Vaccination is an effective population strategy for preventing severe COVID-19 disease and reducing infection transmission.1 The emergence of new variants, particularly more transmissible strains, such as the omicron variants, has resulted in high infection rates in unvaccinated and vaccinated individuals.2 Concerns about more transmissible variants are especially pertinent for immunosuppressed individuals, in whom vaccines are less immunogenic and less effective.3 We and others have shown that patients with inflammatory bowel disease (IBD) established on immunosuppressive drugs, including infliximab (an anti–tumor necrosis factor [TNF] monoclonal antibody) or tofacitinib (a pan–Janus kinase [JAK]-inhibitor), have reduced vaccine-induced humoral responses after 2 and 3 doses of COVID-19 vaccination.3, 4, 5 This study aimed to evaluate functional neutralizing antibody responses against the SARS-CoV-2 ancestral and omicron BA.1 variants in an immunosuppressed population of IBD patients.
Between May 28, 2021, and March 29, 2022, 268 participants without evidence of SARS-CoV-2 infection from the SARS-CoV-2 Vaccination Immunogenicity in Immunosuppressed Inflammatory Bowel Disease Patients (VIP) cohort4 , 5 were included in this study. IBD participants were recruited in specific immunosuppressive treatment regimens, including infliximab (n = 36), thiopurines (n = 51), infliximab and thiopurine combination therapy (n = 39), ustekinumab (n = 39), vedolizumab (n = 38), and tofacitinib (n = 16). We additionally recruited healthy, non-IBD control individuals (n = 49). Participants had received either 2 doses of messenger RNA (mRNA) vaccine (BNT162b2 or mRNA-1273) or adenovirus vector vaccine (ChAdOx1 nCoV-19) as their primary vaccination schedule and received an mRNA vaccine as the third dose. Participant characteristics are shown in Supplementary Table 1.
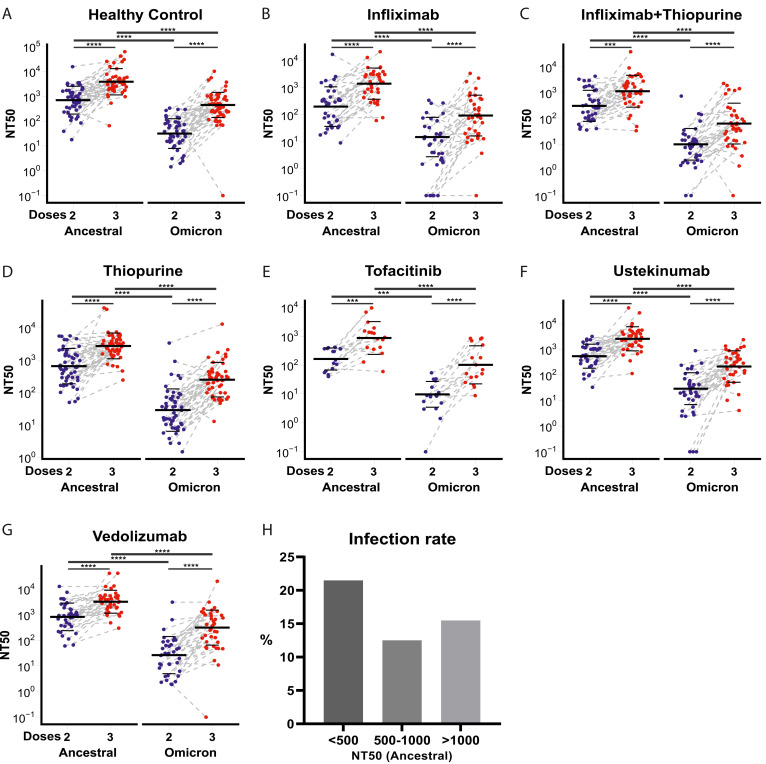
To evaluate vaccine-induced humoral responses, we used a pseudoneutralization assay against the SARS-CoV-2 ancestral strain and omicron BA.1 variant (Supplementary Methods). Reassuringly, neutralizing antibody titers against ancestral and omicron variants significantly increased after a third dose of vaccine compared to titers after a second dose of vaccine in all treatment groups (Figure 1 A–G). However, 50% neutralization titer (NT50) values were significantly lower against omicron than against the ancestral strain in all study groups, irrespective of the immunosuppressive treatment regimen (Figure 1 A–G). After 2 and 3 vaccine doses, patients treated with infliximab, infliximab/thiopurine combination therapy, or tofacitinib mounted significantly lower responses relative to control individuals. A total of 13 patients could not generate NT50 against the omicron variant after 2 doses of vaccine, 7 of whom were treated with infliximab monotherapy, making up 19.4% of this treatment group (Figure 1 B).
