Abstract
Background:
To compare laser ablation (LA) zone dimensions at two predetermined energy parameters to cover a theoretical 10 mm zone + 2 mm margin in a thyroid swine model.
Methods:
Approval of the Institutional Animal Care and Use Committee was obtained. After hydrodissection, an ultrasound-guided LA (Elesta Echolaser X4 with Orblaze technology, 1064nm) was performed in the periphery of the thyroid in 10 swine. Two cohorts were established to ablate a region of 10mm diameter with 2mm margin based on manufacturer’s ex-vivo data (n= 5 at 3W/1400J and n= 5 at 3W/1800J). The ablation zone was measured on contrast-enhanced computed tomography (CT). Euthanasia was performed 48 hours following ablation.
Results:
All ablations in the 3W/1800J group achieved a diameter of 12 mm ± 1 mm in three dimensions. In the 3W/1400J group, 1 ablation reached 12 mm ± 1 mm in 2 dimensions, and 4 ablations reached this size in one dimension. Maximum diameter was higher in the 3W/1800J compared to the 3W/1400J group, both on histology (1.46 cm ± 0.05 vs. 1.1 cm ± 0.0, p< 0.01) and CT (1.52 cm ± 0.04 vs. 1.18 cm ± 0.04, p< 0.01). Similar results were obtained regarding volumes, both on histology (1.12 mL ± 0.13 vs. 0.57 mL ± 0.06, p< 0.01) and CT (1.24 mL ± 0.13 vs. 0.59 mL ± 0.07, p< 0.01). Histology showed coagulation necrosis and correlated well with CT measurements.
Conclusion:
Optimal parameters to obtain a LA zone of 10 mm with 2 mm margin are 3W/1800J.
Keywords: Laser; Thermal ablation; Papillary thyroid microcarcinoma; Radiology, Interventional
Introduction
Percutaneous laser ablation (LA) has been performed in the thyroid for more than 20years [1] with successful clinical results reported for a variety of diseases including benign thyroid nodules, metastatic cervical lymph nodes from papillary thyroid cancer, and parathyroid adenomas [2–6]. Recently, Choi et al. [7] published a systematic review showing that thermal ablation techniques are safe and effective for papillary thyroid microcarcinoma (defined as ≤ 10 mm in diameter) [8], paving the way for its use as a therapeutic option for patients refusing active surveillance [9–13].
Despite a growing body of evidence [14–17], with some centers already using LA as an alternative to surgery as first choice option for patients with papillary thyroid microcarcinoma [18], implementation of LA in clinical practice is still limited [19], due in part to insufficient data on specific reproducible ablation parameters in thyroid [1]. Incomplete ablation can require a second procedure or lead to local recurrence [20, 21]. Ensuring complete coverage of the tumor with adequate ablation margins has been shown to be of major role in decreasing rate of local tumor progression [22]. Whereas algorithms regarding energy delivery and heating time provided by the manufacturers help the operator predict the ablation zone volume, they might lack of precision when they are only based on ex-vivo data [23], because normal in-vivo tissue is subject to arterial and venous perfusion that impact ablation zone shape and size [24].
Based on manufacturer’s ex-vivo data, the energy required during LA to cover a region of 1 cm diameter in thyroid at a constant power of 3W ranges between 1400J and 1800J. Since a balance between the optimal ablation margins and the risks of thermal damage to the thyroid and surrounding structures is needed, the primary objective of this study was to compare dimensions of the ablation zone between 3W/1400J and 3W/1800J in an in-vivo swine model. Secondary aim was to describe histology findings and evaluate correlation and comparability of CT and gross pathology measurements.
Materials and Methods
The study was reviewed and approved by the Institutional Animal Care and Use Committee. A prospective cohort of swine underwent US-guided percutaneous LA of the thyroid gland (Elesta Echolaser X4 with Orblaze technology, 1064nm, Florence, Italy). All animals were housed in an Association for Assessment and Accreditation of Laboratory Animal Care accredited facility.
In vivo porcine thyroid ablation procedure
The Elesta Echolaser X4 with Orblaze technology and necessary dispensable supplies were used in 10 Yorkshire swine (3–4 months old; weight range 25–60 kg) to perform an US-guided LA of the thyroid. The animals were anesthetized and maintained using continuous inhalation of isoflurane throughout the procedure. Veterinarians monitored vitals throughout the procedure. Prior to performing the procedure, the size, shape, and vascularization of the thyroid gland were assessed using an US equipped with a 7.5–13.0 MHz linear transducer (Logic I, GE Healthcare, Milwaukee, WI).
