CEA | / | / | 2.036 (1.069-3.877) | 0.030 | / | 0.059 |
| CA199 | / | 0.062 | / | 0.946 | / | — |
| Postoperative adjuvant chemotherapy | 2.407 (1.056-5.483) | 0.037 | / | 0.067 | / | — |
| With adenomatous polyps | / | 0.058 | / | / | / | — |
| Tumour differentiation | / | 0.125 | / | 0.103 | / | — |
| pN stage | 3.251 (1.856-5.695) | <0.001 | 2.516 (1.689-3.747) | <0.001 | / | 0.233 |
| With lymphovascular invasion | / | 0.369 | / | 0.122 | / | — |
| With extranodal tumor deposits | / | 0.684 | / | 0.383 | / | — |
| With perineural invasion | / | 0.277 | / | 0.079 | 9.275 (2.014–42.721) | 0.004 |
CI indicates confidence interval; HR, hazard ratio
*Cox proportional hazards regression model
Propensity score matching analysis between LS and RS
As the above results showed minimally invasive surgery (MIS) approaches including LS and RS showed better perioperative outcomes but without compromising oncologic principles, the advantage of LS or RS in left colectomy still needs to be validated. To this end, 52 cases in the RS group and 52 cases in the LS group were included after one-to-one nearest-neighbor propensity score matching (PSM) analysis with multiple covariates (all P > 0.05; Supplementary Table 2). Compared with LS for left colectomy after PSM, RS showed a shorter operation time (LS 188.3±46.0 min vs. RS 149.2±41.7 min, P < 0.001; Supplementary Table 3) and LOS for patients without complications (LS 7.2±0.7 days vs. RS 6.8±0.8 days, P = 0.005; Supplementary Table 3), but both exhibited similar perioperative and long-term postoperative complications (all P > 0.05; Supplementary Table 4).
Before PSM, the result of survival analysis showed no statistical difference was found in the overall survival (P = 0.367; Supplementary Fig. 3A1) and by subtype analysis according to TNM stage between LS and RS (all P > 0.05; Supplementary Fig. 3A2, A3, and A4). Similarly, the disease-free survival rate showed no significant differences between LS and RS (P = 0.544; Supplementary Fig. 3B1). Subtype analysis of the disease-free survival still showed no significant difference at stage I (P = 0.472; Supplementary Fig. 3B2) and III (P = 0.767; Supplementary Fig. 3B4) according to the TNM stage between LS and RS. However, the disease-free survival of RS was longer than that of LS at stage II (P = 0.044; Supplementary Fig. 3B3) between LS and RS. Cumulative local recurrence rate (P = 0.665; Supplementary Fig. 3C) and cumulative distant metastatic incidence (P = 0.679; Supplementary Fig. 3D) were similar between LS and RS (Supplementary Fig. 3). After PSM, the result of survival analysis showed overall survival (all P > 0.05; Fig. 3A), and disease-free survival (all P > 0.05; Fig. 3B) was paralleled between RS and LS groups according to the TNM stage. The similar results were for cumulative incidence of local recurrence (P = 0.535, Fig. 3C) and distant metastasis (P = 0.898, Fig. 3D) between LS and RS.(Fig. 3) Univariate and then multivariate Cox regression analysis showed the prognostic factors for overall survival were tumor differentiation (HR [CI] 2.007 (1.009–3.993); P =0.047) and pN stage (HR [CI] 2.920 (1.136–7.509); P = 0.026). The prognostic factors for disease-free survival were tumor differentiation (HR [CI] 2.665 (1.392–5.101); P =0.003), pN stage (HR [CI] 3.211 (1.154–8.934); P = 0.025) and extranodal tumor deposits (HR [CI] 3.881 (1.046–14.403); P = 0.043). The prognostic factors for cumulative local recurrence were tumor differentiation (HR [CI] 3.248 (1.203–8.772); P =0.020) and pN stage (HR [CI] 6.370 (1.384–29.323); P = 0.017). However, the surgical approach (robot or laparoscopy) was not associated with overall survival or disease-free survival or cumulative local recurrence (all P > 0.05; Supplementary Tables 5 & 6).
