ABSTRACT
Opisthorchiasis due to the liver fluke Opisthorchis felineus is highly prevalent in rural regions of Western Siberia, causing severe liver and bile duct maladies. Praziquantel administered as a three-dose regimen is the only drug used to treat O. felineus-infected individuals. A simpler single-dose treatment might serve as an alternative. The aim of this study was to compare the pharmacokinetic (PK) properties of single, ascending doses of praziquantel compared to multiple dosing in patients infected with O. felineus to contribute to updated treatment guidelines. Dried blood spots (DBSs) of 110 adults were collected at 11 time points post-drug administration at single oral doses of 20, 40, and 60 mg/kg, as well as 3× 20 mg/kg (4 h dosing interval). DBS samples were analyzed using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method, and PK parameters were obtained for R-, S-, and R-trans-4-OH-praziquantel employing noncompartmental analysis. We observed the highest drug exposure for all analytes when the triple-dose scheme was used; area under the concentration-time curve from 0 to 24 h (AUC0–24) values of 8.04, 27.75, and 36.38 μg/mL·h were obtained, respectively. Maximal plasma concentrations (Cmax) values of 1.72, 4.89, and 2.69 μg/mL were calculated for R-, S-, and R-trans-4-OH-praziquantel, respectively, when patients were given a single 60-mg/kg dose, and they peaked at 1.5 and 2 h for the enantiomers and at 3 h for the metabolite. The herein-generated PK data, together with results that will be obtained from the integrated efficacy study, lay the groundwork for a possibly optimized treatment scheme for O. felineus-infected patients.
KEYWORDS: praziquantel, pharmacokinetics, Opisthorchis felineus, dried blood spots, anthelmintic, LC-MS/MS, opisthorchiasis
INTRODUCTION
Opisthorchiasis is a major foodborne trematodiasis, which, upon dietary consumption of raw or undercooked fish infested with metacercariae of the liver fluke Opisthorchis felineus, affects the hepatobiliary system of humans (1). The prevalence is especially high in rural areas of Western Siberia (i.e., in the Russian Federation, Siberia, Ukraine, and Kazakhstan), where it is estimated that 12.5 million individuals are at risk of infection with O. felineus, of which 1.6 million are infected (2–4). Due to growing tourist activity and migration, it has been shown that the number of infected humans has increased and spread to new areas outside Western Siberia (5). Humans infected with O. felineus may present with mild or severe symptoms. Left untreated, opisthorchiasis can lead to severe hepatobiliary morbidities such as acute or chronic cholangitis, pancreatitis, cholecystitis, hepatic abscesses, and obstruction of the bile ducts (1). Of concern is the carcinogenic potential of O. felineus; evidence of a risk of acquiring cholangiocarcinoma (CCA) has been reported (5–7). In the Russian Federation, praziquantel is the only drug that is officially registered for the treatment of opisthorchiasis. According to clinical guidelines, different doses of praziquantel are suggested for the treatment of the infection with O. felineus. The detailed treatment scheme is divided into 3 stages, employing pretreatment, anthelmintic therapy (praziquantel), and rehabilitation; the whole cycle takes about 3 months to be accomplished. For adults, praziquantel treatment options are 50, 60, and 75 mg/kg, divided into 3 intakes with dose intervals of 4 to 6 h (8).
Praziquantel is available as a racemic mixture (R and S enantiomer) and is metabolized via the cytochrome P450 system to the main metabolite R-trans-4-OH-praziquantel (9). Recently, the activity of both the enantiomer, as well as the main metabolite, was studied in vitro, and it was shown that R-praziquantel exhibited the highest activity in both juvenile and adult worms of O. felineus (10). There are 17 metabolites of praziquantel that were identified using different in vitro and in vivo techniques, as summarized by Zdesenko and Mutapi in a recent review (9). Of these, eight are distinguishable mono-oxidized metabolites, two are dehydrogenated mono-oxidized metabolites, three are di-oxidized metabolites, and four are glucuronide metabolites. The metabolic pathway of praziquantel to its main metabolite, 4-OH-praziquantel, was described by Nleya and colleagues (11), further highlighting the favorable formation of trans-4-OH-praziquantel to the cis-4-OH-praziquantel isomer. However, it is not known what drug component (enantiomer[s] or metabolite[s]) is driving the efficacy of praziquantel in O. felineus-infected patients.
Metabolism and pharmacokinetic (PK) data of praziquantel are available in the context of schistosomiasis treatment, with a recent review presenting the variable drug exposure in humans and animal models (9). Other PK studies have been conducted in healthy volunteers and Opisthorchis viverrini-infected individuals from Lao PDR, respectively (12–14). However, to date, not a single PK study was conducted in O. felineus-infected individuals. These data would yield important information about optimized, simpler dosing strategies.
In the framework of a randomized, controlled, single-blinded dose-finding trial in Tomsk, Siberia, we conducted a PK study using dried blood spot (DBS) sampling with the aim of elucidating the PK parameters of praziquantel in O. felineus-infected adults in the different treatment groups. We analyzed the kinetic disposition of both praziquantel enantiomers and its main metabolite, R-trans-4-OH-praziquantel, using noncompartmental analysis (NCA).
