Abstract
Mycoplasma bovis induces various clinical manifestations in cattle, such as mastitis, arthritis, and pneumonia. We have evaluated the immunoreactivity of three variable surface proteins (Vsps) of M. bovis, namely VspA, VspB, and VspC, with sera collected from herds with mycoplasmosis or from cattle experimentally infected with M. bovis. Western blot analysis revealed that the Vsps are the predominant antigens recognized by the host humoral response during M. bovis infection. The immunoreactivity of VspA, VspB, and VspC with host antibodies was independent of the clinical manifestations, the geographical origin of the M. bovis isolates, the mode of infection, and the animal’s history. Moreover, the results showed that Vsp-specific host antibodies can be detected about 10 days after experimental infection and for up to several months. The full-length or truncated versions of the VspA product were overexpressed in Escherichia coli as fusion proteins (FP-VspA). Recombinant products showed strong immunoreactivity with the Vsp-specific monoclonal antibodies 1A1 and 1E5, with the corresponding epitopes localized at the VspA N-terminal and C-terminal ends, respectively. Anti-M. bovis sera of cattle naturally or experimentally infected also strongly recognized the full-length FP-VspA. The seroreactivity of sera collected from cattle between 6 and 10 days after experimental infection was weaker with truncated versions of VspA lacking the 1E5 epitope than with the full-length VspA or the truncated versions lacking the 1A1 epitope. Overall, the results indicate that the Vsps, despite their inter- and intraclonal variability, may be applied as target antigens in serodiagnostic assays for epidemiological studies.
Mycoplasma bovis is considered one of the most pathogenic bovine mycoplasmas (18). While mycoplasmosis induced by this pathogen is spread worldwide, it occurs predominantly in Europe and North America, resulting in significant economic losses in areas with intensive dairy and meat production (18, 30). In cattle, M. bovis is associated with diverse clinical manifestations, such as mastitis in cows and arthritis and pneumonia in young animals, as well as genital disorders, abscess, conjunctivitis, otitis, and meningitis (11–13, 18, 28, 32). In most cases, fatal outcomes are due to coinfection with other bacterial pathogens, such as pasteurellas (8, 31). M. bovis may be asymptomatically present as commensal organisms in the upper respiratory tracts of older animals, where the mycoplasmas form a constant source of infection for young animals that are more susceptible to developing clinical symptoms (17, 31). In the absence of an effective antibiotic therapy or vaccination, the only strategy currently available to control infection is the strict segregation of M. bovis-infected animals from healthy herds (18). Rapid detection of animals that have been in contact with the pathogen is therefore a crucial step requiring sensitive and specific diagnostic approaches. The diagnosis of an M. bovis infection is currently based on the identification of the organism in secretions, excretions, or tissues either (i) by cultivation in broth medium followed by colony or dot blot immunostaining methods (6, 14, 19, 21, 26), (ii) by PCR (1, 4, 7, 10, 29), or (iii) by antigen-capture enzyme-linked immunosorbent assay (2, 9). These techniques rely on the presence of organisms in the samples at a detectable concentration that depends on the sensitivity of the test. Assays that assess the presence of anti-M. bovis circulating antibodies offer an improved alternative, because they can identify animals which have been infected within a large herd even in the absence of shedding organisms.
In a preliminary study, serum antibodies from animals naturally infected with M. bovis originating from Northern Germany were shown to predominantly recognize major epitopes carried by a family of abundant, variable surface lipoproteins, designated as Vsps (25). So far, three Vsps, VspA, VspB, and VspC, have been characterized in clonal variants derived from M. bovis type strain PG45. Detailed analysis revealed that each Vsp undergoes high-frequency variation in expression and size, generating extensive surface diversification in a given M. bovis strain or isolate (3). This phenomenon may profoundly affect the outcome of serodiagnostic assays, because their sensitivity may vary, depending on the choice of the target antigen (26).
Development of sensitive and specific serologic tests for the rapid detection of infected animals is bound to the identification of a specific antigen. In this study, we have evaluated the reactivity of M. bovis antigens, and more specifically of Vsp epitopes, with sera obtained from animals experimentally or naturally infected with M. bovis. We describe the expression of recombinant VspA products in Escherichia coli which contain immunodominant epitopes strongly reacting specifically with sera from naturally infected cattle as well as with sera collected 6 days after experimental infection with M. bovis.
MATERIALS AND METHODS
Mycoplasmas, bacterial strains, and plasmids.
M. bovis 1067 was originally isolated from an animal with mastitis in 1983 and propagated as a filter-cloned culture (22). This strain and a clonal variant derived from M. bovis type strain PG45, which expressed a 67-kDa version of VspA (see below) (3) designated VspA 67, were used for experimental infections. Clonal variants used for Western blot analysis were selected from a collection of isogenic variants previously generated from M. bovis type strain PG45 and expressing either 79-kDa VspC, 64-kDa VspA plus 46-kDa VspB, or 67-kDa VspA (3). E. coli DH10B (GIBCO BRL, Life Technologies, Inc., Grand Island, N.Y.) was used as a host for recombinant plasmids derived from the cloning and expression vector pMAL-c2 (New England Biolabs, Inc., Beverly, Mass.).
Serum samples collected from cattle experimentally infected with M. bovis.