Fig. 3.
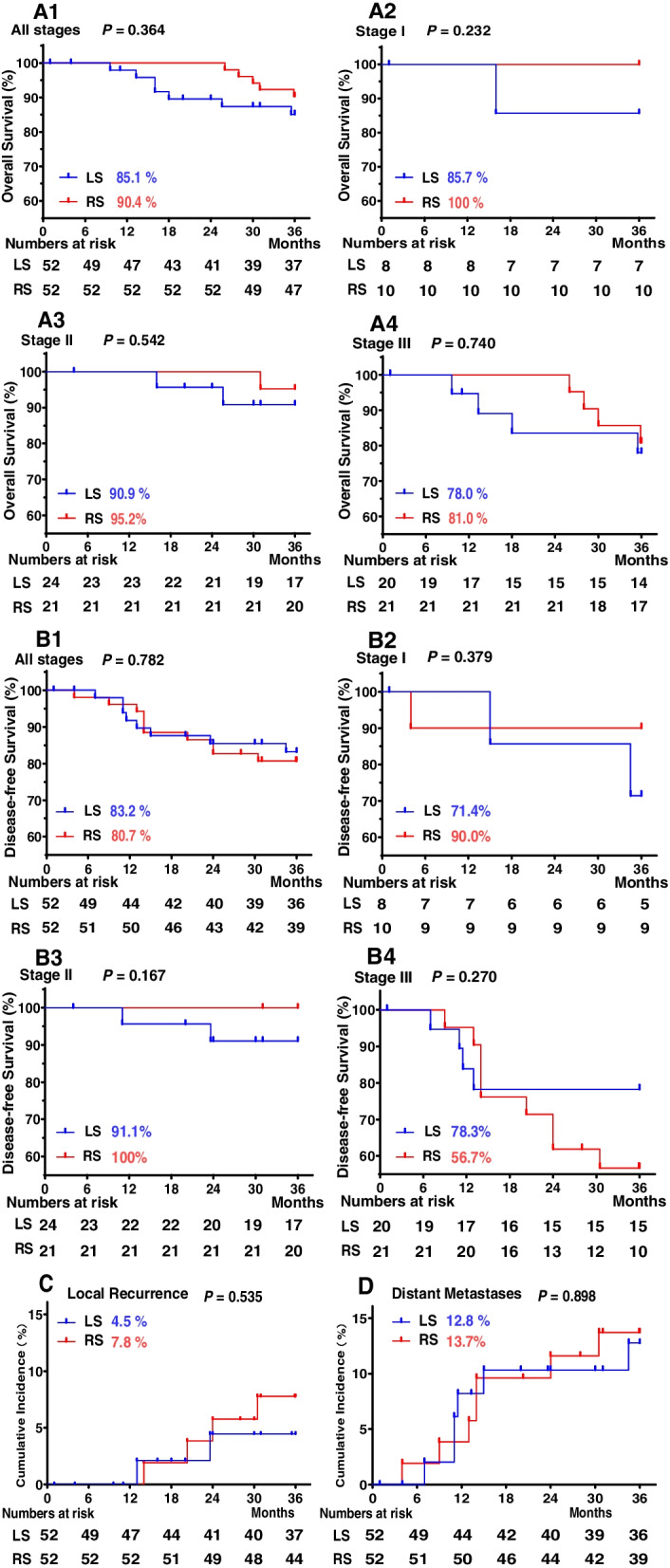
Kaplan–Meier survival curves and cumulative incidence curves (propensity score-matched cohort). A Kaplan–Meier survival curves for overall survival rates according to TNM stage. A1 All stages, A2 Stage I, A3 Stage II, and A4 Stage III. B Kaplan–Meier survival curves for disease-free survival rates according to the TNM stage. B1 All stages, B2 Stage I, B3 Stage II, and B4 Stage III. C Cumulative incidence curves of local recurrence rates. D Cumulative incidence curves of distant metastasis rates
Discussion
Left colectomy is a major method for curing left colonic cancer. Despite the controversy, three techniques including open, laparoscopic, and robotic surgery for left colectomy are commonly used in clinical practice. This retrospective analysis of three techniques for left colectomy demonstrated similar long-term oncologic and survival outcomes but greatly improved perioperative clinical outcomes and decreased occurrence of short-term complications for MIS including laparoscopic or robotic surgery. A large number of clinical trials have shown that short-term outcomes of MIS in CRC were better than that of open surgery [23, 24] but without compromising survival outcomes [9, 25]. Our result also confirmed that MIS, in comparison with OS, was associated with better intraoperative and perioperative clinical outcomes, without compromising the oncology survival outcomes.
