Abstract
Assessment of circulating endotoxin during the perioperative period, which is only demonstrated by the Limulus amebocyte lysate (LAL) test, may be modulated by several endotoxin-binding proteins. Endotoxin-neutralizing capacity (ENC) and the plasma levels of soluble CD14 (sCD14), lipopolysaccharide-binding protein, and bactericidal/permeability-increasing protein (BPI) were determined in 40 patients 6 h prior to skin incision for major abdominal surgery. The bioactivity of plasma endotoxin was tested by the polymyxin B-inhibited stimulatory activity of the plasma samples on healthy monocytes as measured by the release of tumor necrosis factor alpha. Plasma endotoxin levels in almost all patients increased from 0.05 ± 0.01 to 0.23 ± 0.03 experimental units (EU) per ml (P < 0.001); more specifically, 17 of 40 samples showed endotoxin levels of greater than 0.2 EU per ml and corresponding reductions in ENC. Soluble CD14 plasma levels were decreased from 5.6 ± 0.3 to 4.6 ± 0.3 μg per ml (P < 0.05). ENC was strongly correlated with the sCD14 plasma concentration throughout the period of observation. The addition of sCD14-neutralizing monoclonal anti-sCD14 antibodies reduced ENC both pre- and postoperatively. No correlation could be established between ENC and the plasma levels of BPI, high-density lipoproteins, or low-density lipoproteins determined by measuring the concentrations of apoprotein A and apoprotein B. Biologically active endotoxin was found in only 6 of 17 samples with endotoxin levels greater than 0.2 EU per ml in the LAL test. These samples could be characterized by their perioperative loss of at least 35% of their sCD14. No change in sCD14 was detected in the remaining 11 samples. The perioperative loss of ENC is partly caused by the loss of sCD14 resulting from its consumption by endotoxin reaching the bloodstream. This study demonstrated the role of sCD14 on the bioactivity of circulating endotoxin in a human model of endotoxemia after major abdominal surgery.
A number of cell types, including hepatocytes (15, 33), local macrophages (16, 26, 40), and granulocytes (35, 36), have cellular endotoxin-neutralizing activity mediated via well-characterized mechanisms of lipopolysaccharide (LPS) inactivation. In addition to the cellular endotoxin neutralization system, soluble endotoxin-binding and -neutralizing factors that reduce the harmful action of circulating endotoxin are also present in plasma. Early studies showed that plasma itself is a potent inhibitor of endotoxin-mediated phenomena such as pyrogenicity (41, 42). Later experiments showed that several plasma proteins may bind endotoxin either in a specific or unspecific manner, which was assumed to be associated with an alteration of aspects of endotoxin bioactivity (14, 31, 45). Most recently, the soluble form of the endotoxin receptor CD14 (sCD14) was demonstrated to mediate the LPS-neutralizing action of high-density lipoproteins (22, 23, 47). Plasma sCD14 levels are increased during septic diseases (7, 29, 30) as well as after multiple-trauma and burn injuries (28). Bactericidal/permeability-increasing protein (BPI), a neutrophil granule protein, diminishes the bioactivity of LPS in vitro (1, 24) and in vivo (13, 44) and has been shown to increase significantly during sepsis (8, 17). The LPS-binding protein (LBP) first catalytically transfers an LPS monomer to a binding site on sCD14 (20), and the resulting LPS-sCD14 complexes diffuse readily, breaking LPS into lipoprotein particles (47–49). LBP is a classical acute phase protein, which is strongly enhanced during acute inflammatory responses (17, 19).
The endotoxin-neutralizing capacity (ENC) of plasma can be easily determined by a direct Limulus amebocyte lysate (LAL) test without heat inactivation of the inhibitors present in plasma (4). Our previous studies showed that ENC was decreased significantly during aseptic abdominal surgery, which is associated with impending complications due to infection (4). Elective aseptic abdominal surgery represents a human model characterized by a significant and reproducible endotoxemia and a well-defined acute phase reaction (5, 6, 12, 37, 46). Although there are some indications that circulating endotoxin has bioactivity during the postoperative (5, 32) and posttraumatic courses (25), its pathophysiological relevance is far from being generally accepted. The complex nature of cellular and soluble neutralizing mechanisms may account for the observation that high endotoxin levels are not invariably correlated with clinical signs. We propose that the endotoxin-binding proteins, and sCD14 in particular, determine the biological activity of translocated endotoxin during surgery.
In this study, we aimed to (i) evaluate the sCD14, LBP, BPI, and endotoxin plasma levels and the ENC of the plasma during major elective abdominal surgery, (ii) estimate the relationship of sCD14, LBP, and BPI on ENC, and (iii) estimate the biological activity of perioperative plasma assessed by the effect of plasma on monocyte tumor necrosis factor alpha (TNF-α) production in response to LPS.
MATERIALS AND METHODS
The local ethical committee of the University of Ulm approved this study, and blood donors gave informed consent for research.
Patients and a healthy volunteer.
