Abstract
Introduction:
Homebound individuals with advanced Parkinson’s disease (PD) are underrepresented in research and care. We tested the impact of interdisciplinary, telehealth-enhanced home visits (IN-HOME-PD) on patient quality of life (QoL) compared with usual care.
Methods:
Nonrandomized controlled trial of quarterly, structured, telehealth-enhanced interdisciplinary home visits focused on symptom management, home safety, medication reconciliation, and psychosocial needs (ClinicalTrials.gov NCT03189459). We enrolled homebound participants with advanced PD (Hoehn & Yahr (HY) stage ≥3). Usual care participants had ≥2 visits in the Parkinson’s Outcomes Project (POP) registry. We compared within- and between-group one-year change in QoL using the Parkinson’s Disease Questionnaire.
Results:
Sixty-five individuals enrolled in IN-HOME-PD (32.3% women; mean age 78.9 (SD 7.6) years; 74.6% white; 78.5% HY ≥ 4) compared with 319 POP controls, with differences in age, race, and PD severity (37.9% women; mean age 70.1 (7.8) years; 96.2% white; 15.1% HY ≥ 4). Longitudinally, the intervention group’s QoL remained unchanged (within-group p = 0.74, Cohen’s d = 0.05) while QoL decreased over time in POP controls (p < 0.001, Cohen’s d = 0.27). The difference favored the intervention (between-group p = 0.04). POP participants declined in 7/8 dimensions while IN-HOME-PD participants’ bodily discomfort improved and hospice use and death at home—markers of goal-concordant care—far exceeded national data.
Conclusions:
Telehealth-enhanced home visits can stabilize and may improve the predicted QoL decline in advanced PD via continuity of care and facilitating goal-concordant care, particularly among diverse populations. Extrapolating features of this model may improve continuity of care and outcomes in advanced PD.
1. Introduction
Parkinson’s Disease (PD) is the fastest growing neurological disorder globally, projected to double from 6 to 12 million individuals between 2015 and 2040 [1]. While promising research focuses on symptomatic and neuroprotective therapies, the majority of trials enroll individuals with early or moderate PD, leaving those with severe PD nearly absent from research [2,3]. Even large observational studies of symptom burden and quality of life (QoL) frequently combine the two most advanced stages on the five-point Hoehn & Yahr (HY) scale to yield sufficient numbers for reporting [4,5].
Neuropalliative care research demonstrates that interdisciplinary outpatient models influence QoL [6]. Yet these groundbreaking efforts miss a critically vulnerable population. When PD requires a “considerable and taxing effort” and assistive devices, transportation, or caregivers to leave the home, that individual is deemed homebound [7]. Without support, homebound individuals lack access to innovative neuropalliative clinics, let alone routine outpatient visits and clinical trials [8].
In prior work developing an interdisciplinary home visit model of care, we reached the homebound PD population living in New York City and uncoupled the downward trajectory of disease severity from the expected deterioration in QoL [9]. Limitations in that single-site pilot included generalizability, labor intensity, and the lack of a comparison group or PD-specific QoL measure. Telehealth expansion allowed incorporation of team members remotely and relocation to Chicago increased generalizability with a more geographically diverse catchment area. Our aims in this single-center, nonrandomized, controlled study were to determine the efficacy of telehealth-enhanced interdisciplinary home visits for homebound individuals with advanced PD on overall PD-specific QoL and its component dimensions, compared with best possible usual care, over one year.
2. Methods
2.1. Study design
Interdisciplinary Home Visits for Parkinson’s Disease (IN-HOME-PD) is a single-center nonrandomized controlled study comprised of one year of structured, quarterly, telehealth-enhanced home visits for homebound individuals with advanced PD compared with longitudinal data from individuals receiving usual care. The design, methods, and measures are detailed elsewhere, and summarized briefly here [10]. Recruitment began in May 2018 with visits completed by November 2020. Given the ethical considerations of withholding care from homebound individuals, high risk of attrition due to death or institutionalization, and high dropout rates in PD interventions with waitlist controls, we selected a nonrandomized controlled design [11]. We compared IN-HOME-PD data to that of the Parkinson’s Outcomes Project (POP) [12], an active, longitudinal registry of demographic and clinical outcomes data from over 13,000 individuals seen at Parkinson’s Foundation Centers of Excellence (PF COEs). As a secondary comparison of demographic and PD characteristics, we also compared IN-HOME-PD participants to the population of eligible individuals receiving care at the local COE from which we recruited.
2.2. Setting
We recruited IN-HOME-PD dyads from the Rush University Medical Center PF COE between May 2018–October 2019, including referrals from 12 movement disorders specialists (MDS) and six fellows. Study visits occurred in the home within a 30–50-mile radius (≤90-min one-way commute) with urban, suburban, and rural regions. For patients institutionalized before Visit 4, we conducted study visits in the facility.
2.3. Participants and recruitment
We recruited community-dwelling individuals aged ≥40 diagnosed with PD [13] at HY stage 3–5 at the most recent outpatient visit [14]. Participants had to have an informal caregiver willing to be a study partner, meet Medicare homebound criteria [7], and have ≥1 risk factor for caregiver strain and/or institutionalization [10,15–17]. Recruitment included screening the electronic medical record (EMR), contacting potential participants with MDS permission, and fielding direct referrals. If an interested individual had not previously received care at Rush, a visit was expedited to confirm eligibility.
2.4. Screening and informed consent
The Rush University Medical Center Institutional Review Board approved IN-HOME-PD in October 2017. The team made a minimum of three calls or met directly with potential participants to introduce the study and confirm eligibility and interest, after which the team scheduled Visit 1. During this visit, capacity was assessed and participants provided written consent, or assent for those patients with impaired capacity [18].
2.5. Intervention
Participants received four quarterly, structured interdisciplinary home visits over 365 ± 60 days. Visits involved a research nurse, social worker, and MDS supported by a research coordinator. Distinct from our previous model in which all team members were in the home [3,9], the nurse and coordinator were in person for all visits with the MDS remotely present via telemedicine. The coordinator facilitated the telemedicine connection using a wireless hotspot, tablet, and a Health Insurance Portability and Accountability Act (HIPAA)-secure videoconferencing platform.
The social worker visited the home for Visit 1 and joined remotely for Visits 2–4.
The nurse gathered demographics, PD history, comorbidities [19], and the Unified Parkinson’s Disease Rating Scale (UPDRS) Parts I (Mentation, behavior, mood) and & II (Activities of daily living (ADLs)) [20]. She conducted a detailed medication reconciliation and home safety assessment [10,21]. The social worker assessed the dyad’s psychosocial status and needs with a semi-structured interview including but not limited to the following domains: Social Background (name, relationship, contact information of primary care partner; primary language, ethnicity, spiritual background, employment status, living situation, mode of transportation, family structure, insurance coverage); Current Issues (typical daily activities; exercise; mood and cognitive concerns, healthcare power of attorney; presence and details of advance directives); and In-Home Services (paid homemaker or caregiver; cleaning and/or meal services; visiting healthcare professionals; medical alert systems). The team conferred with the MDS while the coordinator administered study questionnaires to which the clinical team was blinded. The MDS completed UPDRS Parts III and IV via telemedicine, supervising the nurse’s in-person rigidity and postural stability assessments. The team provided a health literacy-friendly visit summary to the dyad and sent a comprehensive note to treating healthcare professionals. Visits 2–4 were identical to Visit 1 except for an abbreviated home safety assessment if no changes had occurred, and visits lasted approximately 90–180 min.
