Gene therapy has been a highly anticipated treatment for previously irreversible, blinding retinal disorders. In 2017, the US Food and Drug Administration approved the first gene therapy, voretigene neparvovec (VN), for treatment of retinal dystrophy caused by biallelic mutations in the RPE65 gene (OMIM 180069).1 Given the novel nature of gene therapy, it may be important to continue learning from this first group of treated patients. Herein, we present information gathered from the 6-year follow-up of a patient who was treated with VN.
Report of a Case |
A 9-year-old girl diagnosed as having Leber congenital amaurosis at age 6 months presented to the Harkness Eye Institute at Columbia University, New York, New York, for evaluation. There was no known ocular, medical, or family history of disease. Best-corrected visual acuities (BCVAs) were 20/400 OD and 20/150 OS. Mild horizontal nystagmus was observed. The anterior segment had no abnormalities, and dilated fundus examination revealed salt-and-pepper mottling. Short-wavelength fundus autofluorescence (SW-AF) imaging showed decreased signal with an absence of contrast between the optic nerve and the surrounding fundus. Quantitative fundus autofluorescence (qAF) was performed as previously described.2 Compared with a representative age-matched control patient, the qAF color-coded image revealed near absence of SW-AF in the patient (Figure 1A and B). Spectral-domain optical coherence tomography demonstrated outer retinal and retinal pigment epithelium (RPE) atrophy (Figure 2A and B). Specifically, the foveal region was preserved; however, nasal and temporal to the fovea, the ellipsoid zone was less distinct or absent (Figure 2A and B).
Figure 1. Quantitative Autofluoresence (qAF) Indicative of Visual Cycle Functioning Following RPE65 Gene Augmentation Therapy.
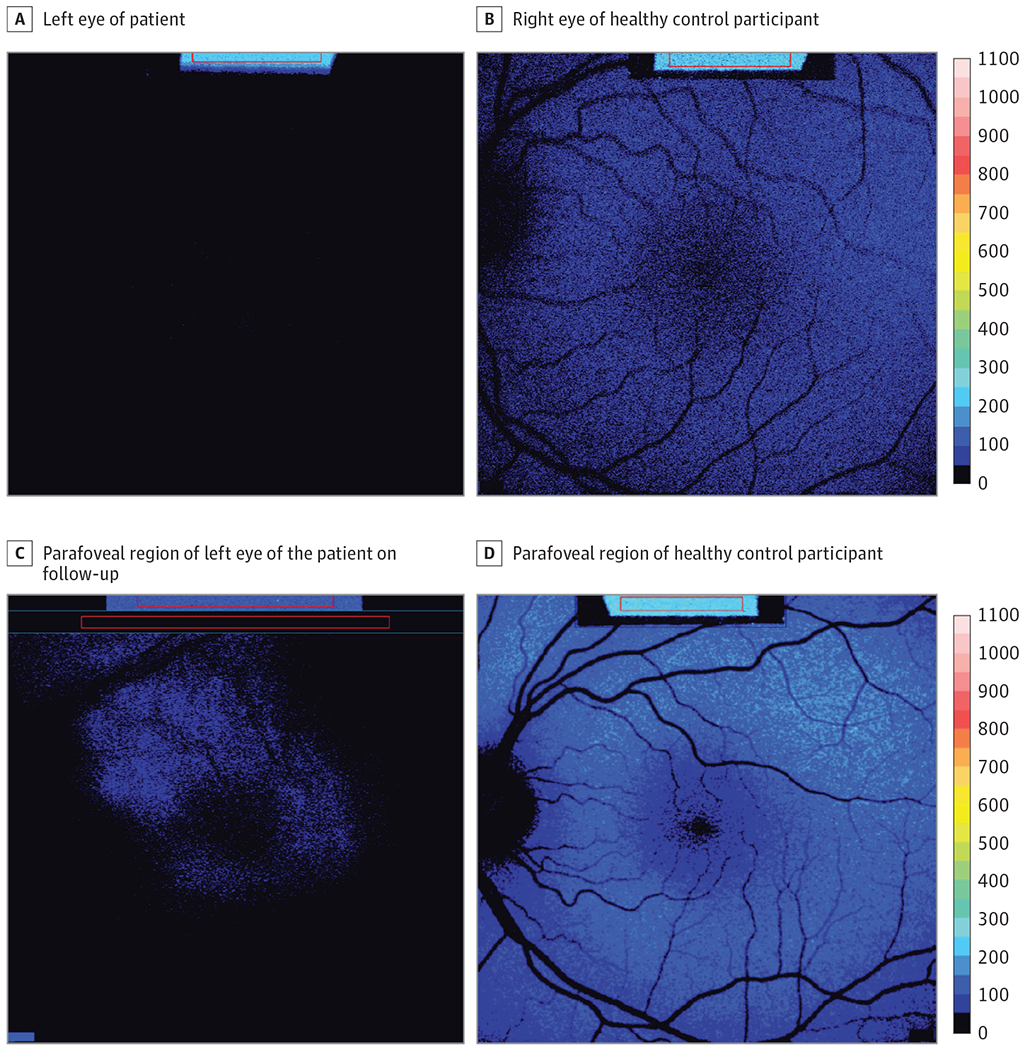
The qAF color-coded images acquired from the left eye of the patient (age 9 years) at the initial visit (A) exhibit a near absence of short-wavelength fundus autofluorescence (SW-AF) relative to the healthy eye of a representative control patient (B). Six years after receiving voretigene neparvovec subretinal injections, SW-AF signal is detected in the parafoveal region of the patient (C). Representative qAF color-coded image of healthy-eye control patient (D). The image in D is left-right reversed.