As LA was planned to be performed in the posterior part of the thyroid lobe, close to the expected location of the recurrent laryngeal nerve, prior hydrodissection was performed. Under US-guidance, normal saline was injected through a needle (Ecochiba, 21G, 11cm) to ensure a thermal barrier and protect the trachea and adjacent neurovascular structures. Hydrodissection was considered optimal when thyroid was lifted off the anterior tracheal wall. After confirming proper hydrodissection and under US-guidance, a second needle (Ecochiba, 21G, 11cm) was advanced to the peripheral posterior part of the gland and a 300-μm diameter laser fiber was introduced coaxially through the needle. The introducer needle was then partially retracted to expose the fiber tip by an additional 5 mm. Ablation was then performed at 2 different settings of energy following manufacturer’s recommendations to obtain a theoretical 1 cm ablation zone in addition to 2 mm margins (Table 1). US was used during the ablation to monitor the extension of the sonographic changes within the thyroid gland and surrounding soft tissues. Description of the procedure is displayed in Figure 1.
Table 1:
Estimated ablation zone size (ex-vivo).
| Maximum Tumor Diameter (mm) | Energy (Joules) | Ablation Time |
|---|---|---|
| < 4 | 600–800 | 200–267 seconds (3.3–4.5 min) |
| ≥ 4 to ≤ 7 | 1000–1200 | 333–400 seconds (5.6–6.7 min) |
| >7 to 10 | 1400–1800 | 467–600 seconds (7.8–10 min) |
Based on manufacturer’s ex-vivo data, the estimated ablation zones to cover a predetermined lesion size were established at a constant power of 3 Watts.
Figure 1: Ablation procedure.
First row: A. General lab set-up. B. Swine positioned supine for US-guided insertion of laser probe and hydrodissection. C. Preplanning US to assess the ablation area and hydrodissection (arrows).
Second row: D. Needle insertion under US guidance. E. Hyperechoic (arrows) area gradually observed with slow enlargement over time. F. Euthanasia and en-bloc thyroid harvest with inspection of adjacent structures.
Based on manufacturer’s ex-vivo work, two cohorts were established to provide an ablation zone that would cover a maximum 10 mm lesion with a 2 mm additional margin (n=5 at 3W/1400J and n=5 at 3W/1800J). The 2 mm margin was selected after expert consensus. All animals were monitored and euthanized at 48 hours post procedure through an intravenous overdose of pentobarbital.
CT imaging and measurements
CT imaging (Lightspeed RTLS, GE Healthcare, Milwaukee, WI) was performed before and after procedure to assess the ablation zone and any adverse events. Unenhanced and dual phase (30 s and 90 s) contrast-enhanced CT scans (Omnipaque 300 mg/ml; GE Healthcare, Princeton, NJ; 4 ml/s; 2 cc/kg; using auto injector) were performed before and immediately following the procedure to assess the ablation zone and technical success (procedure completed according to the planned protocol). In addition, the animals underwent CT imaging prior to euthanasia (approximately 48 hours post-ablation). Parameters for CT scan were: 120 kV tube voltage, tube current as determined by automatic exposure control, 0.8 sec CT gantry rotation time, 0.9375 pitch factor, 0.625 mm image slice thickness. The ablation zone on CT was defined as the non-enhancing region of tissue within the thyroid gland (Figure 2). Three-dimensional measurements of the ablation zone were performed by an interventional radiologist on CT images in the axis of ablation. Ablation zone volume was reported in mL utilizing the ellipsoid volume formula (with measurements expressed in cm):
Figure 2: CT imaging and gross pathology measurements.

First row (During ablation procedure): A. Unenhanced CT of the thyroid before ablation. B. CT of thyroid post hydrodissection. C. Chiba needle position confirmation outside thyroid.
Second row (48 hours post ablation): D. Contrast enhanced CT post-ablation with a well-demarcated ablation zone on axial images. E. Contrast enhanced CT post-ablation with a well-demarcated ablation zone on coronal images. F. Gross macroscopic images of ablation zone.
This formula is used for nodule measurement as proposed in the recent “standardized terminology and reports regarding image-guided thyroid ablations” [25]. Procedure related adverse events (AEs), including hemorrhage, pneumothorax, vessel injury, and organ perforation were recorded.
Gross and Histopathologic analysis
Swine thyroid glands were harvested en-bloc and thyroid tissue containing the ablation area was dissected free from surrounding soft tissues. The thyroid glands were then fixed in formalin. Each thyroid was thinly sliced (3–5 mm thickness) and cross-sections were photographed with an identification label and measurement reference. Each gross image was analyzed using computer image analysis software. Three separate measurements on gross samples were performed and compared to CT imaging data from corresponding animal. The cross sections were stained with Hematoxylin and Eosin (H&E). A board-certified veterinary pathologist evaluated each cross-sectional slice. Correlation with cross-sectional measurements on CT was performed. The histological examination included evaluation of the presence of thermal damage to the thyroid gland.
