Abstract
Background
Estimates of immunity and severity for the SARS-CoV-2 omicron subvariant BA.5 are important to assess the public health impact associated with its rapid global spread despite vaccination. We estimated natural and vaccine immunity and severity of BA.5 relative to BA.2 in Denmark, a country with high mRNA-vaccination coverage and free-of-charge RT-PCR testing.
Methods
This nation-wide population-based study in Denmark included residents aged 18 years or older who had taken an RT-PCR test between 10 April and 30 June, 2022 (ie, the outcome period), and who the national COVID-19 surveillance system identified as having information since February 2020 on RT-PCR tests, whole-genome sequencing, vaccinations, and hospitalisation with a positive RT-PCR test and COVID-19 as the main diagnosis. First, we used a case–control design, in which cases were people infected with BA.5 or BA.2 during the outcome period and controls were people who tested negative for SARS-CoV-2 infection during the outcome period. We calculated the protection provided by a previous PCR-confirmed omicron infection against BA.5 and BA.2 infection and hospitalisation among triple-vaccinated individuals. Second, we compared vaccination status in people infected with BA.5 versus BA.2 and estimated relative vaccine protection against each subvariant. Third, we compared rates of hospitalisation for COVID-19 among people infected with BA.5 versus BA.2. We estimated effects using logistic regression with adjustment for sex, age, region, PCR-test date, comorbidity and, as appropriate, vaccination and previous infection status.
Findings
A total of 210 (2·4%) of 8678 of BA.5 cases, 192 (0·7%) of 29 292 of BA.2 cases, and 33 972 (19·0%) of 178 669 PCR-negative controls previously had an omicron infection, which was estimated in the adjusted analyses to offer 92·7% (95% CI 91·6–93·7) protection against BA.5 infection and 97·1% (96·6–97·5) protection against BA.2 infection. We found similarly high amounts of protection against hospitalisation owing to infection with BA.5 (96·4% [95% CI 74·2–99·5]) and BA.2 (91·2% [76·3–96·7]). Vaccine coverage (three mRNA doses vs none) was 9307 (94·2%) of 9878 among BA.5 cases and 30 581 (94·8%) of 32 272 among BA.2 cases, although in the adjusted analysis, there was a trend towards slightly higher vaccination coverage among BA.5 cases than BA.2 cases (OR 1·18 [95% CI 0·99–1·42]; p=0·064), possibly suggesting marginally poorer vaccine protection against BA.5. The rate of hospitalisation due to COVID-19 was higher among the BA.5 cases (210 [1·9%] of 11 314) than among the BA.2 cases (514 [1·4%] of 36 805), with an OR of 1·34 (95% CI 1·14–1·57) and an adjusted OR of 1·69 (95% CI 1·22–2·33), despite low and stable COVID-19 hospitalisation numbers during the study period.
Interpretation
The study provides evidence that a previous omicron infection in triple-vaccinated individuals provides high amounts of protection against BA.5 and BA.2 infections. However, protection estimates greater than 90% might be too high if individuals with a previous infection were more likely than those without one to come forward for a test for reasons other than suspicion of COVID-19. Our analysis also showed that vaccine protection against BA.5 infection was similar to, or slightly weaker than, protection against BA.2 infection. Finally, there was evidence that BA.5 infections were associated with an increased risk of hospitalisation compared with BA.2 infections.
Funding
There was no funding source for this study.
Introduction
The SARS-CoV-2 omicron BA.5 subvariant has spread rapidly globally, including in Denmark despite high vaccination coverage and a large proportion of the population previously infected with omicron subvariants BA.1 and BA.2. The variant BA.5 was first observed in cocirculation with BA.4 in South Africa,1 where it caused a fifth wave of SARS-CoV-2 infections during April and May 2022; it also caused a large infection surge in May, 2022 Portugal.2
BA.5 has acquired characteristic mutations in the spike protein, including the L452R, F486V mutations, and a Q493 reversion, all in the receptor-binding domain. The L452R mutation was most notably present in the delta variant and has been shown to help SARS-CoV-2 variants to evade cellular and humoral immunity and increase infectivity.3 Although BA.5 is clearly highly transmissible, evidence is less clear about its virulence relative to other omicron subvariants. Experiences from South Africa suggest that BA.5 does not increase COVID-19 disease severity compared with BA.1 and BA.2, as measured by the number of hospital admissions and in-hospital deaths during the BA.4–BA.5 wave.4 A situational report5 from Portugal also found no evidence of increased risk of hospitalisation with BA.5 compared with earlier omicron subvariants (measured as the crude rate ratio of hospital admissions per case notification). At the same time, both South Africa and Portugal have experienced a rise in all-cause excess mortality during the period of BA.5 predominance.5, 6 Overall, omicron (B.1.529) replicates most efficiently in the upper parts of the respiratory tract7 and is associated with less severe disease compared with previous variants of concern.8 However, a study9 published before the emergence of BA.5 showed that the addition of L452R to omicron enhanced its ability to infect lung tissues of humanised ACE2 mice.
Research in context.
Evidence before this study
We searched medRxiv, bioRxiv, and PubMed on Aug 5, 2022, for articles published between Nov 1, 2021 and August 1, 2022, using the search term “(BA.5[Title/Abstract])”, and also searched for reports from national public health institutes in South Africa (National Institute for Communicable Diseases), Portugal (Instituto Nacional de Saúde Doutor Ricardo Jorge), and the UK Health Security Agency. Several studies have shown markedly increased immune escape of omicron BA.4 and BA.5 compared with BA.1 and BA.2 infections, indicating changes in the protection afforded by vaccine and previous-infection immunity. Two studies from Qatar indicated that previous omicron infection affords high protection against infection with BA.1 and BA.2 (85·6–94·9%) and BA.4 and BA.5 (74·3–83·9%). These estimates are higher than those reported in a meta-analysis of protection offered by infection with earlier variants. With regard to vaccine immunity, a preprint study from Portugal found a similar likelihood of being vaccinated among BA.5 and BA.2 cases, which is indicative of similar vaccine effectiveness against both variants, as was also indicated by analysis from UKSHA. With regards to BA.5 severity, in-vitro and in-vivo animal studies have highlighted the potential for increased disease severity of BA.4 and BA.5 compared with BA.1 and BA.2, but population-based results are scarce. However, a study from Portugal reported higher risk of hospitalisation for BA.5 than for BA.2 infection among booster-vaccinated individuals (adjusted OR 3·36 [95% CI 1·18–9·63]). Comparing different infection waves, a study from South Africa published as a preprint reported no difference in the risk of severe hospitalisation and death during the BA.4 and BA.5 wave compared with during the preceding BA.1 wave (HR 1·12 [95% CI 0·93–1·34]). In Denmark, according to data from the national health authorities, the proportion of people hospitalised for COVID-19 and treated for lower respiratory tract infection was lower during the BA.5 wave than during the previous omicron wave.