Sera collected from experimentally infected cattle were selected from four independent M. bovis infections (Table 1, experiments 1 to 4), which were conducted between 1986 and 1998 by the Centre National d’Etude Vétérinaires et Alimentaires (CNEVA) de Lyon, Lyon, France, and the Ecole Nationale Vétérinaire de Lyon, Lyon, France. Before sampling, animals were shown to be free of M. bovis respiratory infection by bacteriological examination of individual bronchoalveolar lavages (BAL) and an indirect hemagglutination test (IHA) (5, 20). This was retrospectively confirmed by Western blot analysis of M. bovis whole-cell antigens as described below by using preimmune sera. Experiment 1 involved 24 young cattle, experiments 2 and 3 involved 8 and 17 calves, respectively, and experiment 4 involved 21 pregnant dairy cows. Cattle were given experimental infections by endobronchial inoculation of approximately 50 ml of fresh culture containing 109 to 1010 CFU of M. bovis strain 1067 per ml (experiments 1, 3, and 4) or of a clonal variant expressing a single VspA of 67 kDa (experiment 2). In experiments 2 and 3, all animals were inoculated, while in experiments 1 and 4, only one-third of the group was inoculated to promote natural infection by contact with the remaining animals. After inoculation, animals of experiments 1, 2, and 3 were examined for 6 to 30 days before slaughtering, while animals of experiment 4 were kept and routinely monitored for an additional 2 years. Routine surveillance consisted of (i) a regular clinical examination (daily for experiment 2), (ii) weekly bacterial testing of BAL in the first month (daily for experiment 2), and (iii) blood sampling for serology before inoculation and then twice a week (monthly for the longest experiment [experiment 4]). Regular respiratory shedding of M. bovis was systematically shown in all infected animals, endobronchially or by contact, for at least few days after exposure. Clinical expression of M. bovis disease was mostly mild (as measured by transient fever, raised respiratory rate, and inappetance), except in experiment 4, in which abortion occurred in two cows with isolation of M. bovis in fetus liver. The extent of macroscopic lung lesions in animals of experiments 1, 2, and 3 largely varied according to the stage of slaughtering, from no lesion of the lung surface to 65% lesion. Animals inoculated with the clonal variant VspA 67 only showed a few microscopic lesions, mainly interstitial pneumonia with occasionally bronchopneumonia areas.
TABLE 1.
Characteristics of bovine sera selected from four experimental M. bovis infections
| Expt (yr)a | Strain | Route of infectionb | Clinical manifestation and pathology | Designation of animal sera tested | No. of days postinoculation | Presence of anti-Vsp antibodiesc |
|---|---|---|---|---|---|---|
| 1 (1989) | 1067 | Endobronchial | Lung lesions | J004 | 10 and 30 | + and + |
| Contactd | Lung lesions | J005 | 10 and 30 | + and + | ||
| 2 (1997) | Clonal variant derived from PG45e | Endobronchial | Lung lesions | A009 | 6 | + |
| A010 | 6 | + | ||||
| 3 (1998) | 1067 | Endobronchial | Lung lesions | M | 6 and 14 | + and + |
| H | 6 and 14 | + and + | ||||
| Yf | 6 and 14 | + and + | ||||
| Vf | 6 and 14 | + and + | ||||
| 4 (1986) | 1067 | Endobronchial | Abortions | F006 | 62 and 524 | + and + |
| E095 | 101 and 532 | + and + |
Experiments were conducted as described in Materials and Methods.
Inoculation was performed with 50 ml of an M. bovis culture containing 109 to 1010 CFU/ml.
All sera were tested by Western blot as described in Materials and Methods and were shown to predominantly contain antibodies directed toward VspA, VspB, and VspC.
Animal J005 (expt 1) was infected by contact with a previously inoculated animal, J004.
Clonal variant derived from strain PG45 expressing a single Vsp, 67-kDa VspA.
Sera from animals Y and V were also taken at day 9 and 21 and shown to react with the fusion protein FP-VspA-I and its truncated versions as described in Results.
Serum samples collected from cattle naturally infected with M. bovis.
Sera from 26 animals were selected during natural outbreaks in four herds. These outbreaks were confirmed by reisolation of M. bovis. They occurred in France (1–3) and in Switzerland (4) and were chosen to be representative of at least one major clinical manifestation of M. bovis infection (i.e., mastitis, pneumonia, or arthritis), as well as to represent different age groups (i.e., calves, young cattle, and adults) (Table 2). Twenty additional serum samples (Table 2, field sampling) were randomly collected from 10 herds showing asymptomatic M. bovis infections. These herds—six in France (1988 to 1990) and four in Switzerland (1997)—contained several animals showing a strong reaction by IHA, suggesting a previous or current asymptomatic infection with M. bovis. Among these 19 serum samples, 17 were IHA positive and 2 were IHA negative.
TABLE 2.
Characteristics of bovine sera selected from natural M. bovis outbreaks
| Outbreak designation | Geographical location and date(s) | Clinical manifestation | No. of animal serum samples testeda | No. of serum samples containing predominantly anti-Vsp antibodiesb | No. of serum samples lacking M. bovis antibodiesb |
|---|---|---|---|---|---|
| Outbreak 1 | France, August 1994 | Arthritis | 7 | 7 | 0 |
| Outbreak 2 | France, April 1997 | Arthritis and pneumonia | 6 | 6 | 0 |
| Outbreak 3 | France, January 1988 | Arthritis, pneumonia, and mastitis | 3 | 3c | 0 |
| Outbreak 4 | Switzerland, April 1997 | Mastitis | 10 | 9 | 0 |
| Field sampling 5d | France, 1988 and 1990; Switzerland, 1997 | No pathology | 19 | 17e | 2f |
Values correspond to one serum sample per animal.
The presence of antibodies directed toward Vsps was assessed by Western blot analysis.
Serum samples from three different animals were tested twice at 20-day intervals and showed a constant similar reaction to Vsps.
