Abstract
Glycoprotein G (gG) of herpes simplex virus type 1 (HSV-1) has been used as a prototype antigen for HSV-1 type-specific serodiagnosis, but data on the sequence variability of the gene coding for this protein in wild-type strains are lacking. In this study, direct DNA sequencing of the gG-1 genes from PCR products was performed with clinical HSV-1 isolates from 11 subjects as well as with strains Syn 17+, F, and KOS 321. The reference strains Syn 17+ and F showed a high degree of conservation, while KOS 321 carried 13 missense mutations and, in addition, 12 silent mutations. Three clinical isolates showed mutations leading to amino acid alterations: one had a mutation of K122 to N, which is a gG-1–to–gG-2 alteration; another contained all mutations which were observed in KOS 321 except two silent mutations; and the third isolate carried five missense mutations. Two clinical isolates as well as strain KOS 321 showed a mutation (F111→V) within the epitope of a gG-1-reactive monoclonal antibody (MAb). When all viruses were tested for reactivity with the anti-gG-1 MAb, the three strains with the F111→V mutation were found to be unreactive. Furthermore, gG-1 antibodies purified from sera from the two patients carrying strains mutated in this epitope were less reactive when they were tested by an HSV-1-infected-cell assay. Therefore, our finding that the sequence variability of the gG-1 gene also affects B-cell epitope regions of this protein in clinical isolates may have consequences for the use of this protein as a type-specific antigen for serodiagnosis.
The two subtypes of herpes simplex virus (HSV), HSV-1 and HSV-2, are genetically highly homologous, but despite this their respective tropisms and clinical pictures of infection differ (23). Type-discriminating diagnostic methods include (i) virus isolation followed by serological typing and (ii) DNA detection, both of which are applicable during active infection, and (iii) serodiagnosis, which is applicable during latent or low-replication phases of infection (1).
A reliable type-specific diagnosis may be of importance for several reasons: (i) for optimal dosage of antiviral treatment since sensitivities to antivirals differ between the two viral subtypes (10), (ii) for counseling of couples where one of the partners has genital herpes, (iii) to provide means for HSV seroepidemiological studies to be based on seroassays of high specificity and sensitivity (13), and (iv) to evaluate efficacy during trials of HSV prophylactic agents, including vaccines, by determining frequencies of type-specific seroconversions.
The development of HSV type-specific diagnostic methods for viral typing and serodiagnosis has been hampered by the reported extensive intertypic cross-reactivity between several of the HSV envelope glycoproteins (1, 5). For viral typing with polyclonal sera, the existence of single cross-reactive epitopes in HSV glycoproteins may disqualify their use as type-specific targets, especially since such epitopes may be present in most HSV strains (9, 32) and may lead to serological cross-reactivity (7).
Glycoprotein G (gG) is the candidate antigen for serological analysis of the type-specific antibody response in individuals infected with HSV-1 and/or HSV-2 (2, 15, 28). Although data on epitope mapping are incomplete, no cross-reactive anti-gG-1 (18) or gG-2 monoclonal antibodies (MAbs) have hitherto been reported (20). Therefore, of all the HSV-1 envelope proteins, gG-1 appears to be the best choice for an HSV-1-specific antigen for clinical serodiagnosis and possibly also for routine typing of viral isolates. Recently, an evaluation of a commercial gG-based enzyme immunoassay (EIA) supported this assumption (3).
When basing type-specific diagnosis on a single antigen such as gG-1, a prerequisite is that the gene coding for this protein is conserved in clinical isolates. The aim of this study was to sequence the HSV-1 gG gene in clinical isolates derived from different localities by a PCR-based system, in order to determine the genetic variability of this gene. In addition, we investigated the clinical isolates for exposure of the gG-1 antigen on infected cells by the use of a gG-1-reactive MAb and purified polyclonal human anti gG-1 antibodies. Here, we detected a genetic gG-1 variant of HSV-1 totally lacking a type-specific epitope.
MATERIALS AND METHODS
Patients and viral strains.
Ten patients (designated patients 1 to 10) with reactivated herpetic cutaneous lesions from different localities of the body (mouth, neck, finger, and genitals), seen at outpatient departments in Göteborg, Sweden, were randomly chosen for investigation. Green monkey kidney (GMK) cells were used for isolation, and all strains were then stored frozen at −70°C. From each patient, one HSV-1 isolate and a simultaneously drawn serum sample were included. In addition, a cerebrospinal fluid (CSF) strain (designated HSV-1 BAN) isolated from a patient during her first attack of multiple sclerosis was included (6). HSV strains were isolated and typed by the use of the type-specific MAbs (24). The HSV-1 reference strains used were Syn 17+, F, and KOS 321 (a plaque-purified isolate of wild-type KOS 321), and the HSV-2 strain used was 333.
All patients were previously found to be seropositive for a type-common HSV antigen, as well as for an HSV-1 type-specific gG-1 antigen (31). Four of the patients were also seropositive for HSV-2 by Western blotting (Table 1). These serum samples were used for the purification of gG-1 antibodies in this study.
TABLE 1.
