Abstract
Medicine food homology (MFH) substances not only provide essential nutrients as food but also have corresponding factors that can prevent and help treat nutritional imbalances, chronic disease, and other related issues. Endophytic fungi associated with plants have potential for use in drug discovery and food therapy. However, the endophytic fungal metabolites from MFH plants and their effects have been overlooked. Therefore, this review focuses on the various biological activities of 108 new metabolites isolated from 53 MFH-derived endophytic fungi. The paper explores the potential nutritional and medicinal value of metabolites of MFH-derived endophytic fungi for food and medical applications. This research is important for the future development of effective, safe, and nontoxic therapeutic nutraceuticals for the prevention and treatment of human diseases.
Keywords: Bioactivities, Endophytic fungi, Medicine food homology, Metabolites
Graphical abstract
Highlights
-
•
The taxonomy of metabolites produced by endophytic fungi isolated from MFH plants.
-
•
The summary of the activities of these metabolites.
-
•
The discovery of potential nutritional and medicinal value of these metabolites for food and medical applications.
-
•
The ideas for further development and utilization of these metabolites for the prevention and treatment of human diseases.
1. Introduction
In the face of a global epidemic of diet-related chronic disease, there is increased experimentation with the concept of “food as medicine” interventions to prevent, manage, and treat illness (Downer et al., 2020). Several studies have identified the potential for nutritional interventions in the prevention, management, treatment, and even in some cases reversal of disease (Feng et al., 2015; Lean et al., 2019). Medicine food homology (MFH) is traditionally defined as substances that have both nutritional and medicinal qualities. Since ancient times, Chinese medicine has been based on the theory that medicine and food are of the same origin, and its origin can be traced back to periods in which both disease and hunger were persistent threats (Liu, C., 2018). MFH substances not only provide essential nutrients but also have corresponding functional factors that can aid in the prevention and treatment of nutritional imbalances, chronic diseases, and other issues. Many crude traditional Chinese medicines (TCM) adhere to the principle of MFH (Chau and Wu, 2006). Because MFH plants occur naturally, show positive effects, and may be associated with fewer adverse reactions, they have become the main components of functional foods and are drawing increasing attention (Zhang, T. T. et al., 2013; Zhao, J. et al., 2011, 2016). MFH plants have become a hotspot for modern food and drug development, and their efficacy may be closely related to endophytic fungi that develop symbiotic relationships with plants.
Endophytes are naturally occurring microbes that inhabit plants but do not cause apparent symptoms in them (Chen et al., 2022). The distribution and community structure of endophytic fungi are influenced by factors such as genetic background, age, and host environmental conditions (Higgins et al., 2014; Oono et al., 2014; Pirttilä et al., 2005). Endophytic fungi also have profound effects on their host plants by promoting their growth, increasing their adaptability, and enhancing their tolerance to abiotic and biotic stresses (Kusari et al., 2012; Mendes et al., 2013; Rho et al., 2018; Sampangi-Ramaiah et al., 2020; Shweta et al., 2010; Waller et al., 2005). Some endophytic fungi have evolved special symbiotic relationships with their host plants and can significantly influence metabolite formation in plants, which in turn affects the quality and quantity of active pharmaceutical ingredients (APIs). They are advantageous sources of novel metabolites, agriculturally important promoters, and stress-resisters. Since 1993, when Stierle first isolated an endophytic fungus from the phloem of Taxus brevifolia capable of producing the novel anticancer substance paclitaxel (Stierle and Strobel G, 1993), Taxomyces andreanae, a growing number of studies have explored endophytic fungi from host plants, uncovering a rich body of metabolites with a variety of biological activities (antioxidant, antimicrobial, anti-inflammatory, and anticancer) (Caicedo et al., 2019; Fu et al., 2019; Nuankeaw et al., 2020; Pan et al., 2019; Xu et al., 2020; Yehia et al., 2020). Endophytic fungal metabolites and their activities have become exciting research topics. Most of the research in this field has focused on medicinal plants (Dalzell, 2020; Gómez et al., 2018; Kamel et al., 2019; Selim et al., 2018; Teimoori-boghsani et al., 2020).
However, the endophytic fungal metabolites from most MFH plants and their effects are neglected. The reported MFH plants have various functions, playing purported roles in anti-aging (Feng et al., 2019; Xing, Y. et al., 2022), antifatigue (Lu et al., 2021; Zhu et al., 2021), enhanced immunity (Diling et al., 2017; Gong et al., 2019), antitumor (Mu et al., 2021; Tang et al., 2021), enhanced memory (Li, T. et al., 2020; Wang, Y. et al., 2022), and reduction of blood fat levels (Gonga et al., 2020; Song, D. xing and Jiang, 2017). If the active effects of such metabolites can be tapped, they could be used for the development of novel therapeutic health products. Therefore, this review summarizes metabolites with important biological activities produced by endophytic fungi isolated from MFH plants to explore their potential nutritional and medicinal value.
2. Endophytic fungi of MFH plants and their metabolites
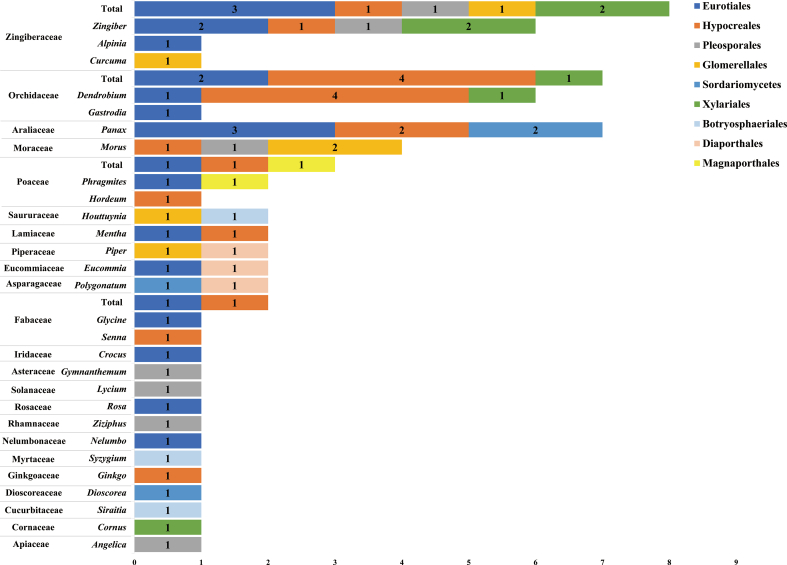
At the time of writing, there was a total of 111 MFH substances described by the Chinese National Health Commission (Ling et al., 2017). Only 53 of them (47.75%), which are sourced from 35 plant species, have been studied for endophytic fungi (Table 1 and Fig. 1). The 35 plant species belong to 23 families, including Zingiberaceae, Orchidaceae, Araliaceae, Moraceae, Poaceae, Saururaceae, Lamiaceae, Piperaceae, Eucommiaceae, Asteraceae, Fabaceae, Iridaceae, Asparagaceae, Solanaceae, Rosaceae, Rhamnaceae, Nelumbonaceae, Myrtaceae, Ginkgoaceae, Dioscoreaceae, Cucurbitaceae, Cornaceae, and Apiaceae (Fig. 1 and S1). Among these families, the number of species associated with Zingiberaceae and Orchidaceae are higher than those of other families and both accounted for 28.30% of all species (Fig. 1 and S1). The Zingiberaceae family includes about 52 genera and more than 1300 species that are dispersed throughout tropical Africa, Asia, and the Americas. This family includes a diversity of aromatic perennial herbs with creeping horizontal or tuberous rhizomes, and many species are economically important as ornamental plants, spices, or for use as a folk medicine. It also includes vital groups of medicinal plants with volatile essential oils and oleoresins of export quality (Chakraborty et al., 2019). The secondary metabolites extracted from different genera of Zingiberaceae, such as Curcuma, Kaempferia, Hedychium, Amomum, Zingiber, Alpinia, and Elettaria, have antimicrobial, antiarthritic, antioxidant, anticancer, anti-inflammatory, and antidiabetic properties (Chakraborty et al., 2019). Endophytic fungi belonging to five fungal orders, including Eurotiales, Hypocreales, Pleosporales, Xylariales, and Glomerellales, have been isolated from three genera (Zingiber, Alpinia, and Curcuma) of the Zingiberaceae (Fig. 1 and S1). Various types of metabolites are produced by these endophytes including alkaloids, terpenoids, esters, phenols, and fungal extracts, and they have antimicrobial, antioxidant, and anticancer properties (Figs. S2 and S3). This indicates that Zingiberaceae species have great potential to possess abundant endophytic fungi with diverse bioactivities. Therefore, Zingiberaceae-associated endophytic fungi and their propertied should be explored further in the future.
Table 1.
Summary of studies on the metabolites of MFH plants-derived endophytic fungi and their activities.
| Endophyte | Host Plant | Pathogen/Cancer Cell (Inhibition rate or IC50 value) | Metabolites | Taxonomy of metabolites | Activity | References |
|---|---|---|---|---|---|---|
| Chaetomium globosum | Dioscorea Opposita | HL-60, A-549, SMMC7721, MCF7, SW480 (51–96%) | Yamchaetoglobosin A (1) | Alkaloids | Anti-cancer | Ruan et al. (2017) |
| Trichoderma harzianum | Zingiber officinale | Mycobacterium tuberculosis (25 mg/mL) | Pretrichodermamide A (2) | Alkaloids | Anti-microbial | Harwoko et al. (2021) |
| Epicoccum nigrum | Zingiber officinale | L5178Y, Ramos, Jurkat J16 (1.3–28 mM) | Epicorazine A (3) | Alkaloids | Anti-cancer | |
| Phomopsis sp. | Polygonatum sibiricum | A2780 | Epoxycytochalasin H (4) | Alkaloids | Anti-cancer | Xu (2020) |
| Phoma sp. JS0228 | Morus alba | MCF-7 (0.29 mM), LNCaP (0.36 mM) | Macrooxazoles C (5) | Alkaloids | Anti-cancer | Ku et al. (2021) |
| Colletotrichum gloeosporioides | Piper nigrum | – | Piperine (6) | Alkaloids | Anti-inflammatory | Krishna et al. (2020) |
| Chaetomium sp. SYP-F7950 | Panax notoginseng | MDA-MB-231 (26.49 μmol L−1) | Chetoseminudin F (7) | Alkaloids | Anti-cancer | Peng et al. (2019) |
| A549, MDA-MB-231 (2.75–8.68 μmol L−1) | Chaetocochin C (8), chetomin A (9), chetomin C (10), chetomin (11) | Alkaloids | Anti-cancer | |||
| Staphylococcus aureus, Bacillus subtilis, Enterococcus faecium, Candida albicans (0.12–9.6 μg mL−1) | Chaetocochin C (8), chetomin A (9), chetomin C (10), chetomin (11) | Alkaloids | Anti-microbial | |||
| Chaetomium globosum 7951 | Panax notoginseng | MCF-7, MDA-MB231, H460, HCT-8 (4.5–65.0 mM) | Demethylchaetocochin C (12), chaetoperazine A (13), 4-formyl-N-(30-hydroxypyridin-20-yl) benzamide (14), chetomin (15) | Alkaloids | Anti-cancer | Wang, F. et al. (2019) |
| Penicillium sp. T2-8 | Gastrodia elata | Candida albicans (128 μg/mL) | Preaustinoid D (16), dihydroxyneogrifolic acid (17) | Terpenoids | Anti-microbial | Duan et al. (2016) |
| Bacillus subtilis (8 μg/mL), Staphylococcus aureus (32 μg/mL) | Dihydroxyneogrifolic acid (17) | Terpenoids | Anti-microbial | |||
| Bacillus subtilis (4 μg/mL) | Preaustinoid A1 (18) | Terpenoids | Anti-microbial | |||
| Staphylococcus aureus (4 μg/mL) | Austin (20), (S)-18,19-dihydroxyneogrifolin (21), | Terpenoids | Anti-microbial | |||
| Escherichia coli (8 μg/mL) | (S)-18,19-dihydroxyneogrifolin (21) | Terpenoids | Anti-microbial | |||
| – | Dehydroaustinol (19), neogrifolin (22) | Terpenoids | – | |||
| Bipolaris sp. L1-2 | Lycium barbarum | NCI–H226, MDA-MB-231 (5.5–9.5 μM) | Bipolahydroquinones A-C (23–25), cochlioquinones I–N (26–30 and 33), isocochlioquinones F and G (31 and 32) | Terpenoids | Anti-cancer | Long et al. (2019) |
| Pestalotiopsis sp. DO14 | Dendrobium officinale | Candida albicans, Cryptococcus neoformans, Trichophyton rubrum, Aspergillus fumigatus (more than 50 μg/mL) | (4S,6S)-6-[(1S,2R)-1, 2-dihydroxybutyl]4-hydroxy-4-methoxytetrahydro-2H-pyran-2-one (34), (6S,2E)-6-hydroxy-3-methoxy-5-oxodec-2-enoic acid (35), LL-P880γ (36), LL-P880α (37) | Terpenoids | Anti-microbial | Wu et al. (2016) |
| Candida albicans, Cryptococcus neoformans, Trichophyton rubrum, Aspergillus fumigatus (higher than 200 μg/mL) | ergosta-5,7,22-trien-3b-ol (38) | Terpenoids | Anti-microbial | |||
| MKN45, LOVO, A549, HL-60 (lower than 200 μM) | (4S,6S)-6-[(1S,2R)-1, 2-dihydroxybutyl]4-hydroxy-4-methoxytetrahydro-2H-pyran-2-one (34), (6S,2E)-6-hydroxy-3-methoxy-5-oxodec-2-enoic acid (35), LL-P880γ (36), LL-P880α (37), ergosta-5,7,22-trien-3b-ol (38) | Terpenoids | Anti-cancer | |||
| Emericella sp. XL 029 | Panax notoginseng | Bacillus subtilis, Bacillus cereus, Escherichia coli (25–50 μg/mL) | Emericellins A (39) and B (40) | Terpenoids | Anti-microbial | Pang et al. (2018) |
| Penicillium sp. | Zingiber officinale | L5278Y, A2780 | Shearilicine (41) | Terpenoids | Anti-cancer | Ariantari et al. (2019) |
| Drechmeria sp. | Panax notoginseng | Candida albicans (12.5 μg/mL) | Drechmerins B (42) | Terpenoids | Anti-microbial | Zhao, J. C. et al. (2018) |
| Hypomontagnella monticulosa Zg15SU | Zingiber griffithii | PANC-1 (0.05 ppm), NBT-T2 (0.75 ppm), HCT116 (0.05 ppm) | Terpenoid-alkaloid (43) | Terpenoids | Anti-cancer | Lut et al. (2021) |
| PANC-1 (0.71 ppm), NBT-T2 (0.30 ppm), HCT116 (0.67 ppm) | Sesterterpenoid (44) | Terpenoids | Anti-cancer | |||
| Colletotrichum sp. JS-0361 | Morus alba | MCF-7 (35.06 and 25.20 μM) | Colletotrichalactones A-Ca (45–47a) | Polyketides | Anti-cancer | Bang et al. (2020) |
