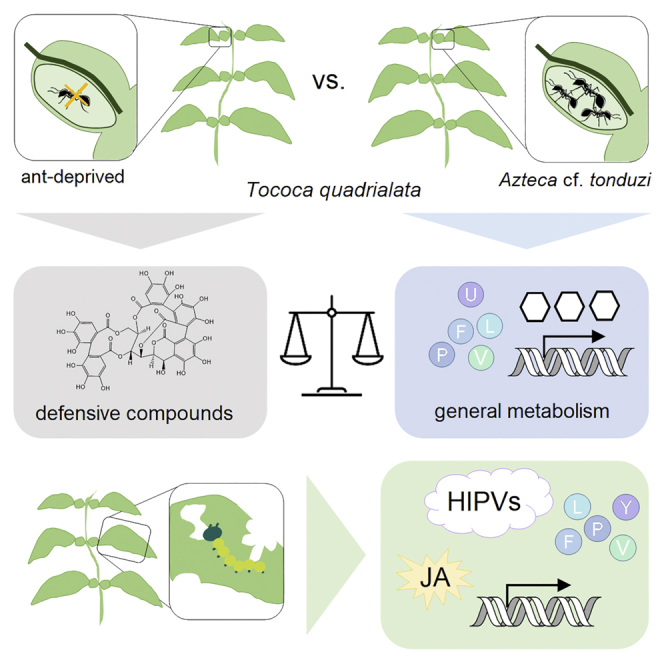
Summary
Ant-plant defensive mutualism is a widely studied phenomenon, where ants protect their host plants (myrmecophytes) against herbivores in return for the provision of nesting sites and food. However, few studies addressed the influence of ant colonization and herbivory on the plant’s metabolism. We chose the Amazonian plant Tococa quadrialata, living in association with Azteca cf. tonduzi ants for an ant-exclusion study to reveal the chemistry behind this symbiosis. We found that colonized plants did not only benefit from protection but also from increased amino acid and nitrogen content, enabling better performance even in an herbivore-free environment. In contrast, ant-deprived T. quadrialata plants accumulated more ellagitannins, a major class of constitutive defense compounds. Moreover, herbivory-induced jasmonate-mediated defense responses, including the upregulation of signaling and defense genes and the emission of volatiles irrespective of colonization status. Altogether, we show how ant-colonization can influence the general and defense-related metabolism and performance of myrmecophytes.
Subject area: Ecology, plant physiology, omics
Graphical abstract
Highlights
-
•
We examine the phytochemistry and transcriptome in myrmecophytic Tococa plants
-
•
Ant-colonization promotes Tococa growth by increasing nitrogen, amino acids, and sugar
-
•
Constitutively produced defensive ellagitannin levels increase in the absence of ants
-
•
Tococa responds to herbivory by phytohormone signaling and emission of HIPVs
Ecology; Plant physiology; Omics
Introduction
Mutualistic relationships between plants and ants have evolved in tropical regions all across the world (Chomicki and Renner, 2015). There are more than 650 plant species classified as obligate ant-plants or myrmecophytes, including species of the genera Acacia, Cecropia, Triplaris, Macaranga, and Tococa (Chomicki and Renner, 2015). All these myrmecophytic plants offer their mutualistic ants pre-formed nesting spaces (domatia), consisting of hollow structures in thorns, petioles, stems, rhizomes, tubers, or modified leaves. Furthermore, they often provide ants with food like extrafloral nectar (EFN) and/or food bodies. In exchange, the ants protect their hosts against insect and vertebrate herbivores, pathogens, and sometimes prevent the growth of competing plants (Gonzalez-Teuber et al., 2014; Heil and McKey, 2003; Morawetz et al., 1992; Renner and Ricklefs, 1998).
The importance of ants as biotic defenders was revealed by several ant-exclusion studies with various myrmecophytic plants. Ant removal generally causes a dramatic increase in herbivore damage (Rosumek et al., 2009) with occasional lethal consequences for the host plant (Heil et al., 2001a). This high susceptibility of ant-plants to herbivory is assumed to be the result of reduced plant defenses, but this has not been well studied. Direct defense against herbivores can be conferred by physical traits like thorns, prickles, or high levels of lignification as well as by the synthesis and accumulation of certain specialized compounds acting as repellents or toxins (Mithöfer and Boland, 2012). Furthermore, some plant traits may attract enemies of the herbivore and thus provide indirect defense (Clavijo McCormick et al., 2014; Mumm and Dicke, 2010; Turlings and Wäckers, 2004). All these types of defense mechanisms are thought to be costly, so plants should produce defenses only when needed and avoid temporal and spatial overlap of individual defenses (McKey, 1979; Rhoades, 1979). As symbiotic ants confer effective resistance against herbivory, ant-plants may not have been selected to invest in additional anti-herbivore defenses (Janzen, 1966). In accordance with this hypothesis, a classic study by Janzen (1966) showed a reduction of cyanogenic glycosides in myrmecophytic acacia (Seigler and Ebinger, 1987), in comparison to non-myrmecophytic acacia. Other studies found negative correlations between the presence of ants and specific defensive compounds e.g. amides in Piper (Dyer et al., 2001), chitinases in Acacia and Macaranga (Heil et al., 1999, 2000) as well as morphological defensive traits in Tococa (Moraes and Vasconcelos (2009); but see Bartimachi et al. (2015)). Overall, however, a general trade-off between ants and other defenses has not been observed in ant-plants (Del Val and Dirzo (2003); Fincher et al. (2008); Heil et al. (2002); Turner (1995); Ward and Young (2002); but see Koricheva and Romero (2012)). Hence, the factors leading to the reduced performance of myrmecophytes when ants are absent remain to be elucidated.
Direct and indirect defensive traits in plants can either be constitutively expressed or inducible upon herbivory. Many plants accumulate defensive compounds only upon tissue damage as toxic or herbivore-deterring substances may result in costs to produce or to maintain, in particular, to avoid autotoxicity (Karban et al., 1997). Previous studies on ant-plants have mainly focused on constitutive defenses, with very few studies looking for inducible direct defenses (Frederickson et al., 2013; Moraes and Vasconcelos, 2009). Jasmonates are known to be the hormones primarily responsible for the induction of plant defenses upon insect herbivory (Gatehouse, 2002). However, Heil et al. (2004) showed that unlike in facultative ant-plants, the EFN production of myrmecophytic Acacia could not be induced by jasmonic acid (JA). In a more recent study, Hernandez-Zepeda et al. (2018) also found that JA treatment was unable to induce a systemic EFN production in a myrmecophyte and could only induce few number of volatiles, which in turn were unable to propagate the defense reaction further. The lack of an inducible defensive response in myrmecophytes might be an important yet unrecognized factor contributing to their performance when ants are not present.
In recent years, mutualist ants have been studied not only for their roles as protectors but also for their function in plant nutrient acquisition. In epiphytic ant-plants, it has long been known that mutualistic ants serve as nitrogen sources rather than as defenders (Gay, 1993). Now, labeling studies with stable nitrogen isotopes (15N) have shown that in myrmecophytic trees like Leonardoxa (Defossez et al., 2011) and Cecropia (Dejean et al., 2012), the nutrient flow between ants and myrmecophytes can be bidirectional (reviewed by Mayer et al. (2014)). Especially in the nutrient-poor soils of the Neotropical rainforests, the supply of nitrogen and other nutrients by ants (“myrmecotrophy”) may be another important aspect affecting the general performance of myrmecophytes.
Tococa (Melastomataceae) is a genus of shrubs and small trees, found all over the Neotropics, from southern Mexico to Bolivia (Michelangeli, 2005). The majority of species are myrmecophytes forming hollow pouches at the base of the leaf blade or at the apex of the petiole. The ant species associated with Tococa vary within its geographic range, with species of the genera Azteca and Allomerus being the most common (Bartimachi et al., 2015; Cabrera and Jaffe, 1994; Dejean et al., 2006; Michelangeli, 2003; Moraes and Vasconcelos, 2009). The protective effect of ants on Tococa species has been demonstrated in several ant-exclusion studies. For instance, in the Canaima National Park in Venezuela, the removal of Azteca ants from Tococa coronata, Tococa macrosperma, or Tococa guianensis resulted in an increase in defoliation from 5% to up to 90% (Michelangeli, 2003). However, while the performance of Tococa spp. was the subject of many studies, little is known about the phytochemicals of these plants, which could serve as alternative defenses to herbivores when ants are absent. Leaves were reported to contain high concentrations of phenolics, mostly hydrolysable tannins (Serna and Martinez, 2015; Svoma and Morawetz, 1992) and anthocyanins (Dejean et al., 2006), but there is no additional information.
Here, we chose to study the myrmecophytic Tococa quadrialata in the Amazonian region of Peru, which associates with Azteca cf. tonduzi ants to investigate the influence of ants on the performance and chemistry of these ant-plants in the field. In order to distinguish the effects of mutualism, we monitored the performance of ant-colonized and uncolonized plants in herbivore-free and natural environments. Combined targeted and untargeted metabolomics and transcriptome analyses revealed how ant-plants are affected by the presence or absence of their symbionts. To understand the high susceptibility of these plants toward herbivore damage in the absence of ants, we investigated the constitutive and inducible defense mechanisms of T. quadrialata, their regulation, and their impact on plant performance upon herbivore stress. We also addressed the possibility that ants improve T. quadrialata performance by enhancing its nutrient supply.
Results
T. quadrialata plants colonized by Azteca cf. tonduzi ants grow much better than uncolonized plants in their natural environment
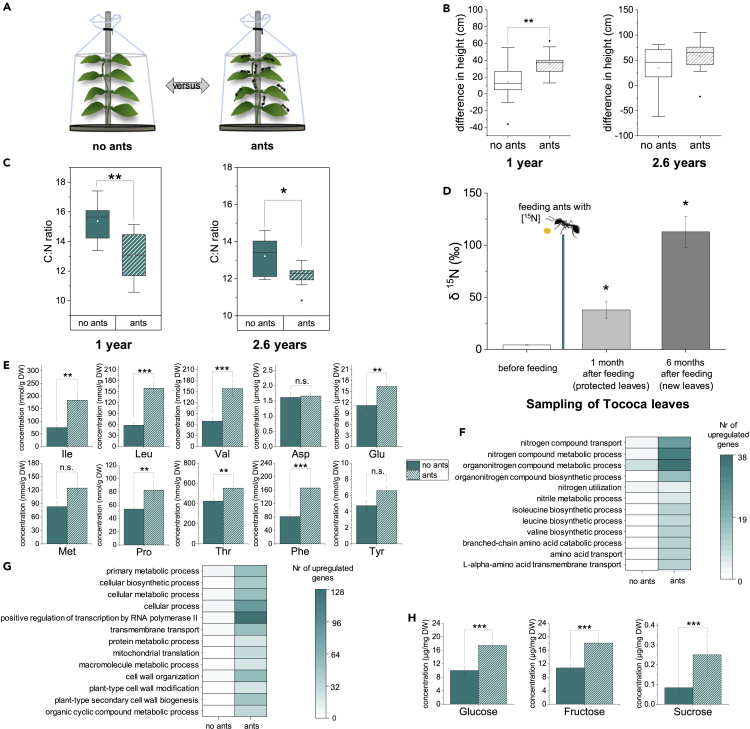
Our field study was centered on a plot of young T. quadrialata plants (0.5–1 m tall) (Figure S1Aa–c) colonized by Azteca cf. tonduzi ants. The ants were observed to constantly patrol the myrmecophytes’ leaves (Figure S1Ad), quickly discovering any invertebrate or other biological material placed on the plants (Figure S1Ae and f), and either attacking the intruding object instantly themselves or first recruiting their nestmates for a joint attack (Data S1). The importance of these aggressive symbionts as biotic defenders was confirmed for this specific interaction when the performances of colonized and uninhabited T. quadrialata plants, naturally occurring within the same plot, were monitored over the course of 2.8 years. As expected, uncolonized plants suffered much more herbivory than ant-colonized plants (p < 0.05) (Figure S1B), whereas ant-colonized plants had increased height (Figure S1C) and gained more leaves (Figure S1D) and domatia (Figure S1E) than uncolonized plants, which suffered on average net losses of leaves and domatia over the monitoring period.
Ant colonization enhances nitrogen availability and general metabolism of T. quadrialata
Ant-colonization often comes with metabolic costs for the myrmecophytes, which are only compensated by the protection against herbivores (Frederickson et al., 2012; Stanton and Palmer, 2011). To see which effects the colonization by A. cf. tonduzi itself has on the metabolism of T. quadrialata, we set up an experiment where we removed ants from half of the young, colonized plants in a plot. To exclude varying levels of herbivory as an influencing factor, all T. quadrialata, both with and without ants, were protected from herbivory by covering them with nets, and the performance and metabolism of these plants over time were measured (Figure 1A). Repeated sampling over a time span of almost 3 years allowed to identify and confirm differences between colonized and ant-deprived plants. Surprisingly, after one year of growing under the nets, ant-colonized plants were taller than ant-deprived plants (p < 0.01; Figure 1B) and had gained more domatia and leaves (p < 0.05; Figure S2). After 2.6 years, there were no longer significant differences between the treatments, likely owing to the fact that the plants reached the maximum height possible below the nets. A possible explanation for the better growth of colonized plants might be that A. cf. tonduzi provides T. quadrialata with nutrients, as reported for some, but not all, ant-plant mutualisms (see e.g. Solano and Dejean, 2004). To determine whether colonization brings nutritional benefits, we compared the carbon:nitrogen (C:N) ratio of ant-colonized and ant-deprived plants. Plants growing with ants for 1 and 2.6 years had significantly lower C:N ratios than plants from which ants were excluded throughout the experiment (p < 0.05; Figures 1C and S3A), suggesting that the presence of ants increased nitrogen availability. To confirm the assumption that the additional foliar nitrogen is the result of myrmecotrophy, a labeling experiment was conducted. T. quadrialata plants outside the main experimental plot colonized by Azteca spp. ants were chosen and a solution of honey mixed with [15N]glycine was fed to the ants (see Figures S3B–S3E for pictures of the experimental setup). To exclude false positives such as from leaves that had direct contact with the labeled honey mixture or with labeled ant feces, we wrapped the youngest leaves in a bag before the labeling and harvested these samples after 1 month. Additionally, new leaves that emerged after the consumption of honey were collected after 6 months. As shown in Figure 1D, there was a clear enrichment of 15N in protected and newly grown leaves compared to those on the plant before ants were fed with [15N]glycine (p < 0.05), demonstrating that T. quadrialata plants can indeed take up and transport nitrogen previously consumed by their ant symbionts.
