Abstract
The emergence of the acute respiratory syndrome coronavirus 2 variant named Omicron has become a global concern. A 74-year-old unvaccinated patient was critically ill infected with the Omicron variant characterised by septic shock, large-scale cerebral embolism, deep vein thrombosis, and multiple organ dysfunction with respiratory failure, acute renal failure, coagulation dysfunction. The clinical symptoms were successfully controlled by active rescue treatment such as anti-infection, anti-shock, implantation of a vena cava filter as well as multi-organ function support. Although there are many complications in critically ill patients with Omicron variant infections, especially coagulation disorders and thrombosis, they can be resolved with a combination of Chinese and Western medicine positive rescue.
Keywords: Omicron, Thrombosis, Septic shock, Critical care
Introduction
Following the outbreak of the novel coronavirus disease 2019 (COVID-19), a series of different types of acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants have appeared. The emerging super strain—B.1.1.529 Omicron which was first detected in November 2021 [1], is becoming the dominant variant worldwide because it is highly infectious. Compared with previous variants, Omicron is characterised by being more transmissible and striking antibody evasion [2]. However, Omicron infections have a 25% lower hospitalisation rate [3], a twofold lower mortality relative to the Delta variant [4], and are less severe. This report describes a critically ill patient with Omicron infection who survived after having septic shock, large-scale cerebral embolism, deep vein thrombosis (DVT), and multiple organ dysfunction.
Case report
A 74-year-old unvaccinated female was admitted to our hospital with a cough on March 20, 2022. The patient had been hospitalised in a medical institution for a long period due to underlying diseases, including left lower extremity disability for 60 years, right limb movement disorder and slurred speech for 3 years due to cerebral haemorrhage, and atrial fibrillation for more than 10 days. The main symptom of the patient who had close contact with Omicron-infected patients was dry cough for 5 days, and her first cycle threshold (Ct) values were 36.21 for nucleocapsid (N) gene, 33.68 for open reading frame (ORF) 1ab. Chest computerised tomography (CT) showed a few exudative lesions and pleural effusion in both lungs, indicative of inflammatory lesions ( Fig. 1A). As per the national protocol used for the diagnosis and treatment of COVID-19 [5], the severity of this patient was moderate. She was treated with arbidol hydrochloride tablets for antiviral therapy and Lianhua Qingwen capsules which had 11 herb components and was involved in T cell activation, viral receptors, and inflammatory responses pathways which were associated with antiviral and anti-inflammatory responses [6], [7].
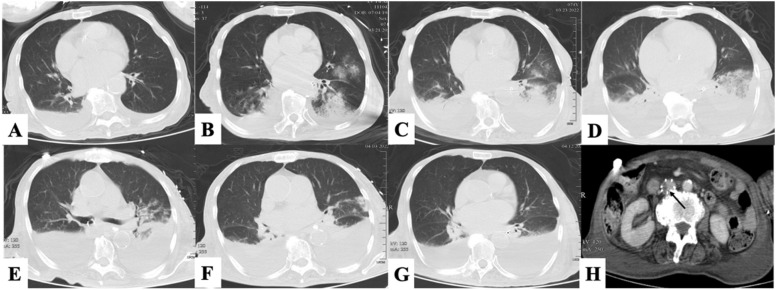
Fig. 1.
The pulmonary imaging of the patient. (A) Admission: There were a few exudative lesions and pleural effusion in both lungs. (B)The 2nd day: Obvious infiltration in both lungs. (C)The 4th day: Multiple infiltration, pulmonary consolidation and pleural effusion. (D) The 7th day: There was an absorption trend in the consolidation of the left lung, and the pleural effusion increased. (E) The 10th day: Both lung lesions improved. (F) The 14th day: The pulmonary lesions were improving. (G) The 24th day: Exudative foci in both lungs were basically absorbed, with a small amount of atelectasis and pleural effusion. (H) The 24th day: The filter in the inferior vena cava trapped thrombus (arrow).
