Abstract
BACKGROUND
The mechanism by which a discrete episode of atrial fibrillation (AF) occurs remains unknown. Alcohol appears to increase the risk for AF, providing an opportunity to study electrophysiologic effects that may render the heart prone to arrhythmia.
OBJECTIVE
To identify acute changes in human atrial electrophysiology during alcohol exposure.
METHODS
We performed a randomized, double-blinded, placebo-controlled trial of intravenous alcohol titrated to 0.08% blood alcohol concentration versus a volume and osmolarity-matched, masked, placebo in patients undergoing AF ablation procedures. Right, left, and pulmonary vein atrial effective refractory periods (AERPs) and conduction times were measured pre- and post-infusion. Isoproterenol infusions and burst atrial pacing were used to assess AF inducibility.
RESULTS
Of 100 participants (50 in each group), placebo recipients were more likely to be diabetic (22% v 4%, p=0.007) and to have undergone a prior AF ablation (36% v 22%, p=0.005). Pulmonary vein AERPs decreased an average of 12 ms (95% CI 1–22 ms, p=0.026) in the alcohol group, with no change in the placebo group (p=0.98). While no statistically significant differences in continuously-assessed AERPs were observed, the proportion of AERP sites tested that decreased with alcohol (median 0.5, IQR 0.6–0.6) was larger than with placebo (median 0.4, IQR 0.2–0.6, p=0.0043). No statistically significant differences in conduction times or in the proportion with inducible AF were observed.
CONCLUSIONS
Acute exposure to alcohol reduces AERP, particularly in the pulmonary veins. These data demonstrate a direct mechanistic link between alcohol, a common lifestyle exposure, and immediate proarrhythmic effects in human atria.
Keywords: Atrial fibrillation, alcohol, ablation, electrophysiology, lifestyle
CONDENSED ABSTRACT
In this randomized, double-blinded, placebo-controlled study of intravenous alcohol titrated to 0.08%, alcohol resulted in a significant reduction in the pulmonary vein atrial effective refractory period and a greater proportion of sites exhibited a lower atrial effective refractory period with alcohol than with placebo. No changes in conduction times or differences in induced AF were observed. These findings demonstrate that alcohol acutely reduces atrial effective refractory periods, a phenomenon known to render atria more prone to fibrillate, providing the first mechanistic evidence that a common lifestyle factor can acute change cardiac electrophysiology to increase the chance of an arrhythmia.
Alcohol is the most commonly consumed drug in the world, and the great majority of Americans drink alcohol on a regular basis (1). While substantial research into the effects of alcohol on heart health has been conducted, there is a dearth of rigorous human subject-based research investigating the acute effects of alcohol on the heart.
Atrial fibrillation (AF) is the most common arrhythmia encountered in clinical practice, expected to affect over 12 million individuals in the US by 2050 (2). Large epidemiologic studies have demonstrated that greater alcohol consumption predicts incident AF (3), and a recent randomized trial showed that alcohol abstinence can reduce the AF burden (measured as frequency and duration of AF episodes) over several months (4). While studies have focused on the more chronic effects of alcohol on AF, patients describe an immediate or near-immediate effect of alcohol on their discrete AF episodes (5,6).
The mechanisms by which alcohol might change cardiac electrophysiology are largely unknown. Animal studies suggest that alcohol may reduce the atrial effective refractory period (AERP) and decrease conduction velocity (7–9), both phenomena that render the atria more prone to fibrillate (10,11). Observational and very small, non-controlled, human studies relying on either self-report of alcohol consumption or oral consumption of various amounts of alcohol have provided inconsistent results (12–14). While the pulmonary veins have proven integral to AF pathophysiology (15,16), the relationship between pulmonary vein electrophysiology and lifestyle factors in particular has not previously been assessed.
METHODS
The HOw ALcohol InDuces Atrial TachYarrhythmias (HOLIDAY) Trial (ClinicalTrials.gov number, NCT01996943) was a single-center, randomized, double-blinded trial of intravenous alcohol versus placebo among patients undergoing pulmonary vein isolation procedures for AF. We included adults age 21–90 presenting in sinus rhythm or AF of recent onset (the latter confirmed by sinus rhythm documented no more than one week prior). Patients were excluded if any of the following were identified: a history of substance abuse or alcoholism; left ventricular ejection fraction <50%; liver dysfunction; pregnancy; inability to give informed consent; amiodarone use within one month prior to procedure; dofetilide use within 24 hours prior to the procedure; or severe intolerance to alcohol.
Participants were asked to hold Vaughn-Williams Class 1 or 3 drugs for at least 5 half-lives and alcohol for at least 48 hours prior the procedure. A breathalyzer (Intoximeters, St. Louis, MO) measurement was taken at baseline to assure all participants had a blood alcohol concentration of 0.0% immediately prior to the procedure. General anesthesia was administered according to a pre-determined protocol to minimize effects on cardiac electrophysiology (described in detail in the Supplemental Material). Intra-cardiac multipolar electrocatheters were placed in the following positions under fluoroscopic guidance: a decapolar catheter in the coronary sinus (CS), a decapolar catheter along the crista terminalis, a quadripolar catheter along the medial tricuspid annulus at the position of the His bundle, a duodecapolar catheter in the left upper pulmonary vein, and a quadripolar ablation catheter in the right upper pulmonary vein (Supplemental Figure 1). If the planned upper pulmonary vein was electrically isolated, the lower vein on the same side was utilized. If both an upper and lower pulmonary vein were electrically isolated, the catheter was placed as close as possible to the target vein where electrical (non-isolated) activity was observed.