Figure 1.
(A–G) NT50 against SARS-CoV-2 ancestral strain and omicron in IBD patients treated with different immunosuppressive medications and healthy control individuals after 2 and 3 doses of vaccine. The horizontal bars represent the geometric mean and standard deviation. Wilcoxon signed-rank tests with Benjamini-Hochberg correction were performed. (H) The percentages of breakthrough infection in participants are stratified by neutralization titer ranges. ∗∗∗P < .001, ∗∗∗∗P < .0001.
The most compelling rationale to justify a third dose of vaccine would be evidence that low antibody titers after a second vaccine dose were associated with increased risk of SARS-CoV-2 infection. Because lower antibody responses are linked to increased risk of breakthrough infection,6 , 7 we investigated the risk of breakthrough infection in our cohort according to different thresholds of neutralizing titer (Figure 1 H). Participants with an NT50 of <500 against the SARS-CoV-2 ancestral strain had a 1.6-fold increased odds ratio of breakthrough infection compared to participants with an NT50 of >500 (P = .066). After 2 vaccine doses, 45.9% (100/218) of IBD patients had an NT50 of >500 against the SARS-CoV-2 ancestral strain. After 3 vaccine doses, 85.3% (186) patients reached the NT50 level of 500. In the healthy control individuals, 34.7% (17/49) had an NT50 of <500 after 2 vaccine doses, and 14.3% (7/49) had breakthrough infections. There was no significant association between NT50 and breakthrough infection in healthy control individuals. Fortunately, the breakthrough infections in our cohort were mild; none of the participants required hospitalization, and there were no deaths.
In this study, neutralizing titers elicited against the omicron variant were generally poor for all individuals and were substantially lower in recipients of infliximab, infliximab/thiopurine combination, or tofacitinib therapy. This raises concerns about whether currently available vaccines will be sufficient to protect against continually evolving SARS-CoV-2 variants, especially in patients established on certain immunosuppressive drugs. Current vaccinations mainly target the ancestral SARS-CoV-2 spike protein. Because many mutations exist in the omicron spike protein, this might lead to a significant escape from immune protection elicited by a COVID-19 vaccine designed against the SARS-CoV-2 ancestral virus.8 Recently, the US Food and Drug Administration approved bivalent omicron-containing vaccines that elicit superior neutralizing antibody responses against the omicron variant.9 Preferential use of bivalent vaccines may be especially valuable in IBD patients taking anti-TNF agents or JAK inhibitors.
Our study has important strengths. We have harnessed more robust functional pseudoneutralization assays than the binding antibody measurement used in most other IBD studies. We also actively recruited patients established on the main IBD drug regimens to get a broad view of the impact of different immunosuppressive mechanisms of action on vaccine-induced immunogenicity. We also prospectively recruited a population of healthy, non-IBD individual as a critical comparison. However, we acknowledge the limitations of this study. First, the sample size for some drug groups, most notably tofacitinib, was small, which might limit the robustness of our findings, although our observations were highly statistically significant. Second, although our study was consistent with a signal for increased risk of breakthrough infection in IBD patients with lower titers of neutralizing antibodies, the study was underpowered to answer this question definitely, and the results should be regarded with caution.
In summary, we have shown that a third dose of vaccination significantly increases neutralizing antibody responses against the SARS-CoV-2 ancestral and omicron variants, but this response, especially to evolving variants, is substantially lower in patients established on infliximab or tofacitinib. As further mutations in the viral genome accumulate over time, with the attendant risk of immune evasion, it remains important to continue to reappraise vaccination strategy, including the implementation of personalized approaches for some patients, such as those treated with anti-TNF drugs and JAK inhibitors.
The data of this study are available under a transfer agreement from the corresponding author based on a reasonable request.
