Abstract
Smilax glabra Roxb. (SGB) is a medicinal plant widely distributed in 17 countries worldwide. It is the primary raw material of the world-famous and best-selling functional food and beneficial tea. SGB was first recorded in Ben Cao Jing Ji Zhu of the Southern and Northern Dynasties (420–589 AD) and was reported for nutritional and medicinal properties for thousands of years. This review searched PubMed, Web of Science, and other databases for relevant literature on SGB species until April 2022. It aims to provide more integrated thinking, detailed awareness, and better knowledge of SGB. More than 200 chemical components have been discovered, including flavonoids, phenolic, phenolic acids, stilbenes, organic acids, phenylpropanoids, and others. Previous studies have demonstrated that SGB and its active ingredients show a wide range of pharmacological effects, including anti-infective, anti-cancer, anti-inflammatory, antioxidant, cardiovascular protection, etc. However, many studies on the biological activity of this plant were mainly based on crude extracts and active ingredients, and there is a lack of clinical studies and toxicity studies to support the development of drug design, development, and therapy. In summary, this review will provide specific and valuable suggestions and guidelines for further research and application of this plant in the medicinal field.
Keywords: Smilax glabra Roxb, astilbin, traditional usages, phytochemical constituents, pharmacological properties, clinical applications
Introduction
Smilax glabra Roxb. (SGB) is the rhizome of the Liliaceae plant, named Tu fuling in Chinese. There are more than 300 species of the Smilax genus in the world, most of which grow on hillsides, barren hills, and semi-shady land near forests.1 They are rich in resources and are widely distributed in 17 countries globally, including Asia, Europe, South America, etc. And it has been used as food and folk medicine in many countries for a long time.
SGB has been widely used worldwide due to its evident pharmacological activity for treating syphilitic, poisoned sores, pathological leucorrhea, eczema itching, carbuncle poisoning, and other diseases.2–4 Modern research has shown that SGB mainly includes flavonoids,5–8 phenolic and phenolic aids,9,10 stilbene and organic acids,11–13 phenylpropanoids,13,14 and others.14–16 Pharmacological studies have shown that the active ingredients in SGB have a variety of pharmacological effects, including anti-infective,17–19 anti-cancer,20–22 anti-inflammatory,16,23,24 antioxidant,25,26 and cardiovascular protection etc.27–29 Simultaneously, SGB is the primary raw material of the world-famous and best-selling functional food, soup and beneficial tea. It has the functions of nourishing yin and detoxification due to heat and humidity. In addition, the rhizome of SGB is rich in starch and can also be used to make cakes or winemaking.30–33
This product is cylindrical, flat or irregular, with nodular swelling, short branches, 5–22cm in length and 2–5cm in diameter. The surface is yellow-brown or gray-brown, uneven, with hard, fibrous root residues, round bud marks on the top of the branches, irregular cracks in the outer skin, and residual scales and leaves. The slices are elliptical or irregular, 1–5mm thick, with rough edges; the cut surface is white to light reddish brown, powdery, punctate vascular bundles.
This study conducted the latest and most comprehensive review of SGB, focusing on its traditional usages, phytochemical constituents, pharmacological properties, and clinical applications. It provides a basis for studying pharmacological and phytochemicals’ biological activity. This review aims to ensure the medicinal safety of SGB and provide guidance for its application in the medicinal field.
Research Methodology
This review searched relevant literature about SGB species from PubMed, Sci-Finder, Google Scholar, Wiley, Web of Science, Springer, Elsevier, Plant List Database, Weipu, and China Knowledge Network. It includes medical books, Ph.D., and MSc published research before April 2022. Data inclusion criteria included (a) published scientific manuscripts; (b) studies on ethnopharmacology; (c) plant extracts in different solvents; (d) mechanisms of action of plant extracts and their plant components; (e) Computational, in vitro, and in vivo studies.
Traditional Usage and the Distribution of Smilax glabra Roxb
Traditional Uses of Smilax glabra Roxb
SGB was first recorded in Ben Cao Jing Ji Zhu of the Southern and Northern Dynasties (420–589 AD) and written by Tao Hongjing. In the Tang Dynasty (618–907 AD), Ben Cao Shi Yi records that SGB could be used as food. The Ben Cao Tu Jing, written by Su Song in the Song Dynasty (AD 960-AD 1279), first recorded that SGB was sweet, calm in nature, non-toxic, and had a record of its efficacy. The Ben Cao Gang Mu, written by Li Shizhen in the Ming Dynasty (AD 1368-AD 1644), recorded that SGB could cure “syphilitic skin lesion”. Another herbal directory in the Ming Dynasty, Dian Nan Ben Cao, also recorded the efficacy of SGB and pointed out that it is the best medicine for treating syphilis. Until the Qing Dynasty (AD1636-AD1912), Ben Cao Bei Yao, written by Wang Ang, further described SGB. The effects were anti-infective, removing rheumatism, facilitating urination, stopping diarrhea, and treating muscles and bones. The modern 2020 edition of Chinese Pharmacopoeia described SGB as the dry rhizome of the Liliaceae family Smilax glabra, treating for syphilis, turbidity, and carbuncle, scrofula, scabies (Chinese Pharmacopoeia Commission, 2020).
The Distribution of Smilax glabra Roxb. Throughout the World
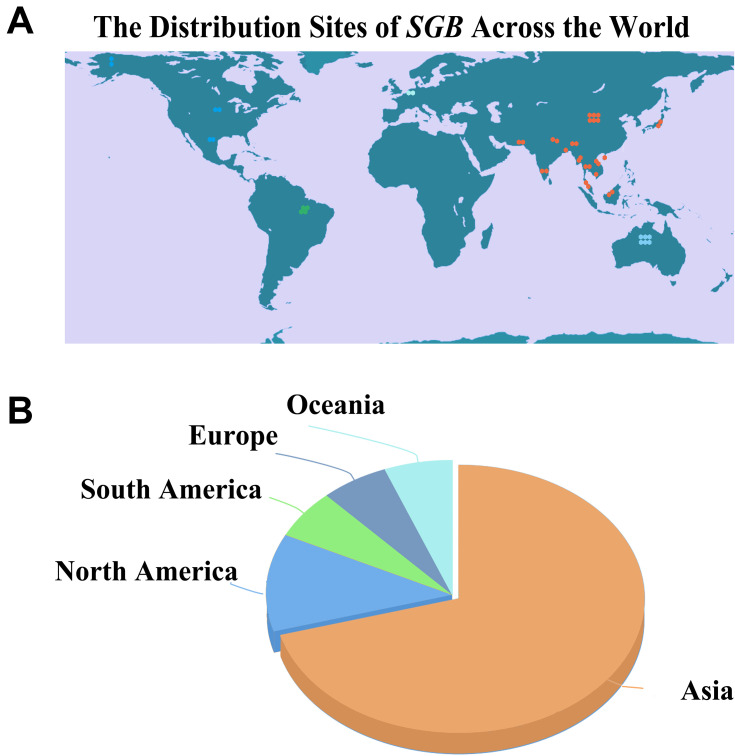
SGB is geographically distributed in China, Nepal, Pakistan, Mexico, Viet Nam, India, Cambodia, Thailand, Bangladesh, Belgium, Lao People’s Democratic Republic, Brazil, Myanmar, Australia, Malaysia, Japan, and the United States of America. In Asian countries, SGB is commonly used in foods, teas, and herbs, while in the West, SGB is an ingredient in draft beer and other beverages due to its foaming properties. And according to the database of the Global Biodiversity Information Facility (https://www.gbif.org/) and consultation of Flora of countries, Asia, including China (Taiwan, Hong Kong, and Macao, etc), harbors the most significant proportion of SGB‐producing sites. In Thailand, traditional practitioners have widely used it to treat cancer, AIDS, and other infectious diseases17,34 (Figure 1).
Figure 1.