RESULTS
Participants’ characteristics are summarized in Table 1. All participants were adults (78% female), aged 27 to 65, with a median body mass index (BMI) of 28. A total of 1,210 DBS samples were analyzed from 110 adults infected with O. felineus. Four hundred seventy-five measurements were under the lower limit of quantification (LLOQ) for R-praziquantel (39.3%), 258 were under the LLOQ for S-praziquantel (21.3%), and 112 were under the LLOQ for R-trans-4-OH-praziquantel (9.3%). Of the DBS samples, 0.7% were randomly chosen for incurred sample reanalysis. For R-praziquantel, 83% deviated less than 20% from the initial analyzed concentration; for S-praziquantel and R-trans-4-OH-praziquantel, 81% and 84% were within the acceptable range, respectively. The PK parameters are summarized in Table 2, and concentration versus time profiles of ascending doses are illustrated in Fig. 1 for all analytes.
TABLE 1.
Participant characteristics for subjects recruited in the Russian Federationa
| Characteristic | Data for patients given praziquantel dose (mg/kg) of: |
Total | |||
|---|---|---|---|---|---|
| 20 | 40 | 60 | 3 × 20 | ||
| No. of participants | 28 | 26 | 28 | 28 | 110 |
| Female (no. [%]) | 71 | 85 | 78 | 82 | 78 |
| Age (yrs) | 49 (39, 58) | 49 (40, 59) | 42 (36, 52) | 54 (47, 58) | 49 (41, 57) |
| Ht (cm) | 164 (158, 172) | 166 (158, 172) | 164 (157, 170) | 165 (160, 171) | 164 (158, 172) |
| Wt (kg) | 75 (66, 84) | 70 (62, 89) | 70 (65, 87) | 80 (74, 90) | 76 (65, 87) |
| BMI | 27 (24, 32) | 25 (23, 35) | 28 (24, 30) | 30 (26, 34) | 28 (24, 32) |
Results are shown as medians with first and third quartiles in parentheses unless indicated otherwise.
TABLE 2.
PK parameters of Praziquantel (PZQ) in O. felineus-infected patientsa
| Dose (mg/kg) | Analyte | Cmax (μg/mL) | AUC0–24h (μg·h/mL) | Tmax (h) | t1/2 (h) |
|---|---|---|---|---|---|
| 20 | R-trans-4-OH-PZQ | 1.18 (0.82–1.29) | 10.29 (6.73–12.32) | 2.50 (2.50–3.00) | 4.59 (3.40–6.37) |
| R-PZQ | 0.75 (0.26–1.28) | 1.38 (0.30–2.49) | 1.00 (0.50–2.00) | 1.21 (0.72–3.40) | |
| S-PZQ | 1.76 (1.01–2.63) | 3.79 (2.36–8.49) | 1.00 (0.50–2.00) | 2.29 (1.62–3.37) | |
| 40 | R-trans-4-OH-PZQ | 2.32 (1.69–2.57) | 19.89 (14.55–29.28) | 3.00 (2.50–3.00) | 5.07 (4.42–7.15) |
| R-PZQ | 1.06 (0.65–1.57) | 2.50 (1.61–4.65) | 1.50 (1.00–2.00) | 1.88 (1.06–2.34) | |
| S-PZQ | 2.91 (1.92–3.78) | 8.96 (5.94–15.18) | 1.50 (1.00–2.00) | 3.23 (1.71–4.09) | |
| 60 | R-trans-4-OH-PZQ | 2.69 (2.32–3.17) | 31.90 (24.54–37.33) | 3.00 (2.88–6.00) | 6.25 (4.82–8.14) |
| R-PZQ | 1.72 (1.18–3.32) | 5.91 (4.22–8.37) | 1.50 (1.00–2.00) | 2.42 (1.55–4.31) | |
| S-PZQ | 4.89 (3.37–6.58) | 19.80 (13.28–31.04) | 2.00 (1.50–2.50) | 3.29 (2.49–4.75) | |
| 3 × 20 | R-trans-4-OH-PZQ | 2.66 (1.98–3.12) | 36.38 (28.47–42.72) | 12.00 (10.00–12.00) | 6.19 (5.38–8.97) |
| R-PZQ | 1.20 (0.69–2.01) | 8.04 (4.23–12.09) | 6.00 (5.00–10.00) | 3.44 (3.02–3.82) | |
| S-PZQ | 3.35 (1.90–4.16) | 27.75 (13.54–37.65) | 5.50 (2.75–10.00) | 3.63 (3.32–4.34) |
Results are shown as medians with interquartile ranges.
FIG 1.