Added value of this study
To our knowledge, there is little available evidence on vaccine effectiveness against, and disease severity of, BA.5 compared with BA.2. Previous studies have largely investigated BA.4 and BA.5 together, some of which used the S-Gene Target Failure test result as a proxy for BA.4 or BA.5 infection. However, BA.5 has consistently displayed higher infection rates than BA.4 across geographical regions. This study combines Danish national COVID-19 surveillance and viral WGS data to estimate the protection afforded by previous infection and vaccination, and the disease severity of BA.5 versus BA.2.
Implications of all the available evidence
The available evidence shows that previous omicron infection offers significant protection against BA.5 in booster-vaccinated individuals. Evidence also points to similar or slightly weaker vaccine effectiveness against infection by BA.5 than by BA.2. The effect of the current BA.5 wave might be small in populations with a high degree of hybrid immunity (ie, via previous infection and vaccines). The increased risk of hospitalisation after BA.5 compared with BA.2 infection merits further investigation into the disease severity of BA.5, as studies from South Africa and Portugal do not suggest an increased risk of severe disease progression and death with BA.5 compared with BA.2. This study, and the others referenced, highlight how WGS continues to have a crucial role in the surveillance of the COVID-19 pandemic.
A study found that BA.4 and BA.5 replicate more efficiently than BA.2 in human lung cells, and are more pathogenic than BA.2 in hamsters.10 A risk assessment11 from Santé Publique in France evaluated syndromic data on 288 people infected with BA.4 or BA.5 and found that the median disease duration was longer in those infected with BA.4 or BA.5 than with BA.1 (median duration 7 days [IQR 3–10 days] vs 4 days [IQR 2–7 days]). The study11 also found that a significantly higher proportion of individuals infected with BA.4 or BA.5 had nasal secretion, nausea, diarrhoea, ageusia, and anosmia than those infected with BA.1. However, these results were unadjusted for higher age among those infected with BA.5 than with BA.1, or for differences in vaccination status between groups.
Given the surge in SARS-CoV-2 infections caused by BA.5, it is important to establish whether infection with this subvariant is more likely to lead to serious disease than with earlier subvariants, and the extent to which vaccination and previous infection protect against infection with BA.5. Using information from whole-genome sequencing (WGS) and national registers in Denmark, we described previously both vaccine effectiveness,12 protection of earlier variants against reinfection,13, 14 and severity of omicron (BA.1 and BA.2),15 delta,15 alpha,16 and other previous variants.17 The aims of the current study were to estimate: first, the protection of a previous infection conveyed against a new infection with BA.5 among triple vaccinated; second, the relative vaccine protection against infection with BA.5 relative to BA.2; and third, the severity of infection with BA.5 compared with infection with BA.2.
Methods
Study design and participants
This nation-wide population-based study in Denmark consisted of three main analyses that pertained to three research questions. The first analysis used a case–control design to assess the degree of protection against a new omicron infection with BA.5 or BA.2 (analysed separately) that is provided by a previous omicron infection in a fully vaccinated population. The second analysis used a case–control design to assess the degree of protection after three mRNA vaccine doses afforded against infection with BA.5 versus BA.2. The third analysis used a cross-sectional design to investigate the relative risk of hospitalisation after infection with BA.5 versus BA.2. None of the analyses included people infected with the omicron BA.2.12.1 strain.
The study population in all three analyses was restricted to people older than 18 years by April 10, 2022 and who had uninterrupted residency in Denmark since February, 2020 to ensure complete SARS-CoV-2 test and vaccination records. Further restrictions on the study populations are detailed subsequently; briefly, analysis 1 involved only (triple) vaccinated individuals, whereas analysis 2 and analysis 3 involved only SARS-CoV-2 infected individuals.
During the COVID-19 pandemic, Denmark had one of the highest PCR-testing capacities per capita globally, with up to a quarter of the population tested every week.18 Tests are registered centrally and free-of-charge for all citizens. The number of these tests dropped during the first half of 2022, from around 1·4 million per week at the start of the year to approximately 60 000 per week on average during the 3-month period from April to June. Close contacts of infected cases no longer required testing and the rate of screening tests in other population segments have also reduced since March 10, 2022.
COVID-19 vaccination coverage is high in Denmark. By April 10, 2022, more than 80% of all adults had completed their primary vaccination series and more than 60% had also received a booster dose.19 Further details of the Danish testing and vaccination strategy are provided in the appendix (p 3).
One of the cornerstones of the pandemic surveillance in Denmark has been the extensive use of WGS through the Danish COVID-19 Genome Consortium, with a capacity to sequence around 15 000 weekly test samples from TestCenter Denmark, in addition to samples from clinics and hospitals (ie, health-care testing provision) that were sequenced regionally at departments of clinical microbiology. Since the first BA.5 case in Denmark was identified on April 10, 2022, the proportion of isolates subjected to WGS has been more than 83% of all positive cases, of which 85% have produced genomic data on which variants were identified. For further details of the WGS methods, see the appendix (p 3).
Data were extracted from the Danish national COVID-19 surveillance system maintained at Statens Serum Institut (Copenhagen, Denmark), described in detail elsewhere.20 Briefly, individual-level information is linked daily between national registers, including the National Patient Register,21 which includes details of all inpatient and outpatient diagnoses and hospital admission and discharge dates. From this surveillance system, we obtained data on hospital admissions, COVID-19 diagnosis codes, and comorbidities based on the International Classification of Diseases 10th revision (ICD-10) diagnosis codes (diabetes, adiposity, haematological and other cancers, neurological diseases, kidney diseases, cardiovascular diseases, chronic pulmonary diseases, respiratory diseases, and immune deficiency conditions). We obtained person-level data on all COVID-19 vaccinations administered from the Danish National Vaccination Registry,22 and details of sex, age, vital status, and address history from the Danish Civil Registration System.23 Finally, we obtained data on all SARS-CoV-2 tests done by PCR in Denmark since the start of the pandemic from the National Microbiology Database.20
Assessment of outcomes
For all three analyses, the outcome period was April 10–June 30, 2022.
Analysis 1 (protection against reinfection) was a case-control study involving only those individuals who had received a complete primary vaccination series and subsequent booster dose (ie, three mRNA doses in total, with BNT162b2 or mRNA-1273 SARS-CoV-2 vaccine, or a combination of the two). Cases were individuals who had tested positive for SARS-CoV-2 during the outcome period with the BA.5 subvariant, as identified through WGS, whereas controls were people who had at least one PCR test during the outcome period without testing positive.24 We then compared the proportions of cases and controls who had a previous omicron infection (defined as a positive SARS-CoV-2 PCR test) during the exposure period. We excluded from the analysis individuals with a positive PCR test from outside the exposure period (ie, Jan 1–Feb 9, 2022, during which BA.1 and BA.2 accounted for virtually all SARS-CoV-2 infections in Denmark) or before the outcome period, those without a third mRNA vaccine dose by March 27, 2022 (ie, 14 days before the start of the outcome period, to allow for the full effect of vaccination), and those who had received a fourth dose by June 30, 2022.
