Abstract
Epoxyeicosatrienoic acids (EETs) are endogenous molecules that exerts effective antinociceptive and resolutive actions. However, because of their rapid metabolism by the soluble epoxide hydrolase (sEH) into a less active diol, EETs are unable to remain bioavailable. Thus, sEH inhibition represents a valuable pharmacological tool to control inflammatory disorders. Therefore, the aim of this study was to investigate whether local treatment with an sEH inhibitor (TPPU) could prevent inflammatory hyperalgesia in the temporomandibular joint (TMJ) of rats. For that, rats were pre-treated with an intra-TMJ injection of TPPU, followed by the noxious stimulus (1.5% of formalin intra-articular) to evaluate nociceptive behavior. Histological analysis was conducted to explore the inflammatory exudate and mast cell degranulation. Periarticular tissue over the TMJ was used to measure inflammatory lipids and cytokines/chemokine by Enzyme-Linked Immunosorbent Assay (ELISA). We demonstrated that peripheral pretreatment with TPPU prevents formalin-induced inflammatory hyperalgesia in the TMJ, and this effect is strictly local. Moreover, TPPU mitigate the leukocyte exudate in the TMJ, as well as inflammatory lipids mediators. Mast cell number and degranulation was abrogated by TPPU, and the inflammatory cytokine levels were decreased by TPPU. On the other hand, TPPU up-regulated the release of interleukin 10 (IL-10), an anti-inflammatory cytokine. In summary, we provide evidences that locally sEH by intra-TMJ injection of TPPU produces an antinociceptive and anti-inflammatory effect on rats’ TMJ.
Keywords: sEH inhibitor, TPPU, pain, mast cell degranulation, temporomandibular joint
1. INTRODUCTION
Inflammation is described as a response to harmful stimuli of a chemical, biological or physical nature. This event occurs in vascularized connective tissue, involving circulating cells, blood vessels, sensory neurons, and cellular constituents of the tissue (Tracey, 2002). During the development of the inflammatory response, several inflammatory mediators such as histamine, bradykinin, serotonin, substance P, interleukins, and eicosanoids are involved in this inflammatory looping (Vanderwall & Milligan 2019). These mediators, besides changing tissue permeability inducing leukocyte migration, also sensitizes primary sensory neurons. Consequently, inflammatory hyperalgesia, allodynia, and pain sensation are elicited (Sherwood & Toliver-Kinsky, 2004; Julius & Basbaum, 2001). Regardless of pain in the orofacial area, around 26% of the worldwide population will is affected (Macfarlane et al., 2002). Specifically, the commonest chronic pain in the orofacial area is attributed to a temporomandibular disorder (TMDs), and 49% will develop persistent pain conditions (Ettlin et al., 2021).
Eicosanoids belong to a group of lipid mediators derived from the metabolism of arachidonic acid (AA) by cyclooxygenases (COX), lipoxygenases (LOX), and cytochrome P450 (CYP450) enzymes (Wagner et al., 2017). Epoxyeicosatrienoic acids (EETs) are eicosanoids derived from the AA cascade, specifically by the CYP450 pathway. EETs are essential bioactive lipids with powerful modulatory effects on pain and inflammation (Wagner et al., 2017). However, due to their rapid metabolism, their properties are short and limited. The enzyme soluble epoxide hydrolase (sEH) is responsible for the conversion of EETs into dihydroxyeicosatrienoic acids (DHETs), losing their anti-inflammatory functions (Kodani & Morisseau 2019).
In the last decade, some sEH inhibitors were developed. For instance, the TPPU (1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl) urea, has demonstrated remarkable effects in a variety of models, including pain, inflammatory and autoimmune experimental models, and has emerged as an established sEH inhibitor in the field (Zhang et al., 2012; Harris et al., 2015; Bettaieb et al., 2015; Goswami et al., 2016, Trindade-da-Silva et al., 2020, Teixeira et al., 2021). However, the impact of the local TPPU effect was scarcely investigated and explored. Although TPPU’s actions as a transition state enzyme inhibitor that increases levels of EET’s and other epoxy-fatty acids (EpFA) and decreases pro-inflammatory diols is clear, how TPPU reduces pain and inflammation in the orofacial area when local delivered, is notable sparse.
In this regard, this study aimed to explore whether peripheral TPPU administration leads to an analgesic state in an inflammatory hypernociception model induced by formalin in the rat’s temporomandibular joint (TMJ). We explore the impact of local TPPU administration in the leukocyte migration and mast cell degranulation in the articular cavity and investigate the ability to control the cytokine storm involved in the uncontrolled inflammation.
2. MATERIALS AND METHODS
2.1. Animals
All animal procedures and experiments were performed under a protocol approved by the Committee on Animal Research of Faculdade São Leopoldo Mandic (#2018/017) and are in accordance with guideline of the National Council for Control of Animal Experimentation (CONCEA, Brazil). Sixty-two male Wistar rats (6–8 weeks, Anilab, Brazil) were housed three per cage in a climate-controlled environment with a 12h dark/light cycle, with food and water ad libitum. Rats were randomly assigned to the different groups. As a note, each rat was used only once. It is important to point out that females were not included in this study due to the hormonal cycle, which represents an important bias to the behavioral response evaluation (Clemente et al., 2004).
2.2. Drugs
The following drugs were used: Formalin solution was prepared from commercially available formalin further diluted in 0.9% NaCl to a final concentration of 1.5% (Sigma-Aldrich, St. Louis, MO, USA); the selective soluble epoxide hydrolase (sEH) inhibitor, 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl) urea (TPPU, doses ng/TMJ) was kindly provided by Dr. Bruce Hammock (University of California, Davis, California, USA) and dissolved in polyethylene glycol (PEG400) (Teixeira et al., 2020; Trindade-da-Silva et al., 2020). After dissolve TPPU in PEG400, the solution must be placed in sonification for about 5 minutes until complete dilution.
