Abstract
Purpose of Review
Parkinson’s disease (PD), the second most common neurodegenerative disease, has a worldwide prevalence projected at 12 million by 2040. While PD has been extensively researched, our understanding of the disease is based on research studies that include mostly participants of European descent. The lack of diversity in clinical trial enrollment has limited the generalizability of scientific discoveries in the field. Here, we discuss contributors to racial and ethnic disparities in PD clinical research enrollment, summarize recently proposed and tested interventions, and propose next steps to increase equity and representation in PD research.
Recent Findings
Enrollment in PD clinical research is vulnerable to upstream disparities and inequities from PD awareness to access to specialized PD centers. While additional research is still needed, recent studies have identified some potential strategies for increasing underrepresented minority (URM) recruitment including increasing the availability of linguistically and culturally diverse research materials and team members, partnering with community organizations, and forming relationships with URM-serving community physicians.
Summary
To move the dial toward equity in PD research, it will be necessary to implement known successful strategies and further investigate additional contributors to the underrepresentation of URMs in PD clinical research while developing and testing interventions to address these factors.
Keywords: Parkinson’s disease, Research, Clinical trials, Equity, Diversity
Introduction
Parkinson’s disease (PD), the second most common neurodegenerative disease, affected over 6 million people worldwide in 2015 and is projected to double in prevalence to an estimated 12 million by 2040 [1, 2]. While PD has been extensively researched and described, our knowledge and management of the disease are based on research studies that include mostly participants of European descent. Clinical trials are necessary to develop, test, and ensure the safety of new therapeutics and interventions. Such research will lead to, and be informed by, additional discoveries in the epidemiology, pathophysiology, and natural history of the disease. In this increasingly diverse society, it is projected that by 2045, less than half of the US population will identify as non-Hispanic White [3]. The lack of diversity in clinical trial enrollment has been identified as a significant moral and scientific problem which has limited the generalizability of scientific discoveries and compounds known, existing disparities in the field [4].
Despite requirements from the US Food and Drug Administration (FDA) and the National Institute of Health (NIH) Revitalization Act of 1993 which requires federally funded clinical trials to include sufficient numbers of women and minority participants, there has been little progress in the diversity of clinical trials over the past several decades [5•, 6, 7]. The low reporting of racial and ethnic demographics in PD randomized controlled trials (RCTs) limits the analysis of the magnitude of the racial and ethnic disparities in PD clinical trial enrollment [5•, 8•].
A study examining all US PD clinical trials from 1985 to 2007 showed that only 17% of the trials reported the racial and ethnic makeup of the participants [9]. Of the 33 US trials that did provide this information, only 6% of the participants were non-White, compared with 20% of the general population, and only 1.7% identified as African American and 1.3% as Hispanic/Latinx. Dedicated research and specific interventions to address this disparity in PD research representation are necessary and require meaningful steps to close the gap between White and minority clinical trial recruitment, and ultimately, use this research to close the gaps in clinical outcomes. The purpose of this paper is to discuss contributors to racial and ethnic disparities in PD clinical research enrollment, summarize existing literature describing interventions to increase diversity and inclusion in PD research, and propose strategies and next steps to increase equity and representation in this area.
Contributors to Disparities in PD Clinical Research Enrollment
Enrollment of PD patients in clinical research is often contingent on the completion of prior steps in the PD journey. Therefore, disparities in PD clinical research are, at least partially, a downstream effect of the “broken ladder” effect of inequities in the field. Disparities in PD among racial and ethnic minorities have been well-documented in the literature spanning from PD diagnosis to the use of advanced therapies like deep brain stimulation (DBS) [10–12]. Most US PD prevalence studies have shown that African Americans are significantly less likely to be diagnosed with PD compared to their White counterparts, even after adjusting for other variables such as sex, age, geography, income, and annual healthcare use [10,13–15]. While biological and genetic differences between groups may exist, this is unlikely to account for the magnitude of the difference. A population-based, door-to-door study investigating PD prevalence in a county in Mississippi found a similar screening prevalence of PD in African American and White residents and a higher rate of missed diagnoses in African American residents, supporting previous research across fields which has shown that it is not biological differences but rather race and ethnicity as a surrogate for socioeconomic inequities leading to differences in education, access, beliefs, and behaviors that account for most health disparities [16, 17].
When diagnosed, African Americans are more likely to have delays in diagnosis and present with greater motor impairment and at a later Hoehn and Yahr stage [18, 19]. This has implications on PD research recruitment as it lowers the total eligible participant pool in this demographic, and later diagnosis can exclude patients from studies investigating potentially disease-modifying therapies requiring enrollment at an earlier clinical stage. With a significant recent focus on neuroprotection and prodromal PD research, we would anticipate an even lower representation of African American PD patients in these clinical trials without an intervention to address the disparities in timely diagnosis. Also, African Americans with PD have a higher all-cause mortality and higher rates of dementia compared to White PD patients, even when controlling for multiple covariates [14]. Therefore, due to the often-strict inclusion criteria of clinical trials, African Americans may be more likely to be excluded from participation because of dementia or other co-morbidities.