Immediately following catheter placement, a one-minute premature atrial contraction (PAC) count was conducted. Atrial effective refractory period measurements were then obtained at the following bipoles: proximal CS, distal bipole of the CS catheter, high right atrium, left upper pulmonary vein, and right upper pulmonary vein. The atrial effective refractory period was obtained using a 600 ms drive cycle length for 8 beats (S1) at twice pacing threshold with incrementally increasing 10 ms steps (S2). The first drive train at each location began with an atrial extrastimulus of 140 ms, which was not expected to result in atrial capture (to avoid electrical effects that might shorten the refractory periods with a “step-down” approach). Once the first atrial capture was achieved, the S2 was decreased by 5 ms in order to provide a resolution of 5 ms. As an estimate of conduction times, we measured activation times “off-line” using digital calipers from the drive trains of these pacing maneuvers between the following locations: proximal CS to high right atrium, proximal CS to left upper pulmonary vein, distal CS to proximal CS, distal right atrium to right atrial septum, high to low right atrium, left upper to right upper pulmonary vein, right upper pulmonary vein to distal CS, and right upper pulmonary vein to right atrial septum. For each, the average of three measurements from different S1’s are reported.
The study infusion was then initiated during creation of the left atrial electroanatomic map (a routine part of the clinical AF ablation procedure) in order to minimize left atrial and total procedure time. Patients were randomized in a 1:1 fashion to alcohol or placebo in blocks of 5 (alternating 2:3 and 3:2). Patients, operators, and investigators were blinded, while the anesthesiologist and research coordinator(s) administering and overseeing the infusion were not. Patients assigned to receive alcohol received a masked 6% volume/volume ethanol solution in 0.45% saline by the breath alcohol concentration quick clamp method as previously described (17,18). This method utilizes an automated algorithm that adjusts the flow of the infusion depending on the individual’s sex, weight and then in a serial fashion in response to breath alcohol measurements. Serial breath alcohol measurements at pre-determined intervals according to the algorithm were obtained using a handheld breathalyzer (Intoximeters, St. Louis, MO) via an adapter and one-way valve placed into the ventilator circuit with an induced artificial breath; those values were then entered into the algorithm to allow for programmed adjustments of the infusion rate to keep the blood alcohol “clamped” at 0.08% during the repeat electrophysiology study. The patients assigned to masked placebo received 5% dextrose in 0.45% saline using the same infusion protocol for a random length of time that was within one standard deviation of the mean time to achieve a steady state using the alcohol protocol (17 ± 4 minutes). To maintain blinding, breath alcohol concentration measurements that were randomly generated within two standard deviations of the predicted values calculated by the algorithm were utilized and verbally communicated for placebo infusions. Once the blood ethanol level was within ± 0.002% of 0.08%, or the predetermined time of placebo infusion was achieved, the electrophysiologic measurements were repeated in the same fashion described above.
Patients then underwent an isoproterenol hydrochloride infusion titrated every 2 minutes from 3 mcg/min, to 6 mcg/min, to 12 mcg/min to 20 mcg/min, with phenylephrine administered as needed to maintain a mean arterial pressure > 60 mmHg. If no sustained AF was observed, the isoproterenol infusion was stopped, and, after the heart rate returned to < 100 beats per minute, the participants underwent up to 3 bursts of rapid atrial pacing at a cycle length of 200 ms from the proximal CS bipole for 5 seconds each.
Episodes of non-sustained and sustained (>30 seconds) of AF were noted during each segment of the study. For AF episodes lasting > 2 minutes, the participant could undergo one electrical cardioversion at the discretion of the operating physician.
The primary outcome was to test the hypothesis that alcohol would reduce the AERP. Pre-specified secondary outcomes included assessing site-specific changes in AERP (grouping the pulmonary veins versus non-pulmonary vein sites), changes in conduction time, and induction of AF.
The study was approved by the University of California, San Francisco Institutional Review Board, and all participants provided informed, witnessed, signed consent.