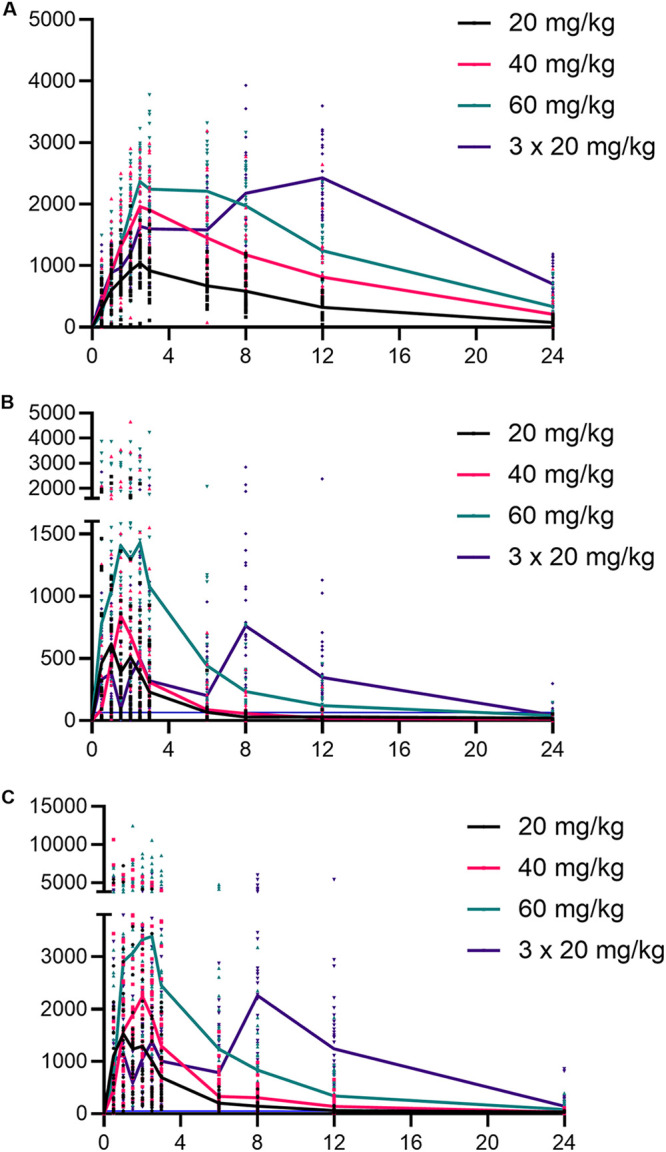
Concentration-time profiles of ascending doses of praziquantel. Graphs are shown for R-trans-4-OH-praziquantel (A), R-praziquantel (B), and S-praziquantel (C), respectively. Symbols capture individual values, while the curve describes the mean value. Table 1 additionally contains interquartile ranges of all PK parameters. The actual LLOQs were 64.5 ng/mL and 51.6 ng/mL for R- and S-praziquantel, respectively, and are indicated by a blue horizontal line.
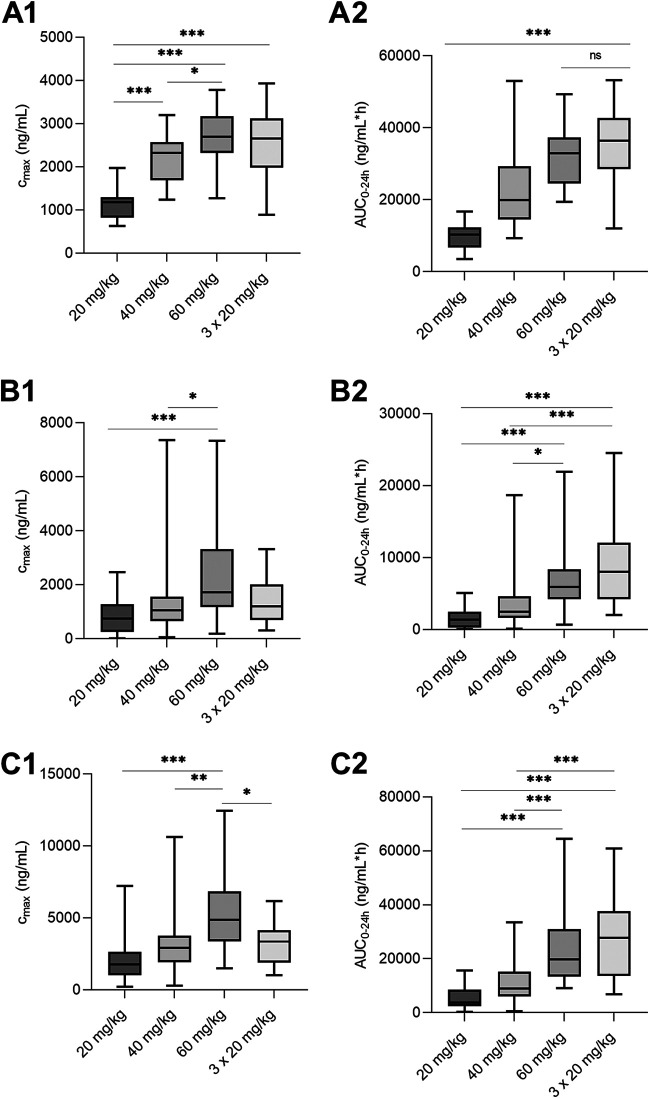
The highest maximal blood concentration (Cmax) values increased with ascending doses, and medians of 1.18, 2.32, and 2.69 μg/mL for R-trans-4-OH-praziquantel; 0.75, 1.06, and 1.72 μg/mL for R-praziquantel; and 1.76, 2.91, and 4.89 μg/mL for S-praziquantel, respectively, were obtained for patients treated with 20, 40, and 60 mg/kg praziquantel, respectively. At a single dose of 60 mg/kg, the Cmax values were observed for S-praziquantel, with a median of 4.89 μg/mL (interquartile range [IQR], 3.37 to 6.58 μg/mL), followed by R-trans-OH-praziquantel at 2.69 μg/mL (IQR, 2.32 to 3.17 μg/mL) and R-praziquantel at 1.72 μg/mL (IQR, 1.18 to 3.32 μg/mL). Patients treated with three doses of 20 mg/kg (total of 60 mg/kg) had lower median Cmax values (2.66, 1.20, and 3.35 for R-trans-4-OH-praziquantel, R-praziquantel, and S-praziquantel, respectively) than those receiving a single oral 60-mg/kg dose. The area under the concentration-time curve (AUC) also correlated with the dose; the highest values were obtained for the group of patients treated with three doses of 20 mg/kg. R-trans-4-OH-praziquantel had the highest AUC, with a median of 36.38 μg/mL (IQR, 28.47 to 42.72 μg/mL), followed by S-praziquantel with 27.75 μg/mL (IQR, 13.54 to 37.65 μg/mL) and R- praziquantel with 8.04 μg/mL (IQR, 4.23 to 12.09 μg/mL). The median time to reach Cmax (Tmax) values were similar in the three groups that received a single dose of praziquantel, ranging between 1 h and 3 h postdosing. However, Tmax values for all analytes were shifted to later time points when the 60-mg/kg dose was given in three single 20-mg/kg intakes (with 4 h intervals). The metabolite R-trans-4-OH-praziquantel reached Cmax at 12 h (IQR, 10.00 to 12.00 h) after the first dose. Large variations in Tmax values were observed for both enantiomers, with medians of 6 h (IQR, 5.00 to 10.00 h) and 5.5 h (IQR, 2.75 to 10.00 h) for R- and S-praziquantel, respectively. For all analytes, the statistical significance of Cmax and AUC was calculated between the treatment groups (Fig. 2), and a significant difference was found for both enantiomers as well as the metabolite when comparing a single 20-mg/kg dose versus a single 60-mg/kg dose.
FIG 2.
Cmax (A1, B1, and C1) and AUC0–24 (A2, B2, and C2) of ascending doses of praziquantel in O. felineus-infected individuals. Graphs are shown for R-trans-4-OH-praziquantel and R- and S-praziquantel, respectively (top down). The median and minimum to maximum are illustrated. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DISCUSSION
