Abstract
Background
Evidence supports therapeutic drug monitoring of polymyxin B, but clinical data for establishing an area under the concentration–time curve across 24 h at steady state (AUCss,24 h) threshold are still limited. This study aimed to examine exposure–response/toxicity relationship for polymyxin B to establish an AUCss,24 h threshold in a real-world cohort of patients.
Methods
Using a validated Bayesian approach to estimate AUCss,24 h from two samples, AUCss,24 h threshold that impacted the risk of polymyxin B-related nephrotoxicity and clinical response were derived by classification and regression tree (CART) analysis and validated by Cox regression analysis and logical regression analysis.
Results
A total of 393 patients were included; acute kidney injury (AKI) was 29.0%, clinical response was 63.4%, and 30-day all-cause mortality was 35.4%. AUCss,24 h thresholds for AKI of > 99.4 mg h/L and clinical response of > 45.7 mg h/L were derived by CART analysis. Cox and logical regression analyses showed that AUCss,24 h of > 100 mg h/L was a significant predictor of AKI (HR 16.29, 95% CI 8.16–30.25, P < 0.001) and AUCss,24 h of ≥ 50 mg h/L (OR 4.39, 95% CI 2.56–7.47, P < 0.001) was independently associated with clinical response. However, these exposures were not associated with mortality. In addition, the correlation between trough concentration (1.2–2.8 mg/L) with outcomes was similar to AUCss,24 h.
Conclusions
For critically ill patients, AUCss,24 h threshold of 50–100 mg h/L was associated with decreased nephrotoxicity while assuring clinical efficacy. Therapeutic drug monitoring is recommended for individualizing polymyxin B dosing.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-022-04195-7.
Keywords: Polymyxin B; AUCss,24 h threshold; Nephrotoxicity; Efficacy; Therapeutic drug monitoring
Background
Polymyxins (i.e. colistin and polymyxin B) are old antibiotics that have been reintroduced in clinical practice because of the increasing incidence rate of carbapenem-resistant gram-negative bacteria (CR-GNB) infections [1, 2]. However, nephrotoxicity is the most common adverse effect of polymyxins, with rates of acute kidney injury (AKI) ranging widely from 20 to 60% [3].
Pharmacokinetics (PK)/pharmacodynamics (PD) theory can effectively promote the rational use of antibiotics [4]. As an agent with wide inter-individual variability in PK and a narrow therapeutic index, polymyxin B is an ideal candidate for therapeutic drug monitoring (TDM) [5, 6]. An area under the concentration–time curve across 24 h at steady state (AUCss,24 h) of 50–100 mg h/L is recommended for the treatment of CR-GNB with minimum inhibitory concentration (MIC) values of ≤ 2 mg/L, focusing on Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacteriaceae [7, 8]. However, it should be pointed out that the upper and lower bound of this therapeutic window was derived from a pharmacometrics meta-analysis of polymyxin B nephrotoxicity data and murine thigh infection PK/PD studies [9, 10]. Therefore, it is necessary to re-evaluate whether this threshold applies to critically ill patients.
Several studies have analyzed predictors for nephrotoxicity/efficacy and population PK of polymyxin B and reported dose as an independent predictor of AKI. Once daily dose is associated with AKI, it is expected that polymyxin B concentrations also be associated with AKI. However, a direct correlation between polymyxin B exposure and response/toxicity has not been well demonstrated [11–15]. Previously, we found that AUCss,24 h of > 100 mg h/L was a good predictor of the probability of nephrotoxicity (P = 0.001) [16]. Ye et al. found that the therapeutic target of AUCss,24 h (odds ratio [OR] = 13.15, P = 0.015) was independently associated with favorable clinical outcomes of polymyxin B treatment [17]. Some studies reported that maintaining a trough concentration (C0h or Cmin) of polymyxin B below 3.13 mg/L or peak concentration (Cmax) of polymyxin B1 below 5.23 mg/L might help reduce the incidence of polymyxin B-related nephrotoxicity [18, 19]. After all, C0h is usually an appropriate choice for antibiotics with AUC/MIC as PK/PD index, such as linezolid, amikacin, and voriconazole [20]. However, considering the small sample size, further research is needed to validate the exposure target extensively.
The primary objective of this study was to validate an AUCss,24h threshold of polymyxin B for predicting nephrotoxicity and efficacy in patients with CR-GNB infections. We also compared the effectiveness of AUCss,24h and C0h. In addition, risk factors for AKI, clinical efficacy, and mortality were evaluated.
Methods
Patient enrollment and data collection
This retrospective observational study was performed at the first affiliated hospital of Zhengzhou University. From April 2018 to March 2022, all patients (≥ 18 years) were included if they had received intravenous polymyxin B (sulfate; polymyxin B injection, Shanghai First Biochemical Pharmaceutical Co., Ltd., China) for the treatment of CR-GNB infections and had TDM. The susceptibility to carbapenems of the causative GNB was determined according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST). Enterobacteriaceae with MIC ≥ 4 mg/L and P. aeruginosa and Acinetobacter spp. with MIC ≥ 8 mg/L were considered resistant to carbapenem [21]. The EUCAST breakpoints of polymyxin B for the above bacterial strains were 2 mg/L or lower for susceptible and > 2 mg/L for resistant [8]. Polymyxin-sensitive bacteria were performed using a VITEK® 2 COMPACT automated system (bioMérieux, Marcy-l’Étoile, France) with VITEK cards (0.5–16 mg/L for polymyxin). Exclusion criteria were (i) less than 18 years of age; (ii) received polymyxin B administration less than 96 h; (iii) received renal replacement therapy prior to polymyxin B therapy; iv) had no pathogenic microorganism result; and (v) had wrong blood drawing time. This study was approved by the Ethics Committees of the First Affiliated Hospital of Zhengzhou University (2020-KY-0318) and waived informed consent given the retrospective nature.
Data were extracted from electronic medical records, including demographics, comorbidities, medication therapy, physiological parameters, and laboratory values on the day of polymyxin B initiation. In addition, severity of illness was quantified using the Acute Physiology and Chronic Health Enquiry (APACHE II) score and the Sequential Organ Failure Assessment (SOFA) score using the worst physiological parameters within 24 h before polymyxin B initiation [22, 23]. Diagnoses of infection site were based on clinical features and positive culture of CR-GNB in sterile localized, and the absence of any bacterial pollution or colonization by two physicians.
Polymyxin B administration and concentration determination
Polymyxin B package insert recommended doses of 1.5–3.0 mg/kg/day in two divided administrations. Therapy management was at the discretion of physicians, including dosage, infusion time, administration period, adjustment treatment, and concomitant antibiotics. Concomitant antibiotics referred to the use of other antibiotics with different mechanisms against pathogenic microorganisms to improve efficacy during polymyxin B treatment. The type and treatment course of concomitant antibiotics were based on pathogen susceptibility results, clinical features, and Chinese consensus statements [24], and only that administered for at least 72 h during polymyxin B treatment were recorded. Moreover, the following medications or medication classes were considered potential nephrotoxic drugs: vancomycin (≥ 1.0 mg/day), aminoglycoside, amphotericin B, furosemide (≥ 20 mg/day), vasoactive drugs, and immunosuppressant, which should be concomitantly used at any time during polymyxin B therapy for at least 72 h.