Protection from a previous infection was estimated with a 95% CI by use of a logistic regression model and was expressed as 1 minus the model-derived odds ratio (OR), which was analogous to the method of estimating vaccine effectiveness. The model was adjusted for sex, age group (18–24, 25–34, 35–44, 45–54, 55–64, 65–74, 75–84, >85 years), geographical area of residency (a five-category variable indicating EU territorial units for statistics-2 region), comorbidity count (a four-category variable indicating the number of comorbidities as none, one, two, or three or more), and time of PCR sampling (a categorical variable indicating the week number). Among controls with multiple negative PCR tests during the outcome period, one was selected using simple random sampling for inclusion in the analysis. Among the few cases who had more than one positive test during the outcome period, only the first positive test was included in the analysis. In a sensitivity analysis to assess the robustness of the findings by use of an alternative analysis approach, the analysis was repeated using a matched case-control design in which cases and controls were pair-matched on test date, sex, and age (for further details about the methods used, see the appendix p 8).
In two extensions of the main analysis, supplementary analyses that instead estimated protection from a previous delta or alpha infection, the exposure definition was changed from infection during a period in which omicron predominated to infection between July 15 and Nov 15, 2021 for delta, and between March 15 and June 30, 2021 for alpha, which was when these variants predominated.
Finally, the analyses were repeated, with the cases being those who tested positive during the outcome period with the BA.2 (instead of the BA.5) subvariant, as identified through WGS, and again the controls were people who had at least one PCR test during the outcome period without testing positive.
Although it is not a requirement for valid inference that previously infected and uninfected individuals should have had the same propensity to come forward for testing (ie, that rates of getting tested were independent of exposure status), the OR will generally be biased if the effect of exposure status on rates of testing is modified by infection status during the period from April 10 to June 30, 2021. The analysis was therefore repeated with hospitalisation (defined under analysis 3) as the outcome, to avoid possible biases owing to differences in the rates of and reasons for testing.
Analysis 2 (vaccine protection) involved infected participants only (ie, those infected with either BA.5 or BA.2 during the outcome period. The analysis compared vaccination status across the two groups with differences interpreted as evidence of reduced vaccine protection against one subvariant versus the other. Given that infection had already occurred, the analysis estimated the association between vaccination status and subvariant: if the vaccines protected equally well against BA.2 and BA.5, the ratio of vaccinated-to-unvaccinated was expected to be identical between BA.2 and BA.5 cases. Only individuals with a complete primary vaccination series and a subsequent booster dose (ie, who received three mRNA vaccine doses in total) by March 27, 2022, or who were completely unvaccinated against COVID-19 by June 30, 2022, were included in the analysis. The analysis excluded people who had received a fourth dose by June 30, 2022. The effect of vaccination on the likelihood of an infection by BA.5 rather than BA.2 was analysed in a logistic regression model and expressed as an OR with a 95% CI. The model was adjusted for previous infection before April 10, 2022 (yes vs no), in addition to the other adjustment variables described previously for analysis 1.
Because only a few people (approximately 9% of those older than 18 years) remain unvaccinated in Denmark, we did a sensitivity analysis that did not rely on comparisons with this age group. In the sensitivity analysis, the reference exposure group was instead people who had completed their primary vaccination series (ie, two mRNA COVID-19 vaccine doses) more than 4·5 months before the start of the outcome period, but with no booster dose by June 30, 2022.
Analysis 3 (severity of BA.5 infection) also involved infected participants only, and compared the proportion hospitalised for COVID-19 among people infected with BA.5 versus BA.2 during the outcome period. The effect of the subvariant (ie, BA.5 vs BA.2) on the risk of hospitalisation was estimated in a logistic regression model with the adjustments described previously, and with additional adjustment for vaccination status (as a categorical variable that indicated the number of doses received at the time of infection) and previous infection (yes vs no). The analysis included all BA.2 or BA.5 cases in the outcome period, irrespective of COVID-19 vaccination history. Hospitalisations included in the analysis were restricted to those that lasted more than 12 h, had associated ICD-10 primary diagnosis codes B342 or B972 (indicating that COVID-19 was the primary reason for hospital admission), and occurred no earlier than 2 days before, and no later than 14 days after, a positive PCR test.
Most of the adult population in Denmark has received three mRNA COVID-19 vaccine doses. We therefore did a subgroup analysis in people who had received three such doses before March 27, 2022, excluding anyone who received a fourth dose before the end of the outcome period. In another supplementary analysis, the outcome period was extended by advancing the start date to Jan 1, 2022. Since the delta variant was still in circulation to a small extent in January, 2022, this analysis enabled simultaneous estimation of the effects of both BA.5 and the delta variant on hospitalisation, using the BA.2 hospitalisation rate as the reference. People with a previous SARS-CoV-2 infection before Jan 1, 2022, were excluded from this analysis to avoid the inclusion of frequently tested long-term or recurrent hospital patients with a delta infection and repeated positive tests during the winter months.
This study was done under the authority task of the Danish National Infectious Disease Control Institute, which allows the Statens Serum Institut to do analyses on data from existing national COVID-19 surveillance systems. According to Danish law, ethical approval or individual consent is not required for anonymised aggregated register-based studies.
Role of funding source
There was no funding source for this study.
Results
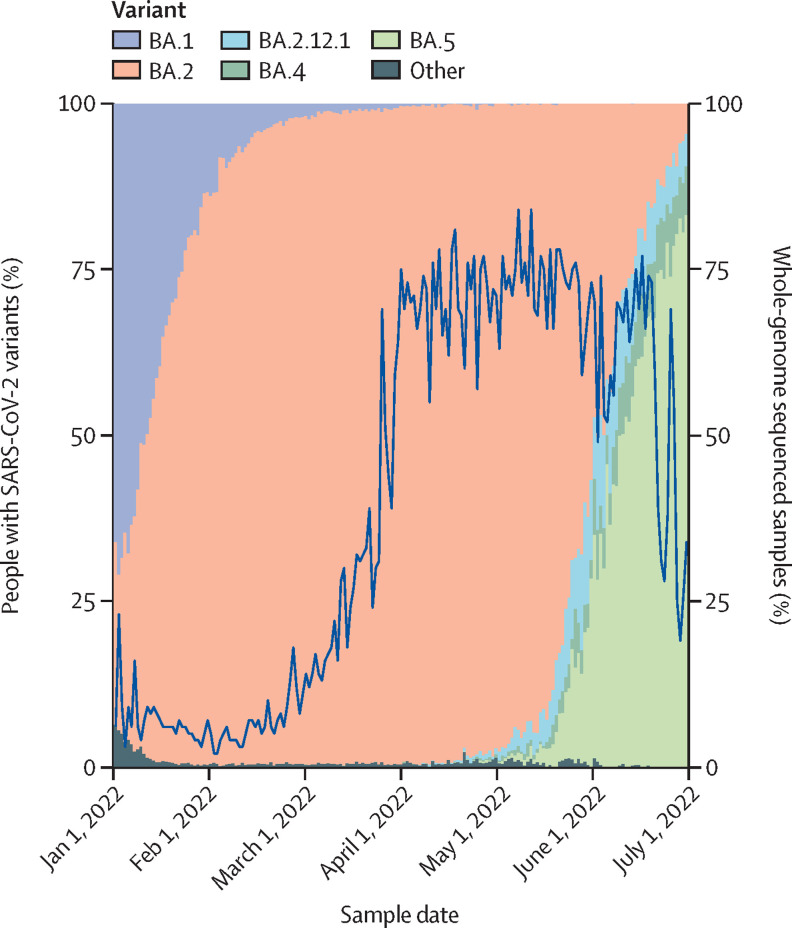
Since the start of 2022, the omicron variant has accounted for virtually all SARS-CoV-2 infections in Denmark (figure 1 ). Similar to many other countries, Denmark had a massive omicron wave between December, 2021 and February, 2022, with around 35% of the adult population testing positive via PCR during this 3-month period (data not shown). Omicron infections were due mainly to the BA.1 subvariant during December 2021 and early January 2022, after which point BA.2 became predominant until the rise of BA.5.