Praziquantel is the drug of choice for the treatment of opisthorchiasis. Clinical guidelines in the Russian Federation suggest the use of different multiple doses of praziquantel, yet it remains unclear which of the suggested doses to apply. Moreover, a single dose of praziquantel is the recommended treatment in preventive chemotherapy, which was shown to be efficacious in O. viverrini infections (15). To our knowledge, the dosing schema has never been truly optimized, and neither the correlation of dose and efficacy nor the PK behavior of praziquantel in O. felineus-infected patients has been studied to date. For the first time, we conducted a PK study in patients infected with O. felineus that were treated with different dosing schema of praziquantel: group 1 received a single 20-mg/kg dose, group 2 received a single 40-mg/kg dose, group 3 received a single 60-mg/kg dose, and group 4 was treated with three doses of 20 mg/kg given in intervals of 4 h. We studied the drug disposition of the two enantiomers, R- and S-praziquantel, as well as the R-trans-OH-praziquantel metabolite in a previously established and validated DBS method (16). Noncompartmental modeling was used in this study; however, the data could be integrated in a next step in a recently established population PK model based on data from O. viverrini-infected adults and Schistosoma mansoni- and Schistosoma haematobium-infected children (17).
High variability between individuals of the same treatment group was observed for all patient samples analyzed. Previous studies observed similar findings and attributed them to host factors such as the involvement of the cytochrome P450 system (CYP) in the first-pass metabolism of praziquantel, as well as external factors like drug-drug interactions or food intake (altered bioavailability) (9, 18, 19). Also, multiple dosages such as given to the participants of group 4 in our study can add to variability, mainly due to differences in the onset of absorption and elimination of the drug.
A previously published study by us examined the PK disposition of praziquantel in O. viverrini-infected patients (14). Nine patients were treated with three doses of 25 mg/kg (total of 75 mg/kg), and sampling was performed within a 24 h frame, similar to our study. Comparing the results of both groups of triple-dosed patients, we observed that the median Cmax values for R-praziquantel (1.20; IQR, 0.69 to 2.01 μg/mL) and S-praziquantel (3.35; IQR, 1.90 to 4.16 μg/mL) were approximately six and four times higher in the O. felineus-infected patients than patients infected with O. viverrini (R-praziquantel, Cmax, 0.2; IQR, 0.1 to 1.1 μg/mL; and S-praziquantel, Cmax, 0.9; IQR, 0.6 to 2.3 μg/mL). Similar observations were made for the AUC values. On the contrary, the metabolite R-trans-OH-praziquantel displayed a 2.5 to 5 times lower Cmax and AUC value, respectively, in the O. felineus cohort. This may be due to differences in genetic polymorphism of liver enzymes that are involved in the metabolism of praziquantel. In this respect, also, differences in ethnic populations may play a role (Lao PDR versus Russian Federation) (9). However, the sample size of both studies limits further interpretation. Half-life values of 3.3 and 6.4 h previously observed for S-praziquantel and R-trans-OH-praziquantel, respectively, in O. viverrini-infected patients is consistent with the half-life of S-praziquantel of 3.6 h and R-trans-OH-praziquantel of 6.2 h in our study (14).
