Abstract
There has been a significant amount of interest in the past two decades in the study of the evolution of the gut microbiota, its internal and external impacts on the gut, and risk factors for cerebrovascular disorders such as cerebral ischemic stroke. The network of bidirectional communication between gut microorganisms and their host is known as the microbiota-gut-brain axis (MGBA). There is mounting evidence that maintaining gut microbiota homeostasis can frequently enhance the effectiveness of ischemic stroke treatment by modulating immune, metabolic, and inflammatory responses through MGBA. To effectively monitor and cure ischemic stroke, restoring a healthy microbial ecology in the gut may be a critical therapeutic focus. This review highlights mechanistic insights on the MGBA in disease pathophysiology. This review summarizes the role of MGBA signaling in the development of stroke risk factors such as aging, hypertension, obesity, diabetes, and atherosclerosis, as well as changes in the microbiota in experimental or clinical populations. In addition, this review also examines dietary changes, the administration of probiotics and prebiotics, and fecal microbiota transplantation as treatment options for ischemic stroke as potential health benefits. It will become more apparent how the MGBA affects human health and disease with continuing advancements in this emerging field of biomedical sciences.
1. Introduction
Stroke is the second leading cause of mortality and a significant contributor to disability globally [1]. Strokes come in two different varieties: ischemic and hemorrhagic. Ischemic stroke (IS) is caused by a thrombus or embolus blocking a cerebral artery, whereas hemorrhagic stroke is caused by a ruptured cerebral vessel [2]. The most prevalent type of stroke worldwide is IS, with 24.9 million cases annually, which imposes a considerable burden on society [1]. Due to its complicated pathogenesis, it exhibits refractory properties, particularly regarding the secondary damage caused by an early ischemia time window and reperfusion [3]. Therefore, the development of measures to lower the prevalence of IS and its detrimental consequences is highly warranted. Recent research has demonstrated that the gut microbiota regulates the pathogenesis of IS via the microbiota-gut-brain axis (MGBA) [4, 5].
The gut microbiota and gut microbiome refer to the collection of all the gastrointestinal (GI) microorganisms and their genetic material, respectively. These commensal microorganisms include eukaryotes (fungi and parasitic helminths), prokaryotes (bacteria and archaea), and viruses [6]. The community of microbes that resides in the GI tract is the largest and most diverse of all the communities of microorganisms and has received a great deal of attention [6]. Naturally, there is mounting evidence for a two-way exchange of information between the central nervous system (CNS) and the GI microbiota, also known as the MGBA, which has been demonstrated to be a significant contributor to the physiology of the brain. The gut microbiota can affect the cardio-cerebral-vascular system, immune system, gut function, and physiological activities through signaling molecules and bioactive metabolites. Additionally, the prevalence of IS are closely related to unchangeable factors (sex, age, and genetic predisposition or pathological alterations) and modifiable factors (hypertension, diet, lifestyle, obesity, hyperlipidemia, smoking, and abnormal blood glucose) [7, 8]. They all significantly impact the diversity and abundance of the gut microbiota.
Here, we concentrate on the gut microbiota's current role in the pathogenesis of IS and how it affects its associated risk factors. We also discuss the potential of the gut microbiota as a novel therapeutic option for the prevention and treatment of IS.
2. Healthy Microbiome and Dysbiosis
The respiratory tract, skin surface, genitourinary systems, and GI tract all include commensal microorganisms. The gut contains commensal microbes, which comprise approximately 95% of the human microbiome. There are over 100 trillion bacteria, representing up to 5000 different species, and they weigh approximately 2 kg in the human gut, which contains ten times more microbial cells than the entire body [9]. More than 100 bacterial phyla constitute the human GI microbiota, with the majority of these species belonging to two phyla, namely, Firmicutes (Ruminococcus, Clostridium) and Bacteroidetes (Prevotella, Porphyromonas), with relatively small amounts of Actinobacteria (Bifidobacterium), Proteobacteria, and Verrucomicrobia [10]. It has been established that the ratio of the bacterial species of Bacteroidetes to Firmicutes significantly impacts health and disease [11]. In addition, it is crucial to emphasize that the gut microbiota is heterogeneous, with microbial density and diversity rising along the GI tract following immunological, chemical, and nutritional gradients [12]. As a result, each species is exceptionally well adapted to carry out particular functions in a specific digestive tract environment [12]. These microorganisms have developed a close, mutually advantageous symbiotic relationship with their hosts during the eons of coevolution rather than passively colonizing their hosts' guts [12]. The host supplies shelter and nutrition for its microbial subtenants, and the microorganisms provide numerous health benefits in exchange [13]. Specifically, the digestion of food, production of metabolites, facilitation of nutrient absorption, and metabolism of xenobiotics and drugs are all positive functions of the intestinal microbiota in a healthy condition that supports host nutrition metabolism [14]. A well-balanced intestinal microbiota maintains a normal intestinal epithelial barrier by maintaining the structural integrity of tight-junction proteins, upregulating mucin genes, and limiting the adhesion of epithelial cells to pathogenic bacteria [15]. It also helps immunological initiation, modulation, and pathogen resistance [16].
Gut dysbiosis, also known as gut microbial dysbiosis, refers to pathological abnormalities in the composition, diversity, and abundance of intestinal flora that affect intestinal metabolism, the immune state, systemic inflammation, and other responses [17]. It is characterized by decreased microbial diversity, fewer beneficial bacteria, or a higher concentration of pathogenic microorganisms. The disruption of MGBA signaling caused by dysbiosis of the intestinal flora usually contributes to alterations in the intestinal structure and increased permeability of the mucosal epithelial barrier, resulting in pathophysiological effects [18]. Specifically, multiple factors inducing gut microbial dysbiosis can lead to leakiness of the intestinal wall, resulting in easier entry of endotoxins, microbial elements, and microbial metabolites into the systemic circulation, ultimately triggering an immune response and exacerbating systemic inflammation [19]. Gut dysbiosis causes T cells to polarize into proinflammatory Th17 (IL-17), Th1 (IFN-γ), or γδcells. These cells then migrate from the small intestine to the ischemic brain, where they cause infarct damage [20, 21]. Therefore, IS may develop when there is an imbalance in the bacterial species.
3. Alterations in the Gut Microbiota during Ischemic Stroke
Microbiome-associated molecular patterns (MAMPs) and metabolites secreted by the microbiome can interact with the mucosal epithelium and intestinal immune cells, stimulate the vagus nerve (VN), or enter the systemic circulation to communicate with the brain and potentially modify neuronal and immune responses [22]. In turn, the parasympathetic and sympathetic nerve fibers of the gut wall convey signals to the brain to influence immune cell and gut motility activity and alter the composition of the gut [23] (this is described in detail below). The commensal microbiota change such that opportunistic pathogens become dominant after IS. This change is most likely caused by the release of cytokines and chemokines produced in the brain, altered intestinal motility and permeability, and mucus production, all of which contribute to dysbiosis. The result of an IS is subsequently worsened by dysbiosis. Acute IS risk factors such as age, hypertension, diabetes, obesity, and vascular dysfunction have also been linked to gut flora dysbiosis [24].
Experimental and clinical research has demonstrated that the gut microbiota composition significantly influences the incidence and outcome of IS [20, 21]. Furthermore, the gut's microbial composition substantially changes during the acute stage of IS [25]. Singh and colleagues showed that cerebral IS results in microbiota dysbiosis, with decreased bacterial diversity in mouse feces including a reduced abundance of Firmicutes and an excessive increase in Bacteroidetes. These changes are connected to decreased intestinal motility and increased intestinal wall permeability. Additionally, they discovered that microbiota transplantation might improve IS outcomes and impact immunity [21]. Yin and colleagues studied the gut microbes of patients with IS. They found that transient ischemic episodes were primarily associated with opportunistic pathogenic bacteria such as Enterobacter, Desulfovibrio, and Megasphaera, while beneficial bacteria such as Faecalibacterium and Prevotella were depleted [26]. Another study found that individuals with IS had higher levels of Atopobium and Lactobacillus ruminans, but Lactobacillus levels were decreased [27]. Chen and colleagues found an increased relative abundance of Bacteroidetes and decreased relative levels of Faecalibacterium, Oscillospira, Lactobacillus, and Streptococcus in a study of monkeys with focal cerebral ischemia, suggesting a correlation with the poststroke inflammatory response [28]. Additionally, they discovered a decline in plasma butyrate concentrations, which may be connected with a reduction in Oscillospira and Faecalibacterium levels. Monkeys with cerebral ischemia for 6-12 months had decreased plasma levels of short-chain fatty acids (SCFAs), suggesting that persistent gut flora dysbiosis may also influence the generation of SCFAs [28]. Li and colleagues' findings imply that the intestinal flora is related to stroke severity. Lachnospiraceae, Pyramidobacter, and Enterobacter were increased in patients with mild stroke, but Ruminococcaceae and Christensenaceae were increased in patients with severe stroke [29]. Therefore, dysbiosis not only develops after stroke but also plays a role in its onset.
Benakis et al. [20] reported the relationship between the gut microbiome and the neuroinflammatory response to IS for the first time. They found that the symbiotic gut microbiota protects the brain by controlling immune cells in the small intestine; bacterial priming of dendritic cells leads to the growth of Treg cells, which suppress IL-17+γδ T cells. Additionally, they showed that T cells move from the intestines to the meninges, where they control the neuroinflammatory reaction [20]. Studies have indicated that lipopolysaccharides (LPS) may play a key role in chronic systemic inflammation after stroke [30], and elevated levels of plasma LPS or inflammatory cytokines are strongly connected with the overgrowth of Bacteroidetes [28]. The higher levels of proinflammatory tumor necrosis factor (TNF-α), interleukin-6 (IL-6), and interferon-gamma (IFN-γ) in the plasma of focal cerebral ischemia monkeys suggest both intestinal microecological dysregulation and chronic systemic inflammation following cerebral infarction [28]. As a result, chronic systemic inflammation and the poststroke gut microbiota may be potential stroke therapeutic targets.
4. Mechanism of the Interactions between the Gut Microbiota and Brain
The CNS, neuroendocrine, immunological, and autonomic nervous system (ANS) are all involved in the MGBA, which links the brain and the gut via direct and indirect channels. What is known is that constant communication within the MGBA maintains particular aspects of homeostasis, partly via signals originating from gut microbes. This exchange is bidirectional; hence, the gut microbiota can influence the host by altering homeostasis components in both directions. The ability of bacteria to produce neuroactive molecules that promote the communication of the MGBA is becoming clearer (Figure 1).
Figure 1.
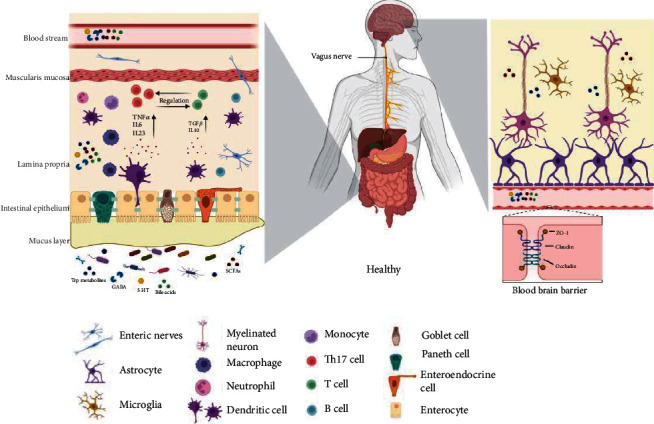
An illustration of the MGBA in the healthy individual. In ENS, the gut microbiota affects how the brain works by releasing various signal molecules that may go to target areas through systemic circulation. Through the ANS, the CNS physiologically controls the GI tract; in turn, the gut provides feedback to the brain to establish a bidirectional link.