Figure 1.
The presence of ants increases nitrogen content and general metabolism in Tococa quadrialata plants in the absence of herbivores
(A) Experimental design: A set of young T. quadrialata plants colonized by Azteca cf. tonduzi ants was split into two groups, and ants were removed from one group. Nets were installed around all plants to prevent herbivore damage and recolonization and ensure the same abiotic conditions. The general performance and metabolism of T. quadrialata plants with (ants) and without ants (no ants) were measured.
(B) Boxplots (25th percentile, median, mean (open circle), and 75th percentile) showing plant growth. 1 year after the start of the experiment, all plants had grown, however, colonized ones had gained more height than their ant-deprived counterparts (∗∗: p < 0.01, df = 33, Student’s t-test, n = 17–18). After 2.6 years, both groups of plants had grown taller, but no significant difference between the treatment groups was detectable (p > 0.05, df = 30, Student’s t-test, n = 13–19). For further growth parameters, see Figure S2.
(C) Boxplots (25th percentile, median, mean (open circle), and 75th percentile) showing carbon-nitrogen (C:N) ratio of the leaves. The C:N ratios of leaves of ant-colonized plants were lower than those of ant-deprived plants at both examined time points (1 year: ∗∗: p < 0.01, F1,16 = 9.789, two-way ANOVA, n = 4–5; see Figure S3; 2.6 years: ∗: p < 0.05, df = 14, Student’s t-test, n = 8).
(D) Feeding 15N-labelled glycine directly to ants increased the relative amount of 15N (δ15N) in T. quadrialata leaves even in leaves inaccessible to ants (protected) and in newly grown leaves (new) (∗:p < 0.05, df = 2, one-tailed t-test, n = 3). Mean and SE are shown. See Figure S3 for details of the experimental setup.
(E) The presence of ants led to a significantly greater accumulation of several amino acids after 2.6 years (∗∗∗: p < 0.001 ∗∗: p < 0.01, df = 14, Student’s t-test, n = 8). Mean and SE are shown.
(F) Transcriptome analysis of ant-colonized and ant-deprived plants (1 year) showed that genes involved in nitrogen and amino acid metabolism are upregulated in colonized plants compared to ant-deprived ones (FDR p value ≤0.05, log2FC ≥ 1). Genes were assigned to the corresponding GO terms for biological processes in which they might play a role.
(G) Genes associated with the general metabolism were found to be induced in ant-colonized vs. ant-deprived plants (FDR p value ≤0.05, log2FC ≥ 1). Genes are grouped by their GO terms (see Data S2).
(H) The presence of ants led to increased amounts of free sugars (2.6 years: ∗∗∗: p < 0.001, df = 14, Student’s t-test, n = 8). Mean and SE are shown.
With increased levels of nitrogen, we expected an increased accumulation of nitrogen-containing compounds in T. quadrialata and thus quantified the free amino acid contents of ant-colonized compared to ant-deprived plants. Indeed, enhanced levels of Ile, Glu, Pro, and Thr (p < 0.01), as well as Leu, Val, and Phe (p < 0.001) were found in ant-colonized plants (Figure 1E). The same trend was observed but without statistical significance for Tyr, Asp, and Met (Figure 1E). Similar differences in the free amino acid content of ant-colonized vs. ant-deprived plants were seen in the results of the herbivory and JA experiments conducted at about the same time (Table S1).
On the molecular level, transcriptome analysis of ant-colonized and ant-deprived T. quadrialata leaves revealed that many genes associated with nitrogen and amino acid metabolism were expressed at higher levels in ant-colonized plants (Data S2). For example, genes involved in nitrogen compound metabolic processes (GO:0006807), nitrogen compound transport (GO:0071705), nitrile metabolic processes (GO:0050898), and valine/leucine/isoleucine biosynthetic processes (GO:0009099, GO:0009098, GO:0009097) were all upregulated in colonized vs. ant-deprived plants (Figure 1F). Besides nitrogen metabolism, other aspects of general metabolism seemed to be influenced by the colonization status. The upregulation of genes associated with GO terms like cellular biosynthetic processes (GO:0044249), primary metabolic processes (GO:0044238), positive regulation of transcription by RNA polymerase II (GO:0045944), organic cyclic compound metabolic processes (GO:1901360), protein metabolic processes (GO:0019538), amino acid transport (GO:0006865), and mitochondrial translation (GO:0032543) indicates that there is enhanced metabolic activity in ant-colonized plants on many fronts (Figure 1G). In addition, increased amounts of the free sugars glucose, fructose, and sucrose were found in ant-colonized T. quadrialata plants compared to ant-deprived plants (p < 0.001, Figure 1H).
T. quadrialata contains ellagitannin defenses that accumulate to higher levels in plants not colonized by symbiotic ants
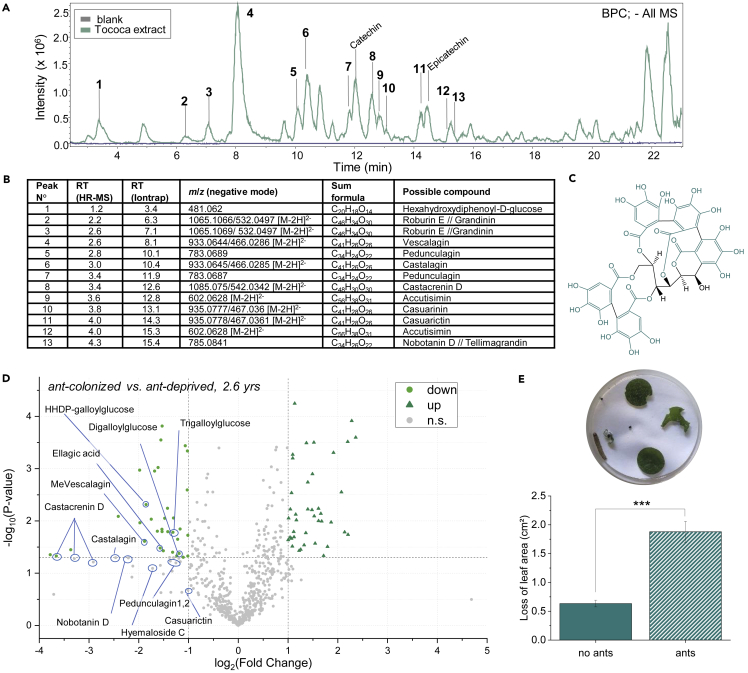
The greater damage suffered by T. quadrialata without its Azteca cf. tonduzi symbionts might be explained by the lack of other forms of anti-herbivore protection. To search for chemical defenses in this species, we employed untargeted metabolome analysis of methanolic leaf extracts and found high levels of phenolic compounds (Figure 2A). Many of the features detected were tentatively identified as ellagitannins—a class of plant defense compounds (Salminen, 2014)—by the comparison of accurate masses, derived sum formulae, and fragmentation patterns to those in databases and the literature (dos Santos et al., 2012; Fracassetti et al., 2013; Moilanen et al., 2013; Serna and Martinez, 2015; Yoshida et al., 1991) (see Table S4), and an authentic standard for vescalagin (Figures 2B, 2C, and S4). Strikingly, many of these ellagitannins were present in greater amounts in ant-deprived vs. ant-colonized plants (2.6 years after ant removal, Figure 2D). This trend applied not only to ellagitannins, but also to their precursors (digalloylglucose, trigalloylglucose) and related polyphenolics (ellagic acid). Similar differences in ellagitannin content between ant-deprived and ant-colonized plants were obtained from an herbivory experiment (1 year after ant removal, Figure S5).
Figure 2.
Ellagitannins, a major class of defense compounds in Tococa quadrialata, increase when symbiotic ants are not present
(A) Base peak chromatogram (BPC) of a methanolic leaf extract run in negative ionization mode on an ion-trap LC-MS -showing that ellagitannins and related compounds are some of the main constituents of the extract (peak numbers correspond to lines in panel B).
(B) Tentative identification of the major peaks of the chromatogram in panel A based on retention time, accurate mass of the most abundant parent ion (usually [M−H]-), predicted sum formula, and fragmentation pattern (not shown) (for more details, see Table S4, Figure S4).
(C) Structure of the ellagitannin vescalagin, derived from 5 gallic acid moieties (highlighted in green) and a central sugar moiety (black).
(D) Volcano plot of untargeted metabolome analysis of T. quadrialata (with MS in negative ionization mode) comparing features of ant-colonized and ant-deprived plants 2.6 years after ant removal. Several features identified as ellagitannins, precursors, and related compounds were found to be more abundant in ant-deprived plants.
(E) Choice assay with Spodoptera larvae offered leaf discs from ant-colonized (ant) and ant-deprived (no ants) plants (2.7–2.8 years after ant-exclusion) in a Petri dish. Larvae allowed to move freely in the Petri dish for 24 h were found to prefer feeding on leaves of ant-colonized plants (∗∗∗:p < 0.001, paired Wilcoxon rank-sum test, n = 41). Mean and SE are shown. RT, retention time; HR-MS, high-resolution mass spectrometry; HHDP, hexahydroxydiphenoyl.
To determine whether the observed differences between ant-colonized and ant-deprived plants in ellagitannin content, as well as in sugars and amino acids, have an impact on resistance to herbivory, a choice experiment was conducted with larvae of Spodoptera spp. These generalist herbivores were allowed to choose whether to feed on leaf discs of ant-colonized or ant-deprived T. quadrialata plants, and were found to exhibit a significant preference for leaves from ant-colonized plants (p < 0.001, Figure 2E). These results suggest that the greater accumulation of ellagitannins and/or the lower amounts of amino acids and sugars in ant-deprived plants (Figure 1) might reduce herbivore feeding in the absence of symbiotic ants.
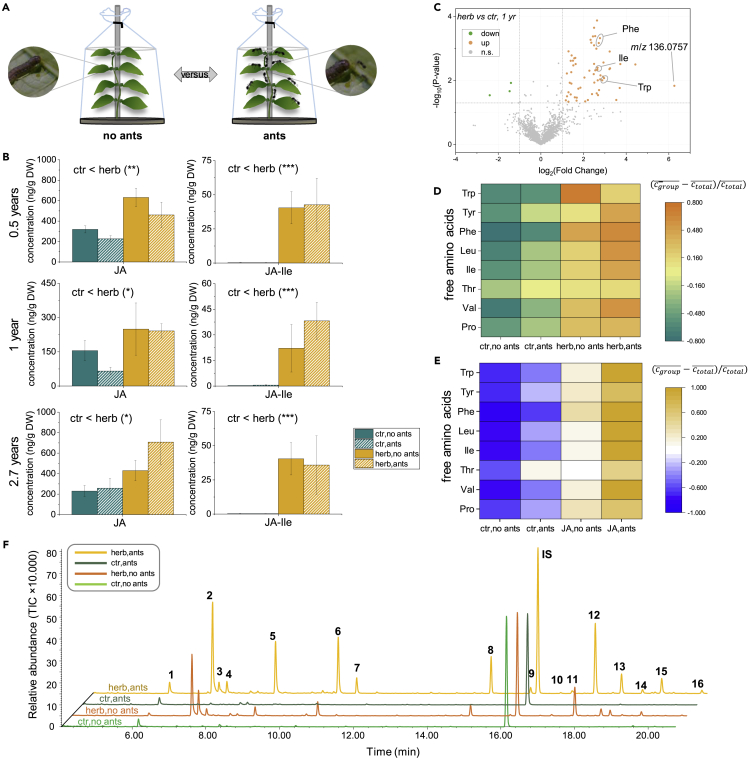
Insect herbivory on T. quadrialata induces extensive changes in defensive hormones, amino acid content, and volatile emission independent of ant colonization
Beside pre-formed defenses, such as ellagitannins, the resistance of plants against herbivores can also be mediated by induced defenses. To study whether induced defense reactions occur in T. quadrialata, we repeatedly (0.5–2.8 years after ant removal, in both rain and dry season) analyzed leaves of ant-colonized and ant-deprived plants after one day of feeding by S. spp. caterpillars (Figure 3A). In response to herbivory, the levels of jasmonic acid (JA) and its bioactive conjugate JA-Ile significantly increased in wounded leaves of both ant-deprived and ant-colonized plants (Figure 3B). Colonization status did not affect this response (p > 0.05 for colonization and interaction in all experiments). Most JA-derivatives followed the same pattern, but two (Sulfo-JA, OH-JA) decreased under ant colonization regardless of herbivory and the phytohormone salicylic acid (SA) increased in the presence of ants regardless of herbivory. No clear trend was observed for abscisic acid (ABA) (Figure S5A, Table S3).
Figure 3.
Insect feeding induces changes in defensive hormones, amino acids, and volatile emission of Tococa quadrialata
(A) Experimental design; leaves of colonized (ants) and ant-deprived (no ants) plants net-protected from uncontrolled herbivory were exposed to insect feeding 0.5 years (n = 6–8), 1 year (n = 4–5) and 2.7 years (n = 5–6) after the installation of the nets.
(B) In all three repetitions, herbivory led to a significant increase of jasmonic acid (JA) (∗: p < 0.05; ∗∗∗: p < 0.001, two-Way ANOVA) and the bioactive conjugate JA-Ile (∗∗∗: p < 0.001, GLS, log-transformed data). Mean and SE are shown. For information about related compounds and statistics see also Figure S5, Table S3.
(C) Untargeted metabolite analysis comparing methanol extracts of control (ctr) and herbivore-induced (herb) leaves using LC-high resolution MS analysis (MS operating in positive ionization mode). Volcano plot depicts the metabolic features that accumulate upon herbivory (1 year) in orange. Features upregulated in all experiments are annotated.
(D) Targeted analysis revealed additional herbivore-inducible amino acids (2.7 years, ptreatment <0.05, two-Way ANOVA, see Table S1).
(E) Treatment of leaves (n = 4–5, 2.7 years) with an aqueous 1 mM solution of jasmonic acid (JA) also induced the accumulation of free amino acids (ptreatment<0.05, two-Way ANOVA, see Table S1). Spraying water served as control (ctr).