Adverse event assessment
AEs were classified according to the Society of Interventional Radiology (SIR) [26]. Major complications included all unexpected events that lead to substantial morbidity and disability, requiring immediate euthanasia. All other complications were considered minor. AEs assessment was performed through regular clinical observation of the animal by experienced veterinarian staff and the investigators.
Statistical analysis
Based on the manufacturer’s ex-vivo data, a 2mm difference was expected between the evaluated parameters (3W/1400J vs. 3W/1800J groups), with a standard deviation of 1 mm. Assuming a power of 80% and an alpha of 0.05 (two-sided), 4 ablations were required in each group. Ablation zone measurements were reported using means ± standard deviations. Normally distributed variables were compared between ablation groups using unpaired t test, and Mann-Whitney test was used otherwise. Correlation between ablation zone maximum diameter and volume measured on CT and on histology was evaluated using simple linear regression analysis. Comparability of volumes measured on gross pathology and CT was assessed using Bland-Altman plot. Normal distribution of differences between CT and pathology volume measurements was evaluated using the Shapiro-Wilk test [27–30]. All statistical analyses were conducted using interface for R (The R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was set at p < 0.05.
Results
Ablation zone dimensions
All five ablations in the 3W/1800J group achieved a diameter of 12 mm ± 1 mm in all three dimensions. In the 3W/1400J group, 1 ablation reached 12 mm ± 1 mm in 2 dimensions, and the other 4 ablations only reached this size in one dimension. Based on CT measurements, maximum diameter of ablation zone was significantly higher in the 3W/1800J group compared to the 3W/1400J, measured respectively at 1.52 cm ± 0.04 vs. 1.18 cm ± 0.04 (p< 0.01). A significant difference was also noted on histology measurements, with a maximum diameter measured at 1.46 cm ± 0.05 in the 3W/1800J group and 1.1 cm ± 0.0 in the 3W/1400J group (p< 0.01). Regarding volumes, ablation zones were also significantly bigger in the 3W/1800J group compared to the 3W/1400J group, both on CT (1.24 mL ± 0.13 vs. 0.59 mL ± 0.07, p< 0.01) and on histology (1.12 mL ± 0.13 vs. 0.57 mL ± 0.06, p< 0.01). CT and gross pathology measurements summary are displayed in Table 2. The procedure was performed according to the protocol in all cases, and no minor or major complications were recorded.
Table 2:
General cohort characteristics.
| Experiment Animal / Energy | Ablation zone CT (cm) | Ablation zone volume CT (mL) | Ablation zone Macro (cm) | Ablation zone volume Macro (mL) |
|---|---|---|---|---|
| 1. 3W/1400J | 1.2×1.0×1.1 | 0.69 | 1.1×1.0×1.1 | 0.63 |
| 2. 3W/1400J | 1.2×1.0×0.9 | 0.56 | 1.1×1.1×1.0 | 0.63 |
| 3. 3W/1400J | 1.1×1.0×1.0 | 0.57 | 1.1×0.9×1.0 | 0.51 |
| 4. 3W/1400J | 1.2×0.9×0.9 | 0.50 | 1.1×1.0×0.9 | 0.52 |
| 5. 3W/1400J | 1.2×1.0×1.0 | 0.62 | 1.1×1.0×1.0 | 0.57 |
| 6. 3W/1800J | 1.6×1.2×1.2 | 1.20 | 1.5×1.2×1.2 | 1.13 |
| 7. 3W/1800J | 1.5×1.4×1.2 | 1.32 | 1.5×1.3×1.1 | 1.12 |
| 8. 3W/1800J | 1.5×1.2×1.2 | 1.13 | 1.4×1.1×1.2 | 0.96 |
| 9. 3W/1800J | 1.5×1.2×1.2 | 1.13 | 1.4×1.2×1.2 | 1.05 |
| 10. 3W/1800J | 1.5×1.4×1.3 | 1.43 | 1.5×1.4×1.2 | 1.32 |
Diameters are expressed in centimeters for length x width x depth. Volumes are calculated with the ellipsoid formula and expressed in milliliters.
Imaging and histological findings
During LA of the thyroid gland, a hyperechoic area with “dirty” shadowing was gradually observed with slow enlargement over time (Figure 1. F). On Ultrasonography, the margin of the ablation zone was irregular and poorly defined. In the immediate post-procedural contrast-enhanced CT, a clearly defined ablation zone with obvious margins was observed (Figure 2. D and E). At necropsy, macroscopic examination demonstrated that all ablation zones showed a central pale well-demarcated zone of necrosis with a surrounding hemorrhagic necrotic halo (Figure 2. F).