Acknowledgments
Members of the VIP study investigators: Imperial College London: Kaixing Le, Xin Zhou, Hajir Ibraheim, Sulak Anandabaskaran, Aamir Saifuddin, Leon R. McFarlane, Nikhil Anand, Laura Constable, Rocio Castro Seoane, Andrea D’Mello, Sharmili Balarajah, Lucy C. Hicks, Horace R.T. Williams, Jonathan W. Lo, Ailsa L. Hart, Daniel M. Altmann, Rosemary J. Boyton, and Julian P. Teare. Royal Devon University Healthcare NHS Foundation Trust: Rachel Nice, Claire Bewshea, James R. Goodhand, Nicholas A. Kennedy, Anna Barnes, John Kirkwood, Marian Parkinson, and Helen Gardner-Thorpe. University of Edinburgh: Charlie W. Lees, Gareth R. Jones, Kate Covil, and Lauranne Derikx. King’s College London: Francesca Fiorentino and Peter M. Irving. University of Cambridge: Miles Parkes and Rachel Linger. Bart’s Health NHS Trust: Klaartje Kok, Irish Lee, and Bessie Cipriano. St George’s Hospital NHS Trust: Kamal V. Patel. Hull University Teaching Hospitals NHS Trust: Shaji Sebastian. King’s College Hospital: Alexandra J. Kent. Imperial College Healthcare NHS Trust: Ijeoma Chukwurah, Sulaimaan Haq, Parita Shah, Stephanie Wilken-Smith, Anitha Ramanathan, Mikin Patel, Lidia Romanczuk, Rebecca King, Jason Domingo, Bridget Knight, Djamila Shamtally, Vivien Mendoza, Joanne Sanchez, Hannah Stark, Louise Bee, Charmaine Estember, Darcy Watkins, Sam Stone, Beatriz Gros Alcalde, Giuseppe Ruocco, Manisha Baden, Graham Cooke, Evgenia Kourampa, Ciro Pasquale, Elena Robisco-Diaz, and Suhaylah Bhatti.
CRediT Authorship Contributions
Zhigang Liu, PhD (Conceptualization: Equal; Data curation: Lead; Formal analysis: Lead; Investigation: Lead; Methodology: Lead; Software: Lead; Validation: Lead; Visualization: Lead; Writing – original draft: Lead; Writing – review & editing: Lead).
James L. Alexander, PhD (Conceptualization: Equal; Data curation: Equal; Resources: Lead; Writing – original draft: Equal; Writing – review & editing: Equal).
Kathy Weitung Lin, BSc (Data curation: Equal; Investigation: Equal).
Tariq Ahmad, PhD (Project administration: Equal; Resources: Equal).
Katrina M. Pollock, PhD (Conceptualization: Lead; Methodology: Lead; Resources: Lead; Supervision: Lead; Writing – review & editing: Equal).
Nick Powell, PhD (Conceptualization: Lead; Data curation: Equal; Formal analysis: Equal; Funding acquisition: Lead; Investigation: Lead; Methodology: Lead; Project administration: Lead; Resources: Lead; Supervision: Lead; Validation: Equal; Visualization: Equal; Writing – original draft: Lead; Writing – review & editing: Lead).