| Pleosporales sp. Sigrf05 | Siraitia grosvenorii | HCT-116, HepG2, BGC-823, NCI–H1650, Daoy (1.26–47.5 μM) | Pleospyrones A-E (48–52), congener (53) | Polyketides | Anti-cancer | Lai et al. (2020) |
| Chaetomium globosum | Polygonatum sibiricum | HepG-2 (38.6 μM) | Chaephilone C (54) | Polyketides | Anti-cancer | Song, C. et al. (2020) |
| Penicillium citrinum | Dendrobium officinale | MG63 (3.49 μmol L−1) | Peptide-polyketide hybrid GKK1032B (55) | Polyketides | Anti-cancer | Na et al. (2022) |
| Alternaria sp. | Ziziphus jujuba | L5178Y (1.7 μM) | Alternariol (56) | Polyketides | Anti-cancer | Orfali et al. (2017) |
| L5178Y (7.8 μM) | Alternariol-5-O-methyl ether (57) | Polyketides | Anti-cancer | |||
| L5178Y (6.8 μM) | Altenusin (58) | Polyketides | Anti-cancer | |||
| L5178Y (6.2 μM) | Altertoxin II (59) | Polyketides | Anti-cancer | |||
| Trichoderma koningiopsis YIM PH3000 | Panax notoginseng | Bacillus subtilis, Salmonella typhimurium, Escherichia coli (128 μg/mL) | Koninginin W (60), koninginin D (61), 7-O-methylkoninginin D (62), koninginin T (63) and koninginin A (64) | Polyketides | Anti-microbial | Wang, Y. L. et al. (2021) |
| Emericella sp. XL 029 | Panax notoginseng | Micrococcus lysodeikticus, Salmonella typhimurium (25–50 μg/mL) | Emericelactones A-D (65–68) | Polyketides | Anti-microbial | Pang et al. (2018) |
| Colletotrichum sp. JS-0367 | Morus alba | – | 1,3-dihydroxy-2,8dimethoxy-6-methylanthraquinone (69) | Quinones | Anti-inflammatory | Fibroblasts et al. (2021) |
| Fusarium solani JS-0169 | Morus alba | HT22 | Fusarubin (70) | Quinones | Anti-cancer | Choi et al. (2020) |
| Fusarium solani | Cassia alata | – | Ergosterol (71) | Quinones | – | Khan et al. (2018) |
| – | Anhydrofusarubin (72), 4-hydroxybenzaldehyde (73), bostrycoidin (74), fusarubin (75) | Quinones | Anti-oxidant | |||
| Bacillus megaterium, Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli | Anhydrofusarubin (72), bostrycoidin (74), fusarubin (75), 3-deoxyfusarubin (76) | Quinones | Anti-microbial | |||
| Vero (25%) | 4-hydroxybenzaldehyde (73), bostrycoidin (74) | Quinones | Anti-cancer | |||
| Vero (35%) | Anhydrofusarubin (72), 3,5,9-trihydroxyergosta-7,22-diene-6-one (77) | Quinones | Anti-cancer | |||
| Alternaria tenuissima F1 | Angelica sinensis | – | EPS (78) | Polysaccharides | Anti-oxidant | Wang, Y. et al. (2019) |
| Fusarium solani DO7 | Dendrobium officinale | – | DGS1 (79) and DGS2 (80) | Polysaccharides | Anti-oxidant | Zeng et al., 2019a, Zeng et al., 2019b |
| Penicillium citreonigrum CSL-27 | Crocus sativus | – | EPS (81) | Polysaccharides | Anti-oxidant | Mag et al. (2018) |
| K562 (46.16%), A549 (44.97%), HL-60 (44.95%), HeLa (33.10%) | EPS (81) | Polysaccharides | Anti-cancer | |||
| Aspergillus terreus-RTN3 | Alpinia chinensis | – | MM2 (82) | Esters | Anti-oxidant | Suhartati (2020) |
| MCF-7 (42.26 μg/mL), Hela (39.21 μg/mL), HepG2 (48.30 μg/mL), NCI–H460 (50.26 μg/mL) | MM2 (82) | Esters | Anti-cancer | |||
| Penicillium sp. | Panax notoginseng | HepG2 (0.024 μM) | Brefeldin A (83) and brefeldin A 7-O-acetate (84) | Esters | Anti-cancer | Xie et al. (2017) |
| Aspergillus austroafricanus CGJ-B3 | Zingiber officinale | – | EAE (85) | Phenols | Anti-oxidant | Danagoudar et al. (2017) |
| Aspergillus flavipes DZ-3 | Eucommia ulmoides | – | 3,4-dihydroxybenzeneacetic acid (86), 3,4-dihydroxyphenylacetic acid methyl ester (87) | Phenols | Anti-oxidant | Liu, W. et al. (2021) |
| Phomopsis sp. XP-8 | Eucommia ulmoides | HepG2 | Pinoresinol (Pin) (88), Pinoresinol monoglucoside (PMG) (89) | Phenols | Anti-cancer | Li, Q. et al. (2016) |
| Fusarium tricinctum | Hordeum sativum | Gram-positive and Gram-negative bacteria | Enniatins (ENs) (90) | Polypeptides | Anti-microbial | Zaher et al. (2015) |
| Fusarium sp. TP-G1 | Dendrobium officinale | Staphylococcus aureus (2 μg/mL), MRSA (2 μg/mL), Acinetobacter baumannii (>128 μg/mL) | Trichosetin (91), beauvericin A (93) | Polypeptides | Anti-microbial | Zaher et al. (2015) |
| Staphylococcus aureus (4 μg/mL), MRSA (4 μg/mL), Acinetobacter baumannii (>128 μg/mL) | Beauvericin (92) | Polypeptides | Anti-microbial | |||
| Staphylococcus aureus (128 μg/mL), MRSA (128 μg/mL), Acinetobacter baumannii (>128 μg/mL) | Enniatin B (94), | Polypeptides | Anti-microbial | |||
| Staphylococcus aureus (32 μg/mL), MRSA (32 μg/mL), Acinetobacter baumannii (>128 μg/mL) | Enniatin H (95) | Polypeptides | Anti-microbial | |||
| Staphylococcus aureus (8 μg/mL), MRSA (16 μg/mL), Acinetobacter baumannii (>128 μg/mL) | Enniatin I (96) | Polypeptides | Anti-microbial | |||
| Staphylococcus aureus (4 μg/mL), MRSA (8 μg/mL), Acinetobacter baumannii (>128 μg/mL) | Enniatin MK1688 (97) | Polypeptides | Anti-microbial | |||
| Staphylococcus aureus (>128 μg/mL), MRSA (>128 μg/mL), Acinetobacter baumannii (64 μg/mL) | Fusaric acid (98) | Polypeptides | Anti-microbial | |||
| Staphylococcus aureus (>128 μg/mL), MRSA (>128 μg/mL), Acinetobacter baumannii (128 μg/mL) | Dehydrofusaric acid (99) | Polypeptides | Anti-microbial | |||
| MCF-7 (12.4 μg/mL) | Beauvericin (92) | Polypeptides | Anti-cancer | |||
| MCF-7 (12.22 μg/mL) | Enniatin B (94) | Polypeptides | Anti-cancer | |||
| MCF-7 (11.46 μg/mL) | Enniatin H (95) | Polypeptides | Anti-cancer | |||
| MCF-7 (10.27 μg/mL) | Enniatin I (96) | Polypeptides | Anti-cancer | |||
| BL16F10 (15.2 μg/mL) | Beauvericin (92) | Polypeptides | Anti-cancer | |||
| BL16F10 (10.64 μg/mL) | Enniatin B (94) | Polypeptides | Anti-cancer | |||
| BL16F10 (8.39 μg/mL) | Enniatin H (95) | Polypeptides | Anti-cancer | |||
| BL16F10 (9.46 μg/mL) | Enniatin I (96) | Polypeptides | Anti-cancer | |||
| Fusarium sp. | Mentha longifolia | Candida albicans (8.3 μg/disc) | Fusaristerol A (100) | Steroids | Anti-microbial | Chester et al. (2017) |
| HCT-116 (0.21 μM) | Fusaristerol A (100) | Steroids | Anti-cancer | |||
| Lasiodiplodia venezuelensis BJA-1 | Syzygium samarangense | – | 5,7-dihydroxy-6,8-dimethyl flavanone (101) | Flavonoids | Anti-oxidant | Budiono et al. (2019) |
| Aspergillus aculeatus MBT 102 | Rosa damascena | MDA-MB-231 (16.6 μM) | Secalonic acid derivative F-7 (102) | Organic acids | Anti-cancer | Farooq et al. (2020) |
| Curvularia papendorfii | Vernonia amygdalina | HCoV 229 E, FCV F9 (128 μg/mL) | The EtOAc extract of Curvularia papendorfii (103) | Fungal Extractives | Anti-microbial | Khiralla et al. (2020) |
| MCF-7 (21.5 ± 5.9 μg/mL) | The EtOAc extract of Curvularia papendorfii (103) | Fungal Extractives | Anti-cancer | |||
| Fusarium sp. CK F05-5 | Dendrobium lindleyi | – | The CK F05-5-EtOAc extract (104) | Fungal Extractives | Anti-oxidant | Bungtongdee et al. (2019) |
| Fusarium oxysporum TY5 | Dendrobium officinale | HepG2 (320 μg/mL) | The TY5-EtOAc extract (105) | Fungal Extractives | Anti-cancer | Ty et al. (2018) |
| Phomopsis heveicola | Piper longum | Pseudomonas aeruginosa (20%), Shigella sonnei (25%), Streptococcus pyogenes (17%), Salmonella typhimurium (23%) | Valproic acid-treated Fungal Extractives of Phomopsis heveicola (106) | Fungal Extractives | Anti-microbial | Ameen et al. (2021) |
| Aspergillus cejpii ST9.1 | Nelumbo nucifera | MRSA (2.5 mg/mL) | The ST9.1-EtOAc extract (107) | Fungal Extractives | Anti-microbial | Techaoei et al. (2020) |
| Fusarium spp. | Ginkgo biloba | Hela | The EtOAc extract of Fusarium spp. (108) | Fungal Extractives | Anti-cancer | Engel (2019) |
| Colletotrichum sp. | Curcuma longa | MCF-7 (500 ppm) | The EtOAc extract of Colletotrichum sp. (109) | Fungal Extractives | Anti-cancer | Line et al. (2016) |
| Nigrospora sphaerica | Cornus florida | U251, A549 (10 μg/mL) | The EtOAc extract of Nigrospora sphaerica (110) | Fungal Extractives | Anti-cancer | Maheshwari et al. (2018) |
| Gaeumannomyces sp. JS0464 | Phragmites communis | – | The JS0464-EtOAc extract (111) | Fungal Extractives | Anti-inflammatory | Lee et al. (2017) |
| Aspergillus sp. AP5 | Phragmites australis | Staphylococcus aureus (53.04%), Escherichia coli (61.23%), Klebsiella sp. (51.16%), Proteus vulgaris (15.44%), Pseudomonas aeruginosa (30.25%) | The AP5-EtOAc extract (112) | Fungal Extractives | Anti-microbial | Abdelgawad et al. (2022) |
| Candida albicans (40.14%) | The AP5-EtOAc extract (112) | Fungal Extractives | Anti-microbial | |||
| Colletotrichum coccodes | Houttuynia cordata | Candida albicans (125 μg/mL), Staphylococcus aureus (125 μg/mL), Escherichia coli (125 μg/mL), Pseudomonas aeruginosa (250 μg/mL) | The EtOAc extract of Colletotrichum coccodes (113) | Fungal Extractives | Anti-microbial | (Talukdar et al., 2021; Talukdar et al., 2021) |
| Staphylococcus aureus (19 mm), Escherichia coli (19 mm), Pseudomonas aeruginosa (18 mm), Candida albicans (21 mm) | The EtOAc extract of Colletotrichum coccodes (114) | Fungal Extractives | Anti-microbial | |||
| Phyllosticta capitalensis | Houttuynia cordata | Staphylococcus aureus (23 mm), Escherichia coli (14 mm), Pseudomonas aeruginosa (14 mm), Candida albicans (22 mm) | The EtOAc extract of Phyllosticta capitalensis (115) | Fungal Extractives | Anti-microbial | Talukdar et al. (2021) |
| Penicillium sp. SNF123 | Panax ginseng | B16F10, MNT-1 (2.5–50 μg/mL) | Acremonidin E (116) | Fungal Extractives | Anti-cancer | Kim et al. (2019) |
| Aspergillus terreus | Glycine max | Anti-COVID-19 | Aspergillide B1 (117), 3a-Hydroxy-3, 5-dihydromonacolin L (118) | Fungal Extractives | Anti-microbial | El-Hawary et al. (2021) |
| Penicillium griseofulvum MPR1 | Mentha pulegium | Escherichia coli (45.5 mm) | The EtOAc extract of Penicillium griseofulvum MPR1 (119) | Fungal Extractives | Anti-microbial | Amina et al. (2018) |
| Escherichia coli (41 mm) | The CHCl2 extract of Penicillium griseofulvum MPR1 (120) | Fungal Extractives | Anti-microbial | |||
| Arthrinium sp. MFLUCC16-1053 | Zingiber cassumunar | Staphylococcus aureus (31.25 μg/mL), Escherichia coli (7.81 μg/mL) | The MFLUCC16-1053-EtOAc extract (121) | Fungal Extractives | Anti-microbial | Pansanit and Pripdeevech (2018) |
Note: “-” indicates the activity of these compounds are not mentioned in the cited references.
Fig. 1.
Metabolites of MFH plant-derived endophytic fungi and their activities. Zin, Zingiber; Alp, Alpinia; Cur, Curcuma; Den, Dendrobium; Gas, Gastrodia; Pan, Panax; Mor, Morus; Phr, Phragmites; Hor, Hordeum; Hou, Houttuynia; Men, Mentha; Pip, Piper; Euc, Eucommia; Pol, Polygonatum; Gly, Glycine; Sen, Senna; Cro, Crocus; Gym, Gymnanthemum; Lyc, Lycium; Ros, Rosa; Ziz, Ziziphus; Nel, Nelumbo; Syz, Syzygium; Gin, Ginkgo; Dio, Dioscorea; Sir, Siraitia; Cor, Cornus; Ang, Angelica; Fus, Fusarium; Tri, Trichoderma; Dre, Drechmeria; Asp, Aspergillus; Pen, Penicillium; Bip, Bipolaris; Alt, Alternaria; Cur, Curvularia; Pho, Phoma; Epi, Epicoccum; Cha, Chaetomium; Pes, Pestalotiopsis; Hyp, Hypomontagnella; Art, Arthrinium; Nig, Nigrospora; Col, Colletotrichum; Ple, Pleosporales; Phy, Phyllosticta; Las, Lasiodiplodia; Phom, Phomopsis; Gae, Gaeumannomyces. The line thickness represents the number.
Known as the Chinese Orchidaceae medicinal families (COMFs), Orchidaceae is one of the largest and most important medicinal families of plants, with more than 28,000 identified species in about 763 genera (Christenhusz and Byng, 2016; Zhang, S. et al., 2018). The chief genera in the Orchidaceae family are Pleurothallis (more than 1000 species), Dendrobium (more than 1400 species), Epidendrum (more than 1500 species), and Bulbophyllum (more than 2000 species) (Sarsaiya et al., 2019). Due to the large number and variety of orchids, they have long been investigated in regard to their biology and associations with diverse fungal endophytes. Recently, endophytic fungi belonging to Eurotiales, Hypocreales, and Xylariales have been isolated from only two genera (Dendrobium and Gastrodia) of Orchidaceae (Fig. 1 and S1). Orchidaceae fungal endophytes (OFEs) can enhance plant growth, increase resistance to disease-triggering pathogens, reduce weed incidence, and improve tolerance capacity to biotic and abiotic stresses (Sturz et al., 2000). Furthermore, they yield vast secondary metabolites (industrially important bioactive natural compounds) with pharmaceutical potential (Demain, 2014). Many endophytic fungi resist an extensive range of pathogens. OFEs also yield an antimicrobial compound that prevents pathogen development and competes with most pathogen species for nutrition and space (Alurappa et al., 2018). At present, more attention is paid to the effect of fungi on plant growth and development. Research on the metabolites and activity of endophytic fungi in Orchidaceae is still extremely limited, having focused mainly on the genus Dendrobium (Jin, Z. et al., 2017; Mei et al., 2010; Sarsaiya et al., 2020; Xing, Y. M. et al., 2011), which is rich in active metabolites.