Figure 1.
Proportion of cases with SARS-CoV-2 variants and whole-genome sequencing between Jan 1 and July 1, 2022 in Denmark
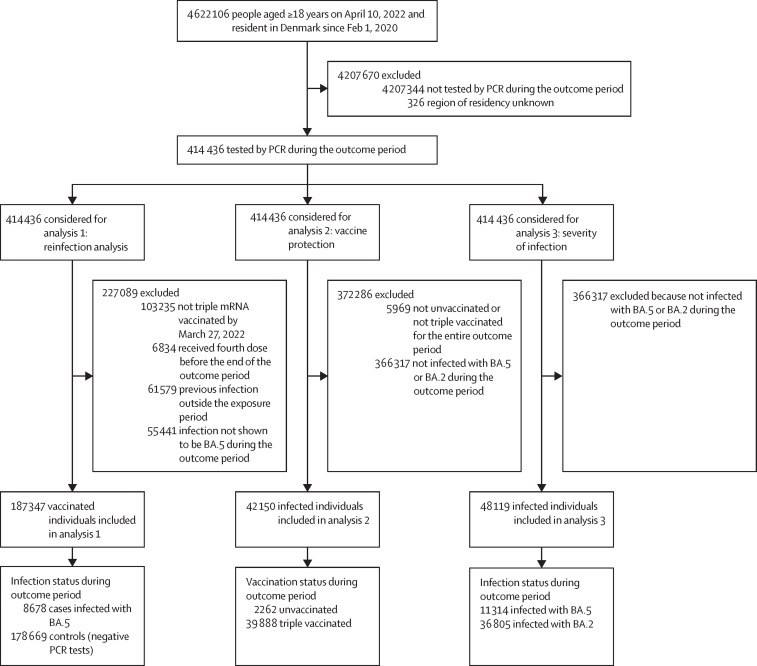
Of the 4 622 106 people aged over 18 years who were resident in Denmark since February 2020, a total of 414 436 (>8·9%) were tested by PCR during the outcome period. Those who were tested during the outcome period were older and more likely to have comorbidities and be without a previous PCR-confirmed SARS-CoV-2 infection compared with those who were not tested (table 1 ). Of the 414 436 people who were tested during the outcome period, 187 347 people were included in analysis 1, 42 150 people were included in analysis 2, and 48 119 people were included in analysis 3 (figure 2 ). In analysis 1, cases were slightly more likely than controls to be without a comorbidity. In analysis 2, unvaccinated people were younger, had less comorbidity, and were more likely to have had a previous PCR-confirmed SARS-CoV-2 infection than vaccinated people. Finally, in analysis 3, BA.5 cases were more likely than BA.2 cases to have had a previous PCR-confirmed SARS-CoV-2 infection (appendix pp 4–6).
Table 1.
Study population characteristics
|
All participants |
Participants tested by PCR during the outcome period |
||||
|---|---|---|---|---|---|
| n | % | n | % | ||
| All | 4 622 106 | 100% | 414 436 | 100% | |
| Sex | |||||
| Female | 2 342 608 | 50·7% | 226 222 | 54·6% | |
| Male | 2 279 498 | 49·3% | 188 214 | 45·4% | |
| Age, years | |||||
| 18–24 | 482 695 | 10·4% | 30 543 | 7·4% | |
| 25–34 | 746 790 | 16·2% | 59 735 | 14·4% | |
| 35–44 | 663 542 | 14·4% | 51 806 | 12·5% | |
| 45–54 | 768 454 | 16·6% | 68 988 | 16·7% | |
| 55–64 | 763 964 | 16·5% | 78 874 | 19·0% | |
| 65–74 | 624 590 | 13·5% | 61 873 | 14·9% | |
| 75–84 | 440 840 | 9·5% | 45 187 | 10·9% | |
| ≥85 | 131 231 | 2·8% | 17 430 | 4·2% | |
| Region of residency | |||||
| Capital | 1 451 806 | 31·4% | 150 372 | 36·3% | |
| Central Denmark | 1 052 396 | 22·8% | 80 397 | 19·4% | |
| Northern Denmark | 472 794 | 10·2% | 36 155 | 8·7% | |
| Zealand | 673 221 | 14·6% | 63 555 | 15·3% | |
| Southern Denmark | 971 563 | 21·0% | 83 957 | 20·3% | |
| Missing data | 326 | 0 | .. | .. | |
| Migration heritage* | |||||
| Denmark | 3 982 616 | 86·2% | 345 707 | 83·4% | |
| Other European country | 295 988 | 6·4% | 25 730 | 6·2% | |
| Middle East and north Africa | 166 071 | 3·6% | 21 304 | 5·1% | |
| Indian subcontinent and southeast Asia | 111 427 | 2·4% | 13 594 | 3·3% | |
| Sub-Saharan Africa | 37 342 | 0·8% | 4654 | 1·1% | |
| Other | 28 559 | 0·6% | 3447 | 0·8% | |
| Missing data | 103 | 0 | .. | .. | |
| Number of comorbidities† | |||||
| 0 | 3 659 772 | 79·2% | 297 644 | 71·8% | |
| 1 | 715 355 | 15·5% | 79 270 | 19·1% | |
| 2 | 186 128 | 4·0% | 26465 | 6·4% | |
| ≥3 | 60 802 | 1·3% | 11 057 | 2·7% | |
| Missing data | 47 | 0 | .. | .. | |
| COVID-19 vaccination status‡ | |||||
| Unvaccinated | 382 479 | 8·3% | 31 880 | 7·7% | |
| Primary vaccination with two mRNA doses completed | 38 558 | 0·8% | 4011 | 1·0% | |
| Primary non-mRNA vaccination completed | 5463 | 0·1% | 596 | 0·1% | |
| Primary mRNA vaccination completed plus one mRNA booster dose | 3 396 426 | 73·5% | 305 731 | 73·8% | |
| Primary non-mRNA vaccination completed plus one mRNA booster dose | 147 970 | 3·2% | 18 055 | 4·4% | |
| Primary vaccination completed plus two booster doses—any types | 36 465 | 0·8% | 6365 | 1·5% | |
| Other§ | 614 745 | 13·3% | 47 798 | 11·5% | |
| PCR-confirmed SARS-CoV-2 infection status by April 10, 2022 | |||||
| No previous infection | 2 457 297 | 53·2% | 256 449 | 61·9% | |
| At least one previous infection | 2 164 809 | 46·8% | 157 987 | 38·1% | |
| Infection likely to have been with omicron¶ | 1 685 557 | 77·9% | 111 047 | 70·3% | |
| Infection likely to have been with an earlier variant than omicron | 479 252 | 22·1% | 46 940 | 29·7% | |
Data are n or %. Participants were older than 18 years on April 10, 2022 and had been resident in Denmark since Feb 1, 2020.