Geographical distribution of SGB throughout the world. (A) Planar map of the distribution of SGB. (B) Distribution in all continents of the world. (A total of 17 countries were searched).
Phytochemical Constituents of the Smilax glabra Roxb
More than 200 compounds, including flavonoids, phenolic and phenolic acids, stilbenes, organic acids, and phenylpropanoids, have been isolated and identified from SGB. Flavonoids, phenolics, and phenylpropanoids are the most widely studied and used biologically active ingredients and are considered essential active ingredients. For example, astilbin, neoastilbin, isoastilbin, and resveratrol may be used as markers for future quality control and quantitative plant verification. It shows the compounds isolated and identified from SGB (Table 1) and the main biologically active compounds.
Table 1.
Chemical Constituents Isolated from SGB
| No. | Compounds | Part | Reference |
|---|---|---|---|
| Flavonoids and flavonoid glycosides | |||
| 1 | Taxifolin | Rhizomes | [5] |
| 2 | Naringenin | Rhizomes | [6] |
| 3 | Dihydrokaempferol | Rhizomes | [7] |
| 4 | Sakuranetin | Rhizomes | [7] |
| 5 | Isoastilbin | Rhizomes | [6] |
| 6 | Astilbin | Rhizomes roots | [14] |
| 7 | Neoisoastilbin | Rhizomes | [60] |
| 8 | Neoastilbin | Rhizomes | [7] |
| 9 | Engeletin | Rhizomes | [12] |
| 10 | Arthromerin B | Rhizomes | [7] |
| 11 | Sinensin | Rhizomes | [7] |
| 12 | (2R,3R)-taxifolin 3’-O-A-D-glucopyranoside | Rhizomes | [60] |
| 13 | (2S,3S)-glucodistylin | Rhizomes | [7] |
| 14 | Isoengeletin | Rhizomes | [14] |
| 15 | Quercetin-4’-O-β-D-pyr-anglucoside | Rhizomes | [61] |
| 16 | (-)-epicatechin | Rhizomes | [11] |
| 17 | (+)-catechin | Rhizomes | [7] |
| 18 | Cinchonain Ib | Rhizomes | [7] |
| 19 | Cinchonain Ia | Rhizomes | [7] |
| 20 | Apigenin | Rhizomes | [7] |
| 21 | Quercetin | Rhizomes | [7] |
| 22 | Luteolin | Rhizomes | [7] |
| 23 | Myricetin | Rhizomes | [7] |
| 24 | Kukulkanin B | Rhizomes | [7] |
| 25 | Smilachromanone | Rhizomes | [7] |
| 26 | 4,4’, 6-trihydroxyaurone | Rhizomes | [7] |
| 27 | Aureusidin | Rhizomes | [7] |
| 28 | 7,6’-dihydroxy-3’-methox-yisoflavone | Roots | [7] |
| 29 | (2R, 3R) -taxifolin-3′-O-β-D-pyr angl-ucoside | Rhizomes | [60] |
| 30 | 2(S)-5-hydroxy-6, 8-dimethoxyflavanone-7-O-beta-D-glucopyranosyl-(16)-O-beta-D-glucopyranoside | Rhizomes | [8] |
| 31 | 5-hydroxy-3, 8-dimethoxyflavone-7-O-beta-D-glucopyranosyl-(16)-O-beta-D-glucopyranoside | Rhizomes | [8] |
| 32 | 3, 7-dihydroxy-8-methoxyflavone-6-O-beta-D-glucopyranosyl-(16)-O-beta-D-glucopyranoside | Rhizomes | [8] |
| 33 | Dihy-droquercetin | Rhizomes | [8] |
| Phenolic and phenolic acids | |||
| 34 | Smiglabrone A | Rhizomes | [7] |
| 35 | Smiglabrone B | Rhizomes | [7] |
| 36 | Smilachromanone | Rhizomes | [7] |
| 37 | Smiglastilbene | Rhizomes | [7] |
| 38 | Smiglactone | Rhizomes | [7] |
| 39 | Smiglabrol | Rhizomes | [7] |
| 40 | Vanillin | Rhizomes | [7] |
| 41 | p-hydroxy-benzaldehyde | Rhizomes | [7] |
| 42 | Acetovanillone | Rhizomes | [7] |
| 43 | (+)-scytalone | Rhizomes | [7] |
| 44 | Glucosyringic acid | Rhizomes | [7] |
| 45 | Protocatechuic acid | Rhizomes | [7] |
| 46 | 3-methoxygallic acid | Rhizomes | [7] |
| 47 | Vanillic acid 1-O-β-D-glucopyranosyl ester | Rhizomes | [7] |
| 48 | Hydroxytyrosol | Rhizomes | [7] |
| 49 | Lasiodiplodin | Rhizomes | [9] |
| 50 | De-O-methyllasiodiplodin | Rhizomes | [9] |
| 51 | Resveratrol-3-O-β-D-gluco-pyranoside | Rhizomes | [10] |
| 52 | 3,4,5-tri-methoxyphenyl-1-O-[β-D-apiofuranosyl-(1-6)]-β-D-glucopyranoside | Rhizomes | [10] |
| 53 | 3,4,5-tri-methoxyphenyl-1-O-β-D-glucopyranoside | Rhizomes | [10] |
| 54 | 3,4-di-hydroxyphenothyl-3-O-β-D-glucopyranoside | Rhizomes | [10] |
| 55 | 2,4,6-trihydroxy acetophenone-2,4-di-O-β-D-glucopyranoside | Rhizomes | [10] |
| 56 | 8,8′-bisdihydrosyringeninglucoside | Rhizomes | [10] |
| Stilbene and organic acids | |||
| 57 | Resveratrol | Rhizomes | [11] |
| 58 | Trans-resveratrol | Rhizomes | [7] |
| 59 | Trans-piceid | Rhizomes | [7] |
| 60 | Piceatannol | Rhizomes | [7] |
| 61 | Syringic acid | Rhizomes | [7] |
| 62 | 5-O-caffeoylshikimic acid | Rhizomes | [12] |
| 63 | 2-methyl-succinic acid | Rhizomes | [7] |
| 64 | Shikimic acid | Rhizomes | [13] |
| 65 | Ferulic acid | Rhizomes | [13] |
| 66 | Palmitic acid | Rhizomes | [14] |
| 67 | Lignoceric acid | Rhizomes | [13] |
| 68 | Succinic acid | Rhizomes | [14] |
| Phenylpropanoids and lignans | |||
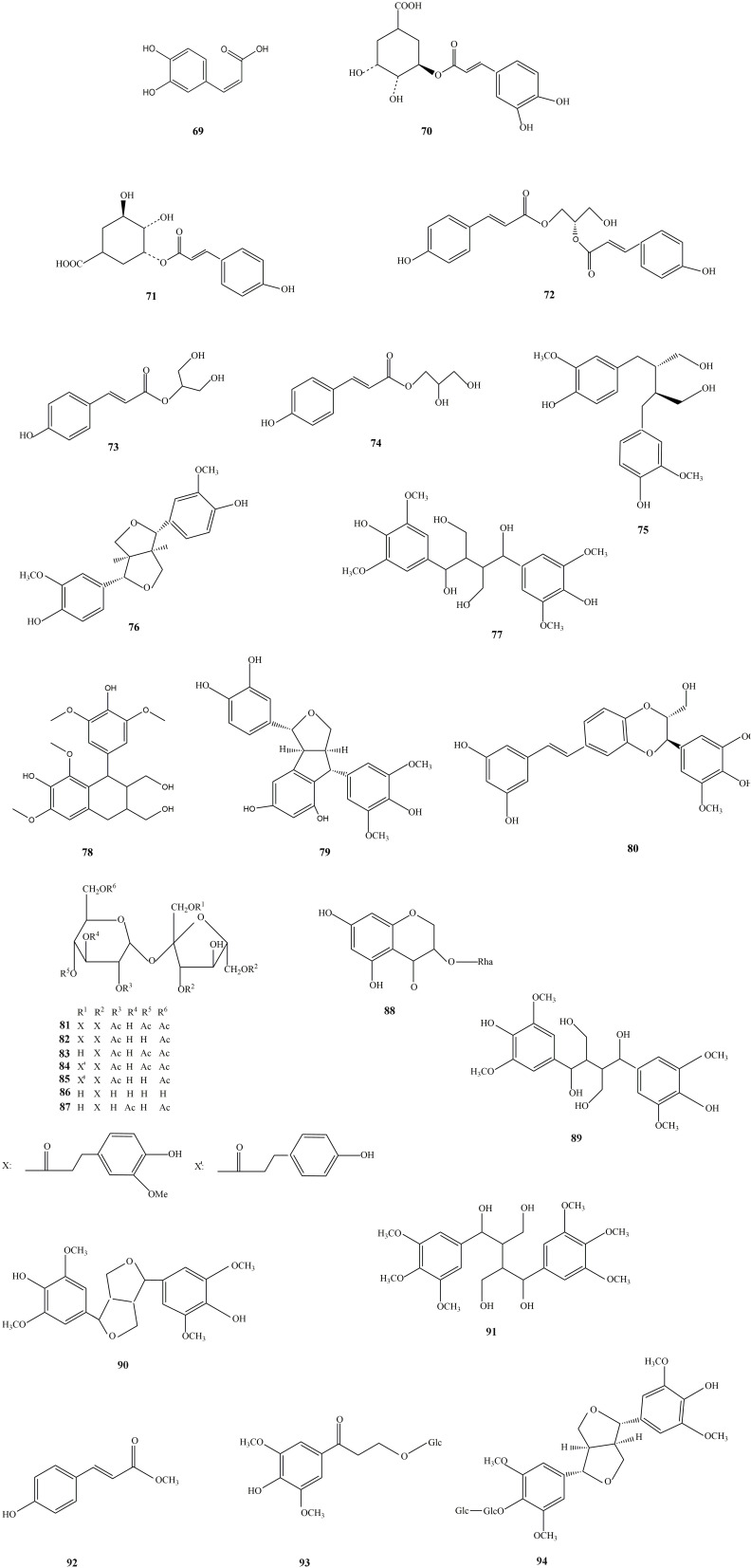
| 69 | Trans-caffeic acid | Rhizomes | [7] |
| 70 | 5-O-caffeoylshikimic acid | Rhizomes | [7] |
| 71 | 3-O-p-coumaroylshikimic acid | Rhizomes | [7] |
| 72 | (2S)-1,2-O-di-trans-p-coumaroylglycerol | Rhizomes | [7] |
| 73 | Juncusyl ester B | Rhizomes | [7] |
| 74 | 1-O-p-coumaroylglycerol | Rhizomes | [7] |
| 75 | (-)-secoisolariciresinol | Rhizomes | [7] |
| 76 | 4-ketopinoresinol | Rhizomes | [7] |
| 77 | Smiglabranol | Rhizomes | [7] |
| 78 | (+)-lyoniresinol | Rhizomes | [7] |
| 79 | KompasinolA | Rhizomes | [7] |
| 80 | Aiphanol | Rhizomes | [7] |
| 81 | Smiglaside A | Rhizomes | [94] |
| 82 | Smiglaside B | Rhizomes | [94] |
| 83 | Smiglaside C | Rhizomes | [94] |
| 84 | Smiglaside D | Rhizomes | [94] |
| 85 | Smiglaside E | Rhizomes | [94] |
| 86 | Helonioside A | Rhizomes | [94] |
| 87 | (3,6-di-O-feruloyl)-b-D-fructofuranosyl-(3,6-di-O-acetyl)-a-D-glu-copyranoside | Rhizomes | [94] |
| 88 | Smiglanin | Rhizomes | [7] |
| 89 | Smiglabranol | Rhizomes | [15] |
| 90 | Syringaresinol | Rhizomes | [9] |
| 91 | 1,4-bis(3,4,5-trimethoxyphenyl)-2,3-bis(hydroxymethyl)-1,4-butanediol | Rhizomes | [94] |
| 92 | Methylcaffeate | Rhizomes | [94] |
| 93 | 3-O-β-D-glucopyranosyl-1-(40-hydroxy-30, 50-dimeth oxyphenyl)-1-propanone | Rhizomes | [96] |
| 94 | (+)-syringaresinol-4-O-β-D-glucopyranosyl-(1-6)-β-D-glucopyranoside | Rhizomes | [94] |
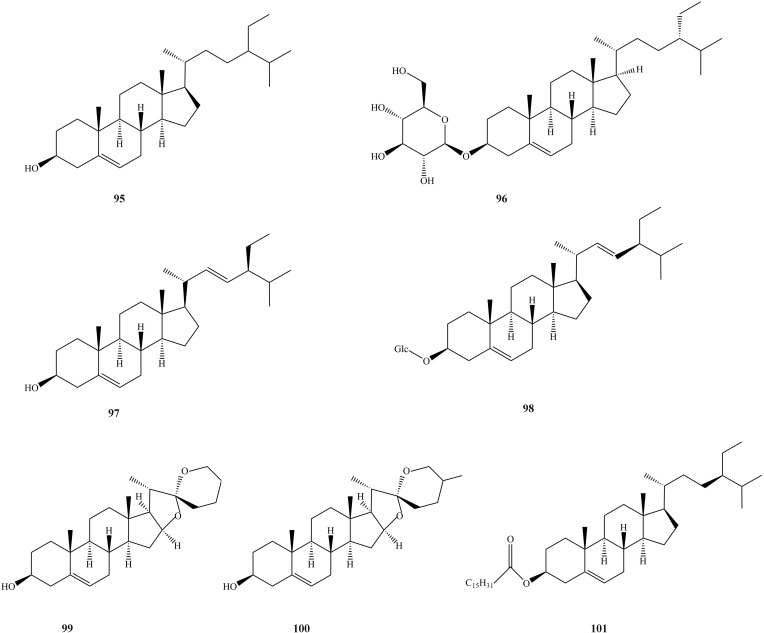
| Steroids and steroid glycosides | |||
| 95 | β-sitosterol | Rhizomes | [14] |
| 96 | Daucosterol | Rhizomes | [14] |
| 97 | Stigmasterol | Rhizomes | [97] |
| 98 | Stigmasterol-3-O-β-D-gluco-pyranoside | Rhizomes | [97] |
| 99 | Diosgenin | Rhizomes | [99] |
| 100 | Smilagenin | Rhizomes | [98] |
| 101 | β-sitosterol palmitate | Rhizomes | [61] |
| Volatile oil | |||
| 102 | Heptanoic acid, isopropyl ester | Rhizomes | [14] |
| 103 | Pentanoic acid, propyl ester | Rhizomes | [14] |
| 104 | Butanedioic acid, diethyl ester | Rhizomes | [14] |
| 105 | Caproic acid, methyl ester | Rhizomes | [14] |
| 106 | Hexanedioic acid, ethylmethyl ester | Rhizomes | [14] |
| 107 | Pentanoic acid 4-oxo-, butyl ester | Rhizomes | [14] |