Forty patients undergoing elective major abdominal surgery (gastrectomy, n = 5; pancreatectomy, n = 28; colectomy, n = 7) were enrolled in the present study (Table 1). Exclusion criteria were as follows: age less than 18 years, liver cirrhosis, pregnancy, preexisting renal insufficiency requiring hemodialysis, immunosuppression, or acute inflammatory disease which was checked by plasma cyclic AMP receptor protein levels (cutoff level of cyclic AMP receptor protein, <100 mg per liter). To rule out the bacteriocidal and bacteriocytic effects of antibiotic therapy, we excluded patients who were given any antibiotics within 6 h before or after the skin incision. We applied monocytes from one healthy volunteer for the stimulation assay. Before starting the experiment, 10 healthy volunteers were checked for responsiveness to endotoxin stimulation by checking TNF-α secretion. Afterwards, we selected a volunteer with average responsiveness as determined by TNF-α secretion.
TABLE 1.
Clinical characteristics of presurgical status in the patients subjects
| Characteristic | Valuea |
|---|---|
| Sex (male/female) | 22/18 |
| Age (average [yr]) | 61 |
| (range [yr]) | 42–77 |
| Body mass index (kg/m2)b | 27.5 ± 0.3 |
| Diagnoses | |
| Chronic pancreatitis | 9 |
| Pancreatic cancer | 19 |
| Gastric cancer | 5 |
| Colon cancer | 7 |
| APACHE II scorec | 5.0 ± 1.0 |
| Total protein (g/dl) | 6.5 ± 0.1 |
| Albumin (g/dl) | 4.1 ± 0.1 |
| Hemoglobin (g/dl) | 13.2 ± 0.1 |
| Total cholesterol (mg/dl) | 220.5 ± 10.2 |
| Choline esterase (U/liter) | 4,382 ± 201 |
| CRP (mg/liter)d | 9.0 ± 1.1 |
Data are presented as means ± SE.
Body mass index = body weight/height2 (kg/m2).
APACHE, Acute Physiology and Chronic Health Evaluation.
C-reactive protein.
Preparation of blood samples.
Blood samples were collected prior to and 6 h after skin incision by puncture of the cubital vein under sterile conditions. To exclude the influence of anesthesia, the preoperative samples were collected after the induction of anesthesia but before the surgery. The time point of 6 h after skin incision was selected because the results of our recent study revealed that this time point would most likely yield peak plasma endotoxin levels (25). The blood was anticoagulated with 10 IU of sodium heparin per ml of blood. Platelet-poor autologous plasma was prepared by centrifugation at 2,000 × g for 10 min. Care was taken to prevent contamination of the plasma samples with polymorphonuclear leukocytes, which may release BPI even after freezing and thawing (9). Hemolytic plasma samples were excluded to minimize artificial BPI release (38). Samples were stored at −70°C for up to 4 weeks in multiple aliquots. All tubes used in blood collection and analysis were certified to be endotoxin free.
Determination of endotoxin content.
Endotoxin plasma levels were determined by using a two-step, endpoint micromethod as described previously in detail (3). The unknown samples were pretreated by heat inactivation for 10 min at 75°C and were incubated with the lysate (Charles River Endosafe, Sulzfeld, Germany) for 30 min at 37°C. After adding 5-mmol/ml chromogenic substrate (Pefachrome from LPS, Sinntal-Oberzell, Germany), samples were further incubated for 3 min at 37°C. The reaction was stopped, and the endotoxin content was quantified according to a simultaneously established standard curve in pyrogen-free plasma.
Estimation of ENC.
The ENC of plasma, expressed as endotoxin recovery, was measured by using the LAL test and has been recently described in detail (4). The method principally relies on the determination of the recovery of exogenously added endotoxin to plasma samples. In contrast to the estimation of endogenous endotoxin, inactivation of the plasma samples was omitted. Ten microliters of a standard endotoxin of Salmonella abortus subsp. equi (1,000 EU/ml) (NP3; Pyroquant Co., Moerfelden-Walldorf, Germany) were added to 90 μl of plasma. After incubation at 24°C for 30 min, the sample was diluted with 900 μl of pyrogen-free water (final concentration, 10 EU/ml). The endotoxin recovery was determined as described above except that the standard curve was established in pyrogen-free water. Intra- and interassay variation coefficients amounted to 6.5 and 7.2%, respectively, as ascertained in 30 single determinations. In experiments designed to determine the role of sCD14, the plasma samples were preincubated with 10 μg of the monoclonal anti-CD14 antibody MEM-18 per ml, (kindly provided by V. Horesji, Institute of Organic Chemistry and Biochemistry, Prague, Czech Republic) (2) for 20 min at 24°C before LPS was added. Endogenous endotoxin levels of all samples were subtracted from the recovery data.
Determination of albumin, apo A, and apo B plasma levels.
The plasma levels of albumin, apoprotein A (apo A), and apoprotein B (apo B) were determined by a nephelometer (200 analyzer; Behring Co., Liederbach, Germany).
ELISA assays.
Plasma TNF-α was quantified with a commercially available enzyme-linked immunosorbent assay (Immunotech, Hamburg, Germany). Soluble CD14 was measured by using a sandwich ELISA with two monoclonal antibodies against CD14 (IBL, Hamburg, Germany). Plasma samples which were diluted 1:200 with phosphate-buffered saline (PBS) were assayed in ELISA following manufacturer’s instructions. Plasma BPI and LBP levels were determined by using a sandwich ELISA as reported elsewhere in detail (9). In short, microtiter plates were coated with human-BPI-specific monoclonal antibody 4E3 or with polyclonal anti-human LBP immunoglobulin G (IgG). Washing and dilution buffers for BPI and LBP determination contained 80 mM and 40 mM MgCl2, respectively. Mg++ ions were added to prevent the influence of LPS on BPI or LBP measurement. Human recombinant BPI or recombinant LBP (provided by M. Marra, InCyte, Palo Alto, Calif.) was used for the standard curve. Samples diluted in the above buffer (1:2 for BPI, 1:2,000 for LBP) were assayed. Biotinylated polyclonal rabbit anti-human BPI IgG and biotinylated rabbit anti-human LBP IgG were used as secondary antibodies, followed by visualization using peroxidase-conjugated streptavidin (Dakopatts, Glostrup, Denmark). The levels of detection of both assays were 200 pg/ml.