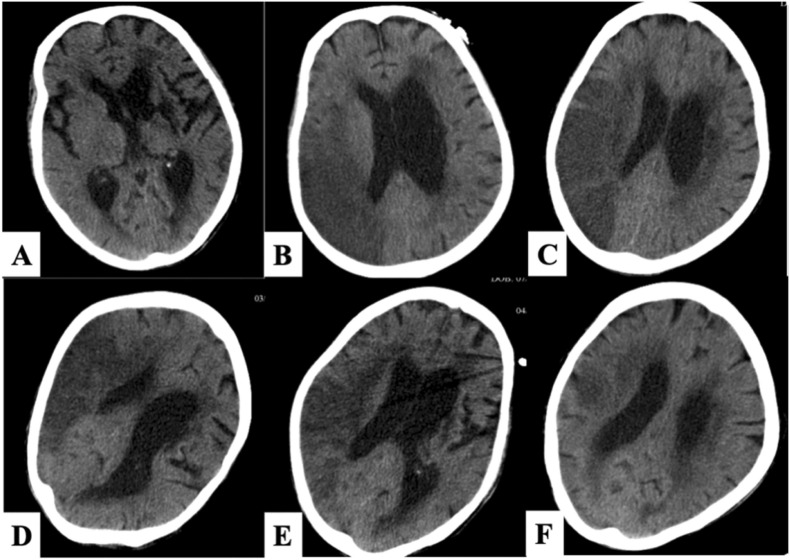
However, the patient’s condition deteriorated rapidly. The next day she developed a drowsy state and gradually became comatose, the score of Glasgow Coma Scale (GCS) was 3–4, bilateral pupils were about 3 mm with a sensitive light response, and Babinski sign was not elicited. Her body temperature rose to 38.6 °C with phlegm sounds in the larynx and shortness of breath; oxygen saturation dropped to 86% (50% oxygen concentration, venturi mask) with cold and cyanosis limbs. At the same time, her heart rate reached 180–200 beats per minute contributing to a downward trend of blood pressure. Review of blood test indicators indicated a significant decrease in white blood cells ( Table 1) and oxygen pressure was 58.8 mmHg. At that time, a flat CT scan of the head did not reveal cerebral haemorrhage or cerebral infarction ( Fig. 2A), and chest CT showed obvious increases in diffuse infiltration of the two lungs (Fig. 1B). Because of atrial fibrillation, she tended to have cerebral infarction which was responsible for aspiration pneumonia. It could not be ruled out that the infection of the Omicron variant was aggravated as Ct values decreased ( Table 2). We immediately performed endotracheal intubation, and the oxygenation index gradually improved from 80 to more than 200. Tracheoscopy showed hyperaemia and oedema of the mucous membrane of the main airway and left bronchi, with no obvious sputum or foreign body. According to changes in inflammatory indicators, she was successively given cefoperazone sodium and sulbactam sodium, meropenem combined with vancomycin anti-infection, and continued arbidol hydrochloride tablets. We also added methylprednisone. Simultaneously, the circulation of the patient fluctuated. After increased rehydration, norepinephrine was reduced from 1.0 ug/kg/min to 0.3 ug/kg/min but there was no significant improvement in the microcirculation status. Lactic acid (LAC) fluctuates trended upward (Table 1), and poor renal perfusion, manifested as anuria and severe metabolic acidosis, was treated with continuous renal replacement therapy (CRRT). In addition, cedilanid was used to improve heart rhythm, as well as other symptomatic treatments were administered. When the patient's circulatory oxygenation was stable, she could tolerate the risk of transport and a cranial CT showed embolism of the middle cerebral artery on the right side causing large cerebral infarction (Fig. 2B).
Table 1.
Changes in various indicators of the patient during the whole treatment.