In a previous study, 200 healthy herds were randomly screened by IHA for the presence of antibodies directed to M. bovis antigens. Among those, 10 were shown to contain several asymptomatic animals presenting a strong reaction by IHA, suggesting an M. bovis infection. From these 10 herds, 17 serum samples of animals positive by IHA and 2 serum samples of animals negative by IHA were selected.
The 17 serum samples were all positive by IHA.
The two serum samples were negative by IHA.
MAbs and hyperimmune sera.
The monoclonal antibodies (MAbs) 1E5 and 1A1 used in this study have been previously described by Rosengarten et al. (3) and LeGrand et al. (15), respectively. Briefly, MAb 1E5 of the immunoglobulin M (IgM) isotype was raised against a M. bovis clonal variant derived from the type strain PG45 and was shown to specifically react with a common epitope to VspA, VspB, and VspC. The MAb 1A1 of the IgG1 isotype was obtained by a similar procedure and was shown to react with VspA and VspC, but not with VspB (3, 15, 25). Both MAbs were shown not to react with ruminant mycoplasma species other than M. bovis. Rabbit hyperimmune sera were raised against those mycoplasma species which are most frequently isolated from cattle (M. bovigenitalium, M. bovirhinis, M. arginini, Acholeplasma laidlawii, and Ureaplasma diversum) and against M. agalactiae, which is closely related to M. bovis, as previously described (21). The serum PAL, kindly provided by D. Bergonier (Ecole Vétérinaire de Toulouse, France), was collected from a sheep naturally infected with M. agalactiae and shown to strongly react with M. agalactiae surface components.
IHA and Western blot analysis.
The IHA was performed as described elsewhere (5, 20) with the type strain PG45 as an antigen. The procedures for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting of mycoplasma proteins have previously been described (25). For Western blot analysis, nitrocellulose membranes were blocked for 50 min with Tris-buffered saline (TBS [0.01 M Tris-HCl, 0.15 M NaCl, pH 7.2]) containing 10% (vol/vol) horse serum, washed once with TBS containing 0.05% (vol/vol) Tween 20 (TBS T20) and once with TBS only, and then incubated for 2 h at 33°C with the primary antibodies diluted in TBS supplemented with 5% (vol/vol) horse serum (bovine sera diluted 1:75, MAb 1E5 diluted 1:100, MAb 1A1 diluted 1:2,000; rabbit hyperimmune sera diluted 1:1,000). After three washes with TBS T20 and one with TBS, the blots were incubated for 1 h at 33°C with the appropriate secondary antibodies. The peroxidase-conjugated rabbit antibovine Igs (Dako, Glostrup, Denmark), sheep anti-bovine IgM and IgG (Bethyl Laboratories, Inc., Montgomery, Tex.), and goat anti-mouse IgM and IgG (Accurate Chemical and Scientific Corporation, Westbury, N.Y.) were diluted in phosphate-buffered saline as recommended by the manufacturer.
Expression of VspA in E. coli as a fusion protein.
The fusion protein FP-VspA-I was generated by using the pMAL-c2 protein fusion system of New England Biolabs. Briefly, oligonucleotides P-5 (5′-GCA GGA TCC TGT GGT GAG ACC AAA G-3′) and P-3 (5′-TAT TAA GCT TAA GAA CTT GTT GGT ATT TT-3′) (boldface letters indicate engineered restriction sites, and underlined letters indicate the codon encoding the first amino acid of the VspA mature protein), which span the exported, mature coding sequence of VspA (Fig. 1A), were used to produce by PCR a DNA fragment from the cloned VspA gene template (16). Engineered BamHI and HindIII restriction sites located in primers P-5 and P-3, respectively, were used to insert the PCR product in frame with the malE gene, which encodes the maltose binding protein (MBP), into the pMAL-c2 plasmid by standard procedures. The fusion protein FP-VspA-I encoded by the resulting recombinant plasmid pFP-VspA-I was overexpressed in transformed E. coli DH10B cells (GIBCO BRL, Life Technologies, Inc., Grand Island, N.Y.) and was purified by affinity chromatography by using maltose binding properties, as prescribed by the manufacturer (New England Biolabs, Inc.). Cleavage of the VspA product from the MBP by the protease factor Xa was achieved as instructed by the manufacturer.
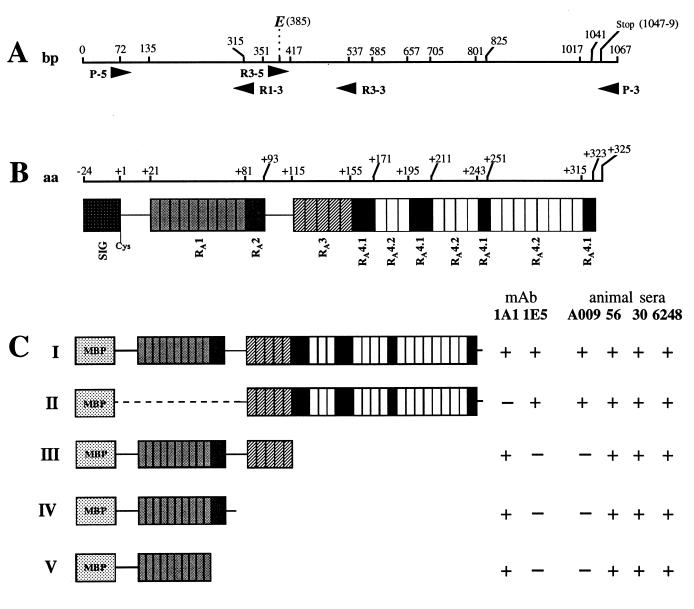
FIG. 1.