| 108 | Nonanoic acid, ethyl ester | Rhizomes | [14] |
| 109 | Butane,1,1,1”-methylidynetris(oxy) tris | Rhizomes | [14] |
| 110 | Octane diacid, dimethyl ester | Rhizomes | [14] |
| 111 | Nonane diacid, dimethyl ester | Rhizomes | [14] |
| 112 | Heptane- diacid, dimethyl ester | Rhizomes | [14] |
| 113 | Nonanediacid, ethyl methyl ester | Rhizomes | [14] |
| 114 | Longipinanes | Rhizomes | [14] |
| 115 | 3-methyldodecane | Rhizomes | [14] |
| 116 | Dodecanoic acid, methyl ester | Rhizomes | [14] |
| 117 | 4-dodecanoic acid, methyl ester | Rhizomes | [14] |
| 118 | Butanedioic acid, dibutylester | Rhizomes | [14] |
| 119 | Pentanedioic acid, dibutyl ester | Rhizomes | [14] |
| 120 | Dodecanoic acid, ethyl ester | Rhizomes | [14] |
| 121 | Butanoic acid, octyl ester | Rhizomes | [14] |
| 122 | 3-hydroxy-dodecanoic acid methyl ester | Rhizomes | [14] |
| 123 | Honandioic acid, diethyl ester | Rhizomes | [14] |
| 124 | Hexadecane | Rhizomes | [14] |
| 125 | Tetradecanoic acid, methyl ester | Rhizomes | [14] |
| 126 | Tetradecanoic acid, ethyl ester | Rhizomes | [14] |
| 127 | 2,4-dimethyl-3-hexanone | Rhizomes | [14] |
| 128 | Pentadecanoic acid, methyl ester | Rhizomes | [14] |
| 129 | Decanedioic acid, diethyl ester | Rhizomes | [14] |
| 130 | 5-hexadecenoic acid, ethyl ester | Rhizomes | [14] |
| 131 | Pentadecanoic acid, ethyl ester | Rhizomes | [14] |
| 132 | 5,9-tetradecadienoic acid, methyl ester | Rhizomes | [14] |
| 133 | Hexadecanoic acid, methyl ester | Rhizomes | [14] |
| 134 | 1,1-benzenedicarboxylic acid, butyl ester | Rhizomes | [14] |
| 135 | 10-hexadecenoic acid, ethyl ester | Rhizomes | [14] |
| 136 | Hexadecenoic acid | Rhizomes | [14] |
| 137 | Heptadecanoic acid | Rhizomes | [14] |
| 138 | Hexadecanoic acid, ethyl ester | Rhizomes | [14] |
| 139 | Eicosane | Rhizomes | [14] |
| 140 | Heptadecanoic acid, methyl ester | Rhizomes | [14] |
| 141 | 9,12-octadecadienoic acid,methyl ester | Rhizomes | [14] |
| 142 | 7-octadecenoic acid, methyl ester | Rhizomes | [14] |
| 143 | 16-octadecenoic acid, methyl ester | Rhizomes | [14] |
| 144 | Octadecanoic acid, methyl ester | Rhizomes | [14] |
| 145 | 9,10-octadecadienoic acid, ethyl ester | Rhizomes | [14] |
| 146 | 8-octadecenoic acid, ethyl ester | Rhizomes | [14] |
| 147 | 9-octadecenoic acid, ethyl ester | Rhizomes | [14] |
| 148 | Octadecanoic acid 2-methyl methyl ester | Rhizomes | [14] |
| 149 | Octadecanoic acid, ethyl ester | Rhizomes | [14] |
| 150 | Nonane | Rhizomes | [15] |
| 151 | L-linalool | Rhizomes | [15] |
| 152 | 1-terpineol | Rhizomes | [15] |
| 153 | L-borneol | Rhizomes | [15] |
| 154 | Terpinen-4-ol | Rhizomes | [15] |
| 155 | Alpha-Terpineol | Rhizomes | [15] |
| 156 | (-)-bormylacetate | Rhizomes | [15] |
| 157 | (E,E)-2,4-decadienal | Rhizomes | [15] |
| 158 | Dihydrop-Ionon | Rhizomes | [15] |
| 159 | a-cedrol | Rhizomes | [15] |
| 160 | B-eudesmol | Rhizomes | [15] |
| 161 | a-eudesmol | Rhizomes | [15] |
| 162 | Myristic acid | Rhizomes | [15] |
| 163 | Methyl palmitate | Rhizomes | [15] |
| 164 | 8,11-octadecadienoic acid | Rhizomes | [15] |
| 165 | Methyl linolenate methyl ester | Rhizomes | [15] |
| 166 | Linoleic acid | Rhizomes | [15] |
| 167 | Stearic acid | Rhizomes | [15] |
| 168 | Docosane | Rhizomes | [15] |
| 169 | Tricosane | Rhizomes | [15] |
| 170 | Hexanal | Rhizomes | [16] |
| 171 | Phenylethylene | Rhizomes | [16] |
| 172 | 7-methyl-7H-Dibenzo[b, g]carbazole | Rhizomes | [16] |
| 173 | Octanal | Rhizomes | [16] |
| 174 | D-Limonene | Rhizomes | [16] |
| 175 | g-Terpinene | Rhizomes | [16] |
| 176 | Nonanal | Rhizomes | [16] |
| 177 | (-)-4-Terpineo | Rhizomes | [16] |
| 178 | Decanal | Rhizomes | [16] |
| 179 | Tridecane | Rhizomes | [16] |
| 180 | Dodecanal | Rhizomes | [16] |
| 181 | 11-Diacetoxydodecane | Rhizomes | [16] |
| 182 | Caryophyllenel | Rhizomes | [16] |
| 183 | Geranylacetone | Rhizomes | [16] |
| 184 | 2,5-bis(1,1-dimethylethyl)-Phenol | Rhizomes | [16] |
| 185 | Nonahexacontanoic acid | Rhizomes | [16] |
| 186 | Hentriacontane | Rhizomes | [16] |
| 187 | 7,9-dimethyl-Hexadecane | Rhizomes | [16] |
| 188 | 2,2,5,5-tetramethylbiphenyl | Rhizomes | [16] |
| 189 | 7-Methyl-octadecane7 | Rhizomes | [16] |
| 190 | Pristane | Rhizomes | [16] |
| 191 | 1-(hexadecyloxy)-Hexadecane | Rhizomes | [16] |
| 192 | 2,6,10,14-tetramethyl-Octadecane | Rhizomes | [16] |
Flavonoids and Flavonoid Glycosides
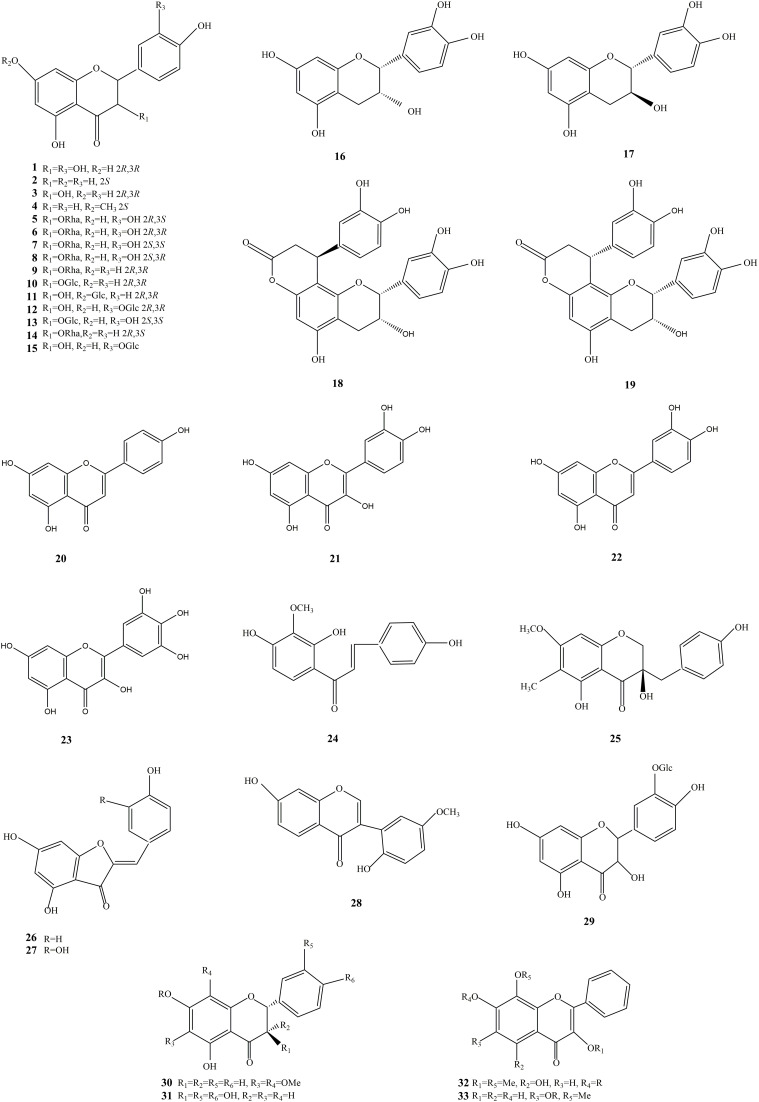
Flavonoids are pigments derived from 2-phenylchromone as the parent nucleus, including the isomers of flavonoids and their hydrogenated and reduced products. Flavonoids can be divided into different categories according to the type of parent nucleus, flavones, flavonols, dihydroflavones, dihydroflavonols, isoflavones, dihydroisoflavones, chalcones, flavones, anthocyanidins, biflavones, and others. Flavonoids in SGB are mainly dihydroflavonols, including taxifolin, astilbin, neoastilbin, isoastilbin, engeletin, isoengeletin, etc (Figure 2). Flavonols include quercetin and dihydro flavonoids include naringenin. Content range of (-)-epicatechin, 5-O-caffeoylshikimic acid, neoastilbin, astillbin, neoisoastilbin, isoastilbin and engeletin in 84 pieces of SGB medicinal samples were in the range of 1.381, 9.913, 3.673, 27.08, 1.181, 4.833, 2.754 mg/g, respectively. The representative component of astilbin content is about 1–2%, accounting for the largest proportion of total flavonoids. And it exhibits pharmacological activities such as antioxidant,35–42 actions on the cardiovascular system,43,44 antimicrobial,45–49 immunosuppressive,50–54 and anti-inflammatory.38,55–59 In the Chinese Pharmacopoeia Commission (2020), astilbin is used for quality inspection of SGB, with a minimum content of 0.45%.11,14,60–62
Figure 2.
The structures of flavonoids and flavonoid glycosides isolated from SGB.
Phenolic and Phenolic Acids
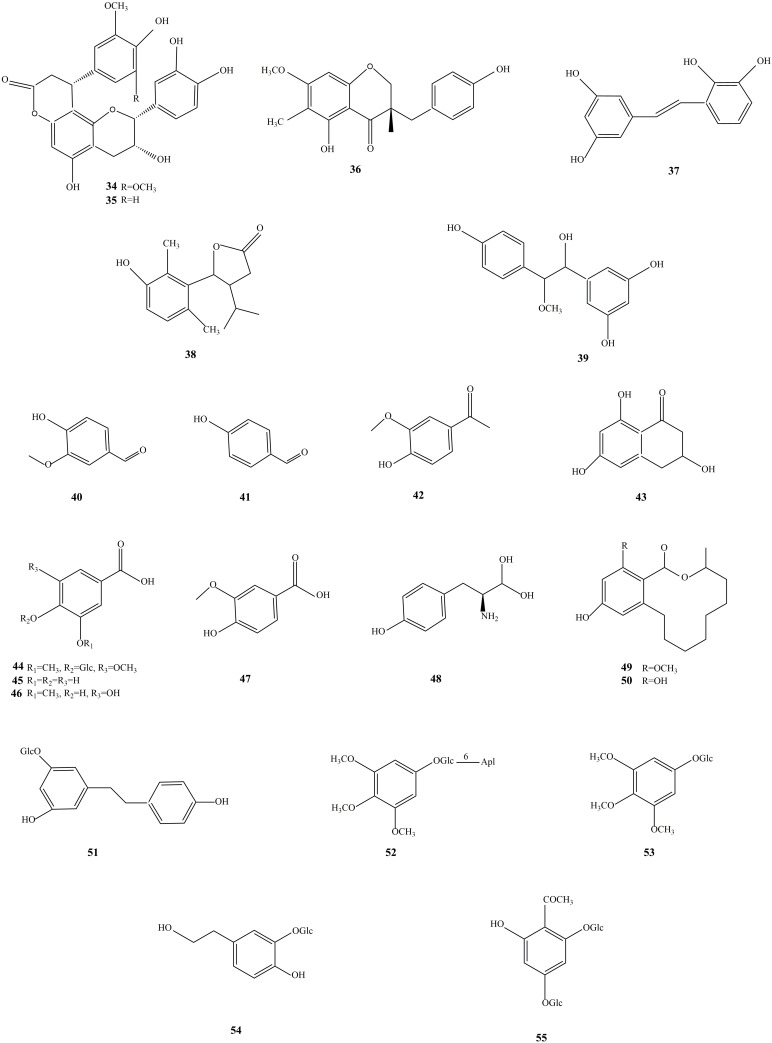
The phenolic acid compound is an aromatic carboxylic acid compound whose benzene ring is substituted by multiple phenolic hydroxyl groups. The structure has more phenolic hydroxyl groups substituted, so its structure is not stable Studies have shown that phenolic acid compounds in SGB include chromones, phenol glycosides, acetophenones, phenethyl alcohols, and caffeic acid esters. Among them, eurryphin, kelampayoside A, osmanthuside F, and syringic acid are the primary phenolic acids in SGB (Figure 3). These compounds have solid pharmacological effects as antioxidants, antibacterial, anti-inflammatory, hypolipidemic, etc.9,10
Figure 3.
The structures of phenolic and phenolic acids isolated from SGB.