2.6. Control population
POP participants received routine PD care at 16 US PF COEs and consented to POP study visits at annual clinic visits, though could be seen more frequently depending on patient need. To be included in the control group, participants had to have ≥2 consecutive annual visits including and following an index visit at which the participant was HY 3. As a secondary cross-sectional comparison group with which to contextualize any differences between the longitudinal IN-HOME-PD and POP groups, we queried the EMR at the Rush University COE for individuals seen by an MDS at least twice, diagnosed with PD, aged ≥40, and at HY stage 3–5 during the first outpatient visit during the time period when IN-HOME-PD was recruiting. After excluding all individuals from this broader sample who enrolled in IN-HOME-PD, this cross-sectional cohort represents the larger population of eligible, potentially homebound individuals at our single COE.
2.7. Measures
Baseline demographics from IN-HOME-PD and POP included age, sex, race, ethnicity, educational attainment, and marital and employment status. Baseline PD characteristics, gathered at IN-HOME-PD Visit 1 and at the POP index visit where HY was ≥3, included HY stage, PD duration, and a brief cognitive assessment involving verbal fluency and five-item immediate- and delayed-recall [12]. Participants completed the Self-Administered Comorbidity Questionnaire which asks whether an individual has, receives treatment for, or is limited in their activities, by 16 comorbidity categories [19]. We measured total days enrolled in the study, counted from visit 1 to either: visit 4, death, or study withdrawal. For participants dying prior to visit 4, we calculated the interval between the most recent study visit and death. We documented the cause of death (if known), place of death, and hospice enrollment status at the time of death.
Primary outcome measures were within-group and between-group differences in health-related QoL as measured by the Parkinson’s Disease Questionnaire-39 (PDQ-39) at home visits 1 and 4 in IN-HOME-PD participants, and at annual POP visits in controls [22]. The PDQ-39 is a validated, 39-item, eight-dimension instrument for which individual dimension and total summary index (SI) scores are calculated, ranging from 0 to 100, where higher scores indicate poorer QoL. We selected the PDQ-39 given its PD-specificity, validation, normative data in advanced PD, and the ability to compare longitudinal change between groups [5]. PDQ-39 dimensions include: mobility, ADLs, emotional wellbeing, stigma, social support, cognitive impairment, communication, and bodily discomfort. We also gathered change in UPDRS Part III (motor examination, ranging from 0 to 108, where higher scores indicate greater motor impairment) and UPDRS total scores (ranging from 0 to 199, where higher scores indicate greater disease severity) over one year as covariates in IN-HOME-PD participants only.
2.8. Statistical analysis
Based on pilot recruitment data [3], we planned to enroll 65 dyads over 16 months with up to 20% attrition, yielding 52 dyads, matched with 4–5 controls per dyad (approximately 300 controls), for 79% power to detect a minimal clinically important difference in the PDQ-SI between groups at one year [23]. All sample size calculations used two-sample, paired-means tests with a significance level of 0.05.
We entered data into a secure, electronic database with quarterly audits for fidelity and analyzed in SAS version 9.4 (SAS Institute). Individual and propensity score matching of IN-HOME-PD and POP participants were not possible due to the scarcity of HY 4–5 POP participants. All analyses were thus conducted as within- and between-group comparisons. We used descriptive statistics to calculate frequencies and percentages, with continuous variables assessed for normality and summarized with parametric or non-parametric statistics, as appropriate. We analyzed both within- and between-group differences in the primary and secondary outcomes based on an intention-to-treat approach using two-sided paired t tests and Cohen’s d for within-group effect sizes, where 0.2, 0.5, and 0.8 signified small, medium, and large effect sizes, respectively. In sensitivity analyses, we stratified the change in PDQ-SI and PDQ-39 dimensions by baseline HY stage.
3. Results
We assessed 255 patients for eligibility and enrolled 65 dyads (25.5% enrollment rate, Fig. 1). Fifty-one dyads completed all visits (78.5% completion rate) for a total of 231 completed visits (65, 61, 54, and 51 dyads completed visits 1–4, respectively) with visits. Of the 14 pairs who withdrew, nine were due to patient death. Table 1 highlights baseline characteristics of the 65 IN-HOME-PD and 319 POP participants used for longitudinal comparisons. Women comprised 32.3% of IN-HOME-PD and 37.9% of POP controls. IN-HOME-PD participants were older (78.9 years (SD 7.6) vs. 70.1 (7.8), p < 0.001) and more likely to be non-White (14.3% vs. 0.3% African American/Black, and 11.1% vs. 2.2% Asian, p < 0.001). Similarly striking differences in age and race exist between IN-HOME-PD participants and the potential recruitment cohort seen at the Rush University COE (n = 1015), shown in the farthest right columns of Table 1. Within the same COE, individuals enrolling in IN-HOME-PD were older and more likely to be non-White than the broader clinic population from which they were recruited.
Fig. 1.
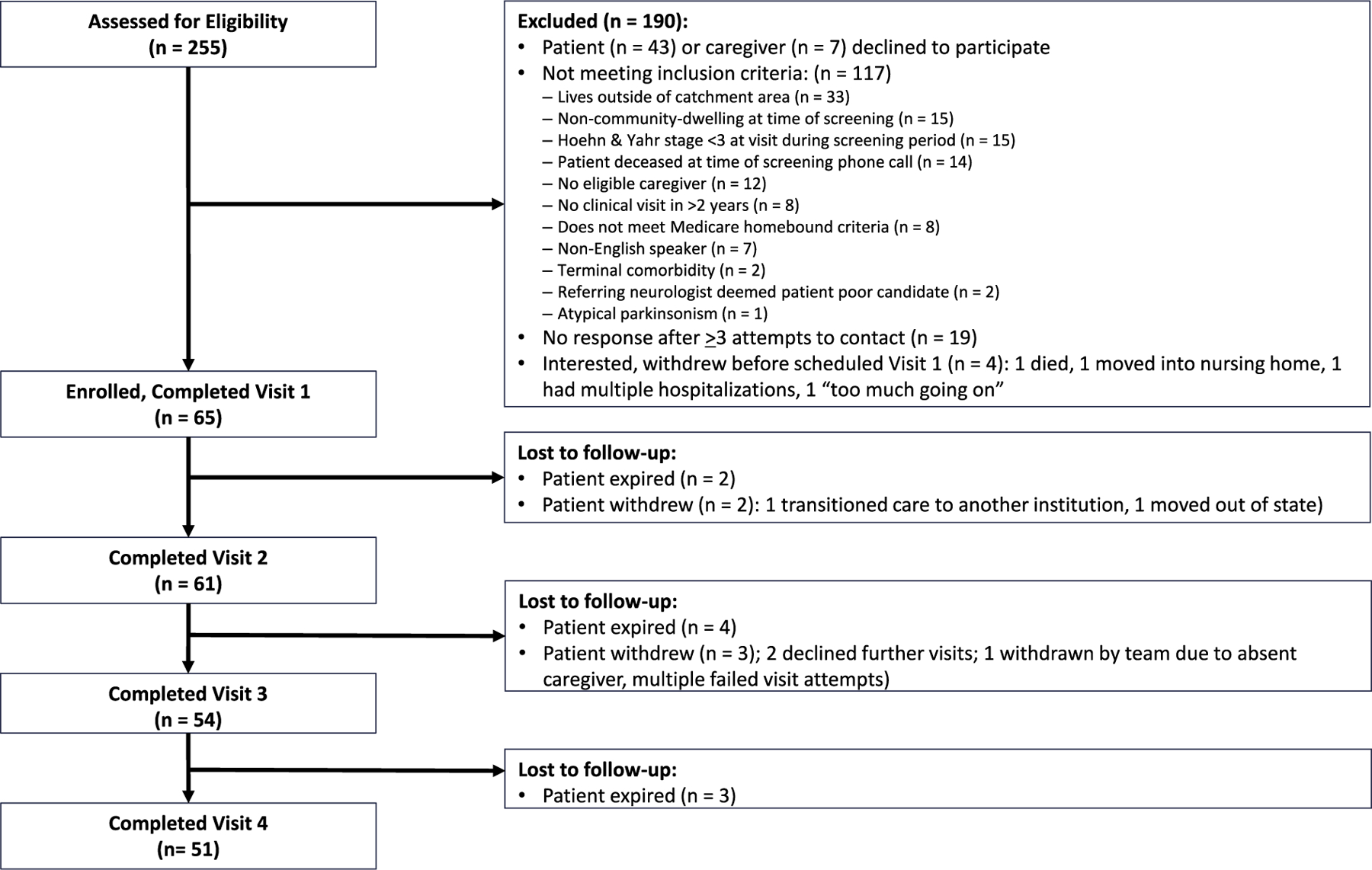
IN-HOME-PD CONSORT diagram.