Statistical Analyses
Normally distributed continuous variables are presented as means ± SD and were compared using t-tests, and continuous variables with skewed distributions are presented as medians and interquartile ranges (IQR) and were compared using Wilcoxon rank sum or signed rank tests as appropriate. Categorical variables were compared using chi squared and Fisher’s exact tests. The proportion of atrial effective refractory periods that decreased with the infusion, the proportion of conduction times that decreased with the infusion, and the continuous change in each individual atrial effective refractory period and conduction time were assessed. Linear mixed models (LMMs) to estimate average and site-specific effects on pre- to post-infusion changes in AERP and conduction times were performed, with site and baseline values modeled as fixed effects and participants as random effects; analyses were repeated after adjusting for covariates statistically significantly different between alcohol and placebo groups. Heterogeneous relationships between pulmonary vein and non-pulmonary vein sites were assessed using formal tests for interaction. In addition, given evidence of potential effect modification by biological sex (19), tests for interaction by sex on the relationships between randomization assignment and outcomes were performed. Based on a review of repeat AERP measurements in 30 AF patients at 600 ms drive cycle length prior to the trial (yielding an average ERP of 230 ms ± 20 ms), we estimated that 22 patients in each group would provide 90% power to detect a statistically significant one standard deviation difference in the overall AERP using a single interim analysis for efficacy (assessed by an independent Data Safety Monitoring Board) and an overall two-sided α = 0.05. We based power calculations for the secondary analysis on 25 paroxysmal AF patients in whom AF induction occurred in 20%; we therefore estimated that 50 patients in each group would provide 84% power to detect a 30% increase in AF induction (20% versus 50%) with ethanol, again using a single interim analysis for efficacy and an overall two-sided α = 0.05. Analyses were conducted using Stata version 16 (College Station, Texas). Two-sided p values < 0.05 were considered statistically significant.
RESULTS
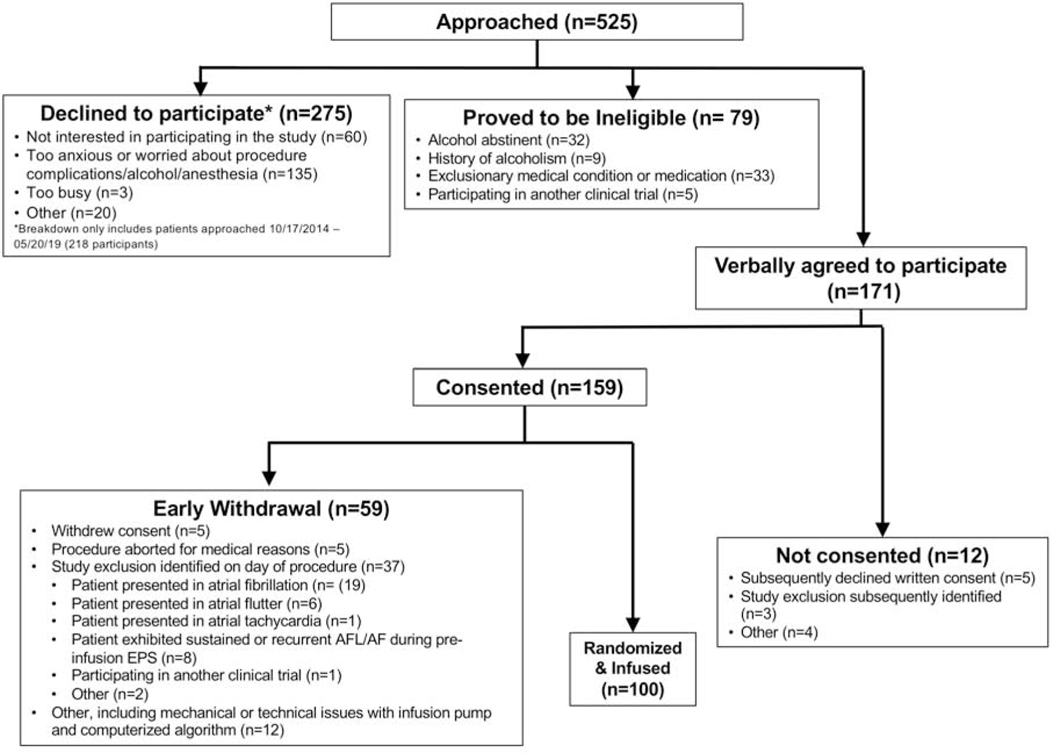
Between February 8, 2013 and May 30, 2019, 100 patients were randomized and received a study infusion (Figure 1). All participants exhibited a baseline breath alcohol concentration of 0.0% prior to procedure initiation. The baseline characteristics of the study participants divided by randomization assignment are shown in Table 1, revealing that the placebo group included a statistically significantly greater proportion of those with diabetes and those who had previously undergone an AF ablation. Among those with echocardiographic measurements, no statistically differences were detected (Supplemental Table 1). While 20 participants discontinued their antiarrhythmic drug within fewer than 3 days of the procedure, no differences relevant to drug type or timing of discontinuation were detected between the groups (Supplemental Table 2). Among the group randomized to alcohol, the median maximum achieved breath alcohol concentration was 0.081% (IQR 0.077–0.0844%).
Figure 1.
Consort Diagram for Trial Enrollment
Table 1.