On histological examination, a well-demarcated area of necrosis within the thyroid tissue was noted and composed of two distinct zones: a central necrotic area, and a peripheral hemorrhagic necrotic rim. These zones display coagulation necrosis of all structures (follicles and blood vessels). Immediately outside the hemorrhagic necrotic zone, there is a thin transition zone (approximately 100 to 300 microns in thickness) that demonstrates viable tissue with occasional necrotic cells in the follicular lumen. An additional lymphocytic infiltrate was noted. The tissue outside the transition zone is normal on histopathology (Figure 3).
Figure 3: Microscopic pathology after H&E staining.
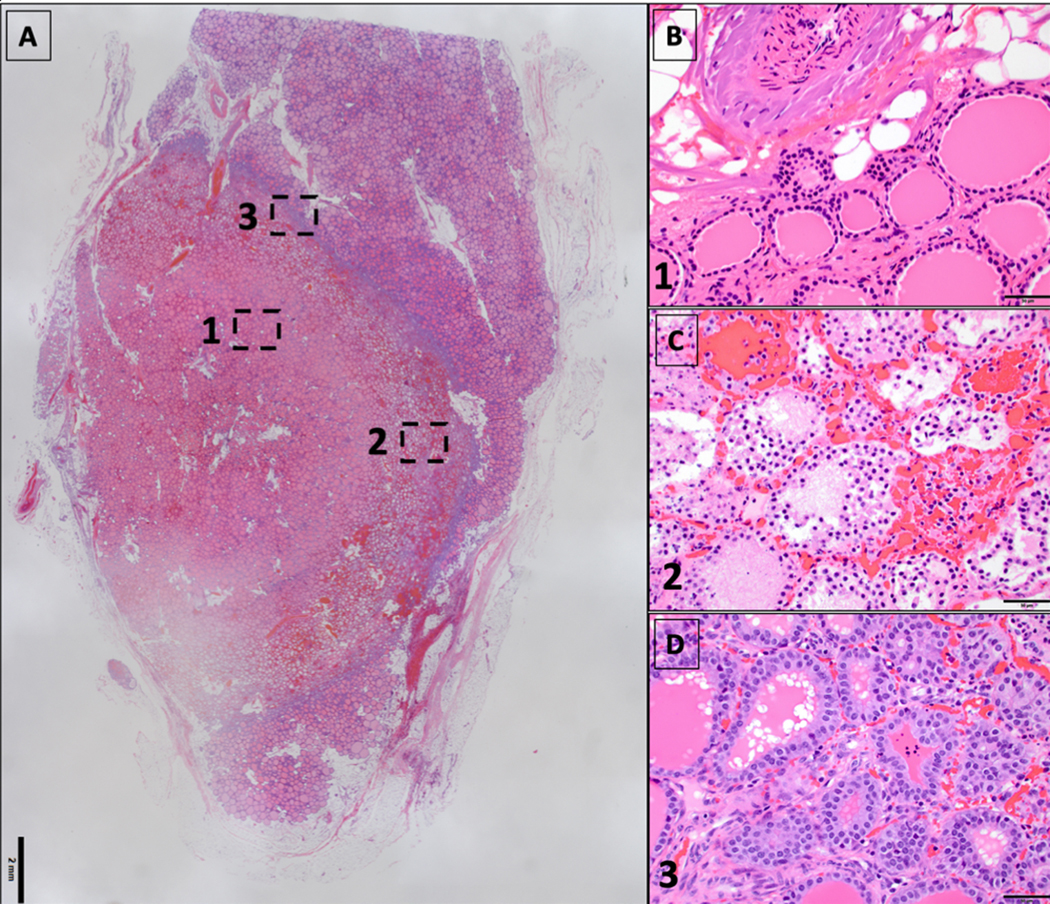
A. Section of thyroid gland with a well demarcated area of coagulation necrosis.
B. Central necrotic zone.
C. Peripheral hemorrhagic necrotic zone.
D. Transition zone (viable, with reactive changes).
Images B, C and D correspond respectively to zones 1, 2 and 3 on image A.
