ABSTRACT
Staphylococcus aureus chronically colonizes up to 30% of the human population on the skin or mucous membranes, including the nasal tract or vaginal canal. While colonization is often benign, this bacterium also has the capability to cause serious infections. Menstrual toxic shock syndrome (mTSS) is a serious toxinosis associated with improper use of tampons, which can induce an environment that is favorable to the production of the superantigen known as toxic shock syndrome toxin-1 (TSST-1). To better understand environmental signaling that influences TSST-1 production, we analyzed expression in the prototype mTSS strain S. aureus MN8. Using transcriptional and protein-based analysis in two niche-related media, we observed that TSST-1 expression was significantly higher in synthetic nasal medium (SNM) than in vaginally defined medium (VDM). One major divergence in medium composition was high glucose concentration in VDM. The glucose-dependent virulence regulator gene ccpA was deleted in MN8, and, compared with wild-type MN8, we observed increased TSST-1 expression in the ΔccpA mutant when grown in VDM, suggesting that TSST-1 is repressed by catabolite control protein A (CcpA) in the vaginal environment. We were able to relieve CcpA-mediated repression by modifying the glucose level in vaginal conditions, confirming that changes in nutritional conditions contribute to the overexpression of TSST-1 that can lead to mTSS. We also compared CcpA-mediated repression to other key regulators of tst, finding that CcpA regulation is dominant compared to other characterized regulatory mechanisms. This study underlines the importance of environmental signaling for S. aureus pathogenesis in the context of mTSS.
IMPORTANCE Menstrual toxic shock syndrome (mTSS) is caused by strains of Staphylococcus aureus that overproduce a toxin known as toxic shock syndrome toxin-1 (TSST-1). This work studied how glucose levels in a model vaginal environment could influence the amount of TSST-1 that is produced by S. aureus. We found that high levels of glucose repress TSST-1 production, and this is done by a regulatory protein called catabolite control protein A (CcpA). The research also demonstrated that, compared with other regulatory proteins, the CcpA regulator appears to be the most important for maintaining low levels of TSST-1 in the vaginal environment, and this information helps to understand how changes in the vaginal environmental can lead to mTSS.
KEYWORDS: Staphylococcus aureus, superantigen, TSST-1, CcpA, glucose, toxic shock syndrome, vaginal environment
INTRODUCTION
Staphylococcus aureus is a Gram-positive bacterium that can both persistently colonize up to 30% of the human population and can also cause numerous diseases ranging from skin or soft tissue infections to severe life-threatening conditions such as pneumonia, sepsis, and endocarditis (1). The main site of human colonization is the nasal passages, with the anterior nares being the primary location for this bacterium (2). S. aureus can also colonize other sites within the human body, including mucosal membranes such as the vaginal canal and rectum (3, 4). S. aureus colonization and disease are aided by an extensive arsenal of factors that contribute to virulence, including adhesives molecules, a wide diversity of toxins, and other proteins that modulate host cell activities (5–8). Furthermore, the bacterium also encodes a complex network of regulators that can respond to various stimuli, including nutritional availability, bacterial density, and stress (9).
One important family of staphylococcal exotoxins are the superantigens (SAgs), which are important for manipulating the immune response and have been associated with a number of diseases (8, 10). These toxins engage with the immune synapse between a T cell and an antigen-presenting cell (APC) to force the activation of the T cell, regardless of the cognitive antigen specificity of the T cell (8). This uncontrolled activation of T cells leads to an increased production of proinflammatory cytokines, which, in severe cases, can lead to a cytokine storm-associated toxinosis, known as toxic shock syndrome (TSS) (10, 11). Symptoms of TSS include fever, hypotension, rash, and multiorgan dysfunction. There are two major forms of TSS caused by S. aureus, including menstrual and nonmenstrual, the latter of which is associated with invasive infections. Menstrual TSS (mTSS) is a rare condition that occurs in women during menstruation, and nearly all cases of this disease are associated with the expression of a single SAg, toxic shock syndrome toxin-1 (TSST-1) (10). This life-threatening toxinosis is linked to the changes in the vaginal environment triggered by the use of some intravaginal devices, such as high-absorbency tampons, that create environmental conditions that result in excessive levels of TSST-1 (12).
In the current study, we aimed to understand how bacterial expression of TSST-1 differed within the vaginal environment from conditions found in the predominant site of colonization, the nose. Using bacterial media defined by these two colonization sites, we established that S. aureus produces the TSST-1 toxin in nasal conditions, whereas production was restricted in the vaginal environment. Furthermore, we established that glucose (through the activity of the carbon catabolite control protein A [CcpA] regulator) was a key nutritional signal that contributed to the bacteria repression of TSST-1 expression in the vaginal conditions. We demonstrate that CcpA likely acts as an independent and dominant repressor of TSST-1 that can override the activity of other characterized repressors of tst and likely functions to restrict S. aureus virulence to maintain commensalism during vaginal colonization.
RESULTS
Expression of TSST-1 is niche specific.
SAg expression has been detected in the nasal passages of S. aureus carriers (13, 14). Conversely, we, among others, have found a multitude of repressors that likely limit SAg expression within the vaginal environment (15–17). To confirm if these observations were consistent for TSST-1, we utilized media that are representative of both environments. Synthetic nasal medium (SNM) is a minimal medium whose composition is based on the metabolic analysis of nasal sections from humans (18). Vaginally defined medium (VDM) is a much richer medium and includes large quantities of several macromolecules, including mucin and peptone (19). To determine if TSST-1 is expressed in a niche-specific manner, we grew S. aureus MN8 in SNM and VDM, along with brain heart infusion (BHI) broth, which is a common rich, but undefined, growth medium. Transcription of tst was measured using the luminescence reporter plasmid pAmilux::Ptst transformed into wild-type S. aureus MN8. Growth curve analysis demonstrated that S. aureus growth in VDM was similar to standard BHI broth; however, S. aureus growth was limited in the minimal conditions of SNM (Fig. 1A to C). Luminescence signal, indicative of tst transcription, was clearly observed in BHI (Fig. 1A) and was also observed in SNM (Fig. 1B) despite the low growth in this medium. In VDM, however, tst transcription was barely detectable, suggesting that this toxin is not readily expressed under these conditions (Fig. 1C). When normalized to growth, Ptst activity in SNM was significantly higher than both BHI and, importantly, VDM (Fig. 1D). We were able to confirm this through protein analysis, detecting a similar level of TSST-1 protein in the supernatants of cultures grown in SNM and BHI by Western blotting for TSST-1 (Fig. 1E). Conversely, we were unable to detect TSST-1 in VDM-grown cultures, which correlated with the reporter analysis of Ptst activity (Fig. 1). Together, these data suggest that TSST-1 is expressed in a niche-specific manner and that this toxin is likely to be produced in larger quantities (in relation to growth) in the nasal environment, whereas production of TSST-1 is strictly repressed in media representing the vaginal niche.
FIG 1.
Expression of TSST-1 is niche specific. S. aureus strain MN8 was transformed with pAmilux::Ptst and analyzed for promoter activity over the course of 19 h. (A to C) Growth curves in brain heart infusion (BHI) (A), synthetic nasal medium (SNM) (B), and vaginally defined medium (VDM) (C). In each analysis, growth was determined by optical density at 600 nm (OD600) (black curve), and promoter activity was measured by luminescence production from the reporter construct (RLU) (pink curve). (D) To compare each medium condition, the area under each curve was determined, and the total production of luminescence was normalized to the total growth to give relative tst transcription in each medium condition. Bars indicate mean ± SD of three biological replicates, and significant differences were determined using an ordinary one-way analysis of variance (ANOVA) test with multiple comparisons (*, P < 0.05; ***, P < 0.001; ****, P < 0.0001). Stationary growth phase cultures of S. aureus MN8 were harvested, and normalized culture supernatants (based on absorbance units) were concentrated by trichloroacetic acid (TCA) precipitation. (E) Following concentration, exoprotein profiles were determined by analysis on 12% SDS-PAGE stained with Coomassie blue (top) and Western blot analysis with anti-TSST-1 rabbit antisera. Molecular weight markers were loaded on the left and are indicated in kilodaltons.