| WBC (4.0–10.0 *10^9/L) |
NEU (50.0–70.0%) |
LY (0.8–4.0 *10^9/L) |
HB (130–175 g/L) |
PLT (100–300 *10^9/L) |
PT (12–15Sec) |
INR (0.91–1.22) |
APTT (30–43Sec) |
FIB (2–4 g/l) |
D2 (≤0.5 mg/l) |
ALT (4–48 U/L) |
TB (5.1–20.5umol/L) |
BUN (35–115 umol/L) |
CR (35–115 umol/l) |
CRP (0.00–5.00 mg/L) |
PCT (< 0.5 ng/ml) |
IL-6 (0–7 pg/ml) |
LAC (0.5–1.6 mmol/l) |
LDH (109–245 U/L) |
CKMB (5–25 U/L) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Admission | 4.2 | 85.4 | 0.42 | 120 | 179 | 13.3 | 1.01 | 34.3 | 3.30 | – | 11.4 | 14.9 | 7.79 | 47.7 | 5.8 | – | – | – | 176.6 | 4 |
| 2nd Day | 1.8 | 84.2 | 0.16 | 121 | 178 | 19.6 | 1.68 | 57.7 | 1.44 | 6.38 | 44.6 | – | 5.84 | 43.3 | – | – | – | 3.9 | 16.7 | 1.1 |
| 3rd Day | 1.1 | 71.4 | 0.26 | 91 | 70 | 19.6 | 1.68 | 57.7 | 1.44 | 3.12 | 6.7 | 14.1 | 6.14 | 53.5 | 64.7 | – | – | 8.1 | 128.3 | 5.8 |
| 4th Day | 4 | 84.1 | 0.59 | 77 | 49 | 17.9 | 1.49 | 67.3 | 2.11 | 1.46 | 29.7 | 27.3 | 3.19 | 30 | 172 | 18.59 | 2370.7 | 9.1 | 261.9 | 16.4 |
| 5th Day | 3.8 | 90.6 | 0.33 | 100 | 23 | 14.2 | 1.1 | 52.3 | 2.6 | – | 27.4 | 28.9 | 3.38 | 41.6 | 255.7 | 8.37 | 796.7 | 6.9 | 213.2 | 7.6 |
| 6th Day am | 2 | 89.5 | 0.18 | 82 | 16 | 13.1 | 0.99 | 58.1 | 2.16 | – | 19.7 | – | 5.8 | 62.1 | 191.9 | 7.02 | 531.2 | 5.7 | 225 | 6.7 |
| 6th Day pm | 2.5 | 83.1 | 0.35 | 97 | 71 | 13.7 | 1.03 | 46.3 | 2.03 | 6.12 | 46.4 | – | 6.92 | 62.8 | – | – | – | 5.1 | 532.8 | 22.5 |
| 7th Day | 4.6 | 87.5 | 0.42 | 100 | 45 | 13.7 | 1.05 | 39.6 | 1.98 | – | 42.2 | 46.7 | 8 | 74.8 | 95.6 | 4.03 | 256.2 | 3.3 | 268.5 | 12.3 |
| 8th Day | 9.9 | 91.6 | 0.37 | 101 | 26 | 15.3 | 1.21 | 38 | 1.41 | 4.09 | 34.3 | 24.3 | 11.81 | 72.3 | 49.7 | 2.15 | – | 2.6 | 331.8 | 4.9 |
| 9th Day am | 12.1 | 90.4 | 0.45 | 94 | 24 | 16 | 1.29 | 37.4 | 1.15 | 3.2 | 50.6 | 17.4 | 14.92 | 64.8 | 29.1 | – | – | 2.3 | 332.3 | 6.1 |
| 9th Day pm | 13.6 | 92.3 | 0.39 | 93 | 71 | 15.6 | 1.21 | 34.6 | 1.69 | – | 61.2 | – | 15.27 | 56.1 | – | – | – | 2.1 | 388.8 | 12.8 |
| 10th Day | 11.5 | 89.8 | 0.46 | 89 | 61 | 16.8 | 1.33 | 35.8 | 1.64 | 3.3 | 78 | 14.6 | 15 | 51.3 | 17.4 | 0.62 | 76.3 | 1.5 | 280.2 | 6.1 |
| 11th Day | 7.1 | 89.7 | 0.31 | 87 | 50 | 15.5 | 1.2 | 36.3 | 2.77 | 1.65 | 49.9 | 12 | 13.07 | 40 | 44.7 | 0.19 | – | 1.5 | 303.8 | 8 |