(F) Representative total ion chromatograms (TIC) of volatiles from each treatment collected for 24 h while insects were feeding. Compounds were identified with GC-MS. IS: internal standard; 1: α-pinene, 2: 1-octen-3-ol, 3: octan-3-one, 4: octan-3-ol, 5: (E)-β-ocimene, 6: (E)-4,8-dimethyl-nonatriene (DMNT), 7: benzyl cyanide, 8: indole, 9: methyl anthranilate, 10: α-copaene, 11: unknown sesquiterpene (fragmentation pattern see Figure S6), 12: (E)-β-caryophyllene, 13: α-humulene, 14: germacrene D, 15: α-farnesene, 16: nerolidol. All compounds were identified with the NIST17 library, Kovats index (see Table S2), and, except for 11 and 16, by comparison to authentic standards.
Untargeted LC-MS analysis of the leaf metabolomes revealed that the features corresponding to ellagitannins were not induced upon herbivory (Figure S5), but those corresponding to amino acids were induced (Figure 3C). Subsequent targeted LC-MS2 analyses demonstrated the accumulation of Trp, Phe, Val, Ile, and Leu upon herbivory at all time points, whereas Thr, Pro, and Tyr were only significantly enriched in herbivore-treated leaves at later time points (1 year and 2.7 years after ant-removal, Figure 3D, Table S1). Ant colonization did not affect the amount of these herbivory-responsive amino acids 0.5 and 1 year after the initiation of the experiment (p > 0.05 for colonization and interaction colonization×treatment, Table S1), but colonized plants had significantly higher concentrations of Val, Leu, Phe, and Tyr after 2.7 years (p < 0.01, Table S1).
To confirm the hypothesis that the induced response to herbivory in T. quadrialata is mediated by jasmonate signaling, 1 mM JA was sprayed onto the leaves of net-covered plants with or without ants (2.8 years after ant removal) and samples were collected after 24 h. Phytohormone analysis showed that levels of the bioactive JA-Ile were increased (p < 0.001, generalized least-squares regression, n = 4–5) similar to concentrations observed upon insect feeding (Table S1) (Figures 3C and 3D). In addition, all of the amino acids found to be inducible by insect feeding—Phe, Trp, Tyr, Val, Thr, Pro, Leu, and Ile—accumulated in leaves after spraying JA (Figure 3E).
Analysis of the volatile organic compounds (VOCs) emitted in response to herbivory by gas chromatography-mass spectrometry (GC-MS) revealed that the volatile bouquet emitted by T. quadrialata plants was significantly altered upon herbivore damage (Figure 3F, Table S2). The most abundant components of the herbivore-induced blend were 1-octen-3-ol and (E)-β-caryophyllene, followed by (E)-β-ocimene, DMNT, and indole. These compounds were present in only trace amounts or were completely undetectable without herbivory. In addition, the C8-compounds octan-3-one and octan-3-ol, the N-containing compounds benzyl cyanide and methyl anthranilate, and several sesquiterpenes (α-copaene, α-humulene, α-farnesene, germacrene D, nerolidol, unknown sesquiterpene) were identified as minor herbivore-induced VOCs. Interestingly, the volatile emission of ant-colonized plants often contained further compounds, like 2-heptanone, 2-heptanol, and several iridoids (Figure S6, Table S2). However, these compounds were only found in substantial amounts when leaves with ants were fed upon by caterpillars (Figure S6), suggesting that they are not part of the plant’s response to herbivory, but actually alarm pheromones of the A. cf. tonduzi ants, in accordance with previous findings (Do Nascimento et al., 1998; Ohmura et al., 2009).
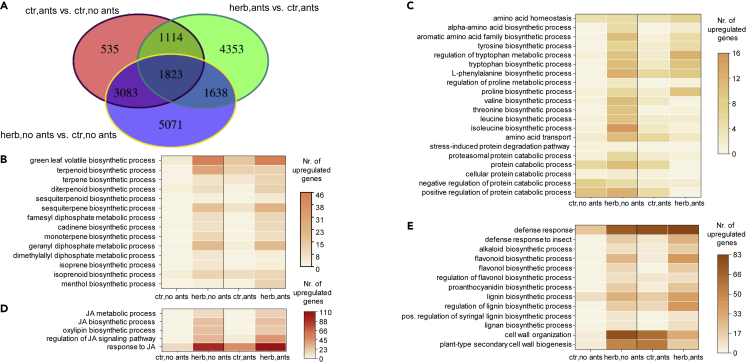
Insect herbivory alters gene expression in T. quadrialata
To learn more about the response of ant-plants to feeding damage, we next sequenced the transcriptomes of leaf samples from the herbivory experiment conducted 1 year after ant-removal and assembled the transcriptome de novo. Statistical analysis showed that herbivory drastically altered the gene expression profile. In ant-colonized plants, 8928 genes were responsive to herbivore treatment, 3004 of them being upregulated. Similarly, 11,615 genes were differentially expressed in ant-deprived plants upon herbivory. Although there was some overlap of differentially expressed genes (DE-Gs) in herbivore-damaged leaves of plants with and without ants, more genes showed specific activation in one interaction or the other (Figure 4A). Interestingly, most genes found to differ upon ant colonization were strongly influenced by herbivory as well. Among the genes induced by herbivory were many involved in the biosynthesis of volatile terpenoids, consistent with the herbivore-triggered emission of terpenoid volatiles by insect feeding (Figure 4B). Similarly, the genes of the tryptophan pathway, associated with the formation of Trp, indole, and methyl anthranilate, were activated by herbivore damage. Several genes involved in the biosynthesis of free amino acids were found to be induced upon herbivory as well, particularly in plants deprived of ants (Figure 4C). On the other hand, analysis of genes associated with protein degradation, another potential source of free amino acids, did not show a clear pattern of regulation by herbivory.
Figure 4.
Insect herbivory alters gene expression in Tococa quadrialata
(A) Venn diagram showing the numbers of differentially expressed genes (DE-Gs, FDR corrected p value ≤0.05, |log2FC| ≥ 1) in different treatment comparisons, indicating how many DE-Gs were unique to a treatment or common to several treatments.
(B–E) Heatmaps showing the number of genes assigned to various gene ontology (GO) terms that are up- or downregulated upon herbivory (herb) in the presence (ants) or absence (no ants) of ants in comparison to the respective control (ctr), see also Data S2. (B) GO terms are associated with the emission of volatile organic compounds. (C) GO terms are associated with the accumulation of free amino acids. D: GO terms linked to jasmonic acid (JA) biosynthesis and signaling. (E) GO terms associated with chemical and physical defense. (B, D, and E) GO terms shown are significantly enriched (FDR corrected p value ≤0.05) for at least one comparison (ants or no ants).
As the JA spraying experiment showed that JA is responsible for signaling and activating defenses after wounding, we monitored genes associated with JA biosynthesis and metabolism. Many of them were upregulated by herbivory, especially genes classified under the GO term “response to JA,” which was among the strongest induced groups of genes (Figure 4D). As typical responses to JA include cell wall reinforcement, production of protease inhibitors and defensive compounds (Maag et al., 2015; Wasternack and Hause, 2013), we investigated GO terms that might correspond to such JA responses and found various genes involved in the formation of aromatic compounds like flavonoids and lignins to be induced upon herbivory (Figure 4E). Strikingly, the expression pattern of protease inhibitors, a major group of defense proteins described in many other plants, was not altered upon herbivory (Data S2).
Discussion
Ant-plants are frequently reported to suffer from reduced growth and survival in the absence of their mutualist ants (Fonseca, 1994; Heil et al., 2001a; Letourneau, 1998; Rosumek et al., 2009; Schupp, 1986). However, it is not known how the presence/absence of ants affects the plant’s metabolism and, thus, if poor plant performance results only from increased herbivory owing to a lack of alternative plant defenses or if the plant misses other ant benefits, such as the provisioning of nutrients, which in turn impairs its metabolism and growth. In this study, we aimed to disentangle the effects of mutualistic ants on a myrmecophytic host plant and characterized the plant’s defense strategies. Based on a combination of metabolomics and transcriptomics, we show that the tropical myrmecophyte T. quadrialata growing in its natural environment does not rely completely on the ant-mediated defenses but possesses a typical repertoire of responses to herbivory, including phytohormone increases and volatile emission, regardless of ant status, and also increased its investment in ellagitannin chemical defenses in the absence of its Azteca cf. tonduzi ants. At the same time, the presence of symbiotic ants makes a significant contribution to the plant nitrogen budget.
Ant colonization provides both protective and nutritional benefits to ant-plants
The absence of ants drastically reduced the performance of T. quadrialata in its natural habitat as described for other myrmecophytic species (Fiala et al., 1989; Fonseca, 1994; Janzen, 1966; Michelangeli, 2003; Schupp, 1986). Uncolonized plants suffered from severe folivory indicating that ants had critical functions in protecting plants from herbivores. Interestingly, ant-deprived plants also grew slower than ant-colonized plants in the absence of herbivory. This suggested the possibility that ants contribute to plant nutrition rather than depriving the plant of resources needed for growth—the expected cost of mutualism (Heil et al., 1997; Heil and McKey, 2003; O'Dowd, 1980). We found that A. cf. tonduzi were directly providing nitrogen to their host plants, increasing the nitrogen content of T. quadrialata, which resulted in higher amounts of free amino acids in ant-colonized plants. An RNA-Seq study revealed that ant-colonized T. quadrialata not only expressed genes of general nitrogen, amino acid, and protein metabolism at higher levels than in ant-deprived plants, but were also upregulated in genes of primary metabolism and had higher levels of free sugars. These differences may well contribute to the greater growth of ant-colonized versus uncolonized T. quadrialata, both with and without herbivores.
Not all ant-plant mutualisms follow this pattern. In a study on Acacia drepanolobium (Stanton and Palmer, 2011), ant-colonization negatively influenced plant growth in an herbivore-free environment, likely owing to the metabolic costs of ant colony maintenance, which in this case are only partly compensated by the benefits of reduced herbivore pressure (Chamberlain and Holland, 2009; Heil et al., 1997). Similar results were also found for Cordia nodosa by Frederickson et al. (2012). The different results can be explained by the varying costs of ant occupancy. Acacia drepanolobium and other myrmecophytes are colonized by plant-ants that feed exclusively on the products of the plants, e.g., food bodies or extrafloral nectar, which require a considerable outlay of plant resources (Heil et al., 1997). Azteca ants, on the other hand, forage on the plant and in the surroundings (Sagers et al., 2000; Vasconcelos and Davidson, 2000), reducing the need for a plant-based diet and thus the costs of the relationship for the plant. This study adds to a growing body of evidence (Chanam et al., 2014; Defossez et al., 2011; Dejean et al., 2017; Gay, 1993; Sagers et al., 2000; Solano and Dejean, 2004) that ant symbionts can provide nitrogen to myrmecophytes (but see McNett et al., 2009; Solano and Dejean, 2004). However, whereas the other studies only demonstrate the bidirectional transfer of nutrients, our study shows the importance of this nitrogen source, as we found that ant colonization increased the total foliar nitrogen content, which probably resulted in the observed growth promotion.
Analysis of the metabolome and transcriptome hint toward the molecular mechanisms behind that. A comparison of the leaf metabolomes showed that ant-colonized plants furthermore contained higher amounts of free amino acids and sugars than ant-deprived plants. This together with the results from the RNA-Seq data suggesting an enhanced protein and nitrogen metabolism indicates that the presence of ants enhances the general metabolism in this ant-plant mutualism. The increased nitrogen content together with the reduced danger of herbivore damage permits the plants to focus on growth, which in turn enables the ant-colony to expand as well—a win-win situation.
T. quadrialata also produces chemical defenses against herbivory, with increased amounts when ants are absent
Using untargeted metabolomics, we found that T. quadrialata produced a diversity of ellagitannins, a class of hydrolyzable tannins that are characteristic anti-herbivore defense compounds of the plant family Melastomataceae (Serna and Martinez, 2015). Ellagitannins are well-known in human medicine for their anti-inflammatory (Gatis-Carrazzoni et al., 2019), anti-bacterial (Araujo et al., 2021; Gontijo et al., 2019), anti-fungal (Klewicka et al., 2020), and anti-viral (Kesharwani et al., 2017) activities. In the alkaline midgut of insects, these compounds act as strong oxidants (Barbehenn et al., 2006) with negative consequences for herbivores. Vescalagin, for example, which was identified here, was shown to inhibit the growth of two generalist caterpillars (Acronicta psi and Amphipyra pyramidea) (Roslin and Salminen, 2008), whereas ellagitannins from Onagraceae reduced survival of S. spp. (Anstett et al., 2019). Hence, the ellagitannins identified in this study likely contribute to the direct defense of T. quadrialata. Their greater accumulation in ant-deprived versus ant-colonized plants implies compensation for the lack of anti-herbivore protection afforded by ants. Such a trade-off between chemical defense and ant protection in myrmecophytes had originally been proposed by Janzen (1966), who found that non-myrmecophytic Acacia produce more cyanogenic glycosides than Acacia colonized by ants. As investment in defense is costly, plants should avoid redundancies. Many studies in ant-plants have addressed this hypothesis with mixed results (see: Koricheva and Romero (2012); (Heil et al., 2002), but some studies compared myrmecophytic with non-myrmecophytic species (Heil et al., 2002), or the same species at different life stages (Del Val and Dirzo, 2003). Of the few studies that compared investment in mechanical, chemical, and ant defenses in myrmecophytes of the same species, most of them indicated a trade-off (Dodson et al., 2000; Dyer et al., 2001, 2004; Moraes and Vasconcelos, 2009), but see Frederickson et al. (2013); Letourneau and Barbosa (1999). Hence, we conclude that ant-plants including T. quadrialata can adjust their level of constitutive anti-herbivore defense to their colonization status.
The choice assay revealed that indeed leaves of ant-deprived plants are less attractive to a generalist herbivore, most likely a combined effect of the higher nutritional value of ant-colonized leaves (higher sugar and amino acid content) and increased defenses in ant-free T. quadrialata plants. Roslin and Salminen (2008) already demonstrated with performance assays on the artificial diet that vescalagin is a strong feeding deterrent for generalist caterpillars, whereas Moraes and Vasconcelos (2009) found enhanced mechanical defenses like leaf toughness and trichome density in ant-free T. guianensis, defensive traits we haven’t analyzed, but may add to the observed feeding preference. Taking into account all the results from the long-term experiment, it can be concluded that ant-deprived plants adjust to their situation over time by the upregulation of their direct defenses and are therefore better defended against insect herbivory than a colonized plant without ant protection.
