Abstract
Electric Arc Furnace Dust (EAFD) is a byproduct of the steelmaking industry, which is one of the most significant and rapidly generated hazardous waste. This material includes recyclable elements such as zinc (Zn) and iron (Fe), exclusively in the form of zinc-ferrite (ZnFe2O4), zincite (ZnO), magnetite (Fe3O4) with some other minor compounds of Si, Mn, Mg, and Pb. A combination of pyro and hydrometallurgical route, also known as hybrid process, has acquired considerable attention to extract Fe and Zn from electric arc furnace dust (EAFD). In the current study, systematic thermodynamic assessments using thermochemical–software FactSage and targeted experimental investigations were carried out to assess the suitability of the caustic-roasting step of this important hybrid process. Thermodynamic calculation of the effect of different parameters on the conversion of EAFD to a suitable feedstock for the next separation stages showed that the major zinc-ferrite (ZnFe2O4) phase of EAFD can be potentially converted to ZnO and sodium-ferrite (NaFeO2) intermediate phase while reacting with NaOH (>2 mol) at temperatures equal or higher than 100 °C. The suitable condition was assessed to an EAFD to NaOH ratio of 1:3 M fraction and a temperature, T > 318 °C at the normal atmospheric condition. Moreover, the selected experimental investigation was also showing a good agreement with the current thermodynamic assessment.
Keywords: Thermodynamics, FactSage, EAFD, Roasting, Recycling
Thermodynamics, FactSage, EAFD, Roasting, Recycling.
1. Introduction
Galvanizing is the process of applying a thin layer of zinc to a steel surface to prevent corrosion. In the process of galvanizing steel products, zinc consumption rises every year. Normally the life span of these products is roughly 5–10 years, afterward, they are discarded as steel melting scrap for recycling purposes (Chairaksa-Fujimoto et al. 2015). During melting of the steel scraps in an electric arc furnace (EAF), a large amount of waste is produced, including slag, sludge, flue dust, and gases. Approximately 15–25 kg of dust is produced per metric ton of smelted steel scrap in an EAF (Dutra et al. 2006). Zinc and iron are the two major components of EAF dust, around 10–20 wt.% and 20–30 wt.%, respectively, and are mostly present in the form of ZnO, zinc-ferrite (ZnFe2O4), and Fe3O4. Other minor but hazardous elements such as lead, cadmium, chromium, and nickel are also present in their respective oxide form with a small concentration of chloride compounds (Polsilapa et al. 2011).
With the declining trend of primary zinc resources, zinc recovery from the electric arc furnace dust (EAFD) has emerged as a key consideration for the conservation of zinc-containing resources (Ma et al. 2011). However, in the case of electric arc furnace dust recycling, several technical and environmental issues need to be addressed. Firstly, the composition of EAF dust varies depending on the type of steel scrap; therefore, it requires a versatile procedure to accommodate this variation. Secondly, a significant number of constituents in EAFD must be taken into account from an environmental standpoint; such as heavy metals like Pb, Cr that must be below a certain limit to minimize the hazardous issues. Lastly, chloride compounds might have negative impacts on the recycling process. Therefore, an effective EAFD recycling process should address all of these aforementioned issues.
