Background: The Omicron (B.1.1.529) variant of SARS-CoV-2 and reports of waning immunity after the first vaccine booster dose (1) have prompted numerous countries to recommend a fourth dose for individuals at higher risk for severe disease. Despite high vaccination and booster coverage, Singapore experienced a resurgence of cases in July 2022 driven by Omicron BA.5. From April 2022, persons 80 years of age and older, living in aged care facilities, or medically susceptible to severe COVID-19 who received their first booster 5 months prior were invited to receive a second booster with Pfizer-BioNTech BNT162b2 or Moderna mRNA-1273.
Objective: To estimate the effectiveness of a fourth mRNA vaccine dose against symptomatic SARS-CoV-2 infection, COVID-19-related hospitalization, and severe COVID-19 in persons 80 years of age or older.
Methods: We used administrative data from the Ministry of Health, Singapore to emulate a target trial of a fourth mRNA vaccine dose versus placebo among persons 80 years of age or older who received their third dose 5 months before the start of the study. Those who received non-mRNA vaccines or had previous documented SARS-CoV-2 infection were excluded. On each day between 6 April and 21 July 2022, persons who had recently received a fourth dose were matched to control participants who were eligible but had not received a fourth dose in a 1:1 ratio, based on age, gender, ethnicity, and housing type (as a socioeconomic status marker and to distinguish nursing home residents). Each person was followed up until the earliest occurrence of an outcome event, receipt of the fourth dose, or study conclusion. Study periods of individuals and their respective controls were matched to account for varying force of infection over time. Unmatched individuals were excluded from analysis.
We assessed vaccine effectiveness (VE) of the fourth dose against symptomatic SARS-CoV-2 infection (VE-I) confirmed by polymerase chain reaction testing or antigen rapid testing, COVID-19-related hospitalization (VE-H), and severe COVID-19 (VE-S). Testing for SARS-CoV-2 was conducted on all persons presenting with acute respiratory symptoms to a health care facility. Physicians assessed need for hospitalization based on clinical presentation and risk factors. Severe COVID-19 was defined as requiring oxygen supplementation, intensive care unit admission, or death.
To allow for sufficient immune response, individuals were classified as having received a booster dose 8 days after vaccination. Classification was dynamic, and those who had not received their fourth dose could serve as controls for those who had. In our secondary analysis, we estimated relative VEs at 8 to 30 days, 31 to 60 days, and more than 60 days from receiving the fourth dose.
After matching, survival curves for both groups were estimated using the Kaplan-Meier estimator, with relative VE calculated as 1 minus the risk ratio, similar to VE analyses by Dagan et al (2) and Iaonnou et al (3). We estimated 95% CI using the percentile bootstrap method with 500 repetitions.
The study was conducted to inform public health policy under the Infectious Diseases Act, Singapore, with exemption from ethics review. Data analysis was performed using Stata version 17.0 (StataCorp).
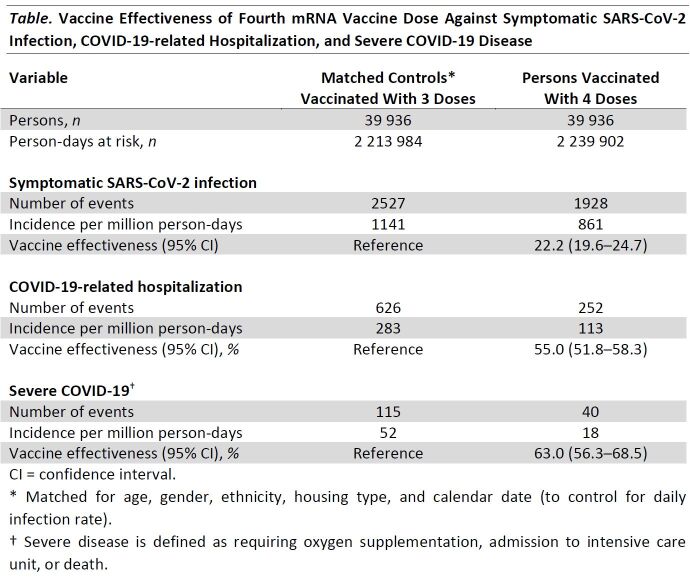
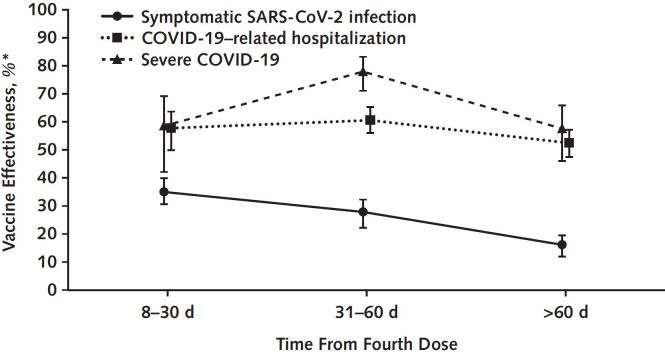
Findings: A total of 40 030 persons 80 years of age or older who received their fourth mRNA vaccine dose were identified and matched to 39 936 control participants who were eligible but had not received their fourth dose (0.2% unmatched). Persons who received 4 doses had lower risk for symptomatic SARS-CoV-2 infection, COVID-19–related hospitalization, and severe disease, with VE estimates at 22.2% (95% CI, 19.6–24.7), 55.0% (CI, 51.8–58.3), and 63.0% (CI, 56.3–68.5), respectively (Table). Analysis stratified by time since vaccination showed that VE-H and VE-S remained high despite a fall in VE-I (Figure).
