Abstract
As the COVID-19 pandemic continues, there is an urgent need to identify clinical and laboratory predictors of disease severity and prognosis. Once the coronavirus enters the cell, it triggers additional events via different signaling pathways. Cellular and molecular deregulation evoked by coronavirus infection can manifest as changes in laboratory findings. Understanding the relationship between laboratory biomarkers and COVID-19 outcomes would help in developing a risk-stratified approach to the treatment of patients with this disease. The purpose of this review is to investigate the role of hematological (white blood cell (WBC), lymphocyte, and neutrophil count, neutrophil-to-lymphocyte ratio (NLR), platelet, and red blood cell (RBC) count), inflammatory (C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and lactate dehydrogenase (LDH)), and biochemical (Albumin, aspartate aminotransferase (AST) and alanine aminotransferase (ALT), blood urea nitrogen (BUN), creatinine, D-dimer, total Cholesterol, low-density lipoprotein (LDL), and high-density lipoprotein (HDL)) biomarkers in the pathogenesis of COVID-19 disease and how their levels vary according to disease severity.
1. Introduction
Since the onset of the new coronavirus (SARS-CoV-2 (severe acute respiratory syndrome coronavirus-2), previously known as 2019-nCoV) pandemic in December 2019 [1, 2], confirmed cases have been reported in countries all over the world. The World Health Organization proclaimed the 2019 coronavirus disease (COVID-19) pandemic on March 11, 2020, mostly because of the disease's pervasive development [3]. Prior to that, it began as an outbreak in mainland China, with the first reports coming from the city of Wuhan in the province of Hubei on February 26, 2020. After the virus' genome was sequenced, the virus was given the name severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) by the International Committee on Taxonomy of Viruses. It shared genetic ancestry with the coronavirus outbreak that caused the SARS epidemic of 2003 [4]. Until July 30, 2022, COVID-19, the disease caused by SARS-CoV-2 virus infection, has posed a very significant threat to global public health with a total of 581,182,629 reported cases and 6,418,043 mortalities documented [5]. With 3,987,543 cases, the American continent was among those with the largest number of cases, with the United States and Brazil as the leading countries (2,137,731 and 923,189, respectively) [5, 6].
SARS-CoV-2 virus belongs to the order Nidovirales, suborder Cornidovirineae, family Coronaviridae, and subfamily Orthocoronavirinae. SARS-CoV-2 is an enveloped and symmetrical virus with spike-like projections on its membrane, giving it the shape of crowns. It has a positive-sense single-stranded RNA genome (Figure 1) [7, 8].
Figure 1.
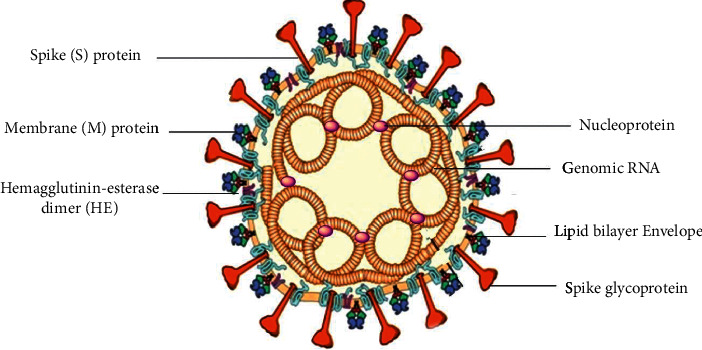
Schematic structure of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), an enveloped virus with single-stranded positive-sense (+sense) RNA taking four principal proteins, including membrane (M) and spike (S) glycoproteins, in addition to nucleocapsid (N) and envelope (E) proteins.
The COVID-19 pathogenesis includes direct cytotoxicity of virus in ACE2 (angiotensin-converting enzyme 2)-expressing cells; Renin-Angiotensin-Aldosterone System (RAAS) dysregulation secondary to virus-mediated ACE2 downregulation; immune response dysregulation; damage to the endothelial cells and thromboinflammation; and tissue fibrosis (Figure 2) [9]. The heterogeneous course of COVID-19 disease is unpredictable, with most patients presenting with mild, self-limiting symptoms. The virus infection commonly starts with flu-like symptoms [10] and can be asymptomatic or may have a minor to severe development [11]. Despite this, up to 30% of patients require hospitalization, and up to 17% of them need intensive care support for acute respiratory distress syndrome (ARDS), hyper-inflammatory responses, and multiorgan failure [12, 13].
Figure 2.
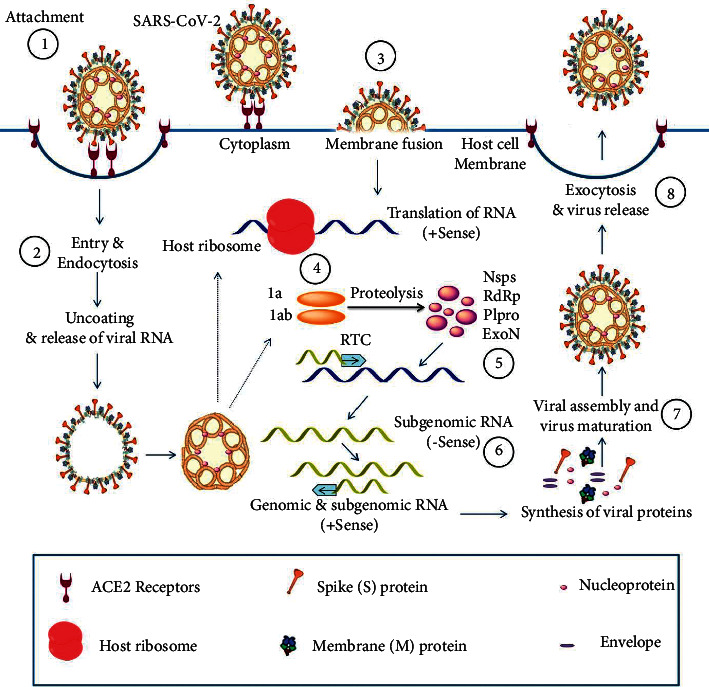
Mode of host entry and infective life cycle of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) virus. (1) The interaction between the angiotensin-converting enzyme 2 (ACE2) receptor and S-protein leads to the attachment of the virus (2) The entry of virus conducted by endocytosis and/or by (3) Membrane fusion of virus (4) Translation of virus RNA leads to produce proteins 1a & 1ab (5) Proteolysis of proteins results in nonstructural proteins and replicase-transcriptase complex (RTC) (6) Synthesizing the new viral RNA (-sense) and the viral proteins (7) The association of the viral particle (8) Release of virus through exocytosis.
The predominant clinical symptoms, presented by individuals infected during the COVID-19 pandemic, are respiratory which is similar to the SARS and Middle East respiratory syndrome (MERS) outbreaks. Roughly 80% of infected people have mild to moderate symptoms. The remaining patients have severe disease that necessitates inpatient care [14]. The overall mortality rate seems to be around 3.8% [2, 15–18].
Fever (83%–98%), cough (50%–82%), fatigue (25%–44%), shortness of breath (19%–55%), and muscle soreness (11%–44%) have been reported to be the most common symptoms of COVID-19 infection. Other symptoms might include headache, sore throat, sputum production, rhinorrhea, chest tightness, nausea, vomiting, diarrhea, ageusia, and anosmia [15, 19].
A small percentage of infected people have no or moderate symptoms, whereas most of the cases have a severe or crucial prognosis, and some people die as a result. Although elderly patients with underlying conditions appear to be more vulnerable to serious sickness and death, there are incidences of life-threatening disease in healthy people. As a result, certain critical issues remained unresolved, as for why does illness intensity differ between individuals? How do some people have a more serious sickness than others? The variability of the COVID-19 clinical phenotype may be addressed by several parameters relating to the host, virus, and environment [20].
The interpretation and understanding of host variables, particularly genetic construction, has largely remained unclear. On the other hand, there is limited information on the pathophysiology of SARS-CoV-2 and only a few educated guesses about the virus' behavior. Scientists are just now starting to understand how host, viral, and environmental variables interplay to impact infection. In fact, people of any age, particularly older adults with comorbidities like chronic bronchitis [21], diabetes [22], hypertension [23], cardiovascular disease [24], lung and liver diseases [25], chronic kidney disease (CKD) [26], and chronic obstructive pulmonary disease (COPD) [21], may present with more severe disease. Furthermore, disorders and therapies that damage the immune system, including cancer therapy, bone marrow or organ transplantation, and long-term use of corticosteroids, may increase the risk of disease, leading to more serious prognosis, and even death [27, 28]. On the other hand, despite the fact that epidemiological data in some research found no indication of a greater transmission of SARS-CoV-2 among asthma patients, it appears that patients with nonallergic asthma had a more severe COVID-19 infection than patients with allergic asthma [29]. Importantly, during metabolic syndrome, the disease's deteriorated prognosis might be attributed to the three factors of this syndrome including hypertension, type 2 diabetes, and obesity, which are all risk factors for COVID-19 severity. Along with these well-known risk factors, there are other host-related variables that might influence COVID-19's result [20]. It has been shown in numerous studies, the SARS-CoV-2 virus affects not only the respiratory tract, but also other systems and organs such as the heart, liver, and gastrointestinal system. Some studies have shown that several important biochemical and hematological indicators are altered in COVID-19 patients [30–32]. These biomarkers may be useful for predicting prognosis and also for treatment management, especially in cases with comorbidities and/or a severe disease course [33].