Polymyxin B plasma concentrations were obtained as part of our hospital's routine clinical practice of TDM. TDM was assessed first on day 4 and then repeated 48 h after dose adjustment. Dose adjustment was determined by physicians based on clinical features, MIC results, and the levels. Only the last TDM results were included in the study. For TDM, two blood samples were collected immediately before the infusion (C0h) and 2 h after the beginning of infusion (C2h). Blood samples were immediately centrifuged at 3500 ×g for 10 min. The supernatant was collected and stored at − 80 °C before analysis within one week.
The plasma concentrations of polymyxin B were determined using a validated ultra-performance liquid chromatography-tandem mass spectrometry previously published in our laboratory [25]. Briefly, the assay was linear over 0.2–10.0 μg/mL for polymyxin B1 and 0.05–2.5 μg/mL for polymyxin B2. The relative standard deviation (% RSD) of intra- and inter-batch assay ranged from 0 to 13.9% for quality control samples, and their corresponding accuracy (% relative error) ranged from − 11.6 to 11.1%. Since polymyxin B1 and B2 had similar structures, molecular weight (Mol.), pharmacological activities, and PK characteristics, the concentration (Conc.) of polymyxin B was derived by the equation as Conc(polymyxin B) = [Conc(polymyxin B1)/Mol(polymyxin B1) + Conc (polymyxin B2)/Mol(polymyxin B2)] × Molavg(polymyxin B).
For each patient, AUCss,24 h was estimated using the Bayesian priors from our previously published population PK model using Phoenix® NLME software (v8.3, Pharsight, Mountain View, CA, USA) [26]. In short, the mean parameter vector and the variance–covariance matrix from a previously published two-compartment population PK model were used as the Bayesian prior, then to estimate the Bayesian conditional posterior PK parameters for each patient using the dosing, concentrations, and creatinine clearance (CrCL) values. Based on Bayesian conditional posterior PK parameters, the AUCss,24 h were estimated. This approach had been validated to assess AUC values with high precision and low bias using C0h and C2h only [26]. CrCL was estimated according to the Cockcroft–Gault formula [27].
Endpoints
The primary endpoint was the occurrence of AKI, which was defined as a serum creatinine increase of 0.3 mg/L (26.5 μmol/L) and 50% from baseline on two consecutive measurements during polymyxin B treatment. Further classification was based on the Kidney Disease Improving Global Outcomes (KDIGO) criteria [28].
Secondary endpoints were clinical efficiency and 30-day all-cause mortality. Clinical response was considered at the end of treatment by two physicians: disappearance or improvement of clinical symptoms (body temperature < 38.0 °C), radiological resolution of signs of infection, and improved biochemistry indicators of infection (a ≥ 30% decrease in the total peripheral white blood cells count or C-reactive protein level). Patients who did not meet all above criteria were classified as clinical failure. Thirty-day mortality was recorded from the start of polymyxin B treatment.
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences version 26.0 (SPSS Inc., Chicago, IL, USA). Data were expressed as median with interquartile range (IQR) for continuous variables and percentages/frequency (%) for categorical variables. Categorical variables were analyzed by the chi-square test. Normally distributed variables were analyzed by the Student’s t test, while non-normally distributed variables were analyzed by Mann–Whitney test. Variables (Table 1 and infection sites) with P values of < 0.1 were included in Cox proportional hazards model to estimate hazard ratios (HRs) for AKI and mortality, and logistic regression model to estimate odds ratios (ORs) for clinical response. The effect of collinearity among variables (tolerance < 0.2 or variance inflation factor > 10) was eliminated in the models. Classification and regression tree (CART) method was used to split the samples into subgroups based on chi-square statistics. Thirty-day mortality and polymyxin B-associated AKI were compared with Kaplan–Meier analysis and log-rank test. P value of < 0.05 was considered significant.
Table 1.
Patient characteristics
| Variable | All (n = 393) |
|---|---|
| Age, years | 56.0 (48.0–65.0) |
| Male, n (%) | 287 (73.0%) |
| Weight, kg | 70.0 (60.0–75.0) |
| BMI, kg/m2 | 23.7 (21.2–25.7) |
| ICU admission, n (%) | 361 (91.9%) |
| Mechanical ventilation, n (%) | 269 (68.4%) |