Another barrier to PD research enrollment is low PD knowledge and education in the general community, which is further compounded by additional barriers in minority communities. African Americans and Chinese Americans are more likely to interpret PD symptoms as normal aging when compared to White Americans [20, 21]. The low awareness of PD and PD symptoms in the community has implications on not only missed and delayed PD diagnoses in these communities, but also on the awareness and access to PD clinical trials for both PD patients and healthy controls [19]. This supports the argument for community engagement, partnership, and education as necessary components of interventions to address disparities in PD clinical research enrollment. African American and Hispanic PD patients are less likely to be cared for by a neurologist compared to White PD patients [22, 23]. This translates to a lower likelihood of being cared for by a movement disorders specialist and PD center, often the hub and recruitment base for many PD clinical trials.
In addition to the “domino effect” from other access and outcome disparities in PD among minorities, there are additional societal, provider/researcher, and institutional barriers that contribute to the underrepresentation of minorities in clinical research [24, 25]. Examples of societal barriers include low healthcare literacy or English language proficiency, mistrust of healthcare and researchers, limited access to caregiver support and transportation, and implications of direct and indirect costs of research participation [25–28]. A systematic review of provider-related barriers to research enrollment included providers’ lack of trust in investigators and sponsors, decreased awareness of available trials, method of trial communication and recruitment, and provider-related stress including limited resources and time [29]. Institution-level obstacles include healthcare access barriers given the often-specialized settings of clinical trial sites, restrictive eligibility criteria, and insufficient participant support infrastructure [26, 27]. Barriers to clinical trial participation include those at the stages of (1) recruitment, (2) screening/enrollment, and (3) retention [26]. Strategies to diversify PD clinical trials must address both barriers and facilitators at these various stages.
As described, the barriers that drive the disparities in care among underrepresented minorities (URMs) in PD are multifaceted, with some obstacles having a downstream “domino effect” compounding the lack of URM clinical trial enrollment (Fig. 1). Therefore, interventions designed to address URM recruitment in PD trials should also account for these upstream factors during project planning. Accordingly, using strategies such as community-based outreach, education, and recruitment, local stakeholder engagement, and diverse research staff may be helpful. Addressing only the downstream effects without a more comprehensive framework of the multiple drivers of these disparities in research engagement may lead to an unsuccessful recruitment intervention.
Fig. 1.
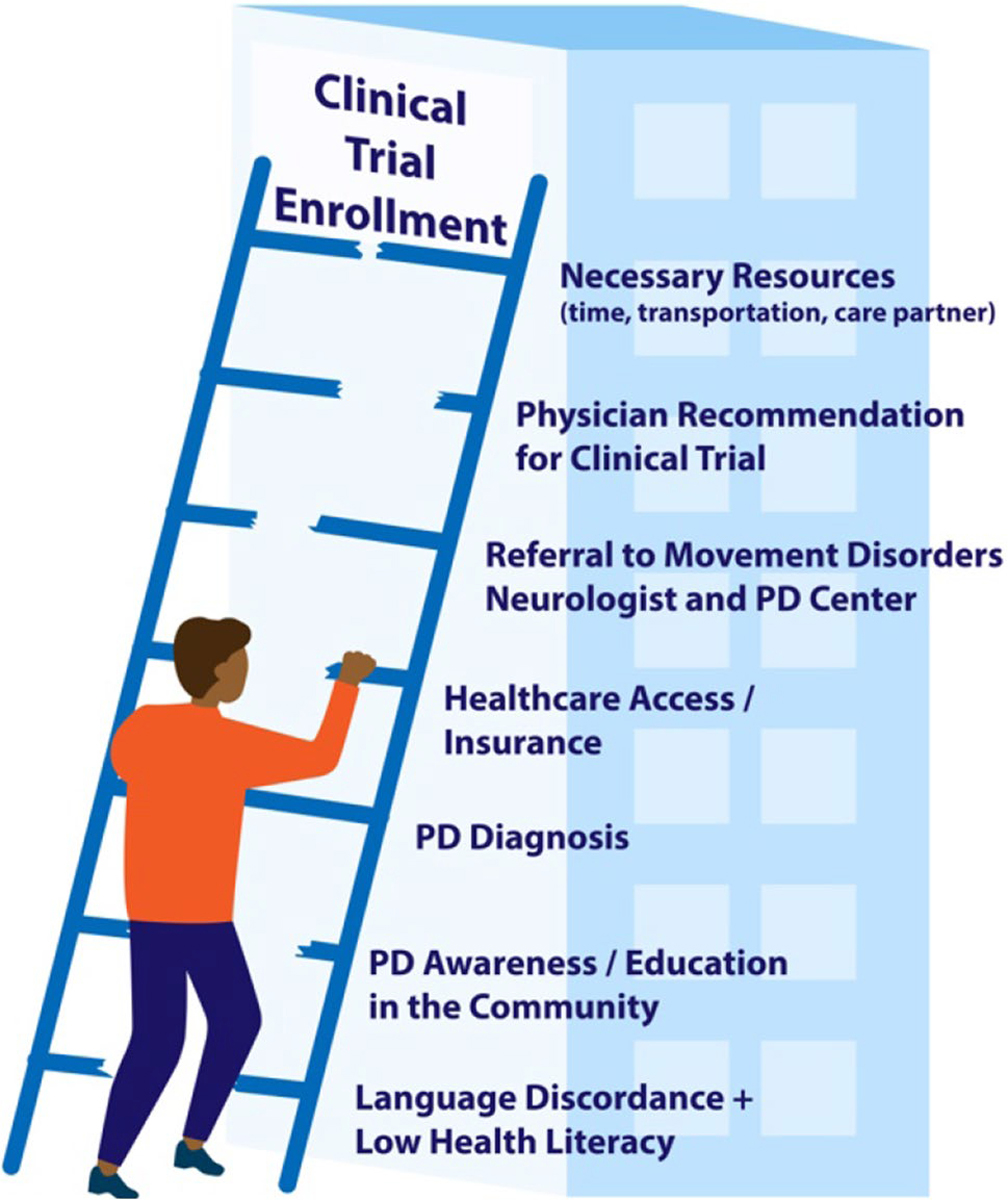
Obstacles to clinical trial enrollment in URMs
Past Research Investigating Diversity in Research Engagement
While there are limited studies specifically in PD, there has been extensive research across fields investigating barriers and facilitators to minority recruitment in clinical trials and proposed interventions [24, 29, 30, 31••, 32, 33]. Here, we discuss the existing studies in PD and related studies across other fields. Past research has shown community support of clinical trial navigation interventions to increase diverse representation in clinical trials [34]. Proposed solutions among African American communities included diversifying research teams, acknowledging past abuses by clinical research, and building community trust [34]. In Latinx communities, proposed solutions included providing easily understandable and Spanish-translated materials, utilizing Spanish-speaking clinicians and advocates, and clarifying potential disclosure and documentation of immigration status [34]. This highlights the importance of tailoring recruitment and retention strategies, and interventions themselves, to the unique needs and barriers of the specific population at the time of study design. A “one-size-fits-all” approach to tackling the disparities in minority enrollment in PD clinical trials may be inadequate.