The clinical value of these three surgical techniques for left colectomy is dependent on oncology survival outcomes which are significantly influenced by pathologic parameters such as the number of retrieved lymph nodes and surgical margin status [26]. The average number of retrieved lymph nodes in the RS group was no less than 12 lymph nodes which were recommended for examination to ensure complete resection and adequate staging [27] and was comparable to the OS and LS groups. No positive margins were found in the three groups, but an anastomotic recurrence was found in the LS group with a survival time of more than 36 months.
Long-term survival is the gold index to evaluate the clinical application in cancer. For left colectomy for radical treatment of CRC, our results showed that overall and disease-free survival rate had no significant difference among OS, LS, and RS, indicating its clinical applicability. Similar survival outcomes among various surgical methods had been also verified. A single-center study [28] showed similar long-term oncologic outcomes in patients undergoing robot-assisted surgery for sigmoid cancer, with no significant differences in the 3-year overall survival rate and 3-year disease-free survival rate. Pinar et al. [29] analyzed 5978 patients for colon cancer and 3206 for rectal cancer and revealed a comparable 3-year disease-free survival rate. However, Mirkin et al. [30] found that RS was associated with improved survival in stage II and III diseases compared with LS. Additionally, according to our analysis by TNM stage, no significant differences were observed between the RS group and the LS group in overall survival rate and, except for stage II, in disease-free survival rate before PSM. The difference of stage II before PSM may be associated with different rates of patients with perineural or lymphovascular invasion at stage II. Indeed, after eliminating the confounding factors by PSM, our study showed RS showed a similar survival compared with LS, indicating the feasibility of RS in colonic cancer.
RS is considered to reduce trauma and improve quality of life while ensuring radical resection [31]. Our study showed that RS showed shorter operation time and LOS for patients without complications, but both exhibited similar perioperative and long-term postoperative complications. Bhama et al. [32] and Ng et al. [14] verified that short-term clinical outcomes for RS were better than those for LS in CRC. However, a multicenter randomized clinical trial, the ROLARR study [33], revealed that robotic surgery did not gain a clinical advantage over conventional laparoscopic surgery in the short-term clinical outcome. The shorter operation time for RS may be as follows: compared with conventional laparoscopic technique, the robotic technique theoretically shows advantages in CRC surgery such as better visualization of fine anatomical structures, the ability to perform a finer and more dexterous dissection of splenic flexure [34], which may be the reason for the differential conversion rate between the RS group and the LS group. Additionally, unlike laparoscopic surgery, which requires tacit cooperation between the chief surgeon and his assistants, robotic surgery is mainly performed by the chief surgeon and the surgical scope is easily exhibited by robotic arms, which allows for a faster and smoother operation for experienced and skilled surgeons.
Conclusions
Among the three techniques for radical left colectomy, MIS including laparoscopic and robotic surgery had significant advantages over open surgery in short-term outcomes, and no significant differences were found in overall, disease-free survival, local recurrence, and distant metastasis incidence rates. Moreover, RS shows better perioperative clinical outcomes but without compromising survival compared with LS.