Stilbene
Stilbene compounds refer to the general term for a class of substances with a stilbene core or its polymers. This type of substance has a low content in the normal tissues of the plant kingdom. Mono- and phenolic hydroxytoluene compounds are mainly found in plant tissues in the parenchyma cells of the xylem. The stilbene compounds in SGB are mostly resveratrol, and resveratrol-3-O-β-D-pyranoglucoside, which have anti-inflammatory,63–67 antioxidant,68–70 anti-cancer,71 and other activities (Figure 4).72–76
Figure 4.
The structures of stilbene and organic acids isolated from SGB.
Organic Acids
Organic acids refer to organic compounds with acidity. Organic acids include natural organic acids and synthetic organic acids. Natural organic acids are mainly organic acids with a specific physiological activity extracted from plants in nature. In contrast, synthetic organic acids are organic acids obtained through chemical synthesis, enzymatic catalysis, and microbial fermentation. SGB contains natural organic acids, including succinic acid, palmitic acid, ferulic acid, shikimic acid, oleic acid, linoleic acid, 2-methyl succinic acid, oxalic acid, etc, which have to protect against cardiovascular disease,77–84 anti-cancer,85–93 antibacterial, anti-inflammatory, hypoglycemic, antioxidant (Figure 4).12,13
Phenylpropanoids
Phenylpropanol is a naturally occurring compound composed of a benzene ring connected with three straight-chain carbons (C6-C3 groups). It generally has a phenol structure and is a phenolic substance. Studies have shown that the phenylpropanoids with a core structure in SGB include Smilax in A, B, C, D, E (Smiglaside A-E), Helonioside A, and so on. Mainly include anti-tumor, anti-oxidation, anti-inflammatory, and other biological activities (Figure 5).13,14
Figure 5.
The structures of phenylpropanoids and lignans isolated from SGB.
Lignans
Lignans are a class of natural compounds polymerized by two molecules of phenylpropanoid derivatives. The lignins in SGB mainly include (-)-secoisolariciresinol, 4-ketopinoresinol, smiglabranol, (+)-lyoniresinol, kompasinolA, Amphenol, including anti-tumor, anti-HIV, anti-oxidation, and liver protection effects, etc (Figure 5).94–96
Steroids and Steroid Glycosides
Sterols are a large group of compounds with cyclopentane perhydrophenanthrene (steroid nucleus) as the basic skeleton. They are widely present in biological tissues and are synthesized by acetyl-CoA and play an essential role in the life process of organisms. In nature, sterols exist in different forms. The sterols isolated from SGB mainly include β-Sitosterol, stigmasterol, stigmasterol-3-O-β-D-gulofuranoside, sitosterol-3-O-β-D-gulofuranoside, etc. The sterols in SGB have pharmacological activities such as cholesterol-lowering, anti-tumor, anti-inflammatory, and immune-regulating effects (Figure 6).97–99
Figure 6.
The structures of steroids and steroid glycosides isolated from SGB.
Volatile Oils
GC-MS is usually used to analyze the volatile oil components in SGB. The chemical components of volatile oil compounds are complex, and terpenoids, small molecular aliphatic, and aromatic compounds are more common. The volatile oil has a wide range of pharmacological activities and has good biological activities in the cardiovascular system, gastrointestinal system, respiratory system and antibacterial, anti-inflammatory, anti-cancer, anti-viral, and promoting drug absorption. The volatile oil compounds isolated are mainly palmitic acid (17.87%), terpinene-4-ol (7.533%), linoleic acid (6.775%), nonane (4.509%), 8, 11-octadecadienoic acid, methyl ester (2.215%), α-cedrol (1.810%), methyl palmitate (1.293%), and L-aromatic alcohols, I-terpineol, etc in SGB.15,100
Others
The experiment found that the content of alkaloids in the rhizomes of SGB is 0.0349%, the content of tannins is 20.77 mg/g, the range of inorganic elements such as Ca, Mg, Fe, Mn, Cd is relatively high, and the content of components such as K, Cu, Zn is relatively high. Low. The study first discovered 14 alkaloids in the supernatant of water-soluble extracts of SGB in 2015 and verified their anti-cancer properties but failed to isolate and identify these 14 compounds.101 The protein components of SGB were heterodimers, non-mannose binding lectins, etc. It shows the effects of SGB extracts and main active compounds (Table 2).
Table 2.
Characteristics of Chemical Constituents Isolated from SGB
| Classes | Chemical Constituents | Biological Activities | References |
|---|---|---|---|
| Flavonoids and flavonoid glycosides | Astilbin, Taxifolin, Neoastilbin, Isoastilbin, Engeletin, Isoengeletin, Taxifolin, Astilbin, Neoastilbin, Isoastilbin, Engeletin, Isoengeletin, Naringenin, Epicatechin | Anti-infective, antibacterial, and antiviral | [11,14,35–62] |
| Phenolic and phenolic acids | Syringic acid, Eurryphin, Kelampayoside A, Osmanthuside F | Anti-inflammatory, antioxidant, and antibacterial | [9,10] |
| Phenylpropanoids and lignans | Smiglaside A, Smiglaside B, Smiglaside C, Smiglaside D, Smiglaside E, Helonioside A | Anti-tumor, antioxidant, and anti-inflammatory | [13,14,94–96] |
| Stilbene and organic acids | Resveratrol, Resveratrol-3-O-β-D-pyranoglucoside, Succinic acid, Palmitic acid, Ferulic Acid, Shikimic acid, Oleic acid, Linoleic acid, 2-Methylsuccinic acid, Oxalic acid | Anti-oxidant, anti-inflammatory, anti-cancer, antibacterial, anti-inflammatory, and hypoglycemic, antioxidant | [63–93] |
| Steroids and steroid glycosides | Stigmasterol, Beta-Sitosterol, Stigmasterol-3-O-β-D-gulofuranoside, Sitosterol-3-O-β-D-gulofuranoside | Lower cholesterol, anti-tumor, anti-inflammatory | [97–99] |
| Volatile oils | N-nonane, 8,11-Methyl octadecadienoate, α-Cedarwood oil, L-Aromatic alcohols, I-Terpineol | Antibacterial, anti-inflammatory, anti-cancer, anti-viral | [15,100] |
| Others | Heterodimeric, Non-mannose-binding agglutinin, Mannose-binding lectin | Support body metabolism, immunity of antibodies, and catalysis of enzymes | [101] |
Pharmacological Properties
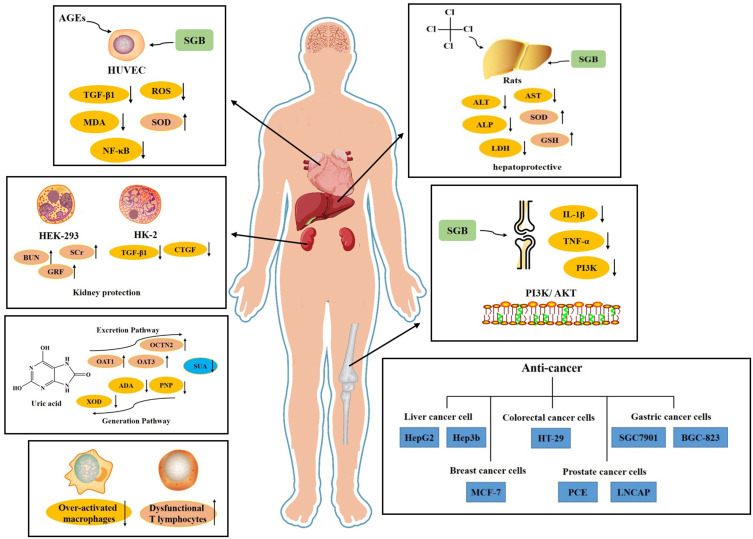
Over the past decades, SGB has been increasingly investigated due to its broad pharmacological activities. SGB extracts (alcoholic extracts, water extracts) and their compounds possess various biological and pharmacological activities, such as anti-infective, anti-cancer, anti-inflammatory, antioxidant, cardiovascular protection, hepatoprotective activity, lower uric acid, and other effects (Figure 7).
Figure 7.
The major pharmacological effects of SGB.
Anti-Infective
There are numerous investigations on the anti-infective activities of the SGB. Studies have explored the inhibitory effects of SGB on HIV-1 protease (HIV-PR) and HIV-1 integrase (HIV-1 IN). The results showed that both the ethanol extract (2μL) and water extract (2μL) of SGB showed anti-HIV-1 IN activity, with IC50 values of 6.7μg/mL and 8.5μg/mL, respectively. In general, ethanol extracts and water extracts of SGB have significant anti-HIV activity.13
It found that the minimum inhibitory concentration (MIC) obtained from the antibacterial evaluation of the SGB extract was effective against Staphylococcus aureus ATCC6538 (50μg/mL) in the ethanol extract ethyl acetate component and n-butanol component. The ethyl acetate component shows considerable activity and has significant antibacterial activity against Candida albicans SC5314 and Staphylococcus aureus ATCC6538, and the MIC value is 200μg/mL. It is the first study on the antifungal activity of SGB.3 To further study the antibacterial activity of SGB, this study used two kinds of gram-positive bacteria, including Staphylococcus aureus and Bacillus subtilis, and it evaluated two types of gram-negative bacteria, including Escherichia coli and Salmonella typhi, and the antibacterial activity of different extracts of SGB rhizome. The research results show that the rhizome extract of SGB is effective against gram-positive bacteria.18
Anti-Viral Activities
Growing research suggests that SGB has antiviral activity. A new mannose-binding lectin was isolated from SGB, called SGM2. Studies have shown that SGM2 has an excellent antiviral effect on herpesvirus type 1 (HSV-1) and respiratory syncytial virus (RSV), and the antiviral activity has the same EC50. HSV-1 was used to increase Vero cells, RSV was used in Hep-2 cells, and the cytopathic effect (CPE) reduction assay was used to determine the antiviral activity. The IC50 of glycoprotein and RSV antiviral activity was 62.5μg/mL, and the IC50 of HSV-1 was 31.3μg/mL, indicating that SGB has a specific antiviral effect.19 Studies have shown that SGB has a better anti-HCMV (human cytomegalovirus) effect in vitro. The cytopathic and MTT methods were used to detect the maximum nontoxic concentration, minimum effective concentration and therapeutic index of SGB against HCMV in vitro and compared with ganciclovir. The cytotoxicity of SGB is much lower than that of ganciclovir, and its anti-HCMV effect is similar to that of ganciclovir. It may become a drug for the treatment of active HCMV infection.102
Anti-Cancer
Various compounds isolated from SGB have been shown to affect the growth and proliferation of cancer cells, including human liver cancer cells (HepG2, Hep3b), colorectal cancer cells (HT-29), gastric cancer cells (SGC7901, BGC-823), breast cancer cells (MCF-7) and gastric mucosal epithelial cells (GES 1).16 Similarly, the study also provides research on the anticancer effects of SGB. SGB can inhibit tumor growth in HT-29 and mouse liver cancer H22 cells. Research results show that cells treated with SGB can inhibit the proliferation of cancer cells, which is related to the apoptosis pathway.17
The extracts isolated from SGB showed anti-proliferative effects on human liver cancer HepG2 and Hep3B cells. The results showed that the extract could cause cell cycle arrest and inhibit the growth of HepG2 and Hep3B cells. SGB-mediated apoptosis pathway is mainly involved in activating P38, JNK, and ERK mitogen-activated protein kinase signal transduction, indicating that SGB has specific anticancer activity in HepG2 and Hep3B cells.98
This study explored the inhibitory effect of SGB water extract on prostate cancer. Research results show that 5-O-caffeoylquinic is one of the active ingredients of SGB to play an anti-prostate cancer effect. In addition, it further explored the molecular mechanism of SGB’s anticancer to provide a reference for the research of SGB in the treatment of prostate cancer.18 It has been reported that two glycoproteins isolated from SG rhizomes also have anticancer effects in human breast cancer cells (MCF-7). They affect the apoptosis of MCF-7 cells, mainly through the in vitro cell cycle, which is the g1 stage.103
Anti-Inflammatory and Cellular Immunosuppressive
Studies have shown that SGB extract has anti-inflammatory and immunomodulatory effects. The study found that rat adjuvant arthritis (AA) induced by Freund’s complete adjuvant (FCA) showed the anti-inflammatory and immunomodulatory effects of SGB. SGB extract showed significant inhibitory activity on primary and secondary paw swelling. By selectively inhibiting the cellular immune response to inflammation and inhibiting the direct anti-inflammatory mechanism of PGE2, SGB extract can be used as a therapeutic agent for immune-inflammatory diseases.19 This research further studied the mechanism of SGB on the inflammatory condition of AA. The results show that SGB can exert a therapeutic effect on AA by regulating over-activated macrophages and dysfunctional T lymphocytes.20
It used molecular biology techniques to analyze and evaluate the effects of SGB polysaccharides on mice. The immunotoxicity and mechanism of Raw264.7 macrophage immunity. The results indicate that SGB polysaccharides can effectively inhibit cell viability by inducing cell apoptosis. SGB polysaccharides can also affect the mRNA expression of apoptotic factors, including Bax and Caspase-8. Studies have shown that SGB polysaccharide has good immunomodulatory activity on Raw264.7 cells.21
Antioxidant Effect