Based on the existing, documented disparities in PD, Ojukwu et al. [35] recommend three overarching strategies in their review Lessons From George Floyd: Racial Inequalities in the Treatment of Parkinson’s Disease: (1) implementation of culture-centered care, (2) enforcement and facilitation of minority recruitment in clinical trials, and (3) incentives for medical and industry professionals who achieve benchmarks and standards that promote diversity and equity in PD care and research.
Strategies to Diversify Clinical Trial Recruitment
Proactive, Targeted Study Design and Advertisement
As recruitment science in PD continues to be an emerging area, it is important to use existing literature to guide intentional study design for PD clinical trials utilizing proactive measures to diversify participant recruitment and address known barriers and facilitators to clinical trial enrollment in underrepresented populations. Research has shown that intentional digital marketing can be used to diversify the pool of study participants. In a study investigating the change in recruitment demographics in a longitudinal, observational trial of PD patients and healthy controls, investigators found that targeted online campaigns were able to significantly increase the representation of participants who were non-White, Hispanic, female, and of older age [36]. They also recruited a higher percentage of participants from lower educational attainment and socioeconomic status. Picillo et al. [27] describe the need for a continuum of targeted passive (newsletters, social media, PD-related websites) to active recruitment methods (networking with local clinicians, outreach at PD support groups, partnerships with community organizations).
Clinical trial design informed by existing research on known barriers to research engagement and methods for decreasing the impact of these obstacles can be helpful at diversifying the participant pool. In a study investigating the feasibility, patient satisfaction, and outcomes of multidisciplinary home visits for advanced PD, the participant enrollment was 15.3% Hispanic/Latinx, 7.1% Black, and 7.1% Asian, significantly more than the usual URM enrollment in PD trials [37, 38]. While increasing URM representation was not an intentional objective of the study, by eliminating the barriers associated with hospital or clinic-based trials and bringing the research to the patient’s home, the study design reduced barriers, many of which disproportionately affect URMs. Bringing research to the patient where they are (home, local community center, etc.) can be a potential method to increase the diversity of PD trial enrollment and should be further explored.
A study of Hispanic people with Parkinson’s (PWPs) found that Hispanic PWPs were more likely to be interested in participating in PD drug trials compared to White PWPs, but they were also more likely to be concerned that research participation would expose their condition and may be associated with a financial burden [31••]. Hispanic PWPs also indicated the importance of family involvement and cultural and language congruence of the research team. Using this information, proactive planning in the study design, recruitment, and staffing addressing these factors of confidentiality and financial concerns as well as a diversity of research team members may increase the recruitment of Hispanic PWPs.
Under-recruitment of URMs in PD clinical trials has led to gaps in our understanding of the genetics, natural history, and management of the disease in diverse populations. To fill these gaps, resources need to be allocated for research trials and programs that address these specific deficits in our knowledge of PD. For example, the Latin American Research Consortium on the Genetics of Parkinson’s disease (LARGE-PD), the Black and African American Connections to Parkinson’s Disease (BLAAC PD) study of the Global Parkinson’s Genetics Program (GP2), and the International Parkinson’s disease Genomics Consortium (IPDGC) Africa project are designed to allocate specific resources to learn more about the genetic and clinical variation in PD within Latinx and Black populations [39, 40]. More tailored trials are needed to fill these knowledge gaps in the field, which will only be accomplished by allocating the necessary resources and time for stakeholder involvement, community engagement, and scientific discovery in these areas.