Footnotes
Conflicts of interest These authors disclose the following: James L. Alexander reports sponsorship from Vifor Pharma for accommodation and travel to the British Society of Gastroenterology annual meeting 2019, outside the submitted work. Nicholas A. Kennedy reports grants from AbbVie, Biogen, Celgene, Celltrion, Galapagos, MSD, Napp, Pfizer, Pharmacosmos, Roche, and Takeda; consulting fees from Amgen, Bristol Myers Squibb, Falk, Janssen, Mylan, Pharmacosmos, Galapagos, Takeda, and Tillotts; personal fees from Allergan, Celltrion, Falk, Ferring, Janssen, Pharmacosmos, Takeda, Tillotts, and Galapagos; and support for attending meetings from AbbVie, Falk, and Janssen, outside the submitted work. Anna Barnes has received travel expense support from Janssen. Shaji Sebastian reports grants from Takeda, AbbVie, Tillotts Pharma, Janssen, Pfizer, and Biogen and personal fees from Takeda, AbbVie, Janssen, Pharmacocosmos, Biogen, Pfizer, Tillotts Pharma, and Falk Pharma, outside the submitted work. Ailsa L. Hart reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AbbVie, AZ, Atlantic, Bristol Myers Squibb, Celltrion, Falk, Galapogos, Janssen, MSD, Napp Pharmaceuticals, Pfizer, Pharmacosmos, Shire, and Takeda; participation on the Global Steering Committee for Genentech; support for attending meetings from AbbVie, Takeda, and Janssen; and participation on a data safety monitoring board or advisory board for AbbVie, AZ, Atlantic, Bristol Myers Squibb, Galapogos, Janssen, Pfizer, and Takeda. Peter M. Irving reports grants from Celltrion, Takeda, MSD, Pfizer, and Galapagos and personal fees from Celltrion, Takeda, Pfizer, Galapagos, Gilead, AbbVie, Janssen, Bristol Myers Squibb, Lilly, and Arena, outside the submitted work. Miles Parkes receives unrestricted educational grants from Pfizer for genetic analyses to support the IBD BioResource and speaker fees from Janssen. Gareth R. Jones has received grants from the Wellcome Trust and ECCO; speaker fees from Takeda, Ferring, and Janssen; and support for attending meetings or travel from Ferring. Klaartje Kok reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Janssen and Ferring; support for attending meetings or travel from Janssen and Takeda; and participation on a data safety monitoring board or advisory board for Janssen and PredictImmune. Kamal V. Patel reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AbbVie, Dr Falk, Janssen, PredictImmune, and Takeda; support for attending meetings or travel from AbbVie, Ferring, Janssen, and Tillotts; and participation on a data safety monitoring board or advisory board for AbbVie, Galapagos, and Janssen. Alexandra J. Kent reports consulting fees from Janssen; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Pfizer and Takeda; support for attending meetings or travel from Janssen, Tillotts, and Norgine; and participation in a data safety monitoring board or advisory board for AbbVie. Lucy C. Hicks reports support for attending meetings or travel from AbbVie. Charlie W. Lees reports a Future Leaders Fellow award from UK Research and Innovation; personal consulting fees from Galapagos, AbbVie, Takeda, Pfizer, Janssen, and Iterative Scopes; institutional consulting fees from Trellus Health; personal fees from Galapagos, AbbVie, Takeda, Pfizer, Janssen, GSK, Gilead, Fresenius Kabi, Ferring, and Dr Falk; and support for attending meetings from Galapagos, AbbVie, Takeda, Pfizer, Janssen, GSK, Gilead, Fresenius Kabi, Ferring, and Dr Falk. Rosemary J. Boyton and Daniel M. Altmann are members of the Global T Cell Expert Consortium and have consulted for Oxford Immunotec outside the submitted work. James R. Goodhand reports grants from F. Hoffmann-La Roche, Biogen, Celltrion Healthcare, and Galapagos and nonfinancial support from Immundiagnostik during the study. Tariq Ahmad reports grant funding from Pfizer to his institution to deliver this study; grants from Celltrion, Roche, Takeda, Biogen, and Galapagos; and honoraria for lectures from Takeda and Roche, outside the submitted work. Katrina M. Pollock is the chief, principal, or coinvestigator for vaccine clinical trials and experimental medicine studies (NCT05007275, NCT04753892, EudraCT 2020-001646-20, NCT04400838, NCT04324606, EudraCT 2017-004610-26, NCT03970993, NCT03816137); is a member of the data safety monitoring board for NCT05249829; has received a fee for speaking from Seqirus and Sanofi Pasteur; and has research funding from the Chan Zuckerberg Initiative, the Medical Research Council (MRC) from the United Kingdom Research Innovation (UKRI), the Vaccine Task Force, and National Institute for Health and Care Research (NIHR) Imperial Biomedical Research Center (BRC) outside the submitted work. Nick Powell is the principal investigator on the research grant from Pfizer that funded the VIP study; has received research grants from Bristol Myers Squibb outside the submitted work; reports personal fees from Takeda, Janssen, Pfizer, Galapagos, Bristol Myers Squibb, AbbVie, Roche, Lilly, Allergan, and Celgene, and AstraZeneca outside the submitted work; and has served as a speaker or advisory board member for AbbVie, Allergan, Bristol Myers Squibb, Celgene, Falk, Ferring, Janssen, Pfizer, Tillotts, Takeda, and Vifor Pharma. The remaining authors disclose no conflicts.