Figure 2. RPE65 Gene Augmentation Therapy.
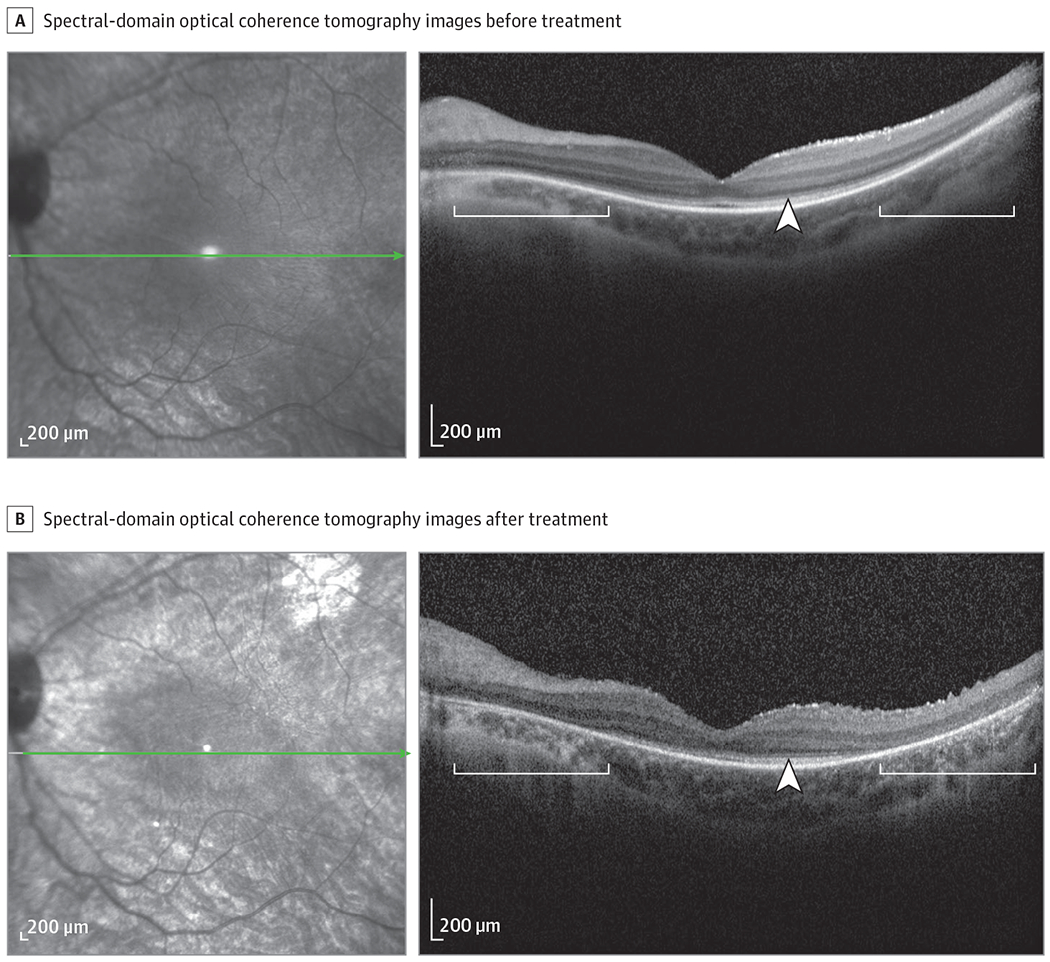
Spectral-domain optical coherence tomography (SD-OCT) scans acquired before (age 9 years) (A) and 6 years after treatment (age 18 years) (B). The axis and horizontal extent of the SD-OCT scan are indicated (green line) in the corresponding infrared reflectance image. Hypertransmission of SD-OCT signal (white brackets) and loss of ellipsoid zone band (white arrowheads) is increased in B relative to A. The green arrow in the infrared reflectance image A indicates the site of the AAV2 injection (B). The difference in quantitative autofluoresence intensity between the 9-year-old vs 10-year-old control patients is owing to the expected age-related increase.