Niche specificity of TSST-1 expression is driven by glucose in the environment.
One major component that differed between the two niche-specific medium conditions was glucose concentration, and an early report demonstrated that glucose could repress TSST-1 production (20). In SNM media, the glucose concentration is 0.7 mM, which is in stark contrast to VDM, which contains nearly 100-fold more glucose at 60 mM (18, 19). In addition to the high glucose, glycogen (a polymer of glucose) is also present in the vaginally defined media. Combined, bacteria growing in VDM have access to an abundance of carbon sources. To determine if glucose could be driving the differing tst transcription, we repeated the growth analysis in VDM and SNM media, modifying the glucose level in each. First, to determine if glucose could repress tst transcription, we increased the concentration of glucose in SNM to 60 mM (the same concentration found in VDM) and observed a significant reduction in Ptst activity (Fig. 2A to C). Furthermore, we observed an increase in tst expression in VDM by replacing glucose with 120 mM sodium pyruvate (modified medium referred to as VDMP) (Fig. 2D to F). Sodium pyruvate was chosen to replace glucose as a carbohydrate in VDM, as it is used directly by the trichloroacetic acid (TCA) cycle and bypasses degradation into glucose that most of the other carbohydrates will undergo during energy metabolism. Together, these analyses demonstrate that a key nutritional signal that supports the niche-specific expression of TSST-1 is glucose, and a high concentration of this carbon source can repress tst expression.
FIG 2.
Niche specificity is driven by glucose in the environment. (A, B, D, and E) S. aureus MN8 was grown in either SNM (A) or modified SNM that contained 60 mM glucose (B), VDM (D), or modified VDM where the 60 mM glucose had been replaced with 120 mM sodium pyruvate (VDMP) (E). (C and F) During the growth analysis, Ptst activity was determined by the pAmilux reporter assay, and this was normalized to bacterial growth to determine relative tst transcription. Bars indicate mean ± SEM of three biological replicates, and significant differences were determined using an ordinary one-way ANOVA test with multiple comparisons (***, P < 0.001).
CcpA regulates niche-specific repression of TSST-1 expression.
A key regulator of glucose-based gene expression in bacteria is the catabolite control protein A (CcpA). This protein has been shown to be an important transcriptional regulator of virulence determinants in many bacterial species, including S. aureus (21–23). The ccpA gene has been implicated in tst regulation previously (21); therefore, to confirm that this regulator was responsible for the glucose-based repression observed in the defined media, we generated a clean, markerless deletion of the ccpA gene from the chromosome in S. aureus MN8. Following ccpA deletion, we repeated the luminescence reporter analysis in BHI, SNM, and VDM. These data indicate that CcpA is a transcriptional repressor of tst, as Ptst activity was found to be significantly increased under all three medium conditions when ccpA was deleted (Fig. 3A). The largest difference observed was between wild-type MN8 and the ccpA-null mutant grown in the VDM medium, with a 10-fold-higher level of tst transcription observed in the mutant, which correlated with the relatively high level of glucose in this medium, suggesting that ccpA is important for niche-specific TSST-1 production.
FIG 3.
CcpA regulates niche-specific repression of TSST-1 expression. (A) A luciferase promoter reporter assay was performed to determine tst transcription in S. aureus MN8 and MN8 ΔccpA in BHI, SNM, and VDM. Bars indicate mean ± SD of three biological replicates, and significant differences were determined using an ordinary one-way ANOVA test with multiple comparisons (*, P < 0.05; ***, P < 0.001). The ccpA gene was cloned into the tetracycline-inducible plasmid pRMC2 and transformed into S. aureus MN8 ΔccpA. SDS-PAGE (top panels) and anti-TSST-1 Western blotting (bottom panels) of TCA-concentrated exoprotein profiles from 18-h cultures of the indicated constructs are shown. The complementation strains were induced with 0, 5, and 50 ng/mL of anhydrotetracycline (aTet) as indicated. The vector control was induced with 50 ng/mL of aTet. (B to D) Each set of constructs was grown in BHI (B), SNM (C), and VDM (D).
To confirm that our observations in the luminescence reporter assay translated to expressed protein, we evaluated TSST-1 production by Western blotting from concentrated supernatants from culture grown under the same medium conditions. We also complemented the deletion of ccpA with an inducible construct (pRMC2::ccpA). In this analysis, we observed that in all three medium conditions, the deletion of ccpA resulted in increased TSST-1 protein (Fig. 3B to D). However, similar to the promoter activity analysis, this difference varied depending on the medium conditions analyzed. Again, the most pronounced difference observed was between wild-type MN8 and MN8 ΔccpA in VDM (Fig. 3D). Complementation of ccpA confirmed our observations, as in each medium condition, the ccpA mutant containing this construct was able to reduce TSST-1 production at 50 ng/mL anhydrotetracycline (ATc) induction (Fig. 3B to D). Together, these data demonstrate that CcpA contributes to the glucose-based niche-specific expression of TSST-1 and is a potent repressor of this toxin.
TSST-1 expression in the vaginal environment is specifically repressed by glucose in a CcpA-dependent manner.
To understand if the loss of glucose repression under vaginal conditions could lead to the erroneous expression of TSST-1, we repeated the transcriptional and protein analysis of wild-type S. aureus and the ccpA-null mutant VDM modified with a range of different glucose concentrations (Fig. 4A and B). Indeed, when we reduced the glucose concentration in VDM to 0.7 mM, production of TSST-1 in wild-type MN8 increased compared to wild-type MN8 grown in unmodified VDM (Fig. 4A). Furthermore, there was a clear indication this was due to derepression by CcpA, as the transcriptional level of tst in MN8 ΔccpA appeared to be similar to wild-type TSST-1 expression under low-glucose conditions (Fig. 4A). This was further supported by the protein-based analysis, as TSST-1 production in both the wild-type MN8 and the ccpA-null mutant was equivalent in VDM containing 0.7 mM glucose (Fig. 4B). As glucose concentration increased, repression of tst also increased, and TSST-1 production was essentially abolished at 5 mM glucose (Fig. 4B). This appears to be driven by CcpA activity, as the null mutant construct still produced significantly more tst than the wild type once glucose concentration (i.e., >2 mM) became repressive (Fig. 4A and B). Together, these data demonstrate that CcpA acts repressively on tst expression at glucose concentrations as low as 2 mM.
FIG 4.
CcpA repression of TSST-1 in the vaginal environment can be modified by varying glucose concentrations. (A) S. aureus MN8 and the ΔccpA mutant were analyzed by luciferase reporter assay, for the Ptst promoter, in VDM and VDM where glucose had been modified from 0.7 to 60 mM. (B) Supernatants from MN8 and ΔccpA mutant constructs, grown in glucose-modified VDM (as indicated) for 18 h, were concentrated by TCA precipitation and analyzed by anti-TSST-1 Western blotting. (C to E) Promoter reporter (C) and protein analysis was also performed on cultures grown in VDMP (D) and VDMP supplemented with 60 mM glucose (E). For panels A and C, bars indicate mean ± SD of three biological replicates, and significant differences were determined using an ordinary one-way ANOVA test with multiple comparisons (*, P < 0.05; **, P < 0.001; ****, P < 0.0001).
