Abstract
MBQ-167 is a novel, small-molecule dual inhibitor of Rac and Cdc42, small GTPases that are involved in cytoskeletal organization, cell cycle progression, and cell migration. In an in vivo mouse model, MBQ-167 has been shown to significantly reduce mammary tumor growth and metastasis and is currently undergoing preclinical studies for the treatment of metastatic cancer. To date, no solubility data have been reported for this compound. For this reason, the present study aims to determine the solubility of this compound in eight neat solvents (acetonitrile, 1-butanol, 2-butanol, ethanol, ethyl acetate, methanol, 1-propanol, and 2-propanol) and two binary solvent mixtures [ethyl acetate (2) + heptane (3) and ethanol (2) + water (3)] between the temperatures of 278.15 and 333.15 K. The results obtained employing the polythermal method show that the solubility of MBQ-167 increases with an increase in temperature in all neat solvents used within this study. Moreover, in the two binary solvent mixtures, the solubility of this compound increases with increasing temperature and decreases with an increasing mass fraction of the antisolvent (heptane or water). The experimental solubility data were correlated using the modified Apelblat and λh model equations. The predicted solubility data acquired from the Apelblat and λh model equations correlate well with the experimental solubility data as indicated by the low ARD % (≤1.8304 and ≤6.5366, respectively). No solvent-mediated polymorphic phase transitions were observed while performing the solubility studies, and no other solid forms were detected after the recrystallization in the solvents and solvent mixtures. The solubility data determined here can offer pathways to develop pharmaceutical crystallization processes that can further the translation of MBQ-167 into a clinical setting.
Graphical Abstract

INTRODUCTION
Rac and Cdc42 are members of the class of Rho GTPases, small regulatory proteins that have key roles in cytoskeletal organization, cell cycle progression, and cell migration.1 Aberrant activity of these proteins is linked to various types of human cancers (e.g., breast, prostate, uterine carcinomas, bladder, and sarcomas) and to the progression of cancer metastasis.1 Therefore, both Rac and Cdc42 represent a possible target for development of new cancer treatments. The compound 9-ethyl-3-(5-phenyl-1H-1,2,3-triazol-3-yl)-9H-carbazole (C22H18N4, Figure 1a), better known as MBQ-167,1,2 was developed in a quest for more potent Rac inhibitors.3 MBQ-167 has been shown to be a 10x more potent Rac inhibitor than N4-(9-ethyl-9H-carbazol-3-yl)-N2-(3-morpholin-4-yl-propyl)-pyrimidine-2,4-diamine (C25H30N6O, EHop-016, Figure 1b).1,2 Besides its activity as a Rac inhibitor, MBQ-167 also inhibits Cdc42 equipotently at ~100 nM and is the most potent dual Rac/Cdc42 inhibitor described in the literature so far.1,2 MBQ-167 has been shown to reduce mammary tumor growth and metastasis by ~90% in an in vivo tumor model in female athymic nude mice, demonstrating its potential to be developed as a novel therapeutic agent for the treatment of metastatic cancer.1,2
Figure 1.

Molecular structures of (a) 9-ethyl-3-(5-phenyl-1H-1,2,3-triazol-3-yl)-9H-carbazole (MBQ-167) and (b) N4-(9-ethyl-9H-carbazol-3-yl)-N2-(3-morpholin-4-yl-propyl)-pyrimidine-2,4-diamine (EHop-016), both inhibitors of Rac and Cdc42 proteins.
To date, no solubility data have been reported for this new preclinical entity. Thus, the present study focuses on the measurement and correlation of the solubility data for MBQ-167 to understand this physicochemical property. The solubility measurements were performed by employing the polythermal method4–10 in eight pure solvents (acetonitrile, 1-butanol, 2-butanol, ethanol, ethyl acetate, methanol, 1-propanol, and 2-propanol) and two binary mixtures [ethyl acetate (2) + heptane (3) and ethanol (2) + water (3)] in a temperature range between 278.15 and 333.15 K. The majority of the solvents selected are classified by the Food and Drug Administration (FDA) as Class 3 solvents (less toxic and lower risk to human health) with the exception of acetonitrile and methanol, which are Class 2 solvents.11 The Apelblat and λh model equations were used to correlate the experimental solubility data obtained. These correlations allow interpolation and extrapolation of the measured solubility data for MBQ-167 within and beyond the temperatures studied. The solubility data determined in this study can offer pathways to develop pharmaceutical crystallization processes that can further the translation of MBQ-167 into a clinical setting.
EXPERIMENTAL SECTION
Materials.
The CAS number, supplier, purity (provided by the chemical supplier), and analytical method for the solute and solvents employed in this study are shown in Table 1. In the case of solvents, the solvent classification11 is also included in Table 1. A water purification system Aries Filter (Gemini) was utilized to obtain nanopurified water (18.23 MOhm/cm, pH = 5.98, and mV = 57.3). MBQ-167 (batch no. MQ7-PD-025–3) was received from MBQ Pharma, Inc. All materials were used “as received” without further purification.
Table 1.
Supplier and Mass Fraction Purity of the Solvents and the Solute Employed in This Solubility Study with Their Corresponding Analysis Method
| chemical name | CAS registry number | supplier | percentage purity (%)a | purification method | analysis method | solvent classification13 |
|---|---|---|---|---|---|---|
| MBQ-167 | 2097938-73-1 | MBQ Pharma | 99.5 | none | HPLCb | |
| acetonitrile | 75-05-8 | Fisher Scientific | 99.9 | none | LC-MSc | class 2 |
| 1-butanol | 71-36-3 | Sigma-Aldrich | ≥99.5 | none | GCd | class 3 |
| 2-butanol | 78-92-2 | Beantown Chemical | 99 | none | GCd | class 3 |
| ethanol (200 proof) | 64-17-5 | Pharmco Aaper | ≥99.9 | none | GCd | class 3 |
| ethyl acetate | 141-78-6 | Fisher Scientific | 99.5 | none | HPLCb | class 3 |
| heptane | 142-82-5 | VWR | 99.9 | none | GCd | class 3 |
| methanol | 67-56-1 | VWR | 99.8 | none | GCd | class 2 |
| 1-propanol | 71-23-8 | Alfa Aesar | 99.5 | none | GCd | class 3 |
| 2-propanol | 67-63-0 | VWR | 99.5 | none | GCd | class 3 |
Provided by the supplier in mass fraction.