2.3. Behavioral assessment
Behavioral assessment was conducted during the light phase (from 8:00 AM to 5:00 PM), in a quiet room. Prior to any procedure, rats were kept in the test chamber for 15 minute to minimize stress related to the new environment. The rats were submitted to handling protocols for 7 days previously of the beginning of the nociceptive analysis. The protocol consisted of holding and manipulate the animals for 3–5minutes daily, during a week. Also, rats were placed into the test chamber for 10 minutes due to the habituation process for the new environment. On the day test, rats were previously anesthetized by inhalation of isoflurane and the intra-TMJ injection was performed as previously described (Clemente et al., 2004). Rats were placed back to the test chamber to completely regain consciousness (30–60s after discontinuing the anesthesia) and then the nociceptive response was evaluated over 45 minutes. This response was defined as the total number of seconds that the rat spent rubbing the orofacial region asymmetrically with the ipsilateral fore or hind paw, plus the number of head flinches counted during the observation period (Roveroni et al., 2001). The recording time was divided into 9 blocks of 5 min each. At the end of each behavior test, the rats were immediately euthanized by deeply anesthetizing and their periarticular tissue was removed as described (Lamana et al., 2017). All the behaviors analysis was conducted in a fashion where the treatments and injections are given were blinded for who analysis the behavior response.
2.4. Experimental Design
To assess whether TPPU could prevent formalin-induced nociception, rats (n=5/per group) were treated with an ipsilateral intra-TMJ injection of TPPU (0, 3, 10, 30, and 90 ng/15 μl/TMJ). Also, to investigate a possibly systemic effect, an intra-TMJ injection of TPPU (30 and 90 ng/15 μl/ TMJ) was performed contralateral. For both set of groups, after 15 minutes of TPPU treatment, rats were challenged with an ipsilateral intra-TMJ injection of 1.5% formalin (30 μl/TMJ). As a negative control, rats received an intra-TMJ injection of saline solution (45 μl/TMJ). As a positive control, rats received an intra-TMJ injection of 1.5% formalin (45 μl/TMJ). All rats received a total volume of 45 μL of solutions into the TMJ. The experimental design is summarized in Figure 1A.
Figure 1. Antinociceptive effect of sEH inhibitor (TPPU) on the formalin-induced nociceptive response in the TMJ.

A) Experimental flowchart. B) Nociceptive behavior assessment. NaCl: animals received an intra-TMJ injection of saline, but no formalin injection. 1.5% Formalin: animals were challenged with an intra-TMJ injection of formalin, allowing nociceptive assessment. PEG: animals were pretreated with an intra-TMJ injection of drug excipient without TPPU, and challenged with formalin injection. TPPU groups: animals were treated with an intra-TMJ injection of TPPU in different concentrations (3, 10, 30, 90 ng/ TMJ), and challenged with formalin injection. TPPU ct: animals were treated with TPPU (30 and 90 ng/ TMJ) into the contralateral TMJ, and challenged with formalin injection. The data are expressed as mean ± S.D. of 5 animals per group. The symbol (*) indicates a nociceptive behavior significantly higher than the saline control group (Intra TMJ saline) (p<0.05: ANOVA, Tukey’s test). The symbol (#) indicates a nociceptive behavior significantly lower than the formalin group (p<0.05: ANOVA, Tukey’s test).
2.5. Protein extraction from TMJ periarticular tissue
Samples of the periarticular tissues over the TMJ were dissected as described by Lamana and collaborators (2017). Briefly, the standard sample size was 1 × 1 × 0.5 cm, which includes temporalis, masseter, and pterygoideus externus muscles, articular cartilage, disc fibrocartilage, and ligaments. Samples were stored at −80°C until processing. Tissues samples were homogenized in 500 μl of the appropriate buffer containing protease inhibitors (Ripa Lyses Buffer, Santa Cruz, Biotechnology, Dallas, Texas, USA) using a specific sample homogenizer (BeadBlaster™ 24, Benchmark, Triple-Pure High Impact Zirconium Beads, Beads, Ø: 1.0mm). After 4 cycles of 30 seconds with resting period of 40 seconds, the samples were centrifuged for 10 min/10.000 rpm or 12298 RCF/ 4°C. The total amount of extracted proteins was colorimetrically measured using the micro bicinchoninic acid (BCA) protein assay kit (Thermo Scientific, Rockford, IL, USA). The supernatants were stored at −20°C until further analysis.
2.6. Cytokine measurement in periarticular tissue
Proteins extracted from periarticular tissues were used to quantify cytokines/chemokines and lipid mediators via Enzyme-linked immunosorbent assay (ELISA). The protein levels of necrosis factor-alpha (TNF-α), interleukin 1 beta (IL-1β), IL-6, IL-10, IL-12, chemokine (C-X-C motif) ligand 1 (CXCL1), and monocyte chemoattractant protein-1 (MCP-1) were purchased from R&D System (Minneapolis, MN, USA). The Leukotriene B4 (LTB4) and Prostaglandin E2 (PGE2) were purchased from RayBiotech Life, Inc. (GA, USA). The assays were performed according to the manufacturer’s protocol.
2.7. Histological analysis
For histological analysis, another set of groups was required (n=3 per group). The histological process was performed as previously described (Basting et al., 2021). Rats were deeply anesthetized with an intraperitoneal injection of α-chloralose (50 mg/kg; Sigma-Aldrich, St. Louis, MO, USA) and urethane (100 mg/kg; Sigma Aldrich, St. Louis, MO, USA), followed by perfusion intracardially with heparinized saline and then perfusion with 4% paraformaldehyde in phosphate-buffered saline (PBS; 0.1 M, pH 7.4, 4 °C). The entire head of the rat was removed and fixed in 10% buffered neutral formalin for 48 hours. The decalcification process was conducted in a solution of ethylenediaminetetraacetic acid (EDTA) 10% for 3 months. After this decalcification period, the head was cut off in half. The TMJ was removed in a block (1 × 1 × 0.5 cm). Samples were washed in running tap water, dehydrated and embedded in paraffin wax. Longitudinal sections (5 μm) were prepared and stained with hematoxylin and eosin (H&E) and toluidine blue. The sections were examined blinded, using light microscopy. The inflammatory score was based on leukocyte infiltration and a qualitative scale was applied of 0–5 as follow: 0 (absence of inflammatory cells); 1 (1 – 10% of inflammatory cells); 2 (11 – 25% of inflammatory cells); 3 (26 – 50% of inflammatory cells); 4 (51 – 75% of inflammatory cells) and 5 (>75% of inflammatory cells). The total number of degranulated or non-degranulated mast cell were counted in 25 microscopic fields. Total mast cells were the sum of both groups (degranulated and non-degranulated cells). Images were taken using 4x (smaller image) and 40x (bigger image), respectively.