Role of Community Engagement
Community engagement and partnerships have been used to create relationships between researchers and underrepresented communities. It has been used as a method to help facilitate trust and access through relationship building and engagement of community stakeholders. Partnerships with organizations in underrepresented communities can also be a resource for the recruitment of healthy controls. Proposed strategies to increase minority enrollment in clinical trials include a multifaceted approach enlisting early community opinion leaders to help with project design and recruitment, using multiple targeted modalities for advertising the study and explaining risks, and intentional recruitment of a diverse, culturally competent research team [25]. Community-based participatory research (CBPR) can be used to foster partnerships and infrastructure for academic researchers and communities to address research questions in mutually beneficial ways, increasing the sustainability and effectiveness of interventions and resources in underserved communities [41].
Community-partnered interventions have been successful in diversifying clinical trials for conditions such as hypertension and diabetes through partnerships with organizations such as religious centers, barbershops, beauty parlors, and community centers [32, 42, 43]. Utilizing patient advocates to advise research teams during the planning, recruitment, and execution of PD clinical trials has been proposed as a method to increase patient engagement in research by considering the interests, needs, and barriers of potential research participants [44]. Using this same model with PWPs from URMs may help guide research teams on optimizing the enrollment of URMs into PD clinical trials by including their input at all stages of trial development and recruitment. Multimodal strategies to increase PD clinical trial recruitment have included (1) engaging with the community through in-person community events, support groups, and physician networks and (2) increasing targeted online presence [45].
The Fostering Inclusion in Research Engagement for Underrepresented Populations in PD (FIRE-UP PD) study recruited four control and four intervention sites to determine best practices for URM research recruitment, assess perspectives toward clinical trials, and increase URM recruitment in the Michael J. Fox Foundation Fox Insight Trial [46]. One of the site’s interventions was the creation of the Chicago Movement Coalition (CMC), an academic-community alliance which recruited a stakeholder advisory board (PWPs, care partners, community leaders, movement disorder specialists) from underrepresented communities of Chicago to help inform and plan community-based educational workshops on PD and PD research [47, 48]. While there was no significant difference in Fox Insight enrollment between sites where they implemented tailored URM recruitment interventions and control sites, they did find an overall increase in URM enrollment in the study across all sites. This suggests an observer effect, such that raising awareness of the lack of URM representation in clinical trials paired with purposeful monitoring of enrollment may inadvertently alter recruitment strategies. Common recruitment barriers highlighted by the study included language discordance, the digital divide, and time.
The underrepresentation of minorities in clinical trials is pervasive and persists across fields. Learning from other areas of medicine in which contributors to disparities have been identified, and relevant interventions implemented, may provide frameworks to adapt and test within PD. For example, a study aimed at increasing the participation of Black cancer patients in clinical trials hired two full-time Black lay people from the community and trained them to be patient navigators to help educate potential Black participants on the trial and help them navigate being a trial participant once enrolled [49]. Through the study, the percentage of Black participants in the trial increased from 9 to 16% and those who used a patient navigator were twice as likely to be retained throughout the study. Utilizing culturally congruent patient navigators may be a useful approach to test and implement in PD trials.
Engaging Minority-Serving Community Physicians
While community partnerships and CBPR practices have proven beneficial at diversifying research enrollment in other fields, there are some limitations in lower prevalence diseases such as PD, whose prevalence is 1% in older adults [50]. Past research has shown that physician advice and referral can increase the likelihood of clinical trial engagement [42, 51]. Studies have also demonstrated a hesitancy of minority-serving community physicians to refer URM patients for clinical trials due to lack of trust in the investigators, perceived lack of time/resources, and concern that patients may leave their practice, superimposed on the decreased awareness of research studies among community physicians [52–54]. Research has shown that although there is interest in clinical trials within Hispanic PWPs, there is a relative lack of awareness of available PD research opportunities, especially among Hispanic PWPs treated in the community, outside of a tertiary center [31••]. Given that PWPs have been shown to have a higher interest in research participation when recommended by their physician, this highlights the importance of physicians who care for PWPs inside and outside of tertiary centers to be aware of ongoing PD trials and avoid assuming that PWPs from URMs are uninterested in participating [31••, 55, 56•].