High-performance liquid chromatography.
Liquid chromatography–mass spectrometry.
Gas chromatography.
Solubility Measurements.
Isothermal12,13 and polythermal4–7 methods are commonly used to measure the solubility of compounds. While the former involves the determination of the solubility at known (isothermal) temperatures, the latter employs known concentrations to determine the temperatures at which solubility is achieved at a constant heating rate.6,7 In this study, the polythermal method was used to determine the solubility of MBQ-167 (1) in eight neat solvents (acetonitrile, 1-butanol, 2-butanol, ethanol, ethyl acetate, methanol, 1-propanol, and 2-propanol) and two binary solvent mixtures [ethyl acetate (2) + heptane (3) and ethanol (2) + water (3)]. An automated multiple reactor system (Crystal16 from Technobis Crystallization Systems) was employed for this study.4–10 The suspensions were prepared in 2 mL sealed glass vials (Fisher Scientific) at predetermined concentrations. A microbalance (XP26, ±0.002 mg) and an analytical balance (MS104S, ±0.1 mg), both from Mettler Toledo, were used to weigh the solute and solvents, respectively. The resulting suspensions were agitated at 700 rpm using rare earth magnetic stir bars and heated from 278.15 to 333.15 K at 0.1 K/min. Assuming that dissolution kinetics can be neglected,9 the clear point (temperature at which the solution is free of crystals and thus saturated) was determined by monitoring the transmission of the light source through the suspensions using CrystalClear software (version 1.0.1.614). The uncertainty of the saturation temperature measurements is within ±0.1 K. The mole fraction solubility (xi) was calculated by utilizing eq 1
| (1) |
where mi represents the mass (g) and Mi the molecular weight (g/mol) of component i; in this case, this refers to MBQ-167 (MW = 338.41 g/mol),2,14 the solvents, or the solvent mixtures.
Powder X-ray Diffraction (PXRD).
Powder diffractograms were collected at 300 K using a Rigaku XtaLAB SuperNova single microfocus Cu Kα radiation (λ = 1.5417 Å, 50 kV, and 1 mA) source equipped with a HyPix3000 X-ray detector in transmission mode. All microcrystalline samples were collected over an angular 2θ range of 6–60° with a step size of 0.01° using the Gandolfi move experiment for powder. The exposure time utilized was 120 s. Before the solubility experiments were performed, the solid form of MBQ-167 was determined (Supporting Information). Once the solubility experiments were concluded, the resulting suspensions were analyzed by PXRD to confirm the solid state of the yielded material (Supporting Information).
Differential Scanning Calorimetry (DSC).
Thermograms were recorded using a DSC from TA Instruments, Inc. (DSC Q2000) equipped with an RCS40 single-stage refrigeration system to determine the onset melting temperature (Tm, onset) and enthalpy of fusion (ΔHfus). Approximately 2.600 mg of microcrystalline MBQ-167 was weighed using an XP26 microbalance from Mettler Toledo (±0.002 mg) and placed on Tzero aluminum pans that were hermetically sealed. Samples were equilibrated at 298.15 K for 5 min before heating to 523.15 K under a N2 atmosphere (50 mL/min) at a rate of 5.0 K/min and a temperature accuracy of 0.1 K. The resulting thermograms were analyzed using TA Universal Analysis 2000 software (version 4.5A). The measurements were performed in duplicates, and the average of the onset melting temperature (Tm, onset) was taken to ensure accuracy (Supporting Information).
Thermogravimetric Analysis (TGA).
TGA thermographs were recorded in a TGA from TA Instruments, Inc. (TGA Q500), calibrated with a calcium oxalate monohydrate (CaC2O4·H2O) standard, to determine if MBQ-167 degrades before melting. About 1.400 mg of the microcrystalline sample were equilibrated at 298.15 K for 5 min before heating to 673.15 K under a N2 atmosphere (60 mL/min) at a rate of 5.0 K/min and a temperature accuracy of 0.1 K. Samples were measured in duplicate, and data was analyzed with TA Universal Analysis 2000 software version 4.5A (Supporting Information).
THERMODYNAMIC MODELS
Thermodynamic models and empirical correlations are approaches that allow extrapolation and interpolation of the solubility data within and beyond the temperatures studied.7,15 The modified Apelblat and λh model equations are frequently used to interpret the solubility behavior of solutes,15,16 in this case MBQ-167.
Modified Apelblat Equation.
The semiempirical modified Apelblat equation (eq 2) allows to correlate the solubility data as a function of temperature and is expressed as7,15–17
| (2) |
where x1 represents the mole fraction solubility of MBQ-167, T is the absolute temperature in Kelvin (K), and A, B, and C are empirical model parameters. The values in parameters A and B represent the variation in the solution activity coefficient, while C represents the effect of the temperature on the enthalpy of fusion.16,18
λh Model Equation.
The λh model equation (eq 3) is also commonly employed to correlate the change in solubility of numerous solutes with temperature.5–7,15,17,19 The λh model equation employed in this study is expressed as
| (3) |
where x1 represents the mole fraction solubility of MBQ-167, T and Tm are the absolute and melting temperature of MBQ-167 in Kelvin (K), and λ and h are parameters that model the nonideal properties of the solution and the excess mixture enthalpy of solution, respectively.
To correlate the modified Apelblat and λh model equations, Origin (OriginLab Corporation, version B95.0.193) was used, and the Levenberg−Marquardt algorithm was applied to solve the nonlinear curve-fitting problem. The relative deviation (RD) and the percent average relative deviation (ARD %) were calculated using eqs 4 and 5, respectively, to estimate the difference between the experimental and calculated solubility data
| (4) |
| (5) |
where and are the ith experimental and calculated mole fraction solubility, respectively. The value of N represents the total number of experiments.