2.8. Statistical Analysis
The statistical analyses were performed using a software program (GraphPad Prism 9.1, La Jolla, CA, USA). To determine if there were significant differences (p < 0.05) among groups, the data were analyzed using the 1-way analysis of variance (ANOVA) with post hoc contrasts using Tukey’s test. Data are presented in figures as mean ± standard deviation (SD).
3. RESULTS
3.1. The sEH inhibitor, TPPU, prevent formalin-induced inflammatory hyperalgesia in TMJ.
Initially, we assessed the antinociceptive effect of TPPU when directly injected into TMJ (Figure 1). For that, titrated doses of TPPU (0, 3, 10, 30, and 90 ng/TMJ) were used. We demonstrated here that intra-TMJ injection of 10, 30, and 90 nanograms of TPPU diminished the formalin-induced inflammatory hyperalgesia (Figure 1B, p < 0.05, one-way ANOVA, post hoc Tukey test). Interestingly, the doses of 30 and 90 nanograms revealed a behavior response equal to a saline injection, remaining in baseline levels (Figure 1B, p < 0.05, one-way ANOVA, post hoc Tukey test). We next investigate whether peripheral TPPU treatment could induce a possible systemic effect due to the high dosages. For that, contralateral (left TMJ) intra-TMJ injections were performed with 30 and 90 nanograms per TMJ, followed by the formalin challenge into the ipsilateral TMJ (right TMJ) (Figure 1B). Herein, we demonstrated that both dosages were ineffective to prevent inflammatory hyperalgesia induced by formalin (Figure 1B, p > 0.05, one-way ANOVA, post hoc Tukey test). These results confirmed that TPPU in 30 and 90 nanograms act locally, not systemically. For further experiments, we fixed the dosage to 30 nanograms per TMJ.
3.2. Peripheral soluble epoxide hydrolase inhibition blockade mast cell degranulation in the TMJ.
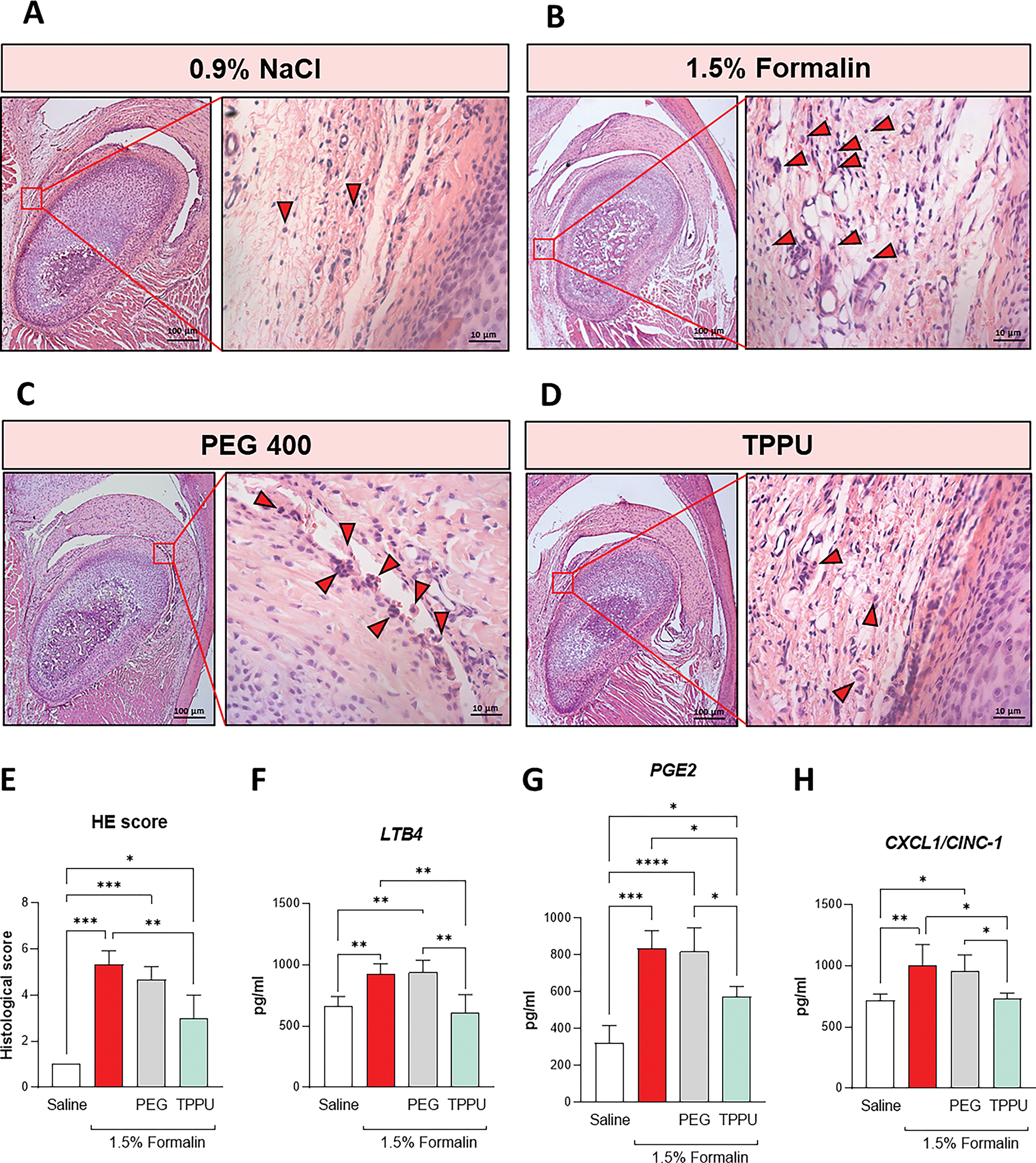
Mast cells have a pivotal role in this formalin-induced pain model, especially in the inflammatory phase (Parada et al., 2001). In this sense, we investigate whether TPPU could affect mast cell degranulation, and consequently, the release of granules carrying bioactive molecules (Figure 2). Histological sections with toluidine blue staining were made in the TMJ (Figure 2A – D). Intense degranulation process of mast cell was observed in formalin and vehicle (PEG400) group (indicated by the redhead arrows), while in the control (saline) and TPPU-treated groups, fewer degranulated mast cells were found (indicated by the blackhead arrows) (Figure 2A – D). Specifically, in terms of total mast cell counts (Figure 2E), pretreatment with TPPU diminished the higher levels induced by formalin (p < 0.05, one-way ANOVA, post hoc Tukey test). In addition, formalin increases the number and percentage of mast cell degranulated (Figures 2F and G, p < 0.05, one-way ANOVA, post hoc Tukey test), and TPPU blocked this inflammatory reaction of mast cell in formalin-induced model (p < 0.05, one-way ANOVA, post hoc Tukey test), preventing the release of bioactive molecules that exacerbates the inflammatory response.