Endophytic fungal species that have been reported in MFH plants belong to nine orders, including Hypocreales, Eurotiales, Pleosporales, Sordariomycetes, Xylariales, Glomerellales, Botryosphaeriales, Diaporthales, and Magnaporthales (Fig. 1 and S2). Hypocreales mainly includes Fusarium, Drechmeria, and Trichoderma, which may produce alkaloids, terpenoids, polyketides, quinones, polysaccharides, polypeptides, and steroids. Eurotiales mainly includes Aspergillus and Penicillium, which may produce terpenoids, polyketides, polysaccharides, esters, phenols, and organic acids. Endophytes of Aspergillus (El-hawary et al., 2020; Hagag et al., 2022), Penicillium (Marie et al., 2020), Fusarium (Manoj and Anil, 2016; Marie and Toghueo, 2019), Drechmeria (Liang et al., 2018; Yu et al., 2018; Zhao, J. et al., 2018b), and Trichoderma (Patel, J. et al., 2019) possess significant antibacterial, antifungal, antioxidant, anti-inflammatory, and anticancer activities.
3. Correlation between MFH plants and their endophytic fungal metabolites
Recent investigations have reported the capacity of multiple endophytic fungi to produce bioactive compounds which are the same or structurally related to those synthesized by their host plants (Bielecka et al., 2022; Sharma et al., 2021; Venieraki et al., 2017; Zhao, J. et al., 2010). Metabolites of the 35 MFH plant species for which endophytes have been reported mainly includes flavonoids, phenols, terpenoids, polysaccharides, glycosides, alkaloids, amino acids, anthocyanins, steroids, vitamins, diarylheptanoids, saponins, organic acids and other compounds (Table S1). Among them, polysaccharides, alkaloids, terpenoids, flavonoids, and phenols were found to be shared by MFH plant and their endophytic fungi (Table S1). However, only a few metabolites of four types (polysaccharides, alkaloids, lignans and terpenoids) produced by the MFH plant-derived endophytic fungi have been reported to have the same or structurally related to those synthesized by their host plants in the recent five years. Crocus sativus petals are rich in polysaccharides (Yang et al., 2018). Two homogeneous polysaccharides were isolated and purified from petals of C. sativus have been documented for their effects of antioxidant activity, which are potentially important for human health (He et al., 2021). Interestingly, the exopolysaccharide isolated from endophytic fungi Penicillium citreonigrum CSL-27 of C. sativus exhibited higher antioxidant activity (Mag et al., 2018). Polysaccharides as the dominatingly active component in Dendrobium officinale have been found due to their nutritional and pharmacological properties (Xing, X. et al., 2013). Recently, an endophytic fungus Fusarium solani DO7 produced polysaccharide showing the similar structure with the host D. officinale polysaccharide (DOP) (Zeng et al., 2019b, Zeng et al., 2019a). Polysaccharides of Angelica sinensis also have been confirmed to have functions of enriching blood, regulating immunity and promoting tumor vaccine's antitumor function (Gu et al., 2019; Jin, M. et al., 2012; Liu, W. et al., 2019; Wang, K. et al., 2016). However, the exopolysaccharide of Alternaria tenuissima F1 isolated from A. sinensis exhibited strong scavenging activity and may be a new source of natural antioxidant (Wang, Y. et al., 2019). Anti-inflammatory and anticancer alkaloid piperine is found in the fruits of Piper longum and P. nigrum and responsible for their pungent taste (Stojanović-Radić et al., 2019). Endophytic fungi (Mycosphaerella sp. PF13, Colletotrichum gloeosporioides and Periconia sp.) isolated from P. nigrum or P. longum could produce piperine (Chithra et al., 2014a; Chithra et al., 2014b; Verma et al., 2011). Pinoresinol diglucoside (PDG), one of the major lignans with various pharmacological functions, is an important antihypertensive compound in Eucommia ulmoides (Shi, J. et al., 2012). Endophytic fungus Phomopsis sp. XP-8 isolated from the bark of E. ulmoides could produce Pin (the precursor of PDG) (Li, Q. et al., 2016). Ginkgolides, one of the main terpenoids found in the leaves and bark of Ginkgo biloba, has potent antagonist effects on platelet activating factors, involved in the development of a number of cardiovascular, renal, respiratory and central nervous system disorders (Liu, X. G. et al., 2021). Ginkgolide B produced endophytic fungus (Fusarium oxysporum) isolated from G. biloba, which is the main phytochemicals of G. biloba (Cui et al., 2012).
Whether other endophytic fungal metabolites of these shared types correlate with their host plants requires further confirmation. To date, the ability of endophytes to produce the same or similar bioactive compounds as their hosts raises several intriguing questions that have yet to be answered, including whether the compound is first synthesized by the plant or by the fungus and whether there is a transfer of genetic information between the two (Caruso et al., 2020). As these questions are addressed, it may be possible in the future to extract more functionalities from endophytic fungi of MFH plants that are similar to the edible and medicinal characteristics of their hosts.
4. Classification of MFH plant-derived endophytic fungal metabolites
We searched the literature published between 2017 and 2022, and 121 metabolites belonging to 12 chemical classes were reported: terpenoids, polyketides, alkaloids, polypeptides, quinones, phenols, polysaccharides, esters, steroids, flavonoids, organic acids, and fungal extracts (Fig. 1 and S3). With respect to biochemistry, terpenoids and polyketides are the most purified antimicrobial secondary metabolites from endophytes (Mousa and Raizada, 2013). Terpenoids are one of the most commonly isolated metabolites of plant endophytic fungi. Trichoderma, Aspergillus, Penicillium, Xylaria, Diaporthe, Pestalotiopsis, and Paraconiothyrium are the principal producers of terpenoids. Cytotoxic, antimicrobial, and anti-inflammatory properties are the most commonly identified biological activities of these terpenoids (Amirzakariya and Shakeri, 2022). Endophytes are a rich source of structurally novel bioactive natural products (Gunatilaka, 2016), predominantly polyketides and non-ribosomal peptides (Fischbach and Walsh, 2006). They represent a structure class that often gives rise to clinically relevant pharmaceuticals (Butler et al., 2014; Clardy et al., 2006; Newman and Cragg, 2012).
4.1. Alkaloids
Many alkaloids have been discovered from the endophytic fungi of MFH plants. For example, a new cytochalasin has been isolated from Chaetomium globosum fermented in Chinese yam (Dioscorea opposita) and named yamchaetoglobosin A (1) (Ruan et al., 2017). A new epidithiodiketopiperazine (ETP), pretrichodermamide A (2), and epicorazine A (3) have been isolated from cultures of Trichoderma harzianum and Epicoccum nigrum, endophytic fungi associated with Zingiber officinale and Salix sp., respectively (Harwoko et al., 2021). A fungal metabolite from Phomopsis, identified as epoxycytochalasin H (4), has been isolated from the flowering plant Polygonatum sibiricum (Xu, 2020). An oxazole-type compound named macrooxazoles C (5) has been isolated from ethyl acetate (EtOAc) extract of Phoma sp. JS0228 cultures, an endophytic fungus of Morus alba (Ku et al., 2021). Colletotrichum gloeosporioides has been isolated from black pepper (Piper nigrum), with alkaloid piperine (6) in its extract (Krishna et al., 2020).
Chaetomium sp. SYP-F7950 isolated from Panax notoginseng produces a large number of alkaloids (7–11) (Peng et al., 2019). In addition, demethylchaetocochin C (12) and chaetoperazine A (13) (two new ETP alkaloids), the novel pyridine benzamide 4-formyl-N-(30-hydroxypyridin-20-yl) benzamide (14), and the ETP derivative chetomin (15) have been isolated from acetate extracts of Chaetomium globosum 7951 solid cultures (Wang, F. et al., 2019).
4.2. Terpenoids
Some endophytic fungi from MFH plants produce fungus-specific components of meroterpenoids. For example, a new meroterpenoid preaustinoid D (16) and a new neogrifolin derivative dihydroxyneogrifolic acid (17), along with other five previously reported meroterpenoids (18–22), have been isolated from the endophytic fungus Penicillium sp. T2–8 associated with Gastrodia elata (Duan et al., 2016). Eleven new meroterpenoids, bipolahydroquinones A–C (23–25), cochlioquinones I–N (26–30 and 33), and isocochlioquinones F and G (31 and 32), along with six known ones, have been obtained from endophytic fungus Bipolaris sp. L1–2 from Lycium barbarum (Long et al., 2019).
Moreover, there are several other endophytic fungi from MFH plants that produce monoterpenoids, sesquiterpenes, indole diterpenes, and heptaterpenes with antibacterial and anticancer activities. For example, two novel cytotoxic and antifungal monoterpenoid constituents (34 and 35), and three known compounds (36–38) have been isolated from the metabolites of endophytic fungi Pestalotiopsis sp. DO14 from Dendrobium officinale (Wu et al., 2016). Two novel sesquiterpenoids, namely, emericellins A (39) and B (40), have been isolated from liquid cultures of endophytic fungus Emericella sp. XL 029 associated with the leaves of Panax notoginseng (Pang et al., 2018a). Shearilicine (41) has been isolated from the endophyte Penicillium sp. (strain ZO-R1-1) isolated from the roots of Zingiber officinale (Ariantari et al., 2019). Drechmerin B (42) has been isolated from fermentation broth of Drechmeria sp. isolated from the root of Panax notoginseng (Zhao, J. C. et al., 2018a). Terpenoid-alkaloid (43) and sesterterpenoid (44) have been isolated from the endophytic fungi Hypomontagnella monticulosa residing within the rhizome of Z. griffithii from northern Sumatra (Lut et al., 2021).
5. Polyketides
Polyketide compounds are also produced by endophytic fungi from MFH plants. For example, three unusual polyketides with a 5/6/10-fused ring system, named colletotrichalactone A-Ca (45–47a), have been isolated from cultures of the endophytic fungus Colletotrichum sp. JS-0361 isolated from a leaf of Morus alba (Bang et al., 2020). Five new chlamydosporol derivatives, named pleospyrone A–E (48–52), along with one known congener (53), have been isolated from a culture of the endophytic fungus Pleosporales sp. Sigrf05 obtained from Siraitia grosvenorii (Lai et al., 2020). A new azaphilone, chaephilone C (54), has been isolated from Chaetomium globosum, an endophytic fungus of Polygonatum sibiricum (Song, C. et al., 2020). Chemical investigation of the culture extract of an endophytic Penicillium citrinum from Dendrobium officinale afforded nine citrinin derivatives and one peptide-polyketide hybrid GKK1032B (55) (Na et al., 2022). In one study, Alternaria sp. was isolated from Ziziphus jujuba, and its liquid extract yielded four natural products (56–59) (Orfali et al., 2017).
Endophytic fungi isolated from Panax notoginseng produce polyketide compounds. For example, a new compound named koninginin W (60) and four known polyketides (61–64), have been isolated from the endophytic fungus Trichoderma koningiopsis YIM PH30002 (Wang, Y. L. et al., 2021). Emericella sp. XL 029 has yielded four novel polyketides, emericelactone A–D (65–68) (Pang et al., 2018b).
5.1. Quinones
Endophytic fungi isolated from Morus alba produce quinones. Colletotrichum sp. JS-0367 produces the quinone 1,3-dihydroxy-2,8dimethoxy-6-methylanthraquinone (69) (Fibroblasts et al., 2021). Chemical investigation of cultures of the fungus F. solani JS-0169 resulted in the isolation of six compounds, including one new γ-pyrone, one known γ-pyrone, fusarester D, and four known naphthoquinones (karuquinone B, javanicin, solaniol, and fusarubin [70]) (Choi et al., 2020). In addition, F. solani has been isolated from Cassia alata and naphthoquinones and aza-anthraquinones (71–77) are present in its metabolites (Khan et al., 2018).
5.2. Polysaccharides
In recent years, the development and use of microbial polysaccharides has received increasing attention due to its potential industrial applications (Donot et al., 2012; Nwodo et al., 2012). Extracellular polysaccharides (EPSs) are a group of microbial polysaccharides biosynthesized mainly by bacteria and fungi through intracellular or extracellular pathways (Freitas et al., 2011; Kumar et al., 2007). Endophytic fungi are important sources of bioactive EPSs, and these structurally novel polysaccharides not only play an important role in plant–endophyte interactions but also have a variety of beneficial biological functions in humans, such as antioxidant, anti-inflammatory, and anticancer properties, with potential application in the health food and pharmaceutical industries (Cultures, 2016; Li, P. et al., 2011; Liu, J. et al., 2016; Zeng, Y. et al., 2019).
However, EPS-producing endophytic fungi from MFH plants have been poorly explored. An EPS (78)-producing endophytic fungus has been isolated from Angelica sinensis and identified as Alternaria tenuissima F1 (Wang, Y. et al., 2019). Two polysaccharides, DGS1 (79) and DGS2 (80), have been obtained via solid-state fermentation (SSF) of Fusarium solani DO7, an endophytic fungus isolated from the orchid Dendrobium officinale (Zeng et al., 2019a, Zeng et al., 2019b). The endophytic fungus Penicillium citreonigrum CSL-27 has been isolated from Crocus sativus and its EPS (81) includes mannose, glucose, galactose xylose, and arabinose at a molar ratio of 25.6:16.5:1.0:3.8:5.4 (Mag et al., 2018).
5.3. Esters
Endophytic fungi of MFH plants produce metabolites that can be employed as antibiotics, some of which are esters. Aspergillus terreus-RTN3, isolated from young stems of Alpinia chinensis, yields the compound MM2 (82) (methyl 2-((4-amino-2-bromo-3-methyl-5-thioxocyclopenta-1,3-dien-1-yl) oxygen)-4-hydroxy-6-methoxybenzoate) (Suhartati, 2020). Penicillium sp. has been isolated from the healthy roots of Panax notoginseng and five macrolide antibiotics have been synthesized, including brefeldin A (83) and brefeldin A 7-O-acetate (84) (Xie et al., 2017).
5.4. Phenols
Studies have shown that phenolic compounds including phenol and phenolic acids are frequently isolated from endophytic fungi of MFH plants, and have strong antioxidant activity.
For example, the ethyl acetate extract of Aspergillus austroafricanus CGJ-B3 (EAE) (85) isolated from Z. officinale is a promising pharmaceutical agent and can be used as an alternative source of polyphenols such as p-coumaric acid, ferulic acid, and cinnamic acid (Danagoudar et al., 2017). Endophytic fungal extracts from the well-known MFH plant Eucommia ulmoides contain phenolic compounds. Furthermore, 3,4-dihydroxybenzeneacetic acid (86) and 3,4-dihydroxyphenylacetic acid methyl ester (87) have been obtained from EtOAc extracts of Aspergillus flavipes DZ-3 (Liu, W. et al., 2021). Phomopsis sp. XP-8 produces the lignan molecules pinoresinol (Pin) (88) and pinoresinol monoglucoside (PMG) (89) (Li, Q. et al., 2016).