| 12th Day | 5.3 | 83.4 | 0.43 | 80 | 60 | 14.6 | 1.11 | 41.6 | 3.46 | 1.4 | 32.8 | 13.8 | 11.5 | 40 | 58.1 | 0.19 | 74.1 | 1.3 | 241.3 | 3 |
| 13th Day | 5.5 | 84.1 | 0.47 | 75 | 79 | 15.2 | 1.17 | 59.2 | 3.48 | 1.43 | 37.2 | 16.9 | 6.6 | 43.4 | 75.7 | 0.19 | 112.1 | 1.4 | – | – |
| 14th Day | 5.8 | 84.4 | 0.45 | 91 | 93 | 15.9 | 1.24 | 51.2 | 4.16 | 1.48 | 47.6 | 24 | 9.3 | 42.1 | 72.3 | <0.1 | 39.3 | 1.1 | 237.2 | 0.4 |
| 15th Day | 5.6 | 83.5 | 0.43 | 86 | 125 | 14.7 | 1.12 | 44.5 | 4.03 | 1.58 | 55.9 | 19.1 | 13.95 | 40.8 | 56.4 | <0.5 | 37.5 | 1.0 | 211.2 | 5 |
| 16th Day | 6.6 | 78.7 | 0.78 | 90 | 164 | 14 | 1.06 | 42.5 | 4.23 | 1.48 | 39.5 | 18.6 | 13.23 | 41.8 | 44.3 | <0.1 | 26.2 | 1.0 | 209.9 | 6.6 |
| 17th Day | 6.8 | 79.9 | 0.73 | 85 | 168 | 14.2 | 1.07 | 45.2 | 4.35 | 1.44 | 32.5 | 13.5 | 13.61 | 41.5 | 48.2 | 0.14 | 47.2 | 1.1 | 214.2 | 8.6 |
| 18th Day | 6.6 | 84.6 | 0.46 | 77 | 187 | 16.3 | 1.28 | 49.9 | 4.72 | 0.81 | 28.2 | 14.5 | 11.89 | 38.1 | 59.1 | 0.1 | 56.1 | 1.1 | 185.9 | 4.4 |
| 19th Day | 6.1 | 81.8 | 0.5 | 77 | 205 | 17.3 | 1.39 | 51.5 | 4.93 | 1.21 | 26.1 | 12.6 | 12.47 | 40.2 | 63.7 | <0.1 | 51.1 | 1.2 | 228.6 | 9.9 |
| 20th day | 5.7 | 74.1 | 0.93 | 91 | 205 | 16.6 | 1.31 | 46.2 | 4.8 | 0.97 | 25.9 | 17.6 | 13.06 | 42.3 | 60.9 | 0.1 | 23.5 | – | 202.3 | 8.1 |
| 21st day | 4.9 | 80.1 | 0.57 | 101 | 199 | 16.2 | 1.28 | 47.9 | 4.68 | 0.86 | 23.0 | 14.9 | 12.49 | 37.2 | 47.3 | <0.1 | 27.5 | 1.1 | 186.5 | 6.5 |
| 22nd Day | 6.0 | 80.3 | 0.71 | 106 | 192 | 15.6 | 1.21 | 47.6 | 5.01 | 0.82 | 25.9 | 11.2 | 11.55 | 31.7 | 45.8 | 0.14 | – | 1.2 | 219.8 | 9.3 |
| 23rd Day | 6.4 | 82.5 | 0.65 | 105 | 187 | 15.6 | 1.21 | 53.3 | 5.11 | 0.73 | 29.4 | 8.3 | 10.77 | 30.6 | 52.7 | 0.1 | – | 1.0 | 219 | 9.3 |
| 1st Month | 6.0 | 77.3 | 0.79 | 91 | 229 | 14.7 | 1.08 | 38.5 | 4.05 | 0.37 | 35.0 | 8.3 | 12.21 | 32.3 | 31.9 | – | – | 1.0 | 194.5 | 13.6 |
WBC white blood cell; NEU neutrophilic granulocyte percentage; LY lymphocyte; Hb haemoglobin; PLT platelet; PT prothrombin time;APTT activated partial thromboplastin time; INR international normalised ratio; FIB fibrinogen; D2 D-Dimer; ALT alanine aminotransferase; TB total bilirubin; BUN blood urea nitrogen; Cr creatinine; CRP C-reactive protein; PCT procalcitonin; IL-6 lnterleukin-6; LAC lactic acid; LDH lactate dehydrogenase; CKMB Creatine kinase isoenzyme; am Ante meridiem; pm Post meridiem.