Figure 2. TPPU abrogated leukocyte infiltration and reduces inflammatory lipids and chemical mediators in the TMJ.

Histological sections of TMJ-affected were staining with hematoxylin and eosin (H&E) stain. Representative images of A) Saline (0,9% NaCl), B) 1.5% Formalin, C) drug vehicle (PEG400), and D) sEH inhibitor (TPPU) were taken magnification of 10 and 40x, respectively. Redhead arrows indicate exudate surrounding the condylar process in the articular cavity. E) Histology score was based on the presence of leukocyte infiltration. Inflammatory lipid mediators F) LTB4 and G) PGE2, and chemokine levels of H) CXCL1/CINC1 were evaluated in the periarticular tissue over the TMJ. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (ANOVA, Tukey’s test). The data are expressed as mean ± S.D.; n = 3 animals per group for histological analysis and 5 for ELISA measurements.
3.3. TPPU mitigates leukocyte exudate in the TMJ and counteracts inflammatory mediators’ release.
To further examine the impact of soluble epoxide hydrolase inhibition on the leukocyte migration into the temporomandibular joint, histological sections stained with H&E were performed (Figures 3A – D). Formalin enhances the leukocyte infiltration when compared to saline injection (Figure 3E, p < 0.05, one-way ANOVA, post hoc Tukey test), and peripheral pretreatment with TPPU mitigated the inflammatory exudate (Figure 3E, p < 0.05, one-way ANOVA, post hoc Tukey test). In addition, the onset of acute inflammation cascade is accompanied by biosynthesis and the sequential release of several chemical mediators (Buckley et al., 2014). Therefore, we quantify the lipids level of LTB4 (Figure 3F), PGE2 (Figure 3G), and the chemokine (C-X-C motif) ligand 1 (CXCL1) (Figure 3H). In agreement with the histological findings, formalin increases LTB4, PGE2, and CXCL1 levels (p < 0.05, one-way ANOVA, post hoc Tukey test), while pretreatment with TPPU abrogated it down (p < 0.05, one-way ANOVA, post hoc Tukey test).
Figure 3. sEH inhibitor (TPPU) prevents mast cell degranulation.
A - D) Representative images. Histological sections of TMJ-affected were staining with toluidine blue stain. Blackhead arrows indicate mast cells with no degranulation process. Redhead arrows indicate mast cell degranulated, indicating their activity on the release of inflammatory mediators. Images were taken in magnification of 10 and 40x, respectively. E) Total mast cell counting, F) Mast cell degranulation, and G) Percentage of mast cell degranulation were performed analyzing 25 microscopic fields. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (ANOVA, Tukey’s test). The data are expressed as mean ± S.D.; n = 3 animals per group.
3.4. Soluble epoxide hydrolase inhibition regulates inflammatory cytokine storm in formalin-induced model in TMJ.
Inflammation is a well-coordinated process characterized by the sequential release of biochemical mediators, culminating in intense cellular leaking and inflammatory cytokine storm. Intra-articular injection of 1.5% formalin statistically increases the cytokine levels of TNF-α, IL-1β, IL-6, IL-12, and MCP-1/CCL2 (Figure 4A – E, p < 0.05, one-way ANOVA, post hoc Tukey test) when compared to saline group. In contrast, peripheral pretreatment with TPPU significantly decreases the formalin-induced cytokines levels (Figure 4A – E, p < 0.05, one-way ANOVA, post hoc Tukey test). Moreover, pretreatment with TPPU significantly increases IL-10, an essential cytokine for the inflammation resolution (Figure 4F, p < 0.05, one-way ANOVA, post hoc Tukey test).
Figure 4. sEH inhibition regulates inflammatory cytokine storm while improves IL-10 releases.

Cytokines levels of A) TNF-α, B) IL-1β, C) IL-6, D) IL-12, E) MCP-1/CCL2, and F) IL-10 were quantitated in periarticular tissue over TMJ. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (ANOVA, Tukey’s test). The data are expressed as mean ± S.D.; n = 5 animals per group.
4. DISCUSSION
Prevention and management of inflammatory pain, especially chronic pain, still is challenging for clinicians and a burden for patients. Many efforts are being made to develop a drug with effective analgesic action and immunomodulatory effects, which is not immunosuppressive and ideally works locally. Here, we showed that locally delivered of TPPU induces antinociceptive effects on rats TMJ. Mechanistically, we demonstrated that peripheral TPPU mitigates leukocyte infiltration and inhibits mast cell degranulation. Moreover, TPPU prevents the ongoing inflammatory cytokine storm, orchestrating the inflammation process to their resolution.