5.5. Polypeptides
Some endophytic fungi from MFH plants, particularly Fusarium sp., also produce polypeptides. Enniatins (ENs) (90), a group of antibiotics commonly produced by various strains of Fusarium, are six-membered cyclic depsipeptides formed by the union of three molecules of D-α-hydroxyisovaleric acid and three N-methyl-L-amino acids. The endophyte Fusarium tricinctum has been isolated from the fruits of Hordeum sativum (Zaher et al., 2015). Trichosetin (91), beauvericins (92–93), enniatins (94–97), fusaric acid (98), and dehydrofusaric acid (99) are produced by the endophytic fungus Fusarium sp. TP-G1 derived from the root of D. officinale (Zaher et al., 2015).
5.6. Steroids, flavonoids, and organic acids
Steroids, flavonoids, and organic acids can also be produced by endophytic fungi of MFH plants. For example, Fusarium sp. isolated from the roots of Mentha longifolia, produce fusaristerol A (100) (Chester et al., 2017). Lasiodiplodia venezuelensis BJA-1 has been isolated from healthy leaves of Syzygium samarangense, and its ethyl acetate extract contains 5,7-dihydroxy-6,8-dimethyl flavanone (101) (Budiono et al., 2019). A new secalonic acid derivative F-7 (102) has been isolated from the endophytic Aspergillus aculeatus MBT 102, associated with Rosa damascena (Farooq et al., 2020).
5.7. Fungal extracts
In addition, mixes of metabolites and unclassified extracts have been reported. In one study, an endophytic fungus isolated from Vernonia amygdalina, an MFH plant from Sudan, was taxonomically characterized as Curvularia papendorfii (Khiralla et al., 2020). A crude extract of ethyl acetate (103) was prepared from it. In another study, Fusarium sp. CK F05-5 was isolated from the flower of Dendrobium lindleyi, and an ethyl acetate extract (104) was obtained from it (Bungtongdee et al., 2019). In yet another study, Fusarium oxysporum TY5 was isolated from D. officinale, and again an ethyl acetate extract (105) was obtained (Ty et al., 2018). In Ameen et al. (2021), seven endophytic fungi were isolated from the tropical MFH plant Piper longum L. After preliminary screening, Phomopsis heveicola was selected for epigenetic activation treatments. Endophytic fungi were treated with different concentrations of valproic acid to obtain extracts (106). In another study, ethyl acetate extract of endophyte Aspergillus cejpii ST9.1 (107) was isolated from the aquatic plant Nelumbo nucifera; the major component of the crude extract was 5-(1H-Indol-3-yl)-4,5-dihydro-[1,2,4]triazin-3-ylamine (C11H11N5) (Techaoei et al., 2020). Three strains of Fusarium species (J-1, J-2, and J-3) have been isolated from Ginkgo biloba, yielding a crude extract (108) (Engel, 2019). Colletotrichum sp. has been isolated from Curcuma longa L., yielding a filtrate (109) (Line et al., 2016). Nigrospora sphaerica has been isolated from Cornus florida, yielding a crude extract (110) (Maheshwari et al., 2018).
A study reported a dark septate endophytic fungal strain (JS0464) identified as Gaeumannomyces sp. isolated from the rhizome of the halophyte Phragmites communis. This strain was cultured on a large scale and extracted with ethyl acetate (111). Chemical investigations of JS0464 extracts have identified two glycosylated dialkylresorcinol derivatives, an anthraquinone derivative, and eight known compounds (Lee et al., 2017). The endophytic fungus Aspergillus sp. AP5 has been isolated from the leaves of Phragmites australis; AP5-EtOAc extract (112) has been prepared with ethyl acetate (Abdelgawad et al., 2022).
Other studies have isolated Colletotrichum coccodes from healthy leaf tissues of Houttuynia cordata Thunb. (yielding ethyl acetate extracts [113] and [114]) (Talukdar et al., 2021a; Talukdar et al., 2021b) and Phyllosticta capitalensis (yielding ethyl acetate extract [115]) (Talukdar et al., 2021a).
Penicillium sp. SNF123 has been isolated from the roots of Panax ginseng, and its extract contains Acremonidin E (116) (Kim et al., 2019). Aspergillus terreus has been isolated from the roots of soybean Glycine max; 18 compounds have been identified in its extract (including aspergillide B1 [117] and 3a-hydroxy-3, 5dihydromonacolin L [118]) (El-Hawary et al., 2021), with the highest content being quinones, polyketides, and isocoumarins.
An endophytic fungus identified as Penicillium griseofulvum, which shows considerable antimicrobial activity, has been isolated from Mentha pulegium Extracts using ethyl acetate (119) and dichloromethane (120) show antimicrobial and anticancer activity (Amina et al., 2018). In one study, Arthrinium sp. MFLUCC16-1053 was isolated from Zingiber cassumunar and identified morphologically; an ethyl acetate extract (121) was obtained and it showed antimicrobial and anticancer activity (Pansanit and Pripdeevech, 2018).
6. Activities of metabolites produced by endophytic fungi from MFH plants
As our review shows, endophytic fungi of MFH plants are diverse, and the wide range of metabolites they produce have many bioactive properties, including antioxidant, antimicrobial, anti-inflammatory, and anticancer activities. They are an important source of biologically active metabolites.
6.1. Antioxidants
During the growth of living organisms, free radicals are continuously produced in the body. If these are not scavenged in time and accumulate in the body to excessive levels, they can cause serious harm to human health (Biswas et al., 2017; Jamshidi-kia et al., 2020; Yashin et al., 2017). There are a variety of endophytic fungal metabolites of MFH plants that demonstrate effective free-radical scavenging and antioxidant functions (Xiaoyue et al., 2020).
Bostrycoidin (74) exhibits significant antioxidant activity with an IC50 value of 1.6 μg/mL, which is comparable to butylated hydroxyanisole (BHA), Trolox, and ascorbic acid. Anhydrofusarubin (72), 4-hydroxybenzaldehyde (73), and fusarubin (75) exhibit good antioxidant activity with IC50 values of 12.4, 28.9, and 34.8 μg/mL, respectively (Khan et al., 2018). EPS (78) slightly affects the scavenging of the superoxide radical. Hydroxyl radical-scavenging activity is concentration-dependent (Wang, Y. et al., 2019). In one study, when the EPS (81) concentration was increased to 0.4 mg/mL, the DPPH radical scavenging activity reached 95.50% (Mag et al., 2018). MM2 (82) has high antioxidant activity, and the ability to scavenge α, α-diphenyl-β-picrylhydrazyl (DPPH) free radicals increases linearly with concentration (Suhartati, 2020). EAE (85) shows a varying degree of antioxidant activity, comparable to ascorbic acid (Danagoudar et al., 2017). 3,4-dihydroxybenzeneacetic acid (86) and 3,4-dihydroxyphenylacetic acid methyl ester (87) show potent antioxidant capacities with IC50 values of 14.4 μM and 27.1 μM, respectively (Liu, W. et al., 2021). 5,7-dihydroxy-6,8-dimethyl flavanone (101) also has high antioxidant activity (IC50 of 49.96 μg/mL) (Budiono et al., 2019). The ethyl acetate extract of CK F05-5 (104) exhibits antioxidant activity with an IC50 value of 89.6 μg/mL (Bungtongdee et al., 2019).
6.2. Antimicrobial compounds
The widespread use of antibiotics has enhance the drug resistance of many microbes including large numbers of pathogens (Fadiji and Babalola, 2020). Therefore, it is essential for new antimicrobials to be developed. Endophytic fungi from MFH plants also produce a wide range of antimicrobial substances that deserve further research (Gao et al., 2010; MT et al., 2018; Pavithra et al., 2020; Rajani et al., 2021; Zabalgogeazcoa, 2008).
6.3. Antibacterial and antifungal compounds
When the body's immune function or resistance decreases and the normal flora is dysregulated, bacteria such as Escherichia coli and Candida albicans can invade cells and cause a variety of local tissue and organ infections such as in the human gastrointestinal tract, resulting in disease (Bischoff et al., 2014; Tlaskalova-Hogenova et al., 2005; Tlaskalová-Hogenová et al., 2004; Wang, Y. hua, 2021).
Pretrichodermamide A (2) shows antimicrobial activity against the human pathogenic bacterium Mycobacterium tuberculosis, with a minimum inhibitory concentration (MIC) value of 25 mg/mL (50 mM) (Harwoko et al., 2021). Chaetocochin C (8), chetomin A (9), chetomin C (10), and chetomin (11) have strong antibacterial activity against Staphylococcus aureus, Bacillus subtilis, and Enterococcus faecium, and antifungal activity against Candida albicans with MIC values of 0.12–9.6 μg mL−1 (Peng et al., 2019). Preaustinoid D (16) and dihydroxyneogrifolic acid (17) show moderate activity against C. albicans (MICs of 128 μg/mL), with the latter also exhibiting activities against Bacillus subtilis and S. aureus. Other compounds that have shown robust antibacterial activity include preaustinoid A1 (18), austin (20), and (S)-18,19-dihydroxyneogrifolin (21) (Duan et al., 2016) (see Table 1 for details). Other compounds that have shown antifungal activities include (4S, 6S)-6-[(1S, 2R)-1, 2-dihydroxybutyl]4-hydroxy-4-methoxytetrahydro-2H-pyran-2-one (34), (6S, 2E)-6-hydroxy-3-methoxy-5-oxodec-2-enoic acid (35), LL-P880γ (36), LL-P880α (37), and ergosta-5,7,22-trien-3b-ol (38) (Wu et al., 2016).
Emericellins A (39) and B (40) displayed moderate activities against three bacterial strains (B. subtilis, Bacillus cereus, and E. coli) with MIC values of 25–50 μg/mL (Pang et al., 2018a). Emericelactones A-D (65–68) showed moderate antimicrobial activities against two human pathogenic bacteria (Micrococcus lysodeikticus and S. typhimurium) with MIC values of 25–50 μg/mL (Pang et al., 2018b). Fusarubin (75), anhydrofusarubin (72) and bostrycoidin (74) exhibited highly significant activity against four tested pathogenic bacteria, including Bacillus megaterium, Staphylococcus aureus, Pseudomonas aeruginosa, and E. coli (Khan et al., 2018). The MIC value of fusaric acid (98) and dehydrofusaric acid (99) against Acinetobacter baumannii were 64 μg/mL and 128 μg/mL, respectively (Shi, S. et al., 2018). Drechmerin B (42) (Zhao, J. C. et al., 2018a) and fusaristerol A (100) (Chester et al., 2017) possessed significant antifungal activity toward Candida albicans, with MIC values of 12.5 μg/mL and 8.3 μg/disc, respectively. The antimicrobial activities of the methanol extract of the fungus F. tricinctum containing Ens (90) were tested against Gram-positive and Gram-negative bacteria and fungi (Zaher et al., 2015). Other compounds that have shown antibacteria activities include koninginin W (60), koninginin D (61), 7-O-methylkoninginin D (62), and koninginin A (64) (Wang, Y. L. et al., 2021).
Some endophytic fungal extracts have shown antibacterial activities. Valproic acid-treated fungal extracts (106) showed significant antibacterial efficiency against the bacteria Pseudomonas aeruginosa, Shigella sonnei, and Streptococcus pyogenes (Ameen et al., 2021). An ST9.1-EtOAc extract (107) had active fractions against methicillin-resistant Staphylococcus aureus (MRSA) with a MIC of 2.5 mg/mL and minimum bactericidal concentration (MBC) concentration of 50.0 mg/mL (Techaoei et al., 2020). An AP5-EtOAc extract (112) displayed pronounced antimicrobial properties against S. aureus, E. coli, and Klebsiella sp. with inhibition ratios of 53.04%, 61.23%, and 51.16%, respectively. However, it had only a low antibacterial activity against Proteus vulgaris and Pseudomonas aeruginosa with an inhibition ratio of 15.44% and 30.25%, respectively. In addition, it had the antifungal activity against C. albicans (Abdelgawad et al., 2022). Ethyl acetate extracts of Colletotrichum coccodes (113) and P. capitalensis (115) exhibited considerable antimicrobial activity against S. aureus, E. coli, C. albicans, and P. aeruginosa (Talukdar et al., 2021a; Talukdar et al., 2021b) (see Table 1 for details). High activities have been reported for EtOAc (119) and CHCl2 (120) crude extracts of Penicillium griseofulvum MPR1 against E. coli with maximal inhibition zones of 45.5 and 41.0 mm, respectively (Amina et al., 2018). The ethyl acetate extract of Arthrinium sp. MFLUCC16–1053 (121) showed activity against S. aureus and E. coli with MIC values of 31.25 and 7.81 μg/mL, respectively (Pansanit and Pripdeevech, 2018).
6.4. Antiviral compounds
Endophytic fungi isolated from MFH plants can produce different metabolites with antiviral activity and thus are considered sources of potential antiviral drugs. Such metabolites are less toxic and milder than traditional modern therapeutic drugs. This could open new paths for the future development of food supplements and nutritional products as alternative therapies for the prevention and treatment of infectious diseases (Patel, B. et al., 2021).
An ethyl acetate crude extract of C. papendorfii (103) showed an important antiviral effect against two viral pathogens, the human coronavirus HCoV 229 E and a norovirus surrogate, the feline coronavirus FCV F9 (Khiralla et al., 2020). Aspergillide B1 (117) and 3a-Hydroxy-3, 5dihydromonacolin L (118) showed the highest binding energy scores towards the main protease (Mpro) of COVID-19 at −9.473 and −9.386, respectively (El-Hawary et al., 2021).
6.5. Anti-inflammatory
Many researchers are now searching for biologically active metabolites that can inhibit the production of inflammatory mediators, with a focus on drugs of natural origin (Abdel-azeem and Khalil, 2019). Numerous studies have shown that metabolites produced by endophytic fungi of MFH plants have significant anti-inflammatory effects.
Piperine (6) has the least binding energy (−104.914) with the active site of efflux protein Rv1258c. In addition, compared to widely used non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin, ibuprofen, and diclofenac piperine (6) showed lower binding energy (−92.383) with the active site of prostaglandin synthesizing enzyme cyclooxygenase 2 (COX2), showing anti-inflammatory activity (Krishna et al., 2020). 1,3-dihydroxy-2,8dimethoxy-6-methylanthraquinone (69) reduces tumor necrosis factor (TNF-α)-stimulated reactive oxygen species (ROS), nitric oxide (NO), and prostaglandin E2 (PGE2), attenuates matrix metalloproteinase (MMP)-1 expression, and enhances collagen synthesis. Furthermore, it inhibits the expression of TNF-α-stimulated proinflammatory cytokine mediators, including inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), and proinflammatory cytokine interleukins (IL-1β), IL-6, and IL-8 (Fibroblasts et al., 2021). The addition of DGS1 (79) and DGS2 (80) significantly increased the levels of TNF-α, IL-6, and NO by activating the expression of TNF-α, IL-6, and iNOs genes, and had significant anti-inflammatory activity (Zeng et al., 2019a, Zeng et al., 2019b). The compounds in a JS0464-EtOAc extract (111) showed significant nitric oxide reduction activity in lipopolysaccharide-stimulated microglia BV-2 cells (Lee et al., 2017).
6.6. Anticancer
Cancer is a leading cause of death worldwide. It is speculated that there will be approximately 4.82 million and 2.37 million new cancer cases, and 3.21 million and 0.64 million cancer deaths in China and the US, respectively, in 2022 (Xia et al., 2022). The prevention and treatment of cancer have become important research fields. There are two traditional ways to obtain anticancer drugs: extraction from plants and chemical synthesis. However, due to the scarcity of plant resources and the tedious chemical synthesis pathways, there is increased research interest in identifying anticancer drugs from endophytic fungi (Wanwan et al., 2018).