Table 1.
Baseline characteristics of IN-HOME-PD participants, Parkinson’s Outcomes Project matched control group, and Rush Center of Excellence outpatient clinic.
| Characteristic | IN-HOME-PD Participants, N = 65 | POP Controls, N = 319 | p-valuea | Rush COE eligibility pool, HY ≥ 3 N = 1015 | p-valueb |
|---|---|---|---|---|---|
| Age at baseline, mean (SD) | 78.94 (7.56) | 70.11 (7.83) | <0.001 | 74.96 | <0.001 |
| Gender, n (%) | 0.39 | 0.09 | |||
| Male | 44 (67.69) | 198 (62.07) | 563 (55.47) | ||
| Female | 21 (32.31) | 121 (37.93) | 452 (44.53) | ||
| Race, n (%) | <0.001 | 0.06 | |||
| Caucasian | 47 (74.60) | 307 (96.24) | 767 (85.32) | ||
| African American | 9 (14.29) | 1 (0.31) | 79 (8.79) | ||
| Asian | 7 (11.11) | 7 (2.19) | 43 (4.78) | ||
| Pacific Islander | 0 | 1 (0.31) | 0 | ||
| Other | 0 | 3 (0.94) | 10 (1.11) | ||
| Missing | 2 | 0 | 116 | ||
| Ethnicity, n (%) | 0.30 | 0.27 | |||
| Hispanic | 4 (6.15) | 11 (3.45) | 104 (10.38)13 | ||
| Non-Hispanic | 61 (93.85) | 308 (96.55) | 898 (89.62) | ||
| Education, n (%) | <0.001 | Data not available | |||
| Less than high school | 9 (13.85) | 6 (1.94) | |||
| High school | 9 (13.85) | 53 (17.15) | |||
| Associate’s degree | 12 (18.46) | 78 (25.24) | |||
| Bachelor’s degree | 10 (15.38) | 82 (26.54) | |||
| Graduate degree | 25 (38.46) | 90 (29.13) | |||
| Missing | 0 | 10 | |||
| Marital status, n (%) | 0.001 | 0.21 | |||
| Single | 3 (4.62) | 19 (5.96) | 109 (10.75)5 | ||
| Married | 44 (67.69) | 268 (84.01) | 699 (68.93) | ||
| Widowed | 14 (21.54) | 17 (5.33) | 130 (12.82) | ||
| Divorced | 4 (6.15) | 15 (4.70) | 72 (7.1) | ||
| Employment status, n (%) | 0.03 | Data not available | |||
| Full time | 0 (0) | 20 (6.27) | |||
| Part time | 0 (0) | 9 (2.82) | |||
| Unemployed | 65 (100) | 290 (90.91) | |||
| Hoehn & Yahr Stage, n (%) | <0.001 | <0.0001 | |||
| 3 | 14 (21.54) | 271 (84.95) | 613 (60.39) | ||
| 4 | 41 (63.08) | 40 (12.54) | 290 (28.57) | ||
| 5 | 10 (15.38) | 8 (2.51) | 112 (11.03) | ||
| PD duration, median (IQR) | 15 (10) | 11 (7)9 | 0.003 | Data not available | |
| MoCA items, mean (SD) Immediate 5-item recall | 3.52 (1.36)4 | 4.37 (0.9)6 | <0.001 | Data not available | |
| Delayed 5-item recall | 1.89 (1.67)4 | 3.48 (1.37)7 | <0.001 | ||
| Verbal fluency | 10.87 (6.07)4 | 17.16 (6.3)8 | <0.001 |
Superscripts numerals indicate number of missing values.
IN-HOME-PD: Interdisciplinary Home Visits for Parkinson’s Disease; IQR: Interquartile range; MoCA: Montreal Cognitive Assessment; POP: Parkinson’s Outcomes Project; SD: Standard deviation.
: Comparison of IN-HOME-PD to POP cohort.
Comparison of IN-HOME-PD to Rush COE eligibility pool of individuals with PD, seen May 2018–October 2019 with HY ≥ 3 in an outpatient visit with a movement disorders specialist, excluding those enrolled in IN-HOME-PD.
IN-HOME-PD participants were staged as 21.5% HY 3, 63.1% HY 4, and 15.4% HY 5 at baseline, while POP participants skewed towards moderate disease (85.0% HY 3, 12.5% HY 4, and 2.5% HY 5, p < 0.001). This finding was recapitulated in the IN-HOME-PD vs. Rush COE eligibility cohort comparison, with the latter comprised of 60.39% HY 3, 28.57% HY 4, and 11.03% HY 5. Median PD duration differed significantly between IN-HOME-PD vs. POP participants: 15 years (interquartile range (IQR) 10) vs. 11 years (IQR 7, p = 0.003), respectively. IN-HOME-PD participants had poorer scores on all cognitive items and more baseline comorbidities including: heart disease (30.8% vs. 13.8%, p = 0.001), diabetes (21.5% vs. 12.2%, p = 0.047), psychological disease (47.7% vs. 15.4%, p < 0.001), and arthritis (60% vs. 41.4%, p = 0.01) (complete comorbidity data in eSupplement 1).