However, this study is a retrospective analysis. The analysis of consecutive patients does represent the “real world” at our large-scale center. Some limitations were inherent to a retrospective analysis, including patients’ selection, inclusion, and recall bias. The findings also need to be further validated by a larger multicenter prospective randomized trial and longer follow-up survival data.
Supplementary Information
Additional file 1: Supplementary Figure 1. Illustrations for the length of bowel resection, location of related arteries ligation and scope of the lymph node dissection for radical left colectomy for tumours located (A) at the distal 1/3 of the transverse colon (TC) and splenic flexure (SF), (B) at the upper segment of descending colon (UDC), (C) at the lower segment of descending colon (LDC).
Additional file 2: Supplementary Figure 2. (A1&B1&C1&D1): The values of hematology examination including of RBC (A1), WBC (B1), TP (C1) and ALB (D1) in OS, LS and RS groups were compared between pre-operation and post-operation by the Kruskal-Wallis test. (A2&B2&C2&D2): The changes of hematology examination including of RBC (A2), WBC (B2), TP (C2) and ALB (D2) from pre-operation to post-operation were compared among OS, LS and OS by the Kruskal-Wallis test. The Delta (Δ) is the postoperative value minus the preoperative value. RS (robotic surgery), LS (laparoscopic surgery), OS (open surgery).
Additional file 3: Supplementary Figure 3. Kaplan–Meier survival curves and Cumulative incidence curves (Before Propensity-score matched cohort). (A) Kaplan–Meier survival curves for overall survival rates according to TNM stage. (A1) All stages; (A2) Stage I; (A3) Stage II; (A4) Stage III. (B) Kaplan–Meier survival curves for disease-free survival rates according to TNM stage. (B1) All stages; (B2) Stage I; (B3) Stage II; (B4) Stage III. (C) Cumulative incidence curves of local recurrence rates. (D) Cumulative incidence curves of distant metastasis rates.
Additional file 4: Supplementary Table 1. Demographics and clinical characteristics of three groups. Supplementary Table 2. Demographics, clinical characteristics and pathologic outcomes before and after PSM. Supplementary Table 3. Perioperative clinical outcomes and short-term oncology outcomes (propensity-score matched cohort). Supplementary Table 4. Perioperative and long-term postoperative complications (propensity-score matched cohort). Supplementary Table 5. Prognostic factors of 3-year survival and local recurrence by univariate analysis (propensity-score matched cohort). Supplementary Table 6. Prognostic factors of 3-year survival and local recurrence by multivariate analysis (propensity-score matched cohort).
Acknowledgements
We appreciated all authors for their efforts on the manuscript.
Abbreviations
- RS
Robotic surgery
- LS
Laparoscopic surgery
- OS
Open surgery
- BMI
Body mass index
- ASA
American Society of Anesthesiology
- CRC
Colorectal cancer
- CEA
Carcinoembryonic antigen
- CA 199
Glucoprotein antigen 199
- RBC
Red blood cells
- WBC
White blood cells
- TP
Total protein
- ALB
Albumin
- Hb
Hemoglobin
- CCI
Charlson Comorbidity Index
- SEMS
Self-expanding metal stent
- LOS
Length of stay
- TC
The distal 1/3 of the transverse colon
- SF
Splenic flexure
- UDC
Upper segment of descending colon
- LDC
Lower segment of descending colon
- pTNM
Pathological tumor-node-metastasis
Authors’ contributions
Study design: Lei X, Li TY, and Zhang GH. Manuscript writing: Lei X and Huang ZX. Data collection: Huang ZX, Shi HR, Zhou Z, Tang C, and Yang LL. Statistical analysis: Huang ZX, Shi HR, and Zhou Z. Huang ZX and Li TY contributed equally to this study. Final approval of manuscript: All authors.