It has been demonstrated in the literature that TPPU shown analgesic properties when orally administrated (Wagner et al., 2017; Teixeira et al., 2020); here we investigated the peripheral effects related to TPPU when locally delivered in the orofacial area, which remains not fully understood. For that, a formalin model was employed to evoke pain. We demonstrated that intra-TMJ injection of TPPU has antinociceptive activity, and this effect initiated with 10 nanograms per TMJ. As a comparative purpose, this dose is 1000-fold lower when compared to systemic doses of different sEH inhibitors, including TPPU (Wagner et al., 2017). Also, it is 50-fold lower when TPPU is compared to intra-TMJ injection of tramadol (500 μg/ TMJ) (Lamana et al., 2017). Glucocorticoids and opioids are the gold standard treatment to manage pain in TMJ (Romero-Reyes and Uyanik, 2014; Gil-Martínezet al., 2018), however, multiple side effects are related to those treatments, such as addiction, a decrease of bone density, adrenal suppression and induction of osteoclastogenic activity, which makes prolonged-use questionable (Romero-Reyes and Uyanik, 2014; Borresen et al., 2015; Gil-Martínezet al., 2018). Herein, by inhibiting the soluble epoxide hydrolase enzyme and subsequently enhancing of endogenous EETs levels, TPPU prevents formalin-induced inflammatory hyperalgesia, being considered a safe and effective candidate to manage orofacial pain state.
Formalin-evoked pain is a well-known model to investigate analgesic and anti-inflammatory properties due to the biphasic action when injected into the tissue. The nociceptive response of formalin occurs firstly in an acute period, due to chemical damage; a second period (inflammatory phase), often after a remission period, due to the release of inflammatory mediators (Parada et al., 2001). It has been shown that histamine (H), serotonin (5-HT), prostaglandins, and bradykinin are fundamental molecules involved in the induction of nociception by formalin (Hong & Abbot 1994). Specifically, co-administration of pyrilamine (a selective H1-receptor antagonist) inhibits both phases of formalin response (Parada et al., 2001). In addition, specific antagonists for 5-HT1A and 3 receptors decrease the nociception induced by formalin (Fischer et al., 2016). In this scenario, mast cell represents the primary source for both inflammatory mediators, and pretreatment with sodium cromoglycate (a mast cell stabilizer) avoids mast cell degranulation and significantly reduces pain-evoked by formalin (Parada et al., 2001). Thus, in agreement with the antinociceptive behavior, we demonstrated that TPPU prevents mast cell degranulation and significantly decreases total mast cells. This is an important finding since formalin leads to a neuroimmune interaction between mast cells and primary sensory neurons. In addition, it is known that inflammatory pain possesses an important sympathomimetic component, and TMJ receives a rich innervation from this component (Parada et al., 2001). This is a reasonable explanation for why non-steroidal anti-inflammatory drugs (NSAIDs) demonstrate less efficacy in controlling pain in TMDs than other body regions (Chichorro et al., 2004). For last, the initial nociception induced by formalin (first phase) results in a self-sustainable mechanism activating TRPA1 in neurons, which in turn release substance P (SP) in an antidromic response (Fischer et al., 2016). Higher amounts of SP in tissue activate the NK1 (Neurokinin 1) receptor in mast cells inducing their degranulation (Fischer et al., 2016). This event is pivotal to the inflammatory phase of formalin action. Therefore, our results suggest that by avoiding mast cell degranulation, the soluble epoxide hydrolase inhibition prevents the activation of the sympathetic component and nociception by consequence.
It is well known that inflammation response results in an influx of polymorphonuclear leukocytes (PMNs) due to increased vascular permeability and blood flow to the wound sites. In terms of pain behavior, it is accepted that pain sensation is closely related to the neuroimmune interaction upon stimulation of immune cell-derived cytokines, growth factors, lipids, and proteases (Pinho-Ribeiro et al., 2017). We demonstrated that pretreatment with TPPU blockade the leukocyte migration induced by formalin in the TMJ, resulting in less pain sensation as previously discussed. Moreover, this decreased leukocyte infiltration is accompanied by the reduction of inflammatory lipids mediators and cytokine-induced neutrophil chemoattractant 1 (CINC-1)/CXCL1 production induced by local TPPU treatment.
For instance, in a carrageenan-induced inflammatory pain (Cunha et al., 2008) and neuropathic model (Kiguchi et al., 2012), it was showed that neutrophils migrate into the damaged tissue, maintaining the pain sensation via cytokines and PGE2 production. Formalin-model follows the same pattern; the neuronal inputs in the first phase are not sufficient to maintain the second phase, indicating that endogenous inflammatory chemical mediators and leukocyte migration must occur (Parada, 2001). Interestingly, unlike PGE2, leukotrienes (LT) seems to not display an essential role in formalin-induced nociception since inhibition of LT synthesis does not affect both phases of formalin behavior (Chichorro et al., 2004). However, it is essential to mention that the authors did not measure LTB4 levels, and formalin was injected into the upper lip while we injected it into the TMJ. Although LTB4 does not directly induce pain, it is a well-known chemoattractant of neutrophils, an important cell source of inflammatory mediators (Verri et al., 2006, Kanashiro et al., 2020). Clinically, PGE2 and LTB4 are elevated in TMJ synovial liquid of patients with arthritis, and PGE2 is also linked with allodynia states (Kopp 2001). Significantly, TPPU reduces both the lipid levels of PGE2 and LTB4 in the formalin-induced group.