Fig. 2.
The cranial imaging of the patient. (A) The 2nd day: There was no obvious cerebral haemorrhage or cerebral infarction. (C)The 4th day: Large cerebral infarction on the right side. (D) The 7th day: Increased area of cerebral infarction with cerebral oedema. (E) The 10th day: The area of cerebral infarction was stable. (F) The 14th day: Cerebral vascular recanalization and reduction of cerebral infarction area. (G) The 24th day: Intracranial condition further improved.
Table 2.
COVID-19 nucleic acid test and Serological test of the patient.
| E | N | ORF1ab | IgM | IgG | |
|---|---|---|---|---|---|
| Admission | 18.5 | 19.01 | 19.19 | – | – |
| 3rd Day | 15.43 | 15.89 | 16.5 | – | – |
| 5th Day | 11.67 | 14.18 | 14.06 | – | – |
| 6th Day | 11.78 | 11.51 | 13.42 | – | +-- |
| 7th Day | 10.39 | 9.37 | 12.93 | – | – |
| 8th Day | 23.95 | 24.46 | 23.93 | – | +-- |
| 9th Day | 20.24 | 20.52 | 20.18 | – | – |
| 10th Day | 21.32 | 21.74 | 21.83 | – | – |
| 11th Day | 25.56 | 26.14 | 26.33 | – | – |
| 12th Day | 21.24 | 23.11 | 20.9 | – | – |
| 13th Day | 24.44 | 26.17 | 26.93 | – | +-- |
| 14th Day | 18.89 | 20.07 | 20.57 | – | +- |
| 15th Day | 21.38 | 22.25 | 21.98 | – | +- |
| 16th Day | 30.67 | 31.57 | 31.69 | – | +-- |
| 17th Day | 28.26 | 30.09 | 29.52 | – | – |
| 18th Day | 32.02 | 32.83 | 32.79 | – | – |
| 19th Day | 32.02 | 32.83 | 32.79 | – | +-- |
| 20th day | 29.63 | 30.44 | 30.56 | – | – |
| 21st day | 31.76 | 32.17 | 32.2 | – | +-- |
| 22nd Day | 35.92 | 36.53 | 35.34 | – | – |
| 23rd Day | 37.08 | 38.6 | 37.73 | – | – |
N: nucleocapsid gene, E: envelope gene, ORF: open reading frame, IgG: immunoglobulin G, IgM immunoglobulin M, ‘-’: negative; ‘+-’: weak positive; ‘+--’: extremely weak positive.
Unfortunately, the patient had evident swelling of the right lower limb on the fifth day, and vascular ultrasound detected DVT, including the right common femoral vein, the right great saphenous vein, and the right external iliac vein. A vena cava filter was implanted on the same day, which trapped thrombus and effectively prevented pulmonary embolism (Fig. 1H). The healthy limb was treated with an air pressure pump, and mirabilite was applied in the elevated affected limb to relieve the water. At the same time, platelets were continuously decreased, and coagulation function was significantly abnormal, which might have led to the appearance of bloody sputum in the airways. According to the patient’s indicators, she was treated with platelets 10 U twice (on the 6th day and on the 9th day) and recombinant human thrombopoietin to increase platelets. She was given daily transfusions of plasma from COVID-19 vaccinators to improve coagulation and immune function. When the novel antiviral drug lopinavir/ritonavir became available, she underwent treatment for a total course of 5 days. Although the efficacy of the novel drug is debatable, and our treatment experience and the curative effect in Chinese people were very limited, we wanted to do as much as possible to save the patient's life. As the patient's condition improved with active rescue, we stopped CRRT and vasoactive drugs owing to improved circulatory status and renal perfusion. Next generation sequencing (NGS) of alveolar lavage suggested streptococcus pneumoniae infection; NGS of blood, sputum culture and blood culture was negative. Meropenem alone was used initially to fight infection, followed by piperacillin sodium and tazobactam sodium as inflammatory indicators improved.