Migration heritage was defined by country of birth or, if known, mother's country of birth.
Comorbidities registered during the past 5 years from among: diabetes, adiposity, haematological and other cancers, neurological diseases, kidney diseases, cardiovascular diseases, chronic pulmonary diseases, respiratory diseases, and immune deficiency conditions.
Vaccinations received by April 10, 2022; mRNA vaccines were either BNT162b2 (tozinameran, Pfizer-BioNtech) or mRNA-1273 (lasomeran, Moderna); non-mRNA vaccines were Jcovden (previously COVID-19 Vaccine Janssen) and ChAdOx1-S [recombinant] COVID-19 vaccine (AstraZeneca).
Incomplete primary vaccination series or non-mRNA vaccine booster doses.
Infections likely to have been with omicron were those in people who tested positive for COVID-19 after Dec 20, 2021.
Figure 2.
Study design
Of the 8678 triple-vaccinated cases who tested positive for SARS-CoV-2 with a BA.5 infection during the outcome period, only 210 (2·4%) had also tested positive for SARS-CoV-2 with an omicron infection previously, between Jan 1 and Feb 9, 2022, at which time the BA.1 and BA.2 omicron subvariants accounted for almost all SARS-CoV-2 infections (table 2 ). By contrast, among the 178 669 triple-vaccinated controls who tested negative for SARS-CoV-2 during the outcome period, 33 972 (19·0%) had tested positive for SARS-CoV-2 between Jan 1 and Feb 9, 2022. The estimated protection against BA.5 infection was 92·7% (95% CI 91·6–93·7), suggesting that a previous omicron infection is highly protective against a new infection with BA.5 in a vaccinated population. By comparison, a previous delta or alpha infection provided a much weaker protection against a new infection with BA.5 (73·4% [95% CI 65·7–79·3%] for delta and 61·2% [49·1–70·4] for alpha).
Table 2.
Protection against BA.5 or BA.2 infection after a previous positive SARS-CoV-2 PCR test in Denmark, by variant strain
| Cases | Controls | OR | Adjusted OR* | Estimated protection | |
|---|---|---|---|---|---|
| Protection against infection with BA.5 | |||||
| Previous omicron infection | |||||
| Exposed | 210 (2·4%) | 33 972 (19·0%) | 0·106 (0·092–0·121) | 0·073 (0·063–0·084) | 92·7% (91·6–93·7) |
| Unexposed | 8468 (97·6%) | 144 697 (81·0%) | 1 | 1 | .. |
| Previous delta infection | |||||
| Exposed | 65 (0·8%) | 3336 (2·3%) | 0·333 (0·261–0·427) | 0·266 (0·207–0·343) | 73·4% (65·7–79·3) |
| Unexposed | 8468 (99·2%) | 144 697 (97·7%) | 1 | 1 | .. |
| Previous alpha infection | |||||
| Exposed | 58 (0·7%) | 1878 (1·3%) | 0·528 (0·406–0·686) | 0·388 (0·296–0·509) | 61·2% (49·1–70·4) |
| Unexposed | 8468 (99·3%) | 144 697 (98·7%) | 1 | 1 | .. |
| Protection against infection with BA.2 | |||||
| Previous omicron infection | |||||
| Exposed | 192 (0·7%) | 33 972 (19·0%) | 0·028 (0·024–0·032) | 0·029 (0·025–0·034) | 97·1 (96·6–97·5) |
| Unexposed | 29 100 (99·3%) | 144 697 (81·0%) | 1 | 1 | .. |
| Previous delta infection | |||||
| Exposed | 100 (0·3%) | 3336 (2·3%) | 0·149 (0·122–0·182) | 0·158 (0·129–0·193) | 84·2 (80·7–87·1) |
| Unexposed | 29 100 (99·7%) | 144 697 (97·7%) | 1 | .. | |
| Previous alpha infection | |||||
| Exposed | 98 (0·3%) | 1878 (1·3%) | 0·259 (0·212–0·318) | 0·262 (0·214–0·322) | 73·8 (67·8–78·6) |
| Unexposed | 29 100 (99·7%) | 144 697 (98·7%) | 1 | 1 | .. |
Data are n (%), OR (95% CI), or estimated % (95% CI). All participants received three doses of mRNA COVID-19 vaccine. The outcome period was April 10–June 30, 2022. Cases were people infected with SARS-CoV-2 omicron subvariants BA.5 or BA.2 during the outcome period. Controls were people who tested negative for SARS-CoV-2 infection during the outcome period. Unexposed individuals had no positive PCR tests before the start of the outcome period on April 10, 2022.
Adjusted for age group, time of infection (week number), sex, region of residency, and comorbidities.
In the supplementary analyses that estimated protection against BA.2 during the outcome period, a previous omicron infection was even more highly protective against BA.2 than was observed in the previous analysis for BA.5. Among the 29 292 tripple-vaccinated individuals who tested positive for SARS-CoV-2 with a BA.2 infection during the outcome period, 192 (0·7%) had tested positive for SARS-CoV-2 between Jan 1, and Feb 9, 2022. The estimated protection against BA.2 infection was 97·1% (95% CI 96·6–97·5). Again, a previous infection with the delta or alpha variant protected less well than a previous omicron infection did against BA.2, with estimated protection of 84·2% (95% CI 80·7–87·1) for delta and 73·8% (67·8–78·6) for alpha. If restricting the case definition to those hospitalised for an infection with BA.5 or BA.2, the extent of protection from a previous omicron infection was 96·4% (74·2–99·5) for BA.5 and 91·2% (95% CI 76·3–96·7%) for BA.2 (appendix p 7).
In the sensitivity analysis using a matched case-control design, the estimates were nearly identical to those in the main analysis. Changing the definition of a reinfection to require a different minimum number of days between repeat positive tests also had little effect on the results. Adjustment for time since vaccination (third dose) also had a minimal effect on the results (appendix pp 8–11).
In the vaccine protection analysis, 9307 (94·2%) of 9878 people with a BA.5 infection and 30 581 (94·8%) of 32 272 with a BA.2 infection were vaccinated against COVID-19 with three mRNA doses (table 3 ). Comparing triple-vaccinated versus unvaccinated individuals, the adjusted OR for the effect of the vaccine on the likelihood of an infection being due to BA.5 rather than BA.2, was 1·18 (95% CI 0·99–1·42; p=0·064). The change from the unadjusted estimated OR of 0·90 (95% CI 0·82–0·99) was driven largely by negative confounding from previous infections, since unvaccinated individuals were more likely to have had a previous infection than those who were vaccinated, and BA.5 cases were more likely than BA.2 cases to have had a previous infection (table 2). Comparing triple-vaccinated cases versus those who had received only two mRNA doses more than 4·5 months earlier, the effects on the chances of an infection being due to BA.5 rather than BA.2 were OR 0·90 (95% CI 0·81–1·00) and adjusted OR 1·12 (95% CI 0·92–1·35; p=0·26). Overall, there was little evidence, therefore, that the mRNA vaccines protect less well against BA.5 than against BA.2.