Community physicians (internists, primary care physicians, neurologists) are potential resources and target populations for interventions to increase PD research diversity. In a large multisite randomized control trial ancillary to the National Institute of Neurologic Disorders and Stroke Exploratory Trials in Parkinson’s Disease Long-Term Study 1, investigators assessed the impact of interventions aimed at increasing community physician trust in trial investigators and decreasing recruitment barriers in underrepresented populations [57]. Interventional sites were provided funds to support a research coordinator and host a continuing medical education (CME) event for local physicians, and they were offered a training session and monthly conference calls to support their efforts. Control sites maintained their own pre-existing recruitment strategies and were provided with trial brochures. The trial was stopped early after a year for lack of efficacy. Key informant interviews from the study showed that low enrolling sites reported more reliance on brochures and newsletters and less direct communication with community physicians and were more likely to place the responsibility of research engagement on the prospective diverse participants. High enrolling sites were more likely to initiate direct communication with community physicians, leverage existing physician relationships, and make extensive efforts to overcome participant barriers. The authors also noted that many principal investigators at low enrolling sites relied heavily on the engagement of the recruitment coordinator and did little direct personal communication with community physicians which highlights the need and role for on-the-ground engagement and investment at multiple levels.
Although a statistically significant difference was not found between intervention and control sites in this study, it raises many questions regarding methodology, study duration, implementation, and endpoints in similar research. How much time is needed to impact underlying issues of trust, perceptions, and relationships in order to move the dial on recruitment and retention of URMs in clinical trials, especially given the centuries-long history and past abuses by the research community? Does a statistically insignificant research outcome negate the potential benefits of the proposed intervention? What are the limitations of a one-size-fits-all RCT design in recruitment science—especially given the effect of observer bias on control sites and the inherent variability of intervention implementation between sites to adapt to intended target populations and settings? Future investigations and discussions regarding the science of recruitment are needed to best design studies and endpoints for trials investigating interventions addressing URM representation in clinical trials. The use of applicable research design and analysis models from other fields may also contribute to project development. Without adequate investment to inform our study designs, we may risk premature study termination or misleading conclusions about interventions that can contribute to the field.
The Randomized Recruitment Intervention Trial (RECRUIT) is a RCT that utilized a trust-based continuous quality improvement intervention in four, parent multisite clinical trials within specialty clinics, including one in PD specifically [58]. RECRUIT aimed to increase trust between study investigators and minority-serving community physicians using a standardized intervention mapping framework that was then tailored to the specific population and disease of interest [58, 59]. While pooled analysis across all trials did not show a statistically significant difference in the minority enrollment between intervention and control sites, analysis separated by trial showed that three of the four studied trials trended in the direction of enrolling more minorities in the intervention versus control sites [60]. The authors noted that the lack of significant findings in the pooled primary analysis was at least partially attributed to the heterogeneity among parent trials and the variations in the implementation of the tailored interventions. The STEADY PD III parent trial recruited 19% minorities in the RECRUIT intervention sites compared to the 10.1% minority enrollment in control sites [60, 61]. One of the factors that investigators cited that may have further improved the results in this cohort was the requirement from the funding organization to enroll at least 10% minorities for the continuation of funding which offered additional motivation to intervention sites and may support the implementation of similar incentives and trial policies that can be investigated in the future. In addition to specific approaches to increase minority enrollment through RECRUIT, the STEADY PD III trial was able to increase the efficiency of overall trial recruitment through strategies to reduce protocol complexity, implement early comprehensive stakeholder and awareness outreach, and rigorous clinical trial site selection [61].
Moving the Dial Toward Equity: Future Directions
Past studies have demonstrated the multifaceted nature of disparities in PD and PD research. However, there remain additional questions and continuing disparities in the field, including inequity in PD clinical trial recruitment. Future directions to pursue equity and justice in PD research representation include (1) investment into the development of best practices in the science of recruitment interventions, specifically in URMs, (2) implementation of measures previously shown to decrease barriers and enhance facilitators to URM research engagement, and (3) increased resource allocation toward the testing and analysis of interventions to diversify PD clinical trial recruitment and retention.
Like other areas of research, the science of URM recruitment requires guidance and methodological frameworks to strengthen both study design and interpretation. Common considerations in this area of research include observer bias, the need for flexible implementation between sites, the strengths and limitations of a standard RCT design, and the identification of attainable outcome measures within a realistic study timeline. There is a need for a dedicated study of interventions to increase the recruitment and retention of URMs. Past research has shown that the lack of a clear conceptual framework and heterogeneity of methodology and analysis both within and across studies has limited the strength and reproducibility of the results [62, 63]. Additionally, a critical limiting factor in interventions aimed at building trust and relationships with the community and community physicians is time [32, 57]. There is no shortcut or quick fix to relationship building; however, we must find ways to adapt our study design to ensure our research question is measurable and answerable. Dedicated research is needed to create methodological and analysis standards in recruitment science which balance reproducibility and interpretability but also allow for the flexibility needed to best implement interventions in different communities/settings.
Secondly, it is important that the PD research community act on the existing results from previous studies that have described some barriers and facilitators to URM clinical trial recruitment and recommendations for real-world application. Picillo et al. [27] describe potential strategies for addressing four factors affecting the recruitment process—infrastructure, nature of the research, recruiter characteristics, and participant characteristics—with examples including those to decrease barriers such as shorter trial duration or fewer study visits to decrease travel burden, use of telemedicine, availability of weekend/evening visits, and availability of translators and culturally congruent research staff. Other measures such as translated and culturally representative recruitment materials, expansion of inclusion criteria to non-English speakers, use of patient advisory boards, and community partnerships have been shown to diversify research engagement. While further work is necessary to identify additional factors and strategies that successfully increase diversity, we also need increased accountability and incentives led by governmental, institutional, and funding organizations to ensure that knowledge gained is intentionally and equitably implemented to improve current PD research representation.