Funding Pfizer Ltd provided financial support for the VIP study as an independent research grant. The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. This research was supported by the NIHR Biomedical Research Centres in Imperial College London and Imperial College Healthcare NHS Trust and Cambridge (BRC-1215–20014) and the NIHR Clinical Research Facility Cambridge. The NIHR IBD BioResource supported recruitment to this study. The NIHR Exeter Clinical Research Facility that supported this project is a partnership between the University of Exeter Medical School College of Medicine and Health and Royal Devon University Healthcare NHS Foundation Trust. The views expressed in this article are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. James L. Alexander is a recipient of an NIHR Academic Clinical Lectureship (CL-2019–21–502), funded by Imperial College London and The Joyce and Norman Freed Charitable Trust. Gareth R. Jones is supported by a Wellcome Trust Clinical Research Career Development Fellowship (220725/Z/20/Z). Rosemary J. Boyton and Daniel M. Altmann are supported by UK Research and Innovation (MR/S019553/1, MR/R02622X/1, MR/V036939/1, and MR/W020610/1), the NIHR Imperial Biomedical Research Centre Institute of Translational Medicine & Therapeutics, the Cystic Fibrosis Trust Strategic Research Centre (2019SRC015), NIHR Efficacy and Mechanism Evaluation Fast Track (NIHR134607), NIHR Long Covid (COV-LT2–0027), Innovate UK (SBRI 10008614), and Horizon 2020 Marie Skłodowska-Curie Innovative Training Network European Training Network (number 860325).
Author names in bold designate shared co-first authorship.
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2022.10.010.
Contributor Information
VIP Study Investigators:
Kaixing Le, Xin Zhou, Hajir Ibraheim, Sulak Anandabaskaran, Aamir Saifuddin, Leon R. McFarlane, Nikhil Anand, Laura Constable, Rocio Castro Seoane, Andrea D’Mello, Sharmili Balarajah, Lucy C. Hicks, Horace R.T. Williams, Jonathan W. Lo, Ailsa L. Hart, Daniel M. Altmann, Rosemary J. Boyton, Julian P. Teare, Rachel Nice, Claire Bewshea, James R. Goodhand, Nicholas A. Kennedy, Anna Barnes, John Kirkwood, Marian Parkinson, Helen Gardner-Thorpe, Charlie W. Lees, Gareth R. Jones, Kate Covil, Lauranne Derikx, Francesca Fiorentino, Peter M. Irving, Miles Parkes, Rachel Linger, Klaartje Kok, Irish Lee, Bessie Cipriano, Kamal V. Patel, Shaji Sebastian, Alexandra J. Kent, Ijeoma Chukwurah, Sulaimaan Haq, Parita Shah, Stephanie Wilken-Smith, Anitha Ramanathan, Mikin Patel, Lidia Romanczuk, Rebecca King, Jason Domingo, Bridget Knight, Djamila Shamtally, Vivien Mendoza, Joanne Sanchez, Hannah Stark, Louise Bee, Charmaine Estember, Darcy Watkins, Sam Stone, Beatriz Gros Alcalde, Giuseppe Ruocco, Manisha Baden, Graham Cooke, Evgenia Kourampa, Ciro Pasquale, Elena Robisco-Diaz, and Suhaylah Bhatti
Supplementary Methods
The SARS-CoV-2 Vaccination Immunogenicity in Immunosuppressed Inflammatory Bowel Disease Patients (VIP) study is a UK multicenter prospective observational study aimed at evaluating the immunogenicity of COVID-19 vaccination in IBD patients on 6 different immunosuppressive treatment regimens (infliximab, thiopurine, infliximab and thiopurine combination therapy, ustekinumab, vedolizumab, or tofacitinib). Participant recruitment as well as inclusion and exclusion criteria have been described previously.1 Participants for the healthy control group were recruited from healthy volunteer databases (National Institute for Health Research National Bioresource, Peninsula Research Bank and Healthy Volunteer Panel of the Imperial Clinical Research Facility) and staff working at the university and medical centers involved in the study. Healthy control individuals were included if they did not have a diagnosis of IBD and were not currently being treated with systemic immunosuppressives for any other indication. Healthy control individuals were not excluded if they had other medical conditions.