After 14 days of treatment, the patient's overall condition was stable and her consciousness recovered with a GCS score of 5–6. Re-examination of cranial CT indicated that the area of cerebral infarction and cerebral oedema had improved (Fig. 2F). Chest CT indicated improvement in lung infection (Fig. 1F). Oedema of the extremities, especially the right lower extremity, had subsided. Based on the patient's stable circulatory status and normal coagulation function, a tracheostomy was performed. After 24 h, she was administered 10 mg Rivaroxaban orally for anticoagulation and there were no bleeding complications. SARS-CoV-2 testing was negative twice with a sampling interval of 24 h on the 23rd day of hospitalisation when the patient was transferred with a long-term tube to rehabilitation in a medical institution.
Discussion
The patient was critically ill infected with the Omicron variant characterised by septic shock, large-scale cerebral embolism, DVT, and multiple organ dysfunction with respiratory failure, acute renal failure, and coagulation dysfunction. The clinical symptoms were successfully controlled with active rescue treatment such as anti-infection, anti-shock, implantation of a vena cava filter as well as multi-organ function support.
There is high risk of developing venous and arterial thromboembolic complications in critically ill patients with COVID-19, causing dysfunction in multiple organs and independently associated with adverse outcomes. A systematic review and meta-analysis reported the pooled incidences of VTE and pulmonary embolism were 17% and 7.1% respectively for hospitalised patients with COVID-19; critically ill patients had significantly higher incidence of VTE than ward patients (27.9% vs 7.1%) [8]. Current research suggests that excessive inflammation, endothelial cell activation and injury, platelet activation, and hypercoagulability contribute to thromboembolic events in the COVID-19 patients [9]. D-dimer levels, fibrinogen levels and APTT, considered as good indicators to identify patients at high risk of VTE and predict disease severity, are recommended as biomarkers of risk of thrombosis during COVID-19 infection [10]. In this case, pro-inflammatory factors were activated as manifested by high levels of CRP, PCT, and IL-6. They were released into the circulatory system and played an essential role in endothelial damage as well as abnormal activation of platelets and coagulation. In the meanwhile, we found that the patient's platelets and fibrinogen significantly decreased, D-dimer increased, and PT was prolonged, which was a typical manifestation of disseminated intravascular coagulation (DIC). COVID-19 combined with sepsis shock was responsible for the consequence. According to the guidelines [11], we added a total of two times platelets. For the first time, we found platelets and fibrinogen dropped rapidly, D2 dimer rose obviously after she was given platelets 10 U. At the same time, the level of LAC remained high, and airway bleeding did not improve. Ultrasonography showed more thrombus in the lower limbs, and cranial CT showed a larger area of cerebral infarction. Combined with the lower Ct value in SARS-CoV-2 testing and decline of inflammation indicators, all of these indicated that the patient was in a state of persistent hypercoagulability, thrombosis aggravation and microcirculation disorder owing to the infection of Omicron. Given the large area of cerebral infarction in the acute stage, airway bleeding as well as low platelets, anticoagulation or antiplatelet therapy was not immediately commenced because of the extremely high risk of bleeding. However, the patient's platelets dropped below 20 again, fibrinogen decreased to 1.15, and the airway bleeding increased slightly. We had to replenish with platelets 10 U and fibrinogen 1 g, and closely monitor the dynamic changes of the indicators. Fortunately, with the Omicron infection under control, the patient’s platelet and coagulation function gradually stabilised, and LAC gradually returned to normal levels in this time.
Early anticoagulation is more conducive to the recovery of patients. According to the current recommendations [11], prophylactic dose low molecular weight heparin is recommended for all hospitalised COVID-19 patients in the absence of active bleeding or except when the platelet count is< 25 × 109/L or fibrinogen levels are< 0.5 g/L. The first apostasis that occurred in this patient was cerebral infarction which was considered as a complications of atrial fibrillation,but COVID-19 may have been an accomplice. Omicron infection may greatly increase the probability of cerebral embolism, and the formation of cerebral embolism, aspiration pneumonia, sepsis shock and VTE are more likely to cause hypercoagulability. Early therapeutic anticoagulation may break this vicious cycle. Based on the change in the patient's indicators after two platelet supplements, perhaps we should not have replenished platelets which may have exacerbated thrombosis. Airway bleeding, which was not mitigated, may be a symptom of severe infection and pulmonary microthrombus formation. When the patient was given rivaroxaban anticoagulation, D-dimer decreased to normal levels. Further studies should be conducted on blood supplements and its related effects. Antiplatelet therapy is controversial. The platelets of this patient with COVID-19 were activated and highly aggregated, which may promote systemic inflammation and coagulation. Consequently, antiplatelet therapy might benefit to antithrombotic and anti-inflammatory effects and is associated with reduced lung injury, intensive care unit needs, and mortality without increased bleeding. A recent prospective study [12] suggests that treatment with an antiplatelet agent may not provide improvement in organ support–free days within 21 days for critically ill patients with COVID-19. More studies are needed to demonstrate the effect of antiplatelet therapy.