Funding
Natural Science Foundation of Jiangxi, China, 20181BBG78043
Natural Science Foundation of Jiangxi, China, 20192ACBL21043
National Health Commission Foundation of Jiangxi, China, 20191016
Natural Science Fund of Education Department of Jiangxi, China, GJJ170007
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Ethical approval was waived by The First Affiliated Hospital of Nanchang University in view of the retrospective nature of the study, and all the procedures being performed were part of the routine care. This study was performed in accordance with the Helsinki Declaration of 1975.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhixiang Huang, Taiyuan Li, and Genghua Zhang contributed equally to this study.
Contributor Information
Zhixiang Huang, Email: huangzhixiang1996@126.com.
Xiong Lei, Email: leixionglinty@126.com.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 3.Dekker E, Tanis PJ, Vleugels J, Kasi PM. Wallace MB: colorectal cancer. Lancet. 2019;394:1467–1480. doi: 10.1016/S0140-6736(19)32319-0. [DOI] [PubMed] [Google Scholar]
- 4.Rentsch M, Schiergens T, Khandoga A, Werner J. Surgery for colorectal cancer - trends, developments, and future perspectives. Visc Med. 2016;32:184–191. doi: 10.1159/000446490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di B, Li Y, Wei K, Xiao X, Shi J, Zhang Y, Yang X, Gao P, Zhang K, Yuan Y, et al. Laparoscopic versus open surgery for colon cancer: a meta-analysis of 5-year follow-up outcomes. Surg Oncol. 2013;22:e39–e43. doi: 10.1016/j.suronc.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Allaix ME, Rebecchi F, Fichera A. The landmark series: minimally invasive (laparoscopic and robotic) colorectal cancer surgery. Ann Surg Oncol. 2020;27:3704–3715. doi: 10.1245/s10434-020-08833-8. [DOI] [PubMed] [Google Scholar]
- 7.Chiu CC, Hsu WT, Choi JJ, Galm B, Lee MG, Chang CN, Liu CC, Lee CC. Comparison of outcome and cost between the open, laparoscopic, and robotic surgical treatments for colon cancer: a propensity score-matched analysis using nationwide hospital record database. Surg Endosc. 2019;33:3757–3765. doi: 10.1007/s00464-019-06672-7. [DOI] [PubMed] [Google Scholar]
- 8.Wells KO, Senagore A. Minimally invasive colon cancer surgery. Surg Oncol Clin N Am. 2019;28:285–296. doi: 10.1016/j.soc.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Kastner C, Reibetanz J, Germer CT, Wiegering A. Evidence in minimally invasive oncological surgery of the colon and rectum. Chirurg. 2021;92:334–343. doi: 10.1007/s00104-020-01320-6. [DOI] [PubMed] [Google Scholar]
- 10.Gehrman J, Angenete E, Björholt I, Lesén E, Haglind E. Cost-effectiveness analysis of laparoscopic and open surgery in routine Swedish care for colorectal cancer. Surg Endosc. 2020;34:4403–4412. doi: 10.1007/s00464-019-07214-x. [DOI] [PubMed] [Google Scholar]
- 11.Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718–1726. doi: 10.1016/S0140-6736(05)66545-2. [DOI] [PubMed] [Google Scholar]
- 12.Gómez RM, Lainez EM, Cagigas FC, Cristobal PL, Santarrufina MS. Robotic surgery for colorectal cancer. Ann Gastroenterol Surg. 2020;4:646–651. doi: 10.1002/ags3.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu M, Zhao Z, Jia B, Liu R, Liu H. Perioperative and long-term outcomes of robot-assisted versus laparoscopy-assisted hemicolectomy for left-sided colon cancers: a retrospective study. Updates Surg. 2021;73:1049–1056. doi: 10.1007/s13304-020-00959-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng KT, Tsia A, Chong V. Robotic versus conventional laparoscopic surgery for colorectal cancer: a systematic review and meta-analysis with trial sequential analysis. World J Surg. 2019;43:1146–1161. doi: 10.1007/s00268-018-04896-7. [DOI] [PubMed] [Google Scholar]