To confirm that the increased expression of TSST-1 in VDMP was due to the removal of the repressive activity of glucose rather than promotion of tst expression by pyruvate, 60 mM glucose was added to the VDMP medium. This resulted in the reduction of tst expression to similar levels as unmodified VDM (Fig. 4C), reinforcing the repressive role of this sugar on TSST-1 expression. Furthermore, we could again confirm that CcpA was responsible for the repression of TSST-1 activity following the addition of 60 mM glucose (Fig. 4C). However, when ccpA was deleted, expression of TSST-1 still increased in VDMP (Fig. 4C). These results further confirmed that when S. aureus was grown in VDMP (Fig. 4D) or modified VDMP with the addition of 60 mM glucose (Fig. 4E), deletion of ccpA resulted in a marked increase in TSST-1, which was repressed by CcpA complementation. Together, these collective data demonstrate that the activity of CcpA is critical in the vaginal niche to suppress production of TSST-1 by S. aureus MN8.
CcpA can modify virulence mechanisms of S. aureus MN8.
CcpA is considered to be a global regulator of virulence in S. aureus and can modify the activity of a number of virulence genes outside TSST-1 (22). To understand how virulence could be impacted under high-glucose conditions, we performed several phenotypic assays. First, as we have been studying the impact of glucose and CcpA on TSST-1 production, we wanted to confirm that this regulator has an impact on the SAg activity of S. aureus MN8. TSST-1 is a highly potent SAg and can activate human T cells (interleukin 2 [IL-2] production was used as a marker of T cell activation), even in subnanogram quantities (Fig. 5A). As a strain that produces TSST-1, MN8 has a high level of SAg activity; however, this is likely tempered in high-glucose conditions. To confirm this, we stimulated human peripheral blood mononuclear cells (PBMCs) with supernatants of MN8 cultures grown in VDM (Fig. 5B). As expected, PBMCs stimulated with wild-type S. aureus MN8 supernatants demonstrated dose-dependent activation. However, when we used the supernatant of the ccpA-null mutant, we were able to observe a significant increase in IL-2 levels. Notably, this assay may also measure the activity of other SAgs produced by S. aureus MN8 but is consistent with an increase in TSST-1 production by the ccpA deletion strain. Again, complementation of ccpA on an inducible plasmid reduced the mitogenic activity back to wild-type levels (Fig. 5B).
FIG 5.
CcpA activity represses the mitogenic capability of S. aureus MN8 of TSST-1 in the vaginal environment but has limited impact on hemolytic or proteolytic capacity. (A) IL-2 production by PBMCs from human blood following stimulation with a titration of TSST-1 protein. (B) IL-2 production by PBMCs from human blood following stimulation with a titration of supernatant from S. aureus MN8 and constructs grown in VDM medium for 8 h. Data shown in panels A and B are mean ± SEM from 10 donors. Significant differences were determined from the area under each curve using a repeated-measures (RM) one-way ANOVA with multiple comparisons (**, P < 0.01; ***, P < 0.001). Hemolytic and proteolytic capability of S. aureus MN8 and mutant constructs was assessed by growth on indicator agar plates. (C and D) BHI, VDM, or modified VDM agar was supplemented with 5% human blood (C) and 1.5% skimmed milk (D). Following inoculation, each colony was grown at 37°C for 24 h. Representative images of 4 experiments are shown.
From the PBMC stimulation analysis, we noted a lower level of production of IL-2 observed in MN8 ΔccpA for the higher supernatant concentrations. Cell toxicity could be responsible for this, either due to the overproduction of superantigens or cytolytic toxins. To attempt to understand if CcpA played a role in cytolytic toxin regulation, we grew MN8 constructs on different agar plates with 5% human blood but containing different base media. In addition to wild-type S. aureus MN8 and the ccpA-null mutant, we also included MN8 Δagr and MN8 Δpsm mutants. The key cytotoxin, α-hemolysin, is truncated in S. aureus MN8; therefore, δ-toxin (from the RNAIII transcript) and α-PSMs are likely the major hemolytic factors in this strain (24, 25). Growth on BHI yielded a hemolytic profile similar to what we have previously reported (17), with the wild-type strain displaying hemolytic activity and a reduced level of hemolysis observed in the two Δagr and Δpsm control strains (Fig. 5C). MN8 ΔccpA exhibited a slightly increased level of hemolysis when grown on BHI as the base medium. When using VDM as the base medium, at high glucose, hemolysis in the wild-type strain was higher than in BHI, and this appeared to be mediated by CcpA, as the null mutant of this regulator exhibited a marked reduction in hemolytic phenotype (Fig. 5C). Interestingly, this did not appear to be due to the activity of PSMs, as both deletion mutants of agr and the α-PSM genes appeared to exhibit wild-type levels of hemolysis. Indeed, when we reduced the glucose to 0.7 mM, a low-level hemolytic phenotype was observed in both the wild-type and MN8 ΔccpA mutant strains. A lack of activity in the MN8 Δagr and MN8 Δpsm mutants suggests that this phenotype, under low-glucose conditions, was primarily due to the PSM peptides. Together, these experiments indicate that CcpA has limited overall impact on driving the expression of PSM toxins but may impact another, but currently unidentified, hemolytic toxin produced from S. aureus MN8. Furthermore, the low level of hemolysis produced by the CcpA mutant grown on VDM suggests that it is the overproduction of TSST-1 that is leading to T cell activation-induced cell death in the higher dilutions of the IL-2 production assays (Fig. 5B).
Protease production is another key virulence characteristic of S. aureus that can be easily assessed, and to test this, we altered the base media in agar plates supplemented with 1.5% skimmed milk to provide a visible substrate for protease-mediated degradation. In this assay, we included the MN8 ΔsarA mutant as a control, as this mutant overproduces proteases (17, 26). We grew MN8 and MN8 ΔccpA in these media; we observed no obvious changes between the deletion or wild-type strains despite the high activity of the MN8 ΔsarA control (Fig. 5D). This analysis suggests that CcpA has a limited role in modulating these proteolytic enzymes in S. aureus MN8.
CcpA is a dominant repressor of TSST-1 expression in the vaginal niche.
CcpA can function as a highly effective repressor of TSST-1 expression in S. aureus MN8 (Fig. 3 and 4). To determine the scope of this regulator in the vaginal environment, we performed transcriptome analysis in VDM, comparing wild-type S. aureus MN8 with the ccpA-null mutant at both 4 and 8 h postinoculation (hpi) (see Table S1 and S2 in the supplemental material). During this analysis, we were able to observe significant upregulation of tst transcription in the MN8 ΔccpA construct at 4 hpi (Fig. 6A); however, this dropped below the significant threshold by 8 hpi (Fig. 6B). What is clear from these data sets is the overall limited changes in the expression of other known major regulators of S. aureus virulence, especially at 4 hpi (Fig. 6 and Table S1 and S2). We did note some changes in vraR and arlR transcription (Fig. 6B), but both occurred at the later 8-hpi time point, and given that peak tst transcription occurred earlier, the impact of these regulator changes on TSST-1 is likely limited. These data suggest that CcpA regulation of TSST-1 is likely direct and independent of other known major regulators of S. aureus.
FIG 6.
CcpA is a dominant repressor of TSST-1 expression within the vaginal environment. (A and B) S. aureus MN8 and MN8 ΔccpA were inoculated into VDM medium and grown at 37°C for 4 h (A) and 8 h (B) before sampling and RNA extraction. The transcriptomic profile was obtained by RNA-seq and transcripts compared against the publicly available MN8 genome (GenBank accession no. CM000952). Volcano plots depicted show the differential analysis of each transcript determined. Each dot represents a single transcript, and red dots indicate transcripts that differed significantly between the wild-type strain and the CcpA-null mutant. Horizontal gray line indicates that comparisons above the line have a P value of <0.001, and vertical dotted lines indicate the fold change of the transcript is higher than 2. Various regulator mutants of S. aureus MN8 were analyzed by Ptst promoter reporter assay as well as anti-TSST-1 Western blotting. (C to F) Bacteria were grown in either VDM (C and D) or VDMP (E and F). For transcriptional analysis, bars indicate mean ± SD of three biological replicates, and significant differences were determined using an ordinary one-way ANOVA test with multiple comparisons (****, P < 0.0001). Western blot analysis was performed on TCA-concentrated supernatants from pictured strain that had been grown in medium for 18 h.