According to studies, severe or fatal COVID-19 cases are associated with significantly increased white blood cell counts (WBCs), liver and kidney function markers, blood urea nitrogen (BUN), creatinine, C-reactive protein (CRP), lactate dehydrogenase (LDH), D-dimer, calprotectin, and interleukin-6 (IL-6). They are also associated with lower lymphocyte and platelet counts, as well as lower albumin levels, compared to milder cases in which the patients survived [34–38]. COVID-19 infection causes great burden of inflammation and neutrophil-to-lymphocyte ratio (NLR) is a novel marker of inflammation in certain conditions such as thyroid conditions [39], irritable bowel disease [40], and COVID-19 infection [41], cardiac conditions [42], and Hashimoto's disease [43].
Understanding the relationship between these biomarkers and COVID-19 outcomes would help in developing a risk-stratified approach to the treatment of patients with this disease. The purpose of this article is to investigate the role of hematological, inflammatory, and biochemical biomarkers in the pathogenesis of COVID-19 disease and how their levels vary according to the disease severity.
2. Biomarkers Associated with COVID-19 Infection and Severity
2.1. Hematological Biomarkers
One of the most commonly requested blood tests in clinical practice is the complete blood count (CBC) [44]. For too long, it has been used to quickly determine if a patient is anemic or infected, as well as to evaluate the blood's ability to coagulate normally [45].
White blood cell (WBC) count, lymphocyte count, neutrophil count, neutrophil-to-lymphocyte ratio (NLR), platelet count, and red blood cell (RBC) count are among the hematological biomarkers applied to stratify COVID-19 patients.
2.1.1. WBC Count
In a study of 140 hospitalized COVID-19 patients confirmed by computed tomography (CT) scan findings, Zhang et al. found that the leukocyte count was within normal range in 68.1% of patients, decreased in 19.6% of them, and increased in 12.3% of them. They also observed that 75.4% of patients had lymphopenia [46]. On the other hand, Qin and colleagues investigated immune response dysregulation markers in 452 confirmed COVID-19 patients. They reported that severe cases had higher leukocyte counts [47]. In a meta-analysis of 21 studies involving 3377 COVID-19 positive patients, Henry et al. discovered that patients with serious and fatal disease had considerably higher WBC in comparison to nonsevere patients [48]. In a study conducted by Mardani et al., hematological biomarkers were compared between 70 COVID-19 positive patients and 130 COVID-19 negative patients. It has been investigated that RT-PCR positive group had significantly lower WBC counts than the negative control group [49].
In a retrospective cohort study of 219 COVID-19 patients, a higher white blood cell (WBC) count was linked to an increased risk of one-month mortality, according to the findings [50]. Moreover, Ferrari et al., in a retrospective study, reported that patients with COVID-19 had significantly higher WBC counts compared to controls [51]. Overall, the current evidence suggests that, while WBC count can be utilized as a predictive factor for severer COVID-19 conditions, the research findings are not consistent and further studies are required.
2.1.2. Lymphocyte Count
Yang et al. [52] found lymphopenia in 80% of severely ill adult COVID-19 patients, while Chen et al. [15] found it in only 25% of mild infected patients. These findings suggest that lymphopenia may be related to the severity of infection. As observed by Henry et al., severe and fatal cases of COVID-19 tend to have a lower lymphocyte count than mild cases [48]. These findings were also confirmed by Qin et al. [47].
Chen et al. in a multicentric, retrospective study of 548 confirmed COVID-19 patients reported that survivors and nonsurvivors had significantly different hematological biomarkers on admission and at the end point. In fact, on admission, severe and critical cases, as well as nonsurvivors, had significant lymphopenia [53]. A meta-analysis of 20 publications found statistically significant decreases in total lymphocytes, CD4+ and CD8+ T-cells, and B-cells in critically ill COVID-19 patients compared to patients with moderate or mild disease [54]. Moutchia et al., in their systematic review, also reported that severe and critical cases of COVID-19 were characterized by lower lymphocyte and CD4 counts [55]. Due to the significant prevalence of lymphopenia in COVID-19 patients and its strong correlation with disease severity, current research suggests that lymphocyte count, particularly CD4+ levels, can be employed as a predictive biomarker for disease severity.
2.1.3. Neutrophil Count
According to the available evidence, neutrophilia is an expression of a cytokine storm and hyperinflammatory state that play a key role in the pathogenesis of COVID-19 [2, 56]. Furthermore, neutrophilia can be caused by a secondary bacterial infection, which is more common in patients with advanced disease [14]. Chen and colleagues, in their study, observed that on admission, severe and critical cases, as well as nonsurvivors, had significantly increased neutrophil count compared with mild cases [53]. As reported by Mardani et al., confirmed COVID-19 patients have a significant increase in neutrophil count in comparison to the control group [49]. Moutchia et al., in a systematic review of 45 studies, observed that compared to nonsevere COVID-19 cases, patients with severe or critical COVID-19 have higher neutrophil counts [55]. In a retrospective cohort study of 201 COVID-19 patients, bivariate Cox regression analysis revealed that neutrophilia was linked to the development of acute respiratory distress syndrome (ARDS) and the progression from ARDS to death [57]. These studies demonstrated that neutrophilia in COVID‐19 patients was associated with the severity of the disease.
2.1.4. Neutrophil-to-Lymphocyte Ratio (NLR)
Neutrophil-to-lymphocyte ratio (NLR) was first considered in the esophageal carcinoma patients under chemotherapeutic treatment, by dividing the relative percentage of neutrophils by lymphocytes. While the normal reference range in healthy population is 1–3, the values greater than 3 reveal an ongoing infection, and a ratio greater than 9 indicates sepsis. Therefore, the NLR value is associated with current inflammation as a prognostic biomarker [58].
As observed by Maet al., patients with a higher neutrophil-to-lymphocyte ratio (NLR>9.8) had a higher incidence of ARDS (P = 0.005), as well as higher rates of nonmechanical and mechanical ventilation (P = 0.002 and P = 0.048, respectively) [38]. Chen et al. also reported that in comparison to milder cases, severe and nonsurvivor COVID-19 cases had a higher neutrophil-to-lymphocyte ratio (NLR) as an inflammatory biomarker and a marker of systemic inflammation [53]. Qin and colleagues also published similar findings in their study [47]. In a retrospective, cross-sectional study, 101 COVID-19 positive patients were examined by means of hematological parameters. The ratio of neutrophils to lymphocytes showed a significant relationship with disease severity (P = 0.001) [59]. Moreover, as observed in a retrospective cohort study of 219 confirmed COVID-19 patients, a significant association between increased neutrophil-to-lymphocyte ratio (NLR) and increased risk of one-month mortality was reported [50]. Due to the relationship between increased neutrophil-to-lymphocyte ratio (NLR) and more severity of disease, NLR can be used as a prognostic marker in COVID-19 patients.
2.1.5. Platelet Count
Platelet count has been suggested as a potential biomarker for COVID-19 patients, because it is a simple, inexpensive, and easily accessible biomarker which has been freely correlated with disease severity and mortality risk in the intensive care unit (ICU). Platelet count was found to be absolutely lower in COVID-19 patients [60], and it was lower in nonsurvivors in comparison to survivors [61]. Waris et al., in a retrospective cross-sectional study, found that the mean platelet count (165.0 × 109/L) was significantly lower (P < 0.001) among critical COVID-19 patients compared to the mild group (217.0 × 109/L) [59]. According to Chen et al., severe and nonsurvivor COVID-19 patients had significant thrombocytopenia, when compared to milder cases [53]. Henry et al., in their study, also discovered similar results and reported that patients with serious and fatal disease had significantly lower platelet counts in comparison to nonsevere patients [48].
Liu et al., in a retrospective study of 383 COVID-19 patients, reported that thrombocytopenia at the time of admission was linked to a nearly threefold increase in mortality rate compared to those who did not have thrombocytopenia. They also found that a 50 × 109/L increase in platelet count was associated with a 40% reduction in mortality [62].
These studies demonstrated that lower platelet count in COVID‐19 cases was associated with disease severity.
2.1.6. RBC Count
Liang et al. performed a study on the laboratory characteristics of 63 COVID-19 patients and investigated their efficacy for the diagnosis of the disease. They observed that, compared to healthy people, COVID-19 patients have lower levels of red blood cell (RBC) counts [11]. Taneri and colleagues conducted a systematic review to evaluate the biomarkers of anemia and iron metabolism. Compared with moderate COVID-19 patients, severe cases had a lower RBC count [63]. In a longitudinal cohort study of 379 COVID-19 patients, Lanini et al. reported that the mean RBC count was significantly lower in nonsurvivor patients compared to survivors. According to the temporal analysis, survivors and nonsurvivors had similar RBC counts at the time of symptom onset (P=0.257). The model predicted that by day 2 after the onset of symptoms, the average level of RBC would be significantly different between survivors and nonsurvivors [64]. The current evidence indicates that severe COVID-19 is associated with lower RBC counts in patients.