| SOFA score | 8.0 (6.0–10.0) |
| APACHE II score | 18.0 (13.0–23.0) |
| Comorbidities, n (%) | |
| Diabetes | 127 (32.3%) |
| Malignancy | 53 (13.5%) |
| Hypertension | 162 (41.2%) |
| Heart disease | 97 (24.7%) |
| Stroke | 92 (23.4%) |
| Sepsis | 186 (47.3%) |
| Septic shock | 153 (38.9%) |
| Pathogen, n (%) | |
| Klebsiella pneumoniae | 177 (42.7%) |
| Acinetobacter baumannii | 168 (40.5%) |
| Pseudomonas aeruginosa | 44 (10.6%) |
| Escherichia coli | 17 (4.1%) |
| Pneumogenic klebsiella | 9 (2.2%) |
| Polymyxin B treatment | |
| Duration days | 13.0 (8.0–18.0) |
| Daily dose, mg | 150.0 (100.0–150.0) |
| Daily dose/weight, mg/kg/day | 2.12 (1.67–2.50) |
| Concomitant antibiotics, n (%) | |
| Carbapenem | 209 (53.2%) |
| Cephalosporin | 184 (46.8%) |
| Tigecycline | 136 (34.6%) |
| Rifampicin | 25 (6.4%) |
| Aminoglycosides | 12 (3.1%) |
| Fosfomycin | 8 (2.0%) |
| Aztreonam | 7 (1.8%) |
| Laboratory data | |
| GFR, mL/min·1.73m2 | 101.2 (71.3–118.3) |
| Scr, µmol/L | 62.0 (45.3–93.4) |
| Albumin, g/L | 30.0 (27.2–34.9) |
| White blood cell, 109/L | 11.2 (8.0–15.7) |
| Platelets, 109/L | 168.5 (88.0–275.3) |
| C-reactive protein, μg/L | 72.2 (34.6–140.0) |
| Procalcitonin, ng/mL | 1.0 (0.4–3.6) |
| Concomitant nephrotoxic drugs, n (%) | |
| Vancomycin | 83 (21.1%) |
| Aminoglycoside | 12 (3.1%) |
| Amphotericin B | 35 (8.9%) |
| Furosemide | 154 (39.2%) |
| Vasoactive drugs | 195 (50.5%) |
| Immunosuppressant | 32 (8.1%) |
| Use of nephrotoxic drugsa | 289 (73.5%) |
| AUCss,24 h, mg h/L | 58.5 (40.6–77.2) |
| C0h, mg L−1 | 1.24 (0.74–1.93) |
Data were n (%) or median (interquartile range, IQR)
AKI acute kidney injury; BMI body mass index; ICU intensive care unit; SOFA Sequential Organ Failure Assessment; APACHE II Acute Physiology, Age, Chronic Health Evaluation II; GFR glomerular filtration rate; Scr serum creatinine; AUCss,24 h, the area under the curve across 24 h at steady state; and C0h concentration pre-dose
anephrotoxic drug including vancomycin, aminoglycoside, amphotericin B, furosemide, vasoactive drugs, and immunosuppressant
Results
Patients Characteristics
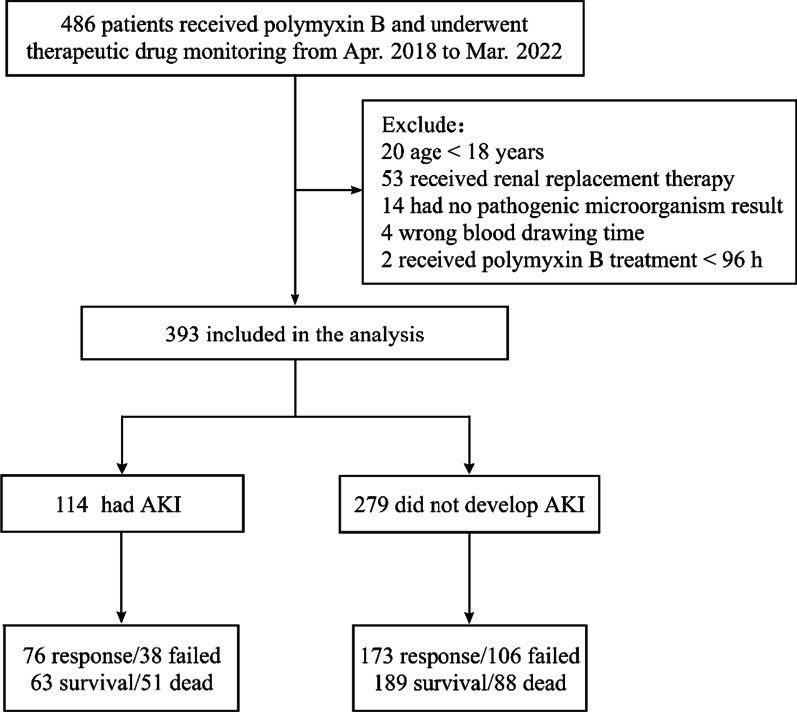
A total of 486 patients received polymyxin B and had TDM during the study period. Ninety-three of them were excluded, and 393 patients were eventually included in the follow-up analysis (Fig. 1). The median age of the patients was 56 years old (IQR, 48–65 years), and 73.0% were male. Among them, 68 patients (17.3%) had multi-site infections. The highest infection site was lung (305/393), followed by bloodstream (96/393), abdomen (26/393), cerebral (17/393), urinary tract (11/393), and skin and soft tissue (6/393). Polymyxin-sensitive bacteria were observed in all patients with MICs ≤ 0.5 mg/L. Information on patient demographics, indications for therapy, and underlying conditions is presented in Table 1.
Fig. 1.
Flow diagram of patient inclusion and exclusion. AKI, acute kidney injury
Polymyxin B exposure and trough concentrations
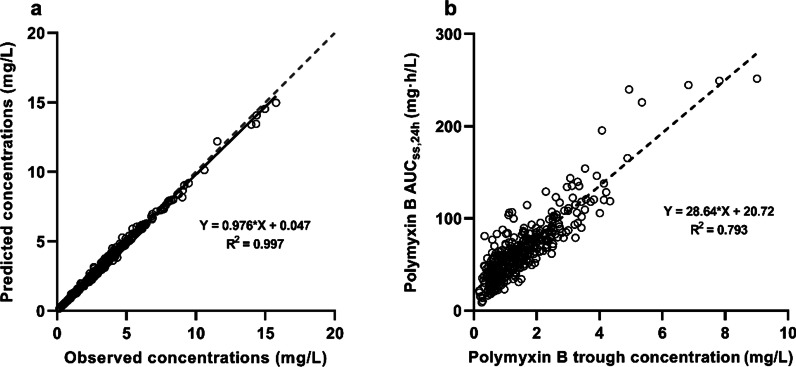
Observed and predicted polymyxin B concentrations from Bayesian estimation are shown in Fig. 2a. The coefficient of determination (R2) of regression line was 0.997, which indicated the predictive performance of Bayesian approach. The median maintenance dose was 2.12 mg/kg/day (IQR, 1.67–2.50 mg/kg/day), which resulted in a median AUCss,24 h of 58.5 mg h/L (IQR, 40.6–77.2 mg h/L) and a median C0h of 1.24 mg/L (IQR, 0.74–1.93 mg/L). Figure 2b shows a positive correlation between C0h and AUCss,24 h with an R2 of 0.793 was detected. Additionally, polymyxin B exposure increased with daily dosage, but it varied largely from patient to patient (Additional file 1: Figure S1).
Fig. 2.
Scatterplot of polymyxin B concentrations. Observed versus predicted polymyxin B concentration for Bayesian estimation approach (a). Bayesian estimated area under the curve across 24 h at steady state (AUCss,24 h) versus trough concentration (b)
AKI
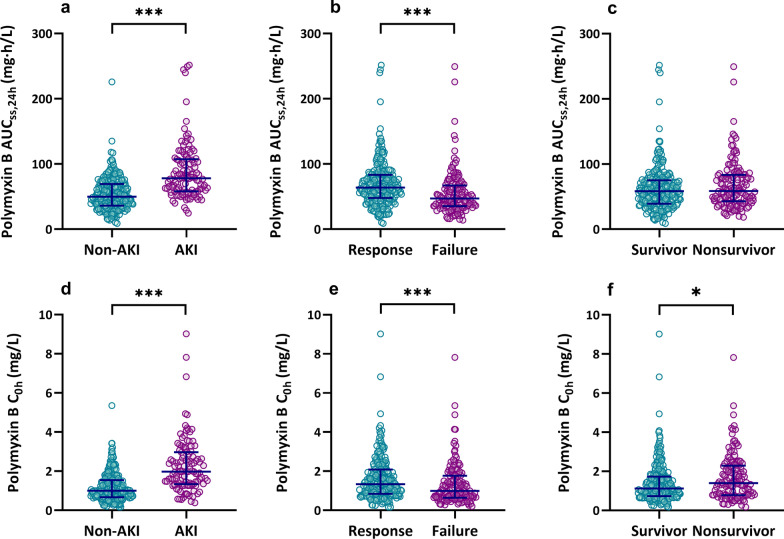
AKI was observed in 29.0% of patients (114/393). Of these, 51 (44.7%) were classified as stage 1; 34 (29.8%) as stage 2; and 29 (25.4%) as stage 3. The median time to develop AKI was 8 days (IQR, 5–14 days). The median AUCss,24 h was significantly higher (78.2 mg h/L; IQR, 58.1–107.5 mg h/L) in patients who developed AKI than in those did not (50.6 mg h/L; IQR, 36.1–69.5 mg h/L; P < 0.001, Fig. 3a). As for AKI degrees (Additional file 1: Figure S2), AUCss,24 h of stage 3 was slightly higher than that of stage 1 (P = 0.044).