To determine if CcpA has a dominant role in TSST-1 production, we performed expression analysis on S. aureus MN8 in VDM and VDMP, along with additional mutants of known repressors of tst expression (Fig. 6C to F). Under high-glucose conditions, it was clear that the dominant repressor was CcpA, as the null mutant of this regulator exhibited significantly higher promoter activity than all the other constructs analyzed (Fig. 6C). Additionally, this construct was the only mutant to exhibit detectable production of TSST-1 by Western blotting (Fig. 6D), confirming that under these conditions, CcpA is the dominant repressor system for TSST-1. Changing the conditions to the more permissive VDMP medium demonstrated that ccpA remained the primary repressive regulator, as the transcriptional analysis again demonstrated much higher tst expression in the ccpA deletion mutant (Fig. 6E). The only mutant, other than MN8 ΔccpA, that exhibited any apparent trend for increased tst transcription was the sarA-null mutant, although this did not reach statistical significance (Fig. 6E). Higher levels of TSST-1 protein production were realized for both the ccpA and sarA mutants by Western blotting than the other constructs tested (Fig. 6F). Together, these findings indicate that CcpA is a dominant repressor of TSST-1 in the vaginal environment and is an important regulator needed to prevent erroneous expression of the toxin within this niche.
DISCUSSION
Staphylococcal superantigens play an important role in the pathogenesis of several severe S. aureus-mediated diseases, promoting inflammation and subverting immune system function (8, 27). The role of these toxins during colonization has yet to be completely understood, but it is likely that the generation of inflammation in colonization environments is still key. However, the occurrence of mTSS would suggest that high levels of SAg production during vaginal colonization are detrimental to the bacteria, and S. aureus colonization must occur in the absence of inflammation mediated by these toxins (8, 10). This is supported in the current study, where we have been able to clearly demonstrate that TSST-1 is produced in a glucose-specific manner by utilizing defined media that reflect the conditions found within the host. Consistent with other reports in the human population, we found that TSST-1 is readily produced in conditions that mimic the nasal environment with glucose as a key nutrient that discriminates this niche from the vaginal canal (13, 18, 19).
Through the use of a comprehensive model such as the defined vaginal medium, this study contributes to our understanding of S. aureus toxin regulation during vaginal colonization, especially during the luteal stage of the menstrual cycle (Fig. 7). At this point in the cycle, Lactobacillus growth is at its peak, vaginal lumen pH is low, and, most importantly, carbohydrate concentration is especially high (28–30). Previous work has demonstrated that lactobacilli such as Lactobacillus reuteri produce cyclic dipeptides that suppress agr function in S. aureus, therefore reducing tst expression (31). Further to this, the lower pH seen in the vaginal tract is below optimal for S. aureus growth, without which the quorum-sensing agr system will not function, effectively limiting tst transcription (17). Our data demonstrate the direct role of glucose through CcpA to repress toxin expression is another mechanism that is exploited at this stage of the menstrual cycle to allow S. aureus to exist commensally without causing severe inflammation (Fig. 7). The literature is unclear regarding the homeostatic concentration of glucose in the vaginal lumen during the luteal stage of the menstrual cycle; however, most reports suggest this is in excess of 5 mM (29, 32–35). Therefore, CcpA plays a critical role in repressing tst at this stage and preventing pathogenic expression.
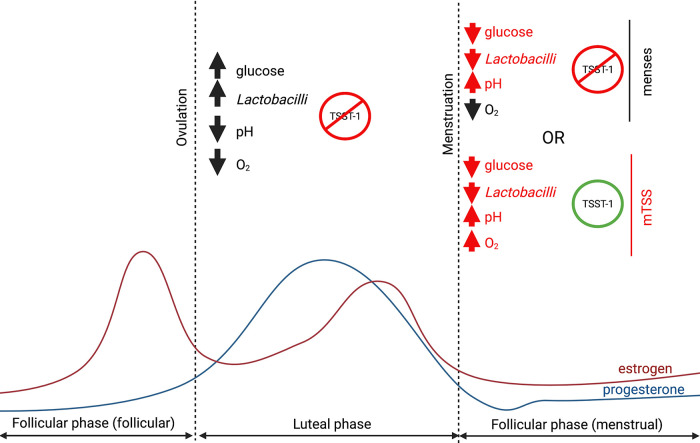
FIG 7.
Environmental signaling and TSST-1 regulation over the menstrual cycle. Over the whole menstrual cycle, the vaginal tract is generally an anaerobic environment. In the late follicular phase (follicular stage), the vaginal tract recovers from menses, and the level of estrogen elevates, leading to an increase of epithelial maturation and glycogen production. The Lactobacillus-dominated microbiota, in the presence of glycogen and glucose, grows and lowers the pH of the tract. Altogether, these environmental cues repress TSST-1 expression. This stage ends with ovulation and the succeeding (luteal) phase, which principally follows the same trend as the follicular stage with a high level of glucose/glycogen and lactobacilli with a low pH and repression of TSST-1. When the levels of estrogen and progesterone drop, the menses start, and the vaginal tract undergoes drastic changes where the glucose and Lactobacillus levels drop, increasing the pH of the niche. However, in normal situations, there is still no availability of oxygen and TSST-1 production from S. aureus is repressed. If oxygen becomes available during menses, S. aureus can grow, and TSST-1 production occurs.
Efforts to understand the pathobiology of mTSS have strongly indicated that vaginal niche modification can lead to an environment where S. aureus can grow and produce TSST-1 (12). Indeed, the environmental changes that occur as menses approach include a drop in carbohydrates, leading to a decrease in vaginal lactobacilli and an increase in pH. This removes the repressive mechanisms of the luteal stage, without which TSST-1 production could occur if S. aureus can grow (Fig. 7). This would support the proposed model of mTSS initiation that occurs due to the introduction of oxygen into the vaginal environment (12, 36). This would suggest that, at this stage in menses, tst repression would require the lack of agr activation (through lack of S. aureus growth) and repression by the oxygen-sensitive SrrAB two-component system (15, 17, 37) (Fig. 7). The high-absorbency tampons associated with the mTSS epidemic of the early 1980s likely allowed for elevated oxygen levels (38). Together, these would create an environment closer to nasal conditions (high oxygen, low carbohydrates, neutral pH) where we have demonstrated TSST-1 is readily produced.