Lastly, using the barriers and facilitators which have been postulated in the research, intervention-based studies and programs should be proposed and supported in order to take steps forward toward action and change. Kilbourne et. al. [64] described a framework of steps needed to address and eventually reduce or eliminate health disparities: (1) define, detect, and measure the disparity, (2) understand the drivers and determinants, and (3) use this information to implement programs and interventions to reduce the disparity [64, 65]. Existing literature in PD predominately addresses the first phase which is detection, a necessary foundation for future work. However, if we are to start moving the dial toward equity and avoid having to report similar dismal statistics of today, we will need to put more attention and resources toward the second two steps which are the why and what now questions addressing contributors, interventions, and next steps.
Conclusion
Representation of URMs in PD clinical research is a matter of both science and ethics. In this era of precision medicine, it is exceedingly important to ensure that the makeup of research participants reflects that of the general PD population. Enrollment in PD clinical research is vulnerable to upstream disparities and inequities from PD education and awareness to access to specialized PD centers. While additional research is still needed, past studies have identified some potential strategies for increasing URM recruitment including increasing the availability of translated recruitment materials, partnering with community organizations and URM-serving community physicians, and diversifying the research team, among others. To move the dial toward equity in PD research, it will be necessary to implement successful strategies while further investigating additional contributors to the underrepresentation of URMs in PD clinical research and developing and testing interventions to address these factors.
Footnotes
Conflict of Interest No funding was received to assist with the preparation of this manuscript. Jennifer Adrissi has no relevant financial or non-financial interests to disclose. Jori Fleisher has received honoraria from the Parkinson’s Foundation and Davis Phinney Foundation for educational or consulting activities and research support from NINDS, NIA/Emory Roybal Center for Caregiving Mastery, Parkinson’s Foundation, CurePSP, Rush University Center for Excellence in Aging, and private philanthropic support from Joyce DeMoose and George Harvey, and Elena Urschel. Jori Fleisher has also received royalties from Wolters Klewer Health/UpToDate.
Declarations
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Nussbaum RL, Ellis CE. Alzheimer’s disease and Parkinson’s disease. N Engl J Med. 2003;348(14):1356–64. [DOI] [PubMed] [Google Scholar]
- 2.Dorsey ER, et al. The emerging evidence of the Parkinson pandemic. J Parkinsons Dis. 2018;8(s1):S3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vespa J, Medina L, Armstrong D. Demographic turning points for the United States: population projections for 2020 to 2060 population estimates and projections. In: Current Population Reports. Washington DC: US Census Bureau; 2020. p. 1–15. [Google Scholar]
- 4.Caplan A, Friesen P. Health disparities and clinical trial recruitment: is there a duty to tweet? PLoS Biol. 2017;15(3):e2002040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lau YH, et al. Does ethnicity influence recruitment into clinical trials of Parkinson’s disease? J Parkinsons Dis. 2022. • A systematic search of PD trials between 2017 and 2021 on the clinicaltrials.gov database revealed that only 42.65% of the 57 trials published in peer-reviewed journals reported participant racial distribution. Of these, only 5 studies had mixed racial representation and only 2 had Black research participants—supporting the need for increased reporting and recruitment of racial diversity in PD clinical trials.
- 6.National Institutes of Health. NIH policy and guidelines on the inclusion of women and minorities as subjects in clinical research. 2017. [cited 2022 January 15]; Available from: https://grants.nih.gov/grants/guide/notice-files/NOT-OD-18-014.html.
- 7.US Food and Drug Administration. Collection of race and ethnicity data in clinical trials: guidance for industry and food and drug administration staff. 2016. [cited 2021 December 09]; Available from: https://www.fda.gov/ucm/groups/fdagov-public/@fdagov-afda-gen/documents/document/ucm126396.pdf.
- 8. Di Luca DG, et al. Minority enrollment in Parkinson’s disease clinical trials: meta analysis and systematic review of studies evaluating treatment of neuropsychiatric symptoms. J Parkinsons Dis. 2020;10(4):1709–1716. • A PubMed and Embase search between 2000 and 2019 of RCTs intending to treat neuropsychiatric symptoms in PD found that only 17.5% of the RCTs reported race/ethnicity information. Within the 11 RCTs reporting this information, 0.2% of the pooled participants were African American, 0.64% Hispanic, and 21.44% Asian, highlighting the underrepresentation of minorities in PD RCTs and the need for reporting of race/ethnicity information in PD RCTs.