Schematic representation of the VspA fusion proteins and localization of immunodominant epitopes 1A1 and 1E5. (A) Localization of the primers on the vspA gene used to generate the recombinant proteins represented in panel C (numbers indicate nucleotide position). (B) Representation of the different domains that compose the VspA product (open boxes) and their localization (numbers represent amino acid position). (C) Representation of the domains that compose the FP-VspA-I to -V fusion proteins and their reactivity with MAbs 1E5 and 1A1 and animal sera A009 (experiment 2), 56 and 30 (from two animals of outbreak 4), and 6248 (from one animal of outbreak 2).
Three truncated versions of the FP-VspA fusion protein (Fig. 1C), namely FP-VspA-II, FP-VspA-III, and FP-VspA-V, were obtained by the same procedure. For this purpose, primers complementary to the junction of two distinct repeated units or to unrepeated sequences located between two blocks of repeated elements were designed: R3-5 (5′-CCC AGG ATC CCC GCA TGA T-3′), R3-3 (5′-CCT GAA GCT TGT TGT GAG TTA G-3′), and R1-3 (5′-GTT TTC CTC AAG CTT TTT AAT TTT C-3′). These primers were used in combination with P-5 (5′) or P-3 (3′), as shown in Fig. 1A. Boldface letters represent engineered BamHI (5′-end primer) and HindIII (3′-end primer) restriction sites for in-frame insertion of the PCR fragment into the pMAL-c2 vector downstream of the malE sequence. Finally, the FP-VspA-IV fusion protein was obtained by subcloning the EcoRI DNA fragment of the plasmid pFP-VspA-I, which contained the 3′ end of the pMAL-c2 polylinker and the first 313 nucleotides of the vspA gene, into EcoRI-pMAL-c2. Expression of the truncated fusion proteins was performed as described above. PCR fragments cloned into pMAL-c2 were sequenced by deoxy terminator cycle sequencing with infrared labelled primers and the DNA sequencer Long Readir 4200 (LI-COR, Lincoln, The Netherlands) and shown to be identical to the previously published vspA gene (16).
RESULTS
Humoral response to M. bovis epitopes in experimentally infected cattle.
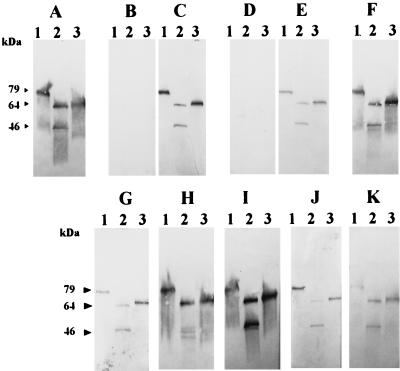
To evaluate the host antibody reactivity to M. bovis antigens, and more specifically to Vsps, identical immunoblots representing whole-cell extracts of selected clonal variants derived from M. bovis type strain PG45 were independently immunostained with the Vsp-specific MAb 1E5 and with sera of experimentally infected animals (Table 1). Analyses were performed with three PG45 clonal variants, each expressing distinct Vsp products easily identified by their size in Western blot analysis by using the MAb 1E5 (Fig. 2A), namely 79-kDa VspC (lane 1), 64-kDa VspA plus 46-kDa VspB (lane 2), and 67-kDa VspA (lane 3). As summarized in Table 1, all sera collected from experimentally infected animals generated immunoprofiles identical to that obtained with MAb 1E5. This is illustrated in Fig. 2, which shows the Vsp-specific reaction obtained with the sera of animals A009 (Fig. 2C), M (Fig. 2E), and E095 (Fig. 2F), while no reaction was observed between M. bovis whole-cell antigens and preimmune sera from the same animals (Fig. 2B and D [not shown for E095]). These results showed that bovine antibodies to Vsp epitopes appeared specifically after infection with M. bovis. Moreover, bovine serum antibodies to M. bovis recognized assorted sizes of Vsp products (Fig. 2C, E, and F; 64- and 67-kDa VspA in lanes 2 and 3, 79-kDa VspC in lane 1, and 46-kDa VspB in lane 2). As well, they reacted with Vsp products expressed by a different strain from the one used for inoculation, since serum antibodies from animals infected with M. bovis 1067 recognized Vsps of the PG45 strain. Interestingly, sera collected from animals inoculated with a clonal variant derived from the type strain PG45 that expressed a single Vsp, 67-kDa VspA (experiment 2), also contained antibodies that reacted with VspC and VspB (see serum A009, Fig. 2C). As shown in Fig. 2, circulating host antibodies directed toward Vsp epitopes were detected in sera as early as 6 days after infection (Fig. 2C and E), but were also detected 532 days postinoculation (Fig. 2F).
FIG. 2.
Selective recognition of defined Vsp products of M. bovis PG45 by serum antibodies from M. bovis-infected cattle. Identical Western blots representing the total proteins of three clonal variants (lanes 1 to 3) derived from M. bovis PG45 were respectively immunostained with MAb 1E5 (A), with sera collected from experimental infection (B to F), or with sera collected from natural outbreaks (G to K). The sera used in this experiment are described in Tables 1 and 2 and correspond to animal A009 before (B) and after (C) infection; animal M before (D) and after (E) infection; sera E095 (F), 30 (G), and 56 (H) from two animals of outbreak 4; serum 6241 (I) from outbreak 2; serum 47 (J) from outbreak 1; and serum 283/17 (K) from the field sampling. Lanes 1 through 3 represent clonal variants expressing 79-kDa VspC (lane 1), 64-kDa VspA plus 46-kDa VspB (lane 2), and 67-kDa VspA (lane 3).
Humoral response to M. bovis epitopes in naturally infected cattle.