Clinical characteristics and IgG titers of 11 patients with recurrent HSV-1 infection and results of a nested-PCR system used for typing of the viral isolates
| Patient | Sexd | Age (yr) | Site of lesions | IgG ELISA end point titer (μg/ml)
ofa:
|
PCR test result
(with gD gene) for:
|
||
|---|---|---|---|---|---|---|---|
| Type-common antigen | gG-1 antigen | HSV-1 | HSV-2 | ||||
| 1c | F | 35 | Lip | 3,200 | ≥6,400 | + | − |
| 2 | F | 67 | Vulva | 6,400 | 800 | + | − |
| 3c | M | 37 | Lip | 12,800 | 3,200 | + | − |
| 4 | F | 32 | Lip | 3,200 | 1,600 | + | − |
| 5c | F | 64 | Lip | ≥6,400 | 3,200 | + | − |
| 6c | M | 76 | Lip | ≥6,400 | 600 | + | − |
| 7 | M | 36 | Neck | 200 | 800 | + | − |
| 8 | F | 61 | Finger | 200 | 400 | + | − |
| 9 | F | 56 | Lip | ≥6,400 | 3,200 | + | − |
| 10 | F | 28 | Lip | 1,600 | 1,600 | + | − |
| 11 | F | 52 | Brainb | 3,200 | ND | + | − |
MAbs.
The following HSV-1 type-specific MAbs were used: a commercially available anti-gG-1 MAb (Advanced Biotechnologies, Cambridge, United Kingdom), which was previously mapped to amino acids A, F, P, and L at positions 110 to 113 (31), and the anti-gC-1 MAb B1C1B4, which is reactive with a type-specific epitope shown to be conserved in clinical isolates (21). The anti-gB-1 MAb 1B11D8, reactive with a type-common epitope (8), was used as a reference antibody. As an HSV-2 type-specific reagent, the anti-gG-2 MAb O1C5B2 (20, 24) was used for subtyping of HSV strains.
Purification of human anti-gG-1 antibodies.
Serum samples were drawn from all patients with the exception of the patient with multiple sclerosis. Human anti-gG-1 antibodies were purified from serum samples collected from the HSV-1 isolation-positive patients 1, 5, 7, and 9 as described previously (31). In brief, purification was achieved by affinity chromatography with a truncated gG-1 antigen prepared in CHO cells (kindly provided by SmithKline Beecham Biologicals, Rixensart, Belgium) as described recently for anti-gG-2 antibodies (20). gG-1 was coupled to CNBr-activated Sepharose 4B (Pharmacia Fine Chemicals) according to the manufacturer’s instructions. The human serum samples were circulated through the column, and the antibodies were eluted with 0.1 M glycine-HCl (pH 2.8).
ELISA with infected cells.
To determine the expression of type-specific epitopes in the 10 cutaneous HSV-1 isolates and the HSV reference strains, an enzyme-linked immunosorbent assay (ELISA) was performed on cells infected with the different virus strains. GMK cells were grown in Eagle’s minimal essential medium supplemented with antibiotics. The cells were infected with the HSV-1 strains at an infectious dose of 106 PFU/ml. The anti-gG-1 MAb was used at a dilution of 1:500, and the purified human anti-gG-1 antibodies as well as the MAbs B1C1B4 (reactive with gC-1), 1B11D8 (reactive with gB-1), and O1C5B2 (reactive with gG-2) were used at a dilution of 1:50. As a conjugate for the MAbs, alkaline phosphatase-conjugated F(ab)2 goat anti-mouse immunoglobulin G (IgG) was used at a dilution of 1:2,000 (Jackson), and as a conjugate for human anti-gG-1 antibodies, alkaline phosphatase-conjugated F(ab)2 goat anti-human IgG conjugate was used at a dilution of 1:2,000 (Jackson). As a substrate, p-nitrophenyl phosphate at a concentration of 1 mg/ml was used. The A405 value was measured with a reference wavelength of 650 nm against a substrate blank.
PCR amplification and sequencing.
For HSV typing we used a nested-PCR system amplifying the type-specific promoter region of the gD-1 or gD-2 gene, previously exploited for diagnosis of HSV-1-induced central nervous system infections (4, 27). For DNA sequencing, we first developed and optimized a PCR system with three pairs of overlapping primers covering the entire gene coding for gG-1. The PCR primers selected for this study are described in Table 2 and were used at a concentration of 10 pmol/μl. DNA extraction of HSV-1 isolates were performed by using a QIAamp blood kit (Qiagen, Göteborg, Sweden).
TABLE 2.
Primers used for PCR DNA sequencing
| Primer | Nucleotide positionsa | Primer length (bp) | Sequence |
|---|---|---|---|
| A | 4046–4068 | 23 | 5′TGTTTCAACAGAAATGACCGCCC3′ |
| B | 4421–4402 | 20 | 5′CTCAAGATGTTCGCCGTCCC3′ |
| C | 4335–4352 | 18 | 5′ACGCCCGACCACACACCC3′ |
| D | 4667–4648 | 20 | 5′TATGTTGAGGCGTCGGAACC3′ |
| E | 4563–4580 | 18 | 5′AGTCGCCCGAAGACACCC3′ |
| F | 4892–4875 | 18 | 5′CCGCATGTGGGCTCTCCC3′ |
According to the gG-1 DNA sequence reported by McGeoch et al. (22).