Participants were enrolled in IN-HOME-PD for a median of 393 days (IQR 357–406 days), with a total of 23,532 person-days enrolled (63.70 person-years). Visits were evenly spaced, with a median of 126, 133, and 131 days between visits 1–2, 2–3, and 3–4, respectively. Among the nine participants who died during the study, two expired between visits 1–2, four between visits 2–3, and three between visits 3–4. A mean of 98 days (SD 44.20) passed between the most recent study visit and death. Causes of death included: Primary attribution to PD (n = 2), urosepsis (n = 2), and 1 each from aspiration pneumonia, COVID infection, hemorrhagic stroke, renal tumor with massive hemorrhage, and unknown cause. Five individuals expired at home, two in a nursing facility, and one each in an inpatient hospital floor and an inpatient hospice unit. Six of nine (66.67%) died while enrolled in hospice.
We present within-group and between-group longitudinal change in overall QoL, signified by the PDQ-39 SI and individual dimensions (Table 2). IN-HOME-PD participants rated their overall and dimension-specific QoL worse than POP participants at baseline. Longitudinally, the intervention group’s QoL remained unchanged (37.99 (14.10) vs. 37.38 (12.85), within-group p = 0.74, Cohen’s d = 0.05) while in the control group, QoL decreased over time (baseline 29.53 (14.61) vs. 32.56 (15.43), within-group p < 0.001, Cohen’s d = 0.27). The difference in favor of the intervention was significant (between-group p = 0.04).
Table 2.
Comparison of overall and domain-specific health-related quality of life between IN-HOME-PD participants and Parkinson’s Outcomes Project matched control group.
| IN-HOME-PD Participants |
POP Control Group |
Between Groups |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Baseline | 1 year | p-valuea | Effect sizeb | N | Baseline | 1 year | p-valuea | Effect sizeb | p-valuec | |
| PDQ-39, mean (SD) | |||||||||||
| Overall quality of life (PDQ-39 SI) | 50 | 37.99 (14.10) | 37.38 (12.85) | 0.74 | 0.05 | 310 | 29.53 (14.61) | 32.56 (15.43) | <0.001 | 0.27 | 0.04 |
| Mobility | 50 | 64.75 (19.82) | 73.75 (20.24) | 0.01 | 0.39 | 313 | 43.39 (27.01) | 49.15 (27.94) | <0.001 | 0.30 | 0.29 |
| Activities of daily living | 51 | 55.07 (22.95) | 61.44 (25.74) | 0.03 | 0.32 | 313 | 37.37 (24.68) | 41.11 (25.26) | 0.001 | 0.19 | 0.38 |
| Emotional well being | 51 | 32.11 (19.33) | 32.35 (20.06) | 0.93 | 0.01 | 313 | 26.72 (18.75) | 30.44 (20.46) | <0.001 | 0.21 | 0.21 |
| Stigma | 51 | 23.04 (24.86) | 17.03 (21.62) | 0.08 | 0.25 | 312 | 17.49 (18.71) | 19.95 (20.68) | 0.02 | 0.14 | 0.02 |
| Social support | 51 | 11.93 (14.73) | 12.75 (15.49) | 0.74 | 0.05 | 311 | 11.79 (15.32) | 13.53 (16.22) | 0.03 | 0.12 | 0.72 |
| Cognitive impairment | 51 | 34.93 (20.12) | 34.07 (20.97) | 0.74 | 0.05 | 312 | 30.97 (19.99) | 32.93 (21.60) | 0.06 | 0.11 | 0.30 |
| Communication | 51 | 38.40 (23.63) | 32.68 (22.66) | 0.09 | 0.24 | 311 | 31.65 (22.60) | 36.63 (23.50) | <0.001 | 0.26 | 0.003 |
| Bodily discomfort | 51 | 41.50 (28.41) | 31.54 (25.73) | 0.03 | 0.31 | 312 | 36.73 (23.26) | 36.70 (22.91) | 0.98 | 0.00 | 0.04 |
p-value for comparison between baseline and 1 year within case and control group.
Cohen’s d used to calculate effect size of baseline to 1 year change within each group.
p-value for comparison of change (from baseline to 1 year) between case and control.
Bolded values indicate statistical significance, two-tailed alpha, p < 0.05.
Italicized values indicate Cohen’s d effect size of small (0.2) or greater.
IN-HOME-PD: Interdisciplinary Home Visits for Parkinson’s Disease; PDQ-39: Parkinson’s Disease Questionnaire; POP: Parkinson’s Outcomes Project.
Additionally, there were between-group differences in individual QoL dimensions: POP participants declined in seven dimensions with only bodily discomfort remaining stable. IN-HOME-PD participants declined in mobility and ADLs (d = 0.39 and 0.32) without changes in emotional well-being, social support, or cognitive impairment. There was a 26% improvement in self-reported stigma and 15% improvement in communication in the IN-HOME-PD group (d = 0.25 and 0.24, between-group p = 0.02 and 0.003, respectively). Although POP participants’ bodily discomfort remained stable, IN-HOME-PD participants began with greater bodily discomfort yet improved 24% (d = 0.31), leading to less bodily discomfort than POP counterparts over time despite greater baseline immobility and pain-associated comorbidities.
Table 3 demonstrates the HY stage-stratified comparison of change in overall and dimension-specific QoL. In exploratory analyses, there was a pattern within each stage of improved stigma, communication, and bodily discomfort among IN-HOME-PD participants compared with decline in POP controls. Although UPDRS ratings were not gathered in the POP cohort, we present them here for better characterization of the IN-HOME-PD participants and comparison with other cohorts in the literature. While 51 individuals completed the study, two final study visits occurred during the COVID lockdown when neither participant had access to videoconferencing technology. These visits were conducted by phone only and UPDRS part III was not assessed, yielding 49 participants with complete data. The mean UPDRS part III motor score was 48.98 (SD 11.09) at baseline and 51.06 (13.30) at visit 4 (p = 0.11). The mean UPDRS total score was consistent with severe disease but did not worsen: 80.76 (SD 17.3) at baseline and 85.63 (21.29) at visit 4 (p = 0.03).
Table 3.
Stratified comparison of longitudinal change in quality of life by Hoehn & Yahr stage among IN-HOME-PD participants and Parkinson’s Outcomes Project matched control group.