To further investigate whether the results obtained with sera from experimentally infected animals reflected the situation occurring in the field, similar experiments were performed with sera collected from animals displaying diverse clinical manifestations during natural M. bovis outbreaks in geographically distant herds. As illustrated in Fig. 2G to K, immunoprofiles obtained with sera representative of outbreaks 1, 2, and 4 (Table 2) were identical to that obtained with MAb 1E5 or with sera collected from animals experimentally infected with M. bovis (Fig. 2). In a few cases, immunoprofiles obtained with field sera were more complex than that presented in Fig. 2, because several mycoplasma components other than the Vsps were also weakly reacting with the host antibodies (data not shown). Sera obtained from asymptomatic animals that were strongly reacting with M. bovis in the IHA displayed a pattern similar to that obtained in Fig. 2, because they only reacted with Vsp epitopes (Table 2, field sampling). In some cases, we observed that the reactivity of the sera with 46-kDa VspB was weaker than that observed with 64-kDa VspA (Fig. 2H, lane 2, and data not shown) indicating that recognition of VspB epitopes by the immune system may vary from one animal to another, while all sera reacted strongly with the 67-kDa VspA product (Fig. 2G to K, lane 3). Finally, hyperimmune sera against other mycoplasmas frequently isolated from cattle (M. bovigenitalium, M. bovirhinis, M. arginini, Acholeplasma laidlawii, and Urealyticum diversum) and against M. agalactiae, a mycoplasma that is phylogenetically closely related to M. bovis and occasionally found in cattle (21), did not react with M. bovis antigens (except for a very weak reaction with M. arginini and uncharacterized antigens of M. bovis whole-cell extract).
Reactivity of VspA overexpressed in E. coli with hyperimmune sera of cattle experimentally and naturally infected with M. bovis.
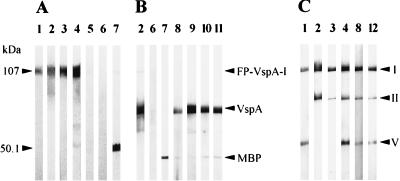
To define whether the VspA product would be a suitable tool for serodiagnostic purposes, for instance, in epidemiological studies, the VspA product of M. bovis PG45 was expressed in E. coli as a nondenatured recombinant product. This was achieved by inserting the DNA sequence encoding the mature VspA product from the +1 Cys to the C-terminal tip, into the pMAL-c2 vector to create an in-frame fusion with the malE gene, which encodes the MBP (Fig. 1). In SDS-PAGE, the resulting recombinant fusion protein, designated FP-VspA-I, had an apparent molecular mass of about 107 kDa (Fig. 3) which corresponds to the mature VspA sequence (67 kDa without the signal peptide) (16) fused to the C-terminal region of the MBP (36 kDa for the MBP). Western blot analysis showed that the FP-VspA-I was antigenically comparable to the native VspA, because it reacted both with the Vsp-specific MAb 1A1 (Fig. 3A, lane 1) and MAb 1E5 (Fig. 3A, lane 2), which binds to VspA and VspC, but not to VspB. Moreover, serum antibodies from cows naturally or experimentally infected with M. bovis also reacted with the recombinant fusion protein FP-VspA-I, as illustrated in Fig. 3A, lanes 3 and 4. In contrast, no reaction of these sera was obtained with the MBP fused with β-galactosidase (Fig. 3A, lanes 3 and 4). The immunoreactivity of FP-VspA-I was also tested with (i) serum collected from sheep infected with M. agalactiae and (ii) serum obtained from an animal which was shown to be free of M. bovis. As shown in Fig. 3A, lanes 5 and 6, none of these sera reacted with FP-VspA-I. Moreover, no reaction was obtained with hyperimmune serum raised against M. arginini (data not shown), which was shown to react weakly with uncharacterized antigens of M. bovis whole-cell extract. Immunoblot analysis of FP-VspA-I digested with the factor Xa revealed that the cleaved 67-kDa VspA product reacted with (i) the MAbs 1E5 (Fig. 3B, lane 2) and 1A1 (data not shown) and (ii) all positive bovine sera corresponding to outbreaks 1, 2, 3, and 4 shown in Table 1 (see Fig. 3B, lanes 8 and 9 for two representative serum samples). By the same procedure, host antibodies to VspA were detected in IHA-negative serum of animal J005 (Table 1, experiment 1) collected at day 13 or 17 following contact with the infected animal J004 (Fig. 3B, lanes 10 and 11). Since M. bovis was first reisolated in BALs of animal J005 at day 6, these results support our previous findings that showed the early appearance of circulating host antibodies to Vsp epitopes in animals infected by endobronchial inoculation (Table 1, experiments 2 and 3). Serum samples collected from animal J005 after day 23 were all positive in IHA and reacted with the VspA product. Similar results obtained with sera collected from two other contact-infected animals (data not shown) indicated that host antibodies to M. bovis are detectable within 10 days with FP-VspA-I, however, not with the IHA.
FIG. 3.
Seroreactivity of the VspA immunogenic domains with Vsp-specific MAbs and animal sera. The FP-VspA-I product and the MBP-β-galactosidase (A) or FP-VspA-I product digested with the factor Xa (B) or the FP-VspA-I, FP-VspA-II, and FP-VspA-V fusion proteins (C) were separated by SDS-PAGE and transferred onto nitrocellulose membrane. Immunostaining of the Western blots was performed with MAb 1A1 (lane 1), MAb 1E5 (lane 2), serum A009 from experiment 2 (lane 3), serum 56 from outbreak 4 (lane 4), hyperimmune serum PAL from sheep infected with M. agalactiae (lane 5), serum from an M. bovis-free animal (lane 6), anti-MBP serum (lane 7), serum 30 from outbreak 4 (lane 8), serum 45 from outbreak 4 (lane 9), IHA-negative sera from animal J005 at days 13 (lane 10) and 17 (lane 11), and serum 6248 from outbreak 2 (lane 12).