Cervical cancer, the most common cancer in women worldwide, is usually prevented or treated with vaccines, radiation, chemotherapy, and short courses of therapy; however, chemotherapy drugs can destroy adjacent normal cells or change the nature of the cells, which can lead to undesirable sequelae (Anorlu, 2008; Siddharthar et al., 2014). To alleviate the side effects caused by chemically synthesized therapeutic agents, numerous researchers have explored new therapies using endophytic fungal metabolites. EPS (81), displays potential cytotoxicity against human cervical cancer (HeLa) with the proliferation of 33.10% (Mag et al., 2018). MM2 (82) has the ability to inhibit HeLa with IC50 values of 39.21 μg/mL (Suhartati, 2020). The growth inhibition rates of crude extracts (108) of strain J-1 and J-3 on HeLa cells were 59.6% and 59.1%, respectively (Engel, 2019).
In addition to cervical cancer, breast cancer is one of the most common cancers among women, causing approximately 450,000 deaths worldwide each year (Cancer and Atlas, 2012). In our study, many compounds produced by endophytic fungi isolated from MFH plants showed significant inhibitory effects on breast cancer cells. For example, yamchaetoglobosin A (1) demonstrated cytotoxicity to human breast cancer MCF-7 cell lines with the inhibition ratio of 58% at a concentration of 40 μM (Ruan et al., 2017). Macrooxazole C (5) showed moderate anti-proliferative activities against MCF-7 cell lines with IC50 values of 0.29 mM (Ku et al., 2021). Chetoseminudin F (7), haetocochin C (8), chetomin A (9), chetomin C (10), and chetomin (11) displayed more potent cytotoxicity against human breast cancer MDA-MB-231 cell lines (Peng et al., 2019). Demethylchaetocochin C (12), chaetoperazine A (13), 4-formyl-N-(30-hydroxypyridin-20-yl) benzamide (14), and chetomin (15) inhibited the growth of MCF-7 and MDA-MB-231 (IC50 from 4.5 to 65.0 mM) (Wang, F. et al., 2019). Bipolahydroquinone C (25), cochlioquinone I (26), and cochlioquinones K–N (28–30and 33) showed cytotoxicity against MDA-MB-231 cells with IC50 values ranging from 5.5 to 9.5 μM (Long et al., 2019). Colletotrichalactones A and Ca (45 and 47a) displayed moderate to potent cytotoxic activities against MCF-7 cells with IC50 values of 35.06 and 25.20 μM, respectively (Bang et al., 2020). Beauvericin (92), enniatin B (94), enniatin H (95), and enniatin I (96) show antitumor activities against MCF-7 cell lines with IC50 values of 12.4, 12.22, 11.46, and 10.27 μg/mL (Shi, S. et al., 2018).
Some endophytic fungal extracts have shown antiproliferative activity, including the EtOAc extract of C. papendorfii (103) (Khiralla et al., 2020) and Colletotrichum sp. (109) (Line et al., 2016), they both reveal antiproliferative activity against the MCF-7 cell line.
Triple-negative breast cancer (TNBC) is a heterogeneous disease characterized by a lack of hormonal receptors and HER2 overexpression. It is the only breast cancer subgroup that does not benefit from targeted therapies, and its prognosis is poor (Prado-vázquez et al., 2019). Patients with TNBC are difficult to treat because of poor prognosis, limited treatment options, and resistance to multiple anticancer drugs. Therefore, the search for novel therapeutic agents to treat this complex malignancy is of great value (Razak et al., 2019). Secalonic acid derivative F-7 (102) possesses strong cytotoxic activity against TNBC cells, inducing mitochondrial damage and reactive oxygen species-mediated apoptosis, arresting the G1 phase of the cells in a dose-dependent manner. In addition, it causes significant microtubule disruption in TNBC cells, and restricts cell migration leading to concomitant increase in the expression of cleaved caspase and PARP (Farooq et al., 2020).
Hepatocellular carcinoma remains the fifth most frequently diagnosed cancer and is the second leading cause of cancer-related mortality globally (Xue et al., 2016). Conventional chemotherapeutic drugs have several disadvantages ranging from limited effectiveness, chemoresistance, treatment-related side effects such as nausea and vomiting, and toxicity to nontumor cells (Chopra et al., 2016; Gheena and Ezhilarasan, 2019). Therefore, the current trend of cancer research is the investigation of medicines of plant origin because of their affordability and accessibility with minimal or no side effects (Abu-darwish and Efferth, 2018; Fridlender et al., 2015; Shareef et al., 2016). Pleospyrone A (48), pleospyrones D-E (51 and 52), and a congener (53) were active against human hepatoma cell lines (HepG2) with IC50 values in the range of 1.26–47.5 μM (Lai et al., 2020). Chaephilone C (54) showed cytotoxicity against HepG2 cells with an IC50 value of 38.6 μM compared to camptothecin (32.3 μM) (Song, C. et al., 2020). Brefeldin A (83) and brefeldin A 7-O-acetate (84) have strong anticancer and antiviral effects and inhibit cell growth by inducing S-phase arrest in HepG2 cells, with an IC50 value of 0.024 μM (Xie et al., 2017). Pin (88) and PMG (89) significantly inhibit the adhesion and migration of HepG2 cells by blocking MMP-9 expression (Li, Q. et al., 2016). The TY5-EtOAc extract (105) induced apoptosis by increasing Bax and decreasing Bcl 2 and had a significant inhibitory effect on HepG2 viability in human hepatoma cells in a dose-dependent manner (Ty et al., 2018).
There are many other cancerous diseases such ovarian cancer, colon cancer, lung cancer, and lymphoma that require considerable chemical treatment (Markowitz et al., 2002; Minna et al., 2002; Mugnaini and Ghosh, 2016; Stewart et al., 2019). This can cause irreversible damage to the human body, while the biological activity of metabolites produced by endophytic fungi from MFH plants can effectively prevent these cancers without harmful effects. For example, epicorazine A (3) exhibited strong to moderate cytotoxicity against mouse lymphoma cells (L5178Y), human B-lymphoma cells (Ramos), and human acute T lymphoblastic leukemia cells (Jurkat J16) with IC50 values ranging from 1.3 to 28 mM (Harwoko et al., 2021). Epoxycytochalasin H (4) inhibits ovarian cancer cell A2780 viability, impairs mitochondrial function, activates mitophagy, and induces the mitochondrial apoptosis pathway (Xu, 2020). (4S, 6S)-6-[(1S, 2R)-1, 2-dihydroxybutyl]4-hydroxy-4-methoxytetrahydro-2H-pyran-2-one (34), (6S, 2E)-6-hydroxy-3-methoxy-5-oxodec-2-enoic acid (35), LL-P880γ (36), and LL-P880α (37) showed significant cytotoxic activities against human gastric cancer (MKN45), human colon cancer (LOVO), human carcinoma cells (A549), and human promyelocytic leukemia (HL-60) cell lines with IC50 values lower than 200 μM. All of these except LL-P880γ (36), as well as Ergosta-5,7,22-trien-3b-ol (38), show strong cytotoxicity against the human colon cancer (LOVO) cell line with IC50 values lower than 100 μM (Wu et al., 2016).
Shearilicine (41) exhibited cytotoxic activity against L5178Y and A2780 (Ariantari et al., 2019). The IC50 values for terpenoid-alkaloid (43) against human pancreatic cancer cells (Panc-1), human lymphoblastoid cells (NBT-T2), and human colon cancer cells (HCT-116) were 0.05, 0.75, and 0.05 parts per million (ppm), respectively. Furthermore, the IC50 values for sesterterpenoid (44) were 0.71, 0.30, and 0.67 ppm against these respective cell lines (Lut et al., 2021). GKK1032B (55) showed significant cytotoxicity against human osteosarcoma cell line MG63 with an IC50 value of 3.49 μmol L−1 (Na et al., 2022). Alternariol (56), alternariol-5-O-methyl ether (57), altenusin (58), and altertoxin II (59) exhibited cytotoxic activity against L5178Y with IC50 values of 1.7, 7.8, 6.8, and 6.2 μM, respectively (Orfali et al., 2017). Fusarubin (70) showed significant neuroprotective activity against glutamate-mediated HT22 (mouse hippocampal neuron cell) death (Choi et al., 2020).
In addition, anhydrofusarubin (72), 4-hydroxybenzaldehyde (73), bostrycoidin (74), and 3,5,9-trihydroxyergosta-7,22-diene-6-one (77) inhibited cell proliferation activity or activate apoptosis or necrosis in transformed kidney cells or kidney (renal) cancer cells (Khan et al., 2018). Fusaristerol A (100) displayed potent cytotoxic potential toward HCT-116 with an IC50 value of 0.21 μM (Chester et al., 2017). Crude extracts of Nigrospora sphaerica (110) exhibited antiproliferative and anti-migratory effects on a solid tumor human glioma cell line (U251) and A549 (Maheshwari et al., 2018). Acremonidin E (116) inhibited melanin synthesis and activation of B16F10 and human melanoma cell line MNT-1 through downregulation of tyrosinase (Kim et al., 2019).
The inhibitory activities of these metabolites summarized above will facilitate the introduction of corresponding anticancer drugs for different cancers. Therefore, MFH plants are an important potential source of new anticancer drugs or precursors, providing a scientific basis and new ideas for the industrial production of such compounds.
7. Conclusion and perspectives
This review highlights the metabolites produced by endophytic fungi isolated from MFH plants that have been studied in recent years, as well as the activities of these compounds for the treatment of human diseases.
MFH plants are not only delicious foods for human consumption but their endophytic fungal metabolites also have a variety of beneficial bioactive properties. Recent studies have shown that endophytic fungi isolated from MFH plants can produce compounds with antioxidant, antimicrobial, anti-inflammatory and anticancer activities, which could enhance human immunity as well as improve human health, and are expected to become effective, safe and nontoxic therapeutic nutraceuticals for the prevention and treatment of diseases.
However, there are still some limitations in the development and utilization of these metabolites. To date, only 48.65% of MFH substances have been studied. In addition, only a small portion of the endophytic fungal resources have been extracted from MFH plants due to limitations associated with isolation and identification technologies. This could be improved using an effective combination of traditional isolation methods and modern advanced technologies, such multilocus sequence typing (MLST), metagenomics, and next-generation sequencing (NGS), thereby enriching the resource library of endophytic fungi.
Most studies on endophytic fungi from MFH plants have focused on their isolation and identification. The structures, compositions, and functions of metabolites produced by endophytic fungi from MFH are far from being explicit. In recent years, there has been great interest in the activity of such metabolites due to their biochemical diversity. The types of compounds consist mainly of alkaloids, terpenoids, polyketides, peptides, polysaccharides, quinones, esters, phenols, flavonoids, and steroids. The activities mainly include antioxidant, antimicrobial, anti-inflammatory, and anticancer activities. However, there is very little information regarding anti-disease mechanisms and medical applications, and more in-depth research is needed. Much of the work reported on bioactive endophytic fungi from MFH plants has remained at the experimental stage and has not been further applied in food or medicine. Effective active strains identified to be beneficial to humans should be further screened, with efforts to carry out large-scale fermentation and production of these strains.
Furthermore, studies on the mechanisms of endophytic fungal resourced-metabolites from MFH plants are not currently sufficient. More in-depth investigations using microbiology, bioinformatics, transcriptome analysis, reverse molecular docking, and network pharmacological technology are needed to scientifically understand their ecological roles and food and medical applications. Such studies would also provide new ideas for the development of such metabolites into mild, safe, and effective therapeutic nutraceuticals with preventive and therapeutic effects on inflammation and cancer.
CRediT authorship contribution statement
Jun Zhang: Data curation, Writing – original draft. Yihui Zhu: Data curation. Jinping Si: Supervision. Lingshang Wu: Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by “Pioneer” and “Leading Goose” R&D Program of Zhejiang [2022C02009] and the Major Science and Technology Projects of Breeding New Varieties of Agriculture in Zhejiang Province [2021C02074].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2022.10.006.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Fig. S1 Taxonomy of 53 MFH plants involved in the reference survey and analysis. The different colored columns represent endophytic fungal orders; Numbers represent the number of endophytic fungi in the corresponding plant genus.
Fig. S2 Taxonomy of 21 endophytes isolated from MFH plants involved in the reference survey and analysis. The different colored columns represent different types of metabolites; Numbers represent the number of metabolites in the corresponding endophytic fungal genus.
Fig. S3 Taxonomy of 108 active metabolites in endophytic fungi isolated from MFH plants involved in the reference survey and analysis. The different colored columns represent different activities of metabolites; Numbers represent the number of metabolites in the corresponding activity.