Fig. 3.
Scatterplot of polymyxin B AUCss,24 h and C0h stratified for outcomes. AUCss,24 h for AKI (a), clinical efficacy (b), and survival (c); C0h for AKI (d), clinical efficacy (e), and survival (f). AUCss,24 h, the area under the plasma concentration–time curve across 24 h at steady state; C0h, trough concentration; AKI, acute kidney injury; ***represent P < 0.001; and *represent P < 0.05
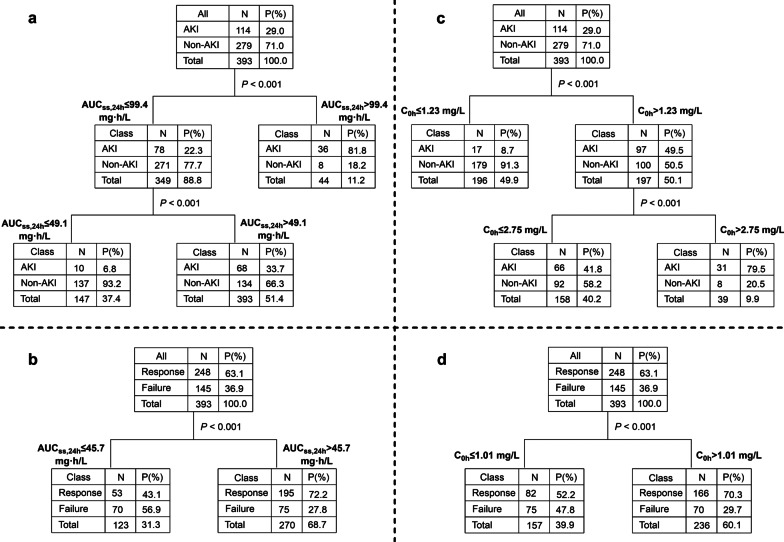
CART analysis (Fig. 4a) revealed that AUCss,24 h of > 99.4 mg h/L was significantly associated with AKI (P < 0.001). Then, subgroup analysis showed that patients with AUCss,24 h of > 49.1 mg h/L had a higher risk of AKI than those with AUCss,24 h of ≤ 49.1 mg h/L (P < 0.001). These results were consistent with the target AUCss,24 h window of 50–100 mg h/L [7], and accordingly, the following analysis took concentrations of 50 mg h/L and 100 mg h/L as cutoff points.
Fig. 4.
Classification and regression tree results for the incidence of AKI and clinical efficacy. AUCss,24 h for AKI (a) and clinical efficacy (b); C0h for AKI (c) and clinical efficacy (d). AKI, acute kidney injury; AUCss,24 h, the area under the plasma concentration–time curve across 24 h at steady state; and C0h, trough concentration
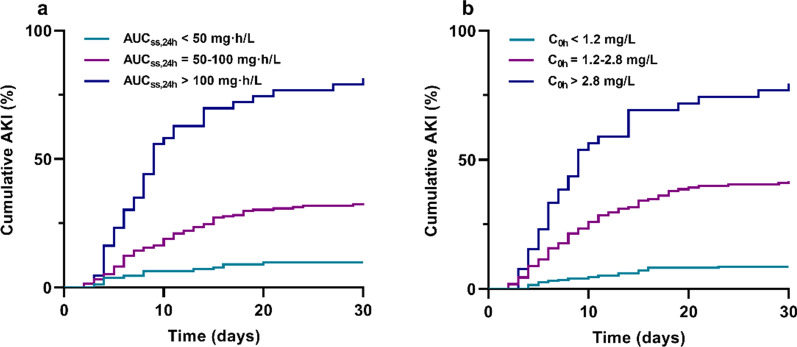
In both of Kaplan–Meier analysis (log-rank, P < 0.001, Fig. 5a) and Cox regression model (Table 2), AKI rate in AUCss,24 h of > 100 mg h/L group (81.4%; HR 16.29, 95% CI 8.16–30.25, P < 0.001) was remarkably higher than that in AUCss,24 h of 50–100 mg h/L group (32.8%; HR 3.89, 95% CI 2.17–6.97, P < 0.001) and AUCss,24 h of < 50 mg h/L group (9.7%, P < 0.001).
Fig. 5.
Kaplan–Meier estimates of the incidence of AKI to day 30 after administration of polymyxin B. Stratified by AUCss,24 h (a) and C0h (b). AKI, acute kidney injury; AUCss,24 h, the area under the plasma concentration–time curve across 24 h at steady state; and C0h, trough concentration
Table 2.
Univariate and Cox regression model for AKI
| Variable | No AKI (n = 279) |
AKI (n = 114) |
Pa | HR (95% CI) | Pb |
|---|---|---|---|---|---|
| Age, years | 54.0 (47.0–64.0) | 58.0 (50.8–66.0) | 0.012 | 1.02 (1.01–1.03) | 0.003 |
| GFR, mL/min·1.73 m2 | 103.1 (74.0–121.3) | 95.5 (66.2–110.6) | 0.025 | ||
| Furosemide | 101 (36.2%) | 53 (46.5%) | 0.058 | ||
| Use of nephrotoxic drugs | 196 (70.3%) | 93 (81.6%) | 0.021 | ||
| Polymyxin B dose and exposure | |||||
| Dose/weight, mg/kg/day | 2.0 (1.67–2.37) | 2.31 (1.94–2.61) | < 0.001 | ||
| Daily dose ≥ 150 mg/day | 178 (63.8%) | 89 (78.1%) | 0.006 | ||
| cAUCss,24 h < 50 mg h/L | 140 (50.2%) | 15 (13.2%) | < 0.001 | – | < 0.001 |
| AUCss,24 h = 50–100 mg h/L | 131 (47.0%) | 64 (56.1%) | 3.89 (2.17–6.97) | < 0.001 | |
| AUCss,24 h > 100 mg h/L | 8 (2.9%) | 35 (30.7%) | 16.29 (8.16–30.25) | < 0.001 | |
| dC0h < 1.2 mg/L | 173 (62.0%) | 17 (14.9%) | < 0.001 | ||
| C0h = 1.2–2.8 mg/L | 98 (35.1%) | 66 (57.9%) | |||
| C0h > 2.8 mg/L | 8 (2.9%) | 31 (27.6%) | |||
AKI acute kidney injury; HR hazard ratio; CI confidence interval; GFR glomerular filtration rate; and AUCss,24 h the area under the curve across 24 h at steady state
aderived from univariate analysis
bderived from Cox regression analysis
cAUCss,24 h < 50 mg h/L was taken as reference
dC0h was not included in the Cox regression model due to collinearity with AUCss,24 h
In the case of C0h, the median C0h was significantly higher (1.98 mg/L; IQR, 1.34–2.97 mg/L) in patients who developed AKI than in those did not (1.01 mg/L; IQR, 0.67–1.54 mg/L; P < 0.001, Fig. 3d). CART analysis showed that two cutoff values of 1.23 mg/L and 2.75 mg/L split C0h levels into three nodes based on AKI rate (Fig. 4c). For the sake of convenient use in the clinic, the values were rounded to 1.2 mg/L and 2.8 mg/L in Kaplan–Meier analysis (log-rank, P < 0.001, Fig. 5b).