Regulation of tst by CcpA has been suggested previously in a surrogate construct of S. aureus (strain Newman) that does not encode the toxin gene (21). Drawing a firm conclusion using this type of approach is risky, especially given that there are examples of regulators that have strain-specific activity for tst regulation, such as SarA (16, 39, 40). Additionally, strain Newman is known to contain an activating mutation within SaeS (41), and SaeRS is the dominant positive regulator of TSST-1 expression (40). In this current study, we were able to confirm robustly that CcpA has a critical role in the regulation of TSST-1 production and have done so in a wild-type S. aureus strain that naturally encodes tst. Additionally, CcpA was the strongest repressor of tst expression under the VDM conditions tested and the only null mutant in our analysis that demonstrated significant derepression (in high-glucose conditions). There are multiple important regulators either proven to play a role in tst regulation or likely to play a role in virulence regulation that were unavailable to us during this study (such as SigB or ArlR/S), which would be informative to analyze in this context in future studies (9, 16). Furthermore, our transcriptome analysis suggests that CcpA activity is likely acting directly on Ptst, as no other major regulator appeared to change in abundance when ccpA was deleted. In addition, a binding site for the CcpA protein (cre-box) can be observed in the promoter region for the tst gene (21, 22) (see Fig. S1 in the supplemental material). A cre-box is a degenerate, pseudopalindrome nucleotide sequence that, when located within promoter regions, will function to repress gene transcription when CcpA binds and hampers transcriptional machinery. Studies in Bacillus subtilis using an inducible CcpA expression system identified high- and low-affinity cre-box consensus sequences (42), and the cre-box sequence within the tst promoter would be consistent with a low-affinity cre-box sequence (based on B. subtilis). This cre-box overlaps with the transcription start site to inhibit transcription but is distinct from the binding sites for the SarA repressor and the SaeR activator (40) (Fig. S1). An additional observation in the context of this study is that BHI yielded robust tst production despite a high level of glucose in this medium. Given that we have previously demonstrated SaeRS has a dominant role in promoting tst under these conditions, it is likely responsible for this observation (40). This would strongly suggest that tst regulation by CcpA can be overridden outside conditions found within the vaginal environment and highlights the need to assess the expression of virulence factors in defined representative media.
While using VDM to reconstruct the nutritional environment of the vagina, we do need to stress the caveat that all our analysis is performed at neutral pH with oxygen introduced by shaking the cultures. As discussed earlier, were these conditions reversed, it would add another layer of repression and prevent us from analyzing the role of glucose in isolation. However, under these conditions, which were highly permissive for TSST-1 production, we were able to demonstrate that glucose alone was able to act as a repressive molecule and may explain why mTSS does not occur outside menses, even when the physiological conditions in the vagina may support it, e.g., bacterial vaginosis (43, 44). From this simplified but comprehensive model of the vaginal niche, it will be important to investigate TSST-1 regulation in a more complex and interactive environment, including microbial interactions with Lactobacillus species and vaginosis indicators such as Gardnerella vaginalis.
The use of defined media in this study highlights the differential expression that can occur with S. aureus virulence factors. Strict CcpA-mediated repression of TSST-1 in vaginal conditions is contrasted with relatively high levels of production in the nasal conditions represented by SNM. This suggests that SAgs like TSST-1 are readily produced in this niche despite low levels of growth. Unlike the vaginal niche, neutral pH and high oxygen are characteristic of the nasal environment and suggest that high SAg production occurs and is likely a desired consequence in this environment, promoting effective colonization by S. aureus. Additionally in this study, the use of defined media also highlighted how conditions can impact other virulence factors, such as cytolytic toxins. Previously, we demonstrated that in the absence of a functional alpha-toxin gene (hla), hemolysis in S. aureus MN8 is mediated by α-PSMs and δ-toxin (using tryptic soy agar with 5% human blood) (17). When performing the similar analysis with VDM, we found a phenotype suggestive of an alternative factor that is positively regulated by CcpA, whose expression is reduced by lower glucose concentration (Fig. 5). It is not clear to us exactly what factor this is, as other potential cytolytic toxins did not appear as significant hits in our transcriptomic analysis, but again, this does highlight the benefit of exploring virulence expression through the utilization of the defined host media.
Together, our observations underline that S. aureus has evolved highly effective regulatory mechanisms to detect nutrients in the environment and modify gene expression to be appropriate for the niche. We have been able to demonstrate that the bacterium is able to discriminate between the colonization niche it is in based on the concentration of a signal nutrient and modify virulence expression accordingly. This furthers our understanding of the pathobiology of mTSS and presents a key target for both feminine product design and antivirulence therapy.
MATERIALS AND METHODS
Ethics statement.
Human venous blood was taken from healthy donors in accordance with a human subject protocol approved by the London Health Sciences Center (LHSC) Research Ethics Boards, University of Western Ontario, London, Ontario, Canada, under protocol 110859. Volunteers were recruited by a passive advertising campaign within the department of microbiology and immunology at the University of Western Ontario, and, following an outline of the risks, written informed consent was given by each volunteer before each sample was taken. Following sampling, blood was fully anonymized, and no information regarding the identity of the donor, including sex and age, was retained.
Bacterial strains, media, and growth conditions.
For general culture, S. aureus strains listed in Table 1 were grown aerobically at 37°C in tryptic soy broth (TSB) (Difco) or brain heart infusion (BHI) broth with shaking (250 rpm) supplemented with the appropriate antibiotics. For solid-phase cultures, tryptic soy agar (TSA) was used (TSB with 1.5% agar; Fisher Scientific), supplemented with the appropriate antibiotics. Escherichia coli strains were used as cloning hosts and were grown in Luria-Bertani (LB) broth (Difco) or LB agar supplemented with appropriate antibiotics at 37°C with shaking (250 rpm). Composition of vaginally defined medium (VDM) and synthetic nasal medium (SNM) was described previously (18, 19). Glucose composition in these media was modified depending on experimental conditions or replaced with sodium pyruvate as needed. S. aureus was grown in these media aerobically with shaking (250 rpm) and could be supplemented with the appropriate antibiotics. For hemolytic and proteolytic activity analysis, BHI and VDM with or without modified glucose levels were used as solid media (1.5% agar added). Media were also supplemented with either 5% human blood or 1.5% skimmed milk powder for hemolytic and proteolytic analysis, respectively.
TABLE 1.
Bacterial strains used in this study
| Strain | Description | Source or reference |
|---|---|---|
| E. coli strains | ||
| XL1-Blue | General cloning strain | Stratagene |
| SA30B | DNA methylation strain | 47 |
| S. aureus strains | ||
| MN8 | Prototypic menstrual TSS strain, tst+ | 53 |
| MN8 ΔccpA | MN8 containing a deletion of the ccpA gene | This study |
| MN8 Δagr | MN8 with the agr operon replaced with a tetR marker | 31 |
| MN8 Δpsmα | MN8 containing a deletion in the psmα operon | 17 |
| MN8 ΔsrrAB | MN8 containing deletions of the srrA and srrB genes | 15 |
| MN8 ΔsarA | MN8 containing a deletion of the sarA gene | 40 |
| MN8 Δrot | MN8 containing a deletion of the rot gene | 17 |
| MN8 ΔccpA (+ccpA) | MN8 ΔccpA containing pRMC2::ccpA | This study |
| MN8 ΔccpA (pRMC2) | MN8 ΔccpA containing pRMC2 | This study |
| MN8 (pAmilux::Ptst) | MN8 containing pAmilux::Ptst | 40 |
| MN8 ΔccpA (pAmilux::Ptst) | MN8 ΔccpA containing pAmilux::Ptst | This study |
| MN8 Δagr (pAmilux::Ptst) | MN8 Δagr containing pAmilux::Ptst | 17 |
| MN8 ΔsrrAB (pAmilux::Ptst) | MN8 ΔsrrAB containing pAmilux::Ptst | 15 |
| MN8 ΔsarA (pAmilux::Ptst) | MN8 ΔsarA containing pAmilux::Ptst | 40 |
| MN8 Δrot (pAmilux::Ptst) | MN8 Δrot containing pAmilux::Ptst | 17 |
Luciferase-based promoter reporter assay.
Strains were grown overnight in TSB and subcultured in the studied media to an optical density at 600 nm (OD600) of 0.01 to be distributed in a 96-well plate. The plate was monitored for the growth (OD600) and activity of the pAmilux::tst reporter (Table 2) (luminescence) for 19 h in a Biotek Synergy H4 multimode plate reader at 37°C with shaking. Following completion of growth curve, promoter activity was normalized to OD600 by taking the area under the curve of the relative light units (RLU) and dividing that by the area under the curve of the growth (i.e., OD600) to give normalized tst transcription.