Another study that examined the kinetic disposition of R-praziquantel, S-praziquantel, and metabolites in nine healthy volunteers after a single oral dose of 1,500 mg (corresponds to 25 mg/kg for an individual weighing 60 kg) observed a 2.5 to 3 times lower exposure of the enantiomers and the R-trans-OH-praziquantel metabolite than we did. Median AUC0–∞ values were 0.52, 1.54, and 4.63 μg/mL·h for R-praziquantel, S-praziquantel, and R-trans-OH-praziquantel, respectively, whereas O. felineus-infected patients in group 1 (single oral 20-mg/kg dose) had median AUC0–∞ values (not shown in Table 1) of 1.67, 4.29, and 10.81, respectively (12). A difference in drug exposure in infected versus noninfected individuals was suggested by several studies (20–22), observing higher exposure in infected patients. Our study supports these findings. However, irrespectively of the dose (and infection status), S-praziquantel exposure was approximately three times higher than R-praziquantel exposure in our study and thus corresponds to previous findings (12, 23–26). Finally, except for R-praziquantel, half-life values were longer in O. felineus-infected versus noninfected individuals. Although our study observed differences in exposure parameters compared to both O. viverrini-infected patients as well as healthy study subjects, it is not fully clear how these findings correlate with praziquantel’s efficacy. More studies (in animal models and opisthorchiasis patients) are needed with respect to connecting exposure values to the pathology of the disease. In particular, the location of the adult worms (biliary duct) needs to be considered, raising the question of whether Cmax or/and AUC levels need to be maintained in the plasma or in the biliary ducts to drive efficacy.
As observed in previous studies, patient samples had double peaks in the PK profiles. This may be due to a possible reuptake of the drug during the passage through the gastrointestinal tract as well as potential precipitation (17, 27). Additionally, from a technical point of view, we were surprised to get different curve shapes of R-trans-OH-praziquantel in patient DBS samples versus laboratory DBS samples that served as calibration line and quality control samples, respectively, and were prepared by spiking venous blood with the analytes. All patient samples had double peaks in the chromatogram, whereas laboratory samples displayed only a single peak. This may be a matrix effect due to (i) differences in composition of capillary versus venous blood (from donors), (ii) the first drop(s) of blood containing fat or other contaminants on the skin, or (iii) the drying of the DBS cards being influenced by temperature and humidity. Such phenomena have been previously reported for, e.g., the drug albendazole (28).
A limitation of our study is that the large majority of our study participants were female (78%) with a median BMI of 28. Gender and weight were found, for many drugs, to influence PK properties (29–31). Though, to our knowledge, no information is available on whether these factors influence praziquantel disposition, it is worth highlighting that the population in this study differs from populations in previous studies, which also might explain the differences observed. Further studies are necessary to elucidate sex-, weight-, and age-related differences in the pharmacokinetics of praziquantel.
Conclusion.
Using four different dosages of praziquantel, we have described the PK parameters in infected O. felineus patients in the Russian Federation. Exposure metrics, AUC, and Cmax were positively correlated with dose. While AUC was highest in the 3× 20-mg/kg dose, the 60-mg/kg dose presented the highest Cmax. Comparing our data with previously studied healthy volunteers, infection status seems to influence drug exposure. Our results in combination with the results from the efficacy study will be pivotal to establishing evidence-based dosing recommendations for praziquantel regarding O. felineus infections.
MATERIALS AND METHODS
Chemicals and reagents.
Racemic (rac) praziquantel was obtained from Sigma-Aldrich (Buchs, Switzerland). R-praziquantel, S-praziquantel, and R-trans-4-OH-praziquantel were the product of Merck KgaA (Darmstadt, Germany). The internal standard praziquantel-d11 was purchased from Toronto Research Chemicals (Toronto, Canada). LC-MS-grade chemicals and solvents, including acetonitrile, isopropanol, formic acid, ammonium formate, and ammonium acetate, were purchased from Sigma-Aldrich (Buchs, Switzerland). Water was purified using a Milli-Q Advantage A10 (Merck, Darmstadt, Germany) purification system. Whatman 903 Proteinsaver cards were purchased from Sigma-Aldrich (Buchs, Switzerland). Human blood for preparation of calibrators and quality control (QC) samples was obtained in lithium heparin-coated vacutainer tubes from the local blood donation center (Basel, Switzerland). Praziquantel tablets (Biltricide; Bayer; 600 mg) were purchased in the local pharmacy.
LC-MS/MS equipment and operating conditions.
A validated LC-MS/MS method described by Meister et al. and revalidated by Kovac et al. was used (16, 27). In brief, chromatographic separation was achieved by first loading the analytes onto a column trapping system (Halo C18; 4.6 by 5 mm; Optimize Technologies, Oregon City, OR, USA) to remove the remaining matrix and then eluting to a chiral Lux Cellulose-2 column (150 by 2 mm, 3 μm; Phenomenex, CA, USA). Mobile phase A, containing 10 mM ammonium acetate with 0.015% formic acid, served as a loading solution at a flow rate of 0.35 mL/min. After a loading time of 1 min, a mixture of 2 mM ammonium formate and acetonitrile (1:4; mobile phase B) was used for the elution to the main column at a flow rate ascending to 0.45 mL/min at 3 min and remaining steady until 9.49 min. From 9.5 to 10.5 min, mobile phase A was used at a flow rate of 1 mL/min to reequilibrate the trapping system. A mixture of isopropanol and purified water (50:50) was used to clean the autosampler syringe, including the injection valve in between sample injections.