Table 3.
Vaccine protection against infection with BA.5 or BA.2 in Denmark, by vaccine status
| Infected with BA.5 during the outcome period | Infected with BA.2 during the outcome period | OR | Adjusted OR* | |
|---|---|---|---|---|
| Three doses versus unvaccinated | ||||
| Three doses† | 9307 (94·2%) | 30 581 (94·8%) | 0·90 (0·82–0·99) | 1·18 (0·99–1·42) |
| Unvaccinated | 571 (5·8%) | 1691 (5·2%) | 1 | 1 |
| Three doses versus two doses | ||||
| Three doses† | 9307 (94·8%) | 30 581 (95·3%) | 0·90 (0·81–1·00) | 1·12 (0·92–1·35) |
| Two doses‡ | 513 (5·2%) | 1515 (4·7%) | 1 | 1 |
Data are n (%) or OR (95% CI). All participants were infected with SARS-CoV-2 omicron subvariants BA.5 or BA.2. The outcome period was April 10–June 30, 2022. The analysis included individuals with or without a previous SARS-CoV-2 infection before the start of the outcome period.
Adjusted for age group, time of infection (week number), sex, region of residence, comorbidities, and previous infection (yes vs no).
Received three doses of either mRNA-1273 (lasomeran, Moderna) or BNT162b2 (tozinameran, Pfizer-BioNtech) before March 27, 2022.
Completed primary vaccination series more than 140 days before the start of the outcome period.
During the outcome period, hospitalisation for COVID-19 occurred in 210 (1·9%) of 11 314 people infected with BA.5 versus 514 (1·4%) of 36 805 of those infected with BA.2 (OR 1·34 [95% CI 1·14–1·57]; adjusted OR 1·69 [1·22–2·33]; table 4 ). The increase in effect size from the unadjusted to the adjusted analysis was largely driven by adjustment for age, as fewer elderly people were infected with BA.5 than with BA.2. The estimate did not change substantially after restricting the analysis to triple-vaccinated individuals only (adjusted OR 1·66 [95% CI 1·16–2·36]). After extending the outcome period by moving the start date back to Jan 1, 2022, and excluding cases with a previous infection, the hospitalisation adjusted OR was 1·83 (95% CI 1·31 to 2·55) for BA.5 versus BA.2, whereas delta cases were substantially more likely to require hospitalisation than BA.2 cases (adjusted OR 2·86 [1·67 to 4·91]).
Table 4.
Risk of hospitalisation after SARS-CoV-2 infection in Denmark, by subvariant
| All | Hospitalised for COVID-19 | Not hospitalised for COVID-19 | OR for difference | Adjusted OR for difference* | |
|---|---|---|---|---|---|
| Main analysis | |||||
| BA.2 | 36 805 | 514 (1·4%) | 36 291 (98·6%) | 1 | 1 |
| BA.5 | 11 314 | 210 (1·9%) | 11 104 (98·1%) | 1·34 (1·14–1·57) | 1·69 (1·22–2·33) |
| Supplementary analysis 1: subgroup analysis in vaccinated individuals† | |||||
| BA.2 | 30 581 | 409 (1·3%) | 30 172 (98·7%) | 1 | 1 |
| BA.5 | 9307 | 178 (1·9%) | 9129 (98·1%) | 1·44 (1·20–1·72) | 1·66 (1·16–2·36) |
| Supplementary analysis 2: extended outcome period‡ | |||||
| BA.2 | 159 943 | 2362 (1·5%) | 157 581 (98·5%) | 1 | 1 |
| BA.5 | 9839 | 203 (2·1%) | 9636 (97·9%) | 1·41 (1·22–1·62) | 1·83 (1·31–2·55) |
| Delta | 526 | 27 (5·1%) | 499 (94·9%) | 3·61 (2·45–5·33) | 2·86 (1·67–4·91) |
Data are n (%), OR (95% CI), or adjusted OR (95% CI). All participants were infected with SARS-CoV-2. The main and supplementary analysis 1 included BA.2 and BA.5 infections that occurred during the outcome period of April 10–June 30, 2022.
Adjusted for age group, time of infection (week number), sex, region of residence, comorbidities, previous infection (except for supplementary analysis 2), and vaccination status (except for supplementary analysis 1).
Received three doses of either mRNA-1273 (lasomeran, Moderna) or BNT162b2 (tozinameran, Pfizer-BioNtech) before March 27, 2022.
Supplementary analysis 2 included BA.2, BA.5, and delta infections that occurred between Jan 1 and June 30, 2022; people with a previous infection before Jan 1, 2022, were excluded.
Discussion
In this study, we investigated the risk of BA.5 infection in a population with hybrid immunity (ie, a previous infection and vaccine immunity), the evidence of reduced vaccine protection, and severity of BA.5 infection, in comparison with earlier SARS-CoV-2 strains.
The analyses of previous infection protection against subsequent infection indicated that a previous omicron infection provided very good protection against subsequent infection with BA.5, and a previous alpha or delta infection offered good protection, although to a lesser extent than omicron, against BA.5 and BA.2. The degree of protection a previous infection provided was higher against BA.2 than BA.5 during the outcome period. In the sensitivity analysis that used a matched case-control design, results were almost identical.
The analysis of vaccine protection against infection with BA.5 versus BA.2 did not provide strong evidence of poorer protection against BA.5 than BA.2. The estimated comparison for the vaccinated against the unvaccinated (OR 1·18 [95% CI 0·99–1·42]), and for the triple-vaccinated versus those who had only received two mRNA vaccine doses more than 4·5 months earlier (1·12 [0·92–1·35], might suggest a slightly heightened ability of BA.5 to escape the vaccine protection compared with BA.2; however, more data are needed to increase precision around the estimates, as both rely on reasonably small comparator populations.
The analysis of disease severity showed evidence of higher hospitalisation rates among BA.5 cases compared with BA.2 cases. As we expected, and consistent with our earlier studies,15, 17 the analysis also showed increased disease severity from a delta infection, with nearly three times the odds of hospitalisation compared with a BA.2 infection.
Real-world evidence on the disease severity of BA.5 is scarce. In a South African study published as a preprint,25 the risk of severe hospitalisation (defined as admission to intensive care, or mechanical ventilation, or an oral or intravenous steroid prescription) or death was similar during the BA.4–BA.5 wave compared with the preceding BA.1 wave. In both Portugal and South Africa, the BA.5 wave passed without overall COVID-19 hospital admissions and deaths exceeding those of the previous omicron wave, although Portugal reported excess mortality for a few weeks.26 A Portuguese study2 found a higher hospitalisation rate among people who had received a booster vaccination among those infected with BA.5 than with BA.2 (OR 3·36 [95% CI 1·18–9·63]).