PCR amplification was carried out over the three regions corresponding to the primers (Table 2). The 50-μl PCR master reaction mixture contained 1.6 mM MgCl2, 0.22 mM deoxynucleoside triphosphate, 0.54 μM each primer, and 1.0 U of Taq polymerase (Boehringer Mannheim, Mannheim, Germany). The amplification program started with an initial denaturation of 2 min 30 s at 94°C, followed by 35 cycles of a two-step amplification consisting of 1 min of denaturation at 94°C, followed by 45 s of annealing and elongation at 70°C. This program was selected due to the high G+C content of the sequence. All PCRs were carried out with a Perkin-Elmer (Göteborg, Sweden) DNA thermal cycler. The amplified DNA products were separated on standard agarose gel, and the correct DNA bands were extracted from the agarose with a QIAEX II gel extraction kit.
PCR cyclic sequencing was carried out with the purified DNA material in a reaction mixture containing the chosen primer and the Dye Terminator Cycle Sequencing mix (Perkin-Elmer) with a fluorescent stop-nucleotide (fluorescent dideoxynucleoside triphosphate) giving chain termination at all positions. The PCR cyclic-sequencing program consisted of 25 cycles of 30 s at 96°C, 15 s at 50°C, and 4 min at 60°C. The sequencing reaction was carried out in both the sense and antisense directions for confirmation and also as an internal control. After cyclic sequencing, the products were precipitated with ethanol, dissolved in template suppression reagent, and then denatured. The sequencing was carried out on an automated sequenser (ABI Prism, 310 Genetic analyzer; Perkin-Elmer). All HSV-1 strains carrying alterations of the DNA sequence were resequenced one to three times for confirmation.
Nucleotide sequence accession numbers.
The nucleotide sequences corresponding to the amino acid sequences, presented in Fig. 1, of the viruses (KOS 321, F, and clinical isolates 1 to 11) were submitted to GenBank and given the accession no. AF116192, AF120934, AF117114, AF117115, AF117116, AF117117, AF117118, AF117119, AF116193, AF117120, AF117121, AF117122, and AF117123.
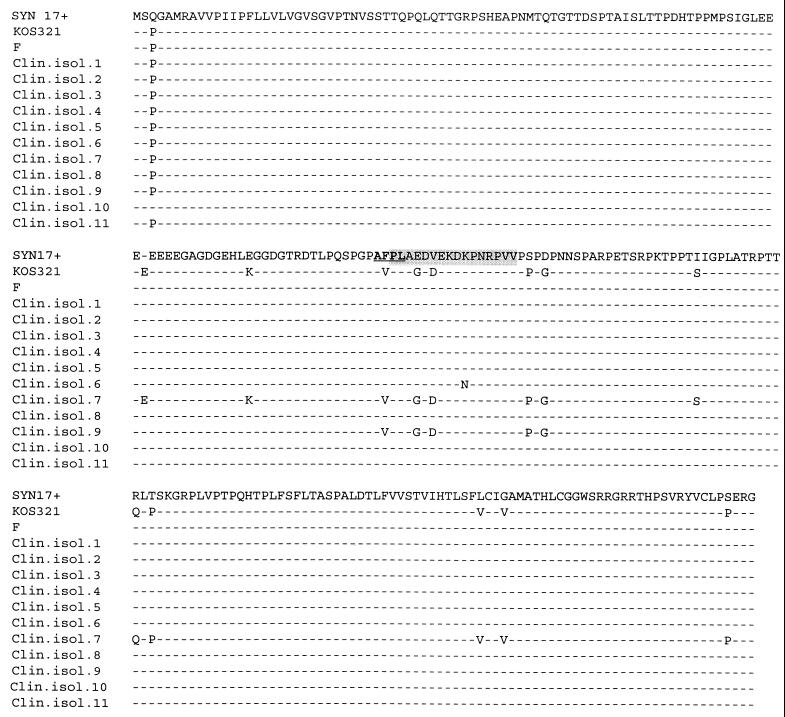
FIG. 1.
Variability in the gG-1 genes of clinical isolates (Clin. isol.) and reference strains, with the variability being reflected in the deduced amino acid sequences. The proposed immunodominant region of gG-1 is shaded, and the sequence of the epitope of an anti-gG-1 MAb is depicted in boldface type and underlined.
RESULTS
Typing of HSV isolates.
All clinical isolates were positive for the HSV-1 type-specific MAb B1C1 reactive with gC (8, 24) but were negative for the HSV-2 anti-gG-2 MAb O1C5 and were therefore classified as HSV-1 (data not shown). Furthermore, typing by a PCR method exploiting the type-specific differences in the promoter region of the gD gene (27) showed that all isolates were positive for HSV-1-specific, but not HSV-2-specific, DNA amplification targeting this region (Table 1).
Sequence alterations in the gG-1 gene.