Study design and ethical considerations.
The DBS sample collection was performed in the frame of a PK and dose-finding randomized controlled, single-blind study of praziquantel against O. felineus in infected adults (18 to 65 years), as assessed by the presence of eggs in the stool (O. S. Fedorova, J. Keiser, unpublished data). Ethical approval of the clinical trial was obtained from the Ethics Committee of Siberian State Medical University, which granted clearance on 29 May 2017 (reference no. 5308). The trial is registered at ISRCTN (https://www.isrctn.com/ISRCTN10577372). All participants gave informed consent. The study was carried out in Tomsk between June 2016 and March 2018 at the clinics of the Siberian State Medical University. The study physician carried out a clinical and physical examination and assessed the medical history of the participants. The safety and tolerability of the interventions were evaluated by clinical examinations and assessment of adverse events. Efficacy and safety data, as well as detailed information on inclusion and exclusion criteria, randomization procedure, and diagnostic methods, will be published elsewhere.
Three groups of each 28, 26, and 28 O. felineus-infected patients were treated with one oral dose of 20 mg/kg, 40 mg/kg, and 60 mg/kg, respectively. Another group of 28 patients was treated with three oral doses of 20 mg/kg praziquantel, with the second and third doses administered 4 and 8 h after the first dose, respectively. A placebo group was included in the study.
DBS sample collection and storage.
A sterile one-way finger pricker (e.g., Accu-Check Softclix Pro; Roche, Switzerland) was used to obtain capillary blood at 0. 0.5, 1, 1.5, 2, 2.5, 3, 6, 8, 12, and 24 h after treatment with 20 mg/kg, 40 mg/kg, and 60 mg/kg of praziquantel (single oral dose) and at 0, 1, 2, 4, 5, 6, 7, 8, 10, 12, and 24 h after treatment with three doses of 20 mg/kg praziquantel given in intervals of 4 h. Quadruplets of a drop of blood at each time point were taken and transferred onto the DBS cards, dried for approximately 1 h, and stored afterward in plastic bags with desiccant. The cards were transferred to Basel, Switzerland, and kept at −20°C until further processing.
Sample procession.
Sample procession was conducted based on previously published methods (14, 16, 27). Stock solutions of the analytes were prepared in acetonitrile to obtain 1.0-mg/mL concentrations for R- and S-praziquantel, a 10-mg/mL concentration for trans-4-OH-praziquantel, and a 1.25-mg/mL concentration for praziquantel-d11. They were stored at −20°C. For R- and S-praziquantel, QC samples at four concentrations of 10 ng/mL (LLOQ), 17.5 ng/mL (low QC [lQC]), 175 ng/mL (medium QC [mQC]), and 1,750 ng/mL (high QC [hQC]), respectively, as well as eight CLs covering the range of 10 ng/mL to 2,500 ng/mL, were prepared by spiking blood with the corresponding working solutions. The calibration line samples of trans-OH-praziquantel covered a range of 100 ng/mL to 25,000 ng/mL, and the QC samples were similarly prepared by spiking six different blanks to obtain LLOQ and low, middle, and high concentrations. The final concentration of organic solvent in the spiked blood samples was <4.2%.
Disks of 6 mm in diameter were punched from the DBS samples and extracted with 200 μL of extraction solution containing 400 ng/mL internal standard. Samples were shaken in a thermomixer for 20 min at 20°C and 1,400 rpm (Eppendorf Thermomixer C; Hamburg, Germany), and sonicated for 40 min. Thereafter, samples were filtered (2-μm polyvinylidene difluoride [PVDF]-membrane 96-well filter plate [Corning Life Sciences, CA, USA]) by centrifugation (10 min at 2,250 × g, 20°C).
Data analysis.
Drug concentration was calculated by interpolation from the calibration curve. The Excel add-in PKSolver (32) using noncompartmental analysis was utilized to calculate PK parameters for each patient. Maximum drug concentrations (Cmax) and the time to reach Cmax (Tmax) were observed values. The area under the curve was determined until the last measurement (AUC0–24) using the linear trapezoidal rule. The elimination half-life (t1/2) was estimated by the equation t1/2 = ln(2)/λz, where lambda was determined by performing a regression of the natural logarithm of the concentration values during the elimination period. The half-life was only estimated for patients who had a single peak in concentration and where the elimination phase was well estimated by the algorithm because of praziquantel’s erratic absorption. The drug oral clearance (CL/F) and the apparent volume of distribution (V/F) were not evaluated. Statistical analysis was performed with Microsoft Excel and GraphPad Prism version 8.2.1 (GraphPad, CA, USA), respectively. Kruskal-Wallis analysis followed by Dunn’s posttest was performed using the software R studio Version 4.0.3 to compare PK parameters (Cmax, AUC0–24) between treatment arms.
ACKNOWLEDGMENTS
This work was supported by the Swiss National Science Foundation (no. 320030_175585).
We are thankful to all patients from the Russian Federation for participating in our study. We further thank the research team from the Siberian State Medical University, and we are grateful to Anna Kovshirina for helping with the sample procession.
We have no conflicts of interest to declare.