In the United Kingdom, vaccines were administered to the most clinically vulnerable and then through progressively lower risk and younger age groups from December 2020. Citizens received either the mRNA-based vaccine (BNT162b2 or mRNA-1273) or the adenovirus-vector vaccine (ChAdOx1 nCoV-19) for the first 2 doses with a 3–12-week interval between the 2 doses. From 13 September 2021, patients deemed to be clinically extremely vulnerable, including those with IBD treated with immunosuppressive therapies, were offered a third primary vaccine dose with an mRNA-based vaccine (BNT162b2 or mRNA-1273) at least 8 weeks after their second dose.
Blood samples were collected from participants 53–92 days after the second vaccine dose and 28–49 days after the third vaccine dose. Participants received either homologous (3 doses of mRNA vaccine) or heterologous (2 doses of adenovirus vector followed by 1 dose of mRNA vaccine) vaccination schedules. The study protocol is available online (https://www.vipstudy.uk).
Anti–SARS-CoV-2 Antibody Electrochemiluminescence Assay
The Roche Elecsys anti-SARS-CoV-2 (N) immunoassay is a sandwich electrochemiluminescence immunoassay that uses a recombinant protein of the nucleocapsid antigen for the determination of antibodies against SARS-CoV-2 infection. The manufacturer reports clinical sensitivity and specificity of 99.5% and 99.8%, respectively, >14 days after polymerase chain reaction–confirmed COVID-19 using a cutoff index of 1. It is reported that anti-N antibody responses after SARS-CoV-2 natural infection are impaired in patients treated with immunosuppressant drugs such as infliximab.2 , 3 Results showed that a threshold of 0.12 times the cutoff index provides 100% specificity for determining prior SARS-CoV-2 infection.4 Therefore, in the current study, individuals with anti-N of ≥0.12 U/mL were deemed to have had SARS-CoV-2 infection.
Pseudoneutralization Assay
SARS-CoV-2 neutralization assay was conducted using pseudotyped viruses (PSV). Briefly, pseudotyped SARS-CoV-2 lentiviruses were produced in HEK293T cells using a SARS-CoV-2 spike plasmid, HIV-1 gag-pol plasmid, and a firefly luciferase reporter.5 Participant sera were serially diluted and incubated with PSV viral supernatant for 1 hour. HEK-ACE2 cells were then coincubated with the sera and PSV for 72 hours before measurement of the luciferase activity using the Bright-Glo Luciferase assay system (Promega). NT50 was calculated as the dilution at which relative luminescence was reduced by 50% compared with control. The First World Health Organization International Standard for anti-SARS-CoV-2 immunoglobulin was included as a positive control, which was determined to have an NT50 of approximately 1:3000.
Outcome Measures
Our primary outcome was anti–SARS-CoV-2 neutralizing response against ancestral virus and omicron variant after the 2 and 3 doses of anti–SARS-CoV-2 vaccine, stratified by baseline immunosuppressive therapy.
The secondary outcome was risk of breakthrough infection. SARS-CoV-2 breakthrough infection was defined by participants who reported a polymerase chain reaction or lateral flow test result confirming SARS-CoV-2 infection or a concentration of Roche Elecsys anti–SARS-CoV-2 nucleocapsid immunoassay nucleocapsid antibodies in the serum above 0.11 U/mL after 2 doses of vaccine.1 , 4
Variables
Demographics were recorded as the variables age, sex, ethnicity, comorbidities, height and weight, smoking status, and postcode; IBD disease activity (defined by patient-reported outcomes [PRO2]),6 , 7 SARS-CoV-2 symptoms aligned to COVID-19 symptoms study (symptoms, previous testing, and hospital admissions for COVID-19), SARS-CoV-2 test date and results, vaccine schedules (type and date of each vaccination), and date of blood collection. Data were entered electronically into a purpose-designed REDCap database hosted at the Royal Devon University Healthcare NHS Foundation Trust.8 Participants without access to the Internet or an electronic device completed their questionnaires on paper case record forms that were subsequently entered by local research teams.