Isolation of monocytes.
Peripheral blood mononuclear cells (PBMCs) were obtained from 10 healthy male donors. Venous blood mixed with 10 IU of sodium heparin was separated in a 50-ml polystyrene tube with a porous filter disk (LuecoSep; Greiner, Frickenhausen, Germany) using Ficoll-Paque solution (Seromed, Berlin, Germany) at 450 × g for 20 min. PBMCs were washed three times with PBS and then finally suspended at 2 × 106 PBMCs per ml of RPMI 1640 (GIBCO, Eggenstein, Germany) containing 10% autologous plasma. Monocytes were separated from lymphocytes by adherence (12 h at 37°C) to 12-well polystyrene plates (Greiner). After removal of nonadherent cells, monocytes were extensively washed with PBS containing 5% autologous plasma to remove residual nonadherent cells.
Assessment of biological activity of LPS in surgical plasma samples.
The bioactivity of LPS in pre- and postsurgical plasma samples was tested by incubating the plasma, diluted to 50% with RPMI 1640, with monocytes of healthy volunteers in the presence or absence of polymyxin B (Pfizer, Karlsruhe, Germany). Plasma samples were incubated for 20 min with or without 5 μg of polymyxin B per ml at room temperature, and subsequently 4 × 105 monocytes were added. The cells were cultured at 37°C in a 5% CO2 atmosphere for 5 h. After incubation, the supernatant was collected and frozen at −70°C. In control experiments, we assessed that polymyxin B abolishes the bioactivity of 50 pg of Escherichia coli O55:B5 (BioWhittaker, Walkersville, Md.) per ml in monocytic cultures. In this experiment, a mixture containing 50% autologus plasma was used.
Statistical analysis.
Data were expressed as means ± standard errors (SEs). Statistical evaluations of continuous data were performed by one-way analyses of variance and unpaired t tests for intergroup differences. Differences were considered significant at P < 0.05.
RESULTS
Endotoxin plasma levels and plasma endotoxin recovery.
We previously demonstrated significant endotoxemia only at the time point of 6 h after surgical stress in patients with multiple trauma (25). Therefore, the time point of 6 h after skin incision was selected for this study design. As shown in Fig. 1A, preoperative endotoxin plasma levels were 0.05 ± 0.01 EU/ml (normal; <0.07 EU/ml). Six hours after the skin incision, a significant increase to 0.23 ± 0.03 EU/ml was observed (P < 0.001). The plasma endotoxin levels did not increase in 4 of 40 patients (10%). The recovery of exogenously added endotoxin (10 EU/ml) to pre- and postoperative plasma samples is given in Fig. 1B. Endotoxin recovery increased from a mean of 0.06 ± 0.01 to 0.31 ± 0.03 EU/ml (P < 0.001) in each of the 40 patients. Endogenous endotoxin levels of all samples were subtracted from the recovery data.
FIG. 1.
(A) Endotoxin plasma levels after major abdominal surgery. Endotoxin plasma levels are demonstrated on the y axis. The time points (preoperative [preop.] and postoperative [postop.]) are given on the x axis. (B) ENC after major abdominal surgery. Levels of endotoxin recovery are depicted on the y axis. The time points (preop. and postop.) are shown on the x axis.
Kinetics of sCD14, LBP, and BPI levels during elective abdominal surgery.
The plasma concentration of sCD14 (Fig. 2A) decreased perioperatively from 5.6 ± 0.3 μg/ml to 4.6 ± 0.3 μg/ml (P < 0.05). A decrease of plasma LBP levels (5.7 ± 0.8 to 4.0 ± 0.5 μg/ml; P < 0.05) was observed (Fig. 2B). Plasma BPI levels did not change during the observation period (Fig. 2C). The plasma levels of albumin, apo A, and apo B were all significantly decreased (Table 2).
FIG. 2.
(A) Plasma sCD14 levels after major abdominal surgery. The plasma sCD14 concentrations are given on the y axis. (B) Plasma LBP levels after major abdominal surgery. The plasma LBP concentrations are depicted on the y axis. (C) Plasma BPI levels after major abdominal surgery. The plasma BPI concentrations are shown on the y axis.
TABLE 2.
Plasma levels of albumin, apo A, and apo B after surgery
| Protein | Avg value for 40 samples
|
|
|---|---|---|
| Preoperative | Postoperativeb | |
| Albumin (g/liter) | 39.2 ± 1.4 | 25.3 ± 1.6 |
| Apo A (g/liter) | 1.4 ± 0.0 | 0.9 ± 0.1 |
| Apo B (g/liter) | 1.0 ± 0.1 | 0.6 ± 0.1 |
Data are presented as means ± SEs.
Each of these values showed significant difference from preoperative values (P < 0.001 by Student’s t test).
Relationship between ENC and endotoxin-binding proteins in plasma.