Cytokines are equally endogenous hyperalgesic mediators like prostaglandins, prostanoids, and sympathomimetic amines and are released by immune cells (e.g., dendritic cells, macrophages, lymphocytes, neutrophils, mast cell). Our results demonstrated that formalin increases the protein levels of TNF-α, IL-1β, IL-6, IL-12, and MCP1, and sEH inhibition reduces these increased levels. The immune system can stimulate neuronal hyperexcitability through cytokine releases by sensitizing the primary sensory neurons, resulting in pain (Pinho-Ribeiro et al., 2017; Zanellato et al., 2018; Gomes et al., 2020; Abdalla et al., 2020). In contrast, IL-10 was enhanced by TPPU. IL-10 was the first anti-inflammatory cytokine described (Fiorentino et al., 1991) and plays a regulatory role in inflammatory diseases by reducing inflammatory mediators release, cellular infiltrate, and joint disruption (Teixeira et al., 2020). These findings point out that by inhibiting sEH, EETs became bioavailable in tissue and regulates the inflammatory response by switching lipid mediator class, limiting the exacerbated response, and driven to homeostasis. As a note, EETs and other lipids mediators (e.g., epoxy fatty acids) stimulate the production of the SPMs (Specialized pro-resolving mediators), which is associated with shifting the biochemical process of inflammation from a massive inflammatory storm toward an active endogenous resolutive pathway (Panigrahy et al., 2020; Hammock et al., 2020).
In summary, the present study provides evidence that local intra-TMJ injection of the sEH inhibitor, TPPU, induces antinociceptive and anti-inflammatory effects in the TMJ. Furthermore, the sEH inhibition prevents mast cell degranulation and leukocyte infiltration, reinforcing the antinociceptive and anti-inflammatory properties. Likewise, inflammatory lipids mediators, cytokines, and chemokines were also diminished, preventing primary sensory neuronal hyperexcitability. Besides, an anti-inflammatory cytokine, IL-10, was enhanced, which indicates the activation of resolving pathways of inflammation and the resumption of hemostasis of the injured tissue. Therefore, local treatments with TPPU seems to be an excellent pharmacological tool to manage TMJ’s inflammatory pain.
ACKNOWLEDGMENTS:
We would like to thank Mariana Franco and Elisangela Juvencio for animal care. We also thank Nadir de Freitas for the histological support. We appreciate Sung Hee Hwang for the synthesis of TPPU.
FUNDING
This work was supported by São Paulo Research Foundation, FAPESP (#2017/22334–9 and #2018/05575–5) and by National Institute of Environmental Health Sciences (NIEH) River Award R35ES030443 and NIEHS/Superfund Research Program P42 ES004699. National Council for Scientific and Technological Development – CNPq by Research Productivity Fellowship to MHN and JTCN, and Coordination of Superior Level Staff Improvement (CAPES) – Financial code 001.
REFERENCES
- 1.Abdalla HB, Napimoga MH, Macedo CG, Bonfante R, De Araujo DR, de Mello NFS, Carvalho LB, Fraceto LF, Clemente-Napimoga JT. Poloxamer micellar system for intra-articular injection of 15-deoxy-Δ12,14-prostaglandin J2 with improved bioavailability and anti-inflammatory properties in the temporomandibular joint of rats. Int J Pharm. 2020. Jun 15; 583:119383. [DOI] [PubMed] [Google Scholar]
- 2.Basting RT, Napimoga MH, de Lima JM, de Freitas NS, Clemente-Napimoga JT. Fast and accurate protocol for histology and immunohistochemistry reactions in temporomandibular joint of rats. Arch Oral Biol. 2021. Jun; 126:105115. [DOI] [PubMed] [Google Scholar]
- 3.Bettaieb A, Chahed S, Bachaalany S, Griffey S, Hammock BD, Haj FG. Soluble epoxide hydrolase pharmacological inhibition ameliorates experimental acute pancreatitis in mice. Mol Pharmacol. 2015; 88:281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouhlel MA, Derudas B, Rigamonti E, Dièvart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, Staels B, Chinetti-Gbaguidi G. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. (2007) Aug; 6(2):137–43. [DOI] [PubMed] [Google Scholar]
- 5.Buckley CD, Gilroy DW, Serhan CN. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity. 2014. Mar 20;40(3):315–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai Z, Zhao G, Yan J, Liu W, Feng W, Ma B, Yang L, Wang JA, Tu L, Wang DW. CYP2J2 overexpression increases EETs and protects against angiotensin II-induced abdominal aortic aneurysm in mice. J Lipid Res. 2013. May; 54(5):1448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM. PPAR-gamma dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med. (2001) Jan; 7(1):48–52. [DOI] [PubMed] [Google Scholar]
- 8.Chichorro JG, Lorenzetti BB, & Zampronio AR (2004). Involvement of bradykinin, cytokines, sympathetic amines and prostaglandins in formalin-induced orofacial nociception in rats. Br J Pharmacol 141(7), 1175–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chima RS, Hake PW, Piraino G, Mangeshkar P, Denenberg A, Zingarelli B. Ciglitazone ameliorates lung inflammation by modulating the inhibitor kappaB protein kinase/nuclear factor-kappaB pathway after hemorrhagic shock. Crit Care Med. 2008. Oct; 36(10):2849–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clemente JT, Parada CA, Veiga MC, Gear RW, Tambeli CH. Sexual dimorphism in the antinociception mediated by kappa opioid receptors in the rat temporomandibular joint. Neurosci Lett. 2004. Dec 6;372(3):250–5. doi: 10.1016/j.neulet.2004.09.048. [DOI] [PubMed] [Google Scholar]