Baseline Characteristics among participants randomly selected to undergo an alcohol or placebo infusion
| Alcohol (N=50) | Placebo (N=50) | P value | |
|---|---|---|---|
| Mean Age (years) | 58.78 ± 11.27 | 61.14 ± 13.08 | 0.34 |
| Female | 14 (28%) | 12 (24%) | 0.65 |
| Race | 0.69 | ||
| White | 44 (88%) | 45 (90%) | |
| African American | 2 (4%) | 1 (2%) | |
| Asian/Pacific Islander | 3 (6%) | 4 (8%) | |
| Other | 1 (2%) | 0 (0%) | |
| Hispanic ethnicity | 1 (2%) | 0 (0%) | 0.31 |
| Body Mass Index (kg/m2) | 27.38 ± 7.47 | 28.19 ± 6.79 | 0.65 |
| History of smoking | 15 (30%) | 19 (38%) | 0.40 |
| Median Pack Years (IQR) | 15 (3–20) | 0.051 | |
| Hypertension | 17 (34%) | 25 (50%) | 0.11 |
| Diabetes | 2 (4%) | 11 (22%) | 0.0074 |
| Coronary artery disease | 1 (2%) | 4 (8%) | 0.17 |
| Congestive heart failure | 2 (4%) | 4 (8%) | 0.40 |
| Stroke | 0 (0%) | 2 (4%) | 0.15 |
| Transient Ischemic Attack | 1 (2%) | 0 (0%) | 0.31 |
| Paroxysmal Atrial Fibrillation | 46 (92%) | 44 (88%) | 0.51 |
| Prior Ablation | 22 (44%) | 20 (40%) | 0.69 |
| Prior Atrial Fibrillation Ablation | 11 (22%) | 18 (36%) | 0.0051 |
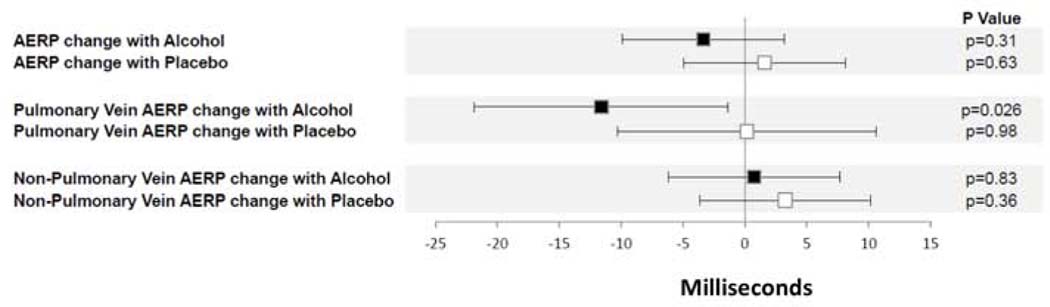
After taking baseline AERP into account, global AERP declined on average by a non-statistically significant 3.35 ms (95% CI reduction of 9.89 to an increase of 3.18, p=0.31) in the alcohol group, while on average it increased by a non-statistically significant 1.59 (95% CI −4.96, 8.14) P=0.63 in the placebo group (Central Illustration). However, the pulmonary vein AERPs decreased by a statistically significant 11.61 ms in the alcohol group, whereas no statistically significant change in the pulmonary vein AERPs were observed in the placebo group (Central Illustration). When assessing non-pulmonary vein AERPs, no significant change was observed in either the alcohol or placebo groups. Within the alcohol group, the overall mean change in AERPs was 25.88 ms lower (95% CI 14.72, 37.04 lower) in the pulmonary veins than at other sites, p for interaction <0.001. All of the above statistically significant relationships were maintained after adjusting for diabetes and a history of AF ablation (Supplemental Figure 2).
Central Illustration. Change in Atrial Effective Refractory Period (AERP) with Study Infusion Compared to Baseline.
Black squares denote changes with the alcohol infusion, and white squares denote changes with the placebo infusion. Analyses utilized linear mixed models and took baseline AERP into account. Y error bars denote 95% confidence intervals.
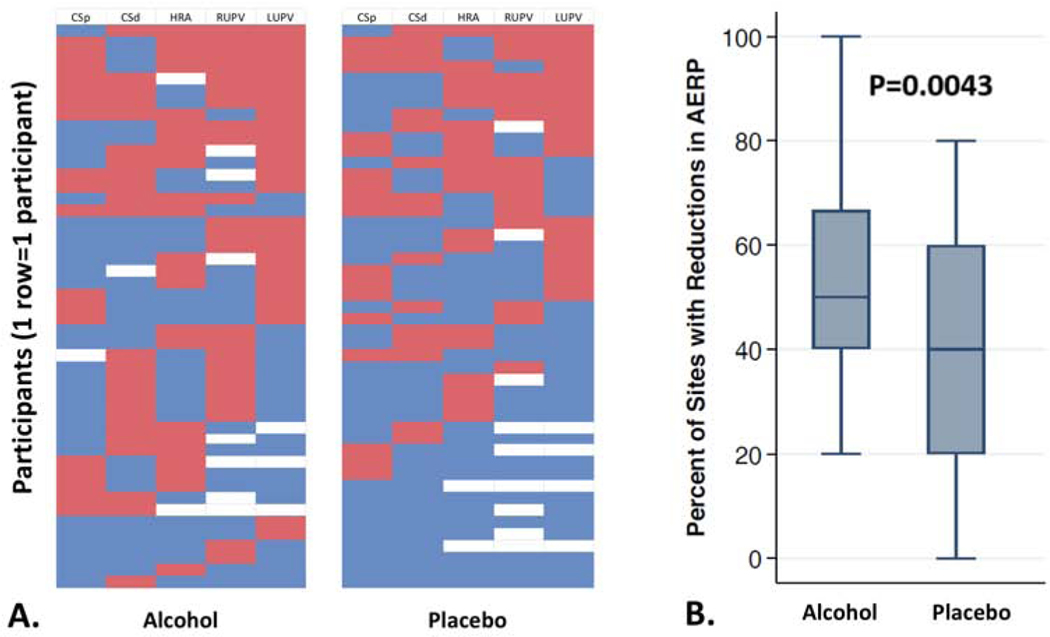
The proportion of AERPs that declined after the infusion among those randomly assigned to receive alcohol was statistically significantly larger than in those receiving placebo (Figure 2, p=0.0043). After adjusting for the two covariates that were statistically significant different between the two groups, history of diabetes and a history of ablation, the alcohol group exhibited on average a 13% greater proportion of tested sites with a decline in AERP compared to the placebo group (95% CI 3–23%, p=0.01; Supplemental Figure 2). No evidence of interactions by biological sex were observed (Supplemental Table 3).