Clinical efficacy
The overall clinical response rate was 63.4% (249/393). The median duration of polymyxin B treatment in patients who achieved clinical response or did not was 11 and 14 days, respectively. A higher AUCss,24 h (63.9 mg h/L; IQR, 47.8–84.0 mg h/L) was observed in patients with clinical response than in those with clinical failure (47.0 mg h/L; IQR, 34.3–62.8 mg h/L; P < 0.001, Fig. 3b). CART analysis (Fig. 4b) revealed that a cutoff value of 45.7 mg h/L split AUCss,24 h levels into two nodes based on clinical response rate (P < 0.001), which was also in agreement with the lower limit of the target therapeutic window (50 mg h/L) [7, 9]. Logistic regression analysis (Table 3) showed that AUCss,24 h ≥ 50 mg h/L (OR 4.39, 95% CI 2.56–7.47, P < 0.001) was an independent factor associated with clinical response, as were concomitant nephrotoxic drugs, age, and septic shock.
Table 3.
Univariate and logistic regression model for clinical response
| Variable | Failure (n = 144) |
Response (n = 249) |
Pa | OR (95% CI) | Pb |
|---|---|---|---|---|---|
| Age, years | 58.0 (51.0–67.0) | 54.0 (47.0–63.0) | 0.009 | 0.98 (0.96–1.0) | 0.012 |
| SOFA score | 8.0 (7.0–12.0) | 8.0 (5.0–10.0) | 0.003 | ||
| APACHE II score | 19.0 (14.0–23.0) | 17.0 (11.0–21.0) | 0.001 | ||
| Septic shock | 69 (47.6%) | 84 (33.9%) | 0.007 | 0.55 (0.33–0.93) | 0.025 |
| Vasoactive drugs | 87 (60.8%) | 108 (44.4%) | 0.002 | ||
| Use of nephrotoxic drugs | 117 (80.7%) | 172 (69.4%) | 0.014 | 0.44 (0.24–0.83) | 0.011 |
| Laboratory data | |||||
| GFR, mL/min·1.73m2 | 96.0 (51.8–114.4) | 102.7 (73.7–118.0) | 0.049 | ||
| Albumin, g/L | 29.2 (26.2–33.2) | 30.5 (27.3–35.0) | 0.080 | ||
| Platelets, 109/L | 139.0(73.0–244.0) | 172.5 (85.5–271.0) | 0.008 | ||
| C-reactive protein, μg/L | 89.4 (45.9–150.0) | 67.6 (30.8–130.7) | 0.072 | ||
| Procalcitonin, ng/mL | 1.2 (0.5–4.0) | 0.9 (0.3–3.5) | 0.080 | ||
| Polymyxin B dose and exposure | |||||
| Dose/weight, mg/kg/day | 2.0 (1.6–2.3) | 2.1 (1.8–2.5) | 0.011 | ||
| Daily dose ≥ 150 mg/day | 82 (56.6%) | 185 (74.6%) | < 0.001 | ||
| AUCss,24 h ≥ 50 mg h/L | 63 (43.4%) | 175 (70.6%) | < 0.001 | 4.39 (2.56–7.47) | < 0.001 |
| cC0h > 1.01 mg/L | 79 (54.9%) | 169 (67.9%) | < 0.001 | ||
OR odds ratio; CI confidence interval; GFR glomerular filtration rate; and AUCss,24 h the area under the curve across 24 h at steady state
aderived from univariate analysis
bderived from logistic regression analysis
cC0h was not included in the logistic regression model due to collinearity with AUCss,24 h
In addition, the median C0h was higher (1.35 mg/L; IQR, 0.85–2.09 mg/L) in patients with clinical response than with clinical failure (0.99 mg/L; IQR, 0.65–1.76 mg/L; P < 0.001, Fig. 3e), and maintenance of C0h > 1.01 mg/L was identified to be correlated with clinical response (Fig. 4d).
Mortality
The 30-day all-cause mortality was 35.4% (139/393). No significant difference in AUCss,24 h between surviving and non-surviving patients was observed (58.3 mg h/L [39.5–75.2 mg h/L] vs. 58.2 mg·h/L [43.1–83.4 mg·h/L]; P = 0.184, Fig. 3c). And, there was no difference within each AUCss,24 h group with regard to 30-day mortality (34.2% vs. 34.9% vs. 41.9%, log-rank, P = 0.785, Additional file 1: Figure S3). In contrast, C0h showed statistic difference between survivors and non-survivors (1.12 mg/L [0.73–1.73 mg/L] vs. 1.40 mg/L [0.78–2.29 mg/L]; P = 0.018, Fig. 3f). Only septic shock, glomerular filtration rate, heart disease, and use of nephrotoxic drugs were associated with 30-day mortality in Cox regression model (Table 4).
Table 4.