4.1. Neural Pathways
The bidirectional connection of the MGBA occurs along two distinct neuroanatomical pathways. One is the direct gut-brain communication that occurs through the spinal cord's VN and ANS. The other is communication between the spinal cord's VN and ANS and the gut's enteric nervous system (ENS) [31]. The VN is a mixed nerve that connects to the brain and gut and has anti-inflammatory capabilities through its afferent and efferent fibers. VN afferent fibers can be stimulated by microbiota components either directly or indirectly via ENS. Through the inflammatory reflex, VN afferent fibers can activate efferent fibers. The vagal efferents in the medullary dorsal motor nucleus of the vagus, which are part of the vago-vagal anti-inflammatory reflex loop, have the ability to regulate the levels of proinflammatory cytokines in the circulation [32]. The VN transports approximately 70% of the parasympathetic fibers and innervates the whole GI system as well as thoracic and abdominal organs [33]. In stroke, the α7 nicotinic acetylcholine receptor (α7-nAchR) plays a vital role as a modulator of cholinergic anti-inflammatory pathways. It has been discovered that endotoxemic mice lacking α7-nAchR have higher amounts of TNF-α, IL-1β, and IL-6 in their systems [34]. VN stimulation activates α7-nAchR, which lowers pyroptosis, a type of programmed cell death, and increases blood–brain barrier (BBB) tight-junction proteins such as occluding junctions [32, 35].
The ANS is composed of sympathetic and parasympathetic branches. The parasympathetic nervous system controls mood, memory, and appetite and senses microbial metabolites [36]. GI transit, secretion, and motility are all affected by altered sympathetic neurophysiology, principally due to changes in cholinergic transmission and the contractions of smooth muscle. The sympathetic and parasympathetic systems can impact the neuronal circuitry of the ENS, leading to altered motility, which can affect the delivery rate of probiotics and other essential microbial nutrition to the small intestine and colon [37]. The ENS is made up of the submucosal plexus, which controls water and electrolyte flow, and the intermuscular plexus, which controls peristalsis [38]. Acetylcholine and 5-hydroxytryptamine (5-HT) are two of the ENS neurotransmitters that enteroendocrine cells (EECs) can release in response to elevated microbiota metabolites (e.g., SCFAs). The communication between the microbiota and the ENS can transform luminal metabolites into neurochemical signals that control intestinal physiology and may be involved poststroke. The administration of the L. rhamnosus strain JB-1 affected mouse behavior via the vagus nerve and caused changes in the expression of γ-aminobutyric acid (GABA) receptors in specific brain regions [39]. Mechanisms for the effects of the L. rhamnosus strain JB-1 on ENS function further demonstrate that intact epithelium is necessary to mediate the action of JB-1-derived microvesicles, which in turn reflects the effects of the strain on the ENS. This finding suggests that epithelial components may be paramount in mediating microbial signaling from the intestinal lumen to the ENS [40]. These findings suggest that the gut microbiota mediates the neuronal pathways of the MGBA.
4.2. Immune Pathways
The immune system and the CNS are intricately organized systems that govern and manage various bodily activities. They both have similar traits in their operational modes and developmental processes. Our intestinal single-cell layer plays an important role in restricting the contact of the gut microbiome with visceral tissue. To do this, the goblet cells of the epithelium secrete a protective viscous mucus layer. Most host-microbe interactions occur at this lumen-mucosal interface, where molecular interactions between mucus layers and epithelial cells facilitate communication between the gut and immune system by distinguishing between self and nonself antigens, enabling the immune system to recognize potentially harmful pathogens [41].
The activity of cells from the myeloid lineage, including neutrophils, macrophages, microglia, mast cells, natural killer T cells, and lymphocytes (T cells), is called innate immunity and is thought to be the body's main line of defense against potentially infectious organisms [42]. It was determined that the maturation of microglia and maintenance of their healthy functional condition require a varied GI microbiome [43]. In contrast, the lack of diverse host microbiota (i.e., GF mice) in the increased microglia population resulted in defects in microglia maturation and differentiation, changed microglia morphology, and impaired immune responses to bacterial infections [44]. Another study revealed that GF mice exhibit impaired microglial cell maturation and that SCFA production by the gut microbiota may affect microglial cell maturation [45]. Cerebral ischemia-induced neuronal cell death releases damage-associated molecular patterns (DAMPs) [46]. The production of proinflammatory cytokines such as IFN-γ, IL-6, IL-1, and TNF-α increases as a result of DAMPs and neurotoxin-mediated activation of M1 microglia, which is linked to secondary neuronal injury caused by BBB breakdown [47]. Activation of M2 macrophage subtypes promotes the release of chemokines like CCL13 and CCL14 or cytokines like TGF-β and IL-10, which improve the outcome poststroke by enhancing BBB integrity, angiogenesis, and tissue healing [48]. Gut microbiota dysbiosis has been linked to increased plasma levels of proinflammatory cytokines caused by loss of BBB integrity [49]. Therefore, a critical therapeutic target for treating ischemic brain injury could be regulating innate immune responses with the aid of the microbiota and its metabolites.
T and B lymphocytes play a pivotal role in mediating the activation of the adaptive immune response. Immune homeostasis is vitally maintained by regulatory T cells (Tregs). Approximately 10% of peripheral CD4+ T cells are CD25+Foxp3+ Treg cells, and they play a crucial role in the induction of immunosuppression by producing cytokines such as IL-10, TGF-β, and IL-35 [50]. IL-10 has anti-inflammatory properties that help to maintain gut homeostasis. According to studies, GF mice have few T helper 1 (Th1) and Th17 cells and IL-22 and IL-17, which causes a reduction in lamina propria- (LP-) associated CD4+ lymphocytes [51]. The ability of dendritic cells to endocytose polysaccharides may help naive T cells expand and differentiate into Th17 and Treg cell subsets. Despite their divergent functional characteristics, the degree of TGF-β expression determines the development of naive T cells into Th17 cells and Treg cells. Naive T cells are transformed into Th17 cells by low levels of TGF-β, IL-23, or IL-6, whereas high levels of TGF-β produce Treg cells [52]. In mice with a proinflammatory microbiota, the poststroke polarization of Tregs by naive T cells is inhibited, and the polarization of proinflammatory IL-17+ γδ T cells is promoted [20]. More IL-17 secretions could be released by the accumulating T cells, thereby aggravating brain damage [53]. Singh et al. [21] transplanted feces from mice with IS into germ-free mice and discovered that both Th1 and Th17 cells produced significantly more IFN-γ or IL-17 after brain damage in mice. Microbial antigens affect B cell activation and differentiation via TLRs [54]. The significance of regulatory T and B lymphocytes in the neuroprotective pathway following the development of IS requires more research.
MAMPs like LPS and peptidoglycans are more likely to enter the bloodstream through a leaky gut, triggering an immune reaction in the host. Toll-like receptor 4 (TLR4) localization and subsequent TLR signaling can both be induced by LPS [55]. Bacterial LPS can bind to TLR4 through a lipid-binding CD14 [56]. Numerous protein kinases, including myeloid differentiation factor 88 (MyD88), can be activated by this process. A range of chemokines, cytokines, and other immune factors can be produced when MyD88 is activated. Recent studies have found that activation of the TLR4/MyD88 signaling pathway promotes cellular injury in cerebral ischemic stroke [57]. Here, we outline the mechanisms driven by the bacteria that induce IS (Figure 2).
Figure 2.
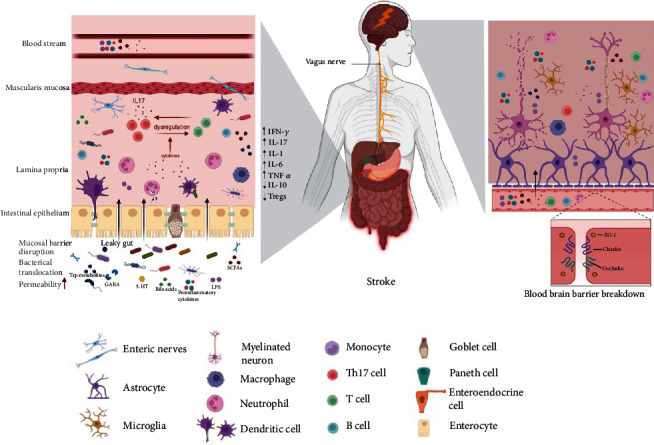
An inflammatory mechanism of the MGBA in IS. By controlling the integrity of the intestinal mucosal barrier, which includes inflammatory cell activation and the production of proinflammatory substances, gut microbiota and their metabolites play a role in the development of intestinal inflammation. Through the expression of TLR, microbial metabolites like TMAO and LPS can also contribute to neuroinflammation and intestinal inflammation. The vagus nerve and blood circulation establish a pathway for intestinal inflammation through which the neurotoxins produced in the gut can reach the brain. The specific manifestations of microbiota-mediated neuroinflammation include the breakdown of the blood-brain barrier, activation of microglia, astrocyte proliferation, and the generation of proinflammatory cytokines.
4.3. Neuroactive Pathways
Several neuroactive substances with host and microbial origins also play a crucial signaling role in host-microbiota interactions at the intestinal interface.
Catecholamines (CChs) include epinephrine, norepinephrine, and dopamine. CChs act as chemical neurotransmitters in the central and peripheral nervous system regulating various physiological processes and functions, including cognitive performance, intestinal motility, and mood [58]. Escherichia species, including commensals found in human guts, are known to produce CChs like norepinephrine. Norepinephrine exerts neuroprotective effects in the brain by reducing the transcription of inflammatory genes and boosting the creation of brain-derived neurotrophic factor (BDNF) by microglia and astrocytes, which can further enhance neuronal survival [59]. The gut produces more than 50% of the dopamine in humans, and gut bacteria can control peripheral dopamine levels. Also discovered in the biomass of Escherichia coli, Bacillus mycoides, and Staphylococcus aureus was dopamine [60]. The generation of cytokines by activated T cells and the activity of effector immune cells are regulated by dopamine [61]. Previous crossover experimental studies have shown that long-term levodopa administration in chronic stroke patients significantly improves motor performance in chronic stroke patients [62]. However, a recent study by Ford et al. showed that co-careldopa does not improve walking after stroke [63]. Therefore, more research is required to fully comprehend CChs dynamics in the gut lumen and its effects on mucosal immunity.
GABA is the main inhibitory neurotransmitter in the CNS. It was found that the introduction of a GABA-producing Bifidobacterium strain was adequate to regulate GABA levels in the gut [64]. GABA-mTORC1 signaling facilitates gut IL-17 expression when GABA levels are elevated in the jejunum of mice [65]. The study found that GABA selectively stimulates mucin-1 expression in epithelial cells. Precisely, GABA exposure to epithelial cells reduced IL-1β-mediated inflammation, increased tight junctions, and transformed growth factor beta (TGF-β) expression, which had a protective impact against the breakdown of the intestinal barrier [66]. Within the ENS, GABA, particularly the GABAA receptor system, plays a role in neuronal excitability. It has been discovered that GABAA receptors, in particular, mediate the suppression of T cell responses, which indicates GABA's potential role as a natural immunomodulator of T lymphocytes [67]. In experimental studies, activation of GABA receptors has been shown to have a neuroprotective effect in animal models of stroke by reducing infarct size and improving functional outcomes [67]. However, clinical trials did not support using GABA receptor agonists to treat acute stroke patients [68]. Further research is necessary to understand glutamate and GABA's relevance and functional dynamics as mediators of host-microbe interaction.