TABLE 2.
Plasmids used in this study
| Plasmid | Description | Reference or source |
|---|---|---|
| pKOR1 | Temp-sensitive integration vector with inducible counter selection; Cmr | 46 |
| pKOR1::ccpA | ccpA deletion plasmid; Cmr | This study |
| pRMC2 | Inducible S. aureus complementation plasmid; Cmr | 48 |
| pRMC2::ccpA | Complementation plasmid containing ccpA; Cmr | This study |
| pAmilux::Ptst | TSST-1 promoter reporter plasmid; Cmr | 31 |
SDS-PAGE and TSST-1 Western blot analyses.
S. aureus MN8 and mutant constructs were grown in various medium compositions. Extracellular proteins were separated from bacterial cells by centrifugation, and a volume of supernatant equivalent to 14 absorbance units (OD600) was harvested to normalize samples for analysis. The resulting exoproteins were precipitated using trichloroacetic acid (TCA) (added at 6% vol/vol) and washed once with acetone prior to resuspension in 8 M urea. For TSST-1 protein expression analysis, protein samples, including a recombinant TSST-1 control (45), were separated using SDS-PAGE performed on Any kD precast gels (Bio-Rad) unless otherwise stated. These gels were run for 40 min at 200 V as per the manufacturer’s instructions and either stained using ReadyBlue stain (Sigma-Aldrich) or transferred to polyvinylidene difluoride (PVDF) membranes (Millipore) at 100 V for 1 h for Western blot analysis. The membrane was blocked overnight at 4°C with phosphate-buffered saline (PBS) containing 5% skimmed milk, 10% normal horse serum (NHS; Gibco), and 10% fetal calf serum (FCS) (Wisent, Inc.). After removal of the blocking solution, the membrane was incubated for 1 h at room temperature with rabbit polyclonal anti-TSST-1 antisera diluted 1:1,000 in PBS containing 2.5% skimmed milk, 5% NHS, and 5% FCS. Anti-TSST-1 polyclonal rabbit antisera were generated by ProSci Incorporated (USA) using recombinant TSST-1 protein. Membranes were washed three times with PBS containing 0.05% Tween 20 (PBST) (Fisher Scientific) followed by incubation with IRDye 800-conjugated donkey anti-rabbit IgG antibody (Rockland) diluted 1:10,000 in PBS containing 2.5% skimmed milk, 5% NHS, and 5% FCS at room temperature for 1 h in the dark. Membranes were washed three times with PBST and imaged with an Odyssey imager (LI-COR Biosciences).
Construction of the MN8 ΔccpA mutant.
Markerless deletion of ccpA in MN8 was performed using the pKOR1 allelic replacement system (see Table 2) (46). DNA fragments consisting of a 986-bp product upstream of the ccpA gene (including the first three codons) and 983-bp product downstream of ccpA (including the last five codons) were amplified by PCR (using primers listed in Table 3) from purified S. aureus MN8 genomic DNA. These fragments were ligated together and cloned into pKOR1 by using the Gateway BP Clonase II system (Life Technologies). The cloned plasmids were transformed into Escherichia coli XL1-Blue and screened for plasmids containing the insert. The confirmed knockout construct was chemically transformed into E. coli SA30B (47) to methylate the plasmid for electrotransformation into S. aureus MN8 (48). The plasmid integration and excision to knock out the ccpA were performed as described previously (46), and candidate constructs were screened by PCR.
TABLE 3.
Primers used in this study
| Primer | Sequence (5′–3′) |
|---|---|
| pKOR-ccpA-upstream-Fora | GGGGACAAGTTTGTACAAAAAAGCAGGCTAAAGAAGCTGGCCGTACGAA |
| pKOR-ccpA-upstream-Rev | AACTGTCATAATTTCCTCCTTG |
| pKOR-ccpA-downstream-Forb | ACAAAATAAATTCACAAAATTAGGC |
| pKOR-ccpA-downstream-Reva | GGGGACCACTTTGTACAAGAAAGCTGGGTGCATATGCACCGCCATAGTG |
| CcpA-complement-KpnI-Forc | ACACGGTACCTGAAAACGTTTACAAGGAGGA |
| CcpA-complement-SacI-Revc | CCCCCGAGCTCTTATTTTGTAGTTCCTCGGTA |
attB sites used for recognition by BP clonase are shown in boldface.
Primer contained a 5′ phosphate group for blunt end ligation.
Restriction sites (indicated in the primer name) are underlined in the primer sequence.
Construction of pRMC2::ccpA complementation plasmid.
A complementation plasmid for ccpA constructed to express the protein in the S. aureus MN8-null mutant was created using the pRMC2 (Table 2)-inducible plasmid previously described, with modifications (48). Briefly, the ccpA coding sequence was amplified by PCR along with the endogenous ribosome binding site (RBS) upstream of the start codon. Primers for this PCR also incorporated cut sites for the KpnI and SacI (NEB) into the PCR product at the 3′ and 5′ ends, respectively (Table 3). The PCR product was digested with these restriction enzymes along with empty pRMC2 vector and ligated together with T4 DNA ligase (NEB). Sequence-positive constructs were transformed into E. coli SA30B (47) for appropriate methylation before transformation of sequence-positive constructs into electrocompetent S. aureus MN8 ΔccpA using a protocol previously described (49).
Human PBMC stimulation assays.
To determine the T cell mitogenic activity of S. aureus toxins and supernatants, IL-2 production assays were performed on peripheral blood mononuclear cells (PBMCs). These cells were isolated from human blood by density-based centrifugation following layering of the blood onto Ficoll-Hypaque Plus (GE Healthcare). Cells were isolated and washed in RPMI three times (Gibco) and then resuspended in complete RPMI (RPMI supplemented with 10% FCS, 1 mM HEPES, 1% penicillin-streptomycin solution [Gibco], 200 mM glutamine, 1% [vol/vol] nonessential amino acids [Gibco], 20 μM sodium pyruvate, and 2 μg/mL polymyxin B). The cell suspension was seeded into 96-well plates to a final concentration of 1.0 × 106 cells/mL. Titrating concentrations of recombinant TSST-1 or S. aureus supernatants were added to cells and incubated for 18 h at 37°C with 5% CO2. Supernatants were assayed for IL-2 by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (Thermo Fisher). Plates were read on a Biotek Synergy H4 multimode plate reader to determine colorimetric absorbance.
RNA-seq analysis.
S. aureus MN8 and MN8 ΔccpA were grown in VDM for 4 or 8 h. At harvest, the bacteria were normalized to an OD600 of 1 and pelleted by centrifugation. The bacterial pellet was treated with RNAprotect bacteria reagent (Qiagen) and frozen until further processing. RNA was extracted from the bacteria by using the RNeasy Plus minikit (Qiagen) according to the manufacturer’s instructions with an additional prelysis step where the bacteria were treated with 100 μg/mL of lysostaphin in 100 mM Tris buffer at 37°C for 1 h prior to use in the extraction kit. Purified RNA was treated with a Turbo DNase Plus kit (Life Technologies) according to the manufacturer’s instructions to remove contaminating DNA. Following purification, RNA integrity was assessed using an Agilent Bioanalyzer; RNA integrity numbers (RIN) above 9 were submitted for transcriptome sequencing (RNA-seq) analysis. RNA sequencing and comparative analysis were performed by the Microbial Genome Sequencing Center (MiGS), Pittsburgh, Pennsylvania, USA. After 12 million paired-end Illumina sequencing, read mapping was performed with HISAT2 (50). Read quantification was performed using Subread’s featureCounts (51) functionality. Read counts were normalized in R (https://www.r-project.org/) using edgeR’s trimmed mean of M values (TMM) algorithm, and the subsequent values were converted to counts per million (cpm) (52). Differential expression analysis was performed using edgeR’s exact test for differences between two groups of negative-binomial counts with an estimated dispersion value of 0.1. Comparisons were performed using the publicly available S. aureus MN8 genome (GenBank accession no. CM000952).