Several investigations have been carried out using the pyrometallurgical and hydrometallurgical routes to recover Zinc from electric arc furnace dust. Most pyrometallurgical methods, such as the Waelz process, Sintering, FASTMET and FASTMELT, OXYCUP, ZincOX, Primus, and ArcFume, are based on the high–temperature reduction of EAFD using a suitable reductant such as carbon, carbon monoxide, or hydrogen gas (McClelland and Metius 2003; Morcali et al. 2012; Lin et al. 2017). Notably, the pyrometallurgical processes have the drawbacks of high labor costs, high energy-intensive, and the production of solid waste. Whereas, in the hydrometallurgical process, acid or alkaline solutions are used to extract the desired elements in the principal extraction step. In acidic media, despite the lack of selectivity for other metals found in steelmaking dust, Zn recovery is higher than that obtained in alkaline media (Zhang et al. 2019). On the other hand, alkaline leaching can provide a comparatively clean and iron-free leach solution due to its selectivity for zinc over iron, which eliminates the very complicated iron removal stages found in most acid–leach methods. However, the solubility of the complex tetrahedral structure of zinc-ferrite (ZnFe2O4) is fairly limited in both acidic and alkaline solutions except in hot strong acid (Martins 2007; Omran and Fabritius 2017). In recent years, many researchers have focused on a combination of the pyro and hydrometallurgical methods, also known as the hybrid process, to increase the leachability of the EAFD. The hybrid process uses a lesser amount of liquid chemicals and is carried out at a lower temperature, therefore overcoming many of the drawbacks of both the pyrometallurgical and hydrometallurgical processes. During this process, the phase composition of the complex–ZnFe2O4 is changed to an intermediate soluble–compound through roasting with additive agents (e.g. NaOH/(NH4)2SO4/CaO/FeCl3) and followed by aqueous leaching in NaOH or NH4Cl solutions to recover Zn and Fe from EAFD (Li et al. 2015; Chairaksa-Fujimoto et al. 2016; Miki et al. 2016). A similar hybrid method using NaOH as an additive for both roasting and leaching has been reported by Xia and Pickles (1999) and Youcai and Stanforth (2000). They described this integrated process into three steps: (1) Hydrolysis: to separate the water-soluble component of EAFD, (2) Fusion: to transform the complex zinc–ferrite to a leachable compound, and (3) Leaching: to dissolve the zinc-rich component obtained from the previous step. Although some experimental work has been carried out in this field, there is a lack of thermodynamic understanding of the caustic–roasting step of this important process.
Therefore, in the current study, the effect of processing parameters on the conversion of electric arc furnace dust (EAFD) to a suitable feedstock for the next separation stages was studied thermodynamically using NaOH as the reducing agent at temperatures ranging from 323 K to 773 K (50–500 °C) at atmospheric pressure (1 atm). Systematic thermodynamic calculations were performed using FactSage 7.2 thermochemical software to evaluate the effect of reactant composition, temperature, and controlled atmosphere on the caustic roasting condition. Selected experimental investigations of using NaOH with raw EAFD from steel plants were also performed to substantiate the thermodynamic calculations.
2. Materials and methods
2.1. Materials
The electric arc furnace dust (EAFD) sample was collected from a Fume Treatment Plant (FTP) and supplied by a steel manufacturing company in Bangladesh. The chemical composition of the as-received EAFD was evaluated using the X-ray Fluorescence (XRF) analysis (Rigaku, Model: ZSX Primus IV) and the results are shown in Table 1.
Table 1.
Chemical composition of the as-received EAFD sample.
| Component | Fe2O3 | ZnO | CaO | MnO | SiO2 | PbO | Al2O3 | MgO | CuO | K2O | Cr2O3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mass % | 42.8 | 34.4 | 3.85 | 2.90 | 2.81 | 1.92 | 1.61 | 1.10 | 0.89 | 0.92 | 0.42 |
The table represents the elemental concentration of the EAFD components as their respective oxide phase. The Zn and Fe are the two predominant components present in the sample with other minor amounts of Ca, Mn, Mg, Si, Pb, and Al impurities. During experiments, sodium hydroxide (NaOH) pellets, 99.9% (Merck, Germany), were used for caustic–roasting reactions.
2.2. Characterization
The structural characterization of the as-received electric arc furnace dust (EAFD) sample was performed using the X-ray Diffractometer (XRD) analysis (Rigaku, Model: SmartLab). X-ray pattern of the powder sample was obtained with a monochromated Cu X-ray source (Kα = 1.540598 Å) in the range from 10° to 80° with a scanning rate of 0.02° per 1.5 s time step. The XRD pattern of the as-received sample is shown in Figure 1 which confirms the presence of zincite (ZnO), zinc-ferrite (ZnFe2O4), and magnetite (Fe3O4) the predominant phases in the sample.
Figure 1.
XRD pattern of as-received EAFD.
