Abstract
The mechanisms responsible for common variable immunodeficiency syndrome (CVID) are as yet unknown. In the present study, we show that the B-cell dysfunction in a subset of CVID patients is caused by defective protein tyrosine phosphorylation (PTP). We demonstrated that the PTP level and immunoglobulin (Ig) secretion malfunctions can be successfully repaired when normal plasma membrane components are implanted into these patients’ B cells. Stimulation of CVID patients’ peripheral blood mononucleated cells with anti-Ig antibody revealed that 7 of 11 patients had lower PTP levels than those found in the normal donor cells. Plasma membrane implantation to the cells of these patients resulted in elevated PTP levels which reached normal levels upon stimulation with anti-human Ig antibody. The results revealed two distinct groups of CVID patients. The first group included patients whose B cells expressed low PTP levels after Ig stimulation. In these patients the plasma membrane implantation restored the normal PTP level as well as the ability to secrete IgM and/or IgG after B-cell stimulation. In the second group, patients whose B cells expressed a normal PTP level after Ig stimulation, with no restoration of their ability to secrete Ig upon plasma membrane implantation and lipopolysaccharide stimulation. We conclude that the first group has an early signal transduction defect located in the B-cell plasma membrane, while in the second group the defect is located elsewhere.
Common variable immunodeficiency syndrome (CVID) is a heterogeneous group of disorders characterized by hypogammaglobulinemia and recurrent bacterial infections, particularly involving the gut and the upper and lower respiratory tracts, which are a direct result of deficiency in antibody production (6, 26, 30).
Most CVID patients have normal numbers of mature B cells in the peripheral blood and lymphoid tissues. However, their B cells are unable to differentiate normally into immunoglobulin (Ig)-secreting plasma cells (26, 30). Thus far, the primary immunologic cause(s) responsible for this defect in B-cell differentiation is not known.
Initial studies on CVID syndrome found that peripheral blood mononuclear cells (PBMCs) from CVID patients were unable to secrete normal amounts of Igs when stimulated in vitro with the lectin pokeweed mitogen (5, 29). In a later study, patients with CVID were classified on the basis of the ability of their B cells to secrete IgM and/or IgG in response to interleukin-2 (IL-2) and anti-Ig antibody stimulation in vitro (4, 7, 10, 11). In addition to B-cell abnormalities, a variety of T-cell functional defects have been described in many patients. These functional defects consist of reduced proliferation rate and IL-2 secretion in response to various T-cell stimuli (24, 27, 28) and an excessive suppressor T-cell function (2, 26).
In the present study, we investigated whether the B-cell defect which may cause this syndrome is related to the membranal receptors or membranal enzymes which participate in signal transduction cascade. We show that the functional defects are caused by defective tyrosine phosphorylation in B-cell signal transduction of a subset of CVID patients. We used plasma membrane (PM) implantation, which provides the patients’ B cells with normal receptors and membranal enzymes (18), in an attempt to restore the protein tyrosine phosphorylation (PTP) level and to repair the Ig secretion malfunctions.
MATERIALS AND METHODS
Patients.
PBMCs were taken from 13 nonrelated CVID patients, eight adults (two males and six females; age range, 20 to 66 years) and five children (two males and three females; age range, 1.5 to 10 years), and 29 normal donors. The serum Ig levels in the adults were <219 mg of IgG, <30 mg of IgM, and <10 mg of IgA per dl and in the children were <50 mg of IgG, <15 mg of IgM, and <8 mg of IgA per dl. Nonetheless, upon counting and staining of their PBMCs with anti-IgM, -CD3, -CD4, -CD8, and -CD19 antibodies, all CVID patients were shown to have normal quantities and phenotypes of B and T cells. Numbers of PBMCs in adults ([1.3 to 2.1] × 106) and in children ([2.4 to 3.2] × 106) were as follows: Ig+ cells, 7 to 14%; CD19+ cells, 8 to 17%; CD3+ cells, 54 to 64%; CD4+ cells, 29 to 54%; CD8+ cells, 15 to 38% (lowest to highest values). All CVID patients had been treated routinely with intravenous injections of Igs.
Cell separation.