References
- Abdel-azeem M.A., Khalil W.F. Endophytic fungi as a new source of antirheumatoid metabolites. Bioact. Food as Diet. Interv. Arthritis Relat. Inflamm. Dis. 2019:355–384. doi: 10.1016/B978-0-12-813820-5.00021-0. [DOI] [Google Scholar]
- Abdelgawad M.A., Hamed A.A., Nayl A.A., Badawy M.S.E.M., Ghoneim M.M., Sayed A.M., Hassan H.M., Gamaleldin N.M. The chemical profiling, docking study, and antimicrobial and antibiofilm activities of the endophytic fungi Aspergillus sp AP5. Molecules. 2022;27:1–13. doi: 10.3390/molecules27051704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-darwish M.S., Efferth T. Medicinal plants from near east for cancer therapy. Front. Pharmacol. 2018;9:1–17. doi: 10.3389/fphar.2018.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alurappa R., Chowdappa S., Radhakrishnan N., Sinniah U.R., Mohanty S.K., Swamy M.K. Endophytic fungi and bioactive metabolites production: an update. Microb. Biotechnol. 2018;2:455–482. doi: 10.1007/978-981-10-7140-9_21. [DOI] [Google Scholar]
- Ameen F., Almansob A., Tami M. Al, Al-enazi N., Al-sabri A., Orfali R. Epigenetic modifiers affect the bioactive compounds secreted by an endophyte of the tropical plant Piper longum. Molecules. 2021;26:1–15. doi: 10.3390/molecules26010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amina Z., Nouari S., Rasime D., Sabrina B., Daoud H. Antibacterial activity of endophytic fungus, Penicillium griseofulvum MPR1 isolated from medicinal plant, Mentha pulegium L. Afr. J. Microbiol. Res. 2018;12:1056–1066. doi: 10.5897/AJMR2018.8887. [DOI] [Google Scholar]
- Amirzakariya B.Z., Shakeri A. Bioactive terpenoids derived from plant endophytic fungi : an updated review (2011-2020) Phytochemistry. 2022;197:1–36. doi: 10.1016/j.phytochem.2022.113130. [DOI] [PubMed] [Google Scholar]
- Anorlu R.I. Cervical cancer: the sub-Saharan African perspective. Reprod. Health Matters. 2008;16:41–49. doi: 10.1016/S0968-8080(08)32415-X. [DOI] [PubMed] [Google Scholar]
- Ariantari N.P., Ancheeva E., Wang C., Mándi A., Knedel T.O., Kurtán T., Chaidir C., Müller W.E.G., Kassack M.U., Janiak C., Daletos G., Proksch P. Indole diterpenoids from an endophytic Penicillium sp. J. Nat. Prod. 2019;82:1412–1423. doi: 10.1021/acs.jnatprod.8b00723. [DOI] [PubMed] [Google Scholar]
- Bang S., Kwon H.E., Baek J.Y., Jang D.S., Kim S., Nam S.J., Lee D., Kang K.S., Shim S.H. Colletotrichalactones A-Ca, unusual 5/6/10-fused tricyclic polyketides produced by an endophytic fungus, Colletotrichum sp. JS-0361. Bioorg. Chem. 2020;105:1–6. doi: 10.1016/j.bioorg.2020.104449. [DOI] [PubMed] [Google Scholar]
- Bielecka M., Pencakowski B., Nicoletti R. Using next-generation sequencing technology to explore genetic pathways in endophytic fungi in the syntheses of plant bioactive metabolites. Agriculture. 2022;12:1–18. doi: 10.3390/agriculture12020187. [DOI] [Google Scholar]
- Bischoff S.C., Barbara G., Buurman W., Ockhuizen T., Schulzke J.D., Serino M., Tilg H., Watson A., Wells J.M. Intestinal permeability - a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:1–25. doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S., Das R., Banerjee E.R. Role of free radicals in human inflammatory diseases. AIMS Biophys. 2017;4:596–614. doi: 10.3934/biophy.2017.4.596. [DOI] [Google Scholar]
- Budiono, Elfita, Muharni, Yohandini H., Widjajanti H. Antioxidant activity of Syzygium samarangense L. and their endophytic fungi. Molekul. 2019;14:48–55. doi: 10.20884/1.jm.2019.14.1.503. [DOI] [Google Scholar]
- Bungtongdee N., Sopalun K., Laosripaiboon W., Iamtham S. The chemical composition, antifungal, antioxidant and antimutagenicity properties of bioactive compounds from fungal endophytes associated with Thai orchids. J. Phytopathol. 2019;167:56–64. doi: 10.1111/jph.12773. [DOI] [Google Scholar]
- Butler M.S., Robertson A.A.B., Cooper M.A. Natural product and natural product derived drugs in clinical trials. Nat. Prod. Rep. 2014;31:1612–1661. doi: 10.1039/c4np00064a. [DOI] [PubMed] [Google Scholar]
- Caicedo N.H., Davalos A.F., Caicedo P.A., Puente P.A., Rodríguez A.Y. Antioxidant activity of exo ‐ metabolites produced by Fusarium oxysporum: an endophytic fungus isolated from leaves of Otoba gracilipes. Microbiol. Open. 2019;8:1–7. doi: 10.1002/mbo3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer T., Atlas G. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso G., Abdelhamid M.T., Kalisz A., Sekara A. Linking endophytic fungi to medicinal plants therapeutic activity. A case study on asteraceae. Agriculture. 2020;10:1–23. doi: 10.3390/agriculture10070286. [DOI] [Google Scholar]
- Chakraborty A., Kundu S., Mukherjee S. In: Endophytes and Secondary Metabolites. Jha S., editor. Springer; Cham: 2019. Endophytism in Zingiberaceae : elucidation of beneficial impact; pp. 187–212. [DOI] [Google Scholar]
- Chau C.F., Wu S.H. The development of regulations of Chinese herbal medicines for both medicinal and food uses. Trends Food Sci. Technol. 2006;17:313–323. doi: 10.1016/j.tifs.2005.12.005. [DOI] [Google Scholar]
- Chen X., Sun M., Chong S., Si J., Wu L. Transcriptomic and metabolomic approaches deepen our knowledge of plant-endophyte interactions. Front. Plant Sci. 2022;12:1–25. doi: 10.3389/fpls.2021.700200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester K., Zahiruddin S., Ahmad A., Khan W., Paliwal S., Ahmad S. Fusaristerol A: a new cytotoxic and antifungal ergosterol fatty acid ester from the endophytic fungus Fusarium sp. associated with Mentha longifolia roots. Phcog. Mag. 2017;13:179–188. doi: 10.4103/pm.pm. [DOI] [Google Scholar]
- Chithra S., Jasim B., Anisha C., Mathew J., Radhakrishnan E.K. LC-MS/MS based identification of piperine production by endophytic Mycosphaerella sp. PF13 from Piper nigrum. Appl. Biochem. Biotechnol. 2014;173:30–35. doi: 10.1007/s12010-014-0832-3. [DOI] [PubMed] [Google Scholar]
- Chithra S., Jasim B., Sachidanandan P., Jyothis M., Radhakrishnan E.K. Piperine production by endophytic fungus Colletotrichum gloeosporioides isolated from Piper nigrum. Phytomedicine. 2014;21:534–540. doi: 10.1016/j.phymed.2013.10.020. [DOI] [PubMed] [Google Scholar]
- Choi H.G., Song J.H., Park M., Kim S., Kim C., Kang K.S., Shim S.H. Neuroprotective γ-pyrones from Fusarium Solani JS-0169 : cell-based identification of active compounds and an informatics approach to predict the mechanism of action. Biomolecules. 2020;10:1–11. doi: 10.3390/biom10010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra D., Rehan H.S., Sharma V., Mishra R. Chemotherapy-induced adverse drug reactions in oncology patients: a prospective observational survey. Indian J. Med. Paediatr. Oncol. 2016;37:42–46. doi: 10.4103/0971-5851.177015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenhusz M.J.M., Byng J.W. The number of known plants species in the world and its annual increase. Phytotaxa. 2016;261:201–217. doi: 10.11646/phytotaxa.261.3.1. [DOI] [Google Scholar]
- Clardy J., Fischbach M.A., Walsh C.T. New antibiotics from bacterial natural products. Nat. Biotechnol. 2006;24:1541–1550. doi: 10.1038/nbt1266. [DOI] [PubMed] [Google Scholar]
- Cui Y., Yi D., Bai X., Sun B., Zhao Y., Zhang Y. Ginkgolide B produced endophytic fungus (Fusarium oxysporum) isolated from Ginkgo biloba. Fitoterapia. 2012;83:913–920. doi: 10.1016/j.fitote.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Cultures S. Effects of polysaccharide elicitors from endophytic Fusarium oxysporum Fat 9 on the growth, flavonoid accumulation and antioxidant property of Fagopyrum tataricum sprout cultures. Molecules. 2016;21:1–13. doi: 10.3390/molecules21121590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalzell G. Antibacterial metabolites from Bipolaris specifera, an endophytic fungus from the endemic medicinal plant Zingiber nimmonii (J. Graham) Dalzell. 3 Biotech. 2020;10:1–8. doi: 10.1007/s13205-020-02307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danagoudar A., Joshi C.G., Sunil Kumar R., Poyya J., Nivya T., Hulikere M.M., Anu Appaiah K. Molecular profiling and antioxidant as well as anti-bacterial potential of polyphenol producing endophytic fungus-Aspergillus austroafricanus CGJ-B3. Mycology. 2017;8:28–38. doi: 10.1080/21501203.2017.1281358. [DOI] [Google Scholar]
- Demain A.L. Importance of microbial natural products and the need to revitalize their discovery. J. Ind. Microbiol. Biotechnol. 2014;41:185–201. doi: 10.1007/s10295-013-1325-z. [DOI] [PubMed] [Google Scholar]
- Diling C., Xin Y., Chaoqun Z., Jian Y., Xiaocui T., Jun C., Ou S., Yizhen X. Extracts from Hericium erinaceus relieve inflammatory bowel disease by regulating immunity and gut microbiota. Oncotarget. 2017;8:85838–85857. doi: 10.18632/oncotarget.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donot F., Fontana A., Baccou J.C., Schorr-galindo S. Microbial exopolysaccharides: main examples of synthesis, excretion, genetics and extraction. Carbohydr. Polym. 2012;87:951–962. doi: 10.1016/j.carbpol.2011.08.083. [DOI] [Google Scholar]
- Downer S., Berkowitz S.A., Berkowitz S.A., Harlan T.S., Olstad D.L., Mozaffarian D. Food is medicine: actions to integrate food and nutrition into healthcare. BMJ. 2020;369:1–6. doi: 10.1136/bmj.m2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan R., Zhou H., Yang Y., Li H., Dong J., Li X., Chen G., Zhao L., Ding Z. Antimicrobial meroterpenoids from the endophytic fungus Penicillium sp. T2-8 associated with Gastrodia elata. Phytochem. Lett. 2016;18:197–201. doi: 10.1016/j.phytol.2016.10.013. [DOI] [Google Scholar]
- El-hawary S.S., Moawad A.S., Bahr H.S. Natural product diversity from the endophytic fungi of the genus Aspergillus. RSC Adv. 2020;10:22058–22079. doi: 10.1039/d0ra04290k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hawary S.S., Mohammed R., Bahr H.S., Attia E.Z., El-Katatny M.H., Abelyan N., Al-Sanea M.M., Moawad A.S., Abdelmohsen U.R. Soybean-associated endophytic fungi as potential source for anti-COVID-19 metabolites supported by docking analysis. J. Appl. Microbiol. 2021;131:1193–1211. doi: 10.1111/jam.15031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel Study on endophytic mycobacterium secondary metabolites and anti-tumor activity of cervical cancer in three strains of crude extracts. Feb-Fresenius Environ. Bull. 2019;28:226–232. [Google Scholar]
- Fadiji A.E., Babalola O.O. Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Front. Bioeng. Biotechnol. 2020;8:1–20. doi: 10.3389/fbioe.2020.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq S., Qayum A., Nalli Y., Lauro G., Chini M.G., Bifulco G., Chaubey A., Singh S.K., Riyaz-Ul-Hassan S., Ali A. Discovery of a secalonic acid derivative from Aspergillus aculeatus, an endophyte of Rosa damascena Mill., triggers apoptosis in MDA-MB-231 triple negative breast cancer cells. ACS Omega. 2020;5:24296–24310. doi: 10.1021/acsomega.0c02505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S., Cheng H., Xu Z., Feng S., Yuan M., Huang Y., Liao J., Ding C. Antioxidant and anti-aging activities and structural elucidation of polysaccharides from Panax notoginseng root. Process Biochem. 2019;78:189–199. doi: 10.1016/j.procbio.2019.01.007. [DOI] [Google Scholar]
- Feng S., LuQi H., Juan G., Min C. History and development of “one root of medicine and food. Chin. Bull. Life Sci. 2015;27:1061–1069. doi: 10.13376/j.cbls/2015146. [DOI] [Google Scholar]
- Fibroblasts D., Lee S., Nguyen Q.N., Phung H.M., Shim S.H., Kim D. Preventive effects of anthraquinones isolated from an endophytic fungus, Colletotrichum sp. JS-0367 in tumor necrosis factor-α-stimulated damage of human dermal fibroblasts. Antioxidants. 2021;10:1–16. doi: 10.3390/antiox10020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach M.A., Walsh C.T. Assembly-line enzymology for polyketide and nonribosomal peptide antibiotics: logic machinery, and mechanisms. Chem. Rev. 2006;106:3468–3496. doi: 10.1021/cr0503097. [DOI] [PubMed] [Google Scholar]
- Freitas F., Alves V.D., Reis M.A.M. Advances in bacterial exopolysaccharides: from production to biotechnological applications. Trends Biotechnol. 2011;29:388–398. doi: 10.1016/j.tibtech.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Fridlender M., Kapulnik Y., Koltai H. Plant derived substances with anti-cancer activity: from folklore to practice. Front. Plant Sci. 2015;6:1–9. doi: 10.3389/fpls.2015.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J., Hu L., Shi Z., Sun W., Yue D., Wang Y., Ma X., Zuo Z., Peng G., Zhong Z., Deng J. Two metabolites isolated from endophytic fungus Coniochaeta sp. F-8 in Ageratina adenophora exhibit antioxidative activity and cytotoxicity. Nat. Prod. Res. 2019;35:1–9. doi: 10.1080/14786419.2019.1675060. [DOI] [PubMed] [Google Scholar]
- Gao F.K., Dai C.C., Liu X.Z. Mechanisms of fungal endophytes in plant protection against pathogens. Afr. J. Microbiol. Res. 2010;4:1346–1351. doi: 10.1111/j.0307-6946.2004.00642.x. [DOI] [Google Scholar]
- Gheena S., Ezhilarasan D. Syringic acid triggers reactive oxygen species–mediated cytotoxicity in HepG2 cells. Hum. Exp. Toxicol. 2019;38:694–702. doi: 10.1177/0960327119839173. [DOI] [PubMed] [Google Scholar]
- Gómez O.C., Honorata J., Luiz H. Endophytic fungi isolated from medicinal plants : future prospects of bioactive natural products from Tabebuia/Handroanthus endophytes. Appl. Microbiol. Biotechnol. 2018;102:1–15. doi: 10.1007/s00253-018-9344-3. [DOI] [PubMed] [Google Scholar]
- Gong X., Chen N., Ren K., Jia J., Wei K., Zhang L., Lv Y., Wang J., Li M. The fruits of siraitia grosvenorii: a review of a Chinese food-medicine. Front. Pharmacol. 2019;10:1–11. doi: 10.3389/fphar.2019.01400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonga X., Jia M., Xua J., Zhanga C., Li M. Hypoglycemic effects of bioactive ingredients from medicine food homology and medicinal health food species used in China. Crit. Rev. Food Sci. Nutr. 2020;60:2303–2326. doi: 10.1080/10408398.2019.1634517. [DOI] [PubMed] [Google Scholar]
- Gu P., Liu Z., Sun Y., Ou N., Hu Y., Liu J., Wu Y., Wang D. Angelica sinensis polysaccharide encapsulated into PLGA nanoparticles as a vaccine delivery and adjuvant system for ovalbumin to promote immune responses. Int. J. Pharm. 2019;554:72–80. doi: 10.1016/j.ijpharm.2018.11.008. [DOI] [PubMed] [Google Scholar]