The semi-quantitative Rietveld refinement of the corresponding XRD pattern reveals that 10 wt.% of zinc oxide and 25 wt.% of magnetite (Fe3O4), with the rest being predominantly zinc-ferrite (ZnFe2O4) in the dust.
2.3. Thermodynamic modeling
The thermodynamic analysis was performed using the FactSage 7.2 thermochemical software with optimized compound and solution databases. FactSage supports the computation and manipulation of phase diagrams, complicated phase equilibria, and predominance diagrams. The species and phases that were either present in the original material or potentially formed during the process were included in the input data. FactSage pure-compound databases: FactPS and solution-databases: FToxide and FTmisc were used in this study. The modules utilized for current studies were ‘Reaction’, ‘Equilib’, and ‘Predom’ with the relevant optimized databases, which were obtained from standard thermodynamic compilation data of NIST-JANAF thermochemical tables and NBS (National Bureau of Standards) tables of chemical thermodynamics (Ahmad et al. 2014). The ‘Reaction’ module calculates the changes in general thermochemical parameters (e.g., H, G, V, S, Cp, A) for a single species, a mixture of species, or a chemical reaction (Bale et al. 2009). This module was used in this study to calculate the Gibbs free energy changes (ΔG) for various caustic reactions with different oxides. The ‘Equilib’ module computes the concentrations of chemical species once they have attained chemical equilibrium using the Gibbs free energy minimization approach. This module was used to examine several equilibrium-reaction products at high temperatures and with various reactant contents. Finally, in this study the ‘Predom’ module was employed to compute a predominance area diagram at a constant temperature. This approach is highly effective for plotting distinct stable phases in different gaseous atmospheres. Few assumptions were made during calculation, which are as follows:
-
●
Calculations were performed at one atmospheric pressure, implying that the concentration of gas species has no influence on the atmospheric temperature.
-
●
The activity of the elements was considered to be 1 in the standard state, and all the atoms/molecules had the potential to interact with one another to obtain the lowest possible energy state.
-
●
The system had been allowed to reach equilibrium, which indicates that reactions with extremely slow reaction kinetics in the system have been completed.
-
●
It was presumed that the system wass closed.
-
●
For simplicity, the zinc–ferrite (ZnFe2O4) of the EAFD (one of the major components) was taken as a basis of weight for the thermodynamic calculations.
The data entered into the thermodynamic modeling was based on the results obtained from the chemical and structural characterization of the EAF dust and is commonly found in the literature (Mehmet et al. 2015). A summary of the equilibrium reaction products predicted from the current study is shown in Table 2.
Table 2.
Thermodynamic stable (product) phases that are utilized in the present study.
| Gas | Solid | Solid | Solid | Solid |
|---|---|---|---|---|
| O2 | Mg(OH)2 | Fe3O4 | CaSiO3 | Al2O3 |
| H2O | Mg3Si2O5(OH)4 | ZnFe2O4 | Na2SiO3 | Al2SiO5 |
| Liquid | Mg2SiO4 | ZnO | ¥Na2xSiyO(2x+y) | MnO |
| NaOH(l) | MgSiO3 | Zn2SiO4 | (x = 1,2,3; y= 1,2) | ¥MnxO(x+1) |
| H2O(l) | H2Si2O5 | Na2ZnO2 | NaFeO2 | (x = 1,2) |
| Solid | H6Si2O7 | CaO | NaFe2O3 | MnSiO3 |
| Ca(OH)2 | H2SiO3 | Na2O | ¥NaxFeO(x-1) | Mn2SiO4 |
| Zn(OH)2 | FeO | Na2O2 | (x = 3,4,7) | PbO |
| NaOH(s) | Fe2O3 | Na2CaSiO4 | MgO | ¥PbxOx+1(x = 1,3) |
¥ Non-stoichiometric compounds.