PBMCs were obtained from heparinized venous blood of CVID patients and age- and sex-matched normal donors. Each patient was bled three to four times at intervals of 0.5 to 1 year. The PBMCs were isolated by gradient centrifugation in Ficoll-Paque (21) and incubated in culture medium containing RPMI 1640 supplemented with 0.01 M HEPES, 0.1 M NaHCO3, 2 mM l-glutamine, 1 μg of kanamycin per ml, 100 U of penicillin per ml, 100 U of neomycin per ml, 100 U of streptomycin per ml, and 10% heat-inactivated fetal calf serum.
Measurements of PTP levels.
PBMCs (7.5 × 106) were cultured in 1 ml of RPMI 1640 in the presence of 20 μg of goat anti-human IgM f(ab′)2 antibody (Jackson Immunoresearch Laboratories ICN, West Grove, Pa.) for various times for up to 30 min at 37°C (maximal response was observed after 10 min). The cells were lysed by the addition of 1 ml of lysis buffer (20 mM Tris-HCl, pH 8; 137 mM NaCl; 10% glycerol; 2 mM EDTA; 1 mM NaVO4; 1% Triton X-100; 1 mM phenylmethylsulfonyl fluoride; 20 μM leupeptin; 0.15 U of aprotinin per ml) and incubated for 45 min at 4°C. Lysates were clarified by centrifugation at 6,000 rpm for 15 min, and the supernatants were collected. The cell lysates (60 μg/lane) were separated on 10% polyacrylamide gels and transferred to nitrocellulose membrane. Tyrosine phosphorylation was detected by immunoblotting with biotinylated antiphosphotyrosine monoclonal antibody (BioMaker; Kiriat Weizmann, Rehovot, Israel), horseradish peroxidase-conjugated streptavidin (Amersham International, Plc., Little Chalfont, Buckinghamshire, United Kingdom), and enzyme-linked chemiluminescence (ECL) (16). Levels of PTP were determined by densitometric analysis and are expressed as a densitometry unit (optical density times the area).
Ig secretion response.
Cells (0.5 × 106) were cultured in flat-bottom microtiter plates with 2 μg of LPS per ml for 7 days at 37°C in humidified 5% CO2. Cell culture supernatants were analyzed for IgM, IgG, and IgA content by enzyme-linked immunosorbent assay (ELISA) by using biotin-conjugated goat anti-human IgM, IgG, or IgA antibodies, streptavidin alkaline phosphatase (Amersham International Plc.), and phosphatase substrate (p-nitrophenylphosphate disodium) (Sigma Chemical Co., St. Louis, Mo.) (25). In order to present the Ig quantities in all experiments performed, we calculated the stimulation index in each experiment by dividing the quantity of Ig secreted in LPS-stimulated cells by that secreted in nonstimulated cells. The isotype levels in the nonstimulated cultures ranged from 150 to 250 ng of IgM, 50 to 150 ng of IgG, and 10 to 45 ng of IgA per ml and in the stimulated cultures were up to 1,000 ng of IgM, 500 ng of IgG, and 90 ng of IgA per ml.
PM implantation.
The implantation of the foreign PM was carried out by fusion of normal functional murine lymphocyte PM vesicles to the patients’ PBMCs by intact noninfectious Sendai virus (SV) (1, 17, 18, 22).
PM vesicles were prepared from murine spleen cells as previously described (1, 18). Briefly, murine spleen cells were suspended in hypotonic solution and were disrupted by use of a glass Teflon Potter homogenizer and a rotor-driven pestle. The disrupted cell suspension was centrifuged on 22% sucrose cushion for 60 min at 100,000 × g, and the interface suspension was collected and stored at −70°C.
Fusion of the PM vesicles to the PBMCs was carried out in a two-step procedure (1, 17, 22). First, 0.06 μg of SV was incubated with 4 μg of PM vesicles in a total volume of 100 μl of phosphate-buffered saline for 10 min in 4°C and then for 30 min in 37°C to allow fusion of the virus to the PM. Second, the PM-SV vesicles were fused to PBMCs under the same conditions as in the first step (18). Immediately after implantation, cells were stimulated, and Ig secretion and the PTP rate were measured as described above. The above quantities, as well as the ratio between viral units and PM, were optimal for maximal PM implantation per cell (18). Implantation of the SV moiety alone was shown before to have no effect on murine and human lymphocyte activation, proliferation, or Ig secretion (3, 23).
Surface staining of implanted normal cells with FITC-labeled anti-mouse Ig antibody.