Comparability of CT and gross pathology measurements
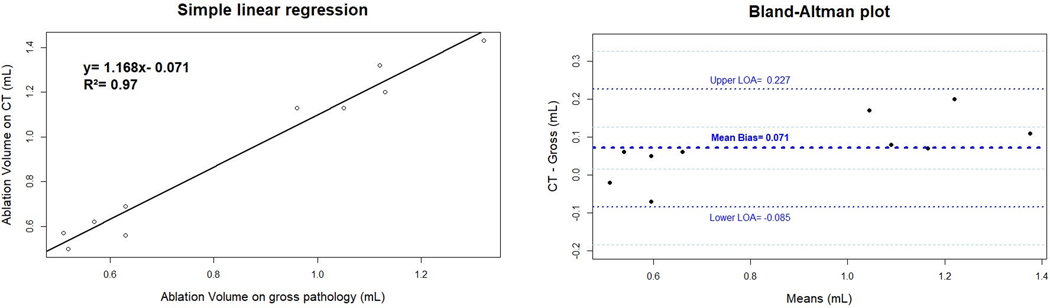
Ablation zone maximal diameter measured on CT showed a good correlation with histology (R2= 0.94). Ablation zone volumes on CT also showed a good correlation with gross pathology, as shown in the simple linear regression model in Figure 4, with a R2= 0.97. Differences of volume measurements between CT and gross pathology were normally distributed (p= 0.73). On average, CT measures were 0.071 mL higher (95% confidence interval [CI]: 0.014– 0.128) than those obtained with gross pathology. The upper limit of agreement (LOA) was 0.227 mL (95% CI: 0.126– 0.328) and the lower LOA was of −0.085 mL (95% CI: −0.186– 0.016).
Figure 4:
Linear Regression and Bland-Altman analysis.
Discussion
In normal swine thyroid, percutaneous laser ablation performed with 3W/1800J achieves a 10 mm diameter coagulation zone with a 2 mm margin in all cases, whereas the 3W/1400J does not. The size of the ablation zone (which can be simulated and planned with ESI - Echolaser Smart Interface manufactured by Elesta) can be measured by contrast CT imaging and correlates with the observed coagulation necrosis zones on histology.
In general, ablation is an accepted minimally invasive technique for the treatment of solid malignancies [31]. Specifically in the thyroid, ablative techniques have been demonstrated to be safe and effective in the management of benign thyroid nodules, metastatic cervical lymph nodes from papillary thyroid cancer, and parathyroid adenomas [2–5, 32–34]. Unlike other percutaneous techniques, ablation can provide controlled regions of coagulation necrosis with a single applicator and has a predictable ablation zone, especially when the target measures less than 3 cm [35].
There is increasing evidence of efficacy and safety of thermal ablation in papillary thyroid carcinoma [7, 36–40] which led to its inclusion as a minimally invasive treatment option for low-risk or incidentally discovered papillary thyroid microcarcinomas in the recent guidelines of the European Thyroid Association and Cardiovascular and Interventional Radiological Society of Europe [41]. However, data on ablation zone predictability and evaluation of reproducibility in the thyroid tissue are still lacking, and uncertainty remains regarding the persistence of viable tumor cells in ablation sites and oncological margins [42]. Because of poor evidence of histologically proven oncologic cure or local control, the American Thyroid Association guidelines do not recognize thermal ablation as primary therapy in microcarcinomas. Therefore, further evidence is required to implement these techniques in clinical practice.
In this study, ex-vivo parameters provided by the manufacturer were corroborated in an in-vivo normal thyroid swine. Ablations were performed with the EchoLaser Evo system (Elesta, Calenzano, Italy) in which a 1064-nm multi-source laser (Echolaser X4) delivers reproducible and accurate energy through disposable 300-micron- flat tip quartz optical fibers, utilizing a multi-fiber guiding system that attaches to the standard clinical ultrasound equipment. This is the first in vivo animal study of Echolaser X4 with the special technology Orblaze. Typically, laser ablation zones are oval instead of spherical. Orblaze technology is a patent pending technology that allows creating a spherical ablation from a flat tip fiber. Ring emission is usually obtained by special fiber tip manufacturing (generally with dimension increase) but in the case of Orblaze, the fiber remains the same (flat tip) but the emission changes from Gaussian beam with slight divergence to a ring shape emission, which increases roundness of the ablation shape into the tissue. In their proprietary ex-vivo work (unpublished data), a power of 3W and energy levels varying between 1400J to 1800J were required to ablate a tumor with a maximum diameter of 7 to 10 mm. Acquired in vivo data confirmed that 3W/1800J parameters can provide a10 mm diameter coagulation zone with an additional 2 mm margin. Furthermore, Bland Altman analyses showed a very low mean bias between CT and histology measurements, with an acceptable and clinically significant range of agreement. Since the swine’s thyroid has increased depth, different orientation and sub sternal location when compared to the human gland, only CT measurements of the ablation zone were performed, without additional assessment with ultrasound or contrast-enhanced ultrasound [43]. By allowing multiplanar reconstructions, CT potentially decreased three-dimensional measurement errors, off plane variability and ultimately allowed a more appropriate correlation with histology.