2.4. Experimental procedure
Experiments were undertaken within a range of temperature from 250 °C to 350 °C as a method of verifying the calculations based on the thermodynamic analysis. A resistive heating muffle furnace (Wincom, Model: TC–5–12) was used for the current experiment. The electric arc furnace dust (EAFD) sample and NaOH were taken at a 1:3 M ratio in a porcelain crucible. The set temperature and molar ratio of EAFD to NaOH were selected based on the thermodynamic study. The furnace was heated to the desired temperature and then the crucible was slowly pushed to the maximum heating zone to avoid cracking due to the thermal shock. The holding time was selected to 1 h as found commonly used in much of the literature (Youcai and Stanforth 2000; Lenz and Martins 2007; Halli et al. 2017). At the end of the roasting process, the sample was taken out from the furnace to allow it to cool directly in natural air.
3. Results and discussion
3.1. Reaction at controlled atmosphere and equilibrium reactant amount
Different caustic roasting conditions for zinc–ferrite dissociation as a function of temperature and NaOH addition were investigated. The reactions as shown in Equation–1 (in absence of O2) and Equation–2 (in presence of O2) represent the influence of atmospheric control on the reactant (ZnFe2O4) dissociation.
| (1) |
| (2) |
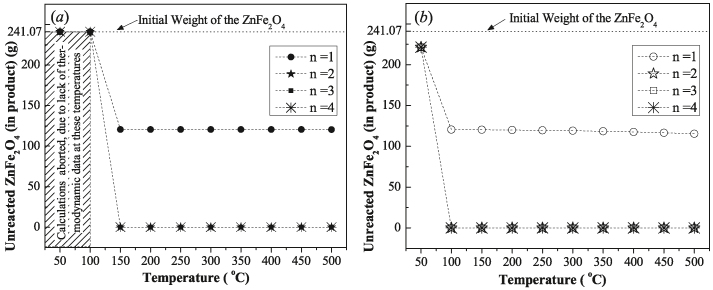
There is no noticeable variation in the dissociation of the ZnFe2O4, at the presence or absence of oxygen as illustrated in Figure 2(a) and Figure 2(b), respectively. At low temperature (T < 100 °C) the calculation for Eq. (1) was aborted due to lack of thermodynamic data at these temperatures as shown in Figure 2(a) whereas, for the rest of the calculations, the amount of unreacted ZnFe2O4 is equal in both cases. Moreover, the effect of change of O2 amount (1–4 mol) had been also investigated and the results were found unchanged with the variation of oxygen content as per Eq. (2). Therefore, it is suggested that the reaction of zinc–ferrite (ZnFe2O4) with NaOH does not require a sealed atmosphere, which means an economical advantage while scaling up the process in practical ways. However the actual atmospheric condition is subjected to experimental validation.
Figure 2.
The effect of the controlled atmosphere in the reaction process: (a) without oxygen and (b) with oxygen.
It is also evident from Figure 2(b) that, at low zinc–ferrite (ZnFe2O4) to NaOH ratios e.g. 1:1 M ratio, the majority of the ZnFe2O4 remained unreacted over the temperature range. Moreover, when the reaction temperature is < 100 °C, a certain amount of ZnFe2O4 is always left unreacted at all quantities of NaOH from n = 1–4 mol. However, at the reaction temperature equal to or more than 100 °C, the minimal amount of NaOH required to complete the dissociation process was determined to be 1:2 M ratios of zinc–ferrite (ZnFe2O4) to NaOH.
3.2. Optimization of temperature and NaOH amount
Figure 3 shows the unreacted NaOH amount present in the equilibrium product phase. It is evident that a minimum of n = 2 mol of NaOH is required to dissociate 1 mol of ZnFe2O4 during caustic–roasting at ≥ 100 °C. However, when the ZnFe2O4 to NaOH ratio increased more than 1:2 (i.e. n > 2 mol), the amount of unreacted NaOH was increased in the product phases. In most cases, the actual EAFD contains additional compounds besides the ZnFe2O4. Therefore a higher amount n = 3 mol of NaOH is chosen as a suitable quantity to complete the reaction process, however the actual amount depends on detailed reaction mechanism and kinetics study. It is assumed that the excess NaOH will aid in dissociating other compounds as well.
Figure 3.