- Gunatilaka A.A.L. Natural products from plant-associated microorganisms: distribution, structural diversity, bioactivity and implication of their occurrence. J. Nat. Prod. 2016;69:6–26. doi: 10.1021/np058128n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagag A., Abdelwahab M.F., El-kader A.M.A., Fouad M.A. The endophytic Aspergillus strains: a bountiful source of natural products. J. Appl. Microbiol. 2022;132:4150–4169. doi: 10.1111/jam.15489. [DOI] [PubMed] [Google Scholar]
- Harwoko H., Daletos G., Stuhldreier F., Lee J., Wesselborg S., Feldbrügge M., Müller W.E.G., Ancheeva E., Proksch P., Harwoko H., Daletos G., Stuhldreier F., Lee J., Wesselborg S., Feldbrügge M., Müller W.E.G., Kalscheuer R. Dithiodiketopiperazine derivatives from endophytic fungi Trichoderma harzianum and Epicoccum nigrum. Nat. Prod. Res. 2021;35:257–265. doi: 10.1080/14786419.2019.1627348. [DOI] [PubMed] [Google Scholar]
- He Y., Peng H., Zhang H., Liu Y., Sun H. Structural characteristics and immunopotentiation activity of two polysaccharides from the petal of Crocus sativus. Int. J. Biol. Macromol. 2021;180:129–142. doi: 10.1016/j.ijbiomac.2021.03.006. [DOI] [PubMed] [Google Scholar]
- Higgins K.L., Arnold A.E., Coley P.D., Kursar T.A. Communities of fungal endophytes in tropical forest grasses: highly diverse host- and habitat generalists characterized by strong spatial structure. Fungal Ecol. 2014;8:1–11. doi: 10.1016/j.funeco.2013.12.005. [DOI] [Google Scholar]
- Jamshidi-kia F., Wibowo J.P., Elachouri M., Masumi R. Battle between plants as antioxidants with free radicals in human body. J. Herbmed Pharmacol. 2020;9:191–199. doi: 10.34172/jhp.2020.25. [DOI] [Google Scholar]
- Jin M., Zhao K., Huang Q., Xu C., Shang P. Isolation, structure and bioactivities of the polysaccharides from Angelica sinensis (Oliv.) Diels: a review. Carbohydr. Polym. 2012;89:713–722. doi: 10.1016/j.carbpol.2012.04.049. [DOI] [PubMed] [Google Scholar]
- Jin Z., Li D., Liu T., Yu F., Zhang Z., Su C., Wang Y., Guo Q., Liu Z. Cultural endophytic fungi associated with Dendrobium officinale: identification, diversity estimation and their antimicrobial potential. Curr. Sci. 2017;112:1690–1697. doi: 10.18520/cs/v112/i08/1690-1697. [DOI] [Google Scholar]
- Kamel N.M., Abdel F.F., Soad M., Zayat A. El. Endophytic fungi from the medicinal herb Euphorbia geniculata as a potential source for bioactive metabolites. Arch. Microbiol. 2019;202:1–9. doi: 10.1007/s00203-019-01740-x. [DOI] [PubMed] [Google Scholar]
- Khan N., Afroz F., Begum M.N., Roy Rony S., Sharmin S., Moni F., Mahmood Hasan C., Shaha K., Sohrab M.H. Endophytic Fusarium solani: a rich source of cytotoxic and antimicrobial napthaquinone and aza-anthraquinone derivatives. Toxicol Rep. 2018;5:970–976. doi: 10.1016/j.toxrep.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khiralla A., Spina R., Varbanov M., Philippot S., Lemiere P., Slezack-Deschaumes S., André P., Mohamed I., Yagi S.M., Laurain-Mattar D. Evaluation of antiviral, antibacterial and antiproliferative activities of the endophytic fungus Curvularia papendorfii, and isolation of a new polyhydroxyacid. Microorganisms. 2020;8:1–20. doi: 10.3390/microorganisms8091353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Jeong H., Yang I., Nam S., Lim K. Acremonidin E produced by Penicillium sp. SNF123, a fungal endophyte of Panax ginseng, has anti-melanogenic activities. J. Ginseng Res. 2019;45:1–34. doi: 10.1016/j.jgr.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna T., Yashwanti K., Surendra U., Ateet S., Kumar K.M. Piperine production from endophytic fungi of Piper nigrum l and in Silico approach for anti-inflammatory and anti- mycobacterial potential. Int. Res. J. Biol. Sci. 2020;8:8–15. [Google Scholar]
- Ku H., Baek J., Kang K.S., Shim S.H. A new anti-proliferative compound from an endophytic fungus, Phoma sp. Nat. Prod. Res. 2021;1–7 doi: 10.1080/14786419.2021.2022663. [DOI] [PubMed] [Google Scholar]
- Kumar A.S., Mody K., Jha B. Bacterial exopolysaccharides-a perception. J. Basic Microbiol. 2007;47:103–117. doi: 10.1002/jobm.200610203. [DOI] [PubMed] [Google Scholar]
- Kusari S., Pandey S.P., Spiteller M. Untapped mutualistic paradigms linking host plant and endophytic fungal production of similar bioactive secondary metabolites. Phytochemistry. 2012;91:1–7. doi: 10.1016/j.phytochem.2012.07.021. [DOI] [PubMed] [Google Scholar]
- Lai D., Mao Z., Zhiyao Z., Siji Z., Xue M., Dai J., Ligang Z., Li D. New chlamydosporol derivatives from the endophytic fungus Pleosporales sp. Sigrf05 and their cytotoxic and antimicrobial activities. Sci. Rep. 2020;10:1–9. doi: 10.1038/s41598-020-65148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lean M.E.J., Leslie W.S., Barnes A.C., Brosnahan N., Thom G., McCombie L., Peters C., Zhyzhneuskaya S., Al-Mrabeh A., Hollingsworth K.G., Rodrigues A.M., Rehackova L., Adamson A.J., Sniehotta F.F., Mathers J.C., Ross H.M., McIlvenna Y., Welsh P., Kean S., et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019;7:344–355. doi: 10.1016/S2213-8587(19)30068-3. [DOI] [PubMed] [Google Scholar]
- Lee C., Kim S., Li W., Bang S., Lee H., Lee H.J., Noh E.Y., Park J.E., Bang W.Y., Shim S.H. Bioactive secondary metabolites produced by an endophytic fungus Gaeumannomyces sp. JS0464 from a maritime halophyte Phragmites communis. J. Antibiot. 2017;70:737–742. doi: 10.1038/ja.2017.39. [DOI] [PubMed] [Google Scholar]
- Li P., Luo C., Sun W., Lu S., Mou Y., Peng Y., Zhou L. In vitro antioxidant activities of polysaccharides from endophytic fungus Fusarium oxysporum Dzf17. Afr. J. Microbiol. Res. 2011;5:5990–5993. doi: 10.5897/AJMR11.1342. [DOI] [Google Scholar]
- Li Q., Zhang Y., Shi J.-L., Wang Y.-L., Zhao H.-B., Shao D., Huang Q.-S., Yang H., Jin M.-L. Mechanism and anticancer activity of the metabolites of an endophytic fungi from Eucommia ulmoides Oliv. Anti Cancer Agents Med. Chem. 2016;17:982–989. doi: 10.2174/1871520616666160923094814. [DOI] [PubMed] [Google Scholar]
- Li T., Xu S., Bi J., Huang S., Fan B., Qian C. Metabolomics study of polysaccharide extracts from Polygonatum sibiricum in mice based on 1H NMR technology. J. Sci. Food Agric. 2020;100:4627–4635. doi: 10.1002/jsfa.10523. [DOI] [PubMed] [Google Scholar]
- Liang J., Huo X., Cheng Z., Sun C., Wu Y., Deng X., Zhang Y. An indole diterpenoid isolated from the fungus Drechmeria sp. and its antimicrobial activity. Nat. Prod. Res. 2018;33:1–7. doi: 10.1080/14786419.2018.1501050. [DOI] [PubMed] [Google Scholar]
- Line T.M.-C., Ningrum R.A., Wisnuwardhani P.H. Inhibitory activity of endophytic fungi isolated from sukabumi turmeric plant (Curcuma longa L.) towards MCF-7 cell line. Indones. J. Pharm. 2016;28:19–25. doi: 10.14499/indonesianjpharm28iss1pp19. [DOI] [Google Scholar]
- Ling Q., Guang Y., Ying L., Feng S., Min C., Lu-qi H. Overview of revision of the catalogue of the substances traditionally considered as both food and Chinese medicine. Chin. Pharmaceut. J. 2017;52:521–524. doi: 10.11669/cpj.2017.07.001. [DOI] [Google Scholar]
- Liu C. Understanding “ medicine and food homology. developing utilization in medicine functions. Chinese Herb. Med. 2018;10:337–338. doi: 10.1016/j.chmed.2018.10.006. [DOI] [Google Scholar]
- Liu J., Wang X., Pu H., Liu S., Kan J., Jin C. Recent advances in endophytic exopolysaccharides: production, structural characterization, physiological role and biological activity. Carbohydr. Polym. 2016;157:1–12. doi: 10.1016/j.carbpol.2016.10.084. [DOI] [PubMed] [Google Scholar]
- Liu W., Li W., Sui Y., Li X.Q., Liu C., Jing H., Zhang H., Cao W. Structure characterization and anti-leukemia activity of a novel polysaccharide from Angelica sinensis (Oliv.) Diels. Int. J. Biol. Macromol. 2019;121:161–172. doi: 10.1016/j.ijbiomac.2018.09.213. [DOI] [PubMed] [Google Scholar]
- Liu W., Liu Y., Yang F., Han S., Zhang J., Yang H., Cheng Z., Li Q. Asperflamides A and B from aspergillus flavipes dz-3, an endophytic fungus of Eucommia ulmoides oliver. Molecules. 2021;26:3–10. doi: 10.3390/molecules26123514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.G., Lu X., Gao W., Li P., Yang H. Structure, synthesis, biosynthesis, and activity of the characteristic compounds from Ginkgo biloba L. Nat. Prod. Rep. 2021;39:474–511. doi: 10.1039/d1np00026h. [DOI] [PubMed] [Google Scholar]
- Long Y., Tang T., Wang L.Y., He B., Gao K. Absolute configuration and biological activities of meroterpenoids from an endophytic fungus of Lycium barbarum. J. Nat. Prod. 2019;82:1–9. doi: 10.1021/acs.jnatprod.9b00288. [DOI] [PubMed] [Google Scholar]
- Lu G., Liu Z., Wang X., Wang C. Recent advances in Panax ginseng C.A. Meyer as a herb for anti-fatigue: an effects and mechanisms review. Foods. 2021;10:1–22. doi: 10.3390/foods10051030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lut A., Munir E., Yurnaliza Y., Basyuni M. Chemical analysis and anticancer activity of sesterterpenoid from an endophytic fungus Hypomontagnella monticulosa Zg15SU and its host Zingiber griffithii Baker. Heliyon. 2021;7(2) doi: 10.1016/j.heliyon.2021.e06292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mag P., Wen L., Xu Y., Wei Q., Chen W., Chen G. Modeling and optimum extraction of multiple bioactive exopolysaccharide from an endophytic fungus of Crocus sativus L. Phcog. Mag. 2018;14:36–43. doi: 10.4103/pm.pm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshwari A., Mmbaga M., Quick Q. Nigrospora sphaerica products from the flowering dogwood exhibit antitumorigenic effects via the translational regulator, pS6 ribosomal protein. Proc. Anticancer Res. 2018;2:8–15. doi: 10.26689/par.v2i3.358. [DOI] [Google Scholar]
- Manoj M.V., Anil B. Fusarium tricinctum, an endophytic fungus exhibits cell growth inhibition and antioxidant activity. Indian J. Microbiol. 2016;56:1–6. doi: 10.1007/s12088-016-0600-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie R., Toghueo K. Bioprospecting endophytic fungi from Fusarium genus as sources of bioactive metabolites metabolites. Mycology. 2019;11:1–21. doi: 10.1080/21501203.2019.1645053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie R., Toghueo K., Fekam F. Endophytic Penicillium species and their agricultural, biotechnological, and pharmaceutical applications. 3 Biotech. 2020;10:1–35. doi: 10.1007/s13205-020-2081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz S.D., Dawson D.M., Willis J., Willson J.K.V. Focus on colon cancer. Cancer Cell. 2002;1:233–236. doi: 10.1016/S1535-6108(02)00053-3. [DOI] [PubMed] [Google Scholar]
- Mei X., Hai C., Dong L., Xing K. Diversity and antimicrobial and plant-growth-promoting activities of endophytic fungi in Dendrobium loddigesii Rolfe. J. Plant Growth Regul. 2010;29:328–337. doi: 10.1007/s00344-010-9139-y. [DOI] [Google Scholar]
- Mendes R., Garbeva P., Raaijmakers J.M. The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013;37:634–663. doi: 10.1111/1574-6976.12028. [DOI] [PubMed] [Google Scholar]
- Minna J.D., Roth J.A., Gazdar A.F. Focus on lung cancer. Cancer Cell. 2002;1:49–52. doi: 10.1016/S1535-6108(02)00027-2. [DOI] [PubMed] [Google Scholar]
- Mousa W.K., Raizada M.N. The diversity of anti-microbial secondary metabolites produced by fungal endophytes: an interdisciplinary perspective. Front. Microbiol. 2013;4:1–18. doi: 10.3389/fmicb.2013.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mt M., S G., A M. Screening of plant endophytes as biological control agents against root rot pathogens of pepper (Capsicum annum L.) J. Plant Pathol. Microbiol. 2018;9:1–9. doi: 10.4172/2157-7471.1000435. [DOI] [Google Scholar]
- Mu C., Sheng Y., Wang Q., Amin A., Li X., Xie Y. Potential compound from herbal food of Rhizoma Polygonati for treatment of COVID-19 analyzed by network pharmacology and molecular docking technology. J. Funct. Foods. 2021;77:1–21. doi: 10.1016/j.jff.2020.104149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugnaini E.N., Ghosh N. Lymphoma. Prim. Care Clin. Off. Pract. 2016;43:661–675. doi: 10.1016/j.pop.2016.07.012. [DOI] [PubMed] [Google Scholar]
- Na L.I.U., Mei-na S., Qian-qian Z., Cong W.U., Kong-kai Z.H.U., Yu-lin S.U.N., Meng-ru L.I., Feng-ying Y., Run-liang F., Yu-ying Z., Hua Z. GKK1032B from endophytic Penicillium citrinum induces the apoptosis of human osteosarcoma MG63 cells through caspase pathway activation. Chin. J. Nat. Med. 2022;20:67–73. doi: 10.1016/S1875-5364(21)60108-5. [DOI] [PubMed] [Google Scholar]
- Newman D.J., Cragg G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuankeaw K., Chaiyosang B., Suebrasri T., Kanokmedhakul S., Lumyong S., Boonlue S. First report of secondary metabolites, Violaceol I and Violaceol II produced by endophytic fungus, Trichoderma polyalthiae and their antimicrobial activity. Mycoscience. 2020;61:16–21. doi: 10.1016/j.myc.2019.10.001. [DOI] [Google Scholar]
- Nwodo U.U., Green E., Okoh A.I. Bacterial exopolysaccharides : functionality and prospects. Int. J. Mol. Sci. 2012;13:14002–14015. doi: 10.3390/ijms131114002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oono R., Lutzoni F., Arnold A.E., Kaye L., U'Ren J.M., May G., Carbone I. Genetic variation in horizontally transmitted fungal endophytes of pine needles reveals population structure in cryptic species. Am. J. Bot. 2014;101:1362–1374. doi: 10.3732/ajb.1400141. [DOI] [PubMed] [Google Scholar]
- Orfali R.S., Ebrahim W., El-Shafae4 A.M. Secondary metabolites from Alternaria sp., a fungal endophyte isolated from the seeds of Ziziphus jujuba. Chem. Nat. Compd. 2017;53:878–880. doi: 10.1007/s10600-017-2195-9. [DOI] [Google Scholar]
- Pan Y., Zheng W., Yang S. Chemical and activity investigation on metabolites produced by an endophytic fungi Psathyrella candolleana from the seed of Ginkgo biloba. Nat. Prod. Res. 2019;34:1–4. doi: 10.1080/14786419.2019.1607335. [DOI] [PubMed] [Google Scholar]
- Pang X.J., Zhang S.B., Chen H.L., Zhao W.T., Yang D.F., Xian P.J., Xu L.L., Tao Y.D., Fu H.Y., Yang X.L. Emericelactones A-D: four novel polyketides produced by Emericella sp. XL 029, a fungus associated the leaves of Panax notoginseng. Tetrahedron Lett. 2018;59:4566–4570. doi: 10.1016/j.tetlet.2018.11.032. [DOI] [Google Scholar]