Normal PBMCs were implanted with murine PM vesicles, stained with fluorescein isothiocyanate (FITC)-conjugated anti-mouse Ig antibody. The stained cells were analyzed by use of a flow cytometer apparatus (FACStar; Becton Dickinson, San Jose, Calif.).
Statistical analysis.
Analysis of variance was used to determine significance.
RESULTS
PTP level in CVID B cells after stimulation through B-cell receptor (BCR).
In an initial set of experiments, we tested the hypothesis that CVID syndrome is related to defects in the early signal transduction events.
We therefore examined the phosphotyrosine kinase function by measuring the PTP level in CVID and normal donor B cells after stimulation with anti-human Ig antibody. Since the kinetics in protein tyrosine phosphorylation were found to be the same in both CVIDs and normal controls, all of the results presented are at 10 min of stimulation, which was found to be the peak response time.
The Western blotting figure of two representative CVID patients and normal control PTP level studies shown in Fig. 1 were from one patient with low and one patient with normal PTP levels. The complete results of these studies in eleven patients are given in densitometry units in Table 1.
FIG. 1.
PTP levels of representative CVID PBMCs stimulated with anti-human IgM antibodies. PBMCs (7.5 × 106) of CVID patients (P) and normal controls (N) were stimulated with goat anti-human IgM f(ab′)2 antibodies (20 μg/ml) for 10 min and lysed in a lysis buffer. PTP levels of cell lysates were visualized by Western blot with antiphosphotyrosine antibodies and ECL.
TABLE 1.
PTP levels of CVID PBMCs stimulated with anti-human IgM antibodya
| Patient | Densitometry units
|
||
|---|---|---|---|
| P | N | P/N ratio | |
| P1 | 110,983 | 161,778 | 0.68 |
| P2 | 294,174 | 643,100 | 0.45 |
| P3 | 362,861 | 372,472 | 0.97 |
| P5 | 198,263 | 300,583 | 0.65 |
| P6 | 265,215 | 226,370 | 1.17 |
| P7 | 204,904 | 196,402 | 1.04 |
| P8 | 727,849 | 1,102,152 | 0.66 |
| P10 | 334,680 | 594,231 | 0.56 |
| P11 | 413,190 | 465,654 | 0.88 |
| P12 | 331,587 | 465,654 | 0.71 |
| P13 | 433,695 | 683,251 | 0.63 |
The results represent the sum of densitometric units of all the bands in each lane. Each row represents the results of one experiment carried out with the cells of one CVID patient (P) and one normal donor (N) after stimulation for 10 min with anti-human Ig antibodies. These data represent one of two repeated experiments. Patients with a P/N ratio of <0.74 were statistically found to be “PTP defective.”
The results demonstrate that B cells of 7 of 11 CVID patients express low PTP levels compared to normal (reduction of more than 30%) after stimulation through BCR, suggesting an early signal transduction defect in B cells in more than one-half of the patients. The maximum variation in the PTP levels of all the examined normal donors was lower than 13%. This value was obtained in an experiment in which the PTP levels were measured in PBMCs of 11 normal donors and were run simultaneously on the same gel. The mean ± the standard deviation (SD) of all densitometry units measurements was 1 ± 0.13. Therefore, measurements that exceeded two SD from the mean (>0.74) indicated a significant reduction in enzyme activity.
PTP levels in implanted CVID B cells after stimulation through BCR.
We next tested the possibility that the signal transduction defect in CVID B cells is related to membranal receptors or enzymes which participate in the signal transduction cascade. This was tested by implanting normal murine PM in CVID PBMCs.
First, we confirmed the success of PM implantation by demonstrating the existence of the foreign murine PM in the normal implanted cell membranes. As shown in Fig. 2, all of the implanted cells contained the murine PM in their cell membrane. The PBMCs of five CVID patients whose B cells had a low PTP level after stimulation through BCR, as well as of normal donors, were implanted with normal murine PM. After verification of implantation in all PBMCs, the implanted cells were stimulated with anti-human Ig antibodies, and their PTP levels were measured. The Western blotting figure of PTP levels in one representative CVID and one normal control is shown in Fig. 3, and the complete results of these measurements carried out in the five patients and five controls are given in densitometry units in Table 2. As shown, almost normal PTP levels were achieved after PM implantation and BCR stimulation in PBMCs of those patients whose levels had been low. These results indicate that B cells of these patients exhibit an early signal transduction defect which is located in their plasma membrane. This defect can be repaired by implantation of normal PM.