Ant-plants still possess some inducible defense mechanisms, such as the emission of volatiles
Based on our observations and numerous literature reports, symbiotic ants can be very effective at preventing herbivory. Hence, we hypothesized that myrmecophytes colonized by ants do not require inducible defense mechanisms. However, we found that insect feeding led to increases in jasmonate levels and the emission of HIPVs. These responses occurred regardless of the status of colonization, suggesting that the presence or absence of ants does not influence such defense reactions. Similar results were found for the myrmecophyte C. nodosa, where herbivory increased leaf toughness, total phenolics, and trichomes, whether or not ants were present (Frederickson et al., 2013). The presence of herbivore-induced defenses in ant-colonized plants is curious as they will never match the reaction speed of the ants. Although ants need only a few minutes to attack intruders (Agrawal, 1998; Agrawal and Dubin-Thaler, 1999; Bruna et al., 2004; Christianini and Machado, 2004; Dejean et al., 2008; Schatz et al., 2009), the JA-mediated production of defensive natural products takes hours to days (Joo et al., 2018; Maffei et al., 2007; McCormick et al., 2014; Schaub et al., 2010) Perhaps ant protection is not sufficiently reliable under all conditions.
The induction of volatiles by herbivory may have several functions in myrmecophytes. For various ant-plant mutualisms, it was demonstrated that volatile cues can attract mutualistic ants to the site of damage within a few minutes (Agrawal, 1998; Agrawal and Dubin-Thaler, 1999; Bruna et al., 2004; Christianini and Machado, 2004; Dejean et al., 2008; Schatz et al., 2009). Thus, any ant-attracting volatile should be released immediately upon wounding, as for instance methyl salicylate or hexanal (McCormick et al., 2014) emitted by Leonardoxa africana to recruit Petalomyrmex phylax ants (Schatz et al., 2009), and Cecropia obtusifolia to attract Azteca spp. ants (Agrawal, 1998). In T. quadrialata, however, only octen-3-ol and its derivatives would qualify as potential wounding signals for ants, as the other released volatiles, including terpenoids and nitrogen-containing compounds, are only emitted hours after induction (Erb et al., 2015; McCormick et al., 2014). The damage-induced emission of octen-3-ol is particularly interesting as it is mainly known as a fungal volatile and, in plants, has only been described in legumes so far (Kigathi et al., 2009). Ants also produce their own volatile alarm pheromones to attract nestmates (Agrawal and Dubin-Thaler, 1999; Bruna et al., 2004; Christianini and Machado, 2004; Dejean et al., 2008), as we observed in our volatile collections (Figure S6).
Many of the herbivore-inducible terpenoids we measured from T. quadrialata have been reported to be emitted upon simulated herbivory in myrmecophytic Piper spp. (α-pinene, β-ocimene, α-copaene, β-caryophyllene, α-humulene, α-farnesene, germacrene D) (Mayer et al., 2018) and from the non-myrmecophytic Acacia cochliacantha (β-ocimene, β-caryophyllene, α-farnesene, germacrene D) (Hernandez-Zepeda et al., 2018). In the latter, this blend was able to induce the secretion of extrafloral nectar in systemic leaves, implying a role for these compounds in intra-plant signaling and induction of plant defense (Hernandez-Zepeda et al., 2018). In non-myrmecophytes, studies have shown that DMNT induces direct defenses in sweet potato (Meents et al., 2019), indole primes defense induction in neighboring maize plants (Erb et al., 2015), and octen-3-ol activates JA-dependent defenses in Arabidopsis (Kishimoto et al., 2007). Hence, one could speculate that ant-plants might use these HIPVs as signals to warn systemic leaves or neighboring plants that ant protection is failing and activate direct plant defenses. Other HIPVs released from T. quadrialata plants may serve as herbivore repellents, as has been shown for (E)-β-caryophyllene with leaf cutter ants (North et al., 2000), for benzyl cyanide with Lymantria dispar caterpillars (Irmisch et al., 2014), and for methyl anthranilate with sparrows (Ahmad et al., 2018).
Besides altering the emission of volatiles, herbivory on T. quadrialata also enhanced the accumulation of free amino acids. Our transcriptome data suggest that this amino acid enrichment is the result of de novo synthesis rather than protein degradation. This response may help provide substrate for other defense reactions.
The response of T. quadrialata to herbivory is mediated by JA signaling
To better understand the inducible defense responses of T. quadrialata, we examined defense hormone levels in response to Spodoptera spp. herbivory. Caterpillar feeding led to the accumulation of JA and its bioactive conjugate JA-Ile as well as increased expression of the respective genes for JA biosynthesis and response to JA, implying that the inducible responses in herbivore-damaged leaves were mainly caused by JA signaling. Indeed, the treatment of T. quadrialata leaves with JA resulted in the accumulation of the same amino acids that were found to be induced upon herbivory. However, not all ant-plants regulate herbivore responses in the same fashion. Myrmecophytic Acacia did not respond to JA treatment at all (Heil et al., 2004), or responded only in a limited way by inducing the enhanced secretion of extrafloral nectar (Heil et al., 2001b; Hernandez-Zepeda et al., 2018) and emission of volatiles (Hernandez-Zepeda et al., 2018) only in the absence of ants and only locally. The effect of JA on direct defenses has not been investigated in ant-plants at all to the best of our knowledge. Typical responses to JA signaling in non-myrmecophytes include increased glucosinolate levels (Wasternack and Hause, 2013), alkaloid biosynthesis (Wasternack and Hause, 2013), and protease inhibitor accumulation (Farmer et al., 1992). Increases in protease inhibitors triggered at the transcript level (Koiwa et al., 1997) seem to be a common JA-inducible defense across the plant kingdom (Habib and Fazili, 2007). However, unlike in other plant species (Eberl et al., 2021), herbivory and subsequent JA signaling in T. quadrialata did not result in a strong upregulation of protease inhibitor genes. The absence of this defense strategy might contribute to the observed high susceptibility of T. quadrialata to herbivory, as protease inhibitors not only have a negative impact on the digestive enzymes of insects but also protect other plant defensive proteins like peroxidases or chitinases from degradation (Mithöfer and Boland, 2012). Nevertheless, the finding that herbivory on T. quadrialata can induce jasmonate biosynthesis and accumulation and downstream JA-mediated defense responses demonstrates that this well-protected myrmecophyte still is able to activate typical plant defense reactions. T. quadrialata neither depends completely on the ant-provided protection nor lost its ability to activate chemical defenses during evolution. However, the obvious loss of protease inhibitor induction might be interpreted as the first step in that direction.
Limitations of the study
We investigated the interaction between the myrmecophytic plant T. quadrialata and Azteca cf. tonduzi on different levels, from ecological to molecular and phytochemical points of view. This included field studies in combination with metabolomics for the identification of defense compounds and RNA-Seq to analyze gene regulation. The protective role of ants and the phenomenon of myrmecotrophy have been shown for other ant-plant mutualisms but needed to be confirmed for this particular system as well. However, the chemical and molecular characterization of the plants in response to the presence/absence of ants on one hand and to herbivory, on the other hand, is a first important step toward a deeper understanding of the underlying molecular mechanisms driving this symbiosis. Combining the chemical and transcriptomic analysis, we were able to draw correlations between ecological observations and metabolic findings; nevertheless, we are still not able to fully explain how the accumulation of certain compounds or genes results in a certain phenotype. Future research will be necessary to fully understand the molecular basis and physiology of ant-plant symbiosis. Conclusive evidence for specific metabolic pathways and their impact on the plant’s performance requires genetic modifications of ant-plants, which hopefully will be possible in the future.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Tococa quadrialata leaves | this paper | NCBI:txid427262 |
| Chemicals, peptides, and recombinant proteins | ||
| n-bromodecane | Sigma-Aldrich | 145785, CAS:112-29-8 |
| Jasmonic acid (JA) | synthesized by Anja David, MPI-CE | adavid@ice.mpg.de, CAS: 77026-92-7 |
| 15N-Glycine | Eurisotope | NLM-202-1 |
| D6-JA | HPC Standards GmbH | 674797 |
| D6-ABA | Toronto Research Chemicals | A110002 |
| D4-SA | Santa Cruz Biotechnology | sc-212908 |
| D6-JA-Ile | HPC Standards GmbH | 674798 |
| algal labelled amino acid mix | Isotec | T81-11001, Sigma-Aldrich:487910 |
| 13C6-glucose | Sigma-Aldrich | 389374 |
| 13C6-fructose | Toronto Research Chemicals | F792546 |
| DNaseI | ZymoResearch | E1010 |
| vescalagin | Sigma-Aldrich | 76418-5MG, CAS: 36001-47-5 |
| α-pinene | Fluka | 80604, CAS: 7785-70-8 |
| (E)-β-ocimene | Firmenich | 948840, CAS: 3779-61-6 |
| (E)-DMNT | synthesized by Stefan Bartram, MPI-CE | bartram@ice.mpg.de, CAS: 19945-61-0 |
| α-copaene | Fluka | 27814, CAS: 3856-25-5 |
| (E)-β-caryophyllene | Sigma-Aldrich | C9653, CAS: 87-44-5 |
| α-humulene | Sigma-Aldrich | 53675, CAS: 6753-98-6 |
| germacrene D | Isolated by Stefan Bartram, MPI-CE | bartram@ice.mpg.de, CAS: 37839-63-7 |
| α-farnesene | Sigma-Aldrich | W383902, CAS: 502-61-4 |
| indole | Sigma-Aldrich | I3408, CAS: 120-72-9 |
| methyl anthranilate | Sigma-Aldrich | M29703, CAS: 134-20-3 |
| 2-heptanone | Sigma-Aldrich | 537683, CAS: 110-43-0 |
| 2-heptanol | Fluka | 51800, CAS: 543-49-7 |
| oct-1-en-3-ol | Fluka | 74950, CAS: 3391-86-4 |
| 3-octanone | Merck | 821860, CAS: 106-68-3 |
| octan-3-ol | Sigma-Aldrich | 218405, CAS: 589-98-0 |
| benzyl cyanide | Merck | 8.01811, CAS: 140-29-4 |
| Critical commercial assays | ||
| Spectrum Plant Total RNA Kit | Sigma-Aldrich | STRN50 |
| One-Step PCR Inhibitor Removal Kit | ZymoResearch | D6030 |
| Deposited data | ||
| Raw reads (transcriptome sequencing) | this paper | PRJNA865704 |
| Experimental models: Organisms/strains | ||
| Tococa quadrialata | this paper | NCBI:txid427262 |
| Azteca spp. | this paper | NCBI:txid121511 |
| Spodoptera spp. | this paper | NCBI:txid7106 |
| Software and algorithms | ||
| ImageJ | Schneider et al. (2012) | https://imagej.nih.gov/ij/ |
| NIST/EPA/NIH EI Mass Spectral Library | National Institute of Standards and Technology | https://chemdata.nist.gov/dokuwiki/doku.php?id=chemdata%3Adownloads%3Astart#nist_epa_nih_mass_spectral_database |
| Analyst 1.6.3 | Applied Biosystems | https://sciex.com/products/software/analyst-software |
| Metaboscape software | Bruker Daltonics | https://www.bruker.com/en/products-and-solutions/mass-spectrometry/ms-software/metaboscape.html |
| CLC Genomics Workbench | Qiagen Bioinformatics | https://www.qiagen.com/us/products/discovery-and-translational-research/next-generation-sequencing/informatics-and-data/analysis-and-visualization/clc-genomics-workbench/ |
| OmicsBox software | biobam | https://www.biobam.com/omicsbox/ |
| R | RStudio_Team (2021) | http://www.rstudio.com/ |
| OriginPro | OriginLab Corporation | https://www.originlab.com/ |
| SciFinder | CAS | https://scifinder.cas.org |
| Other | ||
| PoraPak filter | Material: Alltech, packed filters bought from http://www.volatilecollectiontrap.com/main.sc | VCT-1/4-3-POR-Q-25MG |
| GC-MS | Agilent Technologies | Agilent 6890 GC & 5973 MS |
| Optima-5 column | Macherey-Nagel | 726056.30 |
| PET bags | Toppits® | Bratschlauch |
| HPLC | Agilent Technologies | Agilent 1260 & Agilent 1100 |
| Zorbax Eclipse XDB-C18 column (API6500) | Agilent Technologies | 927975-902 |
| Zorbax Eclipse XDB-C18 column (timsToF) | Agilent Technologies | 981758-902 |
| QTRAP 6500 tandem mass spectrometer | AB Sciex | https://sciex.com/products/mass-spectrometers |
| HILIC column | Supelco | 56401AST |
| ESI iontrap mass spectrometer | Bruker Daltonics | Esquire 6000 https://www.bruker.com/en/products-and-solutions/mass-spectrometry/esi-ion-trap.html |
| Phenyl-Hexyl column | Phenomenex | 00F-4257-E0 |
| Dionex Ultimate 3000 series UHPLC | Thermo Scientific | https://www.thermofisher.com/de/en/home.html |
| Bruker timsToF mass spectrometer | Bruker Daltonics | timsTOF https://www.bruker.com/en/products-and-solutions/mass-spectrometry/timstof/timstof.html |
| isotope ratio mass spectrometer | Thermo Finnigan | Delta + XL |
| elemental analyzer | CE Instruments | NA1110 (now EA1110, part N° 112 110 52) |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Axel Mithöfer (amithoefer@ice.mpg.de).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
Study site and system
The experiments were conducted in the Tambopata Reserve [12° 50’ 10″ S, 69° 17′ 34 ‘’ W] close to the Explorer`s Inn lodge in the lowland Amazon basin of Peru (Madre de Dios province) at an elevation a.s.l. of 210 m. The average annual rainfall is 2377 mm with a dry season from June until October. The maximum monthly temperature is around 30°C whereas the monthly minimum was found to be around 19°C.
For the individual experiments, we worked with several subpopulations of T. quadrialata found at different spots in the reserve and inhabited by distinct ant colonies and/or species. The Tococa species was determined following Michelangeli (2005). All mutualistic ants belonged to the genus Azteca (pers. comm. P.-J. Malé), and the species was identified based on morphological features, following the key for Neotropical Azteca species: https://ants.biology.utah.edu/GENERA/AZTECA/key.html#BM110 (accessed: 22.03.2022).