We found a DNA sequence of strain Syn 17+ identical with a sequence previously published (22). The results from the sequencing of the 11 clinical HSV-1 isolates and strains Syn 17+, KOS 321, and F are shown in Table 3, and the deduced amino acid sequence alterations are depicted in Fig. 1. The DNA sequences of the gG-1 gene from the following eight strains were almost identical: F and clinical isolates 1 to 5, 8, and 11 (HSV-1 BAN) (Table 3). They all differed from the reference strain Syn 17+ at one amino acid position, i.e., position 3, where they had a P rather than a Q, and in addition, strains 6, 7, 9, and KOS 321 also carried this substitution (Table 3 and Fig. 1). Furthermore, these 12 strains all carried two additional nucleotide alterations: the triplet ATC at the postulated noncoding region at codon position −9 was changed to ACC, and the codon ATT (Ile76) was replaced by ATC (Table 3). Clinical isolate 10 shared only the alteration of ATT (Ile76) to ATC but was otherwise completely identical with the DNA sequence of Syn 17+ (Table 3). The triplet CCG coding for proline at codon position 3 as well as the lack of an additional possible methionine codon at −9 might be considered a consensus sequence in the investigated strains. Resequencing of strains KOS 321, 6, 7, and 9 gave results identical to those from the first round of sequencing of those strains.
TABLE 3.
Results of DNA sequencing of the gG-1 gene indicating sites of mutations in 11 clinical HSV-1 isolates and two laboratory strains, compared with Syn 17+
| Codon position(s) | Codon | Mutationa | Amino acid substitution | Mutated HSV-1 strain(s) |
|---|---|---|---|---|
| −9 | ATG | ACC | None | KOS 321, F, 1–9, 11 |
| 3 | CAG | CCG | Q→P | KOS 321, F, 1–9, 11 |
| 20 | GTC | GTT | None | 9 |
| 66 | ACG | ACA | None | 8 |
| 76 | ATT | ATC | None | KOS 321, F, 1–11 |
| 81–82 | CCC | Eb | KOS 321, 7 | |
| 89 | GAC | GAT | None | KOS 321, 7 |
| 94 | GAG | AAG | E→K | KOS 321, 7 |
| 108 | GGC | GGT | None | KOS 321, 7, 9 |
| 111 | TTC | GTC | F→V | KOS 321, 7, 9 |
| 114 | GCT | GCC | None | KOS 321, 7 |
| 115 | GAG | GGG | E→G | KOS 321, 7, 9 |
| 116 | GAC | GAT | None | KOS 321, 7 |
| 117 | GTC | GAC | V→D | KOS 321, 7, 9 |
| 119 | AAG | AAA | None | KOS 321 |
| 121 | AAA | AAC | K→N | 6 |
| 129 | TCC | CCC | S→P | KOS 321, 7, 9 |
| 131 | GAT | GGT | D→G | KOS 321, 7, 9 |
| 143 | CGC | CGA | None | KOS 321, 7, 9 |
| 150 | ATT | AGT | I→S | KOS 321, 7 |
| 157 | CGC | CGA | None | KOS 321, 7 |
| 161 | CGA | CAA | R→Q | KOS 321, 7 |
| 163 | ACC | CCC | T→P | KOS 321, 7 |
| 166 | GGA | GGG | None | KOS 321, 7 |
| 204 | TTG | GTG | L→V | KOS 321, 7 |
| 207 | GGT | GTT | G→V | KOS 321, 7 |
| 208 | GCG | GCT | None | KOS 321 |
| 215 | GGC | GGT | None | KOS 321, 7 |
| 235 | TCC | CCC | S→P | KOS 321, 7 |
Boldface type indicates the mutation.
A triple-C insert was detected at this position in two strains.
Three clinical isolates showed additional sequence alterations (Tables 3 and Fig. 1). Clinical isolate 6 had a single amino acid mutation of K121 to N, which is a gG-1–to–gG-2 substitution, but adhered completely to the consensus sequence with this exception. Two isolates carried extensive mutations: in clinical isolate 7, we found 20 (12 missense and 8 silent) mutations and one triplet substitution (CCC) in comparison with the consensus sequence. Five of the missense mutations, i.e., the amino acid alterations F111 to V, E115 to G, V117 to D, S129 to P, and D131 to G, and two silent mutations were shared with strain 9. At least three of these missense mutations (i.e., F111→V, E115→G, and V117→D) were situated within the suggested immunodominant part of gG-1 (31). Strain KOS 321, which contained all sequence alterations described for strain 7, in addition had two extra silent mutations. Hence, a total of 13 missense and 12 silent mutations and one triplet substitution (CCC) were found in KOS 321 (Table 3 and Fig. 1).
Cell surface expression of a gG-1 epitope in the HSV-1 strains.
When the HSV-1 isolates were investigated for the presence of a type-specific epitope on gG-1, evaluated by reactivity of a gG-1-specific MAb by ELISA of infected GMK cells, the two clinical isolates 7 and 9, as well as the reference strain KOS 321, showed no reactivity at all (Table 4) in parallel with the HSV-2 reference strain 333. As a likely explanation, all three HSV-1 strains contained the F111→V mutation situated within the previously mapped AFPL epitope of the MAb (31). In contrast, the gG-1 MAb showed high reactivity to all of the other eight clinical isolates as well as to the reference strain F (Table 4).
TABLE 4.