The cellular sources of histamine that have been best defined are mast cells, basophils, and histaminergic neurons. Cytokines such as IL-1, IL-12, IL-18, TNF-α, and calcium ionophores affect histidine decarboxylase (HDC) activity [69]. It has been demonstrated that histamine from Lactobacillus reuteri suppresses human monocytoid cells' ability to produce TNF-α in response to TLRs through signaling through the histamine H2 receptor and downstream cAMP and PKA activities [70]. However, a recent study in aged mice following experimental stroke showed that stroke resulted in increased intestinal mast cell numbers and intestinal histamine receptor expression levels. In the peripheral circulation, these gut-centered changes were linked to increased histamine levels and other proinflammatory cytokines (such as IL-6, TNF-α, and IFN-γ) [71]. It is obvious that histamine mediates host-microbe crosstalk as a neuroimmune system; however, it is still unclear how host histamine may affect microbial activity.
It has been demonstrated that serotonin (5-HT) functions as a neuroendocrine signal of host-microbe crosstalk, modulating bacterial motility, biofilm formation, exopolysaccharide production, and inducing the expression of virulence genes in bacteria via a quorum sensing mechanism [72]. Exogenous serotonin treatment worsened pathogenic intestinal symptoms, increased the formation of biofilm on mouse guts, and raised the release of proinflammatory cytokines. Serotonin levels in the blood and colon were lower in GF mice, and the brain's serotonin turnover rate was higher [73]. Serotonin produced by the gut microbiota in mammals can operate locally in the GI tract or reach the bloodstream, but it cannot cross the BBB [74]. However, serotonin has been shown to promote BBB permeability, indirectly affecting brain function [75].
4.4. Hypothalamic–Pituitary–Adrenal Axis Pathways
One of the primary neuroendocrine systems in the human body is the hypothalamic–pituitary–adrenal (HPA) axis, which also functions as a critical nonneuronal communication link in the MGBA [76]. The HPA axis and its neurotransmitter counterpart cause several changes in the neuro-immune system that prepare the body for the “fight or flight” reaction to stress [77]. Interactions between the immunity-HPA axis have been linked to a number of inflammatory and stress-related illnesses. The HPA axis is finally activated when exposure to microbes and antigens outside the epithelial barrier triggers the mucosal immune response [78]. The HPA axis functions can be regulated by stress response by modulating the expression of BDNF, the 2A subtype of N-methyl-D-aspartic acid (NMDA) receptors, and the hippocampus. A key component of the stress response is the cortisol. The HPA axis controls the release of CChs, mineralocorticoids, or glucocorticoids in response to stress to modify the intestinal microenvironment [79]. The corticosterone-releasing factor (CRF) in the hypothalamus can be altered by gut microbiota. Serum cortisol levels have been linked to poststroke mortality and severity [80]. The higher stress response in GF mice was slightly alleviated by fecal microbiota transplantation (FMT) and was entirely reversed over time by Bifidobacterium treatment [81]. Corticosterone or cortisol levels were lower in preclinical and clinical investigations following probiotic or prebiotic administration [82]. As a result, the HPA axis, a crucial regulator of the stress response, can affect how the MGBA is regulated.
4.5. Role of Gut Microbial Metabolites
4.5.1. Trimethylamine N-Oxide (TMAO)
Through the action of intestinal microbial TMA lyases, trimethylamine (TMA) is generated from dietary nutrients (choline, L-carnitine, and phosphatidylcholine) [83]. The host's hepatic flavin-containing monooxygenases (FMOs) convert TMA to trimethylamine N-oxide (TMAO) [84]. Risk factors for recurrent IS and cardiovascular events with elevated TMAO levels were strongly associated with the levels of proinflammatory monocytes (CD14++/CD16+) [85]. When TMAO stimulates platelets, there is a potential for thrombosis due to an increase in the release of Ca2+ from intracellular reserves and platelet-dependent hyperreactivity [86]. Another study showed that after a choline stimulation test, intestinal symbionts transplanted from donors with high or low circulating TMAO levels had different effects on mice with arterial damage in terms of platelet reactivity and thrombosis potential [87]. Zhu et al. [88] discovered by microbial transplantation that the gut microbe CutC (an enzyme source for the conversion of choline-to-TMA) boosted host TMAO synthesis, enlarged the infarct area in the brain and caused functional impairment. In addition, atherosclerosis is a risk factor for IS. Wang et al. [89] reported that dietary supplementation with choline or TMAO increased atherosclerosis in Apoe−/− mice and promoted macrophage cholesterol accumulation and foam cell formation. Studies using animal models have shown that inhibiting FMO3 activity decreases TMAO levels and inhibits atherosclerosis [90]. TMAO increases the inflammatory responses in the vascular wall, causes the generation of reactive oxygen species (ROS), and inhibits the reverse transport of cholesterol, which leads to atherosclerosis [91].
4.5.2. Short-Chain Fatty Acids (SCFAs)
Through bacterial fermentation, resistant starch and dietary fibers are degraded into SCFAs, such as acetate, propionate, butyrate, and other related compounds (5 carbons or less) [92]. SCFAs exert their effects by inhibiting histone deacetylases (HDACs) to influence gene expression and the ligands of the subset of G-protein coupled receptors (GPCRs) in the host epigenome [93]. Peripheral blood monocytes exposed to SCFAs showed reduced proinflammatory TNF-α production, inhibited NF-κB activation [94], and decreased leukocyte adherence to endothelial cells by altering vascular cell adhesion molecules [95]. It has been demonstrated that SCFA stimulation of EECs causes the release of hormones such as glucagon-like peptide 1 (GLP-1) and peptide YY (PYY) [96]. This activity may initiate a signaling cascade that affects brain circuits that regulate appetite and inhibit gastric motility through the systemic circulation or vagal afferents. A prime illustration of a specialized intestinal cell that can act as a sensor for neurochemical signals derived from the microbiota and the CNS is the modulation of GLP-1 signaling by EECs. It enables regulating GI secretory activity, systemic immunity, obesity, diabetes, and other stroke risk factors. PYY affects brain activity and appetite by mechanisms that cross the BBB. Fermentable polysaccharides were added to human diets to raise plasma levels of PYY and GLP-1 [97]. Significantly fewer SCFA-producing bacteria, such as Anaerostipes, Butyriciocus, Faecalibacterium, and Lachnoclostridium, were present in patients with IS [98]. The decrease in SCFA levels may be partly due to poor prognosis in aged mice after stroke. Aged mice received a mixture of probiotics that produce SCFAs, including Lactobacillus fermentum, Bifidobacterium longum, Clostridium symbiosum, Faecalibacterium prausnitzii, and inulin, which resulted in an increased production of SCFAs and attenuated stroke-related neurological deficits and inflammation [99]. Dysbiosis of the intestinal flora linked to atherosclerosis revealed a decrease in butyrate producers such as Eubacterium and Roseburia in the intestine [100]. Experimental investigations have demonstrated that intestinal injection of butyrate lowers inflammation and atherosclerosis [101].
4.5.3. Bile Acids (BA)
The chemical diversification of bile acids (BA) is the result of a cooperative effort between the host (synthesis of primary bile acids) and the gut microbiota (synthesis of secondary bile acids) [102]. BAs have been demonstrated to modulate systemic lipid metabolism, glucose metabolism, and cholesterol as well as immune homeostasis through the interaction of these amphiphilic molecules with membrane and nuclear receptors, including farnesoid X receptors (FXRs) and Takeda G-protein-coupled receptor 5 (TGR5) [103]. The main bacterial species in the gut, such as Lactobacillus and Bifidobacterium taxa, express the enzyme bile salt hydrolase (BSH), which deconjugates BAs from glycine and taurine [104]. According to research, intestinal inflammation [105], metabolic disorders such as diabetes [106], and cardiovascular disease [107] are pathologically determined by gut microorganisms and abundant BSH genes. Metabolite analysis of young stroke patients showed that their serum glycochenodeoxycholic acid (GCDCA) concentration was significantly higher than that of healthy controls [108]. Furthermore, it has been demonstrated that elevated taurocholic acid (TCA) and GCDCA concentrations following liver injury activate the glucocorticoid receptor (GR), which disrupts the hypothalamic–pituitary–adrenal (HPA) axis [109].
As a protective BA in brain diseases, tauroursodeoxycholic acid (TUDCA) has been thoroughly investigated for its anti-inflammatory and antioxidant properties [110]. TUDCA induces anti-inflammatory markers by binding to TGR5 [111]. Moreover, Cheng and colleagues discovered that TUDCA might be linked to suppressing oxidative stress in the brain [112]. Oxidative stress is a pathophysiological process after stroke and is closely associated with neuroinflammation, excitotoxicity, and apoptosis [113]. Compared to the untreated group, IS rats receiving a mixture of hyodeoxycholic acid (HDCA) and cholic acid (CA) had smaller infarcts and considerably lower TNF-α and IL-1 concentrations [114]. It has been noted that specific BAs metabolized by cytotoxic and hydrophobic gut bacteria, such as deoxycholic acid (DCA) and lithocholic acid (LCA), can worsen brain damage [115]. Another study discovered that decreased BA excretion may be an independent risk factor for IS and that greater DCA and LCA concentrations in fecal samples were related to higher poststroke survival [116].
4.5.4. Tryptophan
In the digestive tract, there are three main pathways for tryptophan metabolism: (1) the gut microbiota's direct conversion of tryptophan into several compounds, including aryl hydrocarbon receptor (AHR) ligands [117]; (2) the indoleamine 2,3-dioxygenase (IDO) 1-mediated kynurenine pathway in both immunological and epithelial cells [118]; and (3) the Trp hydroxylase 1- (TpH1-) mediated serotonin (5-HT) synthesis pathway in enterochromaffin cells [117]. Intestinal microorganisms can metabolize tryptophan to produce molecules. For instance, tryptophan is used by Escherichia coli (E. coli) to produce indoles, which have beneficial effects on the intestinal flora and can lessen the biofilm and virulence development of E. coli and other bacteria [119]. In the intestinal microbial environment, indole is a significant intercellular signal that interacts with the intestinal epithelium by enhancing tight-junction resistance and the expression of anti-inflammatory cytokines [120]. The activation of astrocytes and microglia is regulated by tryptophan metabolites, which also govern neuroinflammation via AHR signaling [121]. Patients with acute ischemic stroke (AIS) and carotid stenosis reported lower concentrations of tryptophan and 3-hydroxyanthranilic acid (3-HAA) and higher levels of circulating arachidonic acid (AA) and 3-hydroxykynurenine (3-HK) in their blood than those of controls [122]. The ratio of kynurenine to tryptophan and stroke severity were positively correlated in a study of IS patients [123]. More research is required to fully understand how microorganism-derived tryptophan metabolites are linked to inflammation in brain illness.
5. Gut Dysbiosis and IS Risk Factors
In addition to being closely linked to gastrointestinal disorders such as irritable bowel syndrome and ulcerative colitis, an imbalance in the gut microbiota is also linked to the occurrence and progression of aging, hypertension, diabetes, obesity, and atherosclerosis, all of which are risk factors for IS [124].
5.1. Gut Microbiota and Aging
The incidence of stroke increases with age, with persons over 65 accounting for 70–80% of all IS [125]. Scientists can now examine alterations in the gut microbiota of elderly individuals because of recent developments in next-generation sequencing (NGS) and metagenomic technologies [126]. IS is mostly an aging-related disease, and aged mice do worse than younger mice when they experience an experimental stroke [127].