Spodoptera spp. are well-studied generalist herbivores. Larvae were collected from local fields and starved for 24 h before the experiments. For volatile collections and herbivory treatments, larvae of the 4th and 5th instar were used, whereas choice experiments were conducted with larvae of the 2nd and 3trd instar. All larvae were removed immediately after the respective experiment.
Ant-exclusion
The subpopulation of T. quadrialata used for the ant-exclusion study was found alongside a small path. All individuals were colonized by Azteca cf. tonduzi (subfamily Dolichoderinae), which are obligate plant-ants. It’s noteworthy that Azteca is a polygynous genus so even though multiple ant queens were found within the plot, the whole subpopulation of T. quadrialata seemed to be colonized by a single, large ant colony. The ants mainly lived inside the domatia of the plants, however, in some cases, they also formed carton nests around the stem.
The T. quadrialata population consisted of 70 very young plants, the tallest one measuring 80 cm in height and the largest one having 21 leaves with domatia. Some of the population were transplanted within the plot in May 2018 to assure enough space for each individual. In October 2018, all plants with a minimum height of 20 cm and a minimum number of four domatia were randomly assigned to two groups: ant-colonized and ant-deprived. Ant exclusion from 20 plants was achieved by flushing every domatium several times with water and carefully squeezing the flushed domatia to guarantee complete ant removal. Additional ants found on the leaves and stem were removed manually using forceps. As a control treatment, domatia of colonized plants were squeezed, but not flushed, although this was reported to not cause any effect in previous ant-exclusion experiments. To prevent recolonization and herbivore damage, rain and light permeable nets were put around the plants and dug into the ground to block any access. In addition, plastic fences painted with Tanglefoot, a non-toxic, sticky resin serving as a mechanical barrier, were installed around the individuals.
Both 4 days and 6 weeks after ant removal, all domatia were flushed again and any remaining ants were removed. In the course of the experiment, plants were repeatedly checked for recolonization and whenever it occurred, the ant-removal procedure was repeated, and the individual was excluded from any experiment for the next 6 months.
The 20 ant-colonized plants in this experiment were covered with nets as well to ensure the same light conditions. However, nets were open to the ground allowing free movement of the ants along the plants.
15N-labeling study
For the labeling study, we used isolated individuals from three distinct subpopulations of T. quadrialata associated with Azteca spp. in the same area. The selected plants were 60–120 cm in size with 10–20 leaves. In two of the plants, the ants were living exclusively inside the plant whereas in the other, the colony also formed carton nests.
Method details
Long-term monitoring
To investigate the effect of the presence or absence of ants on the performance of T. quadrialata, the height, leaf and domatium number as well as the herbivore damage of all plants and treatments from the main plot were monitored over time. Data were collected over a time span of three yrs from 2018-2021, but no data could be obtained in 2020 due to the COVID-19 pandemic. Herbivore damage was estimated by two experimenters on a scale of 0-10, where 0 is no leaf damage and 10 no remaining leaf tissue. Only fully opened leaves and domatia with a minimum diameter of 0.5 cm were considered. Since leaves of the protected plants were collected for several experiments, this additional leaf loss was taken into account for growth measurements. These removed leaves appear in the statistics as still belonging to the plants, without considering the possibility of natural loss.
Herbivory experiment
The reaction to herbivory was studied using 10-16 ant-inhabited and 10-16 non-colonized individuals from the ant-exclusion experiment. The plants of each group were divided in half and randomly assigned to the herbivory or control groups. One leaf of the second pair of fully expanded leaves was enclosed with a PET bag (Toppits® Bratschlauch, Minden, Germany) to avoid herbivore escape. Two Spodoptera, spp. larvae were released on all leaf samples of the herbivory group and allowed to feed for 24 hours, whereas the control leaves were enclosed with an empty bag for 24 h. At the end of the experiment, all leaves were excised and photographed to determine the leaf damage, the midrib and domatium were removed and both halves of the leaf lamina were immediately but separately flash-frozen in liquid nitrogen. One half of each leaf sample was stored at -80°C until further processing to extract RNA, the other half was lyophilized for subsequent chemical analysis. Spodoptera larvae were conserved in ethanol. The experiment was conducted 6 months, 12 months, and 33 months after ant-exclusion.
Volatile collection
Simultaneously to the herbivore treatment, volatiles were collected over 24 h hours using a push-pull system, where charcoal purified air was pumped into the bag at a flow rate of 0.4 L min−1 while 0.3 L min−1 were pumped from the plant headspace out of the system passing through a 20 mg PoraPak (Alltech, Deerfield, IL, USA) filter that absorbed the volatiles. As controls, volatiles found in PET bags containing only Spodoptera, only Azteca ants or just purified air were collected in the same way. The experiment was performed in October 2019, 1 y after the ant-exclusion. As the feeding behavior of the larvae, the temperature, and hence the volatile production varied widely, the experiment was repeated in June, July and August 2021. Photographs of the wounded and controlled leaves allowed the determination of leaf size and feeding damage. Herbivory samples with a damage smaller than 1% of leaf area were excluded from further statistical analyses.
Analysis of herbivore induced volatiles
Volatiles were eluted from PoraPak filters using 200 μL dichloromethane containing 10 ng/μL n-bromodecane (Sigma-Aldrich, Taufkirchen, Germany) as an internal standard. Samples were analyzed using a Hewlett-Packard model 6890 gas chromatograph (Agilent Technologies, Santa Clara, CA, USA) equipped with a 30 m × 0.25 mm × 0.25 μm Optima-5 column (Macherey-Nagel, Düren, Germany) with 1 μL splitless injection. The injector was held at 220°C and helium (MS) or H2 (FID) was used as a carrier gas at 2 mL/min. The oven temperature of the GC-MS was held at 45°C for 2 min, then increased to 200°C at a rate of 6°C/min and further increased to 300°C at a rate of 60°C/min where it was hold again for 2 min.
For the identification of the volatiles, the GC was coupled to an Agilent model 5973 mass spectrometer with a quadrupole mass selective detector (transfer line temperature, 270°C; source temperature, 230°C; quadrupole temperature, 150°C; ionization potential, 70 eV; scan range of 50–400 amu). Compounds were identified using the NIST Mass Spectral Search Program (National Institute of Standards and Technology, Gaithersburg, MD, USA) to search the NIST/EPA/NIH EI Mass Spectral Library (NIST 17) and/or authentic standards.
Quantification was performed with the trace of a flame ionization detector (FID) operated at 300°C. Peak areas of the compounds were compared to the peak area of the internal standard n-bromodecane applying equal response factors on a weight basis.
Treatment with jasmonic acid
The role of jasmonic acid (JA, synthesized by Anja David, MPI-CE) in Tococa was studied on 8 ant-inhabited and 8 uninhabited individuals from the ant-exclusion experiment. Plants of each group were divided in half and randomly assigned to the JA or the control group. One leaf per plant was sprayed with approx. 1 mL 1 mM JA aq. (1/200 (v/v) EtOH/H2O) or water (1/200 (v/v) EtOH/H2O). Leaves were allowed to dry for 1 h and then were enclosed with PET bags (Toppits Bratschlauch, Minden, Germany). After 24 h, all leaves were excised, midrib and domatium removed, the lamina flash-frozen in liquid nitrogen and later on lyophilized for subsequent chemical analyses. This experiment was conducted 34 months after ant-removal.
Choice assay
Leaf discs (22 mm diameter) were excised from undamaged leaves of ant-colonized and ant-deprived plants and offered to individual 2nd-3rd instar Spodoptera larvae (n = 41) in custom-made petri dishes (see Figure S6B; for more detail (Boeckler et al., 2014)). After 24 h, the remaining leaf tissue was photographed and the consumed leaf area was determined using ImageJ (Schneider et al., 2012).
Plant tissue for chemical and elemental analysis
After 2.5 yrs following ant exclusion (May 2021), 8 colonized and 8 ant-deprived plants were chosen and one leaf of the second pair of fully expanded leaves of each plant collected. After the removal of midrib and domatia, both halves of the leaf lamina were immediately but separately flash-frozen in liquid nitrogen. One half of each leaf sample was stored at −80°C until further processing to extract RNA, while the other half was lyophilized for chemical analysis.
To analyze the impact of Azteca ants on the plant’s nutrient supply, a part of the lyophilized leaf samples from this sampling and from the herbivory experiments were used for C:N-ratio analysis.
15N pulse-chase experiment
Aqueous solutions of 20 mg of 98% 15N-Glycine (Eurisotope, Saint-Aubin, France) mixed with honey 1:4 (v/v) were prepared. Eppendorf tubes with the 15N-labeled glycine-honey mix were attached at the stem beneath the youngest pair of fully expanded leaves of each plant. As control, 5 cm2 leaf samples of the plants were collected immediately before the start of the experiment. Furthermore, one of the youngest leaves was wrapped into a perforated plastic bag to ensure that no ant could contact the leaf after feeding on the sugar enriched with [15N]glycine and thereby contaminate it. The honey mixtures were consumed by the ants within a few days. Samples of the protected leaves as well as the unprotected neighboring leaves were taken 30 days after the pulse. After 6 and 24 months, newly grown leaves were collected. Leaf samples were dried in the field using silica gel and later in an oven at 60°C for 3 days.
Isotope analysis
1 mg of homogenous leaf powder was weighted in a tin capsule. δ13C and δ15N isotope analyses were conducted on an elemental analyzer (NA1110, CE Instruments, Milan, Italy) coupled to a Delta+XL isotope ratio mass spectrometer (Thermo Finnigan, Bremen, Germany) via a ConFlow III. Sample element amounts were scaled against an in house standard “Ali-j3” (acetanilide) with δ13C and δ15N values of −30.06 ± 0.1‰ and −1.51 ± 0.1‰ on the δ13CVPDB-LSVEC and δ15NAIR-N2 scales, respectively. A caffeine “caf-j3” sample was analyzed multiple times per sequence as quality control with values of −40.46 ± 0.1‰ and −15.46 ± 0.1‰ on the δ13CVPDB-LSVEC and δ15NAIR-N2 scales, respectively. Linearity, blank and drift corrections were done for each sequence according to Werner and Brand, 2001(Werner and Brand, 2001).
Plant defense hormone analysis
Hormones were extracted from 20 mg of freeze-dried ground leaf powder with 0.5 mL of methanol (MeOH) containing the internal standards: 40 ng/mL D6-JA (HPC Standards GmbH, Germany), D6-ABA (Toronto Research Chemicals, Toronto, Canada), D4-SA (Santa Cruz Biotechnology, USA), and 8 ng/mL D6-JA-Ile (HPC Standards GmbH, Germany)]. The homogenate was mixed for 30 min and centrifuged at 16,000 g for 10 min. The filtrated supernatant was used for hormone analysis. Compound separation was achieved by liquid chromatography (Agilent 1260; Agilent Technologies, Waldbronn, Germany) on a Zorbax Eclipse XDB-C18 column (50 mm × 4.6 mm, 1.8 μm, Agilent Technologies). Column temperature was maintained at 20°C, the flow rate was constant at 1.1 mL/min. Both 0.05% formic acid in H2O (A) and 100% acetonitrile (B) were employed as mobile phases A and B. The elution profile was: 0–0.5 min, 5% B; 0.5–6.0 min, 5–37.4% B; 6.02–7.5 min, 80-100% B; 7.5–9.5 min, 100% B; 9.52–12 min, 5% B. An QTRAP 6500 tandem mass spectrometer (AB Sciex, Framingham, MA, USA) was operated in the negative ionization mode, using scheduled multiple reaction monitoring (MRM) to monitor analyte parent ion → product ion formation for detection of JA, JA-Ile, ABA, SA and the respective labeled standards see Heyer et al. (2018). For SO4-JA: m/z 305.0→97.0 (CE -55 V; DP -30 V). For OH-JA: m/z 225.1→59.0 (CE -24 V; DP -30 V). For OH-JA-Ile: m/z 338.1→130.1 (CE -30 V; DP -30 V). For COOH-JA-Ile: m/z 352.1→130.1 (CE -30 V; DP -30 V). Chromatograms were analyzed using the software Analyst 1.6.3 (Applied Biosystems, Bedford, MA, USA) with automated peak integration. The quantification was realized by comparing the sample peak areas with the peak area of the internal standards. The concentrations of OH-JA and SO4-JA were quantified relative to D6-JA applying a theoretical response factor of 1.0 and the determined response factor 6.0, respectively. The levels of 12-hydroxy-JA-Ile (OH-JA-Ile) and 12-carboxy-JA-Ile (COOH-JA-Ile) were determined relative to D6-JA-Ile applying a theoretical response factor of 1.0.
Free amino acid analysis
For the quantification of free amino acids, the raw extracts used for phytohormone analysis were diluted 1:10 with water containing an algal amino acid mix (10 μg mL−1 U-13C, U-15N isotopically labelled amino acid mix; Isotec, Miamisburg, OH, USA) as internal standard. The extracts were analyzed on an Agilent 1260 Infinity high-performance liquid chromatography system (Agilent Technologies) coupled to an API 6500 ESI-Triple Quad mass spectrometer (AB Sciex, Darmstadt, Germany). Separation was achieved on a Zorbax Eclipse XDB-C18 column (50 mm × 4.6 mm, 1.8 μm, Agilent Technologies). Both 0.05% formic acid in H2O and acetonitrile were employed as mobile phases A and B, respectively. The elution profile was 0–1 min 3% B; 1.0–2.7 min 3–100% B; 2.7–3.0 min 100% B; 3.1–6.0 min 3% B. Column temperature was maintained at 20°C, the flow rate was constant at 1.1 mL/min. The mass spectrometer was operated in the positive ionization mode with multiple reaction monitoring (MRM) as described by Jander et al. (2004). The Analyst 1.6.3 software (Applied Biosystems) was used for data acquisition and processing. Individual amino acids in the sample were quantified by the respective 13C, 15N-labelled amino acid internal standard, except for tryptophan that was quantified using 13C, 15N-Phe applying a response factor of 0.42.