Studies have found that phenol-containing extracts in SGB exhibit excellent antioxidant properties. The phenolic extract of SGB shows strong free radical scavenging ability and a dose-dependent manner. At the same time, it also has a significant reduction ability. In this study, the phenolic extract of SGB showed good antioxidant capacity, and the scavenging degree of ABTS was significantly higher than that of ascorbic acid (P<0.01).22
Not only are phenolic compounds but the flavonoids of SGB also show good peroxide properties. The study extracted six flavonoids from SGB and evaluated their antioxidant activity. The results show that the flavonoids of SGB have vigorous antioxidant activity, which is manifested in DPPH and ABTS+ free radical scavenging ability.23
Lower Uric Acid
Studies have shown that the flavonoids in SGB have a good effect on lowering uric acid. The research analyzed the total flavonoids in SGB and studied the effects on serum uric acid indicators and the uric acid production and excretion pathways. It also examined the effects of SGB on renal transporters (OAT1 and OCTN2) and their mRNA. The results showed that SGB could significantly reduce the serum uric acid content in mice (P<0.01) and reduce the activity of XOD (P<0.05), and it indicates that SGB has a uric acid-lowering effect.104
Detoxification Effect
SGB has been proven to have good detoxification effects, mainly including toxicity to heavy metals such as Pb, As, Cd and Hg. The results of acute toxicity test showed that compared with model group, Pb contents in blood, liver and kidney of mice in the total extract from SGB group were significantly lower than that of the model group (P < 0.05). The slow toxicity test showed that compared with the model group, Pb contents in blood, liver and kidney of mice in total flavonoids of SGB group were significantly reduced in a dose-dependent manner (P < 0.05). And liver and kidney damages were ameliorated.105,106 In the screening experiments to reduce the bioavailability of As and Hg, SGB can significantly reduce the bioavailability of total as by 21.1–43.1%, mainly in the gastric digestion stage. SGB can also reduce the bioavailability of Hg (9.3–80.2%), and it mainly occurs in the intestinal digestion stage. It shows that SGB can reduce the impact of heavy metal pollutants on human health.107
Protection of Cardiovascular System
To study the endothelial dysfunction caused by human umbilical vein endothelial cells (HUVECS) exposed to 200μg/mL advanced glycation end products (AGEs). SGB extract significantly reduced the endothelial cell apoptosis induced by AGEs and the down-regulated TGF-β1 protein expression in HUVEC. In addition, SGB extract can reduce ROS, MDA content, and TGF-β1 expression and can significantly increase SOD activity. SGB extract can exert a protective effect on HUVECS by affecting the NF-κB signaling pathway.24
SGB flavonoids have been shown to have anti-cardiac hypertrophy effects. 2μmol/L Ang II was used to induce a rat model of primary cardiomyocyte hypertrophy. Still, after SGB treatment, it restored the protein and mRNA expressions of JP2 and RyR2 in cardiomyocytes, indicating that SGB affects the anti-hypertrophy of cardiomyocytes.16
This study investigated the inhibitory effect of SGB on the intracellular Ca2+ release of rat cardiomyocytes (H9C2). The results show that SGB can significantly inhibit intracellular Ca2+ release induced by phenylephrine or angiotensin II. It can also inhibit the Ca2+ release caused by the ryanodine receptor (RyR) agonist caffeine and the Ca2+ release induced by phenylephrine (PE). The results show that SGB exerts a protective effect by targeting inhibition of intracellular Ca2+ release.25
Hepatoprotective Activity
The protective effect of flavonoids in SGB on the liver has been studied. Carbon tetrachloride (CCL4) is used to induce liver toxicity in rats. The results showed that compared with CCL4, SGB significantly reduced ALT, AST, ALP, and lactate dehydrogenase activities. In addition, flavonoids significantly increase the activities of SOD, glutathione peroxidase, glutathione reductase, glutathione-S-transferase, and glutathione. The results show that SGB has a protective effect on liver injury in rats.108
Protection of Kidney System
Various studies have been conducted to evaluate the potential of SGB to protect the renal system. The effect of total flavonoids from SGB on renal interstitial fibrosis (RIF) and explored the mechanism. After adding TGF-β1 to induce the passage of HK-2 cells after EMT, they were treated with different concentrations of SGB. The results show that SGB treatment can reduce the expression of α-smooth muscle actin and inhibit EMT through the miR-21/PTEN/PI3K/AKT signaling pathway.109
The flavonoid extract in SGB can inhibit the nephrotoxicity of rat and human embryonic kidney (HEK)-293 cells. SGB can increase renal function indexes, BUN, and CRE content. Histopathological results show that SGB has a protective effect on rats with renal injury. SGB can affect caspase-3 mediated apoptosis in HEK-293 cells stimulated by Pb. It indicates that SGB has a specific protective effect on the kidneys and treats lead-induced kidney injury.110
The flavonoid astilbin was isolated from the rhizome of SGB. To observe the therapeutic effect of astilbin on renal injury, astilbin was added to HK-2 cells stimulated by high glucose. The results showed that astilbin could inhibit the expression of TGF-β1 and connective tissue growth factor (CTGF) in HK-2 cells stimulated by high glucose in vivo and in vitro. Histopathological examination showed that astilbin could protect against kidney damage. This study indicates that astilbin in the total flavonoids of SGB may be a potential target for DN treatment.111
Effects of a Joint System
It has been proven that SGB affects joints. The effect of astilbin, the main active ingredient of SGB, on osteoarthritis OA and its mechanism were studied. The rat OA model uses astilbin, PBS, OA, control group, and in vivo experiments. To evaluate the pathological status of cartilage tissue and analyze the expression levels of interleukin (IL)-1β, tumor necrosis factor (TNF)-α, IL-6, AKT, PI3K, and other related proteins. It was found that astilbin can significantly inhibit the expression of IL-1β, TNF-α, and PI3K and play a protective role in joints.112 It studied the mechanism of SGB on secondary inflammation of AA. The results show that SGB can exert a therapeutic effect on AA by regulating macrophages and T lymphocytes. SGB may be used for clinical rheumatoid arthritis to produce beneficial and long-term treatment.24
Clinical Application and Therapeutic Effect
Since 1963, SGB has been listed in the Pharmacopoeia of the People’s Republic of China. And SGB has been useful for treating syphilitic, poisoned sores, pathological leucorrhea, eczema itching, carbuncle poisoning, and other infectious diseases for a long time all over the world.
There are many clinical reports on the treatment of infectious diseases by SGB, and all of them have shown sound therapeutic effects.2 Observe the changes in serum responsive elements in patients with syphilis serum resistance treated by SGB and explore the clinical value of SGB in treating syphilis serum resistance. A total of 42 patients with serum resistance admitted to the STD clinic were randomly divided into treatment group (treated with SGB and benzathine penicillin) and control group (treated with benzathine penicillin), with 21 cases each. The negative conversion rates of serum regaining in the treatment and control groups were 61.90% and 23.81%, respectively. The comparison between the two groups was P<0.05, and the difference was statistically significant. SGB has a good effect in treating syphilis serum resistance and can effectively promote syphilis serum resistance serotonin turns negative.113
Some proprietary Chinese medicines with SGB have been included in the pharmacopeia, including Fuyankang Pian, Shenfukang Jiaonang, Miaoji Wan et al (Table 3). And Fufang Qingdai Wan and Yinxieling Gao have been widely used clinically for infectious skin diseases, etc, and have shown sound therapeutic effects.114–117
Table 3.
The Preparations, in Which SGB is the Main Composition, Used in TCM
| Preparation Name | Types | Main Compositions | Traditional and Clinical Uses | References |
|---|---|---|---|---|
| Fuyankang Pian | Tablet | Smilax glabra Roxb., Paeonia lactiflora Pall., Sparganium stoloniferum, Melia azedarach L., Curcuma longa L., Corydalis yanhusuo, Euryale ferox Salisb., Angelica sinensis (Oliv.) Diels, Sophora flavescens Aiton, Cyperus rotundus L., Phellodendron amurense Rupr., Salvia miltiorrhiza Bunge, Dioscorea oppositifolia L. | Leukorrheal diseases | Chinese Pharmacopoeia Commission (2020) |
| Shenfukang Jiaonang | Capsule | Smilax glabra Roxb., Dioscorea oppositifolia L., Imperata cylindrica (L.), Leonurus cardiaca L., Pogostemon cablin (Blanco) Benth. | Croupous nephritis edema, acute attack of chronic glomerulonephritis | Chinese Pharmacopoeia Commission (2020) |
| Miaoji Wan | Pill |
Smilax glabra Roxb., Pogostemon auricularius (L.), Angelica sinensis (Oliv.), Conioselinum anthriscoides “Chuanxiong”, Chaenomeles lagenaria (Loisel.), Eucommia ulmoides Oliv., Dipsacus asper Wall. ex DC., Achyranthes bidentata Blume, Atractylodes lancea (Thunb.) DC., Foeniculum vulgare Mill., Aucklandia costus Falc., Syzygium aromaticum (L.), Boswellia carteri Birdw., Potentilla reptans L. |
Liver and kidney disease, rheumatoid arthritis | Chinese Pharmacopoeia Commission (2020) |
| Fufang Qingdai Wan | Pill |
Smilax glabra Roxb., Isatis tinctoria L., Prunus mume (Siebold)., Taraxacum officinale F.H.Wigg., Arnebia euchroma (Royle ex Benth.) I.M.Johnst., Angelica dahurica (Hoffm.) Benth., Salvia miltiorrhiza Bunge., Dictamnus dasycarpus Turcz., Silene banksia (Meerb.) Mabb., Dryopteris crassirhizoma Nakai., Portulaca oleracea L., Dioscorea spongiosa, Crataegus monogyna Jacq., Schisandra sphenanthera. |
Universal eczema caused by blood-heat | Chinese Pharmacopoeia Commission (2020) |
| Yinxieling Gao | Ointment | Smilax glabra Roxb., Sophora flavescens Aiton., Glycyrrhiza glabra L., Dictamnus dasycarpus Turcz., Saposhnikovia divaricata (Turcz. ex Ledeb.) Schischk., Cyperus esculentus L., Phellodendron chinense C.K.Schneid., Rehmannia glutinosa (Gaertn.) DC., Lonicera confusa DC., Paeonia lactiflora Pall., Forsythia suspensa (Thunb.) Vahl., Angelica sinensis (Oliv.) Diels. | Lepra alphos and psoriasis | Chinese Pharmacopoeia Commission (2020) |
| Tongfengding Jiaonang | Capsule | Smilax glabra Roxb., Gentiana macrophylla Pall., Phellodendron chinense C.K.Schneid., Corydalis yanhusuo, Paeonia lactiflora Pall., Cyathula officinalis K.C.Kuan., Alisma plantago-aquatica L., Plantago asiatica L. | Bi-diseases caused by damp heat and blood stasis | Chinese Pharmacopoeia Commission (2020) |
| Shiduqing Jiaonang | Capsule | Smilax glabra Roxb., Rehmannia glutinosa (Gaertn.) DC., Angelica sinensis (Oliv.) Diels., Salvia miltiorrhiza Bunge., Cyperus esculentus L., Sophora flavescens Aiton., Dictamnus dasycarpus Turcz., Glycyrrhiza glabra L., Scutellaria baicalensis Georgi. | Pruritus cutanea | Chinese Pharmacopoeia Commission (2020) |
Summary and Perspective
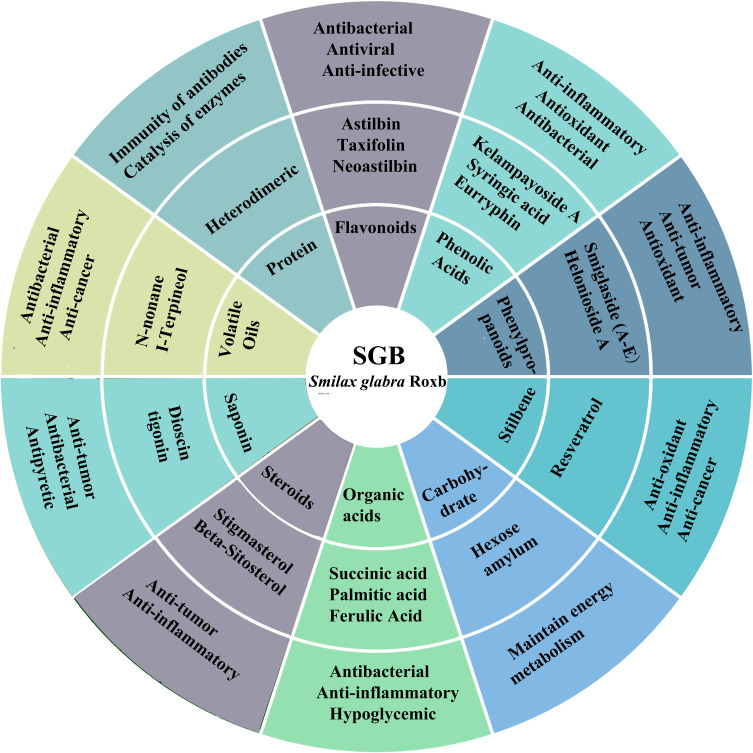
This article reviews the traditional use, chemical composition, pharmacological effects and clinical application of SGB (Figure 8). SGB has complex chemical components and extensive pharmacological effects. It has broad clinical application prospects. However, the relationship between the pharmacological basis and pharmacological activity of Smilax glabra is still unclear. The pharmacologically active ingredients of SGB can be explored, which is also of great significance for the clinical medication and drug development of SGB. In addition, domestic and foreign research on SGB is limited to rhizomes, and the exploration of other parts of SGB, such as above-ground stems, leaves, flowers and fruits, should be intensified to fully interpret the organic integrity of the plant.