| PDQ-39, mean (SD) | IN-HOME-PD Participants |
POP Control Group |
Between Groups |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Baseline | 1 year | p-valuea | Effect sizeb | N | Baseline | 1 year | p-valuea | Effect sizeb | p-valuec | |
| Hoehn & Yahr 3 | |||||||||||
| Summary Index | 13 | 33.19 (11.17) | 33.64 (13.12) | 0.90 | 0.04 | 264 | 27.61 (13.96) | 30.76 (15.12) | <0.001 | 0.27 | 0.41 |
| Mobility | 13 | 47.69 (19.91) | 58.27 (19.93) | 0.23 | 0.35 | 266 | 38.65 (25.05) | 44.76 (26.77) | <0.001 | 0.30 | 0.61 |
| Activities of daily living | 13 | 37.82 (18.36) | 49.04 (24.01) | 0.12 | 0.46 | 266 | 33.54 (22.53) | 38.03 (24.28) | <0.001 | 0.23 | 0.23 |
| Emotional well being | 13 | 28.53 (20.75) | 31.09 (21.08) | 0.69 | 0.11 | 266 | 25.06 (17.73) | 29.06 (20.12) | <0.001 | 0.23 | 0.77 |
| Stigma | 13 | 29.33 (18.82) | 23.56 (27.74) | 0.43 | 0.22 | 266 | 17.06 (18.74) | 19.41 (20.48) | 0.03 | 0.13 | 0.28 |
| Social support | 13 | 9.62 (8.90) | 10.9 (16.8) | 0.77 | 0.08 | 265 | 11.04 (14.62) | 12.61 (15.64) | 0.05 | 0.12 | 0.94 |
| Cognitive impairment | 13 | 29.81 (15.13) | 32.21 (22.37) | 0.67 | 0.12 | 266 | 29.02 (19.17) | 30.99 (21.06) | 0.08 | 0.11 | 0.93 |
| Communication | 13 | 37.18 (21.14) | 32.05 (24.02) | 0.30 | 0.30 | 265 | 29.59 (21.49) | 34.5 (22.99) | <0.001 | 0.26 | 0.06 |
| Bodily discomfort | 13 | 45.51 (27.56) | 32.05 (29.82) | 0.16 | 0.42 | 266 | 36.9 (23.33) | 36.9 (23.15) | 1 | 0.0 | 0.16 |
| Hoehn & Yahr 4 | |||||||||||
| Summary Index | 29 | 37.96 (13.74) | 37.01 (11.39) | 0.66 | 0.08 | 39 | 40.46 (13.51) | 43.42 (13.01) | 0.11 | 0.26 | 0.17 |
| Mobility | 29 | 70.52 (15.92) | 76.55 (18.49) | 0.09 | 0.32 | 40 | 70.13 (21.65) | 75.75 (19.44) | 0.01 | 0.41 | 0.92 |
| Activities of daily living | 30 | 56.94 (19.34) | 60.56 (24.49) | 0.29 | 0.19 | 40 | 56.04 (23.81) | 56.35 (22.94) | 0.92 | 0.02 | 0.49 |
| Emotional well being | 30 | 32.5 (18.61) | 32.78 (20.61) | 0.94 | 0.01 | 40 | 37.92 (21.6) | 40.31 (20.98) | 0.48 | 0.11 | 0.68 |
| Stigma | 30 | 20.21 (25.52) | 16.04 (19.74) | 0.25 | 0.22 | 39 | 19.71 (18.28) | 23.88 (23.42) | 0.20 | 0.21 | 0.08 |
| Social support | 30 | 11.67 (16.89) | 13.06 (14.46) | 0.64 | 0.09 | 39 | 16.45 (19.45) | 19.44 (19.53) | 0.36 | 0.15 | 0.72 |
| Cognitive impairment | 30 | 32.71 (19.67) | 29.79 (17.42) | 0.36 | 0.17 | 39 | 41.35 (21.54) | 44.39 (21.74) | 0.29 | 0.17 | 0.16 |
| Communication | 30 | 37.22 (24.73) | 31.11 (19.93) | 0.23 | 0.23 | 39 | 43.16 (25.43) | 48.93 (21.98) | 0.10 | 0.27 | 0.05 |
| Bodily discomfort | 30 | 38.33 (28.83) | 30.56 (22.57) | 0.12 | 0.29 | 39 | 36.97 (22.6) | 35.9 (22.23) | 0.75 | 0.05 | 0.25 |
| Hoehn & Yahr 5 | |||||||||||
| Summary Index | 8 | 45.92 (17.54) | 44.79 (15.86) | 0.87 | 0.06 | 7 | 41.17 (13.98) | 40.10 (13.42) | 0.63 | 0.19 | 0.99 |
| Mobility | 8 | 71.56 (18.02) | 88.75 (9.16) | 0.09 | 0.71 | 7 | 70.71 (23.17) | 63.93 (25.69) | 0.02 | 1.15 | 0.03 |
| Activities of daily living | 8 | 76.04 (23.75) | 84.9 (18.49) | 0.25 | 0.44 | 7 | 76.19 (28.74) | 70.83 (26.68) | 0.11 | 0.71 | 0.09 |
| Emotional well being | 8 | 36.46 (21.22) | 32.81 (18.69) | 0.59 | 0.20 | 7 | 25.6 (20.04) | 26.79 (16.29) | 0.74 | 0.13 | 0.54 |
| Stigma | 8 | 23.44 (31.65) | 10.16 (16.35) | 0.32 | 0.38 | 7 | 21.43 (12.61) | 18.75 (8.07) | 0.71 | 0.15 | 0.49 |
| Social support | 8 | 16.67 (14.09) | 14.58 (18.77) | 0.82 | 0.08 | 7 | 14.29 (12.47) | 15.48 (12.2) | 0.79 | 0.11 | 0.76 |
| Cognitive impairment | 8 | 51.56 (22.6) | 53.13 (22.9) | 0.84 | 0.08 | 7 | 47.32 (19.05) | 42.86 (21.78) | 0.41 | 0.33 | 0.52 |
| Communication | 8 | 44.79 (25.17) | 39.58 (31.1) | 0.46 | 0.28 | 7 | 45.24 (26.73) | 48.81 (29.04) | 0.57 | 0.23 | 0.35 |
| Bodily discomfort | 8 | 46.88 (30.19) | 34.38 (32.87) | 0.50 | 0.25 | 7 | 28.57 (26.29) | 33.33 (19.84) | 0.39 | 0.35 | 0.38 |
p-value for comparison between baseline and 1 year within case and control group.
Cohen’s d used to calculate effect size of baseline to 1 year change within each group.
p-value for comparison of change (from baseline to 1 year) between case and control.
Bolded values indicate statistical significance, two-tailed alpha, p < 0.05. Italicized values indicate Cohen’s d effect size of small (0.2) or greater.
IN-HOME-PD: Interdisciplinary Home Visits for Parkinson’s Disease; PDQ-39: Parkinson’s Disease Questionnaire; POP: Parkinson’s Outcomes Project.
4. Discussion
In this longitudinal study, we replicated earlier findings that interdisciplinary home visits—here, enhanced by telemedicine—are feasible and acceptable among homebound patients with advanced PD, with minimal attrition aside from patient death [9]. Moreover, homebound participants had stable PD-specific QoL over one year as compared with the longitudinal decline seen in usual care. Bodily discomfort, stigma, and communication also diverged over time: each dimension improved in IN-HOME-PD participants and worsened with usual care.
In prior work, home visits appeared to stabilize declining QoL; without a control group, however, those findings were challenging to interpret. At the outset of this study, POP offered the largest pool of matched controls in the United States, which we deemed critical for ensuring that service and access variability across international healthcare systems were not confounders. Despite this rationale, the IN-HOME-PD and POP cohorts were markedly non-equivalent given the absence of late-stage PD in the latter. In a secondary analysis in which we compared IN-HOME-PD participants with the recruitment pool at our single COE to determine whether differences between our local COE population and the national POP cohort explained the absence of individuals with late-stage parkinsonism receiving care, or particularly, the relative overrepresentation of underrepresented minorities in IN-HOME-PD, we found the opposite. The broader COE eligibility pool and POP cohorts were similarly skewed towards HY 3 and White individuals. Although the Rush COE population more closely approximated the diverse catchment area of Chicago—with 8.8% Black/African American, 4.8% Asian, and 10.4% Hispanic/Latinx participants—IN-HOME-PD participants recruited from that population were even more likely to be non-White than the clinic population itself.