Free sugar analysis
Soluble sugars were analyzed from the methanol extracts (at 1:10 dilution in water containing 5 μg/mL 13C6-glucose (Sigma-Aldrich), and 5 μg/mL 13C6-fructose (Toronto Research Chemicals, Toronto, Canada), by LC-MS/MS as described in (Madsen et al., 2015). The diluted extracts were analyzed on an Agilent 1200 Infinity high-performance liquid chromatography system (Agilent Technologies) coupled to an API 3200 ESI-Triple Quad mass spectrometer (AB Sciex). Separation was achieved on a HILIC column (apHera NH2 Polymer; Supelco, Bellefonte, PA, USA; 150 mm × 4.6 mm, 5 μm). H2O and acetonitrile were employed as mobile phases A and B, respectively. The elution profile was 0–0.5 min 80% B; 0.5–13 min 80–55% B; 13–14 min 80% B; 14–18 min 80% B. Column temperature was maintained at 20°C, the flow rate was constant at 1.0 mL/min. The mass spectrometer was operated in the negative ionization mode with multiple reaction monitoring (MRM) to monitor analyte parent ion → product ion: glucose (m/z 178.8→89.0), fructose (m/z 178.8→89.0), 13C6-glucose (m/z 185.0→92.0), 13C6-fructose (m/z 185.0→92.0), sucrose (m/z 340.9→59.0). The Analyst 1.6.3 software (Applied Biosystems) was used for data acquisition and processing. The concentrations of glucose and fructose were determined relative to the internal standards of 13C6-glucose and 13C6-fructose, respectively. The content of sucrose (Sigma-Aldrich) was calculated based on an external standard curve.
Full scan LC-MS analysis of ellagitannins
MS experiments were carried out using an Esquire 6000 electrospray iontrap mass spectrometer (ESI-MS; Bruker Daltonics, Bremen, Germany) after separation by Agilent 1100 HPLC (Agilent Technologies) using a Luna Phenyl-Hexyl 100 Å column (4.6 × 150 mm, 5 μm; Phenomenex, Aschaffenburg, Germany). The binary mobile phase consisted of 0.2% formic acid in H2O (A) and acetonitrile (B) at the flow rate of 1 mL min−1. The elution profile was: 0–20 min, 5–25% B; 20–20.1 min, 25–100% B; 20.1–23 min 100% B; 23–23.1 min 100–5% B; 23.1–28 min 5% B. The ESI-MS was operated in negative ionization mode, scanning m/z between 100 and 1400, and with an optimal target mass adjusted to m/z 550. The mass spectrometer was operated at the following specifications: capillary exit voltage, −132 eV; capillary voltage, −3,000V; nebulizer pressure, 35 psi; drying gas, 11 L min−1; gas temperature, 330°C. The instrument was further coupled to a diode array detector, which enabled measurement of the absorption spectra of the ellagitannins.
Untargeted metabolomics and HRMS
The methanolic extracts of T. quadrialata leaves were analyzed in an untargeted metabolomic approach as described by Lackus et al. (2020) with a randomized sample order. Ultra-high-performance liquid chromatography–electrospray ionization– high resolution mass spectrometry (UHPLC–ESI–HRMS) was performed with a Dionex Ultimate 3000 series UHPLC (Thermo Scientific) and a Bruker timsToF mass spectrometer (Bruker Daltonics, Bremen, Germany). UHPLC was used applying a reversed-phase Zorbax Eclipse XDB-C18 column (100 mm × 2.1 mm, 1.8 μm, Agilent Technologies, Waldbronn, Germany) with a solvent system of 0.1% formic acid (A) and acetonitrile (B) at a flow rate of 0.3 mL/min. The elution profile was the following: 0 to 0.5 min, 5% B; 0.5 to 11.0 min, 5% to 60% B in A; 11.0 to 11.1 min, 60% to 100% B, 11.1 to 12.0 min, 100% B and 12.1 to 15.0 min 5% B. Electrospray ionization (ESI) in negative/positive ionization mode was used for the coupling of LC to MS. The mass spectrometer parameters were set as follows: capillary voltage 4.5 KV/3.5KV, end plate offset of 500V, nebulizer pressure 2.8 bar, nitrogen at 280°C at a flow rate of 8L/min as drying gas. Acquisition was achieved at 12 Hz with a mass range from m/z 50 to 1500, with data-dependent MS/MS and an active exclusion window of 0.1 min, a reconsideration threshold of 1.8-fold change, and an exclusion after 5 spectra. Fragmentation was triggered on an absolute threshold of 50 counts and acquired on the two most intense peaks with MS/MS spectra acquisition of 12 Hz. Collision energy was alternated between 20 and 50V. At the beginning of each chromatographic analysis 10 μL of a sodium formate-isopropanol solution (10 mM solution of NaOH in 50/50 (v/v%) isopropanol water containing 0.2% formic acid) was injected into the dead volume of the sample injection for re-calibration of the mass spectrometer using the expected cluster ion m/z values. Peak detection was carried out using Metaboscape software (Bruker Daltonik, Bremen, Germany) with the T-Rex 3D algorithm for qTOF data. For peak detection the following parameters were used: intensity threshold of 300 with a minimum of 10 spectra, time window from 0.4 to 11.8 min, peaks were kept if they were detected in at least all replicates of one sample group. Adducts of [M+H]+, [M+Na]+, and [M+K]+ (for positive mode) or [M-H]-, [M+Cl]-, and [M+COOH]- (for negative mode) were grouped as a single bucket if they had an EIC correlation of 0.8. SciFinder (https://scifinder.cas.org) served to predict structures and identify unknown compounds.
RNA extraction and illumina sequencing
The half of the leaf samples that was stored at −80°C was ground in liquid nitrogen. RNA was isolated from 100 mg ground leaf powder utilizing the Spectrum™ Plant Total RNA Kit (50) (Sigma-Aldrich) following the manufacturer’s instructions with an on-column DNA digestion using DNaseI (ZymoResearch, Freiburg/Brsg., Germany). After a further purification step (OneStep PCR Inhibitor Removal Kit, ZymoResearch), RNA concentration, purity, and quality were assessed using a spectrophotometer (NanoDrop, 2000c; Thermo Scientific) and an Agilent 2100 bioanalyzer (Agilent Technologies).
The isolated RNA was sent to Novogene Europe, Cambridge, UK for analysis on an Illumina NovaSeq 6000 instrument (San Diego, CA, USA). There, 20 M paired end reads of 150 bp per sample were generated from the leaf transcriptome libraries of ant-colonized and ant-deprived plants that were subjected to herbivore or control treatment.
RNA-seq data analysis
Trimming of the sequences, quality control, and de novo assembly were performed using CLC Genomics Workbench (Qiagen Bioinformatics). A representative sample per treatment was selected, pooled, and reads randomly reduced by 50%. A total of 145 × 106 paired-end reads were used to generate the transcriptome library (word size 30, bubble size 1200). The consensus assembly contained 67,140 contigs with a N50 value of 1,304. BUSCO (Benchmarking Universal Single-Copy Orthologs) assessment (https://busco.ezlab.org/, last accessed on 04.02.2022 (Simao et al., 2015)), resulted in 66.6% complete BUSCOs and 14.1% missing BUSCOs when comparing our assembled transcripts to the Embryophyta lineage data set. Gene abundances were quantified for each individual sample using RSEM (version 1.2.22). To adjust nuisance technical effects, RNA-Seq data was normalized by R package RUV (remove unwanted variation (Risso et al., 2014)). EdgeR (Robinson et al., 2010) (https://bioconductor.org) was carried out to identify the differentially expressed genes (DEGs) with a cut off p-value ≤ 0.05 and FDR ≤0.05. All DEGs were annotated against Swiss-Prot and GO (Gene Ontology) using OmicsBox software (biobam, Valencia, Spain). GO enrichment analysis of DEGs was performed using R package clusterProfiler (Yu et al., 2012). Contigs potentially encoding for protease inhibitors were identified by GO terms associated with peptidase inhibitor activity (GO:0004866, GO:0004867, GO:0004869, GO:008191, GO:0019828, GO:0030414, GO:0010859) and a positive BLAST hit against a known plant protease inhibitor in the NCBI nr.
Quantification and statistical analysis
The statistical analyses were performed with R (RStudio_Team, 2021): defense hormone, amino acid and C:N data (Figures 1C (1 year), 3B, 3D, 3E, S3A, and S5A; Tables S1 and S3) after herbivore and JA treatments were tested with Two-Way ANOVA or fitted to a linear model (generalized least squares) as a function of treatment × colonization. Whenever possible, the respective model was reduced to the minimal model. All data were tested for statistical assumptions (normal distribution and homogeneity of variances) using diagnostic plots and were log-transformed, if necessary. For the amino acid analysis, outliers were removed if c < (Q1 - 1.5×IQR) or c > (Q3 + 1.5×IQR) with Q as quartile and IQR as interquartile range. Differences in growth (Figures 1B, S1B–S1E, and S2), nutritional value (Figures 1E and 1H) and nitrogen content (Figure 1C, 2.6 yrs) were evaluated by Student’s t-tests or Wilcoxon rank sum tests, whenever the data was not normally distributed. For the 15N-labeling data, one-tailed t-tests were performed, whereas a paired Wilcoxon signed rank test was used to analyze data from the choice assay. As the data from the volatile collections were not normally distributed, nonparametric Kruskal-Wallis tests were utilized to analyze this data (Table S2). Statistical details can be found in the figures (mean ± SE; significance (∗: p < 0.05; ∗∗: p < 0.01, ∗∗∗: p < 0.001)), figure legends and results (n, test, significance) and SI tables (n, test, F-value/L-ratio/χ2, p value). Data for volcano plots (Figure 2D) and heatmaps (Figures 3C and S5B) displaying untargeted metabolomics results were calculated with R using the package MetaboAnalyst(Pang et al., 2021) after preprocessing the data with MetaboScape (Bruker Daltonics). More precisely, the preprocessed data was normalized by weight, filtered by interquartile range and auto scaled. The results of the statistical analysis were visualized with OriginPro, Version 2019 (OriginLab Corporation, Northampton, MA, USA) or R.
Acknowledgments
We thank the Explorer’s Inn for accommodation, especially during the pandemic, Rudi Saul, Fabian Limonchi, and Eliana Esparza (INTE-PUCP) for help with the organization and logistics of the field work, Alfredo Ibañez (ICOBA-PUCP) for lab space and equipment, Daniel Veit and Saskia Gablenz (MPI-CE) for technical equipment, Abel Bernadou and Lina Pedraza (University of Regensburg) for the identification of the ant species, Grit Kunert for statistical advice, AIDER for assistance with the permits, SERNANP and SERFOR for the authorization of this research.
The research was funded by the Max Planck Society, and fellowships of the Friedrich Schiller University Jena as part of the IMPRS, and the German Academic Exchange Service (DAAD) to ATM.
Author contributions
AM, TGK, JG, EGC, and ATM designed the research. ATM, MR, EGC, AN, and NS performed the research. HM and HG carried out the elemental analysis. ATM, MR, and DW analyzed the data. ATM and AM wrote the article with input from all other authors.
Declaration of interests
The authors declare no competing interests.
Published: October 21, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.105261.
Supplemental information
Data and code availability
-
•
The RNA-Seq data generated in this study were deposited at NCBI SRA and are publicly available as of the date of publication. The accession number is listed in the key resources table.