Statistics
A statistical analysis plan was approved by the Study Management Group (available at https://www.vipstudy.uk/info). Analyses were undertaken using R 4.1.0 (R Foundation for Statistical Computing). P values of <.05 with 2-tailed test were considered significant. Antibody concentrations are reported as the geometric mean and standard deviation. Other continuous data are reported as a median and interquartile range, and discrete data are reported as number and percentage unless otherwise stated. The Wilcoxon signed-rank test was performed to test the significance of NT50 between the ancestral strain and omicron variant or between the second and third vaccine doses. For the univariate analysis comparing NT50 among groups treated with different regimens, the Kruskal-Wallis test with Dunn post hoc test was performed.
Ethical Considerations
VIP is an investigator-led UK National Institute for Health Research COVID-19 study. Participants were included after providing written informed consent. The study was registered with the International Standard Randomized Controlled Trial Number (ISRCTN, no. 13495664) registry, and the protocol is available online at https://www.vipstudy.uk.
Supplementary Table 1.
Demographics of the Cohort in This Study
| Characteristics | Control | Infliximab | Infliximab +thiopurine | Thiopurine | Tofacitinib | Ustekinumab | Vedolizumab |
|---|---|---|---|---|---|---|---|
| n (%) | 49 (18.28) | 36 (13.43) | 39 (14.55) | 51 (19.03) | 16 (5.97) | 39 (14.55) | 38 (14.18) |
| Age, y, median (IQR) | 38.30 (30.20–54.30) | 48.55 (39.30–59.55) | 37.30 (30.50–49.85) | 46.10 (35.25–56.25) | 49.30 (43.12–55.57) | 41.80 (33–53.05) | 45.50 (36.45–62.45) |
| Sex, n (%) | |||||||
| Female | 33 (68.75) | 17 (47.22) | 19 (48.72) | 30 (58.82) | 2 (12.50) | 20 (51.28) | 11 (29.73) |
| Male | 15 (31.25) | 19 (52.78) | 20 (51.28) | 21 (41.18) | 14 (87.50) | 19 (48.72) | 26 (70.27) |
| Ethnicity, n (%) | |||||||
| Non-White | 5 (10.42) | 7 (19.44) | 5 (12.82) | 9 (17.65) | 1 (6.25) | 4 (10.26) | 9 (24.32) |
| White | 43 (89.58) | 29 (80.56) | 34 (87.18) | 42 (82.35) | 15 (93.75) | 35 (89.74) | 28 (75.68) |
| BMI, kg/m2, median (IQR) | 22.72 (21.42–24.86) | 25.50 (23.46–28.60) | 25.24 (22.48–27.28) | 24.15 (21.41–26.21) | 26.91 (24.32–30.13) | 25.58 (22.75–29.33) | 24.42 (22.13–27.29) |
| Vaccine, n (%) | |||||||
| AstraZeneca | 26 (53.06) | 16 (44.44) | 27 (69.23) | 32 (62.75) | 13 (81.25) | 26 (66.67) | 23 (60.53) |
| Moderna | 2 (4.08) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Pfizer | 21 (42.86) | 20 (55.56) | 12 (30.77) | 19 (37.25) | 3 (18.75) | 13 (33.33) | 15 (39.47) |
| Diagnosis, n (%) | |||||||
| Crohn’s disease | 0 (0) | 24 (66.67) | 25 (64.10) | 21 (41.18) | 0 (0) | 38 (97.44) | 15 (39.47) |
| IBD unclassified | 0 (0) | 2 (5.56) | 2 (5.13) | 1 (1.96) | 0 (0) | 0 (0) | 0 (0) |
| Ulcerative colitis | 0 (0) | 10 (27.78) | 12 (30.77) | 29 (56.86) | 16 (100) | 1 (2.56) | 23 (60.53) |
| Smoking, n (%) | |||||||
| Currently | 0 (0) | 1 (2.78) | 2 (5.13) | 1 (1.96) | 0 (0) | 3 (7.69) | 4 (10.81) |
| Not currently | 12 (25) | 12 (33.33) | 11 (28.21) | 17 (33.33) | 10 (62.50) | 13 (33.33) | 11 (29.73) |
| Never | 36 (75) | 23 (63.89) | 26 (66.67) | 33 (64.71) | 6 (37.50) | 23 (58.97) | 22 (59.46) |