Equilibrium amount of the NaOH phase remains unreacted in the product phases.
Furthermore, the unreacted NaOH phase was transformed from solid to liquid once the temperature increased above the melting point of NaOH (Tm = 318 °C). From the kinetics points of view, it is apparent that once the reaction set–temperature is higher than the melting point of NaOH (i.e. T > 318 °C), the reaction mechanism will be transformed from solid–solid to solid-liquid reaction mode. Notably, the reactions between solid–liquid mode usually have a lower activation energy barrier as compared to solid–solid reaction process i.e. the former process results in a faster equilibration than the latter (Chen et al. 2022). Moreover, the degree to which the reactants are thoroughly mixed determines the rate of collisions of atomic/molecular; hence if the reactants are thoroughly mixed, the atom/molecules will collide more frequently, and the reaction will be faster. Therefore, the reaction temperature for the further thermodynamic calculations and experimental work is chosen 350 °C, which is above the melting point of NaOH. However, the optimum temperature for the actual caustic–roasting process is subject to the detailed kinetics study which is under planning by the same researcher group. Similar approximations were also reported from the investigation of Youcai and Stanforth (2000) and Halli et al. (2017).
3.3. Gibbs free energy change vs temperature curve
One mole of each possible compound found in electric arc furnace dust (EAFD) was allowed to react with the appropriate amount of NaOH and O2 (calculated from the previous section). The change of Gibbs free energy (ΔG) of different reactions was calculated as per Eq. (3) at temperatures ranging from 50 °C to 500 °C and is presented in Figure 4.
| (3) |
Figure 4.
Change of Gibbs free energy of different reactions with increasing temperatures from 50 °C to 500 °C.
As shown in Figure 4, all conceivable components of EAFD have a positive impact on the increase of roasting temperature (the reactions are more favorable with increasing temperature), except for PbO and FeO, which have a negative impact on temperature increment. The change in Gibbs free energy (ΔG) of the MnSiO3 reaction is more negative in Figure 4, indicating that the reaction of MnSiO3 with NaOH and O2 is more favorable than the others. Therefore, it is expected that MnSiO3 will dissociate first from the mixture of different electric arc furnace dust (EAFD) components during caustic roasting at any temperature of current interest. On the other hand, the ΔG of the ZnO reaction shows a positive value, indicating that the reaction is less favorable and not spontaneous within the existing temperature range. Finally, from the above figure the sequence of most stable phases during caustic roasting at the chosen temperature, 350 °C is as follows: ZnO > PbO > ZnFe2O4 > Fe2O3 > CaSiO3 > MgSiO3 > FeO > Fe3O4 > MnSiO3. This information is beneficial when it is necessary to know the most spontaneous reactant phase from a set of components. Therefore, it is clear from the above calculation that, one of the major components of the collected electric arc furnace dust sample, ZnFe2O4 will preferentially dissolve from the mixture of ZnO and zinc–ferrite (ZnFe2O4).
3.4. Predominance area calculation
The study of the predominance area diagram will help to understand the stable phase(s) present at a certain gaseous condition. The ‘Predom’ module from FactSage 7.2 software was used to perform a systematic predominance calculation of the Zn–Fe–Na–O–H system, and the results are shown in Figure 5.
Figure 5.
Predominance study of Zn–Fe–Na–O–H system at 350 °C.
From Table 2, it has shown that O2 and H2O are the two stable gaseous components in the product phase, therefore, the present predominance calculations utilize the different pO2 and pH2O conditions. An overlay of predominant phase stability diagrams for the Zn–Na–O–H and Fe–Na–O–H systems at 350 °C are presented in Figure 5. The dotted and dashed lines in the illustration represent the Zn–Na–O–H and Fe–Na–O–H systems, respectively. It is evident from Figure 5 that at pO2 = 1 atm and pH2O = 1 atm condition (marked ‘×’ line in the figure), the predicted stable phases are found to be sodium-ferrite (NaFeO2), ZnO, and NaOH(l) in a Zn–Fe–Na–O–H system at 350 °C.