Table.
Vaccine Effectiveness of Fourth mRNA Vaccine Dose Against Symptomatic SARS-CoV-2 Infection, COVID-19-related Hospitalization, and Severe COVID-19 Disease
Figure. Vaccine effectiveness of fourth mRNA vaccine dose against symptomatic SARS-CoV-2 infection, COVID-19-related hospitalization, and severe COVID-19 disease over time, relative to participants vaccinated with 3 doses.
* Vaccine effectiveness was calculated by taking 1 minus the risk ratio.
Discussion: Our study found that 4 doses of BNT162b2 or mRNA-1273 reduced the risk for symptomatic SARS-CoV-2 infection, hospitalization, and severe COVID-19 illness versus 3 doses among persons 80 years of age or older, corroborating studies in a younger cohort in Israel (4, 5). In Singapore, the risk reductions in hospitalization and severe disease were helpful in managing the case surge from BA.5, avoiding the need to tighten control measures as was done for the Omicron BA.1/BA.2 wave in January through April 2022. Consistent with findings of sustained protection against severe disease from Israel (6), we found no waning of this protection after 60 days.
Limitations of this study included residual confounding from comorbid conditions, use of COVID-19 therapeutics and other individual factors affecting vaccination status or disease severity, and underdetection of cases due to persons with mild or asymptomatic illness not seeking medical attention and due to expansion of self-testing with antigen rapid testing. Immunocompromised persons who received 3 primary series doses and 1 booster dose were included under the category of those who received 4 doses. Sequencing to identify the Omicron subvariant was not performed in all cases and the shift from BA.2 toward BA.5 predominance during the study period could have contributed to the waning VE-I observed.
This study, based on comprehensive health care administrative data, provides evidence that a fourth mRNA vaccine dose confers additional sustained protection against hospitalization and severe disease from COVID-19 in an elderly Asian population during Omicron variant epidemic.
Footnotes
This article was published at Annals.org on 13 September 2022.
References
- 1. Andrews N , Stowe J , Kirsebom F , et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med. 2022;386:1532-1546. [PMID: ] doi: 10.1056/NEJMoa2119451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dagan N , Barda N , Kepten E , et al. BNT162b2 mRNA covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412-1423. [PMID: ] doi: 10.1056/NEJMoa2101765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ioannou GN , Locke ER , O'Hare AM , et al. COVID-19 vaccination effectiveness against infection or death in a national U.S. health care system: a target trial emulation study. Ann Intern Med. 2022;175:352-361. [PMID: ] doi: 10.7326/M21-3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bar-On YM , Goldberg Y , Mandel M , et al. Protection by a fourth dose of BNT162b2 against Omicron in Israel. N Engl J Med. 2022;386:1712-1720. [PMID: ] doi: 10.1056/NEJMoa2201570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Magen O , Waxman JG , Makov-Assif M , et al. Fourth dose of BNT162b2 mRNA covid-19 vaccine in a nationwide setting. N Engl J Med. 2022;386:1603-1614. [PMID: ] doi: 10.1056/NEJMoa2201688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gazit S , Saciuk Y , Perez G , et al. Short term, relative effectiveness of four doses versus three doses of BNT162b2 vaccine in people aged 60 years and older in Israel: retrospective, test negative, case-control study. BMJ. 2022;377:e071113. [PMID: ] doi: 10.1136/bmj-2022-071113 [DOI] [PMC free article] [PubMed] [Google Scholar]