In the current model, there was no gross evidence of tissue damage outside the thyroid gland when hydro-dissection was applied. LA was intentionally performed as if a lesion located in the posterior part of the thyroid lobe was to be ablated, close to the expected location of the recurrent laryngeal nerve, without experiencing at necropsy evidence of damage to the tissue planes surrounding the thyroid gland. Hydrodissection is planned to be used in the future clinical trial to protect the adjacent clinical structures. Also, one of the concerns expressed from the surgical perspective suggests the inability of performing surgery after local treatment of the thyroid gland. This study potentially corroborates the hypothesis that it will not be affected by demonstrating that the surrounding tissues are not necessarily damaged facilitating future surgical therapy. However, the dissection was performed within 48 hours following ablation, which is not enough time to establish fibrotic changes.
This study certainly has additional limitations. First, only two energy levels were tested with a small number of cases. Second, anatomically, the swine thyroid is an oblong central structure oriented in a cranio-caudal plane that has no lobes like the human gland. These characteristics may impact the behavior of the thermal energy since large vessels (swine carotid artery and jugular vein) run perfectly parallel to it making it susceptible to heat-sink effect. Third, a functional test such as laryngoscopy was not performed to evaluate recurrent laryngeal nerve paresis. Fourth, ablated thyroid corresponded to non-pathological parenchyma: effect of microcalcifications or tumor density on predictability of the ablation zone could not be evaluated. However, findings suggest that given the small size of microcarcinomas, a balance between the optimal ablation margins and the risks of thermal damage to the thyroid and surrounding structures can be obtained with this technology, which is in agreement with low rates of complications reported in literature [7].
In conclusion, optimal parameters for a 10 mm laser ablation zone with 2 mm margins are 3W/1800J, with a good correlation between computed tomography measurements and the observed coagulation necrosis zones on histology. This data provided the preclinical information necessary to proceed with implementation of a clinical trial to histologically verify complete tumor ablation in a cohort of patients with papillary thyroid microcarcinomas.
Funding
This study was funded by Elesta SpA (Florence, Italy); the Memorial Sloan Kettering Cancer Center Endocrinology Service Research and Development fund; US National Institutes of Health/National Cancer Institute Cancer Center Support [Grant P30 CA008748 to Memorial Sloan Kettering Cancer Center]; and the Special Program of Research Excellence, SPORE in Thyroid Cancer [Grant P50 CA172012-01A1].
Conflict of Interest
Dr. Camacho, Dr. Tuttle and Dr Solomon receive grant support from Elesta. Dr. Ghosn is a grant recipient for a post doc research fellowship from General Electric. Dr. Fagin reports grants from Eisai, personal fees from Loxo Oncology, outside the submitted work.
Footnotes
Compliance with Ethical Standards
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Informed consent
For this type of study informed consent is not required.
Consent for publication
For this type of study consent for publication is not required.
References
- 1.Pacella CM, Bizzarri G, Guglielmi R, et al. (2000) Thyroid tissue: US-guided percutaneous interstitial laser ablation-a feasibility study. Radiology 217:673–677. 10.1148/radiology.217.3.r00dc09673 [DOI] [PubMed] [Google Scholar]
- 2.Andrioli M, Riganti F, Pacella CM, Valcavi R (2012) Long-term effectiveness of ultrasound-guided laser ablation of hyperfunctioning parathyroid adenomas: present and future perspectives. AJR Am J Roentgenol 199:1164–1168. 10.2214/AJR.11.8442 [DOI] [PubMed] [Google Scholar]
- 3.Barile A, Quarchioni S, Bruno F, et al. (2018) Interventional radiology of the thyroid gland: critical review and state of the art. Gland Surg 7:132–146. 10.21037/gs.2017.11.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mauri G, Nicosia L, Della Vigna P, et al. (2019) Percutaneous laser ablation for benign and malignant thyroid diseases. Ultrasonography 38:25–36. 10.14366/usg.18034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nixon IJ, Angelos P, Shaha AR, et al. (2018) Image-guided chemical and thermal ablations for thyroid disease: Review of efficacy and complications. Head Neck 40:2103–2115. 10.1002/hed.25181 [DOI] [PubMed] [Google Scholar]