2.2. Inflammatory Biomarkers
According to the literature, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and lactate dehydrogenase (LDH) are among the most prevalent inflammatory markers related to COVID-19 severity [65].
2.2.1. CRP
C-reactive protein (CRP) is an acute-phase reactant generated by the liver and other organs in response to the release of IL-6, and it is a sensitive biomarker for a variety of inflammatory disorders like infection and tissue injury. Many patients with severe illness have high CRP levels [14]. In severe COVID-19 patients, the CRP marker was shown to be significantly elevated in the early stages of infection. It has also been linked to disease progression and is a predictor of severe COVID-19 [66]. In a retrospective cohort study of 140 confirmed COVID-19 patients, Liu et al. found that the proportion of patients with increased serum CRP levels was significantly higher among severe cases than in mild cases [67]. Another study investigated the association between CRP levels and lung lesions on CT scans. It was observed that, in the early stages of COVID-19, CRP levels were found to be strongly linked with the diameter of lung lesions and the severity of illness [68]. According to Stringer et al., a CRP level of 40 mg/L or above on admission to the hospital should be considered a reliable predictor of disease severity and increased mortality risk in COVID-19 patients [69]. Smilowitz et al., in their study, examined the relationship between serum CRP concentrations and adverse outcomes in hospitalized COVID-19 patients. The results suggested that CRP levels above the median (108 mg/L) were correlated with venous thromboembolism, acute kidney injury, critical illness, and mortality, in comparison with CRP levels below the median [70].
These studies suggest that CRP may be used for risk stratification of COVID-19 patients and early identification of disease severity, adverse outcomes, and mortality risk.
2.2.2. ESR
The ESR is a measurement of the rate at which RBCs settle in a tube of anticoagulated blood over a given period. A wide range of immune and nonimmune factors can affect ESR, including changes in RBC quality and quantity, as well as changes in normal patterns and levels of different plasma proteins [71]. It has been demonstrated that severe COVID-19 is associated with a significant elevation in both ESR and CRP levels in the early stages of the disease. However, CRP changes are more sensitive to the disease condition [66]. Xiong and colleagues, in a study, analyzed the clinical, laboratory, and high-resolution CT scan findings of 42 COVID-19 patients. According to their results, the severity of pneumonia considered on the initial CT scan had a significant positive correlation with ESR. They suggested that an elevation in ESR, CRP, and LDH levels may be an indicator for the severity of inflammation or extensive tissue injury [72].
In a meta-analysis of 17 articles addressing inflammatory biomarkers in COVID-19 patients, Ghahramani et al. also confirmed the correlation between higher ESR levels and severity of the disease [73]. The available data indicate that due to the correlation between elevated ESR and higher disease severity, ESR can be used as a prognostic biomarker in COVID-19 patients.
2.2.3. LDH
Lactate dehydrogenase (LDH) is an intracellular enzyme that catalyzes the oxidation of pyruvate to lactate during anaerobic glycolysis [74]. Serum LDH is routinely tested in clinical settings for a variety of diseases. Elevated serum LDH levels have been linked to a poor prognosis in a variety of diseases, particularly tumors and inflammation [56]. Despite the fact that LDH is an enzyme that originates from many organs, it increases significantly in patients with pulmonary involvement [75]. As noted by Wu et al., at the time of diagnosis, significant differences in serum LDH levels were detected between nonsevere and severe COVID-19 patients. It was also shown that an increase or decrease in LDH levels was a sign of radiographic improvement or progress [76]. Han et al., in a retrospective observational study of 107 confirmed COVID-19 patients, suggested that LDH has the potential to be identified as a powerful predictor for early detection of lung injury and severe cases [77]. According to a meta-analysis by Deng et al., 52% of COVID-19 patients had elevated LDH levels [31]. In a study of 123 hospitalized COVID-19 patients confirmed by RT-PCR, it was reported that serum LDH levels were elevated in 89% of patients. Another finding was the strong negative correlation between LDH and partial pressure of arterial oxygen to the fraction of inspired oxygen ratio (PaO2/FiO2) values, which is an indicator of patients' respiratory function. Therefore, it was concluded from this study that, to avoid a poor prognosis, LDH should be regarded as a useful test for the early identification of cases that need more aggressive supportive therapies and closer respiratory monitoring [78]. On the other hand, in a retrospective case-control study, Li et al. observed that an increased serum LDH level at admission is an independent risk factor for COVID-19 severity and mortality. They concluded that LDH can help with early COVID-19 detection [56]. Akdogan et al. reported that in the early stages of COVID-19, LDH levels were strongly linked to lung lesions, possibly reflecting disease severity [79].
From the current data, it can be concluded that an increased serum LDH level is linked to the severity of COVID-19 and it can be used for early detection of lung involvement, disease severity, and mortality risk.
2.2.4. Inflammatory Cytokines
During SARS-CoV, MERS-CoV, and SARS-CoV-2 infection, scientists routinely focus on our current understanding of innate immune responses, inflammasome activation, inflammatory cell death pathways, and cytokines' release [80]. Cytokines are a type of polypeptide signaling molecule that acts on cell surface receptors to modulate a variety of biological activities [81]. Hyperproduction of mostly proinflammatory cytokines such as interleukin 1 (IL-1), IL-6, interleukin 12 (IL-12), interferon gamma (IFN-ɣ), and tumor necrosis factor alpha (TNF-α), which selectively target lung tissue, can significantly impair the prognosis in the most severe instances [82]. In critically sick COVD-19 patients, the cytokine storm can deteriorate the clinical symptoms or perhaps premature death. To enhance the survival rate of COVID-19 patients, early management of the cytokine storm using immunomodulatory and cytokine antagonists is critical. Table1 offers the most important inflammatory factors during SARS-CoV-2 infection.
Table 1.
The important inflammatory factors associated with COVID-19 infection.
| Biomarker | Function | COVID‐19 cases | Outcome | Ref |
|---|---|---|---|---|
| IL-1 | Main sources of monocyte activation | IL-1β can be activated and maturated during SARS-CoV-2 | IL-1 shows an increase in viral load, mortality, lung damage, and loss of pulmonary function | [83, 84] |
| IL-2 | IL-2 has an important role in the prevention of autoimmune diseases | Elevated levels of IL-2 have been observed in SARS-CoV-2 | Elevated levels of IL-2 in patients with COVID-19 | [85–87] |
| IL-4 | It increases during activation and proliferation of B lymphocytes | Elevated IL-4 levels have been observed in SARS-CoV-2 | IL-4 had negative effects on CD8+ memory T-cells during viral infection | [2, 15, 88] |
| L-6 | IL-6 travels to the liver and induces a large number of acute-phase proteins such as (CRP), (SAA), and α1-antitrypsin | Elevated IL-6 levels have been observed in SARS-CoV-2 | IL-6 induced cytokine storm | [89, 90] |
| IL-7 | IL-7 has an important role in lymphocyte differentiation, peripheral homeostasis development of T-cells, and as a vaccine adjuvant | The role of IL-7 depends on IL-6 activity in SARS-CoV-2 | IL-7 levels related to COVID-19 severity | [56, 91] |
| IL-10 | Inhibits the production of proinflammatory cytokines | IL-10 can have immunostimulatory effects, such as stimulation of IFN-γ production by CD8+ T-cells | IL-10 levels increase in patients with COVID-19 than in those with MERS | [2, 92–94] |
| IL-12 | IL-12 is one of a group of heterodimeric biomolecules with distinctive characteristics | IL-12 has key functions in the development of Th1 and Th17 cells, especially in SARS-CoV-2 | Elevated serum IL-12 levels have been reported in patients infected with SARS-CoV-2 | [15, 95–97] |
| IL-13 | IL-13 is increased by activated Th2 cells | IL-13 has an important role in the development of bronchial asthma by increasing the production of TGF-β | There is association between the increase of IL-13 levels and the viral infection of SARS-CoV-2 | [2, 98] |
| IL-17 | IL-17 elevated in inflammatory processes and can be synthetized by Th17 lymphocytes | IL-17 is a proinflammatory cytokine with important role in tissue damage and infection | The Th17 cells can produce IL-17, which led to therapeutic approach for COVID-19 patients | [91, 99, 100] |
| M-CSF | M-CSF is the primary growth factor modulating the growth and development of hematopoietic lineage cells | Tyrosine-kinase III activated M-CSF, especially in SARS-CoV-2 | M-CSF significantly elevated in patients with COVID-19 which is associated with lung damage and disease severity | [101, 102] |
| G-CSF | G-CSF is essential for the proliferation of polymorphonuclear granulocyte cells (PMNs) | Increased levels of G-CSF have been reported in SARS-CoV-2 infections and in patients with neutropenia | G-CSF levels directly related to the lung damage and viral load of SARS-CoV-2 | [2, 57, 103, 104] |
| IFN-γ | IFN-γ is a type-II IFN produced by a majority type of lymphocyte cells | IFN-γ participates in numerous immunological functions and in inflammatory processes such as SARS-CoV-2 | IFN-γ, IL-6, and IL-10 levels were higher in patients with infection of SARS-CoV-2 | [95, 105, 106] |
| TNF-α | TNF-α is produced by various cell types, such as monocytes, macrophages, and T-cells | The serum TNF-α levels are significantly elevated in patients with COVID-19 | TNF-α was one of the cytokines whose overproduction was related to a poor prognosis in patients with SARS-CoV-2 | [106–111] |
| VEGF | VEGF is essential for vascular endothelial homeostasis and is present in numerous cells | VEGF hyper-regulation is observed in various viral infections, especially COVID-19 | VEGF would be useful in the approach to the regeneration of lung tissue and treatment of lung fibrosis | [2, 107, 112] |
2.3. Biochemical Biomarkers
2.3.1. Albumin
Albumin is a negative acute phase reactant with antioxidant properties. Therefore, under physiological circumstances, plasma albumin is a rich source of free thiols capable of scavenging reactive oxidant species. Under oxidative stress, albumin can undergo irreversible oxidation, impairing antioxidant properties and eventually causing cell and tissue damage [113]. In a systematic review and meta-analysis of 10 studies, in which a total of 1745 COVID-19 patients were evaluated, it was noted that 34% of patients demonstrated serum albumin levels lower than the normal range [31]. Wu et al. reported that at admission, the most common abnormal liver biochemical marker observed in COVID-19 cases was abnormal albumin [114]. In a recent article, Violi et al. investigated the predictive value of serum albumin for COVID-19 mortality. They observed that nonsurvivors had lower values of albumin in comparison to survivors. Cox regression analysis revealed that albumin was independently associated with mortality (hazard ratio: 2.48) after adjusting for sex, heart failure, chronic obstructive pulmonary disease (COPD), and CRP levels [113].