A significant correlation was found between the sCD14 plasma level and the postoperative recovery of endotoxin (Fig. 3B) (r = −0.66; P < 0.0001).
FIG. 3.
Correlation between ENC and sCD14. (A) Preoperative time point. (B) Postoperative time point. Plasma sCD14 levels are given on the x axis. Levels of endotoxin recovery are shown on the y axis.
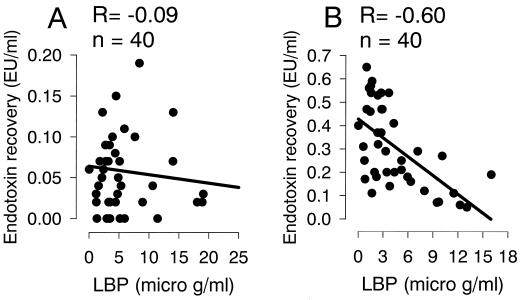
Plasma LBP values showed a negative correlation with endotoxin recovery after surgical stress (Fig. 4B) (r = −0.60; P < 0.0001). Plasma BPI levels did not correlate with endotoxin (data not shown). There was no correlation between albumin, apo A, and apo B plasma levels and the recovery of sCD14, LBP, BPI, or endotoxin.
FIG. 4.
Correlation between ENC and LBP. (A) Preoperative time point. (B) Postoperative time point. Plasma LBP levels are demonstrated on the x axis. Levels of endotoxin recovery are depicted on the y axis.
Figure 5 represents endotoxin recovery in the presence or absence of MEM18. The addition of MEM18 significantly increased endotoxin recovery (P < 0.01; change in endotoxin recovery, 0.05 ± 0.01 EU/ml) in the preoperative plasma samples. A large, but not significant, increase (P = 0.06) in endotoxin recovery compared to the MEM18 negative assay was detected for postoperative samples (change in endotoxin recovery, 0.12 ± 0.04 EU/ml).
FIG. 5.
Effect of anti-CD14 monoclonal antibody (MEM18) on ENC. Ten micrograms of MEM18 per ml was preincubated with plasma before the determination of ENC. Levels of endotoxin recovery are depicted on the y axis. The time points (preop. and postop.) are given on the x axis.
Correlation of plasma sCD14 with the biological activity of postoperative plasma.
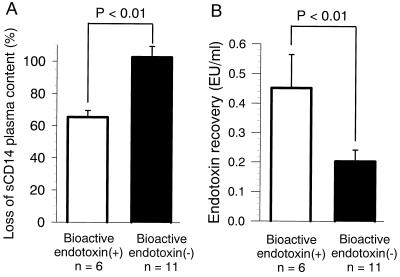
In LAL tests, 17 of 40 (43%) postsurgical plasma samples showed endotoxin levels which were all above 0.20 EU/ml. These 17 samples were used to stimulate healthy monocytes in order to evaluate bioactivity as determined by TNF-α release (Fig. 6). Preoperative plasma did not significantly stimulate the monocyte culture (data not shown). The activity of 6 of the 17 postoperative plasma samples could be blocked by the addition of 5 μg of polymyxin B per ml (P < 0.01) (Fig. 6A), indicating that biologically active endotoxin was responsible for this plasma activity. The observation that the activity of the other 11 plasma samples could not be blocked by polymyxin B (Fig. 6B) indicates that the endotoxin present in these samples is not biologically active but that other plasma components may be responsible for the observed monocyte activation. The sCD14 content of these 6 of the 17 samples decreased more than 65%, whereas the sCD14 levels in the remaining 11 samples did not change (Fig. 7A). The endotoxin recovery was also significantly higher in the six samples containing bioactive endotoxin than in the 11 other samples (P < 0.01) (Fig. 7B).
FIG. 6.
Effect of polymyxin B on biological activity of plasma. Monocytes from healthy volunteers were incubated in a mixture with 50% postoperative plasma with an endotoxin concentration above 0.20 EU/ml in the presence and absence of polymyxin B (5 μg/ml). (A) Six out of 17 plasma samples showed a significant suppression of TNF-α release by polymyxin B. (B) Eleven out of 17 plasma samples showed almost no change of TNF-α release by polymyxin B. The TNF-α level of the supernatant is given on the y axis. Incubation time in hours is depicted on the x axis.
FIG. 7.
Loss of sCD14 and ENC in plasma from six patients with biologically active endotoxin and 11 patients without biologically active endotoxin. (A) The postoperative sCD14 plasma content is given on the y axis as a percentage (postoperative sCD14/preoperative sCD14 × 100). (B) ENC is shown on the y axis as the recovery of LPS exogenously added to the plasma samples.
DISCUSSION
Although the presence of endotoxin in the systemic circulation has been extensively studied in a variety of clinical settings, there is no agreement on the occurrence of endotoxemia due to surgical stress. Many studies have reported significant endotoxemia in humans after trauma (25), major abdominal surgery (5), and cardiac surgery (32). However, other well-designed studies have failed to detect endotoxemia after trauma (27, 34) or hemorrhagic shock (11). These conflicting results were all obtained after making complex measurements of endotoxin content by the LAL test, and the debates over the development of gut-derived endotoxemia have generally been based on the LAL test. We, however, believe that it may be more relevant to focus on the biological activity of circulating endotoxin, which is related to clinical outcome. Recent major studies have corroborated the pathophysiological importance of gut-derived endotoxemia in experiments using antiendotoxin agents, such as polymyxin B (51), a cationic antibiotic that stoichiometrically neutralizes the lipid A moiety of endotoxin, or BPI (50), an endogenous endotoxin-neutralizing protein that reduces endotoxin translocation during experimental hemorrhage in rats and improves clinical outcome. In humans, the selective decontamination of the digestive tract has been demonstrated to reduce perioperative endotoxemia and release of interleukin 6 in cardiac patients (32). In the present study, we assessed the biological activity of the translocated endotoxin after major elective surgery.