Abundant prior research has highlighted the disparities in diagnosis, access to care, access to advanced therapeutics, and participation in clinical research among underrepresented minority populations with PD [24–27]. Here, we raise the concern that even among underrepresented minority patients overcoming these barriers to obtain specialty care at a COE, there may be social determinants of health and other factors contributing to estrangement from care in late-stage disease. This is reflected in the relative absence of such individuals in the POP and local COE cohorts. Furthermore, despite becoming homebound and at risk of losing access to care, IN-HOME-PD enrollment of underrepresented individuals exceeded most US-based observational or interventional studies in PD, and moreover, in a longitudinal and time-intensive study that necessitated a healthcare team repeatedly entering participants’ homes. Therefore individuals with advanced PD, including historically underrepresented minorities, appear open to research participation that is accessible, patient-centered, and responsive to their needs and limitations.
Late-stage PD—HY 4–5 with pronounced motor and non-motor symptoms—has been described by the Care of Late Stage Parkinsonism (CLaSP) study group [28], a multicenter, longitudinal cohort recruited from six European countries with diverse healthcare systems, and which may better contextualize our cohort. A cross-sectional analysis of CLaSP participants without dementia comprised a median age of 76, PD duration of 14 years, 71% HY 4, and 21% HY 5, making this a more apt comparator for our homebound cohort, however we did not exclude those with dementia [28]. In an analysis comparing community-dwelling CLaSP participants with those in nursing homes, the institutionalized individuals more closely mirrored IN-HOME-PD participants by age, PD duration, and mobility. In a cross-sectional study where QoL was assessed by the PDQ-8 (intraclass correlation efficient with PDQ-39 = 0.93–0.96) [29], CLaSP participants’ mean PDQ-8 SI was 44, closer to our cohort’s mean (38), and distinct from POP comparators (29.5) [28]. Thus, the homebound PD population may represent more severe late-stage PD, and if CLaSP findings apply to the US, this suggests that individuals with late-stage PD may be absent from POP due to either homebound status and estrangement from care or institutionalization. This not only leads to underrepresentation in research, but portends a greater loss: only 33% of institutionalized individuals with PD retain access to their outpatient neurologist [30] and lower physician access increases morbidity and mortality in custodial care [31]. Broader efforts are necessary to maintain continuity of care and enroll and retain institutionalized individuals in observational and interventional research. Telehealth-enhanced visits may be one such approach.
There are multiple possible explanations as to why IN-HOME-PD yielded no change in overall QoL and improvements in certain dimensions while POP participants declined. First, the benefits of interdisciplinary care and increasingly interdisciplinary palliative approaches—focused on symptom management and improvement of quality of life in the setting of serious illness—have demonstrated benefits over usual care in PD. Miyasaki demonstrated that pain, mood, constipation, and fatigue were among the most bothersome symptoms and most alterable in an interdisciplinary PD palliative care clinic [32]. In a randomized controlled trial of outpatient interdisciplinary palliative care versus usual care in individuals with parkinsonism and moderate to high palliative needs, those receiving the intervention had better QoL after six months, with greater benefits among high-need individuals [6]. Another randomized controlled trial found that recommendations of an MDS with late-stage PD expertise yielded greater improvements in QoL and mobility [33]. IN-HOME-PD employed such an interdisciplinary palliative care approach, comprehensively assessing and addressing pain, dysautonomia, fatigue, mood, polypharmacy, psychosocial stressors, and standard motor symptom management. The double-digit improvement in bodily discomfort and stabilization of overall QoL via home visits echo and extend prior findings regarding the benefits of interdisciplinary palliative approaches in PD. Furthermore, home visits provided the opportunity to reassess prognosis and preferences such that hospice referrals were not uncommon; 67% of participants who died did so with hospice in place, and 56% died at home. This is in stark contrast to the 4% of Americans with PD dying with hospice and 24% of individuals dying at home in national, retrospective studies, suggesting that IN-HOME-PD affords an opportunity to better align with individuals’ goals of care [34].
Moreover, in a multivariable regression analysis of CLaSP participants controlling for stage, nonmotor symptoms, and healthcare utilization, the only factor independently associated with greater QoL was recent PD nurse involvement [28]. The central role of the IN-HOME-PD nurse may explain improved communication and stigma, contrasted with decline of these dimensions in POP controls who had PD nurse specialists available at their COEs without a schedule or protocol. Additionally, the psychosocial assessment conducted by the IN-HOME-PD social worker—which may be administered by another trained member of the healthcare team in future iterations—provided the opportunity to identify and intervene on social determinants of health.
We acknowledge that interdisciplinary home visits may never feasibly serve the millions affected worldwide. However, these results—representing nearly 64 person-years’ longitudinal experience with this population—may be extrapolated to enhance clinical practice and expand access to care for the homebound and late-stage PD population. Nationally, older adults are at a higher annual risk of becoming homebound than of nursing home placement. Among 35 million community-dwelling Medicare recipients, 4.5 million became homebound, 1.2 million were institutionalized, and 8.3 million died over seven years [8]. Nearly 30% of homebound individuals had dementia, 43% were impoverished, 39% lived alone, and 39% had ≥5 chronic conditions. The authors of that study highlight the cognitive and functional impairment, financial and social vulnerability, and multimorbidity of homebound individuals as critical unmet needs. Underrepresentation of homebound individuals with PD—and particularly homebound underrepresented minority patients—suggests that they may be lost to the healthcare system rather than to disease progression, precisely when specialized, interdisciplinary expertise is pivotal for preventing institutionalization or death. Retention of such patients may require leveraging the exponential uptake of telehealth [35], interim calls by PD nurse specialists [36], expansion of home-based medical care, and other strategies [8].
This study has several limitations, chiefly the single-center, nonrandomized design with a non-equivalent control group. Randomization was ethically and logistically impossible. While POP participants appeared to be the closest US-based comparator, they were able to access outpatient care. Matching failed due to underrepresentation of HY 4 and 5, and to our knowledge, there are no other longitudinal, US-based QoL datasets with ample late-stage populations. The disparate between-group baseline comparisons, preponderance of HY 3, and palliative care availability at PF COEs should mitigate QoL decline in the POP group, making it more difficult to detect between-group longitudinal differences, however we found the opposite. It is also possible that late-stage PD QoL plateaus, either due to individual redefinitions of QoL, cognitive impairment affecting QoL assessments, or other factors. The nature of the intervention prevented blinding. To counter social desirability bias, the coordinator administered the QoL assessments, which were inaccessible to other team members until the study’s conclusion. Additionally, visits were time- and labor-intensive, and many people were excluded for living outside of a reasonable traveling radius for the home visit team, limiting feasibility, generalizability, and sustainability in the current form. While cost-effectiveness analyses are forthcoming, we believe the value of this model lies in its component parts promoting continued access to PD-specialized care: an interdisciplinary palliative approach; motor and non-motor symptom management; and protocolized medication reconciliation, home safety, and psychosocial needs assessments. Future studies are needed to adapt these component parts to a fully-telehealth iteration, to streamline the assessments and necessary team members, and test the approach more broadly.