Figure 8.
Representative chemical constituents isolated from SGB and their corresponding biological activities.
At the same time, for different isomers, their pharmacological activities are different, which may be determined by the positions of substituents on different C bonds, such as astilbin and neoastilbin in SGB. Further discussion on the influence of the changes of different chemical components on the pharmacological effects will help us to carry out more in-depth research on SGB.
SGB has many clinically proven pharmacological activities, including anti-infection, anti-cancer, anti-inflammatory, antioxidant, etc, but there is still a lack of further research to confirm this conclusion and apply it in clinical treatment. For example, in anti-infective activity, SGB was considered a folk medicine against infection. Future research needs to conduct in-depth research on the anti-infective mechanism of SGB and its main active ingredients and carry out relevant clinical trial design, in order to make SGB play a better anti-infective effect. Therefore, the evaluation of clinical trials of SGB anti-infectives needs to be carried out urgently. SGB has the potential for the development of anti-infective drugs. However, there are still few anti-infective studies on SGB and its main components. A large amount of long-term clinical treatment evidence shows that SGB has excellent anti-infective pharmacological activity. Therefore, SGB and its main components should be comprehensively studied, especially the potential anti-infective mechanism, to support its anti-infective use. Finally, SGB is a natural product as a potential treatment for life-threatening infections and helps develop anti-infective drugs from natural resources.
Current research shows that a large number of chemical substances have been isolated and identified from SGB, but there is a lack of further material basic research on SGB pharmacological effects. Therefore, in-depth phytochemical research on SGB and its pharmacological properties, especially the mechanism of its biologically active ingredients, will undoubtedly become the focus of further research. The broad pharmacological properties of SGBs may provide an explorable new avenue for our future drug design, development, therapeutic research, and possibly new perspectives for disease management.
Funding Statement
The study received funding from the National Natural Science Foundation of China (grant number U20A20406, 82104475,); the Beijing Municipal Natural Science Foundation (grant number 7212178).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Li Y, Li Y, Zeng P, Zhang W, Jiang W, Yang Z. Investigation on plant resources of Smilax glabra. Chin Trad Herb Drugs. 2002;33:85–87. [Google Scholar]
- 2.Zhang M, Hu ZZ, Luo HY. Analysis of clinical effects of minocycline combined with Tufuling in the treatment of serum resistance in patients with syphilis. World J Integr Tradit West Med. 2021;16:132–135. doi: 10.13935/j.cnki.sjzx.210129 [DOI] [Google Scholar]
- 3.Ji XM. Brief introduction and origin of the dehumidification and detoxification effects of Smilax glabra in the past dynasties. Guide China Med. 2012;10:217–218. [Google Scholar]
- 4.Liu GH, Zhang YM. Analysis of the application of Smilax glabra in clinical practice. Guangming Tradit Chin Med. 2012;27:1680–1681. [Google Scholar]
- 5.Yi Y, Cao Z, Yang W, Hong W, Cao Y, Leng Z. Chemical studies of Smilax glabra (III): isolation and identification of smiglanin from Smilax glabra. Acta Pharm Sinica. 1995;30:718–720. [Google Scholar]
- 6.Chen G, Shen L, Jiang P. Chemical studies of Smilax glabra. J Beijing Univ TCM. 1996b;5:44. [Google Scholar]
- 7.Xu S, Shang M, Liu G, et al. Chemical constituents from the rhizomes of Smilax glabra and their antimicrobial activity. Molecules. 2013;18:5265–5287. doi: 10.3390/molecules18055265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shu JC, Li LY, Zhou M, Yu JL, Peng CY. Three new flavonoid glycosides from Smilax glabra and their anti-inflammatory activity. Nat Prod Res. 2017. doi: 10.1080/14786419.2017.1402314 [DOI] [PubMed] [Google Scholar]
- 9.Hu M. Studies on The Chemical Constituents and Anti-Inflammatory Activity of Smilax glabra Roxb [Master’s thesis]. Guangzhou University of Chinese Medicine, China; 2014. [Google Scholar]
- 10.Yuan J, Dou D, Chen Y, et al. Phenolic glycosides from rhizome of Smilax glabra. Chin Tradit Herbal Drugs. 2004a;35:12–14. [Google Scholar]
- 11.Zhang M, Li H, Yi Y, Yi Y, Li Y, Yu H. Studies on the chemical constituents of Smilax glabra. J Chin Mater Med. 1995;18:194–196. [Google Scholar]
- 12.Chen T, Li J, Cao J, Xu Q, Komatsu K, Namba T. A new flavanone isolated from rhizoma smilacis glabrae and the structural requirements of its derivatives for preventing immuno-logical hepatocyte damage. Planta Med. 1999;65:56–59. doi: 10.1055/s-1999-13963 [DOI] [PubMed] [Google Scholar]
- 13.Qin R. Researched on Inhibitory Effects of Smilax Glabra on Tyrosinase [Master’s thesis]. Jilin Agricultural University, China; 2007. [Google Scholar]
- 14.Cao Z, Yi Y, Hong W, Lin Y. Studies on the chemical constituents of Smilax glabra Rox. (I) Chin Tradit Herbal Drugs. 1993;24:234–235. [Google Scholar]
- 15.Huo X, Gao Y, Liu J, Liu W, Zhao D. Determination of chemical constituents of the essential oil from Smilax glabra Roxb. Biotechnol. 2006;16:60–62. [Google Scholar]
- 16.Zhao J-W, Zheng C-Y, Wei H, Wang D-W, Zhu W. Proapoptic and immunotoxic effects of sulfur-fumigated polysaccharides from Smilax glabra Roxb. in raw264.7 cells. Chem Biol Interact. 2018;292:84–93. doi: 10.1016/j.cbi.2018.07.009 [DOI] [PubMed] [Google Scholar]
- 17.Tewtrakul S, Itharat A, Rattanasuwan P. Anti-HIV-1 protease- and HIV-1 integrase activities of Thai medicinal plants known as Hua-Khao-Yen. J Ethnopharmacol. 2006;105:312–315. doi: 10.1016/j.jep.2005.11.021 [DOI] [PubMed] [Google Scholar]
- 18.Willy S, Jadhav RN, Pimpliskar M, Vaidya V. Study of bactericidal potency of Smilax glabra rhizome. Int J Pharmacogn Phytochem Res. 2015;1:117–118. [Google Scholar]
- 19.Ooi LSM, Sun SSM, Wang H, Ooi VEC. New mannose-binding lectin isolated from the rhizome of Sarsaparilla Smilax glabra Roxb. (Liliaceae). J Agric Food Chem. 2004;52:6091–6095. doi: 10.1021/jf030837o [DOI] [PubMed] [Google Scholar]
- 20.She T, Zhao C, Feng J, et al. Sarsaparilla (Smilax glabra rhizome) extract inhibits migration and invasion of cancer cells by suppressing TGF-β1 pathway. PLoS One. 2015;10:e118287. doi: 10.1371/journal.pone.0118287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao Y, Su Y, Qu L, et al. Mitochondrial apoptosis contributes to the anti-cancer effect of Smilax glabra Roxb. Toxicol Lett. 2011;207:112–120. doi: 10.1016/j.toxlet.2011.08.024 [DOI] [PubMed] [Google Scholar]
- 22.Kwon OY, Ryu S, Choi JK. Smilax glabra Roxb. inhibits collagen induced adhesion and migration of PC3 and LNCaP prostate cancer cells through the inhibition of beta 1 integrin expression. Molecules. 2020;25:3006. doi: 10.3390/molecules25133006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang J, Wu F, Lu J, Lu Z, Xu Q. Anti-inflammatory activity of the aqueous extract from rhizoma smilacis glabrae. Pharmacol Res. 1997;36:309–314. doi: 10.1006/phrs.1997.0234 [DOI] [PubMed] [Google Scholar]
- 24.Jiang J, Xu Q. Immunomodulatory activity of the aqueous extract from rhizome of Smilax glabra in the later phase of adjuvant-induced arthritis in rats. J Ethnopharmacol. 2003;85:53–59. doi: 10.1016/S0378-8741(02)00340-9 [DOI] [PubMed] [Google Scholar]
- 25.Lu C, Zhu W, Wang M, Xu X, Lu C. Antioxidant and anti-inflammatory activities of phenolic-enriched extracts of Smilax glabra. Evid Based Compl Alt Med. 2014;1–8. doi: 10.1155/2014/910438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao X, Chen R, Shi Y, Zhang X, Xia D. Antioxidant and anti-inflammatory activities of six flavonoids from Smilax glabra Roxb. Molecules. 2020;25:5295. doi: 10.3390/molecules25225295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sang H, Gu J, Yuan J, Zhang M, Jia X, Feng L. The protective effect of Smilax glabra extract on advanced glycation end products-induced endothelial dysfunction in HUVECs via RAGE-ERK1/2-NF-κB pathway. J Ethnopharmacol. 2014;155:785–795. doi: 10.1016/j.jep.2014.06.028 [DOI] [PubMed] [Google Scholar]
- 28.Cai Y, Tu J, Pan S, et al. Medicinal effect and its JP2/RyR2-based mechanism of Smilax glabra flavonoids on angiotensin II-induced hypertrophy model of cardiomyocytes. J Ethnopharmacol. 2015;169:435–440. doi: 10.1016/j.jep.2015.04.026 [DOI] [PubMed] [Google Scholar]
- 29.Shou Q, Pan S, Tu J, et al. Modulation effect of Smilax glabra flavonoids on ryanodine receptor mediated intracellular Ca2+ release in cardiomyoblast cells. J Ethnopharmacol. 2013;150:389–392. doi: 10.1016/j.jep.2013.08.009 [DOI] [PubMed] [Google Scholar]
- 30.Mao QW, Zhou GY. Several food additives optimized the quality and flavor of the Smilax glabra Roxb tea. Food Fermentation Sci Technol. 2020;56(06):39–47. [Google Scholar]
- 31.Li Q, Lan T, He S, et al. A network pharmacology-based approach to explore the active ingredients and molecular mechanism of Lei-gong-gen formula granule on a spontaneously hypertensive rat model. Chin Med. 2021;16(1). doi: 10.1186/s13020-021-00507-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu YF. The study about resource diversity of wild Liliaceae medicinal plants in nature reserve-jiufu mountain. Forest By-Product Speciality China. 2017;05:72–78. [Google Scholar]
- 33.Lan T, Li Q, Chang M, et al. Lei-gong-gen formula granule attenuates hyperlipidemia in rats via cGMP-PKG signaling pathway. J Ethnopharmacol. 2020;260(1):112989. doi: 10.1016/j.jep.2020.112989 [DOI] [PubMed] [Google Scholar]
- 34.Itharat A, Houghton PJ, Eno-Amooquaye E, Burke PJ, Sampson JH, Raman A. In vitro cytotoxic activity of Thai medicinal plants used traditionally to treat cancer. J Ethnopharmacol. 2004;90:33–38. doi: 10.1016/j.jep.2003.09.014 [DOI] [PubMed] [Google Scholar]