Recent research using experimental animal models has revealed that older mice tend to be more susceptible to infection after IS, which is at least partially caused by decreased intestinal barrier integrity and intestinal inflammation, as well as reduced expression of mucin and tight-junction proteins, which facilitates bacterial translocation [71]. Aged mice had a worse prognosis after IS than young mice. Crapser and colleagues demonstrated that IS causes intestinal permeability and bacterial translocation in young and aged mice. Young mice are able to resolve these issues, whereas aged mice experience prolonged sepsis and worse functional recovery following IS [128]. The unavoidable biological aging process significantly increases the risk of stroke and is linked to significant alterations in the gut microbiota composition. Specifically, aging is associated with significant decreases in Firmicutes and Bifidobacterium and increases in Bacteroidetes and Proteobacteria, particularly Gammaproteobacteria [129]. Another study showed that elderly mice had a Bacteroidetes/Firmicutes ratio that was nine times higher than that of younger mice. Young mice that received FMT from an elderly donor had higher levels of systemic proinflammatory cytokines and higher mortality after middle cerebral artery occlusion (MCAO). In contrast, FMT from young to elderly mice increased survival, enhanced motor strength after recovery from proximal MCAO, and improved motor function and anxiety [130].
These findings indicate that aging affects poststroke functional outcomes and survival by increasing gut dysbiosis. To potentially enhance stroke outcomes and recovery, it may be possible to alter the age of the gut microbiota, especially in elderly individuals.
5.2. Hypertension
The development of IS by hypertensive diseases is influenced by endothelial dysfunction, increased shear stress, and stiffness of the large arteries that transport pulsatile flow to the cerebral microcirculation [131]. Experimental studies have validated the role of the intestinal microbiota in the emergence of hypertension. First, giving germ-free recipient mice the gut microbiomes of hypertension patients raises their blood pressure [132]. Furthermore, germ-free mice exhibit decreased renal and vascular immune cell infiltration after receiving angiotensin II injections. They are also resistant to hypertension and vascular dysfunction [133]. Yang et al. [134] discovered an imbalance in the hypertensive rat microbe populations, which decreased overall, while Bacteroides numbers were elevated. Human research suggests that intestinal mucosa shape, gut-derived metabolites, and microbial taxa are all linked to hypertension. Compared to normotensive people, hypertensive people exhibit a microbial shift with an increased abundance of pathogenic taxa and lower microbial richness and diversity [135].
It has recently been found that excess Na+ can even be detected in intestinal microbiota and immune cells, which can promote inflammation and hypertension; an excessive amount of salt causes organ damage to the kidneys, vasculature, and CNS [135]. Low-sodium dietetic control is advised in poststroke therapy to promote healing and lower the chance of stroke recurrence. A high-sodium diet in mice reduces the Firmicutes to Bacteroidetes ratio, fosters gut barrier dysfunction, and damages inflammatory reactions [135]. Inflammatory pathways are controlled by the gut microbiota, which also plays a role in the etiology of hypertension. By raising the number of proinflammatory Th17 cells in the spleen, high salt intake also decreases the population of Lactobacillus murinus. In treated rats, daily injection of Lactobacillus murinus reduces Th17 cells and lowers blood pressure [136].
Gut microbiota-producing metabolites such as TMAO and SCFAs are crucial in developing hypertension. In animal models of hypertension, SCFAs reduce blood pressure and gut dysbiosis and restore the balance between Th17 and Treg cells [137]. SCFAs may modulate the SCFA receptor G-protein coupled receptor 43 (Gpr43) to promote Th1 but restrict Th17 cell differentiation to control blood pressure [138]. An accurate study of individual species and their metabolic products is needed to further our understanding of the gut microbiota and blood pressure management after stroke since the impacts of various metabolites may cause the disease to proceed in different directions.
5.3. Diabetes Mellitus/Obesity
The risk, prognosis, and outcome of IS are often worse for diabetic individuals than for nondiabetic individuals. Many stroke patients also have hyperglycemia even if they have no history of diabetes [139]. Two large-scale metagenome analyses in China and Europe examined the gut microbiota of type 2 diabetes (T2D) patients and healthy individuals. These two studies showed an increase in Clostridium hathewayi and a decrease in Roseburia in patients with T2D [140, 141]. It has been proposed that diabetes may decrease SCFA production or uptake, particularly the anti-inflammatory butyrate [141]. A recent study uncovered a causal link between the insulin response and a genetically driven rise in butyrate synthesis in the host. Conversely, propionate production or absorption irregularities have been linked to a higher incidence of diabetes [142].
GLP-1 produced by EECs in the gut microbiota regulates satiety and hunger. It is possible to distinguish between people with and without diabetes using the transcriptome signature of EECs in obese subjects. Notably, obese diabetic individuals exhibited decreased plasma GLP-1 as well as proglucagon maturation and GLP-1 cell differentiation [143]. An excellent illustration of a specialized intestinal cell that can act as a sensor for neurochemical signals derived from the microbiota and the CNS is the modulation of GLP-1 signaling by EECs. This allows for the reconciliation of known risk factors for IS, such as gastrointestinal secretion function, obesity, and diabetes [124]. Obesity is a significant risk factor for T2D. Experiments with fecal microbiota transplantation have shown that the gut microbiota is crucial for insulin resistance, adipose tissue accumulation, and energy absorption [144]. Several studies have also revealed considerably lower Firmicutes/Bacteroides ratios in the intestines of obese people and rats [145].
5.4. Atherosclerosis
Atherosclerosis is intimately associated with arterial stiffness, which results from the loss of elastic fibers and the thickening of arteriole walls. The accumulation of cholesterol in the arterial wall causes macrophages to phagocytose lipoproteins, forming foam cells characteristic of atherosclerosis [146]. Compared to asymptomatic controls, patients with atherosclerotic stroke had a different gut microbial composition, specifically an increased abundance of opportunistic pathogens (e.g., Enterobacter and Desulfovibrio) and a decreased abundance of commensal or beneficial genera (e.g., Prevotella and Faecalibacterium) [27]. Karlsson and colleagues discovered an increased abundance of the genus Collinsella in stool samples from individuals with symptomatic atherosclerosis (cerebrovascular events), whereas Eubacterium and Roseburia were enriched in controls, implying the existence of dysbiosis in atherosclerosis [147]. Patients with vascular disease frequently have oral Streptococcus mutans in their atherosclerotic plaques [148]. Additionally, it has been discovered that the oral bacterium Porphyromonas gingivalis is linked to the onset of IS [149].
One of the metabolites produced by the gut microbiota, namely, TMAO, has undergone extensive research and has been positively linked to the development of early atherosclerosis [88]. The current study suggests that TMAO can exacerbate brain damage following IS through many pathophysiological mechanisms. It can also induce vascular inflammation and endothelial dysfunction, increasing atherosclerosis and thrombogenesis [150].
Few studies have focused on the connection between atherosclerosis, bacteria in plaque, and the gut microbiota in individuals with IS. There is still much to study about how the gut microbiota influences the pathophysiology of atherosclerosis.
6. Intervention and Management Strategies for Ischemic Stroke That Target the Gut Microbiota
The findings of investigations on both humans and animals point to the possibility that gut dysbiosis may be a risk factor for the occurrence, severity, and prognosis of IS in patients. Emerging treatment approaches aim to reestablish a healthy gut flora to prevent and treat IS. Nutritional therapies that modify the gut microbiota to a healthy condition by diet, probiotics, prebiotics, and FMT through normal donors may aid in preventing the pathogenesis of IS (Figure 3).
Figure 3.
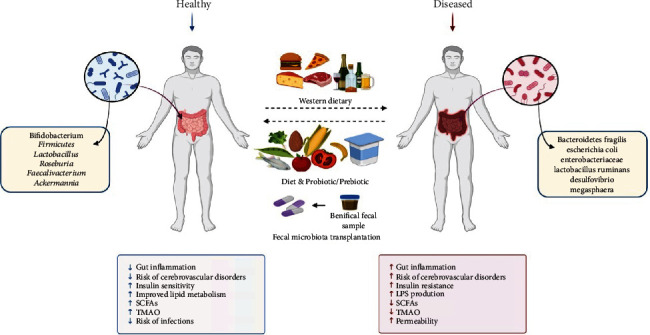
There are several ways to control IS by restoring the dysbiotic gut. A few strategies to treat gut dysbiosis include dietary modification by adding nutritious ingredients boosting the colonization of good bacteria in the gut using probiotics or prebiotics and FMT.
6.1. Diet
It is evident that diet is one of the significant indicators of the intestinal microbiota and that changes in dietary habits can directly impact its composition, variety, and metabolic capacity through the availability of macro- and micronutrients in the intestine [151]. Animal-based protein increases the number of potentially harmful gut microbes in mice, such as Escherichia, Ruminococcaceae, and Streptococcus. In contrast, plant-based proteins increase the abundance of Lactobacillus and Bifidobacterium and decrease the number of pathogens, such as Bacteroides fragilis [152]. In addition, glycated pea protein ingestion increased microbial SCFA synthesis, which is known to have anti-inflammatory and intestinal barrier-protecting properties [153]. More precisely, there were noticeable differences in the relative abundance of specific bacteria related to different protein sources.
It is widely acknowledged that eating a high-fiber diet fosters bacterial diversity, increasing beneficial bacteria, including Roseburia, Prevotella, Faecalibacterium, and Ackermannia, while reducing possibly pathogenic bacteria (such as Enterobacteriaceae) [154]. In their study of 178 older persons, Claesson and colleagues discovered that those who consumed a high-fiber diet produced more butyrate and acetate from SCFAs than those fed a low-fiber diet [155]. Consequently, a high-fiber diet may stimulate the production of anti-inflammatory cytokines in the intestines and reduce brain damage following a stroke [156].
Caesar and colleagues compared the intestinal microorganisms in GF mice fed lard (saturated fatty acids) or fish oil (polyunsaturated fatty acids). They discovered that the fish oil consumption group had higher diversity and abundance of Akkermansia muciniphila and Lactobacillus. In contrast, the lard-fed group had a significant amount of Bilophila wadsworthia and showed evidence of Toll-like receptor 4 (TLR-4) activation [157]. This finding is consistent with earlier studies using saturated fats in clinical and experimental settings [158].
The gut microbiota releases small-molecule metabolites from food derivatives and microbial fermentation into the circulation, where they interact with the host and cause various illnesses, including IS. It is critical to comprehend how certain macronutrients, particularly dietary lipids, carbohydrates, and proteins, affect the gut flora.
6.2. Probiotics and Prebiotics
Live microorganisms known as probiotics, which are beneficial to the host's health, are primarily made up of Bifidobacterium and lactic acid-producing bacteria such as Lactobacillus. Bacteriocins, which are synthesized and secreted by probiotics, prevent bacterial invasion and block pathogens from adhering to epithelial cells [159]. Additionally, they exert a trophic impact on the intestinal mucosa and affect the secretion of cytokines from epithelial cells, which increases barrier integrity and mucus formation [160]. Furthermore, probiotics enhance GLP-1R expression in the brain and GLP-1 and 5-HT secretion in the intestine by maintaining the barrier's integrity [161]. Prestroke treatment of probiotics in mice inhibited TNF-α production, decreased neuronal damage to the hippocampus, and enhanced antioxidant enzyme activity [162]. Probiotics may have an effect by interacting with Toll-like receptors in intestinal epithelial cells to inhibit TNF-α and free radical production [163]. According to a meta-analysis, probiotic supplementation for stroke patients was linked to reduced serum TNF-α, IL-6, and IL-10 levels and positively affected poststroke recovery [164].