RESULTS AND DISCUSSION
Thermal Analysis of MBQ-167.
Thermodynamic data for MBQ-167 have not been reported previously. In this study, the ΔHfus and Tm, onset of MBQ-167 were determined using DSC. The average endotherm Tm, onset in the DSC thermograph was 425.63 ± 0.02 K, while the average area under the curve (ΔHfus) was 30.1 ± 0.1 kJ/mol. A summary of these results and a representative DSC thermograph can be found in the Supporting Information. The degradation of MBQ-167 was determined using TGA. The TGA thermogram shows that MBQ-167 begins to decompose at ~444 K and ends at ~561 K with a mass loss of ~98.4% (Supporting Information). Thus, TGA reveals that MBQ-167 starts to decompose after the melting temperature (425.63 K), which concludes that the average Tm, onset could be reliably employed to calculate using the λh model equation.
Solubility Data.
The experimentally measured solubility data and RD between the experimental and correlated solubility in the neat and binary solvent mixtures are shown in Tables 2–4. The mole fraction solubility of MBQ-167 (1) in the eight neat solvents (acetonitrile, 1-butanol, 2-butanol, ethanol, ethyl acetate, methanol, 1-propanol, and 2-propanol) is listed in Table 2, while data for the ethyl acetate (2) + heptane (3) and ethanol (2) + water (3) binary solvent mixtures are shown in Tables 3 and 4, respectively.
Table 2.
Experimental and Correlated Mole Fraction Solubility of MBQ-167 (x1) in Neat Solvents at Different Temperatures T and at Pressure p = 101.3 kPaa
| Apelblat | λh | Apelblat | λh | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T/K | 103 x1exp | 103 x1cal | 102 RD | 103 x1cal | 102 RD | T/K | 103 x1exp | 103 x1cal | 102 RD | 103 x1cal 1 | 102 RD |
| acetonitrile | 1-butanol | ||||||||||
| 281.7 | 1.88 | 2.00 | −6.40 | 1.58 | 15.73 | 280.1 | 0.585 | 0.588 | −0.50 | 0.500 | 14.52 |
| 287.8 | 2.49 | 2.45 | 1.57 | 2.18 | 12.23 | 283.7 | 0.684 | 0.697 | −1.91 | 0.618 | 9.68 |
| 295.4 | 3.35 | 3.24 | 3.26 | 3.20 | 4.55 | 288.6 | 0.848 | 0.879 | −3.70 | 0.817 | 3.65 |
| 304.8 | 4.85 | 4.77 | 1.55 | 5.00 | −3.15 | 292.7 | 1.11 | 1.07 | 3.77 | 1.03 | 7.73 |
| 312.3 | 6.60 | 6.69 | −1.37 | 7.03 | −6.49 | 298.2 | 1.38 | 1.39 | −1.07 | 1.38 | −0.07 |
| 319.4 | 9.33 | 9.39 | −0.65 | 9.57 | −2.55 | 307.4 | 2.21 | 2.16 | 2.05 | 2.21 | −0.03 |
| 325.0 | 12.48 | 12.43 | 0.39 | 12.11 | 2.94 | 312.6 | 2.76 | 2.77 | −0.32 | 2.85 | −3.03 |
| 320.4 | 4.01 | 4.03 | −0.69 | 4.12 | −2.87 | ||||||
| 331.5 | 6.88 | 6.87 | 0.11 | 6.80 | 1.11 | ||||||
| 2-butanol | ethanol | ||||||||||
| 279.9 | 0.287 | 0.296 | −3.23 | 0.253 | 11.91 | 279.2 | 0.406 | 0.419 | −3.17 | 0.320 | 21.07 |
| 283.2 | 0.366 | 0.362 | 1.29 | 0.320 | 12.69 | 281.5 | 0.428 | 0.454 | −5.99 | 0.365 | 14.81 |
| 291.7 | 0.592 | 0.603 | −1.85 | 0.570 | 3.74 | 285.8 | 0.529 | 0.530 | −0.27 | 0.462 | 12.73 |
| 294.6 | 0.730 | 0.716 | 1.86 | 0.689 | 5.61 | 288.8 | 0.598 | 0.594 | 0.58 | 0.542 | 9.33 |
| 298.4 | 0.892 | 0.897 | −0.61 | 0.879 | 1.48 | 291.9 | 0.685 | 0.671 | 2.02 | 0.638 | 6.85 |
| 306.7 | 1.49 | 1.46 | 2.02 | 1.47 | 1.47 | 297.2 | 0.847 | 0.834 | 1.58 | 0.837 | 1.21 |
| 315.7 | 2.40 | 2.44 | −1.97 | 2.48 | −3.38 | 307.8 | 1.36 | 1.33 | 2.58 | 1.40 | −2.81 |
| 319.1 | 2.95 | 2.96 | −0.58 | 3.00 | −1.78 | 313.0 | 1.70 | 1.69 | 0.75 | 1.79 | −4.77 |
| 321.3 | 3.40 | 3.36 | 1.32 | 3.39 | 0.39 | 318.9 | 2.22 | 2.25 | −1.10 | 2.33 | −4.87 |
| 329.5 | 5.30 | 5.31 | −0.09 | 5.26 | 0.73 | 323.2 | 2.74 | 2.78 | −1.61 | 2.82 | −2.94 |
| 329.0 | 3.76 | 3.74 | 0.74 | 3.62 | 3.88 | ||||||
| ethyl acetate | methanol | ||||||||||
| 278.9 | 4.03 | 4.11 | −2.01 | 3.62 | 10.05 | 279.3 | 0.409 | 0.437 | −6.82 | 0.341 | 16.70 |
| 282.5 | 4.38 | 4.50 | −2.78 | 4.13 | 5.57 | 281.9 | 0.468 | 0.471 | −0.61 | 0.389 | 16.80 |