Li and colleagues found that lower levels of albumin were associated with increased severity of COVID-19 pneumonia. Even after adjusting for confounding factors, plasma albumin values in the critical group continued to have a significant correlation with the risk of mortality [76]. Due to the association between decreased serum albumin levels and higher disease severity, serum albumin can be used as a predictive biomarker for the severity of the disease in COVID-19 patients.
2.3.2. AST & ALT
Abnormal liver function tests (LFTs) including elevated alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are indicators of hepatocyte injury. However, it has been suggested that elevated aminotransferases in COVID-19 could also be caused by myositis rather than liver damage [115]. Deng et al., in their systematic review and meta-analysis, reported that ALT and aspartate AST values were found to be higher than normal in 16% and 20% of COVID-19 patients, respectively [31]. In a systematic review by Wu and colleagues, the incidence, risk factor, and prognosis of abnormal liver biomarkers in patients with COVID-19 were evaluated. They found that at admission, the pooled incidence of abnormal AST and ALT was 21.8% and 20.4%, respectively. Meta-analysis showed that serum AST and ALT levels were significantly increased in severe and critical cases compared to mild and moderate patients [114]. A recent systematic review and meta-analysis of 42 articles reported that, in nonsevere COVID-19 patients, an increase in ALT and AST levels was found in 30% and 21%, respectively, while in severe patients, it was found in 38% and 48% [116]. Canovi et al., in an observational cross-sectional study of 866 COVID-19 patients, observed that circulating concentrations of serum AST and ALT demonstrated a progressive increase with worsening parenchymal lesions [117]. The current evidence suggests that severe COVID-19 is correlated with higher AST and ALT levels in patients.
2.3.3. Blood Urea Nitrogen (BUN)
Mudatsir et al., in a systematic review of 19 articles, observed that elevated levels of BUN were associated with severe COVID-19 [118]. Ghahramani et al., in their systematic review and meta-analysis, also reported that BUN levels showed a significant increase in severe patients compared to nonsevere ones [73]. In another meta-analysis, Danwang et al. confirmed this relation and discovered that severe COVID-19 patients tend to have higher levels of BUN [119]. Zhang and colleagues in a study of 289 COVID-19 patients observed that increased BUN on admission was found in survived severe cases compared to cases with nonsevere disease. They also reported high levels of this biomarker to be associated with in‐hospital death [120]. Moreover, as observed in a multicenter retrospective cohort study of 12,413 confirmed COVID-19 patients, a significant association between increased baseline BUN levels and increased risk of all-cause mortality was reported [121]. A recent study of 266 COVID-19 patients reported that compared with mild cases, severe patients had higher levels of BUN, proposing that BUN could be an independent element for predicting COVID-19 severity [122].
These studies showed that higher BUN concentrations in COVID‐19 cases were associated with higher disease severity and mortality rate.
2.3.4. Serum Creatinine
As reported by Mudatsir et al., elevated levels of serum creatinine were correlated with severe manifestations of COVID-19 [118]. Ghahramani et al. also confirmed the association between higher creatinine levels and severity of disease in COVID 19-patients [73].
Danwang et al., in a meta-analysis, reported that serum creatinine levels are higher among COVID-19 severe cases in comparison to mild cases [119]. Zhang et al. confirmed that high levels of serum creatinine were associated with in-hospital mortality [120]. Liu et al. also observed that there was a relationship between elevated serum creatinine and increased all-cause mortality risk among COVID-19 cases [121]. Ferrando-vivas et al. in an observational cohort study of 10,362 critically ill patients discovered links between increased serum creatinine and higher 30-day mortality among COVID-19 cases, implying that deteriorating renal function was linked to a higher risk of death, as seen in many other types of critical illnesses [123]. Chen et al. in a study evaluated the impact of abnormal renal function on COVID-19 patients' prognosis and the prognostic value of various renal function indicators. They found that when adjusted for several important clinical variables, increased creatinine levels were independent predictors of mortality [124]. Current evidence suggests that serum creatinine can be used as a predictive biomarker for more severe COVID-19 cases and the risk of mortality.
2.3.5. D-Dimer
The plasma cleavage product D-dimer is a breakdown product of cross-linked fibrin. During systemic fibrinolysis after alpha2 depletion, plasma may destroy fibrin monomers, cross-linked fibrin polymers, and perhaps fibrinogen [125]. A fibrin degradation product (FDP) refers to all of these fragments [126]. Although preventive anticoagulation in ICU patients in China was not widespread when these researches were conducted, increased D-dimer in COVID-19 patients has been linked to greater mortality in several publications. While the influence of anticoagulants on D-dimer levels, in the context of COVID-19, is unclear, individuals on anticoagulation therapy frequently have very low D-dimer levels [127]. However, it was demonstrated that there is a dynamic association between COVID-19 progression and the degree of D-dimer [128].
It was indicated that there is a relation between the asymptomatic deep vein thrombosis (DVT) and patients with pneumonia hospitalized due to the COVID-19 disease. Nevertheless, for the diagnosis of DVT in COVID-19 patients, higher D-dimer cut-off levels may be required [129]. Additionally, it was shown that D-dimer levels (above 2.0 g/mL) may reliably predict in-hospital mortality in COVID-19 patients. It is suggesting that D-dimer might be a helpful marker for better COVID-19 patient diagnosis [57, 130]. These results indicated that a higher D-dimer level in COVID-19 patients has been associated with an advanced death rate. Hence, D-dimer characterization can be one of the most valuable options for initial evaluation.
2.3.6. Total Cholesterol
During a retrospective study including 597 hospitalized COVID-19 patients, Wei et al. reported that total cholesterol (TC) levels were significantly lower among COVID-19 cases in comparison to the control group [131]. In a meta-analysis of 22 studies, Zinellu et al. observed that total cholesterol (TC) levels were significantly lower in hospitalized COVID-19 patients with severe disease or nonsurvivor status [132]. Aparisi and colleagues, in a study, evaluated the correlation of lipid biomarkers with 30-days all-cause mortality among COVID-19 patients. They found that nonsurvivor cases had lower TC throughout the entire course of the disease [133]. Li et al. investigated the changes in lipid profile and their association with COVID-19 severity. They observed that TC levels showed an increasing trend in survivor cases, but showed a decreasing trend in nonsurvivor patients [120]. On the other hand, in a retrospective study, Qin et al. found that levels of total cholesterol were negatively correlated with length of hospital (LOS) stay in COVID-19 patients. They reported that at admission, serum levels of TC in the LOS >29 days group were significantly lower than those in the LOS ≤29 days group [134].
From the available literature, it can be stated that a lower total cholesterol (TC) level is linked to the severity of COVID-19, longer length of hospital (LOS) stay, and mortality risk.