FIG. 2.
Flow cytometric analysis of PM implanted cells. PBMCs (106) were implanted as described in Material and Methods with PM originated from murine splenic cells. Implanted (in white) and nonimplanted (in black) PBMCs were stained with FITC-conjugated anti-mouse Ig antibody and analyzed by flow cytometry.
FIG. 3.
PTP levels of representative PM implanted CVID PBMCs stimulated with anti-human IgM antibodies. PBMCs of a CVID patient (P1) and a normal donor (N) were implanted with functional PM and stimulated with anti-human IgM, and PTP levels were measured as described in Fig. 1.
TABLE 2.
PTP levels of PM-implanted CVID PBMCs stimulated with anti-human IgM antibodya
| Patient | Densitometry unitsb
|
|||||
|---|---|---|---|---|---|---|
| P
|
N
|
P/N ratio
|
||||
| − | + | − | + | − | + | |
| P1 | 135,022 | 287,881 | 242,735 | 241,848 | 0.55 | 1.19 |
| P2 | 488,997 | 731,477 | 683,251 | 760,964 | 0.71 | 0.96 |
| P5 | 942,027 | 1,210,743 | 1,290,228 | 1,178,417 | 0.73 | 1.02 |
| P12 | 768,333 | 1,186,863 | 1,290,228 | 1,178,417 | 0.59 | 1.00 |
| P13 | 433,695 | 718,495 | 683,251 | 760,964 | 0.63 | 0.94 |
See Table 1, footnote a.
Results are given as determined with (+) or without (−) PM implantation.
Ig responses of CVID B cells to LPS.
In this set of experiments, we examined whether the implantation of functional PM in CVID PBMCs can not only restore the earliest events of the signal transduction cascade through their BCR but also restore their ability to secrete Ig in vitro in response to LPS stimulation. Implanted and nonimplanted CVID and normal donor PBMCs were stimulated with LPS, and their Ig isotype secretion was assessed.
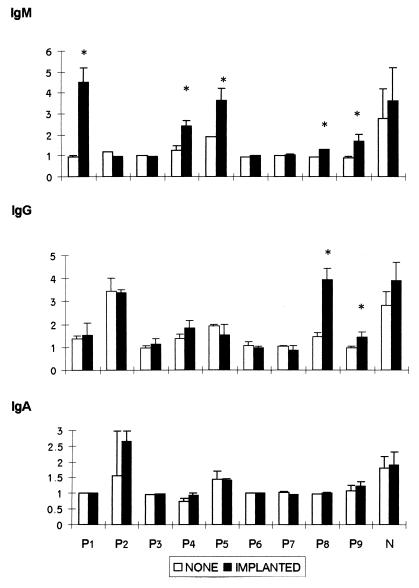
As shown in Fig. 4, upon stimulation with LPS, implanted lymphocytes of 5 of 9 patients exhibited significantly elevated IgM secretion; two of the five also exhibited IgG secretion, while none of the implanted patients exhibited restoration of IgA secretion.
FIG. 4.
In vitro Ig secretion of CVID implanted cells. CVID patients (P1 to P9) and normal control (N) PBMCs were implanted with functional PM as described in Materials and Methods. A total of 5 × 105 implanted and nonimplanted PBMCs were cultured with 2 μg of LPS per ml. Ig titers in 7-day cell culture supernatants were determined by isotype-specific ELISA. The data are presented as the mean ± the SD of the stimulation index from three experiments in each patient, each of which was performed in triplicate. N represents the mean of 14 normal controls. An asterisk indicates statistical significance.
Our overall findings suggest that a membranal defect may be the cause of B-cell dysfunction in a subset of CVID patients.
DISCUSSION
Most patients with CVID have normal numbers of mature B cells in the peripheral blood and lymphoid tissues, but these cells fail to proliferate and/or differentiate into Ig-secreting cells when stimulated with mitogens (26). The study reported here demonstrates that an abnormality exists in the early signal transduction cascade of B cells in a significant group of CVID patients.
We found that, after anti-IgM stimulation, the PTP levels in B cells of more than one-half (7 of 11) of the patients were significantly low compared to normal donors, suggesting abnormality in quantity and/or activity of either PTKases or PTPases located in the plasma membranes of these patients. Implantation of functional PM in the B cells of these patients restored their levels to normal.