| 288.6 | 5.31 | 5.30 | 0.22 | 5.14 | 3.21 | 288.2 | 0.591 | 0.575 | 2.60 | 0.533 | 9.84 |
| 293.8 | 6.20 | 6.14 | 1.10 | 6.15 | 0.90 | 300.7 | 0.949 | 0.915 | 3.59 | 0.958 | −0.99 |
| 296.6 | 6.81 | 6.66 | 2.27 | 6.76 | 0.82 | 306.1 | 1.16 | 1.15 | 1.45 | 1.22 | −4.90 |
| 301.9 | 7.93 | 7.82 | 1.41 | 8.06 | −1.59 | 314.8 | 1.65 | 1.69 | −2.28 | 1.77 | −7.08 |
| 306.3 | 9.03 | 8.97 | 0.60 | 9.29 | −2.91 | 320.7 | 2.22 | 2.24 | −0.86 | 2.26 | −1.71 |
| 310.5 | 10.28 | 10.27 | 0.11 | 10.61 | −3.23 | 325.0 | 2.79 | 2.77 | 0.78 | 2.69 | 3.81 |
| 318.0 | 12.93 | 13.19 | −1.96 | 13.40 | −3.62 | ||||||
| 322.8 | 15.37 | 15.55 | −1.16 | 15.50 | −0.86 | ||||||
| 326.9 | 18.18 | 17.95 | 1.23 | 17.53 | 3.53 | ||||||
| 1-propanol | 2-propanol | ||||||||||
| 283.0 | 0.556 | 0.609 | −9.54 | 0.479 | 13.84 | 279.4 | 0.218 | 0.225 | −3.00 | 0.158 | 27.51 |
| 285.1 | 0.670 | 0.661 | 1.32 | 0.542 | 19.17 | 290.4 | 0.352 | 0.381 | −8.26 | 0.331 | 6.09 |
| 288.8 | 0.774 | 0.768 | 0.71 | 0.670 | 13.38 | 294.2 | 0.467 | 0.461 | 1.45 | 0.421 | 9.78 |
| 297.1 | 1.11 | 1.10 | 1.20 | 1.06 | 4.32 | 298.4 | 0.571 | 0.571 | −0.05 | 0.547 | 4.22 |
| 307.0 | 1.77 | 1.72 | 2.61 | 1.78 | −0.63 | 300.9 | 0.684 | 0.650 | 4.96 | 0.637 | 6.91 |
| 312.2 | 2.23 | 2.21 | 0.74 | 2.31 | −3.68 | 304.6 | 0.794 | 0.790 | 0.45 | 0.794 | 0.02 |
| 319.4 | 3.14 | 3.16 | −0.55 | 3.27 | −4.15 | 307.7 | 0.913 | 0.933 | −2.20 | 0.951 | −4.19 |
| 325.2 | 4.18 | 4.24 | −1.49 | 4.28 | −2.55 | 311.0 | 1.13 | 1.12 | 1.28 | 1.15 | −1.68 |
| 330.2 | 5.54 | 5.50 | 0.69 | 5.38 | 2.90 | 319.5 | 1.81 | 1.78 | 1.55 | 1.84 | −1.44 |
| 324.1 | 2.26 | 2.31 | −2.32 | 2.35 | −3.95 | ||||||
| 330.2 | 3.29 | 3.28 | 0.54 | 3.22 | 2.23 | ||||||
Standard uncertainties u are u(T) = 2 K. Relative standard uncertainties, ur are ur(p) = 0.1, refers to the experimental mole fraction solubility. refers to the calculated solubility data using the modified Apelblat and λh model equations. RD represents the corresponding relative deviation.
Table 4.
Experimental and Correlated Mole Fraction Solubility of MBQ-167 (x1) in Ethanol (2) + Water (3) at Different Temperatures T and at Pressure p = 101.3 kPaa
| Apelblat | λh | Apelblat | λh | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T/K | 103 x1exp | 103 x1cal | 102 RD | 103 x1cal | 102 RD | T/K | 103 x1exp | 103 x1cal | 102 RD | 103 x1cal | 102 RD |
| w3 = 0.24 | w3 = 0.36 | ||||||||||
| 279.8 | 0.0552 | 0.0653 | −18.31 | 0.0410 | 25.72 | 281.4 | 0.0417 | 0.0453 | −8.67 | 0.0358 | 14.12 |
| 295.2 | 0.136 | 0.127 | 6.73 | 0.112 | 18.01 | 289.9 | 0.0672 | 0.0621 | 7.60 | 0.0566 | 15.83 |
| 313.1 | 0.322 | 0.310 | 3.71 | 0.318 | 1.12 | 304.7 | 0.113 | 0.115 | −1.52 | 0.118 | −4.93 |
| 320.8 | 0.451 | 0.468 | −3.93 | 0.482 | −7.04 | 314.8 | 0.181 | 0.181 | 0.23 | 0.189 | −4.34 |
| 326.6 | 0.650 | 0.646 | 0.57 | 0.653 | −0.44 | 325.6 | 0.302 | 0.303 | −0.39 | 0.304 | −0.62 |
| 331.1 | 0.836 | 0.834 | 0.24 | 0.820 | 1.85 | 328.0 | 0.342 | 0.342 | 0.28 | 0.337 | 1.65 |
| w3 = 0.46 | |||||||||||
| 283.9 | 0.0187 | 0.0177 | 5.57 | 0.0131 | 29.76 | ||||||
| 290.0 | 0.0245 | 0.0230 | 6.44 | 0.0192 | 21.63 | ||||||
| 300.5 | 0.0367 | 0.0371 | −1.17 | 0.0358 | 2.53 | ||||||
| 311.8 | 0.0597 | 0.0646 | −8.17 | 0.0668 | −11.87 | ||||||
| 323.4 | 0.121 | 0.118 | 2.66 | 0.122 | −0.69 | ||||||
| 331.0 | 0.180 | 0.177 | 1.74 | 0.177 | 1.71 | ||||||
| 333.0 | 0.194 | 0.197 | −1.72 | 0.195 | −0.47 | ||||||
Standard uncertainties u are u(T) = 2 K. Relative standard uncertainties, ur are ur(p) = 0.1, ur(x1) = 0.01, and ur(w3) = 0.0001. refers to the experimental mole fraction solubility. refers to the calculated solubility data using the modified Apelblat and λh model equations. RD represents the corresponding relative deviation. w3 is the mass fraction of water (3) in a binary ethanol (2) + water (3) mixture.