2.3.7. Low-Density Lipoprotein Cholesterol (LDL-c) and High-Density Lipoprotein Cholesterol (HDL-c)
In their study, Wei et al. reported that COVID-19 cases have significantly lower LDL-c levels in comparison to healthy subjects. Moreover, they reported that when compared to mild and severe cases, high-density lipoprotein cholesterol (HDL-c) levels only decreased significantly in critical cases [131]. According to Zinellu et al., patients with severe COVID-19 have significantly lower levels of HDL-c and LDL-c when compared to patients with milder disease [132]. As noted by Aparisi et al., nonsurvivor COVID-19 patients had lower LDL-c levels than survivors during their disease course [133]. According to Li et al., in severe COVID-19 patients, LDL-c and HDL-c tend to have increased levels in survivors compared with nonsurvivors [120]. Moreover, a cross-sectional retrospective study showed that COVID-19 patients had a serum HDL-c level of 1.02 ± 0.28 mmol/L, which was significantly lower compared to the control group (1.52 ± 0.55 mmol/L). Furthermore, the serum HDL-c quantity in the severe COVID-19 group was 0.83 ± 1.67 mmol/L, considerably lower than that in the other group, nonsevere COVID-19 patients (1.15 ± 0.27 mmol/L) [135]. On the other hand, Qin et al. in their study reported that the length of hospital stay was negatively correlated with serum HDL-c and LDL-c levels in COVID-19 patients. They found that serum HDL-c and LDL-c values were significantly lower in the LOS >29 days group compared to the LOS ≤29 days group at admission [134]. These studies indicate that the levels of serum HDL-c and LDL-c can be utilized for risk stratification of COVID-19 patients, identification of disease severity, length of hospitalization, and mortality risk. Common laboratory biomarkers and their relationship to the severity of COVID-19 are summarized in Table 2.
Table 2.
Biomarkers associated with the severity of COVID-19 infection.
| Biomarker | Normal range | COVID‐19 cases | Outcome | Ref | |
|---|---|---|---|---|---|
| Hematological biomarkers | WBC count | 4.5–11 × 103 cells/mcL | Increase in WBC counts | WBC count can be utilized as a predictive factor for severe COVID-19 conditions | [46–51] |
| Lymphocyte count | 0.77–4.5 × 103 cells/mcL | Lymphopenia, decreases in total lymphocytes, CD4+, CD8+ T-cells, and B-cells | Lymphocyte count, (particularly CD4+) levels, can be employed as a predictive biomarker | [52–55] | |
| Neutrophil count | 0–1.2 × 103 cells/mcL | Neutrophilia | Neutrophilia was linked to the development of ARDS | [14, 57] | |
| Neutrophil-to-lymphocyte ratio (NLR) | 1–3 | Increase in neutrophil-to-lymphocyte ratio | NLR can be used as a prognostic marker in COVID-19 | [50, 53, 59] | |
| Platelet count | 150–350 × 103/mcL | Thrombocytopenia | Lower platelet count in COVID‐19 cases was associated with disease severity | [60–63] | |
| RBC count | 20–30 mL/kg body weight | Decrease in RBC count | Severe COVID-19 is associated with lower RBC counts in patients | [63, 64] | |
|
| |||||
| Inflammatory biomarkers | CRP | <0.8 mg/dL | Increase in CRP | CRP levels above the median (108 mg/L) were correlated with venous thromboembolic disease, acute kidney injury, critical illness, and mortality | [66–70] |
| ESR | Male: <50 years old ≤15 mm/hr Female: <50 years old ≤20 mm/hr | Increase in ESR | ESR can be used as a prognostic biomarker in COVID-19 patients | [71–73] | |
| LDH | 60–160 U/L | Decrease in LDH | Increased serum LDH level is linked to the severity of COVID-19 and it can be used for early detection of lung involvement | [76–79] | |
|
| |||||
| Biochemical biomarkers | Albumin | 3.5 g/dl to 5.4 g/dl. | Decrease in Albumin | Serum albumin can be used as a predictive biomarker for the severity of the disease | [76, 113, 114] |
| AST & ALT | <35 U/L | Increase in AST & ALT | The severe COVID-19 is correlated with higher AST and ALT levels | [115–117] | |
| Blood urea nitrogen (BUN) | 8–20 mg/dL | Increase in BUN | BUN could be an independent element for predicting COVID-19 severity | [119–122] | |
| Serum creatinine (CR) | Men: 0.7–1.2 mg/dL Women: 0.5–1.0 mg/dL | Increase in CR | The serum creatinine can be used as a predictive biomarker for more severe COVID-19 cases and the risk of mortality | [73, 118, 122–124] | |
| D-dimer | <250 ng/mL | Increase in D-dimer | DVT in patients hospitalized with COVID-19 pneumonia and increased D-dimer levels is similar to that reported in previous studies | [57, 126, 127, 129] | |
| Total cholesterol | 150–199 mg/dL | Decrease in total cholesterol | Lower total cholesterol level is linked to the severity of COVID-19, longer length of hospital stay, and mortality risk | [120, 131–134] | |
| HDL-c LDL-c | >40 mg/dl < 100 mg/dL | Decrease in HDL-c LDL-c | HDL-c and LDL-c can be utilized for risk stratification of COVID-19 patients, identification of disease severity, length of hospitalization, and mortality risk. | [120, 133–135] | |
3. Conclusion
Since the beginning of COVID-19 outbreak, the capacity of hematological, biochemical, inflammatory, and immunological factors to predict patients with severe or fatal forms of COVID-19 has been of great scientific importance. To predict the severity of the disease in the early stages, it is critical to obtain a full profile of the laboratory analysis. According to the reviewed literature, hematological, inflammatory, and biochemical parameters are associated with severe prognosis in COVID-19 cases and can thus be used as predictive factors. This issue especially can be facilitated by evaluation of factors such as WBC, RBC platelet, lymphocyte, and neutrophil count, and NLR as hematological; CRP, ESR, and LDH as inflammatory; and Albumin, AST & ALT, BUN, creatinine, D-dimer, total Cholesterol, LDL, and HDL as biochemical biomarkers. However, it is important to know that the disease progression cannot be predicted by relying on only one or two factors, and it is crucial to monitor most of the elements together. On the other hand, while various possible therapeutic options for COVID-19 have been investigated, regulating proinflammatory responses to inactivate the virus could be the best treatment offer.
Thus, this review provided guidance for this prediction on hospital admission of patients to reduce the adverse effects of the disease. Such a prognosis could reduce unnecessary hospitalization of patients and the costs imposed on the health care system. Furthermore, these outcomes should be constantly re-evaluated according to the new findings, since more prospective cohorts with longer follow-up provided more useful and up-to-date data.
Acknowledgments
This work was supported by Mashhad University of Medical Sciences (MUMS) (grant number 99.511.46).
Contributor Information
Vahid Soheili, Email: vahid_so82@yahoo.com.
Amirhossein Sahebkar, Email: sahebkara@mums.ac.ir.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Lu H., Stratton C. W., Tang Y. W. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. Journal of Medical Virology . 2020;92(4):401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet . 2020;395(10223):497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gautret P., Million M., Jarrot P. A., et al. Natural history of COVID-19 and therapeutic options. Expert Review of Clinical Immunology . 2020;16(12):1159–1184. doi: 10.1080/1744666x.2021.1847640. [DOI] [PubMed] [Google Scholar]
- 4.Dos Santos W. G. Natural history of COVID-19 and current knowledge on treatment therapeutic options. Biomedicine & Pharmacotherapy . 2020;129 doi: 10.1016/j.biopha.2020.110493.110493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. 2019. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 .
- 6.Khan M., Adil S. F., Alkhathlan H. Z., et al. COVID-19: a global challenge with old history, epidemiology and progress so far. Molecules . 2020;26(1):p. 39. doi: 10.3390/molecules26010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abebe E. C., Dejenie T. A., Shiferaw M. Y., Malik T. The newly emerged COVID-19 disease: a systemic review. Virology Journal . 2020;17(1):96–98. doi: 10.1186/s12985-020-01363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaideeswar P., Bal A., Agrawal R., Arava S., Jain A. COVID-19: an up-to-date review–from morphology to pathogenesis. Indian Journal of Pathology & Microbiology . 2020;63(3):p. 358. doi: 10.4103/ijpm.ijpm_779_20. [DOI] [PubMed] [Google Scholar]
- 9.Gupta A., Madhavan M. V., Sehgal K., et al. Extrapulmonary manifestations of COVID-19. Nature Medicine . 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 10.Aktas G. A comprehensive review on rational and effective treatment strategies against an invisible enemy; SARS Cov-2 infection. Experimental Biomedical Research . 2020;3(4):293–311. doi: 10.30714/j-ebr.2020463629. [DOI] [Google Scholar]
- 11.Liang Y., Wang Q., Zhao Y., et al. The diagnostic value of multi-index combined detection in patients with COVID-19. Clinical Laboratory . 2020;66(11) doi: 10.7754/clin.lab.2020.200660. [DOI] [PubMed] [Google Scholar]
- 12.McGonagle D., Sharif K., O’Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmunity Reviews . 2020;19(6) doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ICNARC COVID-19 Study Case Mix Programme Database. ICNARC report on COVID-19 in critical care. Technical Report, 2020, https://www.icnarc.org/About/Latest-News/2020/04/04/Report-On2249-PatientsCritically-Ill-With-Covid-19.