Our results demonstrated the presence of significant endotoxemia during major abdominal surgery, which is related to a loss of plasma ENC, as described previously (4). Preoperative patient plasma neutralized almost all exogenously added endotoxin, whereas postoperative plasma did not have enough capacity to neutralize the same quantity of endotoxin, resulting in postoperative loss of ENC. This study demonstrated reduced sCD14 plasma levels in the very early postoperative stage, a finding similar to that of Kruger et al., who observed decreased sCD14 concentrations immediately after multiple trauma (28). Another new finding was a strong correlation between sCD14 levels and ENC as determined by the LAL test. High sCD14 levels were associated with high ENC. The addition of MEM18, a neutralizing anti-CD14 antibody (2), significantly diminished the ENC of preoperative plasma samples. The effect on postoperative plasma was less pronounced and not significant. One explanation for this observation may be the presence of endogenous endotoxin in postoperative plasma, as detected by the conventional LAL test after heat inactivation. Pretreatment by heating is known to destroy sCD14-endotoxin complexes, but ENC levels were determined without any inactivation step. Thus, endogenous endotoxin may block sCD14, resulting not only in loss of ENC but in a less pronounced effect of anti-CD14 antibodies. These antibodies can only bind to s/mCD14 before the addition of LPS (18). Preformed CD14-LPS complexes are no longer accessible to the neutralizing action of MEM18, and thus the ENC of postoperative plasma cannot be influenced by MEM18.
Plasma LBP levels were positively correlated with the ENC and sCD14 values. LBP is thought to facilitate the formation of LPS-s/mCD14 complexes, and it enhances LPS-induced cell activation (21, 43, 52). LBP may also catalyze sCD14-high-density lipoprotein-dependent LPS neutralization (21, 49). Therefore, the positive correlation between LBP and ENC reflects the catalytic effect of LBP on sCD14-LPS binding and is compatible with these LPS-neutralizing mechanisms. However, a more detailed study is required to reveal the role of LBP itself in LPS neutralization.
Plasma BPI was not found to be correlated with ENC or LBP. BPI is a potent LPS-neutralizing factor produced by polymorphonuclear leukocytes (39) and has an antagonistic effect on LBP-related LPS-cell interaction (10, 24). However, BPI did not seem to contribute to ENC, at least during the perioperative period, and BPI release during that period seems to be modest. Therefore, significant secretion of BPI later in the postoperative period may play a role as an LPS-neutralizing mechanism.
The bioactivity of endotoxin in postsurgical plasma was determined by measuring TNF-α release from healthy monocytes after incubation with plasma samples in the presence and absence of polymyxin B. In a preliminary study, there was no procedural activation of monocytes following steps taken to purify them from whole blood. The presence of even very low concentrations of endotoxin (0.05 ng/ml) caused a significant release of TNF-α by monocytes that could be completely abolished by the addition of polymyxin B. For the first time, the biological activity of circulating endotoxin was demonstrated in our study. Almost all of the 17 postoperative plasma samples with endotoxin contents above 0.20 EU/ml stimulated TNF-α secretion by healthy monocytes, whereas no stimulation was observed in the stimulation assay with preoperative plasma. The stimulatory activity of only 6 of the 17 postoperative plasma samples could be inhibited by the addition of polymyxin B, indicating that biologically active endotoxin was present in these six samples. In addition, these six samples had the lowest ENC and the most pronounced decrease in sCD14 compared with preoperative values. It should be noted that the absolute level of sCD14 was not found to be associated with endotoxic bioactivity, despite the relative loss of sCD14 during the perioperative course. These six patients, however, were not distinguished by blood loss, duration of operation, or volume of infusion. Based on these preliminary results obtained in 6 of the 17 patients, we hypothesized that endotoxin present in the blood after major surgical procedures may be bound to and consequently hidden by sCD14. In our series of studies, we found that LPS bioactivity in patients’ plasma affected the clinical outcome assessed by Acute Physiology and Chronic Health Evaluation (APACHE) II scores.
In conclusion, our findings suggest that sCD14 is an important modulator of plasma ENC in the postoperative course, regulating the endotoxin-dependent biological activity of plasma.