Table 3.
Experimental and Correlated Mole Fraction Solubility of MBQ-167 (x1) in Ethyl Acetate (2) + Heptane (3) at Different Temperatures T and at Pressure p = 101.3 kPaa
| Apelblat | λh | Apelblat | λh | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T/K | 103 x1exp | 103 x1cal | 102 RD | 103 x1cal | 102 RD | T/K | 103 x1exp | 103 x1cal | 102 RD | 103 x1cal 1 | 102 RD |
| w3 = 0.08 | w3 = 0.16 | ||||||||||
| 280.5 | 3.54 | 3.70 | −4.40 | 3.13 | 11.62 | 278.5 | 2.83 | 2.74 | 3.22 | 2.63 | 7.19 |
| 285.3 | 4.14 | 4.14 | 0.00 | 3.76 | 9.18 | 281.2 | 3.00 | 2.97 | 0.99 | 2.88 | 3.98 |
| 293.7 | 5.29 | 5.18 | 2.02 | 5.13 | 2.97 | 288.5 | 3.65 | 3.69 | −1.05 | 3.66 | −0.30 |
| 299.8 | 6.38 | 6.20 | 2.91 | 6.37 | 0.24 | 298.0 | 4.84 | 4.90 | −1.15 | 4.95 | −2.09 |
| 308.1 | 8.14 | 8.07 | 0.79 | 8.45 | −3.88 | 305.1 | 5.98 | 6.05 | −1.20 | 6.14 | −2.59 |
| 314.6 | 9.94 | 10.08 | −1.40 | 10.48 | −5.39 | 313.2 | 7.52 | 7.70 | −2.48 | 7.79 | −3.71 |
| 319.0 | 11.66 | 11.80 | −1.23 | 12.08 | −3.64 | 322.2 | 10.45 | 10.05 | 3.82 | 10.09 | 3.45 |
| 328.3 | 16.81 | 16.72 | 0.52 | 16.20 | 3.60 | 330.5 | 12.69 | 12.85 | −1.27 | 12.75 | −0.53 |
| w3 = 0.23 | w3 = 0.34 | ||||||||||
| 282.1 | 2.47 | 2.46 | 0.20 | 2.26 | 8.32 | 278.9 | 1.60 | 1.60 | 0.30 | 1.60 | 0.00 |
| 286.8 | 2.78 | 2.82 | −1.25 | 2.68 | 3.46 | 283.7 | 1.97 | 1.87 | 4.92 | 1.88 | 4.58 |
| 296.5 | 3.79 | 3.76 | 0.80 | 3.77 | 0.45 | 295.7 | 2.57 | 2.73 | −6.39 | 2.74 | −6.61 |
| 300.5 | 4.29 | 4.25 | 0.97 | 4.32 | −0.59 | 302.5 | 3.41 | 3.36 | 1.34 | 3.36 | 1.31 |
| 308.7 | 5.50 | 5.50 | −0.04 | 5.65 | −2.69 | 315.1 | 4.76 | 4.86 | −2.08 | 4.85 | −1.82 |
| 315.8 | 6.82 | 6.93 | −1.67 | 7.08 | −3.87 | 319.8 | 5.72 | 5.56 | 2.79 | 5.54 | 3.05 |
| 324.4 | 9.36 | 9.23 | 1.39 | 9.24 | 1.23 | 331.8 | 7.69 | 7.73 | −0.50 | 7.74 | −0.67 |
| 328.9 | 10.69 | 10.75 | −0.53 | 10.60 | 0.85 | ||||||
| w3 = 0.43 | |||||||||||
| 281.0 | 1.15 | 1.13 | 1.76 | 1.12 | 2.44 | ||||||
| 284.1 | 1.27 | 1.25 | 1.98 | 1.24 | 2.35 | ||||||
| 288.1 | 1.42 | 1.42 | −0.37 | 1.42 | −0.28 | ||||||
| 289.9 | 1.48 | 1.51 | −2.15 | 1.51 | −2.16 | ||||||
| 292.3 | 1.63 | 1.63 | 0.13 | 1.63 | 0.01 | ||||||
| 296.2 | 1.82 | 1.85 | −1.48 | 1.85 | −1.70 | ||||||
| 303.1 | 2.29 | 2.29 | −0.16 | 2.30 | −0.41 | ||||||
| 314.2 | 3.24 | 3.21 | 0.91 | 3.22 | 0.84 | ||||||
| 328.7 | 4.90 | 4.91 | −0.16 | 4.91 | −0.10 | ||||||
Standard uncertainties u are u(T) = 2 K. Relative standard uncertainties, ur, are ur(p) = 0.1, ur(x1) = 0.01, and ur(w3) = 0.0001. refers to the experimental mole fraction solubility. refers to the calculated solubility data using the modified Apelblat and λh model equations. RD represents the corresponding relative deviation. w3 is the mass fraction of heptane (3) in a binary ethyl acetate (2) + heptane (3) mixture.
The modified Apelblat and λh model equations provide direct correlation of the solubility of MBQ-167 in the neat and binary solvent mixtures. Origin was utilized to obtain the optimized values for the correlation parameters that characterize both equations. The correlation parameters and ARD % for both model equations in all neat and binary solvent mixtures are summarized in Table 5. The low ARD % values for the modified Apelblat (≤1.8304) and λh model (≤6.5366) equations demonstrate that the experimental solubility data correlate well with the solubility data calculated by these models. When comparing the two model equations, the Apelblat equation has generally the lower ARD % values. The exception is only one data point determined in ethyl acetate (2) + heptane (3) at w3 = 0.34, which shows a higher ARD % value (0.1471) when compared with the ARD % value (0.0229) of the λh model equation. Thus, it can be stated that the modified Apelblat model equation provides superior correlation results for the solubility of this compound in both the neat and binary solvent mixtures, compared to the λh model equation.
Table 5.