Statistical analysis.
All statistical analysis was performed using GraphPad Prism 9 unless otherwise stated.
Data availability.
Raw RNA read data were deposited at NCBI under BioProject accession no. PRJNA825897.
ACKNOWLEDGMENT
This work was supported by the Kimberly-Clark Corporation.
Footnotes
Supplemental material is available online only.
Contributor Information
John K. McCormick, Email: john.mccormick@uwo.ca.
Stephen W. Tuffs, Email: stuffs@uwo.ca.
Michael J. Federle, University of Illinois at Chicago
REFERENCES
- 1.Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG. 2015. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 28:603–661. 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, Nouwen JL. 2005. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis 5:751–762. 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 3.Gagnaire J, Verhoeven PO, Grattard F, Rigaill J, Lucht F, Pozzetto B, Berthelot P, Botelho-Nevers E. 2017. Epidemiology and clinical relevance of Staphylococcus aureus intestinal carriage: a systematic review and meta-analysis. Expert Rev Anti Infect Ther 15:767–785. 10.1080/14787210.2017.1358611. [DOI] [PubMed] [Google Scholar]
- 4.Deng L, Schilcher K, Burcham LR, Kwiecinski JM, Johnson PM, Head SR, Heinrichs DE, Horswill AR, Doran KS. 2019. Identification of key determinants of Staphylococcus aureus vaginal colonization. mBio 10:e02321-19. 10.1128/mBio.02321-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foster TJ. 2005. Immune evasion by staphylococci. Nat Rev Microbiol 3:948–958. 10.1038/nrmicro1289. [DOI] [PubMed] [Google Scholar]
- 6.Foster TJ, Geoghegan JA, Ganesh VK, Höök M. 2014. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol 12:49–62. 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alonzo F, Torres VJ. 2014. The bicomponent pore-forming leucocidins of Staphylococcus aureus. Microbiol Mol Biol Rev 78:199–230. 10.1128/MMBR.00055-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuffs SW, Haeryfar SMM, McCormick JK. 2018. Manipulation of innate and adaptive immunity by staphylococcal superantigens. Pathogens 7:53. 10.3390/pathogens7020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jenul C, Horswill AR. 2019. Regulation of Staphylococcus aureus virulence. Microbiol Spectr 7. 10.1128/microbiolspec.GPP3-0031-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spaulding AR, Salgado-Pabón W, Kohler PL, Horswill AR, Leung DYM, Schlievert PM. 2013. Staphylococcal and streptococcal superantigen exotoxins. Clin Microbiol Rev 26:422–447. 10.1128/CMR.00104-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCormick JK, Yarwood JM, Schlievert PM. 2001. Toxic shock syndrome and bacterial superantigens: an update. Annu Rev Microbiol 55:77–104. 10.1146/annurev.micro.55.1.77. [DOI] [PubMed] [Google Scholar]
- 12.Dinges MM, Orwin PM, Schlievert PM. 2000. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev 13:16–34. 10.1128/CMR.13.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burian M, Grumann D, Holtfreter S, Wolz C, Goerke C, Bröker BM. 2012. Expression of staphylococcal superantigens during nasal colonization is not sufficient to induce a systemic neutralizing antibody response in humans. Eur J Clin Microbiol Infect Dis 31:251–256. 10.1007/s10096-011-1302-2. [DOI] [PubMed] [Google Scholar]
- 14.Verkaik NJ, de Vogel CP, Boelens HA, Grumann D, Hoogenboezem T, Vink C, Hooijkaas H, Foster TJ, Verbrugh HA, van Belkum A, van Wamel WJ. 2009. Anti-staphylococcal humoral immune response in persistent nasal carriers and noncarriers of Staphylococcus aureus. J Infect Dis 199:625–632. 10.1086/596743. [DOI] [PubMed] [Google Scholar]
- 15.Tiwari N, López-Redondo M, Miguel-Romero L, Kulhankova K, Cahill MP, Tran PM, Kinney KJ, Kilgore SH, Al-Tameemi H, Herfst CA, Tuffs SW, Kirby JR, Boyd JM, McCormick JK, Salgado-Pabón W, Marina A, Schlievert PM, Fuentes EJ. 2020. The SrrAB two-component system regulates Staphylococcus aureus pathogenicity through redox sensitive cysteines. Proc Natl Acad Sci USA 117:10989–10999. 10.1073/pnas.1921307117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrey DO, Jousselin A, Villanueva M, Renzoni A, Monod A, Barras C, Rodriguez N, Kelley WL. 2015. Impact of the regulators sigB, rot, sarA and sarS on the toxic shock tst promoter and TSST-1 expression in Staphylococcus aureus. PLoS One 10:e0135579. 10.1371/journal.pone.0135579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuffs SW, Herfst CA, Baroja ML, Podskalniy VA, DeJong EN, Coleman CEM, McCormick JK. 2019. Regulation of toxic shock syndrome toxin-1 by the accessory gene regulator in Staphylococcus aureus is mediated by the repressor of toxins. Mol Microbiol 112:1163–1177. 10.1111/mmi.14353. [DOI] [PubMed] [Google Scholar]
- 18.Krismer B, Liebeke M, Janek D, Nega M, Rautenberg M, Hornig G, Unger C, Weidenmaier C, Lalk M, Peschel A. 2014. Nutrient limitation governs Staphylococcus aureus metabolism and niche adaptation in the human nose. PLoS Pathog 10:e1003862. 10.1371/journal.ppat.1003862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geshnizgani AM, Onderdonk AB. 1992. Defined medium simulating genital tract secretions for growth of vaginal microflora. J Clin Microbiol 30:1323–1326. 10.1128/jcm.30.5.1323-1326.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlievert PM, Blomster DA. 1983. Production of staphylococcal pyrogenic exotoxin type C: influence of physical and chemical factors. J Infect Dis 147:236–242. 10.1093/infdis/147.2.236. [DOI] [PubMed] [Google Scholar]
- 21.Seidl K, Bischoff M, Berger-Bachi B. 2008. CcpA mediates the catabolite repression of tst in Staphylococcus aureus. Infect Immun 76:5093–5099. 10.1128/IAI.00724-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seidl K, Stucki M, Ruegg M, Goerke C, Wolz C, Harris L, Berger-Bächi B, Bischoff M. 2006. Staphylococcus aureus CcpA affects virulence determinant production and antibiotic resistance. Antimicrob Agents Chemother 50:1183–1194. 10.1128/AAC.50.4.1183-1194.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Titgemeyer F, Hillen W. 2002. Global control of sugar metabolism: a gram-positive solution. Antonie Van Leeuwenhoek 82:59–71. 10.1007/978-94-017-2029-8_4. [DOI] [PubMed] [Google Scholar]