3.5. Equilibrium products
The complex mineralogy of zinc-ferrite (ZnFe2O4) is deemed to transform to a suitable phase after the caustic roasting that can be easily processed on the next separation stage. ZnO and sodium-ferrite (NaFeO2) are the primary product phases obtained from the FactSage equilibrium calculation of ZnFe2O4 with varying molar ratios of NaOH. As the temperature increases, the amount of different product phases also increases. Figure 6 shows that both primary product phases grow with a temperature up to 100 °C and then remain constant over the temperature range. Moreover, both the ZnO and sodium-ferrite (NaFeO2) products have attained their optimum amounts when roasting with n ≥ 2 mol of NaOH at T ≥ 100 °C. However, considering the actual EAFD compositions and faster kinetics of the roasting process the actual NaOH amount and reaction temperature were chosen differently from the calculations as mentioned earlier.
Figure 6.
Amount of different product phases after the reaction (2) for (a) 1 mol NaOH, (b) 2 mol NaOH, (c) 3 mol NaOH and (d) 4 mol NaOH.
3.6. The behavior of other compounds of EAFD
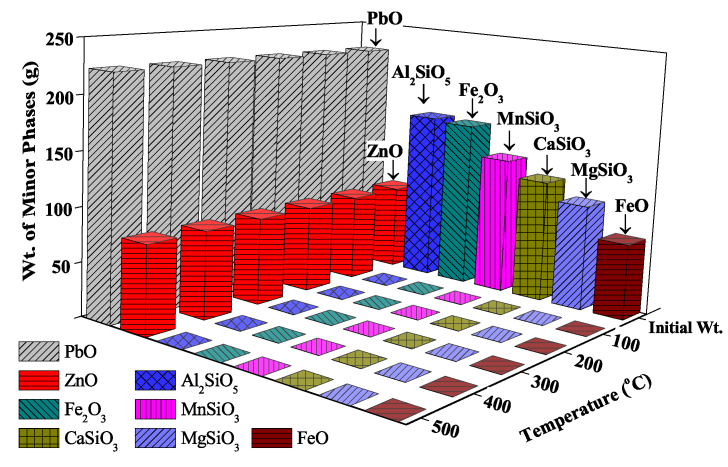
Further thermodynamic investigations were carried out to see the effect of other impurities of electric arc furnace dust (EAFD) during the roasting reaction. Therefore, several equilibrium calculations of caustic roasting were carried out with each possible impurity phase present in the dust sample as per Eq. (2) and the result is shown in Figure 7.
Figure 7.
Effect of other EAFD constituent phases on the caustic roasting process.
Except for ZnO and PbO, most of the EAFD components were dissolved during caustic roasting, as seen in Figure 7. The plot of Gibbs free energy calculation (Figure 4) also yielded similar results. As the ΔG of reactions for ZnO is positive i.e. non-spontaneous and PbO has a positive slope at the conferred temperature range; therefore, the possibilities of their dissolutions are deemed unfavorable at the present conditions. However, the majority of ZnO can be dissolved during alkaline leaching, whereas PbO can be selectively precipitated using sodium sulfide as a precipitant (Youcai and Stanforth 2000).
3.7. Experimental investigation: roasting of EAFD with NaOH
The experimental trials were carried out at two different temperatures (250 °C and 350 °C), a lower and a higher temperature than the set condition (T > 318 °C) and followed the process as described in the experimental methodology section. The post–experimental samples were investigated using the XRD technique and the results are shown in Figure 8.
Figure 8.
XRD pattern of (a) the raw EAF dust and the roasted EAF dust at (b) 250 °C and (c) 350 °C.
It can be seen from the above figure that, after the roasting reaction, several new peaks have appeared in the XRD pattern that corresponds to the major product phase, sodium-ferrite (NaFeO2). Therefore, the experimental analysis also confirms the expected product phase from the equilibrium and predominance calculations. Figure 8 further shows that the major peaks corresponding to the zinc-ferrite (ZnFe2O4) compound (35.34°, 42.97°, 56.76°, 62.28°) in the raw electric arc furnace dust (EAFD) sample were gradually decreasing as the temperature increased, indicating that the dissolution of EAFD is a function of temperature. Moreover, the peaks corresponding to the raw ZnO (31.86°, 34.39°, 36.27°, 47.57°) were increased, indicating that the additional ZnO was formed as per the calculation of equilibrium products in Figure 6.