Absorbance values from an ELISA after binding of HSV type-specific MAbs and purified human anti-gG-1 antibodies to GMK cells infected with different HSV strains
| HSV-1 strains or clinical isolate | Mean
absorbance ± SD
|
|||
|---|---|---|---|---|
| Anti-gG-1 MAb | Purifieda anti-gG-1 antibodies | Anti-gC-1 MAb | Anti-gG-2 MAb | |
| 1 | 1.30 ± 0.16 | 0.71 ± 0.10 | 2.34 ± 0.20 | 0.12 ± 0.01 |
| 2 | 0.90 ± 0.07 | 0.58 ± 0.06 | 2.15 ± 0.03 | 0.12 ± 0.00 |
| 3 | 1.20 ± 0.05 | 0.54 ± 0.01 | 2.52 ± 0.09 | 0.14 ± 0.00 |
| 4 | 1.60 ± 0.10 | 0.85 ± 0.07 | 2.86 ± 0.04 | 0.12 ± 0.02 |
| 5 | 1.20 ± 0.01 | 0.67 ± 0.07 | 1.98 ± 0.08 | 0.13 ± 0.01 |
| 6 | 1.00 ± 0.01 | 0.70 ± 0.02 | 2.16 ± 0.17 | 0.14 ± 0.00 |
| 7 | 0.14 ± 0.01 | 0.55 ± 0.02 | 2.10 ± 0.16 | 0.12 ± 0.01 |
| 8 | 1.60 ± 0.09 | 0.80 ± 0.11 | 2.84 ± 0.12 | 0.13 ± 0.01 |
| 9 | 0.13 ± 0.01 | 0.60 ± 0.02 | 2.47 ± 0.09 | 0.13 ± 0.01 |
| 10 | 1.40 ± 0.02 | 0.60 ± 0.03 | 2.63 ± 0.05 | 0.13 ± 0.01 |
| F | 1.30 ± 0.02 | 0.83 ± 0.12 | 2.41 ± 0.07 | 0.12 ± 0.01 |
| KOS 321 | 0.15 ± 0.01 | 0.52 ± 0.05 | 1.85 ± 0.11 | 0.13 ± 0.01 |
| HSV-2 (333) | 0.20 ± 0.01 | 0.17 ± 0.01 | 0.12 ± 0.01 | 2.36 ± 0.20 |
| Uninfected cells | 0.10 ± 0.01 | 0.11 ± 0.01 | 0.11 ± 0.01 | 0.11 ± 0.00 |
Serum from patient 5.
Reactivities to human polyclonal anti-gG-1 antibodies.
To investigate gross strain differences in the expression of epitopes reactive with human antibodies, purified anti-gG-1 antibodies from one patient (patient 5) were assayed for reactivities to all herein-studied HSV-1 strains. This patient carried an HSV-1 strain displaying a normal gG-1 sequence, and her unpurified serum sample showed strong reactivity in a gG-1 ELISA (Table 1). Due to the small amount of gG-1 antibodies present in human sera and possible losses during extraction (31), the purified antibodies from patient 5 in general showed relatively low reactivities. In addition, the infected-cell assay depends on the expression of gG-1 by different HSV-1 strains, and in all cases, a relatively low expression of this protein was noted in comparison with that of gC-1, as could be deduced from the respective MAb signals (Table 4). However, all HSV-1 strains, including KOS and clinical isolates 7 and 9, showed clear reactivities with the purified human anti-gG-1 antibodies (Table 4). In contrast, strain 333 showed no reactivity, confirming a type-specific behavior of purified human gG-1 antibodies, even when they are purified from a patient showing antibodies also to gG-2 (Table 4).
To further investigate the influence of gG-1 mutations on the seroreactivities of purified anti-gG-1 antibodies, sera from patients 1, 7, and 9 were tested in addition to serum from patient 5 for reactivities to isolates 2, 5, 7, and 9. As a positive control, we included a MAb reactive with gB, which gave high and comparable absorbance values to those of all strains tested. The DNA sequence of the gG-1 gene in the HSV-1 isolates from patients 1 and 2 were identical to the consensus sequence, while isolates 7 and 9 carried several mutations within the immunodominant epitope region (see above). The purified antibodies were sensitive to freezing and thawing since the sample from patient 5 gave a somewhat lower reactivity in two repetitions (data from one of these experiments is shown in Table 5) than it did in the previous experiments (Table 4).
TABLE 5.
Absorbance values from an ELISA after binding of purified human anti-gG-1 antibodies to GMK cells infected with different HSV strainsa
| Isolate or strain | Mean absorbance ± SD
|
||||
|---|---|---|---|---|---|
| Patient 1 serum | Patient 5 serum | Patient 7 serum | Patient 9 serum | Anti-gB MAb | |
| 2 | 0.40 ± 0.03 | 0.49 ± 0.01 | 0.16 ± 0.01 | 0.23 ± 0.01 | 2.4 ± 0.10 |
| 5 | 0.27 ± 0.01 | 0.44 ± 0.04 | 0.13 ± 0.01 | 0.18 ± 0.01 | 2.2 ± 0.20 |
| 7 | 0.14 ± 0.01 | 0.40 ± 0.01 | 0.14 ± 0.01 | 0.19 ± 0.01 | 1.9 ± 0.20 |
| 9 | 0.40 ± 0.01 | 0.45 ± 0.01 | 0.15 ± 0.01 | 0.19 ± 0.01 | 2.3 ± 0.10 |
| KOS 321 | 0.14 ± 0.01 | 0.40 ± 0.02 | 0.12 ± 0.01 | 0.15 ± 0.01 | 2.0 ± 0.20 |
| Syn 17+ | 0.44 ± 0.01 | 0.52 ± 0.01 | 0.14 ± 0.01 | 0.22 ± 0.01 | 2.8 ± 0.10 |
| F | 0.52 ± 0.12 | 0.54 ± 0.05 | 0.13 ± 0.01 | 0.19 ± 0.01 | 2.1 ± 0.30 |
| Uninfected cells | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.02 |
A MAb reactive with gB was used as a positive control.