Figure 2. Proportion of Atrial Effective Refractory Periods (AERPs) that declined during alcohol and placebo infusions.
In Panel A., each row represents an individual participant, each column represents the AERP testing site (CSp denotes proximal coronary sinus; CSd denotes distal coronary sinus; HRA denotes high right atrium; RUPV denotes right upper pulmonary vein; LUPV denotes left upper pulmonary vein). Red denotes an AERP decline with the study infusion compared to baseline, blue denotes no decline, and white means testing was not performed. The hierarchy of these heat maps from top to bottom was dictated first by the proportion red, then red in order from the sites as listed from left to right.
In Panel B., Box Plots represent the median (middle horizontal lines), 25th and 75th percentiles (boxes) and 1.5 times the interquartile ranges (error bars) of the percentages of AERP testing sites that declined during the infusion compared to baseline.
No statistically significant changes in any of the conduction times were observed between the alcohol and placebo groups (Supplemental Figure 3), including in individual analyses restricted to conduction time differences across multi-polar catheters to help assure consistency in distance between the two time points (Table 3).
Table 3.
Median and interquatile range (IQR) change in conduction times among those randomly assigned to alcohol infusion versus placebo.
| Conduction Time | ALCOHOL GROUP Median and IQR Conduction Time Change | PLACEBO GROUP Median and IQR Conduction Time Change | P value |
|---|---|---|---|
| CSp to hRA | 0.33 (−1.67 to 4.33) | 0.67 (−1.33 to 2.67) | 0.79 |
| CSp to LUPV | 2.88 (−3.33 to 9.17) | 3.50 (−2.00 to 12.83) | 0.63 |
| CSd to CSp | 0.33 (−2.00 to 2.33) | 0 (−1.00 to 3.00) | 0.85 |
| hRA to His | −2.00 (−5.00 to 1.33) | −0.50 (−2.50 to 10.67) | 0.073 |
| hRA to lRA | 0.50 (−2.67 to 5.33) | 0.50 (−2.33 to 4.50) | 0.83 |
| LUPV to RUPV | 3.33 (−10.00 to 11.83) | 3.67 (−9.67 to 14.83) | 0.98 |
| RUPV to CSd | 2.33 (−2.33 to 10.50) | 1.00 (−6.00 to 7.67) | 0.18 |
| RUPV to His | 4.33 (−4.00 to 11.00) | 0.33 (−6.00 to 4.67) | 0.64 |
CSp denotes proximal coronary sinus; CSd denotes distal coronary sinus; hRA denotes the high right atrium (distal bipole of the decapolar catheter in the right atrium); lRA denotes the low right atrium (proximal bipole of the decapolar catheter in the right atrium); LUPV denotes the left upper pulmonary vein; RUPV denotes the right upper pulmonary vein
Forty-four percent of participants in the alcohol group exhibited any AF (whether non-sustained or sustained) throughout the procedure compared to 48% of placebo recipients (p=0.69). No statistically significant differences in induction of AF when examining discrete events of the study were observed (Table 2 and Supplemental Figure 4); after adjusting for a history of AF ablation and diabetes, the alcohol group more commonly experienced AF with extrastimuli pacing delivered in the right upper pulmonary vein. With isoproterenol, more sustained AF was observed during the placebo infusion compared to during the alcohol infusion (24% versus 7%, p=0.027), although the statistical significance was no longer present after adjusting for a history of AF ablation and diabetes (p=0.09). No evidence of interactions by biological sex were observed (Supplemental Table 4).
Table 2.
Proportions of participants randomized to alcohol versus placebo infusion exhibiting atrial fibrillation at each time point beginning with initiation of study infusion.