- 14.Frater J. L., Zini G., d’Onofrio G., Rogers H. J. COVID-19 and the clinical hematology laboratory. International Journal of Laboratory Hematology . 2020;42(1):11–18. doi: 10.1111/ijlh.13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet . 2020;395(10223):507–513. doi: 10.1016/s0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan W. J., Ni Z. Y., Hu Y., et al. China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine . 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang C., Horby P. W., Hayden F. G., Gao G. F. A novel coronavirus outbreak of global health concern. The Lancet . 2020;395(10223):470–473. doi: 10.1016/s0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA . 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang B., Ling R., Cheng Y., et al. Characteristics of the coronavirus disease 2019 and related therapeutic options. Molecular Therapy—Methods & Clinical Development . 2020;18:367–375. doi: 10.1016/j.omtm.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jordan R. E., Adab P., Cheng K. K. Covid-19: risk factors for severe disease and death. BMJ . 2020;368:m1198–2. doi: 10.1136/bmj.m1198. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Q., Meng M., Kumar R., et al. The impact of COPD and smoking history on the severity of COVID‐19: a systemic review and meta‐analysis. Journal of Medical Virology . 2020;92(10):1915–1921. doi: 10.1002/jmv.25889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar A., Arora A., Sharma P., et al. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes & Metabolic Syndrome: Clinical Research Reviews . 2020;14(4):535–545. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leiva Sisnieguez C. E., Espeche W. G., Salazar M. R. Arterial hypertension and the risk of severity and mortality of COVID-19. European Respiratory Journal . 2020;55(6):2001148–2001154. doi: 10.1183/13993003.01148-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aggarwal G., Cheruiyot I., Aggarwal S., et al. Association of cardiovascular disease with coronavirus disease 2019 (COVID-19) severity: a meta-analysis. Current Problems in Cardiology . 2020;45(8):100617–100714. doi: 10.1016/j.cpcardiol.2020.100617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rezasoltani S., Hatami B., Yadegar A., Asadzadeh Aghdaei H., Zali M. R. How patients with chronic liver diseases succeed to deal with COVID-19? Frontiers of Medicine . 2020;7:398–399. doi: 10.3389/fmed.2020.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Y., Ren Q., Chen G., et al. Chronic kidney diseases and acute kidney injury in patients with COVID-19: evidence from a meta-analysis. Frontiers of Medicine . 2020;7:588301–588312. doi: 10.3389/fmed.2020.588301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tufan A., Avanoğlu Güler A., Matucci-Cerinic M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turkish Journal of Medical Sciences . 2020;50(SI-1):620–632. doi: 10.3906/sag-2004-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang W., Zhao Y., Zhang F., et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the Perspectives of clinical immunologists from China. Clinical Immunology . 2020;214:108393–108395. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yanai H. Metabolic syndrome and COVID-19. Cardiology Research . 2020;11(6):360–365. doi: 10.14740/cr1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bohn M. K., Lippi G., Horvath A., et al. Molecular, serological, and biochemical diagnosis and monitoring of COVID-19: IFCC taskforce evaluation of the latest evidence. Clinical Chemistry and Laboratory Medicine . 2020;58(7):1037–1052. doi: 10.1515/cclm-2020-0722. [DOI] [PubMed] [Google Scholar]
- 31.Deng X., Liu B., Li J., Zhang J., Zhao Y., Xu K. Blood biochemical characteristics of patients with coronavirus disease 2019 (COVID-19): a systemic review and meta-analysis. Clinical Chemistry and Laboratory Medicine . 2020;58(8):1172–1181. doi: 10.1515/cclm-2020-0338. [DOI] [PubMed] [Google Scholar]
- 32.Lippi G., Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clinical Chemistry and Laboratory Medicine . 2020;58(7):1131–1134. doi: 10.1515/cclm-2020-0198. [DOI] [PubMed] [Google Scholar]
- 33.Thompson S., Bohn M. K., Mancini N., et al. IFCC interim guidelines on biochemical/hematological monitoring of COVID-19 patients. Clinical Chemistry and Laboratory Medicine . 2020;58(12):2009–2016. doi: 10.1515/cclm-2020-1414. [DOI] [PubMed] [Google Scholar]
- 34.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Medicine . 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guan W.-J., Liang W. H., He J. X., Zhong N. S. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. European Respiratory Journal . 2020;55(6):2001227–2001314. doi: 10.1183/13993003.01227-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goyal P., Choi J. J., Pinheiro L. C., et al. Clinical characteristics of Covid-19 in New York city. New England Journal of Medicine . 2020;382(24):2372–2374. doi: 10.1056/nejmc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silvin A., Chapuis N., Dunsmore G., et al. Elevated calprotectin and abnormal myeloid cell subsets discriminate severe from mild COVID-19. Cell . 2020;182(6):1401–1418. doi: 10.1016/j.cell.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma A., Cheng J., Yang J., Dong M., Liao X., Kang Y. Neutrophil-to-lymphocyte ratio as a predictive biomarker for moderate-severe ARDS in severe COVID-19 patients. Critical Care . 2020;24(288):1–4. doi: 10.1186/s13054-020-03007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Afsin H., Aktas G. Platelet to Lymphocyte and Neutrophil to Lymphocyte Ratios are useful in differentiation of thyroid conditions with normal and increased uptake. The Ethiopian Journal of Health Development . 2021;35(3) [Google Scholar]
- 40.Aktaş G., Basaran E. Irritable bowel syndrome is associated with novel inflammatory markers derived from hemogram parameters. Family Medicine & Primary Care Review . 2020;22 [Google Scholar]
- 41.Aktas G. Hematological predictors of novel Coronavirus infection. Revista da Associação Médica Brasileira . 2021;67(1):1–2. doi: 10.1590/1806-9282.67.suppl1.20200678. [DOI] [PubMed] [Google Scholar]
- 42.Maltese A., Cerroni F. Augmentative and alternative communication (AAC) in neurodevelopmental disorders: a MiniReview. Acta Medica Mediterranea . 2018;34:p. 1181. [Google Scholar]
- 43.Aktas G., Sit M., Dikbas O., et al. Elevated neutrophil-to-lymphocyte ratio in the diagnosis of Hashimoto’s thyroiditis. Revista da Associação Médica Brasileira . 2017;63(12):1065–1068. doi: 10.1590/1806-9282.63.12.1065. [DOI] [PubMed] [Google Scholar]
- 44.Coskun A., Braga F., Carobene A., et al. Systematic review and meta-analysis of within-subject and between-subject biological variation estimates of 20 haematological parameters. Clinical Chemistry and Laboratory Medicine . 2019;58(1):25–32. doi: 10.1515/cclm-2019-0658. [DOI] [PubMed] [Google Scholar]
- 45.Dixon L. R. The complete blood count: physiologic basis and clinical usage. Journal of Perinatal and Neonatal Nursing . 1997;11(3):1–18. doi: 10.1097/00005237-199712000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Zhang J. J., Dong X., Cao Y., et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy . 2020;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 47.Qin C., Zhou L., Hu Z., et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clinical Infectious Diseases . 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henry B. M., de Oliveira M. H. S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clinical Chemistry and Laboratory Medicine . 2020;58(7):1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 49.Mardani R., Ahmadi Vasmehjani A., Zali F., et al. Laboratory parameters in detection of COVID-19 patients with positive RT-PCR; a diagnostic accuracy study. Archives of Academic Emergency Medicine . 2020;8(1):e43–5. [PMC free article] [PubMed] [Google Scholar]
- 50.Vafadar Moradi E., Teimouri A., Rezaee R., et al. Increased age, neutrophil-to-lymphocyte ratio (NLR) and white blood cells count are associated with higher COVID-19 mortality. The American Journal of Emergency Medicine . 2021;40:11–14. doi: 10.1016/j.ajem.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferrari D., Motta A., Strollo M., Banfi G., Locatelli M. Routine blood tests as a potential diagnostic tool for COVID-19. Clinical Chemistry and Laboratory Medicine . 2020;58(7):1095–1099. doi: 10.1515/cclm-2020-0398. [DOI] [PubMed] [Google Scholar]
- 52.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The Lancet Respiratory Medicine . 2020;8(5):475–481. doi: 10.1016/s2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen R., Sang L., Jiang M., et al. Longitudinal hematologic and immunologic variations associated with the progression of COVID-19 patients in China. The Journal of Allergy and Clinical Immunology . 2020;146(1):89–100. doi: 10.1016/j.jaci.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang W., Berube J., McNamara M., et al. Lymphocyte Subset counts in COVID-19 patients: a meta-analysis. Cytometry, Part A . 2020;97(8):772–776. doi: 10.1002/cyto.a.24172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moutchia J., Pokharel P., Kerri A., et al. Clinical laboratory parameters associated with severe or critical novel coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. PLoS One . 2020;15(10) doi: 10.1371/journal.pone.0239802.e0239802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mehta P., McAuley D. F., Brown M., Sanchez E., Tattersall R. S., Manson J. J. COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet . 2020;395(10229):1033–1034. doi: 10.1016/s0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu C., Chen X., Cai Y., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Internal Medicine . 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khalid A., Ali Jaffar M., Khan T., et al. Hematological and biochemical parameters as diagnostic and prognostic markers in SARS-COV-2 infected patients of Pakistan: a retrospective comparative analysis. Hematology . 2021;26(1):529–542. doi: 10.1080/16078454.2021.1950898. [DOI] [PubMed] [Google Scholar]