- 15.Lei X, Yang L, Huang Z, Shi H, Zhou Z, Tang C, Li T. No beneficial effect on survival but a decrease in postoperative complications in patients with rectal cancer undergoing robotic surgery: a retrospective cohort study. Bmc Surg. 2021;21:355. doi: 10.1186/s12893-021-01309-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Health Commission of the People's Republic of China. Chinese Protocol of Diagnosis and Treatment of Colorectal Cancer (2020 edition). Zhonghua Wai Ke Za Zhi. 2020;58:561–85. [DOI] [PubMed]
- 17.Ishiguro M, Higashi T, Watanabe T, Sugihara K. Changes in colorectal cancer care in japan before and after guideline publication: a nationwide survey about D3 lymph node dissection and adjuvant chemotherapy. J Am Coll Surg. 2014;218:969–977. doi: 10.1016/j.jamcollsurg.2013.12.046. [DOI] [PubMed] [Google Scholar]
- 18.Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation--technical notes and outcome. Colorectal Dis. 2009;11(354-364):364–365. doi: 10.1111/j.1463-1318.2008.01735.x. [DOI] [PubMed] [Google Scholar]
- 19.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 20.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simillis C, Lal N, Thoukididou SN, Kontovounisios C, Smith JJ, Hompes R, Adamina M, Tekkis PP. Open versus laparoscopic versus robotic versus transanal mesorectal excision for rectal cancer: a systematic review and network meta-analysis. Ann Surg. 2019;270:59–68. doi: 10.1097/SLA.0000000000003227. [DOI] [PubMed] [Google Scholar]
- 22.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 23.Nagasaki T, Akiyoshi T, Fukunaga Y, Tominaga T, Yamaguchi T, Konishi T, Fujimoto Y, Nagayama S, Ueno M. The short- and long-term feasibility of laparoscopic surgery in colon cancer patients with bulky tumors. J Gastrointest Surg. 2019;23:1893–1899. doi: 10.1007/s11605-019-04114-2. [DOI] [PubMed] [Google Scholar]
- 24.Wei D, Johnston S, Goldstein L, Nagle D. Minimally invasive colectomy is associated with reduced risk of anastomotic leak and other major perioperative complications and reduced hospital resource utilization as compared with open surgery: a retrospective population-based study of comparative effectiveness and trends of surgical approach. Surg Endosc. 2020;34:610–621. doi: 10.1007/s00464-019-06805-y. [DOI] [PubMed] [Google Scholar]
- 25.Kitano S, Inomata M, Mizusawa J, Katayama H, Watanabe M, Yamamoto S, Ito M, Saito S, Fujii S, Konishi F, et al. Survival outcomes following laparoscopic versus open D3 dissection for stage II or III colon cancer (JCOG0404): a phase 3, randomised controlled trial. Lancet Gastroenterol Hepatol. 2017;2:261–268. doi: 10.1016/S2468-1253(16)30207-2. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Wei Z, Bie M, Peng X, Chen C. Robot-assisted versus laparoscopic-assisted surgery for colorectal cancer: a meta-analysis. Surg Endosc. 2016;30:5601–5614. doi: 10.1007/s00464-016-4892-z. [DOI] [PubMed] [Google Scholar]
- 27.Yang L, Xiong Z, Xie Q, He W, Liu S, Kong P, Jiang C, Guo G, Xia L. Prognostic value of total number of lymph nodes retrieved differs between left-sided colon cancer and right-sided colon cancer in stage III patients with colon cancer. Bmc Cancer. 2018;18:558. doi: 10.1186/s12885-018-4431-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim DR, Min BS, Kim MS, Alasari S, Kim G, Hur H, Baik SH, Lee KY, Kim NK. Robotic versus laparoscopic anterior resection of sigmoid colon cancer: comparative study of long-term oncologic outcomes. Surg Endosc. 2013;27:1379–1385. doi: 10.1007/s00464-012-2619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinar I, Fransgaard T, Thygesen LC, Gögenur I. Long-term outcomes of robot-assisted surgery in patients with colorectal cancer. Ann Surg Oncol. 2018;25:3906–3912. doi: 10.1245/s10434-018-6862-2. [DOI] [PubMed] [Google Scholar]