The patient was referred for candidate gene testing at the John and Marcia Carver Nonprofit Genetic Testing Laboratory (Iowa City, Iowa), revealing biallelic mutations, p.Lys295Ter and p.His68Tyr, in the RPE65 gene. Two years later, the patient was enrolled in the VN therapy clinical trial and received treatment in the right eye followed 2 weeks later by the left. Six months after treatment, the patient noted subjective improvement in low-light environments and ability to perform daily functions. Two years postoperatively, BCVA in the right eye improved to 20/150. Notably, 6 years after gene augmentation therapy, qAF color-coded images revealed detectable levels of SW-AF with a relatively hyperautofluorescent parafoveal ring in the left eye (Figure 1C, blue speckled fluorescence) and right eye (not shown).2 The healthy eye of an age-matched control patient is shown for comparison (Figure 1D).
Discussion |
RPE65 plays a crucial role within the visual cycle by converting all-trans-retinyl ester to the 11-cis configuration.3 With RPE65 deficiency, 11-cis-retinal levels are profoundly reduced. Concomitantly, bisretinoids that form from random reactions of retinaldehyde form at low levels or not at all. These levels are evidenced by reduced or absent SW-AF signaling,4 an imaging modality that detects autofluorescence from bisretinoid adducts.
Voretigene neparvovec intervenes in this disease process. A recombinant adeno-associated viral vector is used to deliver the RPE65 gene and restore RPE65 synthesis.5 In this case report, we show by qAF analysis with illustration in color-coded images that the visual cycle has been established after VN therapy. Because the qAF approach uses nonnormalized images to avoid histogram stretch, we are able to compare serial SW-AF images acquired years apart. The establishment of a SW-AF signal in qAF color-coded images (Figure 1B and C) is evidence of visual cycle activity followed by nonenzymatic bisretinoid formation and accumulation in RPE as lipofuscin.2 The presence of SW-AF after 6-year follow-up is also indicative of the efficacy of VN as a therapeutic option.6To our knowledge, this is the first report to demonstrate the restoration and maintenance of the visual cycle following gene therapy, as indirectly evidenced by a bisretinoid fluorescent signal in qAF images.
Funding/Support:
This work was supported by National Institutes of Health grant EY024091 (Dr Sparrow) and by Foundation Fighting Blindness (Drs Tsang and Sparrow).
Conflict of Interest Disclosures:
Dr Mahajan reported other support from Spark Therapeutics outside the submitted work and being a surgeon for the phase III gene therapy trial. Dr Sparrow reported grants from National Eye Institute and Foundation Fighting Blindness during the conduct of the study. No other disclosures were reported.
Role of the Funder/Sponsor:
The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions:
We thank the patient for granting permission to publish this information.
Contributor Information
Sarah R. Levi, Department of Ophthalmology, Columbia University Irving Medical Center, New York, New York.
Jin Kyun Oh, Department of Ophthalmology, Columbia University Irving Medical Center, New York, New York.
Jose Ronaldo Lima de Carvalho, Jr, Department of Ophthalmology, Columbia University Irving Medical Center, New York, New York; Department of Ophthalmology, Hospital das Clínicas de Pernambuco, Empresa Brasileira de Serviços Hospitalares, Federal University of Pernambuco, Recife, Pernambuco, Brazil.
Vinit B. Mahajan, Byers Eye Institute, Stanford University, Palo Alto, California.
Stephen H. Tsang, Department of Ophthalmology, Columbia University Irving Medical Center, New York, New York; Department of Pathology and Cell Biology, Columbia University Medical Center, New York, New York.
Janet R. Sparrow, Department of Ophthalmology, Columbia University Irving Medical Center, New York, New York; Department of Pathology and Cell Biology, Columbia University Medical Center, New York, New York.
References
- 1.Maguire AM, Russell S, Wellman JA, et al. Efficacy, safety, and durability of voretigene neparvovec-rzyl in RPE65 mutation-associated inherited retinal dystrophy: results of phase 1 and 3 trials. Ophthalmology. 2019;126(9):1273–1285. doi: 10.1016/j.ophtha.2019.06.017 [DOI] [PubMed] [Google Scholar]
- 2.Sparrow JR, Duncker T, Schuerch K, Paavo M, de Carvalho JRL Jr. Lessons learned from quantitative fundus autofluorescence. Prog Retin Eye Res. 2020; 74:100774. doi: 10.1016/j.preteyeres.2019.100774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Redmond TM, Yu S, Lee E, et al. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20(4):344–351. doi: 10.1038/3813 [DOI] [PubMed] [Google Scholar]
- 4.Lorenz B, Wabbels B, Wegscheider E, Hamel CP, Drexler W, Preising MN. Lack of fundus autofluorescence to 488 nanometers from childhood on in patients with early-onset severe retinal dystrophy associated with mutations in RPE65. Ophthalmology. 2004;111(8):1585–1594. doi: 10.1016/j.ophtha.2004.01.033 [DOI] [PubMed] [Google Scholar]
- 5.Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358(21):2240–2248. doi: 10.1056/NEJMoa0802315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park KS, Lima de Carvalho JR Jr, Tsang SH. Sustained rescue of rod function and probable non-cell-autonomous rescue of cones after gene therapy. Ophthalmology. 2019;126(9):1286–1287. doi: 10.1016/j.ophtha.2019.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]