Univariate and Cox regression model for 30-day mortality
| Variable | Survival (n = 254) |
No survival (n = 139) |
Pa | HR (95% CI) | Pb |
|---|---|---|---|---|---|
| Age, years | 54.0 (48.0–63.0) | 59.0 (48.0–68.0) | < 0.001 | ||
| ICU admission, n (%) | 229 (90.2%) | 132 (95.0%) | 0.096 | ||
| Mechanical ventilation | 163 (64.2%) | 106 (76.3%) | 0.014 | ||
| SOFA score | 8.0 (5.0–10.0) | 9.0 (7.0–12.0) | < 0.001 | ||
| APACHE II score | 17.0 (11.0–21.8) | 19.0 (14.0–24.0) | 0.002 | ||
| Comorbidities | |||||
| Diabetes | 74 (29.1%) | 53 (38.1%) | 0.068 | ||
| Heart disease | 46 (18.1%) | 51 (36.7%) | < 0.001 | 1.92 (1.31–2.83) | 0.001 |
| Sepsis | 106 (41.7%) | 80 (57.6%) | 0.003 | ||
| Septic shock | 75 (29.5%) | 78 (56.1%) | < 0.001 | 2.29 (1.54–3.40) | < 0.001 |
| Laboratory data | |||||
| GFR, mL/min·1.73m2 | 103.1 (80.6–118.9) | 86.5 (41.8–110.9) | < 0.001 | 0.99 (0.99–1.0) | 0.001 |
| Albumin, g/L | 30.2 (27.3–34.9) | 29.2 (26.6–32.9) | 0.021 | ||
| Platelets, 109/L | 178.0(84.3–294.8) | 127.0 (71.0–194.0) | < 0.001 | ||
| C-reactive protein, μg/L | 68.1 (30.4–127.2) | 107.5 (47.3–159.9) | 0.001 | ||
| Procalcitonin, ng/mL | 0.7 (0.3–3.4) | 1.4 (0.6–5.9) | < 0.001 | ||
| Concomitant nephrotoxic drugs | |||||
| Vancomycin | 43 (16.9%) | 40 (28.8%) | 0.006 | ||
| Furosemide | 89 (35.0%) | 65 (47.1%) | 0.020 | ||
| Vasoactive drugs | 110 (43.8%) | 85 (63.0%) | < 0.001 | ||
| Use of nephrotoxic drugs | 166 (65.4%) | 123 (88.5%) | < 0.001 | 2.70 (1.48–4.93) | 0.001 |
| AKI | 63 (24.8%) | 51 (36.7%) | 0.013 | ||
HR hazard ratio; CI confidence interval; and GFR glomerular filtration rate
aderived from univariate analysis
bderived from Cox regression analysis
Discussion
This study investigated and evaluated the association between an AUCss,24 h threshold and polymyxin B exposure–response/toxicity using clinical data from critically ill patients with CR-CNB infections. As a result, a significant relationship between polymyxin B AUCss,24 h and clinical efficacy/AKI was observed. CART analysis identified two cutoff points of 99.4 mg h/L and 49.1 mg h/L as significant predictors of the incidence of AKI, and AUCss,24 h of > 45.7 mg h/L was significantly associated with clinical response (Fig. 4). These results were in accordance with the therapeutic window (50–100 mg h/L) and were also validated by regression analyses and Kaplan–Meier analysis in this study.
In the previous study, we found that an AUCss,24 h of > 100 mg h/L was a good predictor for the probability of nephrotoxicity by both the receiver operating characteristic curve and logistic regression analysis [16]. Unable to estimate AUC, Han et al. reported that C0h > 3.13 mg/L significantly increased the risk of AKI [18]. According to the limited sampling strategy equation (AUCss,24 h = 21.323 + 28.189 × C0h) [29], the C0h value (3.13 mg/L) was equivalent to an AUCss,24 h of 109.6 mg h/L. These results also supported the rationality of the upper bound of AUCss,24 h (100 mg h/L).
For antibiotics with AUC/MIC as PK/PD index, AUC better predicts efficacy and toxicity but is more challenging to get in clinical practice than C0h [30, 31]. Several approaches have been proposed to estimate AUC with sparse samples, such as Bayesian method, limited sampling strategy, and Sawchuk–Zaske equation [26, 29, 32]. Using Bayesian approach, we found that C0h had an apparent relationship with AUC (Fig. 2b). Moreover, CART analysis identified two cutoff points of 1.23 mg/L and 2.75 mg/L as significant predictors of the incidence of AKI (Fig. 4c) and C0h of > 1.01 mg/L as a predictor for clinical response (Fig. 4d). Based on the regression equation (Fig. 2b), these C0h values (1.23 mg/L, 2.75 mg/L, and 1.01 mg/L) were, respectively, equivalent to AUCss,24 h of 55.1 mg h/L, 100.9 mg h/L, and 49.6 mg h/L, which were closed to the AUC target. Since the incidence of AKI was very low when C0h ≤ 1.23 mg/L (8.7%), to avoid the emergence of drug-resistant bacteria caused by low drug exposure, C0h therapeutic target was simplified to 1.2–2.8 mg/L. Alternatively, in the absence of methods to calculate AUC, C0h would be a credible surrogate for dosing adjustment.
Besides drug exposures, older age was also associated with polymyxin B-related AKI in Cox regression model (Table 2). Meta-analyses showed the primary factor affecting nephrotoxicity was polymyxins B dose, along with age, diabetes, and use of nephrotoxins [12, 13, 33]. Most of the above risk factors were found in the univariate analysis but had no effect in Cox regression model. This disparity was attributable to the fact that polymyxin B dose was associated with AUCss,24 h, which showed a more remarkable impact on nephrotoxicity. Therefore, TDM is recommended to decrease the risk of nephrotoxicity.
Compared with AKI, polymyxin B exposures had less effect on clinical response and had no effect on 30-day mortality (Fig. 3). It was also found in the vancomycin exposure–response relationship studies [34–36]. Since this was a retrospective study, these data should be interpreted cautiously. The main reason might be that AUC/MIC ratio, not AUC, was the PK/PD parameter most closely linked to clinical outcomes [4]. In this study, polymyxin-sensitive bacteria were observed in all patients with MICs ≤ 0.5 mg/L by a VITEK® 2 COMPACT automated system rather than broth microdilution (BMD) testing. Nevertheless, Zhu et al. observed that the MICs of polymyxin in several susceptible isolates tested by the VITEK 2 system were onefold to twofold dilutions lower than those of BMD [37]. This may be the reason why the guideline recommends the use of BMD assays to assess the MICs of polymyxins [7]. Additionally, critically ill patients suffered serious underlying diseases and comorbidities, mixed infection, and a combination of multiple drugs, which also affected the eventual efficacy of polymyxin B and mortality.
This study has several limitations to be considered. First, this was a single-center retrospective study of adult, non-dialysis patients. It is unknown whether the observed findings apply to other populations. Second, the AUC threshold was delineated by CART, a useful tool to identify exposure thresholds associated with an increased risk of outcomes. Although it recognized the breakpoint that maximizes the difference in outcomes in a given study sample, it should be validated with external data. Third, this population was identified over 4 years, so unmeasured changes in CR-GNB management may result in improved outcomes over time. Known changes over this period include changes in polymyxin B dosing in accordance with consensus guidelines [7] and novel co-administered agents such as ceftazidime–avibactam [38]. Last, to truly define the polymyxin B exposure–efficacy relationship, larger-scale, multicentered prospective clinical trials are needed with the AUC/MIC analysis, which is under preparation at our center.
Conclusions
In conclusion, the present study confirmed a significant relationship between polymyxin B AUCss,24 h and clinical efficacy/AKI in a real-world cohort of patients treated with polymyxin B for CR-GNB infections. AUCss,24 h threshold of 50–100 mg h/L was a good predictor for clinical response and AKI risk, and C0h (1.2–2.8 mg/L) monitoring was also a credible surrogate for individualized dosing. Accordingly, it is necessary to recommend TDM and AUC-guided polymyxin B dosing.
Supplementary Information
Additional file 1: Figure S1. Scatterplot of polymyxin B AUCss,24h versus dosage. AUCss,24h, the area under the plasma concentration-time curve across 24 hours at steady state. Figure S2. Scatterplot of polymyxin B AUCss,24h (a) and C0h (b) stratified for different stages of acute kidney injury. AUCss,24h, the area under the plasma concentration-time curve across 24 hours at steady state; C0h, trough concentration. Figure S3. Kaplan-Meier estimates of survival to 30 days after administration of polymyxin B. Stratified by AUCss,24h (a) and C0h (b). AUCss,24h, the area under the plasma concentration-time curve across 24 hours at steady state; C0h, trough concentration.