Optimized Values for the Correlation Parameters in the Modified Apelblat and λh Model Equations Used for the Correlation of the Mole Fraction Solubility of MBQ-167 (1) in All Neat and Binary Solvent Mixtures along with the Respective ARD %
| model | |||||||
|---|---|---|---|---|---|---|---|
| Apelblat | λh | ||||||
| solvent | A | B | C | ARD %a | λ | h | ARD %a |
| acetonitrile | −443.1194 | 16425.3698 | 67.1173 | 0.2361 | 0.25545 | 16592.5453 | 3.3217 |
| 1-butanol | −196.1966 | 4822.7228 | 30.4414 | 0.2507 | 0.14561 | 31937.1506 | 3.4100 |
| 2-butanol | −156.6310 | 2204.0637 | 24.9597 | 0.1832 | 0.24305 | 23103.7549 | 3.2872 |
| ethanol | −386.4335 | 13683.1852 | 58.5322 | 0.3538 | 0.0717 | 61290.6492 | 4.9541 |
| ethyl acetate | −255.2047 | 8870.7949 | 38.6984 | 0.0878 | 0.11254 | 24903.2163 | 1.0796 |
| methanol | −454.6354 | 16965.7247 | 68.5615 | 0.2679 | 0.04717 | 85040.3310 | 4.0599 |
| 1-propanol | −362.0488 | 12446.2146 | 55.0297 | 0.4779 | 0.12821 | 36844.6411 | 4.7347 |
| 2-propanol | −344.6907 | 11104.7369 | 52.6479 | 0.5068 | 0.12637 | 43024.7643 | 4.1343 |
| ethyl acetate (2) + heptane (3)b | |||||||
| w3 = 0.08 | −330.2920 | 12212.7433 | 49.8803 | 0.0989 | 0.11557 | 25840.0903 | 1.8384 |
| w3 = 0.16 | −114.1324 | 2591.4733 | 17.5737 | 0.1110 | 0.05911 | 42962.2196 | 0.6763 |
| w3 = 0.23 | −204.1964 | 6521.3163 | 31.0291 | 0.0150 | 0.06593 | 43165.6726 | 0.8950 |
| w3 = 0.34 | −46.1346 | −515.8387 | 7.3783 | 0.1471 | 0.03264 | 75828.3582 | 0.0229 |
| w3 = 0.43 | −61.3324 | 71.5542 | 9.6288 | 0.0513 | 0.02517 | 103636.549 | 0.1114 |
| ethanol (2) + water (3)c | |||||||
| w3 = 0.24 | −407.8217 | 14155.5350 | 61.6953 | 1.8304 | 0.02921 | 183599.167 | 6.5366 |
| w3 = 0.36 | −363.8653 | 12627.8448 | 54.7877 | 0.4128 | 0.00674 | 645411.633 | 3.6174 |
| w3 = 0.46 | −362.2844 | 12133.3526 | 54.6336 | 0.7647 | 0.00537 | 953914.659 | 6.0855 |
ARD % represents the corresponding percentage average relative deviation.
w3 is the mass fraction of heptane (3) in a binary ethyl acetate (2) + heptane (3) mixture.
w3 is the mass fraction of water (3) in a binary ethanol (2) + water (3) mixture.
Figures 2 and 3 illustrate the experimental and correlated solubility data of MBQ-167 in neat and binary solvent mixtures using the modified Apelblat equation. Figures showing the experimental and correlated solubility data employing the λh model equation can be found in the Supporting Information. It can be observed that the solubility of MBQ-167 increases with an increase in temperature in all neat solvents employed in this study. Figure 2 shows that the solubility of MBQ-167 in neat solvents decreases as a function of the solvent employed in the following order: ethyl acetate > acetonitrile > 1-butanol > 1-propanol > methanol > ethanol > 2-butanol > 2-propanol up to 297 K. Above 297 K, the order of solubility changes to ethyl acetate > acetonitrile > 1-butanol > 2-butanol > 1-propanol > ethanol > methanol > 2-propanol.
Figure 2.

Experimental and correlated solubility data of MBQ-167 in neat solvents. (a) green open circle, 1-propanol; light blue solid diamond, 2-butanol; red open square, ethanol; purple solid square, methanol; orange plus sign, 2-propanol; (b) dark blue open diamond, ethyl acetate; pink solid circle, acetonitrile; yellow open triangle, 1-butanol; –, calculated using the modified Apelblat equation. x1 represents the mole fraction solubility of MBQ-167 and T the temperature in Kelvin (K).
Figure 3.
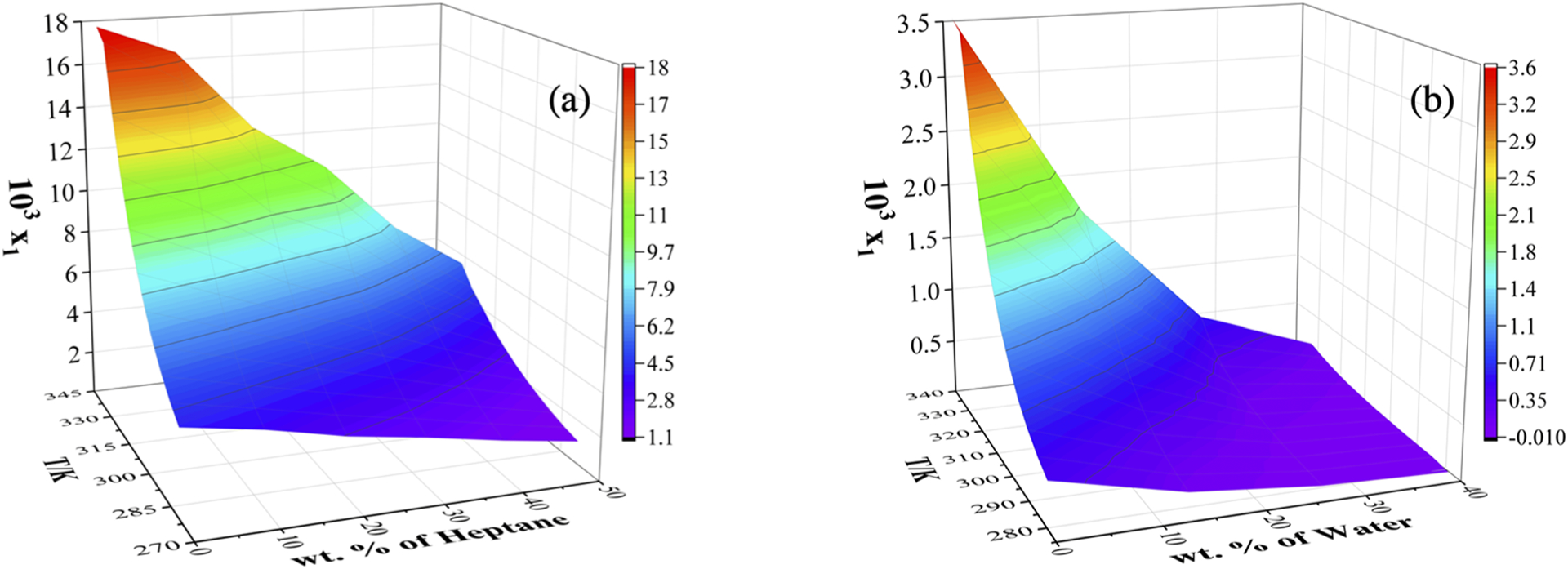
Surface plots for the solubility of MBQ-167 (1) in binary solvent systems: (a) ethyl acetate (2) + heptane (3) and (b) ethanol (2) + water (3) correlated with the modified Apelblat equation. x1 represents the mole fraction solubility of MBQ-167 and T the temperature in Kelvin (K).