- 9.Schneider MG, et al. Minority enrollment in Parkinson’s disease clinical trials. Parkinsonism Relat Disord. 2009;15(4):258–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahodwala N, et al. Racial differences in the diagnosis of Parkinson’s disease. Mov Disord. 2009;24(8):1200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan AK, et al. Disparities in access to deep brain stimulation surgery for Parkinson disease: interaction between African American race and Medicaid use. JAMA Neurol. 2014;71(3):291–9. [DOI] [PubMed] [Google Scholar]
- 12.Willis AW, et al. Disparities in deep brain stimulation surgery among insured elders with Parkinson disease. Neurology. 2014;82(2):163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McInerney-Leo A, Gwinn-Hardy K, Nussbaum RL. Prevalence of Parkinson’s disease in populations of African ancestry: a review. J Natl Med Assoc. 2004;96(7):974–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Wright Willis A, et al. Geographic and ethnic variation in Parkinson disease: a population-based study of US Medicare beneficiaries. Neuroepidemiology. 2010;34(3):143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Den Eeden SK, et al. Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157(11):1015–22. [DOI] [PubMed] [Google Scholar]
- 16.Schoenberg BS, Anderson DW, Haerer AF. Prevalence of Parkinson’s disease in the biracial population of Copiah County. Miss Neurol. 1985;35(6):841–5. [DOI] [PubMed] [Google Scholar]
- 17.Manly JJ. Deconstructing race and ethnicity: implications for measurement of health outcomes. Med Care. 2006;44(11 Suppl 3):S10–6. [DOI] [PubMed] [Google Scholar]
- 18.Hemming JP, et al. Racial and socioeconomic disparities in parkinsonism. Arch Neurol. 2011;68(4):498–503. [DOI] [PubMed] [Google Scholar]
- 19.Dahodwala N, et al. Delayed Parkinson’s disease diagnosis among African-Americans: the role of reporting of disability. Neuroepidemiology. 2011;36(3):150–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Werner P, Korczyn AD. Lay persons’ beliefs and knowledge about Parkinson’s disease: prevalence and socio-demographic correlates. Parkinsonism Relat Disord. 2010;16(6):415–7. [DOI] [PubMed] [Google Scholar]
- 21.Pan S, et al. Knowledge and attitudes about Parkinson’s disease among a diverse group of older adults. J Cross Cult Gerontol. 2014;29(3):339–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willis AW, et al. Neurologist care in Parkinson disease: a utilization, outcomes, and survival study. Neurology. 2011;77(9):851–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saadi A, et al. Racial disparities in neurologic health care access and utilization in the United States. Neurology. 2017;88(24):2268–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.George S, Duran N, Norris K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am J Public Health. 2014;104(2):e16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Branson RD, Davis K Jr., Butler KL. African Americans’ participation in clinical research: importance, barriers, and solutions. Am J Surg. 2007;193(1):32–9; discussion 40. [DOI] [PubMed] [Google Scholar]
- 26.Vaswani PA, Tropea TF, Dahodwala N. Overcoming barriers to Parkinson disease trial participation: increasing diversity and novel designs for recruitment and retention. Neurotherapeutics. 2020;17(4):1724–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Picillo M, et al. Recruitment strategies and patient selection in clinical trials for Parkinson’s disease: going viral and keeping science and ethics at the highest standards. Parkinsonism Relat Disord. 2015;21(9):1041–8. [DOI] [PubMed] [Google Scholar]
- 28.Fleisher J, et al. Health literacy and medication awareness in outpatient neurology. Neurol Clin Pract. 2014;4(1):71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ford JG, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112(2):228–42. [DOI] [PubMed] [Google Scholar]
- 30.UyBico SJ, Pavel S, Gross CP. Recruiting vulnerable populations into research: a systematic review of recruitment interventions. J Gen Intern Med. 2007;22(6):852–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Damron L, et al. Hispanic perspectives on Parkinson’s disease care and research participation. J Alzheimers Dis. 2021;81(2):809–819. •• A mixed-methods study utilizing surveys and interviews revealed factors impacting PD research engagement in the Hispanic community including importance of physician recommendation for research, preference for Spanish-speaking research team, involvement of family members in research process, and lack of awareness of research opportunities.