- 59.Waris A., Din M., Khalid A., et al. Evaluation of hematological parameters as an indicator of disease severity in Covid-19 patients: Pakistan’s experience. Journal of Clinical Laboratory Analysis . 2021;35(6) doi: 10.1002/jcla.23809.e23809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ganji A., Farahani I., Khansarinejad B., Ghazavi A., Mosayebi G. Increased expression of CD8 marker on T-cells in COVID-19 patients. Blood Cells, Molecules, and Diseases . 2020;83:102437–102444. doi: 10.1016/j.bcmd.2020.102437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. Journal of Thrombosis and Haemostasis . 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Y., Sun W., Guo Y., et al. Association between platelet parameters and mortality in coronavirus disease 2019: retrospective cohort study. Platelets . 2020;31(4):490–496. doi: 10.1080/09537104.2020.1754383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taneri P. E., Gomez-Ochoa S. A., Llanaj E., et al. Anemia and iron metabolism in COVID-19: a systematic review and meta-analysis. European Journal of Epidemiology . 2020;35(8):763–773. doi: 10.1007/s10654-020-00678-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lanini S., Montaldo C., Nicastri E., et al. COVID-19 disease—temporal analyses of complete blood count parameters over course of illness, and relationship to patient demographics and management outcomes in survivors and non-survivors: a longitudinal descriptive cohort study. PLoS One . 2020;15(12) doi: 10.1371/journal.pone.0244129.e0244129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodriguez-Morales A. J., Cardona-Ospina J. A., Gutierrez-Ocampo E., et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Medicine and Infectious Disease . 2020;34:101623–101713. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tan C., Huang Y., Shi F., et al. C-reactive protein correlates with computed tomographic findings and predicts severe COVID-19 early. Journal of Medical Virology . 2020;92(7):856–862. doi: 10.1002/jmv.25871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu F., Li L., Xu M., et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. Journal of Clinical Virology . 2020;127:104370–104375. doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang L. C-reactive protein levels in the early stage of COVID-19. Medecine et Maladies Infectieuses . 2020;50(4):332–334. doi: 10.1016/j.medmal.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stringer D., Braude P., Myint P. K., et al. COPE Study Collaborators The role of C-reactive protein as a prognostic marker in COVID-19. International Journal of Epidemiology . 2021;50(2):420–429. doi: 10.1093/ije/dyab012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smilowitz N. R., Kunichoff D., Garshick M., et al. C-reactive protein and clinical outcomes in patients with COVID-19. European Heart Journal . 2021;42(23):2270–2279. doi: 10.1093/eurheartj/ehaa1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bray C., Bell L. N., Liang H., et al. Erythrocyte sedimentation rate and C-reactive protein measurements and their relevance in clinical medicine. Wmj . 2016;115(6):317–321. [PubMed] [Google Scholar]
- 72.Xiong Y., Sun D., Liu Y., et al. Clinical and high-resolution CT features of the COVID-19 infection: comparison of the initial and follow-up changes. Investigative Radiology . 2020;55(6):332–339. doi: 10.1097/rli.0000000000000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ghahramani S., Tabrizi R., Lankarani K. B., et al. Laboratory features of severe vs. non-severe COVID-19 patients in Asian populations: a systematic review and meta-analysis. European Journal of Medical Research . 2020;25(1):30–10. doi: 10.1186/s40001-020-00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Komolafe O., Stephen P P., Davidson B. R. Serum C-reactive protein, procalcitonin, and lactate dehydrogenase for the diagnosis of pancreatic necrosis. Cochrane Database of Systematic Reviews . 2017;4(4):1–53. doi: 10.1002/14651858.CD012645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Serin I., Sari N. D., Dogu M. H., et al. A new parameter in COVID-19 pandemic: initial lactate dehydrogenase (LDH)/Lymphocyte ratio for diagnosis and mortality. Journal of Infection and Public Health . 2020;13(11):1664–1670. doi: 10.1016/j.jiph.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu M.-Y., Yao L., Wang Y., et al. Clinical evaluation of potential usefulness of serum lactate dehydrogenase (LDH) in 2019 novel coronavirus (COVID-19) pneumonia. Respiratory Research . 2020;21(1):171–176. doi: 10.1186/s12931-020-01427-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Han Y., Zhang H., Mu S., et al. Lactate dehydrogenase, an independent risk factor of severe COVID-19 patients: a retrospective and observational study. Aging (Albany NY) . 2020;12(12):11245–11258. doi: 10.18632/aging.103372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Poggiali E., Zaino D., Immovilli P., et al. Lactate dehydrogenase and C-reactive protein as predictors of respiratory failure in CoVID-19 patients. Clinica Chimica Acta . 2020;509:135–138. doi: 10.1016/j.cca.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Akdogan D., Guzel M., Tosun D., Akpinar O. Diagnostic and early prognostic value of serum CRP and LDH levels in patients with possible COVID-19 at the first admission. Journal of Infection in Developing Countries . 2021;15(06):766–772. doi: 10.3855/jidc.14072. [DOI] [PubMed] [Google Scholar]
- 80.Vaninov N. In the eye of the COVID-19 cytokine storm. Nature Reviews Immunology . 2020;20(5):p. 277. doi: 10.1038/s41577-020-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ragab D., Salah Eldin H., Taeimah M., Khattab R., Salem R. The COVID-19 cytokine storm; what we know so far. Frontiers in Immunology . 2020;11:1446–1454. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Darif D., Hammi I., Kihel A., El Idrissi Saik I., Guessous F., Akarid K. The pro-inflammatory cytokines in COVID-19 pathogenesis: what goes wrong? Microbial Pathogenesis . 2021;153:104799–104810. doi: 10.1016/j.micpath.2021.104799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tanaka T., Narazaki M., Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy . 2016;8(8):959–970. doi: 10.2217/imt-2016-0020. [DOI] [PubMed] [Google Scholar]
- 84.Kritas S. K., Ronconi G., Caraffa A., Gallenga C. E., Ross R., Conti P. Mast cells contribute to coronavirus-induced inflammation: new anti-inflammatory strategy. Journal of Biological Regulators & Homeostatic Agents . 2020;34(1):9–14. doi: 10.23812/20-Editorial-Kritas. [DOI] [PubMed] [Google Scholar]
- 85.Huang K. J., Su I. J., Theron M., et al. An interferon-γ-related cytokine storm in SARS patients. Journal of Medical Virology . 2005;75(2):185–194. doi: 10.1002/jmv.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li C. K.-F., Wu H., Yan H., et al. T cell responses to whole SARS coronavirus in humans. Journal of Immunology . 2008;181(8):5490–5500. doi: 10.4049/jimmunol.181.8.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Channappanavar R., Fett C., Zhao J., Meyerholz D. K., Perlman S. Virus-specific memory CD8 T cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection. Journal of Virology . 2014;88(19):11034–11044. doi: 10.1128/jvi.01505-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen C., Zhang X R, Ju Z Y., He W F. Advances in the research of cytokine storm mechanism induced by Corona Virus Disease 2019 and the corresponding immunotherapies. Zhonghua Shaoshang Zazhi . 2020;36:p. E005. doi: 10.3760/cma.j.cn501120-20200224-00088. [DOI] [PubMed] [Google Scholar]
- 89.Gubernatorova E., Gorshkova E. A., Polinova A. I., Drutskaya M. S. IL-6: relevance for immunopathology of SARS-CoV-2. Cytokine & Growth Factor Reviews . 2020;53:13–24. doi: 10.1016/j.cytogfr.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Franco R., Rivas-Santisteban R., Serrano-Marin J., Rodriguez-Perez A. I., Labandeira-Garcia J. L., Navarro G. SARS-CoV-2 as a factor to disbalance the renin–angiotensin system: a suspect in the case of exacerbated IL-6 production. The Journal of Immunology . 2020;205(5):1198–1206. doi: 10.4049/jimmunol.2000642. [DOI] [PubMed] [Google Scholar]
- 91.Wan S., Yi Q., Fan S., et al. Characteristics of Lymphocyte Subsets and Cytokines in Peripheral Blood of 123 Hospitalized Patients with 2019 Novel Coronavirus Pneumonia (NCP) MedRxiv . 2020 [Google Scholar]
- 92.Han H., Ma Q., Li C., et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerging Microbes & Infections . 2020;9(1):1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao Y., Qin L., Zhang P., et al. Longitudinal COVID-19 profiling associates IL-1RA and IL-10 with disease severity and RANTES with mild disease. JCI Insight . 2020;5(13):139834–139911. doi: 10.1172/jci.insight.139834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cheng L., Zhu Z., Wang C., Wang P., He Y. O., Zhang X. COVID-19 induces lower levels of IL-8, IL-10, and MCP-1 than other acute CRS-inducing diseases. Proceedings of the National Academy of Sciences of the USA . 2021;118(21) doi: 10.1073/pnas.2102960118.e2102960118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen G., Wu D., Guo W., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. Journal of Clinical Investigation . 2020;130(5):2620–2629. doi: 10.1172/jci137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wong C. K., Lam C. W. K., Wu A. K. L., et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clinical and Experimental Immunology . 2004;136(1):95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen H.-W., Chen H. Y., Wang L. T., et al. Mesenchymal stem cells tune the development of monocyte-derived dendritic cells toward a myeloid-derived suppressive phenotype through growth-regulated oncogene chemokines. The Journal of Immunology . 2013;190(10):5065–5077. doi: 10.4049/jimmunol.1202775. [DOI] [PubMed] [Google Scholar]