5. Conclusions
We have replicated prior findings that interdisciplinary home visits can stabilize and improve certain QoL domains in the homebound PD population, and that the decline among those receiving usual care may not be inevitable. As with the costly research and development associated with introducing novel therapeutic compounds, IN-HOME-PD and other labor-intensive models of care may serve as proof-of-concept: we can achieve promising engagement and efficacy with a vulnerable, severely affected population. We must refine these models for usual care, drawing upon the resources, interdisciplinary expertise, and technology available. Stabilizing and even improving some aspects of QoL among homebound individuals with PD appears possible.
Supplementary Material
Acknowledgments
The authors thank the study participants for welcoming the team physically and virtually into their homes and lives. The authors acknowledge their colleagues at the Rush University Parkinson’s Disease Program for generously referring and entrusting the home visit team with the care of their patients, and the PF staff and POP coordinators and participants.
Funding sources
Funding: This work was supported by the National Institutes of Health/National Institute of Neurological Disorders and Stroke (K23NS097615, L30NS084235) and by additional philanthropic support from Joyce DeMoose and George Harvey.
Abbreviations
- ADLs
Activities of daily living
- CLaSP
Care of Late Stage Parkinsonism
- COE
Center of Excellence
- EMR
electronic medical record
- HIPAA
Health Insurance Portability and Accountability Act
- HY
Hoehn and Yahr Stage
- IN-HOME-PD
Interdisciplinary Home Visits for Parkinson’s Disease
- MCSI
Multidimensional Caregiver Strain Index
- MDS
Movement disorders specialist
- MoCA
Montreal Cognitive Assessment
- PD
Parkinson disease
- PDQ-39
Parkinson’s Disease Questionnaire
- PDQ-8
Parkinson’s Disease Questionnaire-Short Form
- PDQ-SI
Parkinson’s Disease Questionnaire-Summary Index
- PF
Parkinson’s Foundation
- POP
Parkinson’s Outcome Project
- QoL
quality of life
- REDCap
Research Electronic Data Capture
- SI
summary index
- UPDRS
Unified Parkinson’s Disease Rating Scale
Footnotes
Declaration of competing interest
The authors have no conflicts of interest to declare.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.parkreldis.2022.07.017.
References
- [1].Dorsey ER, Sherer T, Okun MS, Bloem BR, The emerging evidence of the Parkinson pandemic, J. Park. Dis 8 (2018), 10.3233/JPD-181474. S3–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kalia LV, Lang AE, Parkinson’s disease, Lancet Lond. Engl 386 (2015) 896–912, 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- [3].Fleisher J, Barbosa W, Sweeney MM, Oyler SE, Lemen AC, Fazl A, Ko M, Meisel T, Friede N, Dacpano G, Gilbert RM, Di Rocco A, Chodosh J, Interdisciplinary home visits for individuals with advanced Parkinson’s disease and related disorders, J. Am. Geriatr. Soc (2018), 10.1111/jgs.15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, Bottacchi E, Cannas A, Ceravolo G, Ceravolo R, Cicarelli G, Gaglio RM, Giglia RM, Iemolo F, Manfredi M, Meco G, Nicoletti A, Pederzoli M, Petrone A, Pisani A, Pontieri FE, Quatrale R, Ramat S, Scala R, Volpe G, Zappulla S, Bentivoglio AR, Stocchi F, Trianni G, Dotto PD, P. study group, The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease, Mov. Disord. Off. J. Mov. Disord. Soc 24 (2009) 1641–1649, 10.1002/mds.22643. [DOI] [PubMed] [Google Scholar]
- [5].Hagell P, Nygren C, The 39 item Parkinson’s disease questionnaire (PDQ-39) revisited: implications for evidence based medicine, J. Neurol. Neurosurg. Psychiatry 78 (2007) 1191–1198, 10.1136/jnnp.2006.111161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kluger BM, Miyasaki J, Katz M, Galifianakis N, Hall K, Pantilat S, Khan R, Friedman C, Cernik W, Goto Y, Long J, Fairclough D, Sillau S, Kutner JS, Comparison of integrated outpatient palliative care with standard care in patients with Parkinson disease and related disorders, JAMA Neurol (2020), 10.1001/jamaneurol.2019.4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pub 100–02 Medicare Benefit Policy, Clarification of the confined to the home definition in chapter 15, covered medical and other health services, of the Medicare benefit policy manual https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/R192BP.pdf, 2014. (Accessed 15 August 2015).
- [8].Ornstein KA, Garrido MM, Bollens-Lund E, Husain M, Ferreira K, Kelley AS, Siu AL, Estimation of the incident homebound population in the US among older Medicare beneficiaries, 2012 to 2018, JAMA Intern. Med 180 (2020) 1022–1025, 10.1001/jamainternmed.2020.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fleisher JE, Sweeney MM, Oyler S, Meisel T, Friede N, Rocco AD, Chodosh J, Disease severity and quality of life in homebound people with advanced Parkinson disease: a pilot study, Neurol. Clin. Pract 10 (2020) 277–286, 10.1212/CPJ.0000000000000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fleisher J, Hess S, Sennott B, Myrick E, Wallace EK, Lee J, Sanghvi M, Woo K, Ouyang B, Wilkinson J, Beck J, Johnson T, Hall D, Chodosh J, Longitudinal, Interdisciplinary Home Visits vs. Usual Care for Homebound People with Advanced Parkinson’s Disease (IN-HOME-PD): Study Protocol for a Controlled Trial, JMIR Res. Protoc, 2021, 10.2196/31690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Guo L, Jiang Y, Yatsuya H, Yoshida Y, Sakamoto J, Group education with personal rehabilitation for idiopathic Parkinson’s disease, Can. J. Neurol. Sci 36 (2009). [DOI] [PubMed] [Google Scholar]
- [12].Okun MS, Siderowf A, Nutt JG, O’Conner GT, Bloem BR, Olmstead EM, Guttman M, Simuni T, Cheng E, Cohen EV, Parashos S, Marsh L, Malaty IA, Giladi N, Schmidt P, Oberdorf J, Piloting the NPF data-driven quality improvement initiative, Park. Relat. Disord 16 (2010) 517–521, 10.1016/j.parkreldis.2010.06.005. [DOI] [PubMed] [Google Scholar]
- [13].Hughes AJ, Daniel SE, Kilford L, Lees AJ, Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases, J. Neurol. Neurosurg. Psychiatry 55 (1992) 181–184, 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, Giladi N, Holloway RG, Moore CG, Wenning GK, Yahr MD, Seidl L, Movement disorder society task force on rating scales for Parkinson’s, movement disorder society task force report on the Hoehn and Yahr staging scale: status and recommendations, D. Mov. Disord. Off. J. Mov. Disord. Soc 19 (2004) 1020–1028, 10.1002/mds.20213. [DOI] [PubMed] [Google Scholar]