- 32.Curry L, Jackson J. Recruitment and retention of diverse ethnic and racial groups in health research: an evolving science. The science of inclusion: Recruiting and retaining racial and ethnic elders in health research. 2003;1–7 [Google Scholar]
- 33.Sinclair S, et al. Recruiting African Americans for health studies: lessons from the Drew- RAND center on health and aging. J Ment Health Aging. 2000;6(1):39–51. [Google Scholar]
- 34.Ford ME, et al. Unequal burden of disease, unequal participation in clinical trials: solutions from African American and Latino community members. Health Soc Work. 2013;38(1):29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ojukwu DI, Andruska KM, Halpern CH. Lessons from George Floyd: racial inequalities in the treatment of Parkinson’s disease. Mov Disord. 2021;36(3):599–603. [DOI] [PubMed] [Google Scholar]
- 36.Dobkin RD, et al. Innovative recruitment strategies to increase diversity of participation in Parkinson’s disease research: the Fox Insight cohort experience. J Parkinsons Dis. 2020;10(2):665–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fleisher J, et al. Interdisciplinary home visits for individuals with advanced Parkinson’s disease and related disorders. J Am Geriatr Soc. 2018;66(6):1226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nwabuobi L, et al. Sex-related differences in homebound advanced Parkinson’s disease patients. Clin Interv Aging. 2019;14:1371–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zabetian CP, Mata IF, Latin PD American Research Consortium on the Genetics of. LARGE-PD: examining the genetics of Parkinson’s disease in Latin America. Mov Disord. 2017;32(9):1330–1331. [DOI] [PubMed] [Google Scholar]
- 40.Noyce A, et al. Tackling underrepresentation to aid understanding of Parkinson’s disease: progress and further opportunities. Physiology News. 2021;123:32–4. [Google Scholar]
- 41.Jones L, Wells K. Strategies for academic and clinician engagement in community-participatory partnered research. JAMA. 2007;297(4):407–10. [DOI] [PubMed] [Google Scholar]
- 42.Levkoff S, Sanchez H. Lessons learned about minority recruitment and retention from the Centers on Minority Aging and Health Promotion. Gerontologist. 2003;43(1):18–26. [DOI] [PubMed] [Google Scholar]
- 43.Mensah GA, et al. Reducing cardiovascular disparities through community-engaged implementation research: A National Heart, Lung, and Blood Institute Workshop Report. Circ Res. 2018;122(2):213–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feeney M, et al. Utilizing patient advocates in Parkinson’s disease: a proposed framework for patient engagement and the modern metrics that can determine its success. Health Expect. 2020;23(4):722–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siddiqi B Developing an effective multimodal recruitment plan: a case study of a Parkinson’s disease trial demonstrates how one study team met its enrollment goals. Appl Clin Trials. 2019;28(3):16–7. [Google Scholar]
- 46.Jackson J, et al. Importance of diversity in Parkinson’s research. Appl Clin Trials. 2020;29(12):13–5. [Google Scholar]
- 47.Adrissi J, et al. The Chicago Movement Coalition: a community partnered intervention (4671) [Abstract]. Neurology. 2021;96(15 Supplement). [Google Scholar]
- 48.Chicago Movement Coalition. The CMC: making moves for Parkinson’s. 2019. [cited 2021 November 1]; Available from: chicagomovementcoalition.org.
- 49.Fouad MN, et al. Patient navigation as a model to increase participation of African Americans in cancer clinical trials. J Oncol Pract. 2016;12(6):556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5(6):525–35. [DOI] [PubMed] [Google Scholar]
- 51.Jenkins V, Fallowfield L. Reasons for accepting or declining to participate in randomized clinical trials for cancer therapy. Br J Cancer. 2000;82(11):1783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hudson SV, Momperousse D, Leventhal H. Physician perspectives on cancer clinical trials and barriers to minority recruitment. Cancer Control. 2005;12(Suppl 2):93–6. [DOI] [PubMed] [Google Scholar]
- 53.Lynch GF, et al. A pilot survey of African-American physician perceptions about clinical trials. J Natl Med Assoc. 2001;93(12 Suppl):8S–13S. [PMC free article] [PubMed] [Google Scholar]
- 54.Mainous AG 3rd, et al. Factors influencing physician referrals of patients to clinical trials. J Natl Med Assoc. 2008;100(11):1298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guillemin M, et al. Primary care physicians’ views about gatekeeping in clinical research recruitment: a qualitative study. AJOB Empir Bioeth. 2017;8(2):99–105. [DOI] [PubMed] [Google Scholar]
- 56. Nuytemans K, et al. Motivations for participation in Parkinson disease genetic research among Hispanics versus non-Hispanics. Front Genet. 2019;10:658. • Researchers found similar interest and willingness to participate in PD genetic research between Hispanic and non-Hispanic PD patients suggesting underrepresentation of Hispanic patients in PD genetic research may be at least partially attributed to a lower rate of study invitation.
- 57.Tilley BC, et al. A randomized recruitment intervention trial in Parkinson’s disease to increase participant diversity: early stopping for lack of efficacy. Clin Trials. 2012;9(2):188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tilley BC, et al. Design of a cluster-randomized minority recruitment trial: RECRUIT. Clin Trials. 2017;14(3):286–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bartholomew Eldredge LK. Planning health promotion programs : an intervention mapping approach. 2016, Jossey-Bass & Pfeiffer Imprints, Wiley, San Francisco, CA. p. 1 online resource. [Google Scholar]
- 60.Tilley BC, et al. Using increased trust in medical researchers to increase minority recruitment: the RECRUIT cluster randomized clinical trial. Contemp Clin Trials. 2021;109:106519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berk S, et al. Increasing efficiency of recruitment in early Parkinson’s disease trials: a case study examination of the STEADY-PD III trial. J Parkinsons Dis. 2017;7(4):685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gilmore-Bykovskyi AL, et al. Recruitment and retention of underrepresented populations in Alzheimer’s disease research: a systematic review. Alzheimers Dement (N Y). 2019;5:751–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ndumele CD, et al. Publication of recruitment methods in focus group research of minority populations with chronic disease: a systematic review. J Health Care Poor Underserved. 2011;22(1):5–23. [DOI] [PubMed] [Google Scholar]
- 64.Kilbourne AM, et al. Advancing health disparities research within the health care system: a conceptual framework. Am J Public Health. 2006;96(12):2113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gilbert R American Parkinson Disease Association explores the current research in diverse Parkinson’s disease populations. US Neurology. 2019;15(2):63–5. [Google Scholar]