MBQ-167 presents a relatively low solubility in both heptane and water (≤0.2 mg/mL) within the temperature range studied (278.15 to 333.15 K). In light of this finding, heptane and water were used as antisolvents in the determination of the solubility of MBQ-167 in binary solvent mixtures with ethyl acetate and ethanol, respectively. The experimental and correlated mole fraction solubility of MBQ-167 (1) calculated using the modified Apelblat equation in both of the binary solvent mixtures, ethyl acetate (2) + heptane (3) and ethanol (2) + water (3), is presented as surface plots in Figure 3. Additional surface plots depicting the experimental and correlated mole fraction solubility employing the λh model equation can be found in the Supporting Information. Figure 3 shows that in the two binary solvent mixtures, the solubility of this compound increases with increasing temperature. Moreover, the solubility of MBQ-167 decreases with an increasing mass fraction of the antisolvent (heptane or water) in the binary solvent mixtures.
PXRD Analysis.
“As received” MBQ-167 was analyzed by PXRD prior to the solubility measurements, and the solid state was confirmed as that of the only known form to this date for this compound (Supporting Information). Once the solubility experiments were completed, all materials recovered from the resulting suspensions were filtered and characterized by PXRD. PXRD analysis served to confirm that the recrystallized material resulted in that of the only known solid form of MBQ-167 for all neat and binary solvent mixtures (Supporting Information)
CONCLUSIONS
The results presented in this study provide accurate solubility data for MBQ-167 for the first time using the polythermal method between 278.15 and 333.15 K. The experimental solubility data was correlated using the modified Apelblat and λh model equations to provide a better understanding of the solubility of MBQ-167. The low ARD % values for the modified Apelblat (≤1.8304) and λh model (≤6.5366) equations demonstrate that the experimental solubility data correlates well with the solubility data calculated by these models. Once the experiments were completed, the resulting suspensions were analyzed by PXRD, and no other solid forms of MBQ-167 than that of the only known form were observed. The solubility data determined here can offer pathways to develop pharmaceutical crystallization processes that can further the translation of MBQ-167 into a clinical setting.
Supplementary Material
ACKNOWLEDGMENTS
The authors gratefully acknowledge the support of a member of the Crystallization Design Institute, Josmary Colón Vélez, for PXRD collection and analysis and MBQ-Pharma, Inc. for providing the compound studied along with constructive discussions that led to the development of this study.
FUNDING
This work was supported primarily by MBQ Pharma, Inc. and partially by the NIH-NIGMS award no. SC3GM116713 to C.P.V. A Rigaku XtaLAB SuperNova X-ray micro diffractometer was acquired through the support of the National Science Foundation under the Major Research Instrumentation Program (CHE-1626103).
NOMENCLATURE
- A, B, and C
empirical model parameters for the Apelblat equation
- ARD %
percentage average relative deviation
- DSC
differential scanning calorimetry
- PXRD
powder X-ray diffraction
- h
model parameter for the λh equation representing excess
- m
mass (g)
- M
molecular mass (g/mol)
- RD
relative deviation
- T
absolute temperature (K)
- T m
melting temperature (K)
- T m, onset
onset melting temperature (K)
- u
standard uncertainty
- u r
relative standard uncertainty
- w i
mass fraction of solvent mixture compositions
- x 1
mole fraction solubility
calculated mole fraction solubility
experimental mole fraction solubility
Greek Symbols
- λ
model parameter for the λh equation representing nonideal properties of the system
- ΔHfus
molar enthalpy of fusion (kJ/mol)
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jced.0c00908.
Detailed experimental procedure for the solubility curves of MBQ-167 in neat solvents and binary solvent mixtures, calculated surface plots of MBQ-167 in binary solvent mixtures using the λh model equation, PXRD, DSC, and TGA thermographs (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.jced.0c00908
The authors declare the following competing financial interest(s): Dr. Cornelis P. Vlaar is co-inventor on patents awarded to the University of Puerto Rico related to MBQ-167. In addition, he holds shares in MBQ Pharma, which has licensed these patents from the University of Puerto Rico. The other authors declare no competing financial interest.
Contributor Information
Jocelyn M. Jiménez Cruz, Crystallization Design Institute, Molecular Sciences Research Center, University of Puerto Rico, San Juan, Puerto Rico 00926, United States; Department of Pharmaceutical Sciences, University of Puerto Rico - Medical Sciences Campus, San Juan, Puerto Rico 00936, United States.