- 35.Christopher R, Nyandoro SS, Chacha M, De Koning CB. A new cinnamoyl glycoflavonoid, antimycobacterial and antioxidant constituents from Heritiera littoralis leaf extracts. Nat Prod Res. 2014;28:351–358. doi: 10.1080/14786419.2013.863202 [DOI] [PubMed] [Google Scholar]
- 36.Li YP, Li YH, Zhong JD, Li RT. Antioxidant phenolic glycoside and flavonoids from Pieris japonica. J Asian Nat Prod Res. 2013;15:875–879. doi: 10.1080/10286020.2013.803475 [DOI] [PubMed] [Google Scholar]
- 37.Liu J, Li X, Lin J, et al. Sarcandra glabra (Caoshanhu) protects mesenchymal stem cells from oxidative stress: a bioevaluation and mechanistic chemistry. BMC Compl Altern Med. 2016;16:423. doi: 10.1186/s12906-016-1383-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu CL, Zhu W, Wang M, Xu XJ, Lu CJ. Antioxidant and anti-inflammatory activities of phenolic-enriched extracts of Smilax glabra. Evid Based Complement Alternat Med. 2014;2014:910438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petacci F, Freitas SS, Brunetti IL, Khalil NM. Inhibition of peroxidase activity and scavenging of reactive oxygen species by astilbin isolated from Dimorphandra mollis (Fabaceae, Caesalpinioideae). Biol Res. 2010;43:63–74. doi: 10.4067/S0716-97602010000100008 [DOI] [PubMed] [Google Scholar]
- 40.Tung YT, Lin LC, Liu YL, et al. Antioxidative phytochemicals from Rhododendron oldhamii Maxim. Leaf extracts reduce serum uric acid levels in potassium oxonate-induced hyperuricemic mice. BMC Complement Alternat Med. 2015;15:423. doi: 10.1186/s12906-015-0950-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Q-F, Zhang Z-R, Cheung H-Y. Antioxidant activity of Rhizoma Smilacis Glabrae extracts and its key constituent-astilbin. Food Chem. 2009;115:297–303. doi: 10.1016/j.foodchem.2008.11.053 [DOI] [Google Scholar]
- 42.Haraguchi H, Mochida Y, Sakai S, et al. Protection against oxidative damage by dihydroflavonols in Engelhardtia chrysolepis. Biosci Biotechnol Biochem. 1996;60:945–948. doi: 10.1271/bbb.60.945 [DOI] [PubMed] [Google Scholar]
- 43.Diao H, Kang Z, Han F, Jiang W. Astilbin protects diabetic rat heart against ischemia-reperfusion injury via blockade of HMGB1-dependent NF-kappaB signaling pathway. Food Chem Toxicol. 2014;63:104–110. doi: 10.1016/j.fct.2013.10.045 [DOI] [PubMed] [Google Scholar]
- 44.Han LK, Ninomiya H, Taniguchi M, Baba K, Kimura Y, Okuda H. Norepinephrine-augmenting lipolytic effectors from Astilbe thunbergii rhizomes. J Nat Prod. 1998;61:1006–1011. doi: 10.1021/np980107o [DOI] [PubMed] [Google Scholar]
- 45.Dimech GS, Soares LA, Ferreira MA, de Oliveira AG, Carvalho Mda C, Ximenes EA. Phytochemical and antibacterial investigations of the extracts and fractions from the stem bark of Hymenaea stigonocarpa Mart. ex Hayne and effect on ultrastructure of Staphylococcus aureus induced by hydroalcoholic extract. ScientificWorld J. 2013;2013:862763. doi: 10.1155/2013/862763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moulari B, Pellequer Y, Chaumont JP, Guillaume YC, Millet J. In vitro antimicrobial activity of the leaf extract of Harungana madagascariensis Lam. Ex Poir. (Hypericaceae) against strains causing otitis externa in dogs and cats. Acta Vet Hung. 2007;55:97–105. doi: 10.1556/avet.55.2007.1.10 [DOI] [PubMed] [Google Scholar]
- 47.Moulari B, Pellequer Y, Lboutounne H, et al. Isolation and in vitro antibacterial activity of astilbin, the bioactive flavanone from the leaves of Harungana madagascariensis Lam. ex Poir. (Hypericaceae). J Ethnopharmacol. 2006;106:272–278. doi: 10.1016/j.jep.2006.01.008 [DOI] [PubMed] [Google Scholar]
- 48.Wang J, Shi Y, Jing S, Dong H, Wang D, Wang T. Astilbin inhibits the activity of Sortase A from Streptococcus mutans. Molecules. 2019;24:465. doi: 10.3390/molecules24030465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang X, Sun Y, Xu Q, Guo Z. Synthesis and immunosuppressive activity of Lrhamnopyranosyl flavonoids. Org Biomol Chem. 2006;4:2483–2491. doi: 10.1039/b604521a [DOI] [PubMed] [Google Scholar]
- 50.Zheng ZG, Duan TT, He B, et al. Macrophage biospecific extraction and HPLC-ESI-MSn analysis for screening immunological active components in Smilacis Glabrae Rhizoma. JPharmaceut Biomed Anal. 2013;77:44–48. doi: 10.1016/j.jpba.2013.01.003 [DOI] [PubMed] [Google Scholar]
- 51.Zou S, Shen X, Tang Y, Fu Z, Zheng Q, Wang Q. Astilbin suppresses acute heart allograft rejection by inhibiting maturation and function of dendritic cells in mice. Transplant Proc. 2010;42:3798–3802. doi: 10.1016/j.transproceed.2010.06.031 [DOI] [PubMed] [Google Scholar]
- 52.Guo J, Qian F, Li J, Xu Q, Chen T. Identification of a new metabolite of astilbin, 3’-O-methylastilbin, and its immunosuppressive activity against contact dermatitis. Clin Chem. 2007;53:465–471. doi: 10.1373/clinchem.2006.077297 [DOI] [PubMed] [Google Scholar]
- 53.Tang J, Guo J, Fan J, et al. Metabolite profiling of astilbin in rat sera using UPLC/MS(E) and impact of its metabolites on immunosuppressive activity. J Chromatogr B, Analyt Technol Biomed Life Sci. 2013;929:56–62. doi: 10.1016/j.jchromb.2013.04.018 [DOI] [PubMed] [Google Scholar]
- 54.Guo L, Liu W, Lu T, et al. Decrease of functional activated T and B cells and treatment of glomerulonephritis in lupus-prone mice using a natural flavonoid astilbin. PLoS One. 2015;10:e0124002. doi: 10.1371/journal.pone.0124002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen F, Zhu X, Sun Z, Ma Y. Astilbin inhibits high glucose-induced inflammation and extracellular matrix accumulation by suppressing the TLR4/MyD88/NF-κB pathway in rat glomerular mesangial cells. Front Pharmacol. 2018;9:1187. doi: 10.3389/fphar.2018.01187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang H, Cheng Z, Shi H, Xin W, Wang TT, Yu LL. Isolation and characterization of two flavonoids, engeletin and astilbin, from the leaves of Engelhardia roxburghiana and their potential anti-inflammatory properties. J Agric Food Chem. 2011;59:4562–4569. doi: 10.1021/jf2002969 [DOI] [PubMed] [Google Scholar]
- 57.Lu CL, Zhu YF, Hu MM, et al. Optimization of astilbin extraction from the rhizome of Smilax glabra, and evaluation of its antiinflammatory effect and probable underlying mechanism in lipopolysaccharide induced RAW264.7 macrophages. Molecules. 2015;20:625–644. doi: 10.3390/molecules20010625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ruangnoo S, Jaiaree N, Makchuchit S, Panthong S, Thongdeeying P, Itharat A. An in vitro inhibitory effect on RAW 264.7 cells by anti-inflammatory compounds from Smilax corbularia Kunth. Asian Pac J Allergy Immunol. 2012;30:268–274. [PubMed] [Google Scholar]
- 59.Zou W, Zhou H, Hu J, et al. Rhizoma Smilacis Glabrae inhibits pathogen-induced upper genital tract inflammation in rats through suppression of NF-kappaB pathway. J Ethnopharmacol. 2017;202:103–113. doi: 10.1016/j.jep.2017.02.034 [DOI] [PubMed] [Google Scholar]
- 60.Yuan J, Dou D, Chen Y, et al. Studies on dihydroflavonol glycosides from rhizome of Smilax glabra. China J Chin Mater Med. 2004b;29:867–870. [PubMed] [Google Scholar]
- 61.Wu B, Ma Y, Yuan J, Sun Q. Isolation and identification of chemical constituents from Smilax glabra Roxb. J Shenyang Pharm Univ. 2010;2:116–119. [Google Scholar]
- 62.Yi Y, Cao Z, Yang D, Cao Y, Wu Y, Zhao S. Studies on the chemical constituents of Smilax glabra (IV). Acta Pharm Sinica. 1998a;33:74–76. doi: 10.16438/j.0513-4870.1998.11.016 [DOI] [PubMed] [Google Scholar]
- 63.Gao X, Xu YX, Janakiraman N, Chapman RA, Gautam SC. Immunomodulatory activity of resveratrol: suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production. Biochem Pharmacol. 2001;62:1299–1308. doi: 10.1016/S0006-2952(01)00775-4 [DOI] [PubMed] [Google Scholar]
- 64.Fuggetta MP, Bordignon V, Cottarelli A, et al. Downregulation of proinflammatory cytokines in HTLV-1-infected T cells by Resveratrol. J Exp Clin Cancer Res. 2016;35:118. doi: 10.1186/s13046-016-0398-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang C, Zhao XH, Yang L, et al. Resveratrol alleviates heat stress-induced impairment of intestinal morphology, microflora, and barrier integrity in broilers. Poult Sci. 2017;96:4325–4332. doi: 10.3382/ps/pex266 [DOI] [PubMed] [Google Scholar]
- 66.Zhang H, Chen Y, Chen Y, et al. Comparison of the protective effects of resveratrol and pterostilbene against intestinal damage and redox imbalance in weanling piglets. J Anim Sci Biotechnol. 2020;11:52. doi: 10.1186/s40104-020-00460-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xian Y, Gao Y, Lv W, et al. Resveratrol prevents diabetic nephropathy by reducing chronic inflammation and improving the blood glucose memory effect in non-obese diabetic mice. Naunyn Schmiedebergs Arch Pharmacol. 2020;393:2009–2017. doi: 10.1007/s00210-019-01777-1 [DOI] [PubMed] [Google Scholar]
- 68.He S, Chen L, He Y, et al. Resveratrol alleviates heat stress-induced impairment of intestinal morphology, barrier integrity and inflammation in yellow-feather broilers. Anim Prod Sci. 2020;60:1547. [Google Scholar]
- 69.Zhang C, Chen K, Zhao X, Geng Z. Protective effects of resveratrol against high ambient temperature-induced spleen dysplasia in broilers through modulating splenic redox status and apoptosis. J Sci Food Agric. 2018;98:5409–5417. doi: 10.1002/jsfa.9084 [DOI] [PubMed] [Google Scholar]
- 70.He S, Yu Q, He Y, Hu R, Xia S, He J. Dietary resveratrol supplementation inhibits heat stress-induced high-activated innate immunity and inflammatory response in spleen of yellow-feather broilers. Poult Sci. 2019;98:6378–6387. doi: 10.3382/ps/pez471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brockmueller A, Sameri S, Liskova A, et al. Resveratrol’s anti-cancer effects through the modulation of tumor glucose metabolism. Cancers. 2021;13:188. doi: 10.3390/cancers13020188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vestergaard M, Ingmer H. Antibacterial and antifungal properties of resveratrol. Int J Antimicrob Agents. 2019;53(6):716–723. doi: 10.1016/j.ijantimicag.2019.02.015 [DOI] [PubMed] [Google Scholar]