REFERENCES
- 1.Pozio E, Armignacco O, Ferri F, Gomez Morales MA. 2013. Opisthorchis felineus, an emerging infection in Italy and its implication for the European Union. Acta Trop 126:54–62. 10.1016/j.actatropica.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Keiser J, Utzinger J. 2005. Emerging foodborne trematodiasis. Emerg Infect Dis 11:1507–1514. 10.3201/eid1110.050614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keiser J, Utzinger J. 2009. Food-borne trematodiases. Clin Microbiol Rev 22:466–483. 10.1128/CMR.00012-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petney TN, Andrews RH, Saijuntha W, Wenz-Mucke A, Sithithaworn P. 2013. The zoonotic, fish-borne liver flukes Clonorchis sinensis, Opisthorchis felineus and Opisthorchis viverrini. Int J Parasitol 43:1031–1046. 10.1016/j.ijpara.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Fedorova OS, Kovshirina YV, Kovshirina AE, Fedotova MM, Deev IA, Petrovskiy FI, Filimonov AV, Dmitrieva AI, Kudyakov LA, Saltykova IV, Odermatt P, Ogorodova LM. 2017. Opisthorchis felineus infection and cholangiocarcinoma in the Russian Federation: a review of medical statistics. Parasitol Int 66:365–371. 10.1016/j.parint.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 6.Fedorova OS, Fedotova MM, Zvonareva OI, Mazeina SV, Kovshirina YV, Sokolova TS, Golovach EA, Kovshirina AE, Konovalova UV, Kolomeets IL, Gutor SS, Petrov VA, Hattendorf J, Ogorodova LM, Odermatt P. 2020. Opisthorchis felineus infection, risks, and morbidity in rural Western Siberia, Russian Federation. PLoS Negl Trop Dis 14:e0008421. 10.1371/journal.pntd.0008421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pakharukova MY, Mordvinov VA. 2016. The liver fluke Opisthorchis felineus: biology, epidemiology and carcinogenic potential. Trans R Soc Trop Med Hyg 110:28–36. 10.1093/trstmh/trv085. [DOI] [PubMed] [Google Scholar]
- 8.Bronshteĭn AM, Ozeretskovskaia NN, Panteleeva EI, Pliushcheva GL, Gitsu GA. 1988. An analysis of 4 strategies for the praziquantel treatment of persons infested with Opisthorchis felineus under conditions excluding reinfestation. Med Parazitol (Mosk) 19–24. https://pubmed.ncbi.nlm.nih.gov/3252134/. [PubMed] [Google Scholar]
- 9.Zdesenko G, Mutapi F. 2020. Drug metabolism and pharmacokinetics of praziquantel: a review of variable drug exposure during schistosomiasis treatment in human hosts and experimental models. PLoS Negl Trop Dis 14:e0008649. 10.1371/journal.pntd.0008649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avgustinovich DF, Tsyganov MA, Pakharukova MY, Chulakov EN, Dushkin AV, Krasnov VP, Mordvinov VA. 2020. Effectiveness of repeated administration of praziquantel with disodium glycyrrhizinate and two enantiomers of praziquantel on Opisthorchis felineus (Rivolta, 1884). Acta Parasitol 65:156–164. 10.2478/s11686-019-00149-2. [DOI] [PubMed] [Google Scholar]
- 11.Nleya L, Thelingwani R, Li X-Q, Cavallin E, Isin E, Nhachi C, Masimirembwa C. 2019. The effect of ketoconazole on praziquantel pharmacokinetics and the role of CYP3A4 in the formation of X-OH-praziquantel and not 4-OH-praziquantel. Eur J Clin Pharmacol 75:1077–1087. 10.1007/s00228-019-02663-8. [DOI] [PubMed] [Google Scholar]
- 12.Lima RM, Ferreira MAD, de Jesus Ponte Carvalho TM, Dumêt Fernandes BJ, Takayanagui OM, Garcia HH, Coelho EB, Lanchote VL. 2011. Albendazole-praziquantel interaction in healthy volunteers: kinetic disposition, metabolism and enantioselectivity. Br J Clin Pharmacol 71:528–535. 10.1111/j.1365-2125.2010.03874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Na Bangchang K, Karbwang J, Pungpak S, Radomyos B, Bunnag D. 1993. Pharmacokinetics of praziquantel in patients with opisthorchiasis. Southeast Asian J Trop Med Public Health 24:717–723. [PubMed] [Google Scholar]
- 14.Meister I, Kovac J, Duthaler U, Odermatt P, Huwyler J, Vanobberghen F, Sayasone S, Keiser J. 2016. Pharmacokinetic study of praziquantel enantiomers and its main metabolite R-trans-4-OH-praziquantel in plasma, blood and dried blood spots in Opisthorchis viverrini-infected patients. PLoS Negl Trop Dis 10:e0004700. 10.1371/journal.pntd.0004700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sayasone S, Meister I, Andrews JR, Odermatt P, Vonghachack Y, Xayavong S, Senggnam K, Phongluxa K, Hattendorf J, Bogoch II, Keiser J. 2017. Efficacy and safety of praziquantel against light infections of Opisthorchis viverrini: a randomized parallel single-blind dose-ranging trial. Clin Infect Dis 64:451–458. 10.1093/cid/ciw785. [DOI] [PubMed] [Google Scholar]