The results with purified antibodies from patients 7 and 9 showed low reactivities to all tested strains which could not be discriminated from the background of seroreactivity to uninfected cells (absorbance, 0.17 ± 0.04 versus 0.1 ± 0 [means ± standard deviations]). However, purified antibodies from both patients 1 and 5 gave clear reactivities to most strains (absorbance, 0.39 ± 0.13), with the exception of serum from patient 1, which did not react to strain KOS and strain 7.
DISCUSSION
A prerequisite for using gG-1 as a type-specific antigen for serology is the genetic stability of the gene coding for this protein, or at least of its immunogenic regions, in clinical isolates. We found that KOS 321, a strain widely used in different laboratories, surprisingly contained 25 mutations, of which 13 were missense and 1 was a triplet insertion. In addition, 2 of 11 investigated clinical isolates contained mutations resembling those found in KOS 321. One clinical isolate (isolate 7) showed all of the 13 missense mutations and the triplet insertion found in KOS 321 but lacked two silent mutations of the latter. Another clinical isolate (isolate 9) contained 6 of the 13 KOS 321 missense mutations. Since the rest of the analyzed sequences were almost completely conserved, the mutations represented by strains KOS 321, 7, and 9 may suggest a restricted genetic variability of the gG-1 gene outside the genotypic variability represented by KOS 321.
In a previous study, strains isolated from oral lesions exhibited a low genetic variability while the subclinical isolates derived from immunosuppressed patients diverged substantially (29). Although sequence information regarding the gG-1 gene in clinical isolates is currently lacking, data on the genetic variability of other HSV glycoproteins are accumulating. When the gB, gC, and gD genes were sequenced from five isolates of HSV-2, they were generally found to be conserved but different allelic forms were detected (30). Likewise, PCR-generated gB sequences amplified directly from the CSF of patients with encephalitis showed different alleles, especially in the amino-terminal part of the protein (26). In comparison to the minor sequence alterations reported in these studies, the herein described missense mutations of the gG-1 genes in strains KOS 321, 7, and 9 seem to be an uncommon finding.
In neither of the above-mentioned studies could the HSV variants be associated with a particular site of the body or with virulence properties. Likewise, of the gG-1 variants found in our study, one was isolated from the neck of a patient but the other was an ordinary oral isolate. In contrast, our HSV-1 strains derived from the genital tract as well as from CSF showed concensus sequences in their gG-1 genes. Hence, it seems unlikely that the gG-1 variants described here are related to properties of tropism or virulence. In another study describing allelic variants of gB in human herpesvirus 7, the differences were suggested to be related to the geographical origin of the host (12).
One of the missense mutations (F111→V) which were detected in KOS 321 and the two clinical isolates with KOS-like gG-1 sequences was localized within the epitope of a previously mapped anti-gG-1 MAb (31). Accordingly, these three strains showed absorbance values similar to the background value when reactivity was determined by ELISA of cells infected with the respective strains. These results confirmed the previous epitope mapping of the gG-1 MAb to 110AFPL114, of which F111 in mutational analysis of the reactive peptides was found to be of key importance for binding of the MAb, since substitution of any other amino acid for phenylalanine abolished reactivity (19). In addition, this finding indicates that this gG-1 MAb, although reactive with an HSV-1-specific domain of the type-specific gG-1 protein, would not be suitable for typing of HSV-1 isolates due to the genetic variability of the epitope region. However, although the mutated strains showed a slightly lower reactivity to a purified human anti-gG-1 serum, all three viruses were clearly reactive with the polyclonal antibodies derived from the patient harboring a strain with no alteration in the gG-1 sequence.
These findings of the genetic variability of the gG-1 gene raise the question of whether the antibody response to gG-1 is altered in these patients, which might lead to false-negative results in serological assays in which the gG-1 protein is the only antigen. The selected study population with symptomatic, recurrent herpes simplex infections generally showed strong antibody responses both to the type-common membrane antigen and to recombinant gG-1 by ELISA. Also, sera from the two patients (7 and 9) harboring the gG-1 mutation strains displayed a clear reactivity in this assay and would hence not become false-negative in HSV-1 serotesting. In the patients tested in this study, reactivities to remaining epitopes were evidently sufficiently strong to produce a positive reaction in an assay testing reactivity of whole sera with relatively large amounts of gG-1.