| Interval, d, median (IQR) | |||||||
| Second dose to first blood sampling | 80 (78–86) | 77.50 (60.75–88) | 84 (62–88) | 78 (63–85) | 79.50 (63.75–89.25) | 84.50 (65.25–88) | 81 (64–87) |
| Third dose to second blood sampling | 38 (33–42.50) | 42 (32–46) | 40 (38.50–46.50) | 40.50 (30.50–43) | 37 (33.50–42) | 39.50 (33.75–44.25) | 40 (32–42) |
| First to second vaccine dose | 65.50 (56.50–77) | 73.50 (68.75–77) | 77 (76.25–79) | 77 (75–80) | 72.50 (62.75–77) | 77 (70.25–79) | 77 (71–80) |
| Second to third vaccine dose | 174.50 (154.50–184.75) | 176 (167–185) | 171 (151.50–184.50) | 176 (163–187) | 171.50 (152–179) | 177 (152.50–189) | 189 (179–196) |
| Heart disease, n (%) | 0 (0) | 1 (2.78) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.70) |
| Lung disease, n (%) | 1 (2.08) | 6 (16.67) | 5 (12.82) | 6 (11.76) | 3 (18.75) | 3 (7.69) | 3 (8.11) |
| Kidney disease, n (%) | 0 (0) | 2 (5.56) | 0 (0) | 1 (1.96) | 0 (0) | 0 (0) | 1 (2.70) |
| Diabetes, n (%) | 1 (2.08) | 3 (8.33) | 0 (0) | 3 (5.88) | 0 (0) | 3 (7.69) | 3 (8.11) |
| Cancer, n (%) | 0 (0) | 1 (2.78) | 0 (0) | 1 (1.96) | 0 (0) | 0 (0) | 0 (0) |
IQR, interquartile range.
References
- 1.Suthar A.B., et al. BMJ. 2022;377:e069317–e069323. doi: 10.1136/bmj-2021-069317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altarawneh H.N., et al. N Engl J Med. 2022;387:21–34. doi: 10.1056/NEJMoa2203965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kennedy NA, Janjua M, et al. Gut. Published July 28, 2022. https://doi.org/10.1136/gutjnl-2022-327570.
- 4.Alexander J.L., Kennedy N.A., et al. Lancet Gastroenterol Hepatol. 2022;7:342–352. doi: 10.1016/S2468-1253(22)00005-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexander J.L., Liu Z., Muñoz Sandoval D., Reynolds C., et al. Lancet Gastroenterol Hepatol. 2022;7:1005–1015. doi: 10.1016/S2468-1253(22)00274-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coppeta L., et al. Vaccines. 2022;10:141–150. [Google Scholar]
- 7.Takita M., et al. Sci Rep. 2022;12:9147–9157. doi: 10.1038/s41598-022-12834-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hachmann N.P., et al. N Engl J Med. 2022;387:86–88. doi: 10.1056/NEJMc2206576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chalkias S., et al. N Engl J Med. 2022;387:1279–1291. doi: 10.1056/NEJMoa2208343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Supplementary References
- 1.Alexander J.L., Kennedy N.A., et al. Lancet Gastroenterol Hepatol. 2022;7:342–352. doi: 10.1016/S2468-1253(22)00005-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kennedy N.A., Goodhand J.R., et al. Gut. 2021;70:865–875. doi: 10.1136/gutjnl-2021-324388. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy NA, Janjua M, et al. Gut. Published July 28, 2022. https://doi.org/10.1136/gutjnl-2022-327570.
- 4.Lin S.M., Kennedy N.A., Saifuddin A., Sandoval D.M., et al. Nat Commun. 2022;13:1379–1392. [Google Scholar]
- 5.Pollock K.M., et al. EClinicalMedicine. 2022;44 [Google Scholar]
- 6.Khanna R., et al. Aliment Pharmacol Ther. 2015;41:77–86. doi: 10.1111/apt.13001. [DOI] [PubMed] [Google Scholar]
- 7.Jairath V., et al. Aliment Pharmacol Ther. 2015;42:1200–1210. doi: 10.1111/apt.13408. [DOI] [PubMed] [Google Scholar]
- 8.Harris P.A., et al. J Biomed Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]