4. Conclusions
Combined thermodynamic and targeted experimental studies were carried out to optimize the process parameters for electric arc furnace (EAF) dust roasting with NaOH at elevated temperatures. Different modules of FactSage 7.2 thermochemical software were used to assess the suitable reactant amount, temperature, and effect of atmosphere control on caustic–roasting reaction. From the present investigation, the following can be concluded:
-
1)
The roasting reaction of a major component of the EAF dust, namely zinc-ferrite (ZnFe2O4), with NaOH is temperature-dependent and a temperature above 100 °C is thermodynamically sufficient for the reaction to be completed. However, from the kinetics standpoint, the reaction temperature for the current roasting process is chosen T > 318 °C, which is above the melting point of NaOH.
-
2)
Theoretically, the reaction of ZnFe2O4 with NaOH is not selective on O2 atmosphere, therefore the caustic–roasting reaction does not require strict atmospheric control. However, the actual atmospheric condition is subjected to experimental validation.
-
3)
The suitable amount of EAFD to NaOH was found to be a 1:3 M ratio considering other minor compounds present within the dust, however the actual amount depends on detailed reaction mechanism and kinetics study.
-
4)
The ΔG of reaction calculation of EAFD shows that ZnFe2O4 will dissolve preferentially from a mixture of ZnO and ZnFe2O4. Moreover, MnSiO3 was found to decompose first from the dust sample.
-
5)
The predominance study of the Zn–Fe–Na–O–H system showed that the expected equilibrium phases present in the system at 1 atmospheric pressure are sodium-ferrite (NaFeO2), ZnO, and NaOH(l) at 350 °C.
-
6)
The effect of other impurity components of EAFD was investigated through the equilibrium reaction with 3 mol of NaOH in the presence of O2. The majority of the expected phases responded to the experimental condition except for ZnO and PbO. But these phases can be separated easily through a combination of caustic leaching and selective precipitation process.
-
7)
The experimental analysis was also showing the presence of predicted thermodynamically stable phases at the post–reaction XRD graphs.
Nevertheless, the future scope of this research will be conducted on the detailed kinetics study to optimize the reaction time, temperature, and NaOH amount with possible rate‒controlling step and reaction mechanism.
Declarations
Author contribution statement
Sazzad Ahmad: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Wahidur Rahman Sajal: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Fahmida Gulshan: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Mehedi Hasan: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
M Akbar Rhamdhani: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Bangladesh University of Engineering and Technology (BUET).
Data availability statement
Data will be made available on request.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Ahmad S., Rhamdhani M.A., Pownceby M.I., Bruckard W.J. Thermodynamic assessment and experimental study of sulphidation of ilmenite and chromite. Miner. Process. Extr. Metall. (IMM Trans. Sect. C) 2014;123(3):165–177. [Google Scholar]
- Bale C., Bélisle E., Chartrand P., Decterov S., Eriksson G., Hack K., Jung I.-H., Kang Y.-B., Melançon J., Pelton A. FactSage thermochemical software and databases—recent developments. Calphad. 2009;33(2):295–311. [Google Scholar]
- Chairaksa-Fujimoto R., Inoue Y., Umeda N., Itoh S., Nagasaka T. New pyrometallurgical process of EAF dust treatment with CaO addition. Int. J. Minerals, Metall. Mat. 2015;22(8):788–797. [Google Scholar]