It is unclear whether the PM implantation provided a substitute for the receptors or for the membrane-associated enzymes that may be defective in CVID patients’ B cells. However, since the stimulation of the implanted B cells was carried out by anti-human Ig antibody and since the cells were implanted with murine PM, it appears that the membranal defective components in CVID B cells are the membrane-associated enzymes and that the sIg receptors are unimpaired. Moreover, this leads to the assumption that the functional improvement of the implanted cells results from the activity of the murine membranal enzymes. Additional studies to rigorously address these possibilities will be required.
In the PM implantation system one has to take in account that we are using the PM of murine splenic cells which is implanted in both the B and T cells of the PBMCs. However, as the cells are stimulated with anti-human Ig antibodies which do not cross-react with the murine surface Ig, we are thus stimulating only the implanted human B cells.
The results of this study are consistent with those of previous studies demonstrating defects in the signal transduction cascade of B cells of CVID patients: low or no elevation of free intracellular calcium concentration and low expression of c-myc mRNA upon anti-Ig stimulation of the cells (19, 20). Furthermore, similar data on the T cells of CVID patients were obtained by Fischer et al. (13–15), who found a defect in the elevation of free intracellular calcium in response to superantigen in T cells of some CVID patients, whereas the response to phorbol myristate acetate and ionomycin, which bypass receptor-mediated signaling, was unimpaired.
Next, we investigated whether the low PTP levels after stimulation through BCR may be the cause of hypogammaglobulinemia in CVID patients. To this end, we tested whether the implantation of functional PM into the patients’ PBMCs can restore, in addition to PTP levels, the ability to secrete IgM, IgG, and IgA in response to LPS.
The results demonstrate that in a group of CVID patients the Ig secretion defect can be at least partially restored when B cells are implanted with functional PM and stimulated with LPS. These findings are consistent with previous findings from our laboratory demonstrating repair in the secretion of serotonin by murine implanted mast cells (1).
In the present study we have noticed two distinct groups of CVID patients with regard to the ability of PM implantation to restore their B-cell defect(s). The first group included two patients (P1 and P5) expressing low PTP levels in whom PM implantation restored both their PTP levels and their ability to secrete IgM after B-cell stimulation. We suggest that in this group of patients the main defect is an early signal transduction defect which is located in their B-cell plasma membrane. The second group included three patients (P3, P6, and P7) expressing normal levels of PTP in whom PM implantation did not restore their ability to secrete Ig upon LPS stimulation. We suggest that in this group of patients the defect is not located in their B-cell plasma membrane but may be located downstream in their signal transduction pathway.
It seems that the B cells of patient 2 (P2) possess at least two defects in their signal transduction cascade: one located in the plasma membrane and the other located in the cytoplasm or nucleus. This patient’s B cells express low PTP levels that can be restored by PM implantation, with no repair of their Ig secretion upon B-cell stimulation. A possible explanation is that in this patient’s B cells there is more than one defect, one related to receptor-associated enzymes located in the plasma membrane and another located downstream in the signal transduction pathway which therefore cannot be restored by PM implantation.
It is noteworthy that in response to LPS, implanted lymphocytes of 5 of 9 patients significantly elevated IgM levels and two of the five also exhibited IgG secretion, while none of the implanted patients exhibited restoration of IgA secretion. This partial restoration of Ig secretion may indicate an additional defect in the cells’ isotype-switching mechanism which cannot be restored by PM implantation. A possible explanation for the cause of such a defect is a block in CD40-CD40 ligand (8). The CD40-CD40 ligand signal interaction defect can be either common to or different from the BCR signal-transduction defect. This explanation is supported by previous studies which demonstrated a defect in CD40 ligand expression by activated lymphocytes from a group of CVID patients (9, 12). However, further studies are needed to examine this possibility.
In summary, the findings presented here suggest an early signal transduction defect as the cause for the block in B-cell differentiation in a group of CVID patients. Further studies are now being carried out to determine the specific molecular defect in the signal transduction pathway and the role of the receptor itself in these cells.
ACKNOWLEDGMENTS
This work was supported in part through grants from the Israel Academy of Science and Humanity and the Israel Research Fund. This work was done in partial fulfillment of the requirements for the Ph.D. degree of Rivka Schwartz from the Sackler Faculty of Medicine, Tel Aviv University.