-
•
The paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Agrawal A.A. Leaf damage and associated cues induced aggressive and recruitment in a neotropical ant-plant. Ecology. 1998;79:2100–2112. doi: 10.2307/176713. [DOI] [Google Scholar]
- Agrawal A.A., Dubin-Thaler B.J. Induced responses to herbivory in the Neotropical ant-plant association between Azteca ants and Cecropia trees: response of ants to potential inducing cues. Behav. Ecol. 1999;45:47–54. doi: 10.1007/s002650050538. [DOI] [Google Scholar]
- Ahmad S., Saleem Z., Jabeen F., Hussain B., Sultana T., Sultana S., Al-Ghanim K.A., Al-Mulhim N.M.A., Mahboob S. Potential of natural repellents methylanthranilate and anthraquinone applied on maize seeds and seedlings against house sparrow (Passer domesticus) in captivity. Braz. J. Biol. 2018;78:667–672. doi: 10.1590/1519-6984.171686. [DOI] [PubMed] [Google Scholar]
- Anstett D.N., Cheval I., D'Souza C., Salminen J.P., Johnson M.T.J. Ellagitannins from the Onagraceae decrease the performance of generalist and specialist herbivores. J. Chem. Ecol. 2019;45:86–94. doi: 10.1007/s10886-018-1038-x. [DOI] [PubMed] [Google Scholar]
- Araújo A.R., Araújo A.C., Reis R.L., Pires R.A. Vescalagin and castalagin present bactericidal activity toward methicillin-resistant bacteria. ACS Biomater. Sci. Eng. 2021;7:1022–1030. doi: 10.1021/acsbiomaterials.0c01698. [DOI] [PubMed] [Google Scholar]
- Barbehenn R.V., Jones C.P., Hagerman A.E., Karonen M., Salminen J.-P. Ellagitannins have greater oxidative activities than condensed tannins and Galloyl glucoses at high pH: potential impact on caterpillars. J. Chem. Ecol. 2006;32:2253–2267. doi: 10.1007/s10886-006-9143-7. [DOI] [PubMed] [Google Scholar]
- Bartimachi A., Neves J., Vasconcelos H.L. Geographic variation in the protective effects of ants and trichomes in a Neotropical ant–plant. Plant Ecol. 2015;216:1083–1090. doi: 10.1007/s11258-015-0491-7. [DOI] [Google Scholar]
- Boeckler G.A., Towns M., Unsicker S.B., Mellway R.D., Yip L., Hilke I., Gershenzon J., Constabel C.P. Transgenic upregulation of the condensed tannin pathway in poplar leads to a dramatic shift in leaf palatability for two tree-feeding Lepidoptera. J. Chem. Ecol. 2014;40:150–158. doi: 10.1007/s10886-014-0383-7. [DOI] [PubMed] [Google Scholar]
- Bruna E.M., Lapola D.M., Vasconcelos H.L. Interspecific variation in the defensive responses of obligate plant-ants: experimental tests and consequences for herbivory. Oecologia. 2004;138:558–565. doi: 10.1007/s00442-003-1455-5. [DOI] [PubMed] [Google Scholar]
- Cabrera M., Jaffe K. A trophic Mutualism between the myrmecophytic Melastomataceae Tococa guianensis and an Azteca ant species. Ecotropicos. 1994;7:1–10. [Google Scholar]
- Chamberlain S.A., Holland J.N. Quantitative synthesis of context dependency in ant-plant protection mutualisms. Ecology. 2009;90:2384–2392. doi: 10.1890/08-1490.1. [DOI] [PubMed] [Google Scholar]
- Chanam J., Sheshshayee M.S., Kasinathan S., Jagdeesh A., Joshi K.A., Borges R.M., Kay A. Nutritional benefits from domatia inhabitants in an ant-plant interaction: interlopers do pay the rent. Funct. Ecol. 2014;28:1107–1116. doi: 10.1111/1365-2435.12251. [DOI] [Google Scholar]
- Chomicki G., Renner S.S. Phylogenetics and molecular clocks reveal the repeated evolution of ant-plants after the late Miocene in Africa and the early Miocene in Australasia and the Neotropics. New Phytol. 2015;207:411–424. doi: 10.1111/nph.13271. [DOI] [PubMed] [Google Scholar]
- Christianini A.V., Machado G. Induced biotic responses to herbivory and associated cues in the Amazonian ant-plant Maieta poeppigii. Entomol. Exp. Appl. 2004;112:81–88. doi: 10.1111/j.0013-8703.2004.00188.x. [DOI] [Google Scholar]
- Clavijo McCormick A., Irmisch S., Reinecke A., Boeckler G.A., Veit D., Reichelt M., Hansson B.S., Gershenzon J., Köllner T.G., Unsicker S.B. Herbivore-induced volatile emission in black poplar: regulation and role in attracting herbivore enemies. Plant Cell Environ. 2014;37:1909–1923. doi: 10.1111/pce.12287. [DOI] [PubMed] [Google Scholar]
- Defossez E., Djiéto-Lordon C., McKey D., Selosse M.A., Blatrix R. Plant-ants feed their host plant, but above all a fungal symbiont to recycle nitrogen. Proc. Biol. Sci. 2011;278:1419–1426. doi: 10.1098/rspb.2010.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejean A., Delabie J.H.C., Cerdan P., Gibernau M., Corbara B. Are myrmecophytes always better protected against herbivores than other plants? Biol. J. Linn. Soc. Lond. 2006;89:91–98. doi: 10.1111/j.1095-8312.2006.00660.x. [DOI] [Google Scholar]
- Dejean A., Grangier J., Leroy C., Orivel J. Host plant protection by arboreal ants: looking for a pattern in locally induced responses. Evol. Ecol. Res. 2008;10:1217–1223. [Google Scholar]
- Dejean A., Petitclerc F., Compin A., Azémar F., Corbara B., Delabie J.H.C., Leroy C. Hollow internodes permit a neotropical understory plant to shelter multiple mutualistic ant species, obtaining protection and nutrient provisioning (myrmecotrophy) Am. Nat. 2017;190:E124–E131. doi: 10.1086/693782. [DOI] [PubMed] [Google Scholar]
- Dejean A., Petitclerc F., Roux O., Orivel J., Leroy C. Does exogenic food benefit both partners in an ant-plant mutualism? The case of Cecropia obtusa and its guest Azteca plant-ants. C. R. Biol. 2012;335:214–219. doi: 10.1016/j.crvi.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Del Val E., Dirzo R. Does ontogeny cause changes in the defensive strategies of the myrmecophyte Cecropia peltata? Plant Ecol. 2003;169:35–41. doi: 10.1023/A:1026227811685. [DOI] [Google Scholar]
- Do Nascimento R.R., Billen J., Sant'ana A.E.G., Morgan E.D., Harada A.Y. Pygidial gland of Azteca nr. bicolor and Azteca chartifex: morphology and chemical identification of volatile components. J. Chem. Ecol. 1998;24:1629–1637. doi: 10.1023/a:1020864427854. [DOI] [Google Scholar]
- Dodson C.D., Dyer L.A., Searcy J., Wrigh Z., Letourneau D.K. Cenocladamide, a dihydropyridone alkaloid from Piper cenocladum. Phytochemistry. 2000;53:51–54. doi: 10.1016/S0031-9422(99)00446-X. [DOI] [PubMed] [Google Scholar]
- dos Santos A.P.M., Fracasso C.M., Luciene dos Santos M., Romero R., Sazima M., Oliveira P.E. Reproductive biology and species geographical distribution in the Melastomataceae: a survey based on New World taxa. Ann. Bot. 2012;110:667–679. doi: 10.1093/aob/mcs125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer L.A., Dodson C.D., Beihoffer J., Letourneau D.K. Trade-offs in antiherbivore defenses in Piper cenocladum: ant mutualists versus plant secondary metabolites. J. Chem. Ecol. 2001;27:581–592. doi: 10.1023/A:1010345123670. [DOI] [PubMed] [Google Scholar]
- Dyer L.A., Letourneau D.K., Dodson C.D., Tobler M.A., Stireman J.O., III, Hsu A. Ecological causes and consequences of variation in defensive chemistry of a neotropical shrub. Ecology. 2004;85:2795–2803. doi: 10.1890/03-0233. [DOI] [Google Scholar]
- Eberl F., Fabisch T., Luck K., Köllner T.G., Vogel H., Gershenzon J., Unsicker S.B. Poplar protease inhibitor expression differs in an herbivore specific manner. BMC Plant Biol. 2021;21:170. doi: 10.1186/s12870-021-02936-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb M., Veyrat N., Robert C.A.M., Xu H., Frey M., Ton J., Turlings T.C.J. Indole is an essential herbivore-induced volatile priming signal in maize. Nat. Commun. 2015;6:6273. doi: 10.1038/ncomms7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer E.E., Johnson R.R., Ryan C.A. Regulation of expression of proteinase inhibitor genes by methyl jasmonate and jasmonic Acid. Plant Physiol. 1992;98:995–1002. doi: 10.1104/pp.98.3.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala B., Maschwitz U., Pong T.Y., Helbig A.J. Studies of a south East Asian ant-plant association: protection of Macaranga trees by Crematogaster borneensis. Oecologia. 1989;79:463–470. doi: 10.1007/BF00378662. [DOI] [PubMed] [Google Scholar]
- Fincher R.M., Dyer L.A., Dodson C.D., Richards J.L., Tobler M.A., Searcy J., Mather J.E., Reid A.J., Rolig J.S., Pidcock W. Inter- and intraspecific comparisons of antiherbivore defenses in three species of rainforest understory shrubs. J. Chem. Ecol. 2008;34:558–574. doi: 10.1007/s10886-008-9432-4. [DOI] [PubMed] [Google Scholar]
- Fonseca C.R. Herbivory and the long-lived leaves of an Amazonian ant-tree. J. Ecol. 1994;82:833–842. doi: 10.2307/2261447. [DOI] [Google Scholar]
- Fracassetti D., Costa C., Moulay L., Tomás-Barberán F.A. Ellagic acid derivatives, ellagitannins, proanthocyanidins and other phenolics, vitamin C and antioxidant capacity of two powder products from camu-camu fruit (Myrciaria dubia) Food Chem. 2013;139:578–588. doi: 10.1016/j.foodchem.2013.01.121. [DOI] [PubMed] [Google Scholar]
- Frederickson M.E., Ravenscraft A., Arcila Hernández L.M., Booth G., Astudillo V., Miller G.A., Kitzberger T. What happens when ants fail at plant defence? Cordia nodosa dynamically adjusts its investment in both direct and indirect resistance traits in response to herbivore damage. J. Ecol. 2013;101:400–409. doi: 10.1111/1365-2745.12034. [DOI] [Google Scholar]
- Frederickson M.E., Ravenscraft A., Miller G.A., Arcila Hernández L.M., Booth G., Pierce N.E. The direct and ecological costs of an ant-plant symbiosis. Am. Nat. 2012;179:768–778. doi: 10.1086/665654. [DOI] [PubMed] [Google Scholar]
- Gatehouse J.A. Plant resistance towards insect herbivores: a dynamic interaction. New Phytol. 2002;156:145–169. doi: 10.1046/j.1469-8137.2002.00519.x. [DOI] [PubMed] [Google Scholar]
- Gatis-Carrazzoni A.S.S.G., Mota F.V.B., Leite T.C.C., de Oliveira T.B., da Silva S.C., Bastos I.V.A., de Souza Maia M.B., Pereira P.S., Neto P.P.M., de Oliveira Chagas E.C., et al. Anti-inflammatory and antinociceptive activities of the leaf methanol extract of Miconia minutiflora (Bonpl.) DC. and characterization of compounds by UPLC-DAD-QTOF-MS/MS. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019;392:55–68. doi: 10.1007/s00210-018-1561-x. [DOI] [PubMed] [Google Scholar]
- Gay H. Animal-fed plants: an investigation into the uptake of ant-derived nutrients by the far-eastern epiphytic fern Lecanopteris Reinw. (Polypodiaceae) Biol. J. Linn. Soc. Lond. 1993;50:221–233. doi: 10.1111/j.1095-8312.1993.tb00928.x. [DOI] [Google Scholar]
- Gontijo D.C., Gontijo P.C., Brandão G.C., Diaz M.A.N., de Oliveira A.B., Fietto L.G., Leite J.P.V. Antioxidant study indicative of antibacterial and antimutagenic activities of an ellagitannin-rich aqueous extract from the leaves of Miconia latecrenatal. J. Ethnopharmacol. 2019;236:114–123. doi: 10.1016/j.jep.2019.03.007. [DOI] [PubMed] [Google Scholar]
- González-Teuber M., Kaltenpoth M., Boland W. Mutualistic ants as an indirect defence against leaf pathogens. New Phytol. 2014;202:640–650. doi: 10.1111/nph.12664. [DOI] [PubMed] [Google Scholar]
- Habib H., Fazili K.M. Plant protease inhibitors: a defense strategy in plants. Biotechnol. Mol. Biol. Rev. 2007;2:68–85. [Google Scholar]
- Heil M., Delsinne T., Hilpert A., Schürkens S., Andary C., Linsenmair K.E., Sousa S M., McKey D. Reduced chemical defence in ant-plants? A critical re-evaluation of a widely accepted hypothesis. Oikos. 2002;99:457–468. doi: 10.1034/j.1600-0706.2002.11954.x. [DOI] [Google Scholar]
- Heil M., Fiala B., Linsenmair K.E., Boller T. Reduced chitinase activities in ant plants of the genus Macaranga. Naturwissenschaften. 1999;86:146–149. doi: 10.1007/s001140050589. [DOI] [Google Scholar]
- Heil M., Fiala B., Linsenmair K.E., Zotz G., Menke P. Food body production in Macaranga triloba (Euphorbiaceae): a plant investment in anti-herbivore defence via symbiotic ant partners. J. Ecol. 1997;85:847–861. doi: 10.2307/2960606. [DOI] [Google Scholar]
- Heil M., Fiala B., Maschwitz U., Linsenmair K.E. On benefits of indirect defence: short- and long-term studies of antiherbivore protection via mutualistic ants. Oecologia. 2001;126:395–403. doi: 10.1007/s004420000532. [DOI] [PubMed] [Google Scholar]
- Heil M., Greiner S., Meimberg H., Krüger R., Noyer J.-L., Heubl G., Linsenmair K.E., Boland W. Evolutionary change from induced to constitutive expression of an indirect plant resistance. Nature. 2004;430:205–208. doi: 10.1038/nature02703. [DOI] [PubMed] [Google Scholar]
- Heil M., Koch T., Hilpert A., Fiala B., Boland W., Linsenmair K. Extrafloral nectar production of the ant-associated plant, Macaranga tanarius, is an induced, indirect, defensive response elicited by jasmonic acid. Proc. Natl. Acad. Sci. USA. 2001;98:1083–1088. doi: 10.1073/pnas.031563398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil M., McKey D. Protective ant-plant interactions as model systems in ecological and evolutionary research. Annu. Rev. Ecol. Evol. Syst. 2003;34:425–553. doi: 10.1146/annurev.ecolsys.34.011802.132410. [DOI] [Google Scholar]
- Heil M., Staehelin C., McKey D. Low chitinase activity in Acacia myrmecophytes: a potential trade-off between biotic and chemical defences? Naturwissenschaften. 2000;87:555–558. doi: 10.1007/s001140050778. [DOI] [PubMed] [Google Scholar]
- Hernández-Zepeda O.F., Razo-Belman R., Heil M. Reduced responsiveness to volatile signals creates a modular reward provisioning in an obligate food-for-protection mutualism. Front. Plant Sci. 2018;9:1076. doi: 10.3389/fpls.2018.01076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer M., Reichelt M., Mithöfer A. A holistic approach to analyze systemic jasmonate accumulation in individual leaves of Arabidopsis rosettes upon wounding. Front. Plant Sci. 2018;9:1569. doi: 10.3389/fpls.2018.01569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irmisch S., Clavijo McCormick A., Günther J., Schmidt A., Boeckler G.A., Gershenzon J., Unsicker S.B., Köllner T.G. Herbivore-induced poplar cytochrome P450 enzymes of the CYP71 family convert aldoximes to nitriles which repel a generalist caterpillar. Plant J. 2014;80:1095–1107. doi: 10.1111/tpj.12711. [DOI] [PubMed] [Google Scholar]