However, when gG-1 antibodies, purified against the consensus sequence gG-1 antigen, from patients 7 and 9, who carry gG-1 mutation strains, were tested on cells infected with a variety of strains, the reactivities did not differ from the background. This finding indicates a difference in the levels of antigen presentation of the two gG-1 variants, leading to lower yields of purified gG-1-reactive antibodies from the sera of patients carrying KOS-like variants. This antigenic difference between the gG-1 variants might have consequences for serotesting. Although an initial report indicated a sensitivity of 94% for patients with recurrent oral herpes (18) by a highly efficient gG-1 immunodot assay, a recent study has indicated a lower sensitivity (89%) when gG-1 was used as the EIA antigen for testing of sera collected from isolation-proven patients (14). In the latter study, the performance of the gG-1 EIA was clearly worse than that of the gG-2 EIA for HSV-2-specific serology. Another problem recently reported with the gG-1 ELISA is an inconsistency of results from serial samples from the same patient (25). The eventual role of viral genetic variability in these limitations in the use of gG-1 as a seroantigen may be considered.
We suggest that, in addition to influence from a possibly lower IgG response due to low expression of gG-1 during natural infection, the lower sensitivity of gG-1-based seroassays might be influenced by the variability of the gene coding for this protein among clinical isolates. It should be of interest to perform further sequencing of the gG-1 gene in asymtomatically shedded viruses isolated from immunosuppressed patients and to define the seroresponses to this protein in this category of hosts as part of attempts to improve HSV-1-specific serology.
ACKNOWLEDGMENTS
We thank Maria Johansson for technical advice.
Financial support was received from the Swedish Medical Research Council (grant 11225), the LUA Foundation at the Sahlgren’s University hospital, the Göteborg, Medical Society of Göteborg, Sweden, and the Swedish Society for Medical Research.
REFERENCES
- 1.Ashley R L, Benedetti J, Corey L. Humoral immune response to HSV-1 and HSV-2 viral proteins in patients with primary genital herpes. J Med Virol. 1985;17:153–166. doi: 10.1002/jmv.1890170208. [DOI] [PubMed] [Google Scholar]
- 2.Ashley R L, Militoni J, Lee F, Nahmias A, Corey L. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunoblot enzyme assay for detecting antibodies to herpes simplex virus type 1 and 2 in human sera. J Clin Microbiol. 1988;26:662–667. doi: 10.1128/jcm.26.4.662-667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashley R L, Wu L, Pickering J W, Tu M C, Schnorenberg L. Premarket evaluation of a commercial glycoprotein G-based enzyme immunoassay for herpes simplex virus type-specific antibodies. J Clin Microbiol. 1998;36:294–295. doi: 10.1128/jcm.36.1.294-295.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aurelius E, Johansson B, Sköldenberg B, Staland Å, Forsgren M. Rapid diagnosis of herpes simplex encephalitis by nested polymerase chain reaction assay of cerebrospinal fluid. Lancet. 1991;337:189–192. doi: 10.1016/0140-6736(91)92155-u. [DOI] [PubMed] [Google Scholar]
- 5.Balachandran N, Oba D E, Hutt-Fletcher L. Antigenic cross-reactions among herpes simplex viruses types 1 and 2, Epstein-Barr virus, and cytomegalovirus. J Virol. 1987;61:1125–1135. doi: 10.1128/jvi.61.4.1125-1135.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergström T, Andersen O, Vahlne A. Isolation of herpes simplex type 1 during first attack of multiple sclerosis. Ann Neurol. 1989;26:283–285. doi: 10.1002/ana.410260218. [DOI] [PubMed] [Google Scholar]
- 7.Bergström T, Trybala E. Antigenic differences between HSV-1 and HSV-2 glycoproteins and their importance for type-specific serology. Intervirology. 1996;39:176–184. doi: 10.1159/000150493. [DOI] [PubMed] [Google Scholar]
- 8.Bergström T, Sjögren-Jansson E, Jeansson S, Lycke E. Mapping neuroinvasiveness of the herpes simplex virus encephalitis-inducing strain 2762 by the use of monoclonal antibodies. Mol Cell Probes. 1992;6:41–49. doi: 10.1016/0890-8508(92)90070-E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eis-Hubinger A M, Kleim J P, Mohr K, Schneweis K E. A related epitope is consistently present on glycoprotein C of herpes simplex virus type 1 and 2. Acta Virol. 1991;35:276–281. [PubMed] [Google Scholar]
- 10.Elion G B. Mechanism of action and selectivity of acyclovir. Am J Med. 1982;73:7–13. doi: 10.1016/0002-9343(82)90055-9. [DOI] [PubMed] [Google Scholar]
- 11.Frank R. Spot-synthesis: an easy technique for the positionally addressable, parallel chemical synthesis on a membrane support. Tetrahedron. 1992;48:9217–9232. [Google Scholar]
- 12.Franti M, Aubin J T, Poirel L, Gautheret-Dejean A, Candotti D, Huraux J M, Agut H. Definition and distribution analysis of glycoprotein B gene alleles of human herpesvirus 7. J Virol. 1998;72:8725–8730. doi: 10.1128/jvi.72.11.8725-8730.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forsgren M, Skoog E, Jeansson S, Olofsson S, Giesecke J. Prevalence of antibodies to herpes simplex virus in pregnant women in Stockholm in 1969, 1983 and 1989: implications for STD epidemiology. Int J STD AIDS. 1994;5:113–116. doi: 10.1177/095646249400500207. [DOI] [PubMed] [Google Scholar]
- 14.Hashido M, Lee F K, Inouye S, Kawana T. Detection of herpes simplex virus type-specific antibodies by an enzyme-linked immunosorbent assay based on glycoprotein G. J Med Virol. 1997;53:319–323. doi: 10.1002/(sici)1096-9071(199712)53:4<319::aid-jmv2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 15.Ho D W, Field P R, Sjögren-Jansson E, Jeansson S, Cunningham A L. Indirect ELISA for the detection of HSV-2 specific IgG and IgM antibodies with glycoprotein G (gG-2) J Virol Methods. 1992;36:249–264. doi: 10.1016/0166-0934(92)90056-j. [DOI] [PubMed] [Google Scholar]
- 16.Ho D W, Field P R, Irving W L, Packham D R, Cunningham A L. Detection of immunoglobulin M antibodies to glycoprotein G-2 by Western blot (immunoblot) for diagnosis of initial herpes simplex virus type 2 genital infections. J Clin Microbiol. 1993;31:3157–3164. doi: 10.1128/jcm.31.12.3157-3164.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kramer A, Schuster A, Reineke U, Malin R, Volkmer-Engert R, Landgraf C, Schneider-Mergener J. Combinatorial peptide libraries: screening tools for the identification of peptides that bind ligands with predefined specificity. Methods (Orlando) 1994;6:388–395. [Google Scholar]
- 18.Lee F K, Pereira L, Griffin C, Reid E, Nahmias A J. A novel glycoprotein for detection of herpes simplex virus type 1-specific antibodies. J Virol Methods. 1986;14:111–118. doi: 10.1016/0166-0934(86)90041-8. [DOI] [PubMed] [Google Scholar]
- 19.Liljeqvist, J. Å. Personal communication.