As in our analysis, a study27 from Qatar estimated that previous BA.1 infection gave very high protection against infection with BA.2, and vice versa. Studies,28, 29, 30 including our own,13, 14 on the protective effect of a previous SARS-CoV-2 infection have generally found good protection of around 80% or higher against reinfection, although a lower degree of protection against a subsequent omicron infection has generally been reported after infection with earlier variants. In the current study, the protective effect of an earlier alpha or delta infection against omicron was considerably higher than that in our cohort analysis14 of the protective effect of an earlier variant infection against omicron in an unvaccinated population (estimated protection ranged from 19% to 51%), and also higher than estimates from elsewhere.28 Although the reasonably high estimates of protection in the current study might reflect a genuine hybrid immunity effect in the vaccinated population, it is possible that those with a previous infection were much more likely than the previously uninfected to have been tested for reasons other than a suspicion of having COVID-19, which in turn would inflate the estimated amounts of protection of a previous infection in our study. Nonetheless, assuming that the number of BA.5 infections observed in our study among people with a previous infection is only half of that which would have been observed in the absence of such a bias, the resulting OR estimate would be around 2 × 0·073 = 0·146, and the extent of protection would still be high, at around 85%. Importantly, the analysis that was restricted to cases who were hospitalised for COVID-19, and which was not subject to biases owing to testing, still showed that a previous omicron infection gave very high amounts (>90%) of protection against hospitalisation owing to infection with BA.5 or BA.2.
For analysis 2 (vaccine protection), it is important to note that, because the remaining unvaccinated people make up such a small proportion of the population, we were unable to assess vaccine effectiveness directly as the ratio of infection rates in vaccinated and unvaccinated individuals. Instead, basing the analysis upon infected individuals only, the analysis compared the vaccination status in those infected with BA.5 versus with BA.2, providing a relative measure of vaccine protection against BA.5 versus BA.2.
In a preliminary analysis from the UK Health Security Agency,31 a similar analysis strategy was followed, comparing people who had been recently vaccinated with a second, third, or fourth dose versus a baseline group of people vaccinated with a second or third dose more than 25 weeks before infection. The study did not find differences in vaccination status between those infected with BA.5 compared with BA.2 (OR 0·83 [95% CI 0·64–1·08]). Importantly, this type of analysis relies on there being some degree of vaccine protection against infection with BA.2, or the relative measure will be uninformative, as the OR will be 1.
Our study was made possible due to the intensive WGS efforts at the Statens Serum Institut. However, it is possible that some bias exists in the selection of samples for sequencing, as not all sequenced samples were selected at random. Second, not all positive cases during the outcome period would have been identified, as many were no longer being tested. Also, the study did not include results from the Danish national free-of-charge rapid antigen test programme; however, these only accounted for approximately 8% of all (ie, rapid antigen test and PCR) registered test results during the overall study period.18 Third, as described previously, estimates of protection from a previous infection might be too high if exposed people were more likely than unexposed people to come forward for testing owing to reasons other than suspected COVID-19. We believe bias from test procedures were largely mitigated in analysis 2 and 3, as they included only PCR-confirmed cases (either BA.2 or BA.5). Fourth, the analysis did not attempt to take into account the order in which vaccine and natural immunity were acquired, therefore some participants would have been unvaccinated at the time of their previous infection, whereas others would have received one, two, or three vaccine doses. Individuals with a previous omicron infection were much more likely to have had a breakthrough infection than those previously infected with delta or alpha during periods in which the vaccination coverage, and number of doses given per person, were much lower. Also, the analysis did not attempt to assess the effect of waning immunity as a function of time from vaccination or past infection. It was therefore not possible to attribute the weaker protection observed among those with a previous alpha or delta infection, relative to those with a previous omicron infection, to reduced cross-reactive immunity with different variant strains rather than to a waning effect.
Finally, infection rates varied considerably throughout the first half of 2022, affecting rates of testing and the age profile of cases and, in turn, the proportion of PCR-confirmed cases that were hospitalised. By evaluating the adjustment variables, we confirmed that age and time (week number) of infection contributed to confounding of the relationship between subvariant and risk of hospitalisation, which explains why a stronger effect was apparent from the adjusted estimate. Importantly, the observation that BA.5 is more severe compared with BA.2 occurred in the context of stable and low absolute numbers of hospitalisations of people who tested positive for SARS-CoV-2 in Denmark during the study period.
In conclusion, our study found that a previous omicron infection in triple mRNA-vaccinated individuals offers significant protection against BA.5 infection, including against infection leading to hospitalisation. Our analysis also indicated a similar or slightly weaker vaccine protection against BA.5 infection than against BA.2 infection. Overall, the effect of the current BA.5 wave might be small in populations with a high degree of hybrid immunity and might be similar to that of the previous BA.1–BA.2 wave. The increased risk of hospitalisation after a BA.5 infection found in our study merits further investigation into the disease severity of BA.5. This study also highlights how WGS continues to be a cornerstone in surveillance of the SARS-CoV-2 pandemic.
Data sharing
Deidentified participant-level data are available for access to members of the scientific and medical community for non-commercial use only. Applications for data access should be submitted to Forskerservice at The Danish Health Data Authority and will be reviewed on the basis of relevance and scientific merit. Data are available now, with no defined end date. For information about submitting an application, see the Forskerservice website (https://sundhedsdatastyrelsen.dk/da/forskerservice). Consensus sequences from the Danish WGS surveillance are routinely made available at both GISAID (https://gisaid.org/) and the European Nucleotide Archive (https://www.ebi.ac.uk/ena/browser/home).
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
We thank the staff at the test sites, Danish departments of clinical microbiology, the virology laboratory, and TestCenter Denmark at the Statens Serum Institut (SSI), and the staff of the Data Integration and Analysis Secretariat at SSI. We also thank Richard Hayes at the London School of Hygiene & Tropical Medicine for his useful insights on the analysis methods.
Contributors
All authors contributed to the conception and design of the study, the acquisition of data, or the data analysis and interpretation. CHH, NUF, and PB drafted the manuscript and all authors provided critical revisions. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. CHH and PB accessed and verified the data. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supplementary Material
References
- 1.Tegally H, Moir M, Everatt J, et al. Emergence of SARS-CoV-2 omicron lineages BA.4 and BA.5 in South Africa. Nat Med. 2022 doi: 10.1038/s41591-022-01911-2. published online June 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kislaya I, Casaca P, Borges V, et al. SARS-CoV-2 BA.5 vaccine breakthrough risk and severity compared with BA.2: a case–case and cohort study using electronic health records in Portugal. [DOI]
- 3.Motozono C, Toyoda M, Zahradnik J, et al. SARS-CoV-2 spike L452R variant evades cellular immunity and increases infectivity. Cell Host Microbe. 2021;29:1124. doi: 10.1016/j.chom.2021.06.006. 36.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Institute for Communicable Diseases, South Africa COVID-19 hospital surveillance update—week 22, 2022. June 2022. https://www.nicd.ac.za/wp-content/uploads/2022/06/NICD-COVID-19-Weekly-Sentinel-Hospital-Surveillnace-update-Week-22-2022.pdf
- 5.Instituto Nacional de Saúde Doutor Ricardo Jorge Relatório de Monitorização da Situação Epidemiológica da COVID-19. June 17, 2022. https://www.insa.min-saude.pt/wp-content/uploads/2022/06/20220615_Monitorizacao_COVID-19.pdf (in Portuguese).