| Study Time Period | Unadjusted Proportion with AF | Adjusted Proportion with AF* | ||||
|---|---|---|---|---|---|---|
| Alcohol | Placebo | p value | Alcohol | Placebo | p value | |
| Infusion | 0.14 | 0.12 | 0.77 | 0.16 | 0.10 | 0.41 |
| Infusion PAC count † | 0 | 0 | -- | -- | -- | -- |
| Extrastimuli pacing during infusion at site: | ||||||
| CSp | 0 | 0.04 | 0.49 | -- | -- | -- |
| CSd | 0.02 | 0.04 | 0.56 | 0.03 | 0.03 | 0.84 |
| hRA | 0.02 | 0.02 | 1.00 | 0.1 | 0.08 | 0.85 |
| LUPV | 0.06 | 0.08 | 0.69 | 0.07 | 0.07 | 0.86 |
| RUPV | 0.10 | 0.02 | 0.09 | 0.13 | 0.02 | 0.025 |
| Isoproterenol | 0.16 | 0.26 | 0.26 | 0.18 | 0.24 | 0.48 |
| Burst Pacing | 0.19 | 0.18 | 0.87 | 0.20 | 0.17 | 0.70 |
Adjusted for history of atrial fibrillation ablation and diabetes
During the 2 minute period after the infusion achieved steady state and the number of premature atrial contractions were determined
CSp denotes proximal coronary sinus; CSd denotes distal coronary sinus; hRA denotes the high right atrium; LUPV denotes the left upper pulmonary vein; RUPV denotes the right upper pulmonary vein
DISCUSSION
In this double-blinded, placebo-controlled study, pulmonary vein atrial refractory periods declined significantly only in those randomly assigned to a blood alcohol concentration of approximately 0.08%. In addition, among all sites tested, the atrial refractory period more commonly shortened during the alcohol infusion compared to the placebo infusion. No acute differences in conduction time, a marker of conduction velocity, nor in overall AF inducibility were observed between the alcohol and placebo groups. These data suggest that alcohol exerts direct and acute electrophysiologic effects that may render the atria, and particularly the pulmonary veins, more prone to arrhythmogenesis, but failed to show immediate effects on AF occurrence.
Alcohol is the most commonly consumed drug in the world (1), but remarkably little is understood about the acute effects on fundamental human physiology. AF is the most common sustained arrhythmia, expected to affect one out of every fourth person that reaches that age of 40, is responsible for a reduced quality of life, and is associated with a substantially increased risk of stroke, dementia, chronic kidney disease, myocardial infarction, heart failure and death (2). There is a growing interest in identifying modifiable lifestyle factors that are directly accessible to the lay public that influence the occurrence of a discrete AF episode (20).
Large epidemiological studies have demonstrated fairly consistent, albeit not uniform, relationships between increased alcohol consumption and the risk of incident AF (3). While reliance on self-report of alcohol consumption has been a common limitation, we have demonstrated that both physician coding for evidence of excess alcohol consumption (21) as well as residing in jurisdictions with greater access to alcohol (22) are both associated with a heightened AF risk. Our observational evidence that abstinence from alcohol can reduce AF risk (23) was recently validated by a prospective trial demonstrating a reduction in AF recurrence among those randomly assigned to alcohol abstention (4). While left atrial remodeling may explain chronic effects (24,25), the mechanism underlying acute relationships between alcohol and AF remain poorly understood. Because AF itself may contribute to left atrial enlargement and fibrosis (26), it may be that AF itself contributes to the remodeling, leaving the true proximate cause as the true etiology.
A recent survey of nearly 1,000 AF patients revealed that alcohol was the most common trigger reported for discrete AF episodes (5). Such a dynamic and immediate association cannot be explained by chronic changes such as left atrial fibrosis or, presumably, left atrial enlargement. A recent animal study utilizing optical mapping demonstrated that 0.08% alcohol concentration in the perfusate of rat heart Langendorff preparations resulted in a slowing of conduction velocity and shortening of the right atrial effective refractory period (7)—of note, these sort of experiments do not allow for an assessment of pulmonary vein electrophysiology.
In the current study, patients randomly assigned to alcohol infusion more often experienced a decline in their atrial effective refractory period. A shorter atrial effective refractory period has been shown to be the mechanism by which “AF begets AF” in animal models (27). Whether due to classic reentry (including in the setting of rotors (28)) or partial reentry, a shorter atrial effective refractory period is an enabler, increasing the excitable gap between head and tail of the electrical wavefront, thereby enhancing its perpetuation (10). The pulmonary veins seemed to exhibit amplified effects in our study, fitting with the observation that the pulmonary veins are integral to AF initiation (16). It is possible that the relative insulation of myocardial sleeves (29) in the pulmonary veins result in reduced electrotonic interaction, rendering them more prone to alcohol’s effects, or perhaps some interaction with autonomic ganglia, also in closer proximity to the pulmonary veins (30), is operative. Regardless, given a triggered beat from a pulmonary vein, shortening of the refractory period would allow faster or more tightly coupled premature beats to interact with the atria and induce AF.
Contrary to recent evidence from the Langendorff-prepared rat hearts (7), we failed to observe any differences in conduction times between patients randomly assigned to alcohol versus placebo. It was notable that, despite the changes in atrial effective refractory period, no differences in actual AF occurrences occurred between the groups. It is difficult to conclude that the anesthesia had a meaningful effect on AF inducibility given the high rate of AF events that were observed in both groups. Perhaps this group of individuals undergoing AF ablations at a tertiary referral center (many having failed previous AF ablations) were overly sensitive to AF induction in general, with the mechanisms responsible for any AF induction in this setting overwhelming effects due solely to alcohol.
Our findings provide biological plausibility in humans to bolster observations in epidemiologic studies that alcohol makes the human atria more prone to fibrillate (3). Why a given individual experiences a discrete AF episode at a particular time has long been a mystery. To our knowledge, this is the first time that a common modifiable exposure has been shown to acutely change human atrial and pulmonary vein electrophysiology, providing evidence that a lifestyle factor can immediately influence the atria in a way expected to enhance the likelihood of an AF event.