- 73.Wang J, Zhang X, Gao L, et al. The synergistic antifungal activity of resveratrol with azoles against Candida albicans. Lett Appl Microbiol. 2021;72(6):688–697. doi: 10.1111/lam.13458 [DOI] [PubMed] [Google Scholar]
- 74.Huang HH, Liao D, Zhou GH, et al. Antiviral activities of resveratrol against rotavirus in vitro and in vivo. Phytomedicine. 2020;77:153230. doi: 10.1016/j.phymed.2020.153230 [DOI] [PubMed] [Google Scholar]
- 75.Hui Y, Tang Cyluo C, Cheng L, et al. Resveratrol attenuates the cytotoxicity induced by amyloid-B1-42 in PC12 cells by upregulating heme oxygenase-1 via the PI3K/Akt/Nrf2 pat hway. Neurochem Res. 2018;43(2):297–305. doi: 10.1007/s11064-017-2421-7 [DOI] [PubMed] [Google Scholar]
- 76.Sarroca S, Gatius A, Rodriguez-Farre E, et al. Resveratrol confers neuroprotection against high-fat diet in a mouse model of Alzheimer’s disease via modulation of proteolytic mechanisms. J Nutr Biochem. 2021;89:108569. doi: 10.1016/j.jnutbio.2020.108569 [DOI] [PubMed] [Google Scholar]
- 77.Qiao Q, Gao W, Zhang L, et al. Metabolic syndrome and cardiovascular disease. Ann Clin Biochem. 2007;44:232–263. doi: 10.1258/000456307780480963 [DOI] [PubMed] [Google Scholar]
- 78.Wilson PW, D’Agostino RB, Parise H, et al. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528 [DOI] [PubMed] [Google Scholar]
- 79.Dokken BB. The Pathophysiology of cardiovascular disease and diabetes: beyond blood pressure and lipids. Diabetes Spectr. 2008;21(3):160–165. doi: 10.2337/diaspect.21.3.160 [DOI] [Google Scholar]
- 80.Leon BM, Maddox TM. Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes. 2015;6(13):1246–1258. doi: 10.4239/wjd.v6.i13.1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mozaffarian D. Saturated fatty acids and type 2 diabetes: more evidence to re-invent dietary guidelines. Lancet Diabetes Endocrinol. 2014;2(10):770–772. doi: 10.1016/S2213-8587(14)70166-4 [DOI] [PubMed] [Google Scholar]
- 82.Ebbesson SO, Voruganti VS, Higgins PB, et al. Fatty acids linked to cardiovascular mortality are associated with risk factors. Int J Circumpolar Health. 2015;74:28055. doi: 10.3402/ijch.v74.28055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Briggs MA, Petersen KS, Kris-Etherton PM. Saturated fatty acids and cardiovascular disease: replacements for saturated fat to reduce cardiovascular risk. Healthcare. 2017;5(2):29. doi: 10.3390/healthcare5020029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Knowles CJ, Cebova M, Pinz IM, et al. Palmitate diet-induced loss of cardiac caveolin-3: a novel mechanism for lipid-induced contractile dysfunction. PLoS One. 2013;8(4):e61369. doi: 10.1371/journal.pone.0061369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kwan HY, Fu X, Liu B, et al. Subcutaneous adipocytes promote melanoma cell growth by activating the Akt signaling pathway: role of palmitic acid. J Biol Chem. 2014;289(44):30525–30537. doi: 10.1074/jbc.M114.593210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wong RH, Chang I, Hudak CSS, et al. A role of DNA-PK for the metabolic gene regulation in response to insulin. Cell. 2009;136:1056–1072. doi: 10.1016/j.cell.2008.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Y, Viscarra J, Kim S-J, et al. Transcriptional regulation of hepatic lipo-genesis. Nature Reviews Mol Cell Biol. 2015;16:678–689. doi: 10.1038/nrm4074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.DeBerardinis RJ, Mancuso A, Daikhin E, et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kwan HY, Chao X, Su T, et al. Dietary lipids and adipocytes: potential therapeutic targets in cancers. J Nutr Biochem. 2015;26:303–311. doi: 10.1016/j.jnutbio.2014.11.001 [DOI] [PubMed] [Google Scholar]
- 90.Little JL, Kridel SJ. Fatty acid synthase activity in tumor cells. Subcell Biochem. 2008;49:169–194. [DOI] [PubMed] [Google Scholar]
- 91.Rohrig F, Schulze A. The multifaceted roles of fatty acid synthesis in cancer. Nat Rev Cancer. 2016;16:732–749. doi: 10.1038/nrc.2016.89 [DOI] [PubMed] [Google Scholar]
- 92.Kwan HY, Yang Z, Fong W-F, et al. The anticancer effect of oridonin is mediated by fatty acid synthase suppression in human colorectal cancer cells. J Gastroenterol. 2013;48:182–192. doi: 10.1007/s00535-012-0612-1 [DOI] [PubMed] [Google Scholar]
- 93.Ventura R, Mordec K, Waszczuk J, et al. Inhibition of de novo palmitate synthesis by fatty acid synthase induces apoptosis in tumor cells by remodeling cell membranes, inhibiting signaling pathways, and reprogramming gene expression. EBioMedicine. 2015;2:808–824. doi: 10.1016/j.ebiom.2015.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen T, Li J, Xu Q. Phenylpropanoid glycosides from Smilax glabra. Phytochemistry. 2000;53:1051–1055. doi: 10.1016/S0031-9422(99)00522-1 [DOI] [PubMed] [Google Scholar]
- 95.Lu C, Zhu W, Wang D, et al. Inhibitory effects of chemical compounds isolated from the rhizome of Smilax glabra on nitric oxide and tumor necrosis factor-α production in lipopolysaccharide-induced RAW264.7 cell. Evid Based Compl Alt Med. 2015;1–9. doi: 10.1155/2015/602425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yuan J. Studies on the Constituents of Smilax glabra Roxb. and Tinospora sinensis (Lour) [Merr.Ph.D. thesis]. Shenyang Pharmaceutical University, China; 2005. [Google Scholar]
- 97.Li Y, Yi Y, Tang H, Xiao K. Chemical studies of Smilax glabra. Chin Tradit Herbal Drugs. 1996;12:712–714. [Google Scholar]
- 98.Sa F, Gao J, Fung K, Zheng Y, Lee SM, Wang Y. Anti-proliferative and pro-apoptotic effect of Smilax glabra Roxb. extract on hepatoma cell lines. Chem Biol Interact. 2008;171:1–14. doi: 10.1016/j.cbi.2007.08.0122 [DOI] [PubMed] [Google Scholar]
- 99.Su H, Liu Y. Determination of yam in Rhizoma Smilacis glabrae, by HPLC. J Liaoning Univ TCM. 2008;10:159–160. [Google Scholar]
- 100.Zhou Y, Lu JQ, Cui L, Meng JM, Xiao YS. Analysis of volatile components in Smilax glabra Roxb and its adulterant. China Pharmacist. 2018;21:1865–1867. [Google Scholar]
- 101.She T, Qu L, Wang L, et al. Sarsaparilla (Smilax Glabra rhizome) extract inhibits cancer cell growth by S phase arrest, apoptosis, and autophagy via redox-dependent ERK1/2 pathway. Cancer Prev Res. 2015b;8:464–474. doi: 10.1158/1940-6207.capr-14-0372 [DOI] [PubMed] [Google Scholar]
- 102.Feng Y, Chen JJ, Fang JG, et al. Experimental study on the anti-cytomegalovirus effect of Smilax glabra in vitro. Maternal Child Health Care China. 2010;25(36):5457–5459. [Google Scholar]
- 103.Ooi LS, Wong EY, Chiu LC, Sun SS, Ooi VE. Antiviral and anti-proliferative glycoproteins from the rhizome of Smilax glabra Roxb (Liliaceae). Am J Chin Med. 2008;36:185–195. doi: 10.1142/s0192415x08005692 [DOI] [PubMed] [Google Scholar]
- 104.Huang LP, Deng J, Chen GT, et al. The anti-hyperuricemic effect of four astilbin stereoisomers in Smilax glabra on hyperuricemic mice. J Ethnopharmacol. 2019;238:111777. doi: 10.1016/j.jep.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 105.Li J, Cheng S, Dong DY, et al. Primary investigation for material basis of Smilax glabra for detoxification of heavy metal Pb. Chin Trad Herbal Drugs. 2022;53(01):117–125. [Google Scholar]
- 106.Chang S. Study on the substance basis of toxicity of Pb heavy metal detoxicate by Smilax glabra Roxb. Jiangxi University of Chinese Medicine; 2021. [Google Scholar]
- 107.Liao W. Study of speciation and bioaccessibility of arsenic and mercury in food. Chin Acad Sci. 2019;45:e34. [Google Scholar]
- 108.Xia D, Fan Y, Zhang P, Fu Y, Ju M, Zhang X. Protective effects of the flavonoid-rich fraction from rhizomes of Smilax glabra Roxb. on carbon tetrachloride-induced hepatotoxicity in rats. J Membrane Biol. 2013;246:479–485. doi: 10.1007/s00232-013-9560-9 [DOI] [PubMed] [Google Scholar]
- 109.Luo Q, Cai Z, Tu J, Ling Y, Wang DJ, Cai Y. Total flavonoids from Smilax glabra Roxb blocks epithelial‐mesenchymal transition and inhibits renal interstitial fibrosis by targeting mir‐21/PTEN signaling. J Cell Biochem. 2019;120:12. doi: 10.1002/jcb.27668 [DOI] [PubMed] [Google Scholar]
- 110.Shi Y, Tian C, Yu X, et al. Protective effects of Smilax glabra Roxb. against lead-induced renal oxidative stress, inflammation and apoptosis in weaning rats and HEK-293 cells. Front Pharmacol. 2020;11:556248. doi: 10.3389/fphar.2020.556248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li GS, Jiang WL, Yue XD, et al. Effect of astilbin on experimental diabetic nephropathy in vivo and in vitro. Planta Med. 2009;75:1470–1475. doi: 10.1055/s-0029-1185802 [DOI] [PubMed] [Google Scholar]
- 112.Chen CL, Yang M, Chen YJ, et al. Astilbin-induced inhibition of the pi3k/akt signaling pathway decelerates the progression of osteoarthritis. Exp Ther Med. 2020. doi: 10.3892/etm.2020.9048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yue S. Observation on curative effect of Smilax glabra combined with penicillin in the treatment of 26 cases of syphilis serum resistance. Nei Mongol J Trad Chin Med. 2017;06:87. [Google Scholar]
- 114.Wang T, Ye L. Observation of therapeutic effect of compound Qingdai pills and BCG polysaccharide nucleic acid injection in treating psoriasis of wind-heat and blood dryness and the influence of serum inflammatory factors. World Latest Med Info. 2018;18:7–8. doi: 10.19613/j.cnki.1671-3141.2018.05.004 [DOI] [Google Scholar]
- 115.He YM, Yang Y, Liao CQ, Li CX, Xiong X. A clinical study of compound Qingdai Pill combined with Acitretin and calcipotriol in the treatment of patients with moderate to severe psoriasis vulgaris. Chin J Derm. 2016;30:769–770. doi: 10.13735/j.cjdv.1001-7089.201512007 [DOI] [Google Scholar]
- 116.Han FH, Li SH, Li GD. Clinical study of compound Qingdai pill combined with compound antaisu in treatment of psoriasis. Med Innov China. 2014;11:097–099. doi: 10.3969/j.issn.1674-4985.2014.29.035 [DOI] [Google Scholar]
- 117.Ping L, Yang F, Gao SP. Clinical observation on Xiegan Liangxue Jiedu decoction in the treatment of plaque psoriasis. Acad J Shanghai Univ Trad Chin Med. 2018;32:38–40. doi: 10.16306/j.1008-861x.2018.04.009 [DOI] [Google Scholar]