- 6.Bradshaw D, Laubscher R, Dorrington R, Groenewald P, Moultrie T. Report on weekly deaths in South Africa—12–18 June 2022 (week 24) June 21, 2022. https://www.samrc.ac.za/sites/default/files/files/2022-06-22/weekly18June2022.pdf
- 7.Hui KPY, Ho JCW, Cheung MC, et al. SARS-CoV-2 omicron variant replication in human bronchus and lung ex vivo. Nature. 2022;603:715–720. doi: 10.1038/s41586-022-04479-6. [DOI] [PubMed] [Google Scholar]
- 8.Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399:1303–1312. doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Zhang T, Fang Y, Liu J, Ye Q, Ding L. SARS-CoV-2 spike L452R mutation increases Omicron variant fusogenicity and infectivity as well as host glycolysis. Signal Transduct Target Ther. 2022;7:76. doi: 10.1038/s41392-022-00941-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimura I, Yamasoba D, Tamura T, et al. Virological characteristics of the novel SARS-CoV-2 omicron variants including BA.2.12.1, BA.4 and BA.5. BioRxiv. 2022 doi: 10.1101/2022.05.26.493539. published online May 26. (preprint). [DOI] [Google Scholar]
- 11.Santé Publique France Analyse de risque sur les variants émergents du SARS-CoV-2 réalisée conjointement par Santé publique France et le CNR Virus des infections respiratoires. May 18, 2022. https://www.santepubliquefrance.fr/content/download/445484/file/analyse_risque_variants_20220615.pdf (in French).
- 12.Gram MA, Emborg HD, Schelde AB, et al. Vaccine effectiveness against SARS-CoV-2 infection or COVID-19-related hospitalization with the alpha, delta and omicron SARS-CoV-2 variants: a nationwide Danish cohort study. PLoS Med. 2022;19 doi: 10.1371/journal.pmed.1003992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen CH, Michlmayr D, Gubbels SM, Mølbak K, Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet. 2021;397:1204–1212. doi: 10.1016/S0140-6736(21)00575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michlmayr D, Hansen CH, Gubbels SM, et al. Observed protection against SARS-CoV-2 reinfection following a primary infection: a Danish cohort study among unvaccinated using two years of nationwide PCR-test data. Lancet Reg Health Eur. 2022;20 doi: 10.1016/j.lanepe.2022.100452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bager P, Wohlfahrt J, Bhatt S, et al. Risk of hospitalisation associated with infection with SARS-CoV-2 omicron variant versus delta variant in Denmark: an observational cohort study. Lancet Infect Dis. 2022;22:967–976. doi: 10.1016/S1473-3099(22)00154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bager P, Wohlfahrt J, Fonager J, et al. Risk of hospitalisation associated with infection with SARS-CoV-2 lineage B.1.1.7 in Denmark: an observational cohort study. Lancet Infect Dis. 2021;21:1507–1517. doi: 10.1016/S1473-3099(21)00290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bager P, Wohlfahrt J, Rasmussen M, Albertsen M, Krause TG. Hospitalisation associated with SARS-CoV-2 delta variant in Denmark. Lancet Infect Dis. 2021;21 doi: 10.1016/S1473-3099(21)00580-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Statens Serum Institut COVID-19 dashboard (scroll through the figures shown on the bottom right of the screen to: PCR- og antigen test pr. uge [PCR and antigen tests per week]) https://experience.arcgis.com/experience/aa41b29149f24e20a4007a0c4e13db1d
- 19.Our World in Data Coronavirus pandemic (COVID-19). COVID-19 data explorer vaccine doses, people vaccinated, and booster doses for Denmark. 2020. https://ourworldindata.org/coronavirus
- 20.Schønning K, Dessau RB, Jensen TG, et al. Electronic reporting of diagnostic laboratory test results from all healthcare sectors is a cornerstone of national preparedness and control of COVID-19 in Denmark. APMIS. 2021;129:438–451. doi: 10.1111/apm.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(suppl):30–33. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 22.Grove Krause T, Jakobsen S, Haarh M, Mølbak K. The Danish vaccination register. Euro Surveill. 2012;17 doi: 10.2807/ese.17.17.20155-en. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29:541–549. doi: 10.1007/s10654-014-9930-3. [DOI] [PubMed] [Google Scholar]
- 24.Ayoub HH, Tomy M, Chemaitelly H, et al. Estimating protection afforded by prior infection in preventing reinfection: applying the test-negative study design. medRxiv. 2022 doi: 10.1101/2022.01.02.22268622. published online January 3. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies M, Morden E, Rosseau P, et al. Outcomes of laboratory-confirmed SARS-CoV-2 infection during resurgence driven by Omicron lineages BA.4 and BA.5 compared with previous waves in the Western Cape Province, South Africa. medRxiv. 2022 doi: 10.1101/2022.06.28.22276983. published online July 1. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinto Leite P, Fernandes E, Casaca P, Peralta Santos A, Oliveira AL. Monitoring of COVID-19. June 29, 2022. https://www.insa.min-saude.pt/wp-content/uploads/2022/07/20220629_Monitorizacao_COVID-19.pdf
- 27.Chemaitelly H, Ayoub HH, Coyle P, et al. Protection of omicron sub-lineage infection against reinfection with another omicron sub-lineage. Nat Commun. 2022 doi: 10.1038/s41467-022-32363-4. published online Aug 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.COVID-19 Forecasting Team. Lim SS. Past SARS-CoV-2 infection protection against reinfection: a systematic review and meta-analysis. SSRN. 2022 doi: 10.1016/S0140-6736(22)02465-5. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4155225 published online July 6. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kashir J, AlKattan K, Yaqinuddin A. COVID-19: cross-immunity of viral epitopes may influence severity of infection and immune response. Signal Transduct Target Ther. 2021;6:102. doi: 10.1038/s41392-021-00490-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altarawneh HN, Chemaitelly H, Hasan MR, et al. Protection against the omicron variant from previous SARS-CoV-2 infection. N Engl J Med. 2022;386:1288–1290. doi: 10.1056/NEJMc2200133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.UK Health Security Agency SARS-CoV-2 variants of concern and variants under investigation in England—technical briefing 43. 2022. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1086494/Technical-Briefing-43-28.06.22.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified participant-level data are available for access to members of the scientific and medical community for non-commercial use only. Applications for data access should be submitted to Forskerservice at The Danish Health Data Authority and will be reviewed on the basis of relevance and scientific merit. Data are available now, with no defined end date. For information about submitting an application, see the Forskerservice website (https://sundhedsdatastyrelsen.dk/da/forskerservice). Consensus sequences from the Danish WGS surveillance are routinely made available at both GISAID (https://gisaid.org/) and the European Nucleotide Archive (https://www.ebi.ac.uk/ena/browser/home).