Prebiotics are a group of carbohydrates, such as resistant starch, oligosaccharides, especially fructose (such as inulin and fructooligosaccharides (FOS)), and galactose (such as galactooligosaccharides (GOS)), that are not digested by the host but can selectively alter the microbiome's activities and composition [165]. Rats treated with probiotics (B-GOS) exhibited improved cognitive performance, inhibition of microglial activation, and decreased expression of proinflammatory cytokines [166]. Similarly, the elderly population who consumed probiotics had significantly higher amounts of Bacteroides and Bifidobacterium, elevated lactate levels in their feces, a significant decrease in proinflammatory cytokines, and an increase in anti-inflammatory cytokines (IL-10 and IL-8) [167]. Studies have demonstrated that inulin boosts butyrate synthesis while promoting the production of Bifidobacterium and Faecalibacterium [168].
Therefore, it appears that probiotics and prebiotics are positive therapeutic alternatives for neurological illnesses, as suggested by these studies.
6.3. Fecal Microbiota Transplantation
Fecal microbiota transplantation (FMT) transfers intestinal microbiota from one healthy donor to another by oral intake of fecal matter in rodents or colonoscopy in humans. The FMT method has gained much attention for its significant percentage of success in the treatment of recurrent Clostridium difficile infection [169]. In a recent study, the effectiveness of FMT was compared using three different pretreatment strategies: antibiotics, bowel cleansing solution, and no pretreatment. The study indicated that antibiotic pretreatment increased the efficacy of FMT [170]. Recolonization by FMT in mice with MCAO with either healthy sham control gut microbiota or gut microbiota treated with antibiotics lessened brain injuries following an experimental stroke [20]. Germ-free mice developed larger infarcts after a stroke, but when their guts were colonized with normal gut microbiota, this condition began to improve [171].
Previous research has demonstrated that leaner donor FMT improves microbial diversity, insulin sensitivity, and butyric acid-producing bacteria in obese patients [172]. Diabetes is one of the major risk factors for IS. FMT of T2D-related gut microbiota in GF mice showed that dysbiosis of the gut flora increased brain damage aggregation and poststroke infarct size. It also caused intestinal barrier dysfunction in GF mice, including increased serum LPS and impaired tight-junction protein distribution [173].
FMT has the advantage of being coupled with other techniques for gut microbiota remodeling. However, FMT also has drawbacks, such as the fact that most studies use only animal models and that study designs vary widely, using various techniques, donors, and antibiotics. The FMT investigations did not identify any solid mechanisms underlying these advancements, so further research is needed to determine the mechanisms underlying this advantageous effect.
7. Conclusion and Future Perspectives
The importance of MGBA in a person's health status has been highlighted by numerous studies that found imbalances in the GI microflora composition to be related to particular abnormal physiological situations. The biological relationship between the gut microbiota, the CNS, and immune signaling suggests that systemic microbial signaling or microbial-derived metabolites may indirectly or directly impact immunological and neurological activity in IS. More research is necessary on the potential function and precise mechanism of MGBA in IS. It will be essential to enhance preclinical studies of novel therapeutics for the prevention and treatment of IS and to help understand the high risk of IS and its tendency for recurrence. Overall, it must be appreciated that the GI microflora could be viewed as a new organ and denoted the “second brain,” and that it plays a significant part in the pathogenesis of IS. Future research in neurotherapeutics will provide crucial information about the gut microbiota as a new dividing line between human health and disorders.
On the one hand, fundamental science research should employ randomization, blinding, and data analysis techniques similar to clinical trials to more accurately replicate the complexity of human trials. On the other hand, clinical studies should be sufficiently powered to evaluate efficacy in stroke subtypes, establish salvageable tissue and target engagement, and be mindful of the therapeutic window. Proteomics, metabolomics, and 16S microbiome sequencing should be used in future gut microbiome studies and IS to thoroughly understand the pathways, microbes, and metabolites involved and to clarify the effect of their interactions on IS. To conclude, the translatability of the results will increase if recent guidelines for bettering the quality of the design, collection, and analysis of microbiological datasets are followed, which is vital to advance the field and shift from association to causation.
Acknowledgments
We acknowledge funds from the National Natural Science Foundation of China (82174477) and the Outstanding Cultivation Fund of Heilongjiang University of Traditional Chinese Medicine (no. 2019JC03).
Data Availability
No data were used to support this study.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Authors' Contributions
Wenjing Huang and Luwen Zhu share first authorship. All authors approved the final version.
References
- 1.Virani S. S., Alonso A., Aparicio H. J., et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation . 2021;143(8):e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 2.Paul S., Candelario-Jalil E. Emerging neuroprotective strategies for the treatment of ischemic stroke: an overview of clinical and preclinical studies. Experimental Neurology . 2021;335, article 113518 doi: 10.1016/j.expneurol.2020.113518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moskowitz M. A., Lo E. H., Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron . 2010;67(2):181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin H., Chen S., Shen L., et al. Integrated analysis of the cecal microbiome and plasma metabolomics to explore NaoMaiTong and its potential role in changing the intestinal flora and their metabolites in ischemic stroke. Frontiers in Pharmacology . 2021;12, article 773722 doi: 10.3389/fphar.2021.773722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng Y., Zhang D., Zhao Y., et al. Effect of intestinal microbiota transplantation on cerebral ischemia reperfusion injury in aged mice via inhibition of IL-17. Neurogastroenterology and Motility: the Official Journal of the European Gastrointestinal Motility Society . 2022;34(7, article e14313) doi: 10.1111/nmo.14313. [DOI] [PubMed] [Google Scholar]
- 6.Cryan J. F., O'Riordan K. J., Cowan C. S. M., et al. The microbiota-gut-brain axis. Physiological Reviews . 2019;99(4):1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 7.Avery E. G., Bartolomaeus H., Maifeld A., et al. The gut microbiome in hypertension. Circulation Research . 2021;128(7):934–950. doi: 10.1161/CIRCRESAHA.121.318065. [DOI] [PubMed] [Google Scholar]
- 8.Baothman O. A., Zamzami M. A., Taher I., Abubaker J., Abu-Farha M. The role of gut microbiota in the development of obesity and diabetes. Lipids in Health and Disease . 2016;15(1):p. 108. doi: 10.1186/s12944-016-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferranti E. P., Dunbar S. B., Dunlop A. L., Corwin E. J. 20 things you didn't know about the human gut microbiome. The Journal of Cardiovascular Nursing . 2014;29(6):479–481. doi: 10.1097/JCN.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lloyd-Price J., Mahurkar A., Rahnavard G., et al. Strains, functions and dynamics in the expanded human microbiome project. Nature . 2017;550(7674):61–66. doi: 10.1038/nature23889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adak A., Khan M. R. An insight into gut microbiota and its functionalities. Cellular and Molecular Life Sciences : CMLS . 2019;76(3):473–493. doi: 10.1007/s00018-018-2943-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorboni S. G., Moghaddam H. S., Jafarzadeh-Esfehani R., Soleimanpour S. A comprehensive review on the role of the gut microbiome in human neurological disorders. Clinical Microbiology Reviews . 2022;35(1, article e0033820) doi: 10.1128/CMR.00338-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sommer F., Bäckhed F. The gut microbiota--masters of host development and physiology. Nature Reviews. Microbiology . 2013;11(4):227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 14.Carabotti M., Scirocco A., Maselli M. A., Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Annals of Gastroenterology . 2015;28(2):203–209. [PMC free article] [PubMed] [Google Scholar]
- 15.Mallesh S., Ten Hove A. S., Schneider R., et al. Sympathetic innervation modulates mucosal immune homeostasis and epithelial host defense. Cells . 2022;11(16):p. 2606. doi: 10.3390/cells11162606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou W., Cheng Y., Zhu P., Nasser M. I., Zhang X., Zhao M. Implication of gut microbiota in cardiovascular diseases. Oxidative Medicine and Cellular Longevity . 2020;2020 doi: 10.1155/2020/5394096.5394096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chidambaram S. B., Essa M. M., Rathipriya A. G., et al. Gut dysbiosis, defective autophagy and altered immune responses in neurodegenerative diseases: tales of a vicious cycle. Pharmacology & Therapeutics . 2022;231, article 107988 doi: 10.1016/j.pharmthera.2021.107988. [DOI] [PubMed] [Google Scholar]
- 18.Liu L., Huh J. R., Shah K. Microbiota and the gut-brain-axis: implications for new therapeutic design in the CNS. eBioMedicine . 2022;77, article 103908 doi: 10.1016/j.ebiom.2022.103908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang C., Li Q., Ren J. Microbiota-immune interaction in the pathogenesis of gut-derived infection. Frontiers in Immunology . 2019;10:p. 1873. doi: 10.3389/fimmu.2019.01873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benakis C., Brea D., Caballero S., et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nature Medicine . 2016;22(5):516–523. doi: 10.1038/nm.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh V., Roth S., Llovera G., et al. Microbiota dysbiosis controls the neuroinflammatory response after stroke. The Journal of neuroscience : the official journal of the Society for Neuroscience . 2016;36(28):7428–7440. doi: 10.1523/JNEUROSCI.1114-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schroeder B. O., Bäckhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nature Medicine . 2016;22(10):1079–1089. doi: 10.1038/nm.4185. [DOI] [PubMed] [Google Scholar]
- 23.Seguella L., Gulbransen B. D. Enteric glial biology, intercellular signalling and roles in gastrointestinal disease. Nature Reviews. Gastroenterology & Hepatology . 2021;18(8):571–587. doi: 10.1038/s41575-021-00423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Battaglini D., Pimentel-Coelho P. M., Robba C., et al. Gut microbiota in acute ischemic stroke: from pathophysiology to therapeutic implications. Frontiers in Neurology . 2020;11:p. 598. doi: 10.3389/fneur.2020.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu D.-J., Wang K.-C., Yuan L.-B., et al. Compositional and functional alterations of gut microbiota in patients with stroke. Nutrition, Metabolism, and Cardiovascular Diseases : NMCD . 2021;31(12):3434–3448. doi: 10.1016/j.numecd.2021.08.045. [DOI] [PubMed] [Google Scholar]