- 16.Meister I, Leonidova A, Kovač J, Duthaler U, Keiser J, Huwyler J. 2016. Development and validation of an enantioselective LC-MS/MS method for the analysis of the anthelmintic drug praziquantel and its main metabolite in human plasma, blood and dried blood spots. J Pharm Biomed Anal 118:81–88. 10.1016/j.jpba.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Falcoz C, Guzy S, Kovač J, Meister I, Coulibaly J, Sayasone S, Wesche D, Lin YW, Keiser J. 2022. R-praziquantel integrated population pharmacokinetics in preschool- and school-aged African children infected with Schistosoma mansoni and S. haematobium and Lao adults infected with Opisthorchis viverrini. J Pharmacokinet Pharmacodyn 49:293–310. 10.1007/s10928-021-09791-8. [DOI] [PubMed] [Google Scholar]
- 18.Olliaro P, Delgado-Romero P, Keiser J. 2014. The little we know about the pharmacokinetics and pharmacodynamics of praziquantel (racemate and R-enantiomer). J Antimicrob Chemother 69:863–870. 10.1093/jac/dkt491. [DOI] [PubMed] [Google Scholar]
- 19.Murray M. 2006. Altered CYP expression and function in response to dietary factors: potential roles in disease pathogenesis. Curr Drug Metab 7:67–81. 10.2174/138920006774832569. [DOI] [PubMed] [Google Scholar]
- 20.Mandour ME, el Turabi H, Homeida MM, el Sadig T, Ali HM, Bennett JL, Leahey WJ, Harron DW. 1990. Pharmacokinetics of praziquantel in healthy volunteers and patients with schistosomiasis. Trans R Soc Trop Med Hyg 84:389–393. 10.1016/0035-9203(90)90333-a. [DOI] [PubMed] [Google Scholar]
- 21.el Guiniady MA, el Touny MA, Abdel-Bary MA, Abdel-Fatah SA, Metwally A. 1994. Clinical and pharmacokinetic study of praziquantel in Egyptian schistosomiasis patients with and without liver cell failure. Am J Trop Med Hyg 51:809–818. 10.4269/ajtmh.1994.51.809. [DOI] [PubMed] [Google Scholar]
- 22.Ofori-Adjei D, Adjepon-Yamoah KK, Lindström B. 1988. Oral praziquantel kinetics in normal and Schistosoma haematobium-infected subjects. Ther Drug Monit 10:45–49. 10.1097/00007691-198810010-00008. [DOI] [PubMed] [Google Scholar]
- 23.Bagchus WM, Bezuidenhout D, Harrison-Moench E, Kourany-Lefoll E, Wolna P, Yalkinoglu O. 2019. Relative bioavailability of orally dispersible tablet formulations of Levo- and Racemic praziquantel: two phase I studies. Clin Transl Sci 12:66–76. 10.1111/cts.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meister I, Ingram-Sieber K, Cowan N, Todd M, Robertson MN, Meli C, Patra M, Gasser G, Keiser J. 2014. Activity of praziquantel enantiomers and main metabolites against Schistosoma mansoni. Antimicrob Agents Chemother 58:5466–5472. 10.1128/AAC.02741-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonate PL, Wang T, Passier P, Bagchus W, Burt H, Lüpfert C, Abla N, Kovac J, Keiser J. 2018. Extrapolation of praziquantel pharmacokinetics to a pediatric population: a cautionary tale. J Pharmacokinet Pharmacodyn 45:747–762. 10.1007/s10928-018-9601-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lima RM, Ferreira MAD, Ponte T, Marques MP, Takayanagui OM, Garcia HH, Coelho EB, Bonato PS, Lanchote VL. 2009. Enantioselective analysis of praziquantel and trans-4-hydroxypraziquantel in human plasma by chiral LC–MS/MS: application to pharmacokinetics. J Chromatogr B Analyt Technol Biomed Life Sci 877:3083–3088. 10.1016/j.jchromb.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 27.Kovač J, Meister I, Neodo A, Panic G, Coulibaly JT, Falcoz C, Keiser J. 2018. Pharmacokinetics of praziquantel in Schistosoma mansoni- and Schistosoma haematobium-infected school- and preschool-aged children. Antimicrob Agents Chemother 62:e02253-17. 10.1128/AAC.02253-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schulz JD, Neodo A, Coulibaly JT, Keiser J. 2019. Pharmacokinetics of albendazole, albendazole sulfoxide, and albendazole sulfone determined from plasma, blood, dried-blood spots, and Mitra samples of hookworm-infected adolescents. Antimicrob Agents Chemother 63:e02489-18. 10.1128/AAC.02489-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenblatt DJ, von Moltke LL. 2008. Gender has a small but statistically significant effect on clearance of CYP3A substrate drugs. J Clin Pharmacol 48:1350–1355. 10.1177/0091270008323754. [DOI] [PubMed] [Google Scholar]
- 30.Floridia M, Giuliano M, Palmisano L, Vella S. 2008. Gender differences in the treatment of HIV infection. Pharmacol Res 58:173–182. 10.1016/j.phrs.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz JB. 2007. The current state of knowledge on age, sex, and their interactions on clinical pharmacology. Clin Pharmacol Ther 82:87–96. 10.1038/sj.clpt.6100226. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Huo M, Zhou J, Xie S. 2010. PKSolver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed 99:306–314. 10.1016/j.cmpb.2010.01.007. [DOI] [PubMed] [Google Scholar]