- 20.Liljeqvist J Å, Trybala E, Svennerholm B, Jeansson S, Sjögren-Jansson E, Bergström T. Localization of type-specific epitopes of herpes simplex virus type 2 glycoprotein G by human and mouse antibodies. J Gen Virol. 1998;79:1215–1224. doi: 10.1099/0022-1317-79-5-1215. [DOI] [PubMed] [Google Scholar]
- 21.Liljeqvist J Å, Svennerholm B, Bergström T. Typing of clinical herpes simplex virus type 1 and 2 isolates with monoclonal antibodies. J Clin Microbiol. 1999;37:2717–2718. doi: 10.1128/jcm.37.8.2717-2718.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGeoch D J, Dolan A, Donald S, Rixon F J. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type 1. J Mol Biol. 1985;181:1–13. doi: 10.1016/0022-2836(85)90320-1. [DOI] [PubMed] [Google Scholar]
- 23.Nahmias A J, Dowdle W R. Antigenic and biological differences in herpesvirus hominis. Prog Med Virol. 1968;10:119–159. [PubMed] [Google Scholar]
- 24.Nilheden E, Jeansson S, Vahlne A. Typing of herpes simplex virus by an enzyme-linked immunosorbent assay with monoclonal antibodies. J Clin Microbiol. 1983;17:677–680. doi: 10.1128/jcm.17.4.677-680.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmid D S, Brown D R, Nisenbaum R, Burke R L, Alexander D, Ashley R L, Pellett P E, Reeves W C. Limits in reliability of glycoprotein G-based type-specific serologic assays for herpes simplex virus types 1 and 2. J Clin Microbiol. 1999;37:376–379. doi: 10.1128/jcm.37.2.376-379.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sivadon V, Lebon P, Rozenberg F. Variations of HSV-1 glycoprotein B in human herpes simplex encephalitis. J Neurovirol. 1998;4:106–114. doi: 10.3109/13550289809113488. [DOI] [PubMed] [Google Scholar]
- 27.Studahl, M., L. Hagberg, E. Rekabdar, and T. Bergström. Herpesvirus DNA detection in CSF samples—differences in clinical presentation between alpha-, beta-, and gamma-herpesviruses. Scand. J. Infect. Dis., in press. [DOI] [PubMed]
- 28.Svennerholm B, Olofsson S, Jeansson S, Vahlne A, Lycke E. Herpes simplex virus type-selective enzyme-linked immunosorbent assay with Helix pomatia lectin-purified antigens. J Clin Microbiol. 1984;19:235–239. doi: 10.1128/jcm.19.2.235-239.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terasaki S I. Latent multiple infections by herpes simplex virus type 1. Kurume Med J. 1996;43:127–136. doi: 10.2739/kurumemedj.43.127. [DOI] [PubMed] [Google Scholar]
- 30.Terhune S S, Coleman K T, Sekulovich R, Burke R L, Spear P G. Limited variability of glycoprotein gene sequences and neutralizing targets in herpes simplex virus type 2 isolates and stability on passage in cell culture. J Infect Dis. 1998;178:8–15. doi: 10.1086/515590. [DOI] [PubMed] [Google Scholar]
- 31.Tunbäck, P., J. Å. Liljeqvist, G. B. Löwhagen, and T. Bergström. Glycoprotein G of herpes simplex virus type 1—identification of type-specific human epitopes. Submitted for publication. [DOI] [PubMed]
- 32.Zweig M, Showalter S D, Bladen S V, Heilman C J, Hampar B. Herpes simplex virus type 2 glycoprotein gF and type 1 glycoprotein gC have related antigenic determinants. J Virol. 1983;47:185–192. doi: 10.1128/jvi.47.1.185-192.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]