- 24.DeLeo FR, Kennedy AD, Chen L, Wardenburg JB, Kobayashi SD, Mathema B, Braughton KR, Whitney AR, Villaruz AE, Martens CA, Porcella SF, McGavin MJ, Otto M, Musser JM, Kreiswirth BN. 2011. Molecular differentiation of historic phage-type 80/81 and contemporary epidemic Staphylococcus aureus. Proc Natl Acad Sci USA 108:18091–18096. 10.1073/pnas.1111084108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGavin MJ, Arsic B, Nickerson NN. 2012. Evolutionary blueprint for host- and niche-adaptation in Staphylococcus aureus clonal complex CC30. Front Cell Infect Microbiol 2:48. 10.3389/fcimb.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zielinska AK, Beenken KE, Mrak LN, Spencer HJ, Post GR, Skinner RA, Tackett AJ, Horswill AR, Smeltzer MS. 2012. sarA-mediated repression of protease production plays a key role in the pathogenesis of Staphylococcus aureus USA300 isolates. Mol Microbiol 86:1183–1196. 10.1111/mmi.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuffs SW, Goncheva MI, Xu SX, Craig HC, Kasper KJ, Choi J, Flannagan RS, Kerfoot SM, Heinrichs DE, McCormick JK. 2022. Superantigens promote Staphylococcus aureus bloodstream infection by eliciting pathogenic interferon-gamma production. Proc Natl Acad Sci USA 119:e2115987119. 10.1073/pnas.2115987119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mirmonsef P, Hotton AL, Gilbert D, Gioia CJ, Maric D, Hope TJ, Landay AL, Spear GT. 2016. Glycogen levels in undiluted genital fluid and their relationship to vaginal pH, estrogen, and progesterone. PLoS One 11:e0153553. 10.1371/journal.pone.0153553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mirmonsef P, Hotton AL, Gilbert D, Burgad D, Landay A, Weber KM, Cohen M, Ravel J, Spear GT. 2014. Free glycogen in vaginal fluids is associated with Lactobacillus colonization and low vaginal pH. PLoS One 9:e102467. 10.1371/journal.pone.0102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Draper CF, Duisters K, Weger B, Chakrabarti A, Harms AC, Brennan L, Hankemeier T, Goulet L, Konz T, Martin FP, Moco S, van der Greef J. 2018. Menstrual cycle rhythmicity: metabolic patterns in healthy women. Sci Rep 8:14568. 10.1038/s41598-018-32647-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J, Wang W, Xu SX, Magarvey NA, McCormick JK. 2011. Lactobacillus reuteri-produced cyclic dipeptides quench agr-mediated expression of toxic shock syndrome toxin-1 in staphylococci. Proc Natl Acad Sci USA 108:3360–3365. 10.1073/pnas.1017431108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Owen DH, Katz DF. 1999. A vaginal fluid simulant. Contraception 59:91–95. 10.1016/S0010-7824(99)00010-4. [DOI] [PubMed] [Google Scholar]
- 33.Ehrström S, Yu A, Rylander E. 2006. Glucose in vaginal secretions before and after oral glucose tolerance testing in women with and without recurrent vulvovaginal candidiasis. Obstet Gynecol 108:1432–1437. 10.1097/01.AOG.0000246800.38892.fc. [DOI] [PubMed] [Google Scholar]
- 34.Sumawong V, Gregoire AT, Johnson WD, Rakoff AE. 1962. Identification of carbohydrates in the vaginal fluid of normal females. Fertil Steril 13:270–280. 10.1016/s0015-0282(16)34507-1. [DOI] [PubMed] [Google Scholar]
- 35.Stingley RL, Liu H, Mullis LB, Elkins CA, Hart ME. 2014. Staphylococcus aureus toxic shock syndrome toxin-1 (TSST-1) production and Lactobacillus species growth in a defined medium simulating vaginal secretions. J Microbiol Methods 106:57–66. 10.1016/j.mimet.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Yarwood JM, Schlievert PM. 2000. Oxygen and carbon dioxide regulation of toxic shock syndrome toxin 1 production by Staphylococcus aureus MN8. J Clin Microbiol 38:1797–1803. 10.1128/JCM.38.5.1797-1803.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yarwood JM, McCormick JK, Schlievert PM. 2001. Identification of a novel two-component regulatory system that acts in global regulation of virulence factors of Staphylococcus aureus. J Bacteriol 183:1113–1123. 10.1128/JB.183.4.1113-1123.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagner G, Bohr L, Wagner P, Petersen LN. 1984. Tampon-induced changes in vaginal oxygen and carbon dioxide tensions. Am J Obstet Gynecol 148:147–150. 10.1016/s0002-9378(84)80165-9. [DOI] [PubMed] [Google Scholar]
- 39.Andrey DO, Renzoni A, Monod A, Lew DP, Cheung AL, Kelley WL. 2010. Control of the Staphylococcus aureus toxic shock tst promoter by the global regulator SarA. J Bacteriol 192:6077–6085. 10.1128/JB.00146-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baroja ML, Herfst CA, Kasper KJ, Xu SX, Gillett DA, Li J, Reid G, McCormick JK. 2016. The SaeRS two-component system is a direct and dominant transcriptional activator of toxic shock syndrome toxin 1 in Staphylococcus aureus. J Bacteriol 198:2732–2742. 10.1128/JB.00425-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mainiero M, Goerke C, Geiger T, Gonser C, Herbert S, Wolz C. 2010. Differential target gene activation by the Staphylococcus aureus two-component system saeRS. J Bacteriol 192:613–623. 10.1128/JB.01242-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marciniak BC, Pabijaniak M, de Jong A, Dűhring R, Seidel G, Hillen W, Kuipers OP. 2012. High- and low-affinity cre boxes for CcpA binding in Bacillus subtilis revealed by genome-wide analysis. BMC Genomics 13:401. 10.1186/1471-2164-13-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vitali B, Pugliese C, Biagi E, Candela M, Turroni S, Bellen G, Donders GGG, Brigidi P. 2007. Dynamics of vaginal bacterial communities in women developing bacterial vaginosis, candidiasis, or no infection, analyzed by PCR-denaturing gradient gel electrophoresis and real-time PCR. Appl Environ Microbiol 73:5731–5741. 10.1128/AEM.01251-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pierson JD, Hansmann MA, Forney LJ. 2018. The effect of vaginal microbial communities on colonization by Staphylococcus aureus with the gene for toxic shock syndrome toxin 1 (TSST-1): a case–control study. Pathog Dis 76:fty015. 10.1093/femspd/fty015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moza B, Buonpane RA, Zhu P, Herfst CA, Nur-Ur Rahman AKM, McCormick JK, Kranz DM, Sundberg EJ. 2006. Long-range cooperative binding effects in a T cell receptor variable domain. Proc Natl Acad Sci USA 103:9867–9872. 10.1073/pnas.0600220103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bae T, Schneewind O. 2006. Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid 55:58–63. 10.1016/j.plasmid.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 47.Monk IR, Tree JJ, Howden BP, Stinear TP, Foster TJ. 2015. Complete bypass of restriction systems for major Staphylococcus aureus lineages. mBio 6:e00308-15. 10.1128/mBio.00308-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corrigan RM, Foster TJ. 2009. An improved tetracycline-inducible expression vector for Staphylococcus aureus. Plasmid 61:126–129. 10.1016/j.plasmid.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 49.Monk IR, Shah IM, Xu M, Tan M-W, Foster TJ. 2012. Transforming the untransformable: application of direct transformation to manipulate genetically Staphylococcus aureus and Staphylococcus epidermidis. mBio 3:e00277-11. 10.1128/mBio.00277-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim D, Paggi JM, Park C, Bennett C, Salzberg SL. 2019. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol 37:907–915. 10.1038/s41587-019-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liao Y, Smyth GK, Shi W. 2014. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30:923–930. 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 52.Robinson MD, McCarthy DJ, Smyth GK. 2010. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26:139–140. 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blomster-Hautamaa DA, Kreiswirth BN, Kornblum JS, Novick RP, Schlievert PM. 1986. The nucleotide and partial amino acid sequence of toxic shock syndrome toxin-1. J Biol Chem 261:15783–15786. 10.1016/S0021-9258(18)66787-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 and Tables S1 and S2. Download jb.00269-22-s0001.pdf, PDF file, 0.5 MB (475KB, pdf)
Data Availability Statement
Raw RNA read data were deposited at NCBI under BioProject accession no. PRJNA825897.