Approximately 80% of the VspA amino sequence is composed by two distinct stretches of repeated sequences separated from each other by 22 amino acids (Fig. 1B). The first block, localized at the N-terminal portion, is composed of two distinct motifs, designated RA1 and RA2, while the second block, localized at the C-terminal portion, contains three repeated motifs, RA3, RA4.1, and RA4.2 (16). In order to better define which portion of the VspA molecule is involved in stimulating the host humorale immune response, four truncated versions of the FP-VspA-I, designated FP-VspA-II, FP-VspA-III, FP-VspA-IV, and FP-VspA-V, were generated by cloning independently selected vspA regions into the pMAL-c2 vector, as described in Materials and Methods. Figure 1C illustrates the different regions encoded by the genes coding for these truncated products. Localization of the 1A1 and 1E5 epitopes was achieved by Western blot analysis. This showed that the first 106-amino-acid sequence contains the 1A1 epitope (Fig. 3C, lane 1), while the C-terminal region from amino acid 155 to amino acid 325 encodes the 1E5 epitope (Fig. 3C, lane 2). The four truncated fusion proteins reacted with sera 56 and 30, collected from outbreak 4, and with serum 6248, collected from outbreak 2. Interestingly, serum A009, collected 6 days after experimental infection with the VspA 67 clonal variant (experiment 2), only reacted with fusion proteins (FP-VspA-I and -II) that contain the 1E5 epitope (Fig. 1C and 3C, lane 3). Similarly, sera collected 9 days after infection with M. bovis 1067 (experiment 3, calves Y and V [Table 2]) only recognized the FP-VspA-I and -II, while sera taken at day 21 from the same calves (experiment 3, calves Y and V) did react as well with FP-VspA-III (data not shown).
DISCUSSION
The results presented in this report show that the Vsps represent those components that predominantly elicit the bovine humoral immune response in cattle after experimental or natural infection with M. bovis, independently of the clinical manifestations, the geographic location and origin of the agent, the mode of infection, and the animal’s history. In experimentally infected calves, circulating host antibodies directed toward Vsp epitopes appeared within an average of 10 days following inoculation with M. bovis, but also as early as 6 days, and were still detectable for several months after infection. Results obtained with contact-infected animals indicated that a similar situation is likely to occur in the field. Serum antibodies collected from cattle naturally infected with M. bovis of unknown Vsp phenotype and genotype were shown to recognize the three Vsps expressed by the type strain PG45. Inoculation of animals with strain 1067 also resulted in the appearance of antibodies that cross-reacted with the Vsps of strain PG45. This implies that despite their clonal variability, the Vsps or at least some members of the Vsp family are persistently expressed by M. bovis in the bovine host during infection and that immunodominant epitopes are highly conserved among strains and isolates. The presence of anti-VspB and anti-VspC antibodies in addition to anti-VspA antibodies during infection with a clonal variant expressing VspA (experiment 2) indicated that common epitopes shared by the three Vsps (VspA, VspB, and VspC) are strongly immunogenic in the host and/or that oscillation in Vsp expression occurs in vivo, generating subpopulations expressing VspB and VspC. In some cases, the reactivity of bovine serum antibodies was stronger with the VspA and VspC products than with VspB. This can be explained by (i) the absence of the 1A1 epitope on VspB which is shared by both the VspA and the VspC proteins and (ii) the fact that the number of repeated elements which constitute 80% of the molecule and are thought to contain the immunodominant epitopes is lower in the 46-kDa VspB product than in the 64-kDa VspA and 79-kDa VspC molecules. On the other hand, previous data suggested that the VspA and the VspC proteins may be the products of two distinct allelic versions of the same vsp gene (16), explaining their similar reactivity with the MAbs 1A1 and 1E5 and the animal sera.
In light of these findings and the proven nonreactivity of the Vsps to sera raised against closely related mycoplasmas commonly isolated from cattle, the surface-exposed VspA product of M. bovis was overexpressed in E. coli as a recombinant protein. This product was shown to be antigenically comparable to the native VspA, because it reacted with two MAbs directed to Vsps, 1A1 and 1E5, as well as with all sera used in this study, collected from cattle experimentally or naturally infected with M. bovis. Interestingly, recognition of the VspA immunodominant domains by the host immune system was slightly different, because truncated recombinant VspA products lacking the RA4 repeated region, FP-VspA-III, FP-VspA-IV, and FP-VspA-V, failed to react with sera taken between 6 to 10 days, but were recognized by sera of the same animals collected at a later stage. As shown in this study, the RA4 region contains the target epitope of MAb 1E5, which is an IgM isotype, while the N-terminal RA1 repeated motif, encoded by the genes coding for all of the truncated VspA products, is recognized by MAb 1A1, which is an IgG isotype. This suggests that detection of the N-terminal region of VspA, which contains the RA1 motif, may require the seroconversion of IgM to IgG, due to either a low concentration of IgM reacting with the 1A1 target epitope or due to a conformational structure that temporarily masks the target epitope. Nevertheless, these data indicate that the recombinant product containing the entire VspA sequence is suitable for the early and late detection of animals infected with M. bovis.