REFERENCES
- 1.Abramovitz B, Altboum I, Lapidot M, Loyter A, Zan Bar I. Induction of serotonin release from mast cells by lymphocyte activators is dependent upon implantation of lymphocyte plasma membrane components. Exp Cell Res. 1991;194:228–231. doi: 10.1016/0014-4827(91)90358-2. [DOI] [PubMed] [Google Scholar]
- 2.Baumert E, Vorbeck G W, Schlesier M, Peter H H. Immunophenotypical alterations in a subset of patients with common variable immunodeficiency (CVID) Clin Exp Immunol. 1992;90:25–30. doi: 10.1111/j.1365-2249.1992.tb05826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben Anat-Porat Y, Zan-Bar I. Repair of immunoglobulin response in B cell line (JK32.1) originating from immunodeficient patient via implantation of functional plasma membranes. Clin Immunol Immunopathol. 1995;74:151–155. doi: 10.1006/clin.1995.1022. [DOI] [PubMed] [Google Scholar]
- 4.Bryant A, Calver N C, Toubi E, Webster A D, Farrant J. Classification of patients with common variable immunodeficiency by B cell secretion of IgM and IgG in response to anti-IgM and interleukin-2. Clin Immunol Immunopathol. 1990;56:239–248. doi: 10.1016/0090-1229(90)90145-g. [DOI] [PubMed] [Google Scholar]
- 5.Choi Y S, Biggar W D, Good R A. Biosynthesis and secretion of immunoglobulins by peripheral-blood lymphocytes in severe hypogammaglobulinaemia. Lancet. 1972;i:1149–1152. doi: 10.1016/s0140-6736(72)91374-8. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham-Rundles C. Clinical and immunologic analyses of 103 patients with common variable immunodeficiency. J Clin Immunol. 1989;9:22–33. doi: 10.1007/BF00917124. [DOI] [PubMed] [Google Scholar]
- 7.De Gast G C, Wilkins S R, Webster A D, Rickinson A, Platts-Mills T A. Functional “immaturity” of isolated B cells from patients with hypogammaglobulinaemia. Clin Exp Immunol. 1980;42:535–544. [PMC free article] [PubMed] [Google Scholar]
- 8.Durie F H, Foy T M, Masters S R, Laman J D, Noelle R J. The role of CD40 in the regulation of humoral and cell-mediated immunity. Immunol Today. 1994;15:406–411. doi: 10.1016/0167-5699(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 9.Eisenstein E M, Chua K, Strober W. B cell differentiation defects in common variable immunodeficiency are ameliorated after stimulation with anti-CD40 antibody and IL-10. J Immunol. 1994;152:5957–5968. [PubMed] [Google Scholar]
- 10.Farrant J. T and B cell defects in common variable immunodeficiency. Immunol Invest. 1991;20:143–150. doi: 10.3109/08820139109050782. [DOI] [PubMed] [Google Scholar]
- 11.Farrant J, Bryant A, Franz A, Webster A D B. Biological spectrum of CVID. In: Bayer J R, editor. Auances en el diagno’stico y tratamiento de las immunodeficiencias primarias. J. R. Barcelona, Spain: Prous Editors; 1994. pp. 31–39. [Google Scholar]
- 12.Farrington M, Grosmaire L S, Nonoyama S, Fischer S H, Hollenbaugh D, Ledbetter J A, Noelle R J, Aruffo A, Ochs H D. CD40 ligand expression is defective in a subset of patients with common variable immunodeficiency. Proc Natl Acad Sci USA. 1994;91:1099–1103. doi: 10.1073/pnas.91.3.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer M B, Hauber I, Eggenbauer H, Thon V, Vogel E, Schaffer E, Lokaj J, Litzman J, Wolf H M, Mannhalter J W, et al. A defect in the early phase of T-cell receptor-mediated T-cell activation in patients with common variable immunodeficiency. Blood. 1994;84:4234–4241. [PubMed] [Google Scholar]
- 14.Fischer M B, Hauber I, Vogel E, Wolf H M, Mannhalter J W, Eibl M M. Immunodeficiency and other clinical immunology: defective interleukin-2 and interferon-γ gene expression in response to antigen in a subgroup of patients with common variable immunodeficiency. J Allergy Clin Immunol. 1993;92:340. doi: 10.1016/0091-6749(93)90178-i. [DOI] [PubMed] [Google Scholar]