- 26.Yin J., Liao S.-X., He Y., et al. Dysbiosis of gut microbiota with reduced trimethylamine-N-oxide level in patients with large-artery atherosclerotic stroke or transient ischemic attack. Journal of the American Heart Association . 2015;4(11) doi: 10.1161/JAHA.115.002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamashiro K., Tanaka R., Urabe T., et al. Gut dysbiosis is associated with metabolism and systemic inflammation in patients with ischemic stroke. PLoS One . 2017;12(2, article e0171521) doi: 10.1371/journal.pone.0171521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y., Liang J., Ouyang F., et al. Persistence of gut microbiota dysbiosis and chronic systemic inflammation after cerebral infarction in cynomolgus monkeys. Frontiers in Neurology . 2019;10:p. 661. doi: 10.3389/fneur.2019.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li N., Wang X., Sun C., et al. Change of intestinal microbiota in cerebral ischemic stroke patients. BMC Microbiology . 2019;19(1):p. 191. doi: 10.1186/s12866-019-1552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hakoupian M., Ferino E., Jickling G. C., et al. Bacterial lipopolysaccharide is associated with stroke. Scientific Reports . 2021;11(1):p. 6570. doi: 10.1038/s41598-021-86083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spencer N. J., Hu H. Enteric nervous system: sensory transduction, neural circuits and gastrointestinal motility. Nature Reviews. Gastroenterology & Hepatology . 2020;17(6):338–351. doi: 10.1038/s41575-020-0271-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang H., Li J., Zhou Q., et al. Vagus nerve stimulation alleviated cerebral ischemia and reperfusion injury in rats by inhibiting pyroptosis via α7 nicotinic acetylcholine receptor. Cell Death Discovery . 2022;8(1):p. 54. doi: 10.1038/s41420-022-00852-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fülling C., Dinan T. G., Cryan J. F. Gut microbe to brain signaling: what happens in vagus…. Neuron . 2019;101(6):998–1002. doi: 10.1016/j.neuron.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Kimura I., Dohgu S., Takata F., et al. Activation of the α7 nicotinic acetylcholine receptor upregulates blood-brain barrier function through increased claudin-5 and occludin expression in rat brain endothelial cells. Neuroscience Letters . 2019;694:9–13. doi: 10.1016/j.neulet.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 35.Ramos-Castaneda J. A., Barreto-Cortes C. F., Losada-Floriano D., Sanabria-Barrera S. M., Silva-Sieger F. A., Garcia R. G. Efficacy and safety of vagus nerve stimulation on upper limb motor recovery after stroke. A systematic review and meta-analysis. Frontiers In Neurology . 2022;13, article 889953 doi: 10.3389/fneur.2022.889953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borgmann D., Ciglieri E., Biglari N., et al. Gut-brain communication by distinct sensory neurons differently controls feeding and glucose metabolism. Cell Metabolism . 2021;33(7) doi: 10.1016/j.cmet.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tilocca B., Pieroni L., Soggiu A., et al. Gut-brain axis and neurodegeneration: state-of-the-art of meta-omics sciences for microbiota characterization. International Journal of Molecular Sciences . 2020;21(11) doi: 10.3390/ijms21114045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joly A., Leulier F., De Vadder F. Microbial modulation of the development and physiology of the enteric nervous system. Trends in Microbiology . 2021;29(8):686–699. doi: 10.1016/j.tim.2020.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Lomasney K. W., Cryan J. F., Hyland N. P. Converging effects of a Bifidobacterium and Lactobacillus probiotic strain on mouse intestinal physiology. American Journal of Physiology-Gastrointestinal and Liver Physiology . 2014;307(2):G241–G247. doi: 10.1152/ajpgi.00401.2013. [DOI] [PubMed] [Google Scholar]
- 40.Al-Nedawi K., Mian M. F., Hossain N. Gut commensal microvesicles reproduce parent bacterial signals to host immune and enteric nervous systems. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology . 2015;29(2):684–695. doi: 10.1096/fj.14-259721. [DOI] [PubMed] [Google Scholar]
- 41.Paray B. A., Albeshr M. F., Jan A. T., Rather I. A. Leaky gut and autoimmunity: an intricate balance in individuals health and the diseased state. International Journal of Molecular Sciences . 2020;21(24) doi: 10.3390/ijms21249770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Q., Cao X. Epigenetic remodeling in innate immunity and inflammation. Annual Review of Immunology . 2021;39:279–311. doi: 10.1146/annurev-immunol-093019-123619. [DOI] [PubMed] [Google Scholar]
- 43.Erny D., Hrabě de Angelis A. L., Jaitin D., et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nature Neuroscience . 2015;18(7):965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mossad O., Erny D. The microbiota-microglia axis in central nervous system disorders. Brain Pathology . 2020;30(6):1159–1177. doi: 10.1111/bpa.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erny D., Prinz M. How microbiota shape microglial phenotypes and epigenetics. Glia . 2020;68(8):1655–1672. doi: 10.1002/glia.23822. [DOI] [PubMed] [Google Scholar]
- 46.Malone K., Amu S., Moore A. C., Waeber C. The immune system and stroke: from current targets to future therapy. Immunology and Cell Biology . 2019;97(1) doi: 10.1111/imcb.12191. [DOI] [PubMed] [Google Scholar]
- 47.Chen A.-Q., Fang Z., Chen X.-L., et al. Microglia-derived TNF-α mediates endothelial necroptosis aggravating blood brain-barrier disruption after ischemic stroke. Cell Death & Disease . 2019;10(7):p. 487. doi: 10.1038/s41419-019-1716-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu J., Cao D., Guo C., et al. Berberine facilitates angiogenesis against ischemic stroke through modulating microglial polarization via AMPK signaling. Cellular and Molecular Neurobiology . 2019;39(6):751–768. doi: 10.1007/s10571-019-00675-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng Q., Chen Y., Zhai Y., et al. Gut dysbiosis is associated with the severity of cryptogenic stroke and enhanced systemic inflammatory response. Frontiers in Immunology . 2022;13, article 836820 doi: 10.3389/fimmu.2022.836820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang W., Yu T., Cong Y. CD4 T cell metabolism, gut microbiota, and autoimmune diseases: implication in precision medicine of autoimmune diseases. Precision Clinical Medicine . 2022;5(3, article pbac018) doi: 10.1093/pcmedi/pbac018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schiweck C., Edwin Thanarajah S., Aichholzer M., et al. Regulation of CD4 and CD8 T cell biology by short-chain fatty acids and its relevance for autoimmune pathology. International Journal of Molecular Sciences . 2022;23(15):p. 8272. doi: 10.3390/ijms23158272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gagliani N., Vesely M. C. A., Iseppon A., et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature . 2015;523(7559):221–225. doi: 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gelderblom M., Weymar A., Bernreuther C., et al. Neutralization of the IL-17 axis diminishes neutrophil invasion and protects from ischemic stroke. Blood . 2012;120(18):3793–3802. doi: 10.1182/blood-2012-02-412726. [DOI] [PubMed] [Google Scholar]
- 54.Brioschi S., Wang W. L., Peng V., et al. Heterogeneity of meningeal B cells reveals a lymphopoietic niche at the CNS borders. Science . 2021;373(6553) doi: 10.1126/science.abf9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li R., Fang H., Shen J., et al. Curcumin alleviates LPS-induced oxidative stress, inflammation and apoptosis in bovine mammary epithelial cells via the NFE2L2 signaling pathway. Toxins . 2021;13(3):p. 208. doi: 10.3390/toxins13030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo S., Al-Sadi R., Said H. M., Ma T. Y. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. The American Journal of Pathology . 2013;182(2):375–387. doi: 10.1016/j.ajpath.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen W., Wang L., Liu Z. MicroRNA-155 influences cell damage in ischemic stroke via TLR4/MYD88 signaling pathway. Bioengineered . 2021;12(1):2449–2458. doi: 10.1080/21655979.2021.1935066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mittal R., Debs L. H., Patel A. P., et al. Neurotransmitters: the critical modulators regulating gut–brain axis. Journal of Cellular Physiology . 2017;232(9):2359–2372. doi: 10.1002/jcp.25518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li C., Wang Y., Xing Y., et al. Regulation of microglia phagocytosis and potential involvement of exercise. Frontiers in Cellular Neuroscience . 2022;16, article 953534 doi: 10.3389/fncel.2022.953534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roshchina V. V. New trends and perspectives in the evolution of neurotransmitters in microbial, plant, and animal cells. Advances in Experimental Medicine and Biology . 2016;874:25–77. doi: 10.1007/978-3-319-20215-0_2. [DOI] [PubMed] [Google Scholar]
- 61.Thomas Broome S., Louangaphay K., Keay K. A., Leggio G. M., Musumeci G., Castorina A. Dopamine: an immune transmitter. Neural Regeneration Research . 2020;15(12):2173–2185. doi: 10.4103/1673-5374.284976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Acler M., Fiaschi A., Manganotti P. Long-term levodopa administration in chronic stroke patients. A clinical and neurophysiologic single-blind placebo-controlled cross-over pilot study. Restorative Neurology and Neuroscience . 2009;27(4):277–283. doi: 10.3233/RNN-2009-0477. [DOI] [PubMed] [Google Scholar]
- 63.Ford G. A., Bhakta B. B., Cozens A., et al. Safety and efficacy of co-careldopa as an add-on therapy to occupational and physical therapy in patients after stroke (DARS): a randomised, double-blind, placebo-controlled trial. The Lancet. Neurology . 2019;18(6):530–538. doi: 10.1016/S1474-4422(19)30147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matsumoto M., Ooga T., Kibe R., Aiba Y., Koga Y., Benno Y. Colonic absorption of low-molecular-weight metabolites influenced by the intestinal microbiome: a pilot study. PLoS One . 2017;12(1, article e0169207) doi: 10.1371/journal.pone.0169207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ren W., Yin J., Xiao H., et al. Intestinal microbiota-derived GABA mediates interleukin-17 expression during enterotoxigenic infection. Frontiers in Immunology . 2016;7:p. 685. doi: 10.3389/fimmu.2016.00685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sokovic Bajic S., Djokic J., Dinic M., et al. GABA-producing natural dairy isolate from artisanal Zlatar cheese attenuates gut inflammation and strengthens gut epithelial barrier in vitro. Frontiers in Microbiology . 2019;10:p. 527. doi: 10.3389/fmicb.2019.00527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Michalettos G., Ruscher K. Crosstalk between GABAergic neurotransmission and inflammatory cascades in the post-ischemic brain: relevance for stroke recovery. Frontiers in Cellular Neuroscience . 2022;16, article e807911 doi: 10.3389/fncel.2022.807911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu J., Wang L.-N., Ma X., Ji X. Gamma aminobutyric acid (GABA) receptor agonists for acute stroke. The Cochrane Database of Systematic Reviews . 2016;10:p. CD009622. doi: 10.1002/14651858.CD009622.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sánchez-Pérez S., Comas-Basté O., Duelo A., et al. Intestinal dysbiosis in patients with histamine intolerance. Nutrients . 2022;14(9):p. 1774. doi: 10.3390/nu14091774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barcik W., Wawrzyniak M., Akdis C. A., O'Mahony L. Immune regulation by histamine and histamine-secreting bacteria. Current Opinion in Immunology . 2017;48:108–113. doi: 10.1016/j.coi.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 71.Blasco M. P., Chauhan A., Honarpisheh P., et al. Age-dependent involvement of gut mast cells and histamine in post-stroke inflammation. Journal of Neuroinflammation . 2020;17(1):p. 160. doi: 10.1186/s12974-020-01833-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Knecht L. D., O'Connor G., Mittal R., et al. Serotonin activates bacterial quorum sensing and enhances the virulence of Pseudomonas aeruginosa in the host. eBioMedicine . 2016;9:161–169. doi: 10.1016/j.ebiom.2016.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hata T., Asano Y., Yoshihara K., et al. Regulation of gut luminal serotonin by commensal microbiota in mice. PLoS One . 2017;12(7, article e0180745) doi: 10.1371/journal.pone.0180745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Donovan M. H., Tecott L. H. Serotonin and the regulation of mammalian energy balance. Frontiers in Neuroscience . 2013;7:p. 36. doi: 10.3389/fnins.2013.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Whelan R., Hargaden G. C., Knox A. J. S. Modulating the blood-brain barrier: a comprehensive review. Pharmaceutics . 2021;13(11) doi: 10.3390/pharmaceutics13111980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hou K., Wu Z.-X., Chen X.-Y., et al. Microbiota in health and diseases. Signal Transduction and Targeted Therapy . 2022;7(1):p. 135. doi: 10.1038/s41392-022-00974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frankiensztajn L. M., Elliott E., Koren O. The microbiota and the hypothalamus-pituitary-adrenocortical (HPA) axis, implications for anxiety and stress disorders. Current Opinion in Neurobiology . 2020;62:76–82. doi: 10.1016/j.conb.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 78.Yamashiro K., Kurita N., Urabe T., Hattori N. Role of the gut microbiota in stroke pathogenesis and potential therapeutic implications. Annals of Nutrition & Metabolism . 2021;77(Supplement 2):36–44. doi: 10.1159/000516398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martin C. R., Osadchiy V., Kalani A., Mayer E. A. The brain-gut-microbiome axis. Cellular and Molecular Gastroenterology and Hepatology . 2018;6(2):133–148. doi: 10.1016/j.jcmgh.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barugh A. J., Gray P., Shenkin S. D., MacLullich A. M. J., Mead G. E. Cortisol levels and the severity and outcomes of acute stroke: a systematic review. Journal of Neurology . 2014;261(3):533–545. doi: 10.1007/s00415-013-7231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao L., Xiong Q., Stary C. M., et al. Bidirectional gut-brain-microbiota axis as a potential link between inflammatory bowel disease and ischemic stroke. Journal of Neuroinflammation . 2018;15(1):p. 339. doi: 10.1186/s12974-018-1382-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schmidt K., Cowen P. J., Harmer C. J., Tzortzis G., Errington S., Burnet P. W. J. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology . 2015;232(10):1793–1801. doi: 10.1007/s00213-014-3810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hou L., Zhang Y., Zheng D., et al. Increasing trimethylamine N-oxide levels as a predictor of early neurological deterioration in patients with acute ischemic stroke. Neurological Research . 2020;42(2):153–158. doi: 10.1080/01616412.2019.1710416. [DOI] [PubMed] [Google Scholar]