REFERENCES
- 1.Arditi M, Zhou J, Huang S H, Luckett P M, Marra M N, Kim K S. Bactericidal/permeability-increasing protein protects vascular endothelial cells from lipopolysaccharide-induced activation and injury. Infect Immun. 1994;62:3930–3936. doi: 10.1128/iai.62.9.3930-3936.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bazil V, Horejsi V, Baudys M, Kristofova H, Strominger J L, Kostka W, Hilgert I. Biochemical characterization of a soluble form of the 53-kDa monocyte surface antigen. Eur J Immunol. 1986;16:1583–1589. doi: 10.1002/eji.1830161218. [DOI] [PubMed] [Google Scholar]
- 3.Berger D, Bolke E, Huegel H, Seidelmann M, Hannekum A, Beger H G. New aspects concerning the regulation of the post-operative acute phase reaction during cardiac surgery. Clin Chim Acta. 1995;239:121–130. doi: 10.1016/0009-8981(95)06105-m. [DOI] [PubMed] [Google Scholar]
- 4.Berger D, Ott S, Schmidt U M, Bolke E, Seidelmann M, Beger H G. Determination of endotoxin-neutralizing capacity of plasma in postsurgical patients. Eur Surg Res. 1996;28:130–139. doi: 10.1159/000129450. [DOI] [PubMed] [Google Scholar]
- 5.Berger D, Schmidt U M, Ott S, Seidelmann M, Martin R, Beger H G. Incidence and pathophysiological relevance of postoperative endotoxemia. FEMS Immunol Med Microbiol. 1995;11:285–290. doi: 10.1111/j.1574-695X.1995.tb00158.x. [DOI] [PubMed] [Google Scholar]
- 6.Brinkmann A, Wolf C F, Berger D, Kneitinger E, Neumeister B, Buchler M, Radermacher P, Seeling W, Georgieff M. Perioperative endotoxemia and bacterial translocation during major abdominal surgery: evidence for the protective effect of endogenous prostacyclin? Crit Care Med. 1996;24:1293–1301. doi: 10.1097/00003246-199608000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Burgmann H, Winkler S, Locker G J, Presterl E, Laczika K, Staudinger T, Knapp S, Thalhammer F, Wenisch C, Zedwitz, Liebenstein K, Frass M, Graninger W. Increased serum concentration of soluble CD14 is a prognostic marker in gram-positive sepsis. Clin Immunol Immunopathol. 1996;80:307–310. doi: 10.1006/clin.1996.0128. [DOI] [PubMed] [Google Scholar]
- 8.Calvano S E, Thompson W A, Marra M N, Coyle S M, de Riesthal H F, Trousdale R K, Barie P S, Scott R W, Moldawer L L, Lowry S F. Changes in polymorphonuclear leukocyte surface and plasma bactericidal/permeability-increasing protein and plasma lipopolysaccharide binding protein during endotoxemia or sepsis. Arch Surg. 1994;129:220–226. doi: 10.1001/archsurg.1994.01420260116016. [DOI] [PubMed] [Google Scholar]
- 9.Dentener M A, Francot G J, Smit F T, Froon A H, Pennings H J, Wouters E F, Buurman W A. Presence of bactericidal/permeability-increasing protein in disease: detection by ELISA. J Infect Dis. 1995;171:739–743. doi: 10.1093/infdis/171.3.739. [DOI] [PubMed] [Google Scholar]
- 10.Dentener M A, Von Asmuth E J, Francot G J, Marra M N, Buurman W A. Antagonistic effects of lipopolysaccharide binding protein and bactericidal/permeability-increasing protein on lipopolysaccharide-induced cytokine release by mononuclear phagocytes. Competition for binding to lipopolysaccharide. J Immunol. 1993;151:4258–4265. [PubMed] [Google Scholar]
- 11.Endo S, Inada K, Yamada Y, Takakuwa T, Kasai T, Nakae H, Yoshida M, Ceska M. Plasma endotoxin and cytokine concentrations in patients with hemorrhagic shock. Crit Care Med. 1994;22:949–955. doi: 10.1097/00003246-199406000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Engstrom L, Torngren S, Rohdin Alm C. Peroperative endotoxin concentrations in portal and peripheral venous blood in patients undergoing right hemicolectomy for carcinoma. Eur J Surg. 1992;158:301–305. [PubMed] [Google Scholar]
- 13.Fisher C J, Jr, Marra M N, Palardy J E, Marchbanks C R, Scott R W, Opal S M. Human neutrophil bactericidal/permeability-increasing protein reduces mortality rate from endotoxin challenge: a placebo-controlled study. Crit Care Med. 1994;22:553–558. doi: 10.1097/00003246-199404000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Flegel W A, Wölpl A, Männel D N, Northoff H. Inhibition of endotoxin-induced activation of human monocytes by human lipoproteins. Infect Immun. 1989;57:2237–2245. doi: 10.1128/iai.57.7.2237-2245.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox E S, Thomas P, Broitman S A. Clearance of gut-derived endotoxins by the liver. Release and modification of 3H, 14C-lipopolysaccharide by isolated rat Kupffer cells. Gastroenterology. 1989;96:456–461. doi: 10.1016/0016-5085(89)91571-0. [DOI] [PubMed] [Google Scholar]
- 16.Freudenberg M A, Kleine B, Galanos C. The fate of lipopolysaccharide in rats: evidence for chemical alteration in the molecule. Rev Infect Dis. 1984;6:483–487. doi: 10.1093/clinids/6.4.483. [DOI] [PubMed] [Google Scholar]