It is important to acknowledge several potential limitations. As mentioned, all patients were under general anesthesia. While this enabled double-blinding and enhanced the feasibility of the study and although we employed a specific anesthesia drug protocol to minimize electrophysiologic effects, we cannot exclude the possibility that these anesthetic agents influenced our electrophysiologic findings or propensities to AF. Similarly, not all patients stopped their antiarrhythmic drugs three days before the procedure—however, no meaningful differences between the groups were observed in this regard. Although different individuals may metabolize alcohol differently, an advantage of the current approach was the use of the computerized pharmacokinetic model that assured equal blood alcohol concentrations during the electrophysiology assessments among those randomized to alcohol. Of note, these limitations would likely have reduced our power to detect differences, but it appears unlikely any them would have led to false positive results. While insufficient power may explain the lack of association between alcohol and conduction time, there was no evidence of any signal favoring such an association observed. The type of AF induction may have also had opposing effects in the presence or absence of alcohol, such as some evidence that simulated pulmonary vein premature atrial contractions (particularly the right upper pulmonary vein) may more readily induce AF in the setting of alcohol whereas the presence of isoproterenol (or enhanced sympathetic tone) may reduce the likelihood of AF in the setting of alcohol. The targeted blood alcohol concentration of 0.08%, generally requiring 4–6 standard drinks and itself considered evidence of binge drinking (31), represented a level to optimally strike the balance between a sufficient amount of alcohol versus the ability to obtain Institutional Review Board approval, assure participant safety, and achieve patient acceptability to enable recruitment. It is important to note that while binge drinking has been anecdotally associated with discrete episodes of AF, the precise amounts necessary to trigger AF remain unknown. It is possible that higher concentrations of alcohol may have yielded different results. As shown in our Consort diagram, many patients declined participation for various reasons, often because of anxiety about the procedure itself or about study participation, and it remains possible that those individuals may have exhibited different alcohol-related effects. Although we were unable to detect statistically significant interactions by biologic sex, we cannot exclude the possibility that insufficient power precluded observing truly different effects among men versus women. Indeed, alcohol likely exerts heterogeneous effects on different individuals, fitting with the observation that many have AF in the absence of alcohol and potentially further reducing our power to detect uniformly manifest relationships.
CONCLUSIONS
Random, double-blind assignment to 0.08% blood alcohol concentration was associated with a greater likelihood of a reduced atrial effective refractory period, with evidence that the pulmonary veins exhibited the greatest magnitude of that effect. We could detect no statistically significant changes in conduction times or overall acute occurrence of AF between the two groups. These observations suggest that alcohol has direct and acute electrophysiologic effects on human in vivo atria that would be expected to render them more prone to AF, substantiating evidence that alcohol avoidance or cessation may directly influence the risk of a discrete AF episode.
Supplementary Material
CLINICAL PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
Alcohol acutely changes cardiac electrophysiology in a fashion that renders the atria more prone to fibrillate.
COMPETENCY IN PATIENT CARE:
Patients consuming alcohol should be made aware that such an exposure can have immediate effects on the heart that increase the risk of arrhythmias.
TRANSLATIONAL OUTLOOK:
Identifying the molecular mechanisms underlying the relationship between alcohol and a shortening in atrial effective refractoriness may identify novel targets for atrial fibrillation prevention and treatment.
Funding:
This study was funded by R01AA022222 (to Dr. Marcus) from the NIAAA.
Disclosures:
Dr. Marcus has received research support from the NIH, PCORI, Medtronic, Eight, Jawbone, and Baylis and is a consultant and holds equity interest in InCarda.