- 30.Mirkin KA, Kulaylat AS, Hollenbeak CS, Messaris E. Robotic versus laparoscopic colectomy for stage I-III colon cancer: oncologic and long-term survival outcomes. Surg Endosc. 2018;32:2894–2901. doi: 10.1007/s00464-017-5999-6. [DOI] [PubMed] [Google Scholar]
- 31.Park EJ, Baik SH. Robotic surgery for colon and rectal cancer. Curr Oncol Rep. 2016;18:5. doi: 10.1007/s11912-015-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhama AR, Obias V, Welch KB, Vandewarker JF, Cleary RK. A comparison of laparoscopic and robotic colorectal surgery outcomes using the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database. Surg Endosc. 2016;30:1576–1584. doi: 10.1007/s00464-015-4381-9. [DOI] [PubMed] [Google Scholar]
- 33.Jayne D, Pigazzi A, Marshall H, Croft J, Corrigan N, Copeland J, Quirke P, West N, Rautio T, Thomassen N, et al. Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: the ROLARR randomized clinical trial. JAMA. 2017;318:1569–1580. doi: 10.1001/jama.2017.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsuda S, Oleynikov D, Gould J, Azagury D, Sandler B, Hutter M, Ross S, Haas E, Brody F, Satava R. SAGES TAVAC safety and effectiveness analysis: da Vinci ® Surgical System (Intuitive Surgical, Sunnyvale, CA) Surg Endosc. 2015;29:2873–2884. doi: 10.1007/s00464-015-4428-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Figure 1. Illustrations for the length of bowel resection, location of related arteries ligation and scope of the lymph node dissection for radical left colectomy for tumours located (A) at the distal 1/3 of the transverse colon (TC) and splenic flexure (SF), (B) at the upper segment of descending colon (UDC), (C) at the lower segment of descending colon (LDC).
Additional file 2: Supplementary Figure 2. (A1&B1&C1&D1): The values of hematology examination including of RBC (A1), WBC (B1), TP (C1) and ALB (D1) in OS, LS and RS groups were compared between pre-operation and post-operation by the Kruskal-Wallis test. (A2&B2&C2&D2): The changes of hematology examination including of RBC (A2), WBC (B2), TP (C2) and ALB (D2) from pre-operation to post-operation were compared among OS, LS and OS by the Kruskal-Wallis test. The Delta (Δ) is the postoperative value minus the preoperative value. RS (robotic surgery), LS (laparoscopic surgery), OS (open surgery).
Additional file 3: Supplementary Figure 3. Kaplan–Meier survival curves and Cumulative incidence curves (Before Propensity-score matched cohort). (A) Kaplan–Meier survival curves for overall survival rates according to TNM stage. (A1) All stages; (A2) Stage I; (A3) Stage II; (A4) Stage III. (B) Kaplan–Meier survival curves for disease-free survival rates according to TNM stage. (B1) All stages; (B2) Stage I; (B3) Stage II; (B4) Stage III. (C) Cumulative incidence curves of local recurrence rates. (D) Cumulative incidence curves of distant metastasis rates.
Additional file 4: Supplementary Table 1. Demographics and clinical characteristics of three groups. Supplementary Table 2. Demographics, clinical characteristics and pathologic outcomes before and after PSM. Supplementary Table 3. Perioperative clinical outcomes and short-term oncology outcomes (propensity-score matched cohort). Supplementary Table 4. Perioperative and long-term postoperative complications (propensity-score matched cohort). Supplementary Table 5. Prognostic factors of 3-year survival and local recurrence by univariate analysis (propensity-score matched cohort). Supplementary Table 6. Prognostic factors of 3-year survival and local recurrence by multivariate analysis (propensity-score matched cohort).