- 17.Froon A H, Dentener M A, Greve J W, Ramsay G, Buurman W A. Lipopolysaccharide toxicity-regulating proteins in bacteremia. J Infect Dis. 1995;171:1250–1257. doi: 10.1093/infdis/171.5.1250. [DOI] [PubMed] [Google Scholar]
- 18.Gallay P, Jongeneel C V, Barras C, Burnier M, Baumgartner J D, Glauser M P, Heumann D. Short time exposure to lipopolysaccharide is sufficient to activate human monocytes. J Immunol. 1993;150:5086–5093. [PubMed] [Google Scholar]
- 19.Geller D A, Kispert P H, Su G L, Wang S C, Di Silvio M, Tweardy D J, Billiar T R, Simmons R L. Induction of hepatocyte lipopolysaccharide binding protein in models of sepsis and the acute-phase response. Arch Surg. 1993;128:22–27. doi: 10.1001/archsurg.1993.01420130026005. [DOI] [PubMed] [Google Scholar]
- 20.Hailman E, Lichenstein H S, Wurfel M M, Miller D S, Johnson D A, Kelley M, Busse L A, Zukowski M M, Wright S D. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J Exp Med. 1994;179:269–277. doi: 10.1084/jem.179.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hailman E, Vasselon T, Kelley M, Busse L A, Hu M C, Lichenstein H S, Detmers P A, Wright S D. Stimulation of macrophages and neutrophils by complexes of lipopolysaccharide and soluble CD14. J Immunol. 1996;156:4384–4390. [PubMed] [Google Scholar]
- 22.Haziot A, Rong G W, Bazil V, Silver J, Goyert S M. Recombinant soluble CD14 inhibits LPS-induced tumor necrosis factor-alpha production by cells in whole blood. J Immunol. 1994;152:5868–5876. [PubMed] [Google Scholar]
- 23.Haziot A, Rong G W, Lin X Y, Silver J, Goyert S M. Recombinant soluble CD14 prevents mortality in mice treated with endotoxin (lipopolysaccharide) J Immunol. 1995;154:6529–6532. [PubMed] [Google Scholar]
- 24.Heumann D, Gallay P, Betz-Corradin S, Barras C, Baumgartner J D, Glauser M P. Competition between bactericidal/permeability-increasing protein and lipopolysaccharide-binding protein for lipopolysaccharide binding to monocytes. J Infect Dis. 1993;167:1351–1357. doi: 10.1093/infdis/167.6.1351. [DOI] [PubMed] [Google Scholar]
- 25.Hiki N, Berger D, Buttenschoen K, Boelke E, Seidelmann M, Strecker W, Kinzl L, Beger H G. Endotoxemia and specific antibody behavior against different endotoxins following multiple injuries. J Trauma. 1995;38:794–801. doi: 10.1097/00005373-199505000-00020. [DOI] [PubMed] [Google Scholar]
- 26.Hiki N, Berger D, Prigl C, Boelke E, Wiedeck H, Seidelmann M, Staib L, Oohara T, Kaminishi M, Beger H G. Endotoxin binding and elimination by monocytes: the secretion of soluble CD14 represents an inducible mechanism counteracting reduced expression of membrane CD14 during sepsis and in a patient with paroxysmal nocturnal hemoglobinuria. Infect Immun. 1998;66:1135–1141. doi: 10.1128/iai.66.3.1135-1141.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoch R C, Rodriguez R, Manning T, Bishop M, Mead P, Shoemaker W C, Abraham E. Effects of accidental trauma on cytokine and endotoxin production. Crit Care Med. 1993;21:839–845. doi: 10.1097/00003246-199306000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Kruger C, Schutt C, Obertacke U, Joka T, Muller F E, Knoller J, Koller M, Konig W, Schonfeld W. Serum CD14 levels in polytraumatized and severely burned patients. Clin Exp Immunol. 1991;85:297–301. doi: 10.1111/j.1365-2249.1991.tb05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landmann R, Reber A M, Sansano S, Zimmerli W. Function of soluble CD14 in serum from patients with septic shock. J Infect Dis. 1996;173:661–668. doi: 10.1093/infdis/173.3.661. [DOI] [PubMed] [Google Scholar]
- 30.Landmann R, Zimmerli W, Sansano S, Link S, Hahn A, Glauser M P, Calandra T. Increased circulating soluble CD14 is associated with high mortality in gram-negative septic shock. J Infect Dis. 1995;171:639–644. doi: 10.1093/infdis/171.3.639. [DOI] [PubMed] [Google Scholar]
- 31.Levine D M, Parker T S, Donnelly T M, Walsh A, Rubin A L. In vivo protection against endotoxin by plasma high density lipoprotein. Proc Natl Acad Sci USA. 1993;90:12040–12044. doi: 10.1073/pnas.90.24.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez Pellus A E, Merino P, Bru M, Conejero R, Seller G, Munoz C, Fuentes T, Gonzalez G, Alvarez B. Can selective digestive decontamination avoid the endotoxemia and cytokine activation promoted by cardiopulmonary bypass? Crit Care Med. 1993;21:1684–1691. doi: 10.1097/00003246-199311000-00017. [DOI] [PubMed] [Google Scholar]
- 33.Mimura Y, Sakisaka S, Harada M, Sata M, Tanikawa K. Role of hepatocytes in direct clearance of lipopolysaccharide in rats. Gastroenterology. 1995;109:1969–1976. doi: 10.1016/0016-5085(95)90765-3. [DOI] [PubMed] [Google Scholar]