ABBREVIATIONS
- AERP
atrial effective refractory period
- AF
atrial fibrillation
- CS
coronary sinus
- IQR
interquartile range
- LMM
linear mixed model
- PAC
premature atrial contraction
Footnotes
Trial Registration: ClinicalTrials.gov number, NCT01996943; The HOLIDAY (HOw ALcohol InDuces Atrial TachYarrhythmias) Study (HOLIDAY); https://clinicaltrials.gov/ct2/show/NCT01996943
None of the other authors have any potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Substance Abuse and Mental Health Services Administration, Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series, H-41, HHS Publication No (SMA) 11–4658. Rockville, MD: Substance Abuse and Mental Health Services Administration., 2011. [Google Scholar]
- 2.Benjamin EJ, Blaha MJ, Chiuve SE et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kodama S, Saito K, Tanaka S et al. Alcohol consumption and risk of atrial fibrillation: a meta-analysis. J Am Coll Cardiol 2011;57:427–436. [DOI] [PubMed] [Google Scholar]
- 4.Voskoboinik A, Kalman JM, De Silva A et al. Alcohol Abstinence in Drinkers with Atrial Fibrillation. N Engl J Med 2020;382:20–28. [DOI] [PubMed] [Google Scholar]
- 5.Groh CA, Faulkner M, Getabecha S et al. Patient-reported triggers of paroxysmal atrial fibrillation. Heart Rhythm 2019. [DOI] [PubMed] [Google Scholar]
- 6.Mandyam MC, Vedantham V, Scheinman MM et al. Alcohol and vagal tone as triggers for paroxysmal atrial fibrillation. Am J Cardiol 2012;110:364–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang H, Ruan H, Rahmutula D et al. Effect of acute and chronic ethanol on atrial fibrillation vulnerability in rats. Heart Rhythm 2019. [DOI] [PubMed] [Google Scholar]
- 8.Goodkind MJ, Gerber NH Jr., Mellen JR, Kostis JB. Altered intracardiac conduction after acute administration of ethanol in the dog. J Pharmacol Exp Ther 1975;194:633–8. [PubMed] [Google Scholar]
- 9.Fisher VJ, Kavaler F. The action of ethanol upon the action potential and contraction of ventricular muscle. Recent Adv Stud Cardiac Struct Metab 1975;5:415–22. [PubMed] [Google Scholar]
- 10.Moe GK, Rheinboldt WC, Abildskov JA. A Computer Model of Atrial Fibrillation. Am Heart J 1964;67:200–20. [DOI] [PubMed] [Google Scholar]
- 11.Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res 2002;54:230–46. [DOI] [PubMed] [Google Scholar]
- 12.Engel TR, Luck JC. Effect of whiskey on atrial vulnerability and “holiday heart”. J Am Coll Cardiol 1983;1:816–818. [DOI] [PubMed] [Google Scholar]
- 13.Greenspon AJ, Schaal SF. The “holiday heart”: electrophysiologic studies of alcohol effects in alcoholics. Ann Intern Med 1983;98:135–139. [DOI] [PubMed] [Google Scholar]
- 14.Gould L, Reddy CV, Becker W, Oh KC, Kim SG. Electrophysiologic properties of alcohol in man. J Electrocardiol 1978;11:219–226. [DOI] [PubMed] [Google Scholar]
- 15.Calkins H, Hindricks G, Cappato R et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017;14:e275–e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haissaguerre M, Jais P, Shah DC et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659–66. [DOI] [PubMed] [Google Scholar]
- 17.Ramchandani VA, Bolane J, Li TK, O’Connor S. A physiologically-based pharmacokinetic (PBPK) model for alcohol facilitates rapid BrAC clamping. Alcohol Clin Exp Res 1999;23:617–623. [PubMed] [Google Scholar]
- 18.Ramchandani VA, Plawecki M, Li T-K, O’Connor S. Intravenous ethanol infusions can mimic the time course of breath alcohol concentrations following oral alcohol administration in healthy volunteers. Alcohol Clin Exp Res 2009;33:938–944. [DOI] [PubMed] [Google Scholar]
- 19.Gallagher C, Hendriks JML, Elliott AD et al. Alcohol and incident atrial fibrillation - A systematic review and meta-analysis. Int J Cardiol 2017;246:46–52. [DOI] [PubMed] [Google Scholar]
- 20.January CT, Wann LS, Calkins H et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019;140:e125–e151. [DOI] [PubMed] [Google Scholar]
- 21.Whitman IR, Agarwal V, Nah G et al. Alcohol Abuse and Cardiac Disease. J Am Coll Cardiol 2017;69:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dukes JW, Dewland TA, Vittinghoff E et al. Access to alcohol and heart disease among patients in hospital: observational cohort study using differences in alcohol sales laws. BMJ 2016;353:i2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dixit S, Alonso A, Vittinghoff E, Soliman EZ, Chen LY, Marcus GM. Past alcohol consumption and incident atrial fibrillation: The Atherosclerosis Risk in Communities (ARIC) Study. PLoS One 2017;12:e0185228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McManus DD, Yin X, Gladstone R et al. Alcohol Consumption, Left Atrial Diameter, and Atrial Fibrillation. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiao Y, Shi R, Hou B et al. Impact of Alcohol Consumption on Substrate Remodeling and Ablation Outcome of Paroxysmal Atrial Fibrillation. J Am Heart Assoc 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanfilippo AJ, Abascal VM, Sheehan M et al. Atrial enlargement as a consequence of atrial fibrillation. A prospective echocardiographic study. Circulation 1990;82:792–7. [DOI] [PubMed] [Google Scholar]
- 27.Wijffels MCEF, Kirchhof CJHJ, Dorland R, Allessie MA. Atrial Fibrillation Begets Atrial Fibrillation : A Study in Awake Chronically Instrumented Goats. Circulation 1995;92:1954–1968. [DOI] [PubMed] [Google Scholar]
- 28.Krummen DE, Swarup V, Narayan SM. The role of rotors in atrial fibrillation. J Thorac Dis 2015;7:142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verheule S, Wilson EE, Arora R, Engle SK, Scott LR, Olgin JE. Tissue structure and connexin expression of canine pulmonary veins. Cardiovasc Res 2002;55:727–38. [DOI] [PubMed] [Google Scholar]
- 30.Stavrakis S, Nakagawa H, Po SS, Scherlag BJ, Lazzara R, Jackman WM. The role of the autonomic ganglia in atrial fibrillation. JACC Clin Electrophysiol 2015;1:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fillmore MT, Jude R. Defining “binge” drinking as five drinks per occasion or drinking to a .08% BAC: which is more sensitive to risk? Am J Addict 2011;20:468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.