Abbreviations
- CR-GNB
Carbapenem-resistant gram-negative bacteria
- AKI
Acute kidney injury
- PK
Pharmacokinetics
- PD
Pharmacodynamics
- TD
Toxicodynamic
- TDM
Therapeutic drug monitoring
- AUCss,24 h
Area under the concentration–time curve across 24 h at steady state
- MIC
Minimum inhibitory concentration
- C0h or Cmin
Trough concentration
- EUCAST
European Committee on Antimicrobial Susceptibility Testing
- APACHE II
Acute Physiology and Chronic Health Enquiry
- SOFA
Sequential Organ Failure Assessment
- C2h
Concentration after 2 h dose
- CrCL
Creatinine clearance
- KDIGO
Kidney Disease Improving Global Outcomes
- IQR
Interquartile range
- HR
Hazard ratio
- OR
Odds ratios
- CART
Classification and regression tree
- R2
Coefficient of determination
- BMD
Broth microdilution
Author contributions
JY, PW, and SL contributed to data acquisition, analysis, and interpretation. JY contributed to manuscript preparation. JL performed the experiments. PW supervised the research and revised the manuscript. TS and XZ designed the research. All authors approved the final manuscript.
Funding
This work was supported by the National Key R&D Program of China (Grant No. 2020YFC2008304).
Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committees of the First Affiliated Hospital of Zhengzhou University (2020-KY-0318) and waived informed consent.
Consent for publication
All authors have consented to the publication of the present manuscript.
Competing interests
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Peile Wang, Email: comwpl5876@163.com.
Xiaojian Zhang, Email: zhangxj6686@163.com.
References
- 1.Velkov T, Roberts KD, Nation RL, Thompson PE, Li J. Pharmacology of polymyxins: new insights into an ‘old’ class of antibiotics. Future Microbiol. 2013;8(6):711–724. doi: 10.2217/fmb.13.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paul M, Carrara E, Retamar P, Tängdén T, Bitterman R, Bonomo RA, et al. European society of clinical microbiology and infectious diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant gram-negative bacilli (endorsed by European society of intensive care medicine) Clin Microbiol Infect. 2022;28(4):521–547. doi: 10.1016/j.cmi.2021.11.025. [DOI] [PubMed] [Google Scholar]
- 3.Rigatto MH, Oliveira MS, Perdigão-Neto LV, Levin AS, Carrilho CM, Tanita MT, et al. Multicenter prospective cohort study of renal failure in patients treated with colistin versus polymyxin B. Antimicrob Agents Ch. 2016;60(4):2443–2449. doi: 10.1128/AAC.02634-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tängdén T, Ramos Martín V, Felton T, Nielsen EI, Marchand S, Brüggemann RJ, et al. The role of infection models and PK/PD modelling for optimising care of critically ill patients with severe infections. Intensive Care Med. 2017;43(7):1021–1032. doi: 10.1007/s00134-017-4780-6. [DOI] [PubMed] [Google Scholar]
- 5.Sandri AM, Landersdorfer CB, Jacob J, Boniatti MM, Dalarosa MG, Falci DR, et al. Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens. Clin Infect Dis. 2013;57(4):524–531. doi: 10.1093/cid/cit334. [DOI] [PubMed] [Google Scholar]
- 6.Tran TB, Velkov T, Nation RL, Forrest A, Tsuji BT, Bergen PJ, et al. Pharmacokinetics/pharmacodynamics of colistin and polymyxin B: Are we there yet? Int J Antimicrob Ag. 2016;48(6):592–597. doi: 10.1016/j.ijantimicag.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsuji BT, Pogue JM, Zavascki AP, Paul M, Daikos GL, Forrest A, et al. International consensus guidelines for the optimal use of the polymyxins: endorsed by the American college of clinical pharmacy (ACCP), European society of clinical microbiology and infectious diseases (ESCMID), infectious diseases society of America (IDSA), international society for anti-infective pharmacology (ISAP), society of critical care medicine (SCCM), and society of infectious diseases pharmacists (SIDP) Pharmacotherapy. 2019;39(1):10–39. doi: 10.1002/phar.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satlin MJ, Lewis JS, Weinstein MP, Jean P, Humphries RM, Gunnar K, et al. Clinical and laboratory standards institute and European committee on antimicrobial susceptibility testing position statements on polymyxin B and colistin clinical breakpoints. Clin Infect Dis. 2020;71(9):e523–e529. doi: 10.1093/cid/ciaa121. [DOI] [PubMed] [Google Scholar]
- 9.Lakota EA, Landersdorfer CB, Nation RL, Li J, Kaye KS, Rao GG, et al. Personalizing polymyxin B dosing using an adaptive feedback control algorithm. Antimicrob Agents Ch. 2018;62(7):e00483–e418. doi: 10.1128/AAC.00483-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alosaimy S, Jorgensen SC, Rybak MJ. The optimal use of the polymyxins before their time is up. Pharmacotherapy. 2019;39(1):7–9. doi: 10.1002/phar.2207. [DOI] [PubMed] [Google Scholar]
- 11.Tuon FF, Rigatto MH, Lopes CK, Kamei LK, Rocha JL, Zavascki AP. Risk factors for acute kidney injury in patients treated with polymyxin B or colistin methanesulfonate sodium. Int J Antimicrob Ag. 2014;43(4):349–352. doi: 10.1016/j.ijantimicag.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Wagenlehner F, Lucenteforte E, Pea F, Soriano A, Tavoschi L, Steele VR, et al. Systematic review on estimated rates of nephrotoxicity and neurotoxicity in patients treated with polymyxins. Clin Microbiol Infect. 2021;27(5):671–686. doi: 10.1016/j.cmi.2020.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Sisay M, Hagos B, Edessa D, Tadiwos Y, Mekuria AN. Polymyxin-induced nephrotoxicity and its predictors: a systematic review and meta-analysis of studies conducted using RIFLE criteria of acute kidney injury. Pharmacol Res. 2021;163:105328. doi: 10.1016/j.phrs.2020.105328. [DOI] [PubMed] [Google Scholar]
- 14.Zhang XJ, Qi SY, Duan XG, Han B, Zhang SG, Liu SH, et al. Clinical outcomes and safety of polymyxin B in the treatment of carbapenem-resistant gram-negative bacterial infections: a real-world multicenter study. J Transl Med. 2021;19:431. doi: 10.1186/s12967-021-03111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen N, Guo J, Xie J, Xu M, Hao X, Ma K, et al. Population pharmacokinetics of polymyxin B: a systematic review. Ann Transl Med. 2022;10(4):231. doi: 10.21037/atm-22-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang PL, Zhang QW, Zhu ZF, Pei H, Feng M, Sun TW, et al. Comparing population pharmacokinetics and acute kidney injury of polymyxin B in Chinese patients with and without renal insufficiency. Antimicrob Agents Ch. 2021;62(2):e01900–01920. doi: 10.1128/AAC.01900-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye Q, Wang Q, Chen Z, Chen W, Zhan Q, Wang C. Effectiveness, nephrotoxicity, and therapeutic drug monitoring of polymyxin B in nosocomial pneumonia among critically ill patients. Clin Respir J. 2022;16(5):402–412. doi: 10.1111/crj.13493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han L, Xu FM, Zhang XS, Zhang CH, Dai Y, Zhou ZY, et al. Trough polymyxin B plasma concentration is an independent risk factor for its nephrotoxicity. Br J Clin Pharmacol. 2022;88(3):1202–1210. doi: 10.1111/bcp.15061. [DOI] [PubMed] [Google Scholar]
- 19.Deng Y, Gu JY, Li X, Tong H, Guo SW, Xu B, et al. Does monitoring total and free polymyxin B1 plasma concentrations predict polymyxin B-induced nephrotoxicity? A retrospective study in critically ill patients. Infect Dis Ther. 2022;11(4):1591–1608. doi: 10.1007/s40121-022-00655-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdul-Aziz MH, Alffenaar JWC, Bassetti M, Bracht H, Dimopoulos G, Marriott D, et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a position paper. Intensive Care Med. 2020;46(6):1127–1153. doi: 10.1007/s00134-020-06050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.EUCAST. European Committee on antimicrobial susceptibility testing breakpoint tables for interpretation of MICs and zone diameters. Version 7.1. In. 2017.