- 6.Papini E, Monpeyssen H, Frasoldati A, Hegedüs L (2020) 2020 European Thyroid Association Clinical Practice Guideline for the Use of Image-Guided Ablation in Benign Thyroid Nodules. Eur Thyroid J 9:172–185. 10.1159/000508484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi Y, Jung S-L (2020) Efficacy and Safety of Thermal Ablation Techniques for the Treatment of Primary Papillary Thyroid Microcarcinoma: A Systematic Review and Meta-Analysis. Thyroid 30:720–731. 10.1089/thy.2019.0707 [DOI] [PubMed] [Google Scholar]
- 8.Davies L, Ouellette M, Hunter M, Welch HG (2010) The increasing incidence of small thyroid cancers: where are the cases coming from? Laryngoscope 120:2446–2451. 10.1002/lary.21076 [DOI] [PubMed] [Google Scholar]
- 9.Haugen BR, Alexander EK, Bible KC, et al. (2016) 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26:1–133. 10.1089/thy.2015.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito Y, Miyauchi A, Kudo T, et al. (2018) Trends in the Implementation of Active Surveillance for Low-Risk Papillary Thyroid Microcarcinomas at Kuma Hospital: Gradual Increase and Heterogeneity in the Acceptance of This New Management Option. Thyroid 28:488–495. 10.1089/thy.2017.0448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito Y, Oda H, Miyauchi A (2016) Insights and clinical questions about the active surveillance of low-risk papillary thyroid microcarcinomas [Review]. Endocr J 63:323–328. 10.1507/endocrj.EJ15-0637 [DOI] [PubMed] [Google Scholar]
- 12.Tuttle RM, Fagin JA, Minkowitz G, et al. (2017) Natural History and Tumor Volume Kinetics of Papillary Thyroid Cancers During Active Surveillance. JAMA Otolaryngol Head Neck Surg 143:1015–1020. 10.1001/jamaoto.2017.1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuttle RM, Zhang L, Shaha A (2018) A clinical framework to facilitate selection of patients with differentiated thyroid cancer for active surveillance or less aggressive initial surgical management. Expert Rev Endocrinol Metab 13:77–85. 10.1080/17446651.2018.1449641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papini E, Guglielmi R, Gharib H, et al. (2011) Ultrasound-guided laser ablation of incidental papillary thyroid microcarcinoma: a potential therapeutic approach in patients at surgical risk. Thyroid 21:917–920. 10.1089/thy.2010.0447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Persichetti A, Bizzarri G, Guglielmi R, et al. (2018) Ultrasound-guided laser ablation for local control of neck recurrences of medullary thyroid cancer. A feasibility study. Int J Hyperthermia 35:480–492. 10.1080/02656736.2018.1508759 [DOI] [PubMed] [Google Scholar]
- 16.Valcavi R, Piana S, Bortolan GS, et al. (2013) Ultrasound-guided percutaneous laser ablation of papillary thyroid microcarcinoma: a feasibility study on three cases with pathological and immunohistochemical evaluation. Thyroid 23:1578–1582. 10.1089/thy.2013.0279 [DOI] [PubMed] [Google Scholar]
- 17.Zhou W, Jiang S, Zhan W, et al. (2017) Ultrasound-guided percutaneous laser ablation of unifocal T1N0M0 papillary thyroid microcarcinoma: Preliminary results. Eur Radiol 27:2934–2940. 10.1007/s00330-016-4610-1 [DOI] [PubMed] [Google Scholar]
- 18.Mauri G, Orsi F, Carriero S, et al. (2020) Image-Guided Thermal Ablation as an Alternative to Surgery for Papillary Thyroid Microcarcinoma: Preliminary Results of an Italian Experience. Front Endocrinol (Lausanne) 11:575152. 10.3389/fendo.2020.575152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hegedüs L, Frasoldati A, Negro R, Papini E (2020) European Thyroid Association Survey on Use of Minimally Invasive Techniques for Thyroid Nodules. Eur Thyroid J 9:194–204. 10.1159/000506513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji L, Wu Q, Gu J, et al. (2019) Ultrasound-guided percutaneous laser ablation for papillary thyroid microcarcinoma: a retrospective analysis of 37 patients. Cancer Imaging 19:16. 10.1186/s40644-019-0204-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanis E, Nordlinger B, Mauer M, et al. (2014) Local recurrence rates after radiofrequency ablation or resection of colorectal liver metastases. Analysis of the European Organisation for Research and Treatment of Cancer #40004 and #40983. Eur J Cancer 50:912–919. 10.1016/j.ejca.2013.12.008 [DOI] [PubMed] [Google Scholar]
- 22.Kim Y-S, Lee WJ, Rhim H, et al. (2010) The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (> 2 and < 5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR Am J Roentgenol 195:758–765. 10.2214/AJR.09.2954 [DOI] [PubMed] [Google Scholar]