- 11.Cunha TM, Verri WA Jr, Schivo IR, Napimoga MH, Parada CA, Poole S, Teixeira MM, Ferreira SH, Cunha FQ. Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. J Leukoc Biol. 2008. Apr;83(4):824–32. [DOI] [PubMed] [Google Scholar]
- 12.Cuzzocrea S, Mazzon E, Dugo L, Patel NS, Serraino I, Di Paola R, Genovese T, Britti D, De Maio M, Caputi AP, Thiemermann C. Reduction in the evolution of murine type II collagen-induced arthritis by treatment with rosiglitazone, a ligand of the peroxisome proliferator-activated receptor gamma. Arthritis Rheum. 2003. Dec; 48(12):3544–56. [DOI] [PubMed] [Google Scholar]
- 13.Dai M, Wu L, He Z, Zhang S, Chen C, Xu X, Wang P, Gruzdev A, Zeldin DC, Wang DW. Epoxyeicosatrienoic acids regulate macrophage polarization and prevent LPS-induced cardiac dysfunction. J Cell Physiol. 2015. Sep; 230(9):2108–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandes TL, Gomoll AH, Lattermann C, Hernandez AJ, Bueno DF, Amano MT. Macrophage: A Potential Target on Cartilage Regeneration. Front Immunol. 2020. Feb 11; 11:111. doi: 10.3389/fimmu.2020.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’Garra A, 1991. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 147 (11), 3815–3822. [PubMed] [Google Scholar]
- 16.Fischer L, Lavoranti MI, de Oliveira Borges M, Miksza AF, Sardi NF, Martynhak BJ, Tambeli CH, Parada CA. TRPA1, substance P, histamine and 5-hydroxytryptamine interact in an interdependent way to induce nociception. Inflamm Res. 2017. Apr;66(4):311–322. [DOI] [PubMed] [Google Scholar]
- 17.Gil-Martínez A, Paris-Alemany A, López-de-Uralde-Villanueva I, and La Touche R (2018) Management of pain in patients with temporomandibular disorder (TMD): challenges and solutions. J Pain Res 11:571–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goswami SK, Wan D, Yang J, et al. Anti-ulcer efficacy of soluble epoxide hydrolase inhibitor TPPU on diclofenac-induced intestinal ulcers. J Pharmacol Exp Ther. 2016; 357:529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammock BD, Wang W, Gilligan MM, Panigrahy D. Eicosanoids: The Overlooked Storm in Coronavirus Disease 2019 (COVID-19)? Am J Pathol. 2020. Sep; 190(9):1782–1788. doi: 10.1016/j.ajpath.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris TR, Bettaieb A, Kodani S, et al. Inhibition of soluble epoxide hydrolase attenuates hepatic fibrosis and endoplasmic reticulum stress induced by carbon tetrachloride in mice. Toxicol Appl Pharmacol. 2015; 286:102–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong Y, Abbott FV. Behavioural effects of intraplantar injection of inflammatory mediators in the rat. Neuroscience. 1994. Dec; 63(3):827–36. [DOI] [PubMed] [Google Scholar]
- 22.Kanashiro A, Hiroki CH, da Fonseca DM, Birbrair A, Ferreira RG, Bassi GS, Fonseca MD, Kusuda R, Cebinelli GCM, da Silva KP, Wanderley CW, Menezes GB, Alves-Fiho JC, Oliveira AG, Cunha TM, Pupo AS, Ulloa L, Cunha FQ. The role of neutrophils in neuroimmune modulation. Pharmacol Res. 2020. Jan; 151:104580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiguchi N, Kobayashi Y, Maeda T, Fukazawa Y, Tohya K, Kimura M, Kishioka S. Epigenetic augmentation of the macrophage inflammatory protein 2/C-X-C chemokine receptor type 2 axis through histone H3 acetylation in injured peripheral nerves elicits neuropathic pain. J Pharmacol Exp Ther. 2012. Mar;340(3):577–87. [DOI] [PubMed] [Google Scholar]
- 24.Kodani SD, Morisseau C. Role of epoxy-fatty acids and epoxide hydrolases in the pathology of neuro-inflammation. Biochimie. (2019) Apr; 159:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neuroendocrine Kopp S., immune, and local responses related to temporomandibular disorders. J Orofac Pain. 2001. Winter;15(1):9–28. [PubMed] [Google Scholar]
- 26.Lamana SMS, Napimoga MH, Nascimento APC, Freitas FF, de Araujo DR, Quinteiro MS, Macedo CG, Fogaça CL, and Clemente-Napimoga JT (2017). The antiinflammatory effect of tramadol in the temporomandibular joint of rats. Eur J Pharmacol 807:82–90. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Zhang Y, Schmelzer K, Lee TS, Fang X, Zhu Y, Spector AA, Gill S, Morisseau C, Hammock BD, Shyy JY. The antiinflammatory effect of laminar flow: the role of PPARgamma, epoxyeicosatrienoic acids, and soluble epoxide hydrolase. Proc Natl Acad Sci U S A. 2005. Nov 15; 102(46):16747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macedo CG, Napimoga MH, Rocha-Neto LM, Abdalla HB, Clemente-Napimoga JT. The role of endogenous opioid peptides in the antinociceptive effect of 15-deoxy(Δ12,14)-prostaglandin J2 in the temporomandibular joint. Prostaglandins Leukot Essent Fatty Acids. 2016. Jul; 110:27–34. [DOI] [PubMed] [Google Scholar]
- 29.Macfarlane TV, Blinkhorn AS, Davies RM, Kincey J, Worthington HV, 2002. Orofacial pain in the community: prevalence and associated impact. Community Dent. Oral Epidemiol. 30, 52–60. [DOI] [PubMed] [Google Scholar]
- 30.Naito Y, Takagi T, Higashimura Y. Heme oxygenase-1 and anti-inflammatory M2 macrophages. Arch Biochem Biophys. (2014) Dec 15; 564:83–8. [DOI] [PubMed] [Google Scholar]
- 31.Napimoga MH, Rocha EP, Trindade-da-Silva CA, Demasi APD, Martinez EF, Macedo CG, Abdalla HB, Bettaieb A, Haj FG, Clemente-Napimoga JT, Inceoglu B, Hammock BD. Soluble epoxide hydrolase inhibitor promotes immunomodulation to inhibit bone resorption. J Periodontal Res. 2018. Oct; 53(5):743–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Napimoga MH, Vieira SM, Dal-Secco D, Freitas A, Souto FO, Mestriner FL, Alves-Filho JC, Grespan R, Kawai T, Ferreira SH, Cunha FQ. Peroxisome proliferator-activated receptor-gamma ligand, 15-deoxy-Delta12,14-prostaglandin J2, reduces neutrophil migration via a nitric oxide pathway. J Immunol. 2008. Jan 1; 180(1):609–17. [DOI] [PubMed] [Google Scholar]