Cornelis P. Vlaar, Department of Pharmaceutical Sciences, University of Puerto Rico - Medical Sciences Campus, San Juan, Puerto Rico 00936, United States
Vilmalí López-Mejías, Crystallization Design Institute, Molecular Sciences Research Center, University of Puerto Rico, San Juan, Puerto Rico 00926, United States; Department of Chemistry, University of Puerto Rico - Río Piedras Campus, San Juan, Puerto Rico 00931, United States.
Torsten Stelzer, Crystallization Design Institute, Molecular Sciences Research Center, University of Puerto Rico, San Juan, Puerto Rico 00926, United States; Department of Pharmaceutical Sciences, University of Puerto Rico - Medical Sciences Campus, San Juan, Puerto Rico 00936, United States.
REFERENCES
- (1).del Mar Maldonado M; Dharmawardhane S Targeting Rac and Cdc42 GTPases in Cancer. Cancer Res. 2018, 78, 3101–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Humphries-Bickley T; Castillo-Pichardo L; Hernandez-O’Farrill E; Borrero-Garcia LD; Forestier-Roman I; Gerena Y; Blanco M; Rivera-Robles MJ; Rodriguez-Medina JR; Cubano LA; Vlaar CP; Dharmawardhane S Characterization of a Dual Rac/Cdc42 Inhibitor MBQ-167 in Metastatic Cancer. Mol. Cancer Ther 2017, 16, 805–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Dharmawardhane S; Hernandez E; Vlaar C Development of EHop-016. In Inhibitors of the Ras Superfamily G-proteins, Part A; Tamanoi FBT-TE, Ed.; Academic Press, 2013; Vol. 33, pp. 117–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Nývlt J Kinetics of Nucleation in Solutions. J. Cryst. Growth 1968, 3–4, 377–383. [Google Scholar]
- (5).Zorrilla-Veloz RI; Stelzer T; López-Mejías V Measurement and Correlation of the Solubility of 5-Fluorouracil in Pure and Binary Solvents. J. Chem. Eng. Data 2018, 63, 3809–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).George De la Rosa MV; Santiago R; Malavé Romero J;Duconge J; Monbaliu J-C; López-Mejías V; Stelzer T Solubility Determination and Correlation of Warfarin Sodium 2-Propanol Solvate in Pure, Binary, and Ternary Solvent Mixtures. J. Chem. Eng. Data 2019, 64, 1399–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Vázquez Marrero VR; Piñero Berríos C; De Dios Rodríguez L; Stelzer T; López-Mejías V In the Context of Polymorphism: Accurate Measurement, and Validation of Solubility Data. Cryst. Growth Des 2019, 19, 4101–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Reus MA; van der Heijden AEDM; ter Horst JH Solubility Determination from Clear Points upon Solvent Addition. Org. Process Res. Dev 2015, 19, 1004–1011. [Google Scholar]
- (9).Vellema J; Hunfeld NGM; Van Den Akker HEA; ter Horst JH Avoiding Crystallization of Lorazepam during Infusion. Eur. J. Pharm. Sci 2011, 44, 621–626. [DOI] [PubMed] [Google Scholar]
- (10).Kaemmerer H; Jones MJ; Lorenz H; Seidel-Morgenstern A Selective Crystallisation of a Chiral Compound-Forming System-Solvent Screening, SLE Determination and Process Design. Fluid Phase Equilib. 2010, 296, 192–205. [Google Scholar]
- (11).International Council for Harmonisation. Guidance for Industry Q3C; US Health & Human Services Department- Food and Drug Adminsitration, 2017; Food and Drug Administration: Vol. 9765, pp. 1–8. 2017. [Google Scholar]
- (12).Cabrera AL; Toledo AR; del Valle JM; de la Fuente JC Measuring and Validation for Isothermal Solubility Data of Solid 2-(3,4-Dimethoxyphenyl)-5,6,7,8-Tetramethoxychromen-4-One (Nobiletin) in Supercritical Carbon Dioxide. J. Chem. Thermodyn 2015, 91, 378–383. [Google Scholar]
- (13).Shiflett MB; Harmer MA; Junk CP; Yokozeki A Solubility and Diffusivity of 1,1,1,2-Tetrafluoroethane in Room-Temperature Ionic Liquids. Fluid Phase Equilib. 2006, 242, 220–232. [Google Scholar]
- (14).Vlaar CP; Dharmawardhane Flanagan S; Hernandez-O’Farrill E; Castillo-Pichardo L 1,5-Disubstituted 1,2,3-Triazoles Are Inhibitores of RAC/CDC42 GTPases. US 9,981,980 B2, 2018. [Google Scholar]
- (15).Pascual GK; Donnellan P; Glennon B; Kamaraju VK; Jones RC Experimental and Modeling Studies on the Solubility of 2-Chloro-N-(4-Methylphenyl)Propanamide (S1) in Binary Ethyl Acetate + Hexane, Toluene + Hexane, Acetone + Hexane, and Butanone + Hexane Solvent Mixtures Using Polythermal Method. J. Chem. Eng. Data 2017, 62, 3193–3205. [Google Scholar]
- (16).Wei T; Wang C; Du S; Wu S; Li J; Gong J Measurement and Correlation of the Solubility of Penicillin V Potassium in Ethanol + Water and 1-Butyl Alcohol + Water Systems. J. Chem. Eng. Data 2015, 60, 112–117. [Google Scholar]
- (17).Wang X; Qin Y; Zhang T; Tang W; Ma B; Gong J Measurement and Correlation of Solubility of Azithromycin Monohydrate in Five Pure Solvents. J. Chem. Eng. Data 2014, 59, 784–791. [Google Scholar]
- (18).Guo Y; Yin Q; Hao H; Zhang M; Bao Y; Hou B; Chen W; Zhang H; Cong W Measurement and Correlation of Solubility and Dissolution Thermodynamic Properties of Furan-2-Carboxylic Acid in Pure and Binary Solvents. J. Chem. Eng. Data 2014, 59, 1326–1333. [Google Scholar]
- (19).Buchowski H; Ksiazczak A; Pietrzyk S Solvent Activity along a Saturation Line and Solubility of Hydrogen-Bonding Solids. J. Phys. Chem 1980, 84, 975–979. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