- 98.Liu Y., Yang Y., Zhang C., et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Science China Life Sciences . 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Faure E., Poissy J., Goffard A., et al. Distinct immune response in two MERS-CoV-infected patients: can we go from bench to bedside? PLoS One . 2014;9(2) doi: 10.1371/journal.pone.0088716.e88716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Josset L., Menachery V. D., Gralinski L. E., et al. Cell host response to infection with novel human coronavirus EMC predicts potential antivirals and important differences with SARS coronavirus. mBio . 2013;4(3):e00165–e00113. doi: 10.1128/mbio.00165-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chockalingam S., Ghosh S. S. Macrophage colony-stimulating factor and cancer: a review. Tumor Biology . 2014;35(11):10635–10644. doi: 10.1007/s13277-014-2627-0. [DOI] [PubMed] [Google Scholar]
- 102.Chiba Y., Mizoguchi I., Hasegawa H., et al. Regulation of myelopoiesis by proinflammatory cytokines in infectious diseases. Cellular and Molecular Life Sciences . 2018;75(8):1363–1376. doi: 10.1007/s00018-017-2724-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu D., Yang X. O. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor Fedratinib. Journal of Microbiology, Immunology, and Infection . 2020;53(3):368–370. doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Del Rio C., Malani P. N. 2019 novel coronavirus—important information for clinicians. JAMA . 2020;323(11):1039–1040. doi: 10.1001/jama.2020.1490. [DOI] [PubMed] [Google Scholar]
- 105.Sun D., Li H., Lu X. X., et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center’s observational study. World Journal of Pediatrics . 2020;16(3):251–259. doi: 10.1007/s12519-020-00354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Karki R., Sharma B. R., Tuladhar S., et al. Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell . 2021;184(1):149–168.e17. doi: 10.1016/j.cell.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Leng Z., Zhu R., Hou W., et al. Transplantation of ACE2(-) mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging and Disease . 2020;11(2):216–228. doi: 10.14336/ad.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang R., Wang X., Ni L., et al. COVID-19: Melatonin as a potential adjuvant treatment. Life Sciences . 2020;250 doi: 10.1016/j.lfs.2020.117583.117583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mahallawi W. H., Khabour O. F., Zhang Q., Makhdoum H. M., Suliman B. A. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine . 2018;104:8–13. doi: 10.1016/j.cyto.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cheung C. Y., Poon L. L. M., Ng I. H. Y., et al. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. Journal of Virology . 2005;79(12):7819–7826. doi: 10.1128/jvi.79.12.7819-7826.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Law H. K. W., Cheung C. Y., Ng H. Y., et al. Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells. Blood . 2005;106(7):2366–2374. doi: 10.1182/blood-2004-10-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Korobelnik J. F., Loewenstein A., Eldem B., et al. Guidance for anti-VEGF intravitreal injections during the COVID-19 pandemic. Graefes Archive for Clinical and Experimental Ophthalmology . 2020;258(6):1149–1156. doi: 10.1007/s00417-020-04703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Violi F., Cangemi R., Romiti G. F., et al. Is albumin predictor of mortality in COVID-19? Antioxidants and Redox Signaling . 2021;35(2):139–142. doi: 10.1089/ars.2020.8142. [DOI] [PubMed] [Google Scholar]
- 114.Wu Y., Li H., Guo X., et al. Incidence, risk factors, and prognosis of abnormal liver biochemical tests in COVID-19 patients: a systematic review and meta-analysis. Hepatology International . 2020;14(5):621–637. doi: 10.1007/s12072-020-10074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bertolini A., Peppel I. P., Bodewes F. A., et al. Abnormal liver function tests in patients with COVID-19: relevance and potential pathogenesis. Hepatology . 2020;72(5):1864–1872. doi: 10.1002/hep.31480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zahedi M., Yousefi M., Abounoori M., et al. The interrelationship between liver function test and the coronavirus disease 2019: a systematic review and meta-analysis. Iranian Journal of Medical Sciences . 2021;46(4):237–255. doi: 10.30476/ijms.2021.87555.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Canovi S., Besutti G., Bonelli E., et al. The association between clinical laboratory data and chest CT findings explains disease severity in a large Italian cohort of COVID-19 patients. BMC Infectious Diseases . 2021;21(1):157–159. doi: 10.1186/s12879-021-05855-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mudatsir M., Fajar J. K., Wulandari L., et al. Predictors of COVID-19 severity: a systematic review and meta-analysis. F1000Res . 2020;9:1–26. doi: 10.12688/f1000research.26186.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Danwang C., Endomba F. T., Nkeck J. R., Wouna D. L. A., Robert A., Noubiap J. J. A meta-analysis of potential biomarkers associated with severity of coronavirus disease 2019 (COVID-19) Biomarker Research . 2020;8:37–13. doi: 10.1186/s40364-020-00217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang J. J., Cao Y., Tan G., et al. Clinical, radiological, and laboratory characteristics and risk factors for severity and mortality of 289 hospitalized COVID-19 patients. Allergy . 2021;76(2):533–550. doi: 10.1111/all.14496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu Y. M., Xie J., Chen M. M., et al. Kidney function indicators predict adverse outcomes of COVID-19. Med (NY) . 2021;2(1):38–48.e2. doi: 10.1016/j.medj.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Qu J., Zhu H. H., Huang X. J., et al. Abnormal indexes of liver and kidney injury markers predict severity in COVID-19 patients. Infection and Drug Resistance . 2021;14:3029–3040. doi: 10.2147/idr.s321915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ferrando-Vivas P., Doidge J., Thomas K., et al. Prognostic factors for 30-day mortality in critically ill patients with coronavirus disease 2019: an observational cohort study. Critical Care Medicine . 2021;49(1):102–111. doi: 10.1097/ccm.0000000000004740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen S., Li J., Liu Z., et al. Comparing the value of cystatin C and serum creatinine for evaluating the renal function and predicting the prognosis of COVID-19 patients. Frontiers in Pharmacology . 2021;12 doi: 10.3389/fphar.2021.587816.587816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oudkerk M., Buller H. R., Kuijpers D., Oudkerk S. F., van Beek E. J. R. d-Dimer and COVID-19. Radiology . 2020;297(3):E343–E344. doi: 10.1148/radiol.2020203481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yao Y., Cao J., Wang Q., et al. D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study. Journal of Intensive Care Medicine . 2020;8(1):49–11. doi: 10.1186/s40560-020-00466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schutgens R. E. D-dimer in COVID-19: a guide with pitfalls. Hemasphere . 2020;4(4):p. e422. doi: 10.1097/hs9.0000000000000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang L., Yan X., Fan Q., et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. Journal of Thrombosis and Haemostasis . 2020;18(6):1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Demelo-Rodríguez P., Cervilla-Munoz E., Ordieres-Ortega L., et al. Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thrombosis Research . 2020;192:23–26. doi: 10.1016/j.thromres.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Naymagon L., Zubizarreta N., Feld J., et al. Admission D-dimer levels, D-dimer trends, and outcomes in COVID-19. Thrombosis Research . 2020;196:99–105. doi: 10.1016/j.thromres.2020.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wei X., Zeng W., Su J., et al. Hypolipidemia is associated with the severity of COVID-19. Journal of Clinical Lipidology . 2020;14(3):297–304. doi: 10.1016/j.jacl.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zinellu A., Paliogiannis P., Fois A. G., Solidoro P., Carru C., Mangoni A. A. Cholesterol and triglyceride concentrations, COVID-19 severity, and mortality: a systematic review and meta-analysis with meta-regression. Frontiers in Public Health . 2021;9 doi: 10.3389/fpubh.2021.705916.705916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Aparisi Á., Iglesias-Echeverria C., Ybarra-Falcon C., et al. Low-density lipoprotein cholesterol levels are associated with poor clinical outcomes in COVID-19. Nutrition, Metabolism, and Cardiovascular Diseases . 2021;31(9):2619–2627. doi: 10.1016/j.numecd.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Qin C., Minghan H., Ziwen Z., Yukun L. Alteration of lipid profile and value of lipids in the prediction of the length of hospital stay in COVID-19 pneumonia patients. Food Sciences and Nutrition . 2020;8(11):6144–6152. doi: 10.1002/fsn3.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Huang S., Zhou C., Yuan Z., Xiao H., Wu X. The clinical value of high-density lipoprotein in the evaluation of new coronavirus pneumonia. Advances in Clinical and Experimental Medicine . 2021;30(2):153–156. doi: 10.17219/acem/130606. [DOI] [PubMed] [Google Scholar]