The presence of vsp gene homologues in field isolates or strains other than the PG45 type strain was recently assessed in 250 M. bovis field isolates collected in France, Germany, Italy, Spain, and Switzerland (23). All were shown to contain DNA sequences homologous to vsp genes and to express, to various degrees, epitopes that reacted with either the 1A1 or the 1E5 MAb. Interestingly, the few isolates that did not react with MAb 1E5 failed to react in Southern blot analysis with the oligonucleotide probe corresponding to the RA4 motif. In contrast, all isolates contained multiple copies of the sequence encoding the motif RA1 and were reacting with MAb 1A1 (23). This corresponds to the results obtained in this study with the truncated recombinant Vsps revealing the location of the 1A1 and 1E5 epitopes within the RA1 and RA4 repeated motifs, respectively.
Even though the Vsp proteins were shown to participate in adhesion to the host cell (27), their exact role during the process of the disease remains to be elucidated. However, if the presence of Vsp epitopes at the surface of the mycoplasma depends on the on and off status of the corresponding gene or genes, it also depends on the number of vsp genes that dictate the Vsp repertoire in a given strain. For the type strain PG45, which contains eight distinct vsp genes (24) that may all be subjected to on and off oscillation in expression, the likelihood that each cell expresses at least one Vsp is rather high. In fact, immunostaining of M. bovis PG45 colonies with MAbs 1E5 and 1A1 revealed that nearly all colonies do express the target epitopes. It is likely that a similar situation occurs in M. bovis field isolates, and this argument is supported by the results presented in this study that showed the presence of anti-Vsp antibodies in sera of animals infected with M. bovis of unknown Vsp phenotypes.
Thus, based on the data obtained in this study, we propose the utilization of the VspA recombinant fusion protein FP-VspA-I as an immunologic reagent for the rapid identification of cattle infected with M. bovis.
ACKNOWLEDGMENTS
We thank J. Nicolet (University of Bern, Bern, Switzerland), F. Marty (France), and Sanofi Santé Nutrition Animal for providing sera, K. Sachse for providing the clonal VspA gene, and K. Siebert-Gulle and M. Solsona for technical assistance.
This study is part of the European COST action 826 “Ruminants’ mycoplasmoses” and was supported by a grant by the French Ministry of Agriculture (E.N.V.L. Lyon-454-1997) to D.L.G. and a grant by the Austrian Ministry of Health and Consumer Protection (353.024/2-III/9/96) to R.R.
M. Brank and D. Le Grand contributed equally to this paper.
REFERENCES
- 1.Ayling R D, Nicholas R A, Johansson K E. Application of the polymerase chain reaction for the routine identification of Mycoplasma bovis. Vet Rec. 1997;141:307–308. doi: 10.1136/vr.141.12.307. [DOI] [PubMed] [Google Scholar]
- 2.Ball H J, Finlay D, Reilly G A. Sandwich ELISA detection of Mycoplasma bovis in pneumonic calf lungs and nasal swabs. Vet Rec. 1994;135:531–532. doi: 10.1136/vr.135.22.531. [DOI] [PubMed] [Google Scholar]
- 3.Behrens A, Heller M, Kirchhoff H, Yogev D, Rosengarten R. A family of phase- and size-variant membrane surface lipoprotein antigens (Vsps) of Mycoplasma bovis. Infect Immun. 1994;62:5075–5084. doi: 10.1128/iai.62.11.5075-5084.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chavez Gonzalez Y R, Bascunana C R, Bölske G, Mattson J G, Fernandez Molina C, Johansson K E. In vitro amplification of 16S rRNA genes from Mycoplasma bovis and Mycoplasma agalactiae. Vet Microbiol. 1995;47:183–190. doi: 10.1016/0378-1135(95)00058-i. [DOI] [PubMed] [Google Scholar]
- 5.Cho H J, Ruhnke H L, Langford E V. The indirect hemagglutination test for the detection of antibodies in cattle naturally infected with mycoplasmas. Can J Comp Med. 1976;40:20–29. [PMC free article] [PubMed] [Google Scholar]
- 6.Gardella R S, Delguidice R A, Tully J G. Immunofluorescence. In: Razin S, Tully J G, editors. Methods in mycoplasmology. I. Mycoplasma characterization. New York, N.Y: Academic Press; 1983. pp. 431–439. [Google Scholar]
- 7.Ghadersohi A, Coelen R J, Hirst R G. Development of a specific DNA probe and PCR for the detection of Mycoplasma bovis. Vet Microbiol. 1997;56:87–98. doi: 10.1016/S0378-1135(96)01343-0. [DOI] [PubMed] [Google Scholar]
- 8.Gourlay R N, Houghton S B. Experimental pneumonia in conventionally reared and gnotobiotic calves by dual infection with Mycoplasma bovis and Pasteurella haemolytica. Res Vet Sci. 1985;38:377–382. [PubMed] [Google Scholar]
- 9.Heller M, Berthold E, Pfützner H, Leirer R, Sachse K. Antigen capture ELISA using a monoclonal antibody for the detection of Mycoplasma bovis in milk. Vet Microbiol. 1993;37:127–133. doi: 10.1016/0378-1135(93)90187-c. [DOI] [PubMed] [Google Scholar]
- 10.Hotzel H, Sachse K, Pfützner H. Rapid detection of Mycoplasma bovis in milk samples and nasal swabs using the polymerase chain reaction. J Appl Bacteriol. 1996;80:505–510. doi: 10.1111/j.1365-2672.1996.tb03249.x. [DOI] [PubMed] [Google Scholar]