- 84.Fang S., Wang M., Zheng Y., Zhou S., Ji G. Acupuncture and lifestyle modification treatment for obesity: a meta-analysis. The American Journal of Chinese Medicine . 2017;45(2):239–254. doi: 10.1142/S0192415X1750015X. [DOI] [PubMed] [Google Scholar]
- 85.Komaroff A. L. The microbiome and risk for atherosclerosis. JAMA . 2018;319(23):2381–2382. doi: 10.1001/jama.2018.5240. [DOI] [PubMed] [Google Scholar]
- 86.Zhu W., Gregory J. C., Org E., et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell . 2016;165(1):111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Skye S. M., Zhu W., Romano K. A., et al. Microbial transplantation with human gut commensals containing CutC is sufficient to transmit enhanced platelet reactivity and thrombosis potential. Circulation Research . 2018;123(10):1164–1176. doi: 10.1161/CIRCRESAHA.118.313142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu W., Romano K. A., Li L., et al. Gut microbes impact stroke severity via the trimethylamine N-oxide pathway. Cell Host & Microbe . 2021;29(7):1199–1208. doi: 10.1016/j.chom.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Z., Klipfell E., Bennett B. J., et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature . 2011;472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shih D. M., Wang Z., Lee R., et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. Journal of Lipid Research . 2015;56(1):22–37. doi: 10.1194/jlr.M051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sun X., Jiao X., Ma Y., et al. Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochemical and Biophysical Research Communications . 2016;481(1-2):63–70. doi: 10.1016/j.bbrc.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 92.Martin-Gallausiaux C., Marinelli L., Blottière H. M., Larraufie P., Lapaque N. SCFA: mechanisms and functional importance in the gut. The Proceedings of the Nutrition Society . 2021;80(1):37–49. doi: 10.1017/S0029665120006916. [DOI] [PubMed] [Google Scholar]
- 93.Li G., Lin J., Zhang C., et al. Microbiota metabolite butyrate constrains neutrophil functions and ameliorates mucosal inflammation in inflammatory bowel disease. Gut Microbes . 2021;13(1, article 1968257) doi: 10.1080/19490976.2021.1968257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aguilar E. C., Leonel A. J., Teixeira L. G., et al. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFκB activation. Nutrition, Metabolism, and Cardiovascular Diseases : NMCD . 2014;24(6):606–613. doi: 10.1016/j.numecd.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 95.Usami M., Kishimoto K., Ohata A., et al. Butyrate and Trichostatin A Attenuate Nuclear Factor KappaB Activation and Tumor Necrosis Factor Alpha Secretion and Increase Prostaglandin E2 Secretion in Human Peripheral Blood Mononuclear Cells. Nutrition Research . 2008;28(5):321–328. doi: 10.1016/j.nutres.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 96.Larraufie P., Martin-Gallausiaux C., Lapaque N., et al. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Scientific Reports . 2018;8(1):p. 74. doi: 10.1038/s41598-017-18259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tolhurst G., Heffron H., Lam Y. S., et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes . 2012;61(2):364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Haak B. W., Westendorp W. F., van Engelen T. S. R., et al. Disruptions of anaerobic gut bacteria are associated with stroke and post-stroke infection: a prospective case-control study. Translational Stroke Research . 2021;12(4):581–592. doi: 10.1007/s12975-020-00863-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee J., d’Aigle J., Atadja L., et al. Gut microbiota-derived short-chain fatty acids promote poststroke recovery in aged mice. Circulation Research . 2020;127(4):453–465. doi: 10.1161/CIRCRESAHA.119.316448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chan Y. K., Brar M. S., Kirjavainen P. V., et al. High fat diet induced atherosclerosis is accompanied with low colonic bacterial diversity and altered abundances that correlates with plaque size, plasma A-FABP and cholesterol: a pilot study of high fat diet and its intervention with Lactobacillus rhamnosus GG (LGG) or telmisartan in ApoE−/− mice. BMC Microbiology . 2016;16(1):p. 264. doi: 10.1186/s12866-016-0883-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kasahara K., Krautkramer K. A., Org E., et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nature Microbiology . 2018;3(12):1461–1471. doi: 10.1038/s41564-018-0272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Winston J. A., Theriot C. M. Diversification of host bile acids by members of the gut microbiota. Gut Microbes . 2020;11(2):158–171. doi: 10.1080/19490976.2019.1674124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jia W., Wei M., Rajani C., Zheng X. Targeting the alternative bile acid synthetic pathway for metabolic diseases. Protein & Cell . 2021;12(5):411–425. doi: 10.1007/s13238-020-00804-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gadaleta R. M., Cariello M., Crudele L., Moschetta A. Bile salt hydrolase-competent probiotics in the management of IBD: unlocking the "bile acid code". Nutrients . 2022;14(15) doi: 10.3390/nu14153212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Das P., Marcišauskas S., Ji B., Nielsen J. Metagenomic analysis of bile salt biotransformation in the human gut microbiome. BMC Genomics . 2019;20(1):p. 517. doi: 10.1186/s12864-019-5899-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chávez-Talavera O., Tailleux A., Lefebvre P., Staels B. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology . 2017;152(7) doi: 10.1053/j.gastro.2017.01.055. [DOI] [PubMed] [Google Scholar]
- 107.Ding Q. Y., Tian J. X., Li M., et al. Interactions between therapeutics for metabolic disease, cardiovascular risk factors, and gut microbiota. Frontiers In Cellular and Infection Microbiology . 2020;10, article 530160 doi: 10.3389/fcimb.2020.530160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu J., Yuan J., Zhao J., Zhang L., Wang Q., Wang G. Serum metabolomic patterns in young patients with ischemic stroke: a case study. Metabolomics : Official Journal of the Metabolomic Society . 2021;17(2):p. 24. doi: 10.1007/s11306-021-01774-7. [DOI] [PubMed] [Google Scholar]
- 109.McMillin M., Frampton G., Tobin R., et al. TGR5 signaling reduces neuroinflammation during hepatic encephalopathy. Journal of Neurochemistry . 2015;135(3):565–576. doi: 10.1111/jnc.13243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zangerolamo L., Vettorazzi J. F., Rosa L. R. O., Carneiro E. M., Barbosa H. C. L. The bile acid TUDCA and neurodegenerative disorders: an overview. Life Sciences . 2021;272, article 119252 doi: 10.1016/j.lfs.2021.119252. [DOI] [PubMed] [Google Scholar]
- 111.Yanguas-Casás N., Barreda-Manso M. A., Nieto-Sampedro M., Romero-Ramírez L. TUDCA: an agonist of the bile acid receptor GPBAR1/TGR5 with anti-inflammatory effects in microglial cells. Journal of Cellular Physiology . 2017;232(8):2231–2245. doi: 10.1002/jcp.25742. [DOI] [PubMed] [Google Scholar]
- 112.Cheng L., Huang C., Chen Z. Tauroursodeoxycholic acid ameliorates lipopolysaccharide-induced depression like behavior in mice via the inhibition of neuroinflammation and oxido-nitrosative stress. Pharmacology . 2019;103(1-2) doi: 10.1159/000494139. [DOI] [PubMed] [Google Scholar]
- 113.Zhu G., Wang X., Chen L., et al. Crosstalk between the oxidative stress and glia cells after stroke: from mechanism to therapies. Frontiers in Immunology . 2022;13, article 852416 doi: 10.3389/fimmu.2022.852416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hua Q., Zhu X. L., Li P. T., et al. The inhibitory effects of cholalic acid and hyodeoxycholalic acid on the expression of TNFalpha and IL-1beta after cerebral ischemia in rats. Archives of Pharmacal Research . 2009;32(1):65–73. doi: 10.1007/s12272-009-1119-z. [DOI] [PubMed] [Google Scholar]
- 115.Perez M.-J., Briz O. Bile-acid-induced cell injury and protection. World Journal of Gastroenterology . 2009;15(14):1677–1689. doi: 10.3748/wjg.15.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Charach G., Karniel E., Novikov I., et al. Reduced bile acid excretion is an independent risk factor for stroke and mortality: a prospective follow-up study. Atherosclerosis . 2020;293:79–85. doi: 10.1016/j.atherosclerosis.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 117.Yano J. M., Yu K., Donaldson G. P., et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell . 2015;161(2):264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Badawy A. A. B. Kynurenine pathway and human systems. Experimental Gerontology . 2020;129, article 110770 doi: 10.1016/j.exger.2019.110770. [DOI] [PubMed] [Google Scholar]
- 119.Li X., Yang Q., Dierckens K., Milton D. L., Defoirdt T. RpoS and indole signaling control the virulence of Vibrio anguillarum towards gnotobiotic sea bass (Dicentrarchus labrax) larvae. PLoS One . 2014;9(10, article e111801) doi: 10.1371/journal.pone.0111801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Whitfield-Cargile C. M., Cohen N. D., Chapkin R. S., et al. The microbiota-derived metabolite indole decreases mucosal inflammation and injury in a murine model of NSAID enteropathy. Gut Microbes . 2016;7(3):246–261. doi: 10.1080/19490976.2016.1156827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rothhammer V., Borucki D. M., Tjon E. C., et al. Microglial control of astrocytes in response to microbial metabolites. Nature . 2018;557(7707):724–728. doi: 10.1038/s41586-018-0119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hajsl M., Hlavackova A., Broulikova K., et al. Tryptophan metabolism, inflammation, and oxidative stress in patients with neurovascular disease. Metabolites . 2020;10(5) doi: 10.3390/metabo10050208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Brouns R., Verkerk R., Aerts T., et al. The role of tryptophan catabolism along the kynurenine pathway in acute ischemic stroke. Neurochemical Research . 2010;35(9):1315–1322. doi: 10.1007/s11064-010-0187-2. [DOI] [PubMed] [Google Scholar]
- 124.Honarpisheh P., Bryan R. M., McCullough L. D. Aging microbiota-gut-brain axis in stroke risk and outcome. Circulation Research . 2022;130(8):1112–1144. doi: 10.1161/CIRCRESAHA.122.319983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Saini V., Guada L., Yavagal D. R. Global epidemiology of stroke and access to acute ischemic stroke interventions. Neurology . 2021;97(20) Supplement 2 doi: 10.1212/WNL.0000000000012781. [DOI] [PubMed] [Google Scholar]