- 22.Larvin M, Mcmahon MJ. APACHE-II score for assessment and monitoring of acute pancreatitis. Lancet. 1989;334(8656):201–205. doi: 10.1016/S0140-6736(89)90381-4. [DOI] [PubMed] [Google Scholar]
- 23.Vincent JL, De Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Crit Care Med. 1998;26(11):1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Guan X, He L, Hu B, Hu J, Huang X, Lai G, et al. Laboratory diagnosis, clinical management and infection control of the infections caused by extensively drug-resistant gram-negative bacilli: a Chinese consensus statement. Clin Microbiol Infect. 2016;22:S15–S25. doi: 10.1016/j.cmi.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Wang PL, Zhang QW, Qin ZF, Xing H, Xu M, Pei H, et al. A simple and robust LC-MS/MS analytical method for therapeutic drug monitoring of plasma and cerebrospinal fluid polymyxin B1 and B2. Ther Drug Monit. 2020;42:716–723. doi: 10.1097/FTD.0000000000000754. [DOI] [PubMed] [Google Scholar]
- 26.Wang PL, Zhang QW, Zhu ZF, Feng M, Sun TW, Yang J, et al. Population pharmacokinetics and limited sampling strategy for therapeutic drug monitoring of polymyxin B in Chinese patients with multidrug-resistant Gram-negative bacterial infections. Front Pharmacol. 2020;11:829. doi: 10.3389/fphar.2020.00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levey AS, Coresh J, Bolton K, Culleton B, Harvey KS, Ikizler TA, et al. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 SUPPL. 1):i-ii+S1-S266. [PubMed] [Google Scholar]
- 28.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 29.Chen W, Liu H, Wang Q, Wang X, Kong X, Wang X, et al. Estimation of the area under concentration-time curve of polymyxin B based on limited sampling concentrations in Chinese patients with severe pneumonia. Eur J Pharm Sci. 2021;77:95–105. doi: 10.1007/s00228-020-02986-x. [DOI] [PubMed] [Google Scholar]
- 30.Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American society of health-system pharmacists, the infectious diseases society of America, the pediatric infectious diseases society, and the society of infectious diseases pharmacists. Clin Infect Dis. 2020;71(6):1361–1364. doi: 10.1093/cid/ciaa303. [DOI] [PubMed] [Google Scholar]
- 31.Wicha SG, Märtson AG, Nielsen EI, Koch BC, Friberg LE, Alffenaar JW, et al. From therapeutic drug monitoring to model-informed precision dosing for antibiotics. Clin Pharmacol Ther. 2021;109(4):928–941. doi: 10.1002/cpt.2202. [DOI] [PubMed] [Google Scholar]
- 32.Pai MP, Neely M, Rodvold KA, Lodise TP. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 2014;77:50–57. doi: 10.1016/j.addr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 33.Rigatto MH, Behle TF, Falci DR, Freitas T, Lopes NT, Nunes M, et al. Risk factors for acute kidney injury (AKI) in patients treated with polymyxin B and influence of AKI on mortality: a multicentre prospective cohort study. J Antimicrob Chemoth. 2015;70(5):1552–1557. doi: 10.1093/jac/dku561. [DOI] [PubMed] [Google Scholar]
- 34.Ueda T, Takesue Y, Nakajima K, Ichiki K, Ishikawa K, Yamada K, et al. Validation of vancomycin area under the concentration-time curve estimation by the Bayesian approach using one-point samples for predicting clinical outcomes in patients with methicillin-resistant Staphylococcus aureus infections. Antibiotics. 2022;11:96. doi: 10.3390/antibiotics11010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dalton B, Rajakumar I, Langevin A, Ondro C, Sabuda D, Griener T, et al. Vancomycin area under the curve to minimum inhibitory concentration ratio predicting clinical outcome: a systematic review and meta-analysis with pooled sensitivity and specificity. Clin Microbiol Infect. 2020;26(4):436–446. doi: 10.1016/j.cmi.2019.10.029. [DOI] [PubMed] [Google Scholar]
- 36.Dilworth TJ, Schulz LT, Rose WE. Vancomycin advanced therapeutic drug monitoring: Exercise in futility or virtuous endeavor to improve drug efficacy and safety? Clin Infec Dis. 2021;72(10):e675–e681. doi: 10.1093/cid/ciaa1354. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Y, Jia P, Zhou M, Zhang J, Zhang G, Kang W, et al. Evaluation of the clinical systems for polymyxin susceptibility testing of clinical Gram-negative bacteria in China. Front Microbiol. 2021;11:610604. doi: 10.3389/fmicb.2020.610604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doi Y. Treatment options for carbapenem-resistant gram-negative bacterial infections. Clin Infec Dis. 2019;69(Supplement_7):S565–S575. doi: 10.1093/cid/ciz830. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Scatterplot of polymyxin B AUCss,24h versus dosage. AUCss,24h, the area under the plasma concentration-time curve across 24 hours at steady state. Figure S2. Scatterplot of polymyxin B AUCss,24h (a) and C0h (b) stratified for different stages of acute kidney injury. AUCss,24h, the area under the plasma concentration-time curve across 24 hours at steady state; C0h, trough concentration. Figure S3. Kaplan-Meier estimates of survival to 30 days after administration of polymyxin B. Stratified by AUCss,24h (a) and C0h (b). AUCss,24h, the area under the plasma concentration-time curve across 24 hours at steady state; C0h, trough concentration.
Data Availability Statement
The datasets used during the current study are available from the corresponding author on reasonable request.