Conclusion
There are many complications in critically ill patients with Omicron variant infections, especially coagulation disorders and thrombosis. Further studies are needed to clarify the optimal therapy for such patients.
Declaration of Competing Interest
The authors declare they have no conflict of interest.
References
- 1.Omicron variant: what you need to know. CDC. 〈https://www.cdc.gov/coronavirus/2019-ncov/variants/omicron-variant.html〉 [Accessed 15 December 2021].
- 2.Liu L., Iketani S., Guo Y., Chan J.F., Wang M., Liu L., et al. Striking antibody evasion manifested by the omicron variant of SARS-CoV-2. Nature. 2022;602(7898):676–681. doi: 10.1038/s41586-021-04388-0. [DOI] [PubMed] [Google Scholar]
- 3.Menni C., Valdes A.M., Polidori L., Antonelli M., Penamakuri S., Nogal A., et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet. 2022;7 doi: 10.1016/S0140-6736(22)00327-0. S0140-6736(22)00327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sigal A., Milo R., Jassat W. Estimating disease severity of Omicron and Delta SARS-CoV-2 infections. Nat Rev Immunol. 2022;12:1–3. doi: 10.1038/s41577-022-00720-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Health Commissionof the People's Republic of China, State Administration ofTraditional Chinese Medicine of the People's Republic of China Diagnosis and treatment plan for novel coronavirus pneumonia (trial version 9) China Med. 2022;04:481–487. doi: 10.3760/j.issn.1673-4777.2022.04.001. [DOI] [Google Scholar]
- 6.Shen X., Yin F. The mechanisms and clinical application of Traditional Chinese Medicine Lianhua-Qingwen capsule. Biomed Pharmacother. 2021;142 doi: 10.1016/j.biopha.2021.111998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ling X.Y., Tao J.L., Sun X., Yua B. Exploring material basis and mechanism of Lianhua Qingwen prescription against coronavirus based on network pharmacology. Chin Tradit Herb Drugs. 2020;51(07):1723–1730. doi: 10.7501/j.issn.0253-2670.2020.07.006. [DOI] [Google Scholar]
- 8.Jiménez D., García-Sanchez A., Rali P., Muriel A., Bikdeli B., Ruiz-Artacho P., et al. Incidence of VTE and bleeding among hospitalized patients with Coronavirus Disease 2019: a systematic review and meta-analysis. Chest. 2021;159(3):1182–1196. doi: 10.1016/j.chest.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorog D.A., Storey R.F., Gurbel P.A., Tantry U.S., Berger J.S., Chan M.Y., et al. Current and novel biomarkers of thrombotic risk in COVID-19: a consensus statement from the international COVID-19 thrombosis biomarkers colloquium. Nat Rev Cardiol. 2022;13:1–21. doi: 10.1038/s41569-021-00665-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salabei J.K., Fishman T.J., Asnake Z.T., Ali A., Iyer U.G. COVID-19 coagulopathy: current knowledge and guidelines on anticoagulation. Heart Lung. 2021;50(2):357–360. doi: 10.1016/j.hrtlng.2021.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.REMAP-CAP Writing Committeefor the REMAP-CAP Investigators, Bradbury C.A., Lawler P.R., Stanworth S.J., McVerry B.J., McQuilten Z., et al. Effect of antiplatelet therapy on survival and organ support-free days in critically Ill patients with COVID-19: a randomized clinical trial. JAMA. 2022;5(327(13)):1247–1259. doi: 10.1001/jama.2022.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]