- Chairaksa-Fujimoto R., Maruyama K., Miki T., Nagasaka T. The selective alkaline leaching of zinc oxide from Electric Arc Furnace dust pre-treated with calcium oxide. Hydrometallurgy. 2016;159:120–125. [Google Scholar]
- Chen X., Feng Y., Zhang S., Kou W., Ji H., Yang G. Comparison study on regeneration of spent ternary materials by molten salt solid-liquid method and traditional solid-solid method. J. Alloys Compd. 2022;900 [Google Scholar]
- Dutra A., Paiva P., Tavares L. Alkaline leaching of zinc from electric arc furnace steel dust. Miner. Eng. 2006;19(5):478–485. [Google Scholar]
- Halli P., Hamuyuni J., Revitzer H., Lundström M. Selection of leaching media for metal dissolution from electric arc furnace dust. J. Clean. Prod. 2017;164:265–276. [Google Scholar]
- Lenz D., Martins F. Lead and zinc selective precipitation from leach electric arc furnace dust solutions. Materia. 2007;12(3):503–509. [Google Scholar]
- Li Y., Liu H., Peng B., Min X., Hu M., Peng N., Yuang Y., Lei J. Study on separating of zinc and iron from zinc leaching residues by roasting with ammonium sulphate. Hydrometallurgy. 2015;158:42–48. [Google Scholar]
- Lin X., Peng Z., Yan J., Li Z., Hwang J.-Y., Zhang Y., Li G., Jiang T. Pyrometallurgical recycling of electric arc furnace dust. J. Clean. Prod. 2017;149:1079–1100. [Google Scholar]
- Ma H.-w., Matsubae K., Nakajima K., Tsai M.-S., Shao K.-H., Chen P.-C., Lee C.-H., Nagasaka T. Substance flow analysis of zinc cycle and current status of electric arc furnace dust management for zinc recovery in Taiwan. Resour. Conserv. Recycl. 2011;56(1):134–140. [Google Scholar]
- Martins D.M.L. Lead and zinc selective precipitation from leach electric arc furnace dust solutions. Materia. 2007;12:503–509. [Google Scholar]
- McClelland J.M., Metius G.E. Recycling ferrous and nonferrous waste streams with FASTMET.". JOM (J. Occup. Med.) 2003;55(8):30–34. [Google Scholar]
- Mehmet K., Oskay K.O., Şimşir M., Sübütay H., Kirgezen H. Optimization of selective leaching of Zn from electric arc furnace steelmaking dust using response surface methodology. Trans. Nonferrous Metals Soc. China. 2015;25(8):2753–2762. [Google Scholar]
- Miki T., Chairaksa-Fujimoto R., Maruyama K., Nagasaka T. Hydrometallurgical extraction of zinc from CaO treated EAF dust in ammonium chloride solution. J. Hazard Mater. 2016;302:90–96. doi: 10.1016/j.jhazmat.2015.09.020. [DOI] [PubMed] [Google Scholar]
- Morcali M.H., Yucel O., Aydin A., Derin B. Carbothermic reduction of electric arc furnace dust and calcination of waelz oxide by semi-pilot scale rotary furnace. J. Min. Metall. B Metall. 2012;48(2):173–184. [Google Scholar]
- Omran M., Fabritius T. Effect of steelmaking dust characteristics on suitable recycling process determining: ferrochrome converter (CRC) and electric arc furnace (EAF) dusts. Powder Technol. 2017;308:47–60. [Google Scholar]
- Polsilapa S., Sadedin D.R., Wangyao P. Thermodynamics analysis for the zinc ferrite reduction by hydrogen. High Temp. Mater. Process. 2011;30(6):587–592. [Google Scholar]
- Xia D., Pickles C. Caustic roasting and leaching of electric arc furnace dust. Can. Metall. Q. 1999;38(3):175–186. [Google Scholar]
- Youcai Z., Stanforth R. Integrated hydrometallurgical process for production of zinc from electric arc furnace dust in alkaline medium. J. Hazard Mater. 2000;80(1-3):223–240. doi: 10.1016/s0304-3894(00)00305-8. [DOI] [PubMed] [Google Scholar]
- Zhang D., Ling H., Yang T., Liu W., Chen L. Selective leaching of zinc from electric arc furnace dust by a hydrothermal reduction method in a sodium hydroxide system. J. Clean. Prod. 2019;224:536–544. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.