- 126.Gao B., Chi L., Zhu Y., et al. An introduction to next generation sequencing bioinformatic analysis in gut microbiome studies. Biomolecules . 2021;11(4) doi: 10.3390/biom11040530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Manwani B., Liu F., Scranton V., Hammond M. D., Sansing L. H., McCullough L. D. Differential effects of aging and sex on stroke induced inflammation across the lifespan. Experimental Neurology . 2013;249:120–131. doi: 10.1016/j.expneurol.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Crapser J., Ritzel R., Verma R., et al. Ischemic stroke induces gut permeability and enhances bacterial translocation leading to sepsis in aged mice. Aging . 2016;8(5):1049–1063. doi: 10.18632/aging.100952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ceylani T., Teker H. T. The effect of young blood plasma administration on gut microbiota in middle-aged rats. Archives of Microbiology . 2022;204(9):p. 541. doi: 10.1007/s00203-022-03154-8. [DOI] [PubMed] [Google Scholar]
- 130.Spychala M. S., Venna V. R., Jandzinski M., et al. Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Annals of Neurology . 2018;84(1):23–36. doi: 10.1002/ana.25250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cipolla M. J., Liebeskind D. S., Chan S.-L. The importance of comorbidities in ischemic stroke: impact of hypertension on the cerebral circulation. Journal of Cerebral Blood Flow and Metabolism : Official Journal of the International Society of Cerebral Blood Flow and Metabolism . 2018;38(12):2129–2149. doi: 10.1177/0271678X18800589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Guo J., Guo X., Sun Y., Li Z., Jia P. Application of omics in hypertension and resistant hypertension. Hypertension Research: Official Journal of the Japanese Society of Hypertension . 2022;45(5):775–788. doi: 10.1038/s41440-022-00885-5. [DOI] [PubMed] [Google Scholar]
- 133.Karbach S. H., Schönfelder T., Brandão I., et al. Gut microbiota promote angiotensin II-induced arterial hypertension and vascular dysfunction. Journal of the American Heart Association . 2016;5(9) doi: 10.1161/JAHA.116.003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang T., Santisteban M. M., Rodriguez V., et al. Gut dysbiosis is linked to hypertension. Hypertension . 2015;65(6):1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ferguson J. F., Aden L. A., Barbaro N. R., et al. High dietary salt-induced dendritic cell activation underlies microbial dysbiosis-associated hypertension. JCI Insight . 2019;5 doi: 10.1172/jci.insight.126241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wilck N., Matus M. G., Kearney S. M., et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature . 2017;551(7682):585–589. doi: 10.1038/nature24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang L., Zhu Q., Lu A., et al. Sodium butyrate suppresses angiotensin II-induced hypertension by inhibition of renal (pro)renin receptor and intrarenal renin-angiotensin system. Journal of Hypertension . 2017;35(9):1899–1908. doi: 10.1097/HJH.0000000000001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen L., Sun M., Wu W., et al. Microbiota metabolite butyrate differentially regulates Th1 and Th17 Cells' differentiation and function in induction of colitis. Inflammatory Bowel Diseases . 2019;25(9):1450–1461. doi: 10.1093/ibd/izz046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Diener H.-C., Hankey G. J. Primary and secondary prevention of ischemic stroke and cerebral hemorrhage: Journal of the American College of Cardiology . 2020;75(15):1804–1818. doi: 10.1016/j.jacc.2019.12.072. [DOI] [PubMed] [Google Scholar]
- 140.Karlsson F. H., Tremaroli V., Nookaew I., et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature . 2013;498(7452) doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 141.Qin J., Li Y., Cai Z., et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature . 2012;490(7418):55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 142.Sanna S., van Zuydam N. R., Mahajan A., et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nature Genetics . 2019;51(4):600–605. doi: 10.1038/s41588-019-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Osinski C., Le Gléau L., Poitou C., et al. Type 2 diabetes is associated with impaired jejunal enteroendocrine GLP-1 cell lineage in human obesity. International Journal of Obesity . 2021;45(1):170–183. doi: 10.1038/s41366-020-00694-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ridaura V. K., Faith J. J., Rey F. E., et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science . 2013;341(6150, article 1241214) doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Turnbaugh P. J., Ley R. E., Mahowald M. A., Magrini V., Mardis E. R., Gordon J. I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature . 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 146.Paganelli F., Mottola G., Fromonot J., et al. Hyperhomocysteinemia and cardiovascular disease: is the adenosinergic system the missing link? International Journal of Molecular Sciences . 2021;22(4) doi: 10.3390/ijms22041690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Karlsson F. H., Fåk F., Nookaew I., et al. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nature Communications . 2012;3:p. 1245. doi: 10.1038/ncomms2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fernandes C. P., Oliveira F. A. F., Silva P. G. . B., et al. Molecular analysis of oral bacteria in dental biofilm and atherosclerotic plaques of patients with vascular disease. International Journal of Cardiology . 2014;174(3):710–712. doi: 10.1016/j.ijcard.2014.04.201. [DOI] [PubMed] [Google Scholar]
- 149.Hosomi N., Aoki S., Matsuo K., et al. Association of serum anti-periodontal pathogen antibody with ischemic stroke. Cerebrovascular Diseases (Basel, Switzerland) . 2012;34(5-6):385–392. doi: 10.1159/000343659. [DOI] [PubMed] [Google Scholar]
- 150.Li T., Chen Y., Gua C., Li X. Elevated circulating trimethylamine N-oxide levels contribute to endothelial dysfunction in aged rats through vascular inflammation and oxidative stress. Frontiers in Physiology . 2017;8:p. 350. doi: 10.3389/fphys.2017.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sheflin A. M., Melby C. L., Carbonero F., Weir T. L. Linking dietary patterns with gut microbial composition and function. Gut Microbes . 2017;8(2):113–129. doi: 10.1080/19490976.2016.1270809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tomova A., Bukovsky I., Rembert E., et al. The effects of vegetarian and vegan diets on gut microbiota. Frontiers In Nutrition . 2019;6:p. 47. doi: 10.3389/fnut.2019.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dominika Ś., Arjan N., Karyn R. P., Henryk K. The study on the impact of glycated pea proteins on human intestinal bacteria. International Journal of Food Microbiology . 2011;145(1):267–272. doi: 10.1016/j.ijfoodmicro.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 154.Vanegas S. M., Meydani M., Barnett J. B., et al. Substituting whole grains for refined grains in a 6-wk randomized trial has a modest effect on gut microbiota and immune and inflammatory markers of healthy adults. The American Journal of Clinical Nutrition . 2017;105(3):635–650. doi: 10.3945/ajcn.116.146928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Claesson M. J., Jeffery I. B., Conde S., et al. Gut microbiota composition correlates with diet and health in the elderly. Nature . 2012;488(7410):178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 156.Li M., van Esch B. C. A. M., Henricks P. A. J., Folkerts G., Garssen J. The anti-inflammatory effects of short chain fatty acids on lipopolysaccharide- or tumor necrosis factor α-stimulated endothelial cells via activation of GPR41/43 and inhibition of HDACs. Frontiers in Pharmacology . 2018;9:p. 533. doi: 10.3389/fphar.2018.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Caesar R., Tremaroli V., Kovatcheva-Datchary P., Cani P. D., Bäckhed F. Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling. Cell Metabolism . 2015;22(4):658–668. doi: 10.1016/j.cmet.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Devkota S., Wang Y., Musch M. W., et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in _Il10_ _− /−_ mice. Nature . 2012;487(7405):104–108. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cryan J. F., O'Riordan K. J., Sandhu K., Peterson V., Dinan T. G. The gut microbiome in neurological disorders. The Lancet. Neurology . 2020;19(2):179–194. doi: 10.1016/S1474-4422(19)30356-4. [DOI] [PubMed] [Google Scholar]
- 160.Neunlist M., Schemann M. Nutrient-induced changes in the phenotype and function of the enteric nervous system. The Journal of Physiology . 2014;592(14):2959–2965. doi: 10.1113/jphysiol.2014.272948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Brenner L. A., Stearns-Yoder K. A., Hoffberg A. S., et al. Growing literature but limited evidence: a systematic review regarding prebiotic and probiotic interventions for those with traumatic brain injury and/or posttraumatic stress disorder. Brain, Behavior, and Immunity . 2017;65:57–67. doi: 10.1016/j.bbi.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 162.Rahmati H., Momenabadi S., Vafaei A. A., Bandegi A. R., Mazaheri Z., Vakili A. Probiotic supplementation attenuates hippocampus injury and spatial learning and memory impairments in a cerebral hypoperfusion mouse model. Molecular Biology Reports . 2019;46(5):4985–4995. doi: 10.1007/s11033-019-04949-7. [DOI] [PubMed] [Google Scholar]
- 163.Akhoundzadeh K., Vakili A., Shadnoush M., Sadeghzadeh J. Effects of the oral ingestion of probiotics on brain damage in a transient model of focal cerebral ischemia in mice. Iranian Journal of Medical Sciences . 2018;43(1):32–40. [PMC free article] [PubMed] [Google Scholar]
- 164.Zhong D.-Y., Li L., Ma R.-M., Deng Y.-H. The effect of probiotics in stroke treatment. Evidence-based Complementary and Alternative Medicine : ECAM . 2021;2021, article 4877311:10. doi: 10.1155/2021/4877311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Stephen A. M., Champ M. M. J., Cloran S. J., et al. Dietary fibre in Europe: current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutrition Research Reviews . 2017;30(2):149–190. doi: 10.1017/S095442241700004X. [DOI] [PubMed] [Google Scholar]
- 166.Yang X.-D., Wang L.-K., Wu H.-Y., Jiao L. Effects of prebiotic galacto-oligosaccharide on postoperative cognitive dysfunction and neuroinflammation through targeting of the gut-brain axis. BMC Anesthesiology . 2018;18(1):p. 177. doi: 10.1186/s12871-018-0642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Vulevic J., Juric A., Walton G. E., et al. Influence of galacto-oligosaccharide mixture (B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. The British Journal of Nutrition . 2015;114(4):586–595. doi: 10.1017/S0007114515001889. [DOI] [PubMed] [Google Scholar]
- 168.Renye J. A., White A. K., Hotchkiss A. T. Identification of strains capable of fermenting fructo-oligosaccharides and inulin. Microorganisms . 2021;9(10) doi: 10.3390/microorganisms9102020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dawwas G. K., Brensinger C. M., Vajravelu R. K., et al. Long-term outcomes following multiply recurrent clostridioides difficile infection and fecal microbiota transplantation. Clinical Gastroenterology and Hepatology : the Official Clinical Practice Journal of the American Gastroenterological Association . 2022;20(4) doi: 10.1016/j.cgh.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ji S. K., Yan H., Jiang T., et al. Preparing the gut with antibiotics enhances gut microbiota reprogramming efficiency by promoting xenomicrobiota colonization. Frontiers in Microbiology . 2017;8:p. 1208. doi: 10.3389/fmicb.2017.01208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Singh V., Sadler R., Heindl S., et al. The gut microbiome primes a cerebroprotective immune response after stroke. Journal of Cerebral Blood Flow and Metabolism : Official Journal of the International Society of Cerebral Blood Flow and Metabolism . 2018;38(8):1293–1298. doi: 10.1177/0271678X18780130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.de Groot P., Scheithauer T., Bakker G. J., et al. Donor metabolic characteristics drive effects of faecal microbiota transplantation on recipient insulin sensitivity, energy expenditure and intestinal transit time. Gut . 2020;69(3):502–512. doi: 10.1136/gutjnl-2019-318320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chen X., Wu Q., Gao X., et al. Gut microbial dysbiosis associated with type 2 diabetes aggravates acute ischemic stroke. MSystems . 2021;6(6, article e0130421) doi: 10.1128/msystems.01304-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used to support this study.