- 34.Moore F A, Moore E E, Poggetti R, McAnena O J, Peterson V M, Abernathy C M, Parsons P E. Gut bacterial translocation via the portal vein: a clinical perspective with major torso trauma. J Trauma. 1991;31:629–636. doi: 10.1097/00005373-199105000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Munford R S, Hall C L. Detoxification of bacterial lipopolysaccharides (endotoxins) by a human neutrophil enzyme. Science. 1986;234:203–205. doi: 10.1126/science.3529396. [DOI] [PubMed] [Google Scholar]
- 36.Munford R S, Hall C L. Purification of acyloxyacyl hydrolase, a leukocyte enzyme that removes secondary acyl chains from bacterial lipopolysaccharides. J Biol Chem. 1989;264:15613–15619. [PubMed] [Google Scholar]
- 37.Naito Y, Tamai S, Shingu K, Shindo K, Matsui T, Segawa H, Nakai Y, Mori K. Responses of plasma adrenocorticotropic hormone, cortisol, and cytokines during and after upper abdominal surgery [see comments] Anesthesiology. 1992;77:426–431. doi: 10.1097/00000542-199209000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Newman S L, Chaturvedi S, Klein B S. The WI-1 antigen of Blastomyces dermatitidis yeasts mediates binding to human macrophage CD11b/CD18 (CR3) and CD14. J Immunol. 1995;154:753–761. [PubMed] [Google Scholar]
- 39.Ooi C E, Weiss J, Doerfler M E, Elsbach P. Endotoxin-neutralizing properties of the 25 kD N-terminal fragment and a newly isolated 30 kD C-terminal fragment of the 55-60 kD bactericidal/permeability-increasing protein of human neutrophils. J Exp Med. 1991;174:649–655. doi: 10.1084/jem.174.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peterson A A, Munford R S. Dephosphorylation of the lipid A moiety of Escherichia coli lipopolysaccharide by mouse macrophages. Infect Immun. 1987;55:974–978. doi: 10.1128/iai.55.4.974-978.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rall D P, Gaskins J R, Kelly M G, Rudbach J A, Johnson A G. Reduction of febrile response to bacterial polysaccharide following incubation with serum. Nature. 1964;23:811–812. doi: 10.1152/ajplegacy.1957.188.3.559. [DOI] [PubMed] [Google Scholar]
- 42.Rudbach J A, Johnson A G. Restoration of endotoxin activity following alteration by plasma. Nature. 1964;23:811–812. doi: 10.1038/202811a0. [DOI] [PubMed] [Google Scholar]
- 43.Tobias P S, Soldau K, Gegner J A, Mintz D, Ulevitch R J. Lipopolysaccharide binding protein-mediated complexation of lipopolysaccharide with soluble CD14. J Biol Chem. 1995;270:10482–10488. doi: 10.1074/jbc.270.18.10482. [DOI] [PubMed] [Google Scholar]
- 44.von der Mohlen M A, Kimmings A N, Wedel N I, Mevissen M L, Jansen J, Friedmann N, Lorenz T J, Nelson B J, White M L, Bauer R, et al. Inhibition of endotoxin-induced cytokine release and neutrophil activation in humans by use of recombinant bactericidal/permeability-increasing protein. J Infect Dis. 1995;172:144–151. doi: 10.1093/infdis/172.1.144. [DOI] [PubMed] [Google Scholar]
- 45.Warren H S, Novitsky T J, Ketchum P A, Roslansky P F, Kania S, Siber G R. Neutralization of bacterial lipopolysaccharides by human plasma. J Clin Microbiol. 1985;22:590–595. doi: 10.1128/jcm.22.4.590-595.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wortel C H, van Deventer S J, Aarden L A, Lygidakis N J, Buller H R, Hoek F J, Horikx J, ten Cate J W. Interleukin-6 mediates host defense responses induced by abdominal surgery. Surgery. 1993;114:564–570. [PubMed] [Google Scholar]
- 47.Wurfel M M, Hailman E, Wright S D. Soluble CD14 acts as a shuttle in the neutralization of lipopolysaccharide (LPS) by LPS-binding protein and reconstituted high density lipoprotein. J Exp Med. 1995;181:1743–1754. doi: 10.1084/jem.181.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wurfel M M, Kunitake S T, Lichenstein H, Kane J P, Wright S D. Lipopolysaccharide (LPS)-binding protein is carried on lipoproteins and acts as a cofactor in the neutralization of LPS. J Exp Med. 1994;180:1025–1035. doi: 10.1084/jem.180.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wurfel M M, Wright S D. Lipopolysaccharide (LPS) binding protein catalyzes binding of LPS to lipoproteins. Prog Clin Biol Res. 1995;392:287–295. [PubMed] [Google Scholar]
- 50.Yao Y M, Bahrami S, Leichtfried G, Redl H, Schlag G. Pathogenesis of hemorrhage-induced bacteria/endotoxin translocation in rats. Effects of recombinant bactericidal/permeability-increasing protein. Ann Surg. 1995;221:398–405. doi: 10.1097/00000658-199504000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yao Y M, Tian H M, Sheng Z Y, Wang Y P, Yu Y, Sun S R, Xu S H. Inhibitory effects of low-dose polymyxin B on hemorrhage-induced endotoxin/bacterial translocation and cytokine formation in rats. J Trauma. 1995;38:924–930. doi: 10.1097/00005373-199506000-00018. [DOI] [PubMed] [Google Scholar]
- 52.Yu B, Wright S D. Catalytic properties of lipopolysaccharide (LPS) binding protein. Transfer of LPS to soluble CD14. J Biol Chem. 1996;271:4100–4105. doi: 10.1074/jbc.271.8.4100. [DOI] [PubMed] [Google Scholar]