- 33.Panigrahy D, Gilligan MM, Huang S, Gartung A, Cortés-Puch I, Sime PJ, Phipps RP, Serhan CN, Hammock BD. Inflammation resolution: a dual-pronged approach to averting cytokine storms in COVID-19? Cancer Metastasis Rev. 2020. Jun; 39(2):337–340. doi: 10.1007/s10555-020-09889-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parada CA, Tambeli CH, Cunha FQ, Ferreira SH. The major role of peripheral release of histamine and 5-hydroxytryptamine in formalin-induced nociception. Neuroscience. (2001); 102(4):937–44. [DOI] [PubMed] [Google Scholar]
- 35.Pena-dos-Santos DR, Severino FP, Pereira SA, Rodrigues DB, Cunha FQ, Vieira SM, Napimoga MH, Clemente-Napimoga JT. Activation of peripheral kappa/delta opioid receptors mediates 15-deoxy-(Delta12,14)-prostaglandin J2 induced-antinociception in rat temporomandibular joint. Neuroscience. 2009. Nov 10;163(4):1211–9. [DOI] [PubMed] [Google Scholar]
- 36.Pinho-Ribeiro FA, Verri WA Jr, Chiu IM. Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation. Trends Immunol. 2017. Jan;38(1):5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romero-Reyes M and Uyanik JM (2014) Orofacial pain management: current perspectives. J Pain Res 7:99–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roveroni RC, Parada CA, Cecília M, Veiga FA, Tambeli CH. Development of a behavioral model of TMJ pain in rats: the TMJ formalin test. Pain. (2001) Nov; 94(2):185–91. [DOI] [PubMed] [Google Scholar]
- 39.Borresen SW, Klose M, Rasmussen AK, Feldt-Rasmussen U, Adrenal insufficiency caused by locally applied glucocorticoids-myth or fact? Curr. Med. Chem. 22 (2015) 2801–2809. [DOI] [PubMed] [Google Scholar]
- 40.Shan ZZ, Masuko-Hongo K, Dai SM, Nakamura H, Kato T, Nishioka K. A potential role of 15-deoxy-delta (12,14)-prostaglandin J2 for induction of human articular chondrocyte apoptosis in arthritis. J Biol Chem. 2004. Sep 3; 279(36):37939–50. Epub 2004 Jun 22. [DOI] [PubMed] [Google Scholar]
- 41.Sherwood ER, Toliver-Kinsky T. Mechanisms of the inflammatory response. Best Pract Res Clin Anaesthesiol. 2004. Sep; 18(3):385–405. [DOI] [PubMed] [Google Scholar]
- 42.Teixeira JM, Abdalla HB, Basting RT, Hammock BD, Napimoga MH, Clemente-Napimoga JT. Peripheral soluble epoxide hydrolase inhibition reduces hypernociception and inflammation in albumin-induced arthritis in temporomandibular joint of rats. Int Immunopharmacol. 2020. Oct; 87:106841. doi: 10.1016/j.intimp.2020.106841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trindade-da-Silva CA, Bettaieb A, Napimoga MH, Lee KSS, Inceoglu B, Ueira-Vieira C, Bruun D, Goswami SK, Haj FG, Hammock BD. Soluble Epoxide Hydrolase Pharmacological Inhibition Decreases Alveolar Bone Loss by Modulating Host Inflammatory Response, RANK-Related Signaling, Endoplasmic Reticulum Stress, and Apoptosis. J Pharmacol Exp Ther. 2017. Jun; 361(3):408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trindade-da-Silva CA, Clemente-Napimoga JT, Abdalla HB, Rosa SM, Ueira-Vieira C, Morisseau C, Verri WA Jr, Montalli VAM, Hammock BD, Napimoga MH. Soluble epoxide hydrolase inhibitor, TPPU, increases regulatory T cells pathway in an arthritis model. FASEB J. 2020. Jul; 34(7):9074–9086. doi: 10.1096/fj.202000415R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vanderwall AG, Milligan ED. Cytokines in Pain: Harnessing Endogenous Anti-Inflammatory Signaling for Improved Pain Management. Front Immunol. 2019. Dec 23; 10:3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verri WA Jr, Cunha TM, Parada CA, Poole S, Cunha FQ, Ferreira SH. Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development? Pharmacol Ther. 2006. Oct;112(1):116–38. [DOI] [PubMed] [Google Scholar]
- 47.Wagner KM, McReynolds CB, Schmidt WK, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for pain, inflammatory and neurodegenerative diseases. Pharmacol Ther. 2017. Dec; 180:62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wahli W, Braissant O, and Desvergne B (1995). Peroxisome proliferator activated receptors: transcriptional regulators of adipogenesis, lipid metabolism and more. Chem Biol; 2(5):261–6. [DOI] [PubMed] [Google Scholar]
- 49.Zanelatto FB, Dias EV, Teixeira JM, Sartori CR, Parada CA, Tambeli CH Anti-inflammatory effects of propranolol in the temporomandibular joint of female rats and its contribution to antinociceptive action. Eur. J. Pain 2018. 22 (3), 572–582. [DOI] [PubMed] [Google Scholar]
- 50.Zhang W, Yang AL, Liao J, et al. Soluble epoxide hydrolase gene deficiency or inhibition attenuates chronic active inflammatory bowel disease in IL-10(−/−) mice. Dig Dis Sci. 2012; 57:2580–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao G, Tu L, Li X, Yang S, Chen C, Xu X, Wang P, Wang DW. Delivery of AAV2-CYP2J2 protects remnant kidney in the 5/6-nephrectomized rat via inhibition of apoptosis and fibrosis. Hum Gene Ther. 2012. Jul;23(7):688–99. [DOI] [PubMed] [Google Scholar]
- 52.Zhao G, Wang J, Xu X, Jing Y, Tu L, Li X, Chen C, Cianflone K, Wang P, Dackor RT, Zeldin DC, Wang DW. Epoxyeicosatrienoic acids protect rat hearts against tumor necrosis factor-α-induced injury. J Lipid Res. 2012. Mar;53(3):456–66. [DOI] [PMC free article] [PubMed] [Google Scholar]