- 15.Fischer M B, Hauber I, Wolf H M, Vogel E, Mannhalter J W, Eibl M M. Impaired TCR signal transduction, but normal antigen presentation, in a patient with common variable immunodeficiency. Br J Haematol. 1994;88:520–526. doi: 10.1111/j.1365-2141.1994.tb05068.x. [DOI] [PubMed] [Google Scholar]
- 16.Heinicke E, Kumar U, Munoz D G. Quantitative dot-blot assay for proteins using enhanced chemiluminescence. J Immunol Methods. 1992;152:227–236. doi: 10.1016/0022-1759(92)90144-i. [DOI] [PubMed] [Google Scholar]
- 17.Hoekstra D, de Boer T, Klappe K, Wilschut J. Fluorescence method for measuring the kinetics of fusion between biological membranes. Biochemistry. 1984;23:5675–5681. doi: 10.1021/bi00319a002. [DOI] [PubMed] [Google Scholar]
- 18.Jakobovits A, Sharon N, Zan-Bar I. Acquisition of mitogenic responsiveness by nonresponding lymphocytes upon insertion of appropriate membrane components. J Exp Med. 1982;156:1274–1279. doi: 10.1084/jem.156.4.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kondo N, Inoue R, Yano M, Hayashi T, Miwa Y, Kasahara K, Yamasaki M, Utsumi M, Shinbara M, Orii T. Defective calcium-dependent signal transduction in B lymphocytes of a certain common variable immunodeficiency. Exp Clin Immunogenet. 1993;10:16–20. [PubMed] [Google Scholar]
- 20.Kondo N, Motoyoshi F, Kasahara K, Inoue Y, Mori S, Orii T. Failure of c-myc gene expression in B cells of some patients with common variable immunodeficiencies. Exp Clin Immunogenet. 1992;9:109–116. [PubMed] [Google Scholar]
- 21.Mosmann T R, Cherwinski H, Bond M W, Giedlin M A, Coffman R L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 22.Peretz H, Toister Z, Laster Y, Loyter A. Fusion of intact human erythrocytes and erythrocyte ghosts. J Cell Biol. 1974;63:1–11. doi: 10.1083/jcb.63.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porat Y B, Levy D, Levy J, Zan-Bar I. Intrinsic defect in B cells of patients with hyper-immunoglobulin M syndrome. Clin Diagn Lab Immunol. 1995;2:412–416. doi: 10.1128/cdli.2.4.412-416.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rump J A, Jahreis A, Schlesier M, Drager R, Melchers I, Peter H H. Possible role of IL-2 deficiency for hypogammaglobulinaemia in patients with common variable immunodeficiency. Clin Exp Immunol. 1992;89:204–210. doi: 10.1111/j.1365-2249.1992.tb06933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snapper C M, Pecanha L M, Levine A D, Mond J J. IgE class switching is critically dependent upon the nature of the B cell activator, in addition to the presence of IL-4. J Immunol. 1991;147:1163–1170. [PubMed] [Google Scholar]
- 26.Sneller M C, Strober W, Eisenstein E, Jaffe J S, Cunningham-Rundles C. NIH conference. New insights into common variable immunodeficiency. Ann Intern Med. 1993;118:720–730. doi: 10.7326/0003-4819-118-9-199305010-00011. [DOI] [PubMed] [Google Scholar]
- 27.Stagg A J, Funauchi M, Knight S C, Webster A D, Farrant J. Failure in antigen responses by T cells from patients with common variable immunodeficiency (CVID) Clin Exp Immunol. 1994;96:48–53. doi: 10.1111/j.1365-2249.1994.tb06228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Webster A B D, North M E, Funauchi M, Farrant J. T cell defects in cvid, and the role of IL-2 deficiency. In: Bayer J R, editor. Auances en el diagno’stico y tratamiento de las immunodeficiencias primarias. J. R. Barcelona, Spain: Prous Editors; 1994. p. 13. [Google Scholar]
- 29.Wu L Y, Lawton A R, Cooper M D. Differentiation capacity of cultured B lymphocytes from immunodeficient patients. J Clin Invest. 1973;52:3180–3189. doi: 10.1172/JCI107518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yocum M W, Kelso J M. Common variable immunodeficiency: the disorder and treatment. Mayo Clin Proc. 1991;66:83–96. doi: 10.1016/s0025-6196(12)61176-8. [DOI] [PubMed] [Google Scholar]