- [15].Hassan A, Wu SS, Schmidt P, Dai Y, Simuni T, Giladi N, Bloem BR, Malaty IA, Okun MS, Investigators N-Q, High rates and the risk factors for emergency room visits and hospitalization in Parkinson’s disease, Park. Relat. Disord 19 (2013) 949–954, 10.1016/j.parkreldis.2013.06.006. [DOI] [PubMed] [Google Scholar]
- [16].Aarsland D, Larsen JP, Tandberg E, Laake K, Predictors of nursing home placement in Parkinson’s disease: a population-based, prospective study, J. Am. Geriatr. Soc 48 (2000) 938–942. [DOI] [PubMed] [Google Scholar]
- [17].Abendroth M, Lutz BJ, Young ME, Family caregivers’ decision process to institutionalize persons with Parkinson’s disease: a grounded theory study, Int. J. Nurs. Stud 49 (2012) 445–454, 10.1016/j.ijnurstu.2011.10.003. [DOI] [PubMed] [Google Scholar]
- [18].Alzheimer’s A, Research consent for cognitively impaired adults: recommendations for institutional review boards and investigators, Alzheimer Assoc. Disord 18 (2004) 171–175. [DOI] [PubMed] [Google Scholar]
- [19].Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN, The self-administered comorbidity questionnaire: a new method to assess comorbidity for clinical and health services research, Arthritis Rheum 49 (2003) 156–163, 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- [20].Martínez-Martín P, Gil-Nagel A, Gracia LM, Gómez JB, Martínez-Sarriés J, Bermejo F, Unified Parkinson’s disease rating scale characteristics and structure. The cooperative multicentric group, Mov. Disord. Off. J. Mov. Disord. Soc 9 (1994) 76–83, 10.1002/mds.870090112. [DOI] [PubMed] [Google Scholar]
- [21].Fleisher JE, Klostermann EC, Hess SP, Lee J, Myrick E, Chodosh J, Interdisciplinary palliative care for people with advanced Parkinson’s disease: a view from the home, Ann. Palliat. Med. Publ (2019). Ahead Print. http://apm.amegroups.com/article/view/30264. (Accessed 1 January 2019). [DOI] [PMC free article] [PubMed]
- [22].Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N, The Parkinson’s Disease Questionnaire (PDQ-39): development and validation of a Parkinson’s disease summary index score, Age Ageing 26 (1997) 353–357. [DOI] [PubMed] [Google Scholar]
- [23].Peto V, Jenkinson C, Fitzpatrick R, Determining minimally important differences for the PDQ-39 Parkinson’s disease questionnaire, Age Ageing 30 (2001) 299–302. [DOI] [PubMed] [Google Scholar]
- [24].Willis AW, Schootman M, Tran R, Kung N, Evanoff BA, Perlmutter JS, Racette BA, Neurologist-associated reduction in PD-related hospitalizations and health care expenditures, Neurology 79 (2012) 1774–1780, 10.1212/WNL.0b013e3182703f92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Willis AW, Schootman M, Kung N, Wang XY, Perlmutter JS, Racette BA, Disparities in deep brain stimulation surgery among insured elders with Parkinson disease, Neurology 82 (2014) 163–171, 10.1212/WNL.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sanchez AV, Ison JM, Hemley H, Willis A, Siddiqi B, Macklin EA, Ulysse C, Reynolds M, Schwarzschild MA, Jackson JD, Designing the fostering inclusivity in research engagement for underrepresented populations in Parkinson’s disease study, Contemp. Clin. Trials 115 (2022), 106713, 10.1016/j.cct.2022.106713. [DOI] [PubMed] [Google Scholar]
- [27].Adrissi J, Fleisher J, Moving the dial toward equity in Parkinson’s disease clinical research: a review of current literature and future directions in diversifying PD clinical trial participation, Curr. Neurol. Neurosci. Rep 22 (2022) 475–483, 10.1007/s11910-022-01212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rosqvist K, Odin P, Lorenzl S, Meissner WG, Bloem BR, Ferreira JJ, Dodel R, Schrag A, Care of late stage parkinsonism (CLaSP) consortium, factors associated with health-related quality of life in late-stage Parkinson’s disease, Mov. Disord. Clin. Pract 8 (2021) 563–570, 10.1002/mdc3.13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jenkinson C, Clarke C, Gray R, Hewitson P, Ives N, Morley D, Rick C, Wheatley K, Williams A, Comparing results from long and short form versions of the Parkinson’s disease questionnaire in a longitudinal study, Park. Relat. Disord 21 (2015) 1312–1316, 10.1016/j.parkreldis.2015.09.008. [DOI] [PubMed] [Google Scholar]
- [30].Safarpour D, Thibault DP, DeSanto CL, Boyd CM, Dorsey ER, Racette BA, Willis AW, Nursing home and end-of-life care in Parkinson disease, Neurology (2015), 10.1212/WNL.0000000000001715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Heckman GA, Crizzle AM, Chen J, Pringsheim T, Jette N, Kergoat M-J, Eckel L, Hirdes JP, Clinical complexity and use of antipsychotics and restraints in long-term care residents with Parkinson’s disease, J. Park. Dis 7 (2017) 103–115, 10.3233/JPD-160931. [DOI] [PubMed] [Google Scholar]
- [32].Miyasaki JM, Long J, Mancini D, Moro E, Fox SH, Lang AE, Marras C, Chen R, Strafella A, Arshinoff R, Ghoche R, Hui J, Palliative care for advanced Parkinson disease: an interdisciplinary clinic and new scale, the ESAS-PD, Park. Relat. Disord 18 (Suppl 3) (2012) S6–S9, 10.1016/j.parkreldis.2012.06.013. [DOI] [PubMed] [Google Scholar]
- [33].Hommel ALAJ, Meinders MJ, Weerkamp NJ, Richinger C, Schmotz C, Lorenzl S, Dodel R, Coelho M, Ferreira JJ, Tison F, Boraud T, Meissner WG, Rosqvist K, Timpka J, Odin P, Wittenberg M, Bloem BR, Koopmans RT, Schragand A, CLaSP consortium, optimizing treatment in undertreated late-stage parkinsonism: a pragmatic randomized trial, J. Park. Dis 10 (2020) 1171–1184, 10.3233/JPD-202033. [DOI] [PubMed] [Google Scholar]
- [34].Moens K, Houttekier D, Van den Block L, Harding R, Morin L, Marchetti S, Csikos A, Loucka M, Naylor WA, Wilson DM, Teno J, Cardenas-Turanzas M, Rhee Y, Garcia-Leon FJ, Deliens L, Cohen J, Place of death of people living with Parkinson’s disease: a population-level study in 11 countries, BMC Palliat. Care 14 (2015) 28, 10.1186/s12904-015-0021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shivkumar V, Subramanian T, Agarwal P, Mari Z, Mestre TA, Parkinson Study Group, Uptake of telehealth in Parkinson’s disease clinical care and research during the COVID-19 pandemic, Park. Relat. Disord 86 (2021) 97–100, 10.1016/j.parkreldis.2021.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sennott B, Woo K, Hess S, Mitchem D, Klostermann EC, Myrick E, Anderson S, Savica R, Fleisher JE, Novel outreach program and practical strategies for patients with parkinsonism in the COVID-19 pandemic, J. Park. Dis 10 (2020) 1383–1388, 10.3233/JPD-202156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