- Jander G., Norris S.R., Joshi V., Fraga M., Rugg A., Yu S., Li L., Last R.L. Application of a high-throughput HPLC-MS/MS assay to Arabidopsis mutant screening; evidence that threonine aldolase plays a role in seed nutritional quality. Plant J. 2004;39:465–475. doi: 10.1111/j.1365-313X.2004.02140.x. [DOI] [PubMed] [Google Scholar]
- Janzen D.H. Coevolution of mutualism between ants and acacias in centra America. Evolution. 1966;20:249–275. doi: 10.2307/2406628. [DOI] [PubMed] [Google Scholar]
- Joo Y., Schuman M.C., Goldberg J.K., Kim S., Yon F., Brütting C., Baldwin I.T. Herbivore-induced volatile blends with both “fast” and “slow” components provide robust indirect defence in nature. Funct. Ecol. 2018;32:136–149. doi: 10.1111/1365-2435.12947. [DOI] [Google Scholar]
- Karban R., Agrawal A.A., Mangel M. The benefits of induced defenses against herbivores. Ecology. 1997;78:1351–1355. doi: 10.1890/0012-9658(1997)078[1351:TBOIDA]2.0.CO;2. [DOI] [Google Scholar]
- Kesharwani A., Polachira S.K., Nair R., Agarwal A., Mishra N.N., Gupta S.K. Anti-HSV-2 activity of Terminalia chebula Retz extract and its constituents, chebulagic and chebulinic acids. BMC Compl. Alternative Med. 2017;17:110. doi: 10.1186/s12906-017-1620-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigathi R.N., Unsicker S.B., Reichelt M., Kesselmeier J., Gershenzon J., Weisser W.W. Emission of volatile organic compounds after herbivory from Trifolium pratense (L.) under laboratory and field conditions. J. Chem. Ecol. 2009;35:1335–1348. doi: 10.1007/s10886-009-9716-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto K., Matsui K., Ozawa R., Takabayashi J. Volatile 1-octen-3-ol induces a defensive response in Arabidopsis thaliana. J. Gen. Plant Pathol. 2007;73:35–37. doi: 10.1007/s10327-006-0314-8. [DOI] [Google Scholar]
- Klewicka E., Sójka M., Ścieszka S., Klewicki R., Milczarek A., Lipińska L., Kołodziejczyk K. The antimycotic effect of ellagitannins from raspberry (Rubus idaeus L.) on Alternaria alternata ŁOCK 0409. Eur. Food Res. Technol. 2020;246:1341–1349. doi: 10.1007/s00217-020-03493-0. [DOI] [Google Scholar]
- Koiwa H., Bressan R.A., Hasegawa P.M. Regulation of protease inhibitors and plant defense. Trends Plant Sci. 1997;2:379–384. doi: 10.1016/S1360-1385(97)90052-2. [DOI] [Google Scholar]
- Koricheva J., Romero G.Q. You get what you pay for: reward-specific trade-offs among direct and ant-mediated defences in plants. Biol. Lett. 2012;8:628–630. doi: 10.1098/rsbl.2012.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackus N.D., Müller A., Kröber T.D.U., Reichelt M., Schmidt A., Nakamura Y., Paetz C., Luck K., Lindroth R.L., Constabel C.P., et al. The occurrence of sulfated salicinoids in poplar and their formation by Sulfotransferase1. Plant Physiol. 2020;183:137–151. doi: 10.1104/pp.19.01447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneau D.K. Ants, stem-borers, and fungal pathogens: experimental tests of a fitness Advantage in piper ant-plants. Ecology. 1998;79:593–603. doi: 10.2307/176956. [DOI] [Google Scholar]
- Letourneau D.K., Barbosa P. Ants, stem borers, and pubescence in Endospermum in Papua New Guinea. Biotropica. 1999;31:295–302. doi: 10.1111/j.1744-7429.1999.tb00141.x. [DOI] [Google Scholar]
- Maag D., Erb M., Köllner T.G., Gershenzon J. Defensive weapons and defense signals in plants: some metabolites serve both roles. Bioessays. 2015;37:167–174. doi: 10.1002/bies.201400124. [DOI] [PubMed] [Google Scholar]
- Madsen S.R., Kunert G., Reichelt M., Gershenzon J., Halkier B.A. Feeding on leaves of the glucosinolate transporter mutant gtr1gtr2 reduces fitness of Myzus persicae. J. Chem. Ecol. 2015;41:975–984. doi: 10.1007/s10886-015-0641-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei M.E., Mithöfer A., Boland W. Before gene expression: early events in plant–insect interaction. Trends Plant Sci. 2007;12:310–316. doi: 10.1016/j.tplants.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Mayer V.E., Frederickson M.E., McKey D., Blatrix R. Current issues in the evolutionary ecology of ant-plant symbioses. New Phytol. 2014;202:749–764. doi: 10.1111/nph.12690. [DOI] [PubMed] [Google Scholar]
- Mayer V.E., Nepel M., Blatrix R., Oberhauser F.B., Fiedler K., Schönenberger J., Voglmayr H. Transmission of fungal partners to incipient Cecropia-tree ant colonies. PLoS One. 2018;13:e0192207. doi: 10.1371/journal.pone.0192207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick A.C., Boeckler G.A., Köllner T.G., Gershenzon J., Unsicker S.B. The timing of herbivore-induced volatile emission in black poplar (Populus nigra) and the influence of herbivore age and identity affect the value of individual volatiles as cues for herbivore enemies. BMC Plant Biol. 2014;14:304. doi: 10.1186/s12870-014-0304-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKey D. In: Herbivores: their interaction with secondary plant metabolites. Rosenthal G.A., Janzen D.H., editors. Academic Press; 1979. The distribution of plant secondary compounds within plants; pp. 55–133. [Google Scholar]
- McNett K., Longino J., Barriga P., Vargas O., Phillips K., Sagers C.L. Stable isotope investigation of a cryptic ant-plant association: myrmelachista flavocotea (Hymenoptera, Formicidae) and Ocotea spp. (Lauraceae) Insectes Soc. 2009;57:67–72. doi: 10.1007/s00040-009-0051-z. [DOI] [Google Scholar]
- Meents A.K., Chen S.P., Reichelt M., Lu H.H., Bartram S., Yeh K.W., Mithöfer A. Volatile DMNT systemically induces jasmonate-independent direct anti-herbivore defense in leaves of sweet potato (Ipomoea batatas) plants. Sci. Rep. 2019;9:17431. doi: 10.1038/s41598-019-53946-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelangeli F.A. Ant protection against herbivory in three species of Tococa (Melastomataceae) occupying different environments. Biotropica. 2003;35:181–188. doi: 10.1111/j.1744-7429.2003.tb00277.x. [DOI] [Google Scholar]
- Michelangeli F.A. Tococa (Melastomataceae) Flora Neotrop. 2005;98:1–114. doi: 10.2307/4393950. [DOI] [Google Scholar]
- Mithöfer A., Boland W. Plant defense against herbivores: chemical aspects. Annu. Rev. Plant Biol. 2012;63:431–450. doi: 10.1146/annurev-arplant-042110-103854. [DOI] [PubMed] [Google Scholar]
- Moilanen J., Sinkkonen J., Salminen J.-P. Characterization of bioactive plant ellagitannins by chromatographic, spectroscopic and mass spectrometric methods. Chemoecology. 2013;23:165–179. doi: 10.1007/s00049-013-0132-3. [DOI] [Google Scholar]
- Moraes S.C., Vasconcelos H.L. Long-term persistence of a Neotropical ant-plant population in the absence of obligate plant-ants. Ecology. 2009;90:2375–2383. doi: 10.1890/08-1274.1. [DOI] [PubMed] [Google Scholar]
- Morawetz W., Henzl M., Wallnöfer B. Tree killing by herbicide producing ants for the establishment of pure Tococa occidentalis populations in the Peruvian Amazon. Biodivers. Conserv. 1992;1:19–33. doi: 10.1007/BF00700248. [DOI] [Google Scholar]
- Mumm R., Dicke M. Variation in natural plant products and the attraction of bodyguards involved in indirect plant defense. Can. J. Zool. 2010;88:628–667. doi: 10.1139/z10-032. [DOI] [Google Scholar]
- North R.D., Howse P.E., Jackson C.W. Agonistic behavior of the leaf-cutting ant Atta sexdens rubropilosa elicited by caryophyllene. J. Insect Behav. 2000;13:1–13. doi: 10.1023/A:1007749723868. [DOI] [Google Scholar]
- O'Dowd D.J. Pearl bodies of a neotropical tree, Ochroma pyramidale: ecological implications. Am. J. Bot. 1980;67:543–549. doi: 10.2307/2442294. [DOI] [Google Scholar]
- Ohmura W., Hishiyama S., Nakashima T., Kato A., Makihara H., Ohira T., Irei H. Chemical composition of the defensive secretion of the longhorned beetle, Chloridolum loochooanum. J. Chem. Ecol. 2009;35:250–255. doi: 10.1007/s10886-009-9591-y. [DOI] [PubMed] [Google Scholar]
- Pang Z., Chong J., Zhou G., de Lima Morais D.A., Chang L., Barrette M., Gauthier C., Jacques P.É., Li S., Xia J. MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021;49:W388–W396. doi: 10.1093/nar/gkab382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner S.S., Ricklefs R.E. Herbicidal activity of domatia-inhabiting ants in patches of Tococa guianensis and Clidemia heterophylla. Biotropica. 1998;30:324–327. doi: 10.1111/j.1744-7429.1998.tb00067.x. [DOI] [Google Scholar]
- Rhoades D.F. In: Herbivores: their interaction with secondary metabolites. Rosenthal G.A., Janzen D.H., editors. Academic Press; 1979. Evolution of plant defense against herbivores; pp. 1–55. [Google Scholar]
- Risso D., Ngai J., Speed T.P., Dudoit S. Normalization of RNA-seq data using factor analysis of control genes or samples. Nat. Biotechnol. 2014;32:896–902. doi: 10.1038/nbt.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M.D., McCarthy D.J., Smyth G.K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roslin T., Salminen J.P. Specialization pays off: contrasting effects of two types of tannins on oak specialist and generalist moth species. Oikos. 2008;117:1560–1568. doi: 10.1111/j.2008.0030-1299.16725.x. [DOI] [Google Scholar]
- Rosumek F.B., Silveira F.A.O., de S Neves F., de U Barbosa N.P., Diniz L., Oki Y., Pezzini F., Fernandes G.W., Cornelissen T. Ants on plants: a meta-analysis of the role of ants as plant biotic defenses. Oecologia. 2009;160:537–549. doi: 10.1007/s00442-009-1309-x. [DOI] [PubMed] [Google Scholar]
- RStudio_Team . RStudio PBC; 2021. RStudio: Integrated Development Environment for R.http://www.rstudio.com/ [Google Scholar]
- Sagers C.L., Ginger S.M., Evans R.D. Carbon and nitrogen isotopes trace nutrient exchange in an ant-plant mutualism. Oecologia. 2000;123:582–586. doi: 10.1007/PL00008863. [DOI] [PubMed] [Google Scholar]
- Salminen J.P. In: Recent Advances in Polyphenol Research. Romani A., Lattanzio V., Quideau S., editors. Wiley-Blackwell; 2014. The chemistry and chemical ecology of ellagitannins in plant–insect interactions: from underestimated molecules to bioactive plant constituents; pp. 83–113. [Google Scholar]
- Schatz B., Djieto-Lordon C., Dormont L., Bessière J.M., McKey D., Blatrix R. A simple non-specific chemical signal mediates defence behaviour in a specialised ant-plant mutualism. Curr. Biol. 2009;19:R361–R362. doi: 10.1016/j.cub.2009.03.026. [DOI] [PubMed] [Google Scholar]
- Schaub A., Blande J.D., Graus M., Oksanen E., Holopainen J.K., Hansel A. Real-time monitoring of herbivore induced volatile emissions in the field. Physiol. Plant. 2010;138:123–133. doi: 10.1111/j.1399-3054.2009.01322.x. [DOI] [PubMed] [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp E.W. Azteca protection of Cecropia: ant occupation benefits juvenile trees. Oecologia. 1986;70:379–385. doi: 10.1007/BF00379500. [DOI] [PubMed] [Google Scholar]
- Seigler D.S., Ebinger J.E. Cyanogenic glycosides in ant-acacias of Mexico and Central America. SW. Nat. 1987;32:499–503. doi: 10.2307/3671484. [DOI] [Google Scholar]
- Serna D.M.O., Martínez J.H.I. Phenolics and polyphenolics from Melastomataceae species. Molecules. 2015;20:17818–17847. doi: 10.3390/molecules201017818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simão F.A., Waterhouse R.M., Ioannidis P., Kriventseva E.V., Zdobnov E.M. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31:3210–3212. doi: 10.1093/bioinformatics/btv351. [DOI] [PubMed] [Google Scholar]
- Solano P.J., Dejean A. Ant-fed plants: comparison between three geophytic myrmecophytes. Biol. J. Linn. Soc. Lond. 2004;83:433–439. doi: 10.1111/j.1095-8312.2004.00381.x. [DOI] [Google Scholar]
- Stanton M.L., Palmer T.M. The high cost of mutualism: effects of four species of East African ant symbionts on their myrmecophyte host tree. Ecology. 2011;92:1073–1082. doi: 10.1890/i0012-9658-92-5-1073. [DOI] [PubMed] [Google Scholar]
- Svoma E., Morawetz W. Drüsenhaare, Emergenzen und Blattdomatien bei der Ameisenpflanze Tococa occidentalis (Melastomataceae) Bot. Jahrb. Syst. 1992;114:185–200. [Google Scholar]
- Turlings T.C.J., Wäckers F. In: Advances in Insect Chemical Ecology. Millar J.G., Cardé R.T., editors. Cambridge University Press; 2004. Recruitment of predators and parasitoids by herbivore-injured plants; pp. 21–75. [DOI] [Google Scholar]
- Turner I.M. Foliar defences and habitat Adversity of three Woody plant communities in Singapore. Funct. Ecol. 1995;9:279–284. doi: 10.2307/2390574. [DOI] [Google Scholar]
- Vasconcelos H.L., Davidson D.W. Relationship between plant size and ant associates in two Amazonian ant-plants. Biotropica. 2000;32:100–111. doi: 10.1111/j.1744-7429.2000.tb00452.x. [DOI] [Google Scholar]
- Ward D., Young T.P. Effects of large mammalian herbivores and ant symbionts on condensed tannins of Acacia drepanolobium in Kenya. J. Chem. Ecol. 2002;28:921–937. doi: 10.1023/A:1015249431942. [DOI] [PubMed] [Google Scholar]
- Wasternack C., Hause B. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review. Ann. Bot. 2013;111:1021–1058. doi: 10.1093/aob/mct067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner R.A., Brand W.A. Referencing strategies and techniques in stable isotope ratio analysis. Rapid Commun. Mass Spectrom. 2001;15:501–519. doi: 10.1002/rcm.258. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Ohbayashi H., Ishihara K., Ohwashi W., Haba K., Okano Y., Shingu T., Okuda T. Tannins and related polyphenols of melastomataceous plants. I. Hydrolyzable tannins from Tibouchina semidecandra COGN. Chem. Pharm. Bull. 1991;39:2233–2240. doi: 10.1248/cpb.39.2233. [DOI] [PubMed] [Google Scholar]
- Yu G., Wang L.-G., Han Y., He Q.-Y. clusterProfiler: an R Package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The RNA-Seq data generated in this study were deposited at NCBI SRA and are publicly available as of the date of publication. The accession number is listed in the key resources table.
-
•
The paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.