- 11.Kinde H, Daft B M, Walker R L, Charlton B R, Petty R. Mycoplasma bovis associated with decubital abscesses in Holstein calves. J Vet Diagn Investig. 1993;5:194–197. doi: 10.1177/104063879300500209. [DOI] [PubMed] [Google Scholar]
- 12.Kirby F D, Nicolas R A. Isolation of Mycoplasma bovis from bullocks’ eyes. Vet Rec. 1996;138:552. . (Letter.) [PubMed] [Google Scholar]
- 13.Kirk J H, Lauermann L H. Mycoplasma mastitis in dairy cows. The compendium. Compend Edu Prat Vet. 1994;16:541–551. [Google Scholar]
- 14.Kotani H, McGarrity G J. Identification of mycoplasma colonies by immunobinding. J Clin Microbiol. 1986;23:783–785. doi: 10.1128/jcm.23.4.783-785.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Grand D, Solsona M, Rosengarten R, Poumarat F. Adaptive surface antigen variation in Mycoplasma bovis to the host immune response. FEMS Microbiol Lett. 1996;144:267–275. doi: 10.1111/j.1574-6968.1996.tb08540.x. [DOI] [PubMed] [Google Scholar]
- 16.Lysnyansky I, Rosengarten R, Yogev D. Phenotypic switching of variable surface lipoproteins in Mycoplasma bovis involves high-frequency chromosomal rearrangements. J Bacteriol. 1996;178:5395–5401. doi: 10.1128/jb.178.18.5395-5401.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfützner H. Epizootiology of Mycoplasma bovis infection of cattle. In: Stanek G, Cassell G H, Tully J H, Whitcomb R F, editors. Recent advances in mycoplasmology, Proceedings of the 7th Congress of the International Organization for Mycoplasmology. Vol. 20. Stuttgart, Germany: Gustav Fisher Verlag; 1990. pp. 394–399. [Google Scholar]
- 18.Pfützner H, Sachse K. Mycoplasma bovis as an agent of mastitis, pneumonia, arthritis and genital disorders in cattle. Rev Sci Technol. 1996;15:1477–1494. doi: 10.20506/rst.15.4.987. [DOI] [PubMed] [Google Scholar]
- 19.Polak-Vogelzang A A, Hagenaars R, Nagel J. Evaluation of an indirect immunoperoxidase test for identification of Acholeplasma and Mycoplasma. J Gen Microbiol. 1978;106:241–249. doi: 10.1099/00221287-106-2-241. [DOI] [PubMed] [Google Scholar]
- 20.Poumarat F, Perrin M, Belli P, Martel J L. Recherche des anticorps anti-Mycoplasma bovis dans les serum des bovins a l’aide de la réaction d’hemagglutination passive: valeur et limites de la réaction. Rev Med Vet. 1987;138:981–989. [Google Scholar]
- 21.Poumarat F, Perrin M, Longchambon D. Identification of ruminant mycoplasmas by dot immunobinding on membrane filtration (MF dot) Vet Microbiol. 1991;29:329–338. doi: 10.1016/0378-1135(91)90140-b. [DOI] [PubMed] [Google Scholar]
- 22.Poumarat F, Perrin M, Martel J L, Lacombe J P. Etude d’un foyer de mammites àMycoplasma bovis. Rev Med Vet. 1985;161:649–654. [Google Scholar]
- 23.Poumarat F, Le Grand D, Solsona M, Rosengarten R, Citti C. Vsp antigen and vsp-related sequences in field isolates of M. bovis. FEMS Microbiol Lett. 1999;173:103–110. doi: 10.1111/j.1574-6968.1999.tb13490.x. [DOI] [PubMed] [Google Scholar]
- 24.Razin S, Yogev D, Naot Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev. 1998;62:1094–1156. doi: 10.1128/mmbr.62.4.1094-1156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosengarten R, Behrens A, Stetefeld A, Heller M, Ahrens M, Sachse K, Yogev D, Kirchhoff H. Antigen heterogeneity among isolates of Mycoplasma bovis is generated by high-frequency variation of diverse membrane surface proteins. Infect Immun. 1994;62:5066–5074. doi: 10.1128/iai.62.11.5066-5074.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosengarten R, Yogev D. Variant colony surface antigenic phenotypes within mycoplasma strain populations: implications for species identification and strain standardization. J Clin Microbiol. 1996;34:149–158. doi: 10.1128/jcm.34.1.149-158.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sachse K, Pfützner H, Heller M, Hänel I. Inhibition of Mycoplasma bovis cytadherence by a monoclonal antibody and various carbohydrate substances. Vet Microbiol. 1993;36:307–316. doi: 10.1016/0378-1135(93)90097-q. [DOI] [PubMed] [Google Scholar]
- 28.Stipkovits L, Rady M, Glavits R. Mycoplasmal arthritis and meningitis in calves. Acta Vet Hung. 1993;41:73–88. [PubMed] [Google Scholar]
- 29.Subramaniam S, Bergonier D, Poumarat F, Capaul S, Schlatter Y, Nicolet J, Frey J. Species identification of Mycoplasma bovis and Mycoplasma agalactiae based on the uvrC genes by PCR. Mol Cell Probes. 1998;12:161–169. doi: 10.1006/mcpr.1998.0160. [DOI] [PubMed] [Google Scholar]
- 30.Ter Laak E A, Wentink G H, Zimmer G M. Increased prevalence of Mycoplasma bovis in The Netherlands. Vet Q. 1992;15:100–104. doi: 10.1080/01652176.1992.9694342. [DOI] [PubMed] [Google Scholar]
- 31.Trevor R. Dairy calf pneumonia, the disease and its impacts. Vet Clin N Am Small Anim Pract. 1997;13:379–391. doi: 10.1016/S0749-0720(15)30303-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walz P H, Mullaney T P, Render J A, Walker R D, Mosser T, Baker J C. Otitis media in preweaned Holstein dairy calves in Michigan due to Mycoplasma bovis. J Vet Diagn Investig. 1997;9:250–254. doi: 10.1177/104063879700900305. [DOI] [PubMed] [Google Scholar]