- 23.Ruiter SJS, Heerink WJ, de Jong KP (2019) Liver microwave ablation: a systematic review of various FDA-approved systems. Eur Radiol 29:4026–4035. 10.1007/s00330-018-5842-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu DSK, Raman SS, Vodopich DJ, et al. (2002) Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the “heat sink” effect. AJR Am J Roentgenol 178:47–51. 10.2214/ajr.178.1.1780047 [DOI] [PubMed] [Google Scholar]
- 25.Mauri G, Pacella CM, Papini E, et al. (2019) Image-Guided Thyroid Ablation: Proposal for Standardization of Terminology and Reporting Criteria. Thyroid 29:611–618. 10.1089/thy.2018.0604 [DOI] [PubMed] [Google Scholar]
- 26.Khalilzadeh O, Baerlocher MO, Shyn PB, et al. (2017) Proposal of a New Adverse Event Classification by the Society of Interventional Radiology Standards of Practice Committee. J Vasc Interv Radiol 28:1432–1437.e3. 10.1016/j.jvir.2017.06.019 [DOI] [PubMed] [Google Scholar]
- 27.Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310 [PubMed] [Google Scholar]
- 28.Giavarina D (2015) Understanding Bland Altman analysis. Biochem Med (Zagreb) 25:141–151. 10.11613/BM.2015.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bland JM, Altman DG (1995) Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet 346:1085–1087. 10.1016/s0140-6736(95)91748-9 [DOI] [PubMed] [Google Scholar]
- 30.Abu-Arafeh A, Jordan H, Drummond G (2016) Reporting of method comparison studies: a review of advice, an assessment of current practice, and specific suggestions for future reports. Br J Anaesth 117:569–575. 10.1093/bja/aew320 [DOI] [PubMed] [Google Scholar]
- 31.Cho YK, Kim JK, Kim MY, et al. (2009) Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology 49:453–459. 10.1002/hep.22648 [DOI] [PubMed] [Google Scholar]
- 32.Pacella CM, Mauri G, Achille G, et al. (2015) Outcomes and Risk Factors for Complications of Laser Ablation for Thyroid Nodules: A Multicenter Study on 1531 Patients. J Clin Endocrinol Metab 100:3903–3910. 10.1210/jc.2015-1964 [DOI] [PubMed] [Google Scholar]
- 33.Appelbaum L, Goldberg SN, Ierace T, et al. (2020) US-guided laser treatment of parathyroid adenomas. Int J Hyperthermia 37:366–372. 10.1080/02656736.2020.1750712 [DOI] [PubMed] [Google Scholar]
- 34.Mauri G, Cova L, Ierace T, et al. (2016) Treatment of Metastatic Lymph Nodes in the Neck from Papillary Thyroid Carcinoma with Percutaneous Laser Ablation. Cardiovasc Intervent Radiol 39:1023–1030. 10.1007/s00270-016-1313-6 [DOI] [PubMed] [Google Scholar]
- 35.Lin S-M, Lin C-J, Lin C-C, et al. (2005) Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut 54:1151–1156. 10.1136/gut.2004.045203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen J, Cao J, Qiu F, Huang P (2019) The Efficacy and The Safety of Ultrasound-guided Ablation Therapy for Treating Papillary Thyroid Microcarcinoma. J Cancer 10:5272–5282. 10.7150/jca.36289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho SJ, Baek SM, Lim HK, et al. (2020) Long-Term Follow-Up Results of Ultrasound-Guided Radiofrequency Ablation for Low-Risk Papillary Thyroid Microcarcinoma: More Than 5-Year Follow-Up for 84 Tumors. Thyroid. 10.1089/thy.2020.0106 [DOI] [PubMed] [Google Scholar]
- 38.Yue W, Wang S, Yu S, Wang B (2014) Ultrasound-guided percutaneous microwave ablation of solitary T1N0M0 papillary thyroid microcarcinoma: initial experience. Int J Hyperthermia 30:150–157. 10.3109/02656736.2014.885590 [DOI] [PubMed] [Google Scholar]
- 39.Zhang M, Luo Y, Zhang Y, Tang J (2016) Efficacy and Safety of Ultrasound-Guided Radiofrequency Ablation for Treating Low-Risk Papillary Thyroid Microcarcinoma: A Prospective Study. Thyroid 26:1581–1587. 10.1089/thy.2015.0471 [DOI] [PubMed] [Google Scholar]
- 40.Zhou W, Ni X, Xu S, et al. (2019) Ultrasound-guided laser ablation versus surgery for solitary papillary thyroid microcarcinoma: a retrospective study. Int J Hyperthermia 36:897–904. 10.1080/02656736.2019.1649475 [DOI] [PubMed] [Google Scholar]
- 41.Mauri G, Hegedüs L, Bandula S, et al. (2021) European Thyroid Association and Cardiovascular and Interventional Radiological Society of Europe 2021 Clinical Practice Guideline for the Use of Minimally Invasive Treatments in Malignant Thyroid Lesions. ETJ 10:185–197. 10.1159/000516469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hegedüs L, Miyauchi A, Tuttle RM (2020) Nonsurgical Thermal Ablation of Thyroid Nodules: Not if, but Why, When, and How? Thyroid. 10.1089/thy.2020.0659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim J, Baek JH, Lim HK, Na DG (2019) Summary of the 2017 thyroid radiofrequency ablation guideline and comparison with the 2012 guideline. Ultrasonography 38:125–134. 10.14366/usg.18044 [DOI] [PMC free article] [PubMed] [Google Scholar]