- Pang X.J., Zhang S.B., Xian P.J., Wu X., Yang D.F., Fu H.Y., Yang X.L. Emericellins A and B: two sesquiterpenoids with an unprecedented tricyclo[4,4,2,1]hendecane scaffold from the liquid cultures of endophytic fungus Emericella sp. XL 029. Fitoterapia. 2018;131:55–58. doi: 10.1016/j.fitote.2018.10.022. [DOI] [PubMed] [Google Scholar]
- Pansanit A., Pripdeevech P. Antibacterial secondary metabolites from an endophytic fungus, Arthrinium sp. MFLUCC16-1053 isolated from Zingiber cassumunar. Mycology. 2018;9:264–272. doi: 10.1080/21501203.2018.1481154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel B., Sharma S., Nair N., Majeed J., Goyal R.K., Dhobi M. Therapeutic opportunities of edible antiviral plants for COVID-19. Mol. Cell. Biochem. 2021:1–20. doi: 10.1007/s11010-021-04084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J., Teli B., Bajpai R., Meher J., Rashid M., Mukherjee A., Yadav S.K. Role of Plant Growth Promoting Microorganisms in Sustainable Agriculture and Nanotechnology. Woodhead Publishing; 2019. Trichoderma-mediated biocontrol and growth promotion in plants: an endophytic approach; pp. 219–239. [DOI] [Google Scholar]
- Pavithra G., Bindal S., Rana M., Srivastava S. Role of endophytic microbes against plant pathogens: a review. Asian J. Plant Sci. 2020;19:54–62. doi: 10.3923/ajps.2020.54.62. [DOI] [Google Scholar]
- Peng F., Hou S.Y., Zhang T.Y., Wu Y.Y., Zhang M.Y., Yan X.M., Xia M.Y., Zhang Y.X. Cytotoxic and antimicrobial indole alkaloids from an endophytic fungus: Chaetomium sp. SYP-F7950 of Panax notoginseng. RSC Adv. 2019;9:28754–28763. doi: 10.1039/c9ra04747f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirttilä A.M., Pospiech H., Laukkanen H., Myllylä R., Hohtola A. Seasonal variations in location and population structure of endophytes in buds of Scots pine. Tree Physiol. 2005;25:289–297. doi: 10.1093/treephys/25.3.289. [DOI] [PubMed] [Google Scholar]
- Prado-vázquez G., Gámez-pozo A., Trilla-fuertes L., Arevalillo J.M., Zapater-moros A., Ferrer-gómez M., Díaz-al M., López-vacas R., Navarro H., Maín P., Feliú J., Zamora P., Espinosa E., Ángel J., Vara F. A novel approach to triple- negative breast cancer molecular classification reveals a luminal immune-positive subgroup with good prognoses. Sci. Rep. 2019;9:1–12. doi: 10.1038/s41598-018-38364-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajani P., Rajasekaran C., Vasanthakumari M.M., Olsson S.B., Ravikanth G., Uma Shaanker R. Inhibition of plant pathogenic fungi by endophytic Trichoderma spp. through mycoparasitism and volatile organic compounds. Microbiol. Res. 2021;242 doi: 10.1016/j.micres.2020.126595. [DOI] [PubMed] [Google Scholar]
- Razak N.A., Abu N., Ho W.Y., Zamberi N.R., Tan S.W. Cytotoxicity of eupatorin in MCF-7 and MDA-MB-231 human breast cancer cells via cell cycle arres, anti-angiogenesis and induction of apoptosis. Sci. Rep. 2019;9:1–12. doi: 10.1038/s41598-018-37796-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho H., Hsieh M., Kandel S.L., Cantillo J., Doty S.L., Kim S.H. Do endophytes promote growth of host plants under stress? A meta-analysis on plant stress mitigation by endophytes. Microb. Ecol. 2018;75:407–418. doi: 10.1007/s00248-017-1054-3. [DOI] [PubMed] [Google Scholar]
- Ruan B., Yu Z., Yang X., Yang Y., Hu M. New bioactive compounds from aquatic endophyte Chaetomium globosum. Nat. Prod. Res. 2017;32:1–6. doi: 10.1080/14786419.2017.1378210. [DOI] [PubMed] [Google Scholar]
- Sampangi-Ramaiah M.H., Jagadheesh Dey P., Jambagi S., Vasantha Kumari M.M., Oelmüller R., Nataraja K.N., Venkataramana Ravishankar K., Ravikanth G., Uma Shaanker R. An endophyte from salt-adapted Pokkali rice confers salt-tolerance to a salt-sensitive rice variety and targets a unique pattern of genes in its new host. Sci. Rep. 2020;10:1–14. doi: 10.1038/s41598-020-59998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarsaiya S., Shi J., Chen J. A comprehensive review on fungal endophytes and its dynamics on Orchidaceae plants: current research, challenges, and future possibilities. Bioengineered. 2019;10:316–334. doi: 10.1080/21655979.2019.1644854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarsaiya S., Shi J., Chen J. Current progress on endophytic microbial dynamics on Dendrobium plants. Fungal Biotechnol. Bioeng. 2020:397–418. doi: 10.1007/978-3-030-41870-0_20. [DOI] [Google Scholar]
- Selim K.A., Elkhateeb W.A., Tawila A.M., El-beih A.A., Abdel-rahman T.M., El-diwany A.I., Ahmed E.F. Antiviral and antioxidant potential of fungal endophytes of egyptian medicinal plants. Fermentation. 2018;4:1–11. doi: 10.3390/fermentation4030049. [DOI] [Google Scholar]
- Shareef M., Ashraf M.A., Sarfraz M. Natural cures for breast cancer treatment: a review. Saudi Pharmaceut. J. 2016;24:1–23. doi: 10.1016/j.jsps.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma H., Rai A.K., Dahiya D., Chettri R., Singh P. Exploring endophytes for in vitro synthesis of bioactive compounds similar to metabolites produced in vivo by host plants. AIMS Microbiol. 2021;7:175–199. doi: 10.3934/microbiol.2021012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Liu C., Liu L., Yang B., Zhang Y. Structure identification and fermentation characteristics of pinoresinol diglucoside produced by Phomopsis sp. isolated from Eucommia ulmoides Oliv. Appl. Microbiol. Biotechnol. 2012;93:1475–1483. doi: 10.1007/s00253-011-3613-8. [DOI] [PubMed] [Google Scholar]
- Shi S., Li Y., Ming Y., Li C., Li Z., Chen J., Luo M. Biological activity and chemical composition of the endophytic fungus Fusarium sp. TP-G1 obtained from the root of Dendrobium officinale Kimura et Migo. Record Nat. Prod. 2018;6:549–556. [Google Scholar]
- Shweta S., Zuehlke S., Ramesha B.T., Priti V., Mohana Kumar P., Ravikanth G., Spiteller M., Vasudeva R., Uma Shaanker R. Endophytic fungal strains of Fusarium solani, from Apodytes dimidiata E. Mey. ex Arn (Icacinaceae) produce camptothecin, 10-hydroxycamptothecin and 9-methoxycamptothecin. Phytochemistry. 2010;71:117–122. doi: 10.1016/j.phytochem.2009.09.030. [DOI] [PubMed] [Google Scholar]
- Siddharthar J., Rajkumar B., Deivasigamani K. Knowledge awareness and prevention of cervical cancer among women attending a tertiary care hospital in Puducherry, India. J. Clin. Diagn. Res. 2014;8:8–11. doi: 10.7860/JCDR/2014/8115.4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C., Ding G., Wu G., Yang J., Zhang M., Wang H., Wei D., Qin J., Guo L. Identification of a unique azaphilone produced by Chaetomium globosum isolated from Polygonatum sibiricum. Chem. Biodivers. 2020;17 doi: 10.1002/cbdv.201900744. [DOI] [PubMed] [Google Scholar]
- Song D., xing Jiang, guo J. Hypolipidemic components from medicine food homology species used in China: pharmacological and health effects. Arch. Med. Res. 2017;48:569–581. doi: 10.1016/j.arcmed.2018.01.004. [DOI] [PubMed] [Google Scholar]
- Stewart C., Ralyea C., Lockwood S. Ovarian cancer: an integrated review. Semin. Oncol. Nurs. 2019;35:151–156. doi: 10.1016/j.soncn.2019.02.001. [DOI] [PubMed] [Google Scholar]
- Stierle A., Strobel G S.D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific Yew. Science. 1993;260:214–216. doi: 10.1126/science.8097061. [DOI] [PubMed] [Google Scholar]
- Stojanović-Radić Z., Pejčić M., Dimitrijević M., Aleksić A., Anil Kumar N.V., Salehi B., Cho W.C., Sharifi-Rad J. Piperine-A major principle of black pepper: a review of its bioactivity and studies. Appl. Sci. 2019;9:1–29. doi: 10.3390/app9204270. [DOI] [Google Scholar]
- Sturz A.V., Christie B.R., Nowak J. Bacterial endophytes: potential role in developing sustainable systems of crop production. CRC Crit. Rev. Plant Sci. 2000;19:1–30. [Google Scholar]
- Suhartati R. Determination of structure and anticancer activity of MM2 compound, isolated from endophytic fungus Aspergillus terreus-RTN3 of Alpinia chinensis Rosc. IOP Conf. Ser. 2020;991:1–7. doi: 10.1088/1757-899X/991/1/012045. [DOI] [Google Scholar]
- Talukdar R., Padhi S., Rai A.K., Masi M., Evidente A., Jha D.K., Cimmino A., Tayung K. Isolation and characterization of an endophytic fungus Colletotrichum coccodes producing tyrosol from Houttuynia cordata Thunb. using ITS2 RNA secondary structure and molecular docking study. Front. Bioeng. Biotechnol. 2021;9:1–12. doi: 10.3389/fbioe.2021.650247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar R., Wary S., Mili C., Roy S., Tayung K. Antimicrobial secondary metabolites obtained from endophytic fungi inhabiting healthy leaf tissues of Houttuynia cordata Thunb., an ethnomedicinal plant of Northeast India. J. Appl. Pharmaceut. Sci. 2021;10:99–106. doi: 10.7324/JAPS.2020.10912. [DOI] [Google Scholar]
- Tang K., Wang Z., Gao S., Jiao Y., Li R., Tong Y., Yang Y. Study on anti-tumor mechanism of Poria cocos based on network pharmacology. TMR Pharmacol. Res. 2021;1:1–11. doi: 10.12032/TMRPR20210429011. [DOI] [Google Scholar]
- Techaoei S., Jirayuthcharoenkul C., Jarmkom K. Chemical evaluation and antibacterial activity of novel bioactive compounds from endophytic fungi in Nelumbo nucifera. Saudi J. Biol. Sci. 2020;27:1–7. doi: 10.1016/j.sjbs.2020.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teimoori-boghsani Y., Ganjeali A., Cernava T., Müller H. Endophytic fungi of native Salvia abrotanoides plants reveal high taxonomic diversity and unique profiles of secondary metabolites. Front. Microbiol. 2020;10:1–12. doi: 10.3389/fmicb.2019.03013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlaskalová-Hogenová H., Štěpánková R., Hudcovic T., Tučková L., Cukrowska B., Lodinová-Žádníková R., Kozáková H., Rossmann P., Bártová J., Sokol D., Funda D.P., Borovská D., Řeháková Z., Šinkora J., Hofman J., Drastich P., Kokešová A. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol. Lett. 2004;93:97–108. doi: 10.1016/j.imlet.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Tlaskalova-Hogenova H., Tuckova L., Mestecky J., Kolinska J., Rossmann P., Stepankova R., Kozakova H., Hudcovic T., Hrncir T., Frolova L., Kverka M. Interaction of mucosal microbiota with the innate immune system. Scand. J. Immunol. Suppl. 2005;62:106–113. doi: 10.1111/j.1365-3083.2005.01618.x. [DOI] [PubMed] [Google Scholar]
- Ty F., Li R., Li J., Zhou Z., Guo Y., Zhang T. Antibacterial and antitumor activity of secondary metabolites of endophytic fungi Ty5 from Dendrobium officinale. J. Biobased Mater. Bioenergy. 2018;12:1–10. doi: 10.1166/jbmb.2018.1769. [DOI] [Google Scholar]
- Venieraki A., Dimou M., Katinakis P. Endophytic fungi residing in medicinal plants have the ability to produce the same or similar pharmacologically active secondary metabolites as their hosts. Hell. Plant Prot. J. 2017;10:51–66. doi: 10.1515/hppj-2017-0006. [DOI] [Google Scholar]
- Verma V.C., Lobkovsky E., Gange A.C., Singh S.K., Prakash S. Piperine production by endophytic fungus Periconia sp. isolated from Piper longum L. J. Antibiot. (Tokyo) 2011;64:427–431. doi: 10.1038/ja.2011.27. [DOI] [PubMed] [Google Scholar]
- Waller F., Achatz B., Baltruschat H., Fodor J., Becker K., Fischer M., Heier T., Hückelhoven R., Neumann C., Von Wettstein D., Franken P., Kogel K.H. The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc. Natl. Acad. Sci. USA. 2005;102:13386–13391. doi: 10.1073/pnas.0504423102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Zhao W., Zhang C., Chang S., Shao R., Xing J., Chen M., Zhang Y., Si S. Cytotoxic metabolites from the endophytic fungus: Chaetomium globosum 7951. RSC Adv. 2019;9:16035–16039. doi: 10.1039/c9ra02647a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Cao P., Wang H., Tang Z., Wang N., Wang J., Zhang Y. Chronic administration of Angelica sinensis polysaccharide effectively improves fatty liver and glucose homeostasis in high-fat diet-fed mice. Sci. Rep. 2016;6:1–11. doi: 10.1038/srep26229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. hua. Current progress of research on intestinal bacterial translocation. Microb. Pathog. 2021;152:1–9. doi: 10.1016/j.micpath.2020.104652. [DOI] [PubMed] [Google Scholar]
- Wang Y.L., Hu B.Y., Qian M.A., Wang Z.H., Zou J.M., Sang X.Y., Li L., Luo X.D., Zhao L.X. Koninginin W, a new polyketide from the endophytic fungus Trichoderma koningiopsis YIM PH30002. Chem. Biodivers. 2021;18:1–5. doi: 10.1002/cbdv.202100460. [DOI] [PubMed] [Google Scholar]
- Wang Y., Li Y., Li S., Li Q.Y., Fan W., Kiatoukosin L., Chen J. Extracellular polysaccharides of endophytic fungus Alternaria tenuissima F1 from Angelica sinensis: production conditions, purification, and antioxidant properties. Int. J. Biol. Macromol. 2019;133:172–183. doi: 10.1016/j.ijbiomac.2019.03.246. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhao Y., Liu X., Li J., Zhang J., Liu D. Chemical constituents and pharmacological activities of medicinal plants from Rosa genus. Chinese Herb. Med. 2022;14:187–209. doi: 10.1016/j.chmed.2022.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanwan L., Ming Z., Guangqiang L., Guanghui Z., Shengchao Y., Junwen C. Research advances of endophytic fungi active substances in medicinal plants. Chinese Arch. Tradit. Chinese Med. 2018;36:654–658. [Google Scholar]
- Wu L.S., Jia M., Chen L., Zhu B., Dong H.X., Si J.P., Peng W., Han T. Cytotoxic and antifungal constituents isolated from the metabolites of endophytic fungus DO14 from Dendrobium officinale. Molecules. 2016;21:1–14. doi: 10.3390/molecules21010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C., Dong X., Li H., Cao M., Sun D., He S., Yang F., Yan X., Zhang S., Li N., Chen W. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin. Med. J. 2022;135:584–590. doi: 10.1097/CM9.0000000000002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiaoyue W., Lihong Z., Na F., Mingqiong T. Research progress on application of endophytic fungi in Chinese medicinal materials. Shandong Chem. Ind. 2020;49:62–64. doi: 10.19319/j.cnki.issn.1008-021x.2020.22.023. [DOI] [Google Scholar]
- Xie J., Wu Y.Y., Zhang T.Y., Zhang M.Y., Zhu W.W., Gullen E.A., Wang Z.J., Cheng Y.C., Zhang Y.X. New and bioactive natural products from an endophyte of Panax notoginseng. RSC Adv. 2017;7:38100–38109. doi: 10.1039/c7ra07060h. [DOI] [Google Scholar]
- Xing X., Cui S.W., Nie S., Phillips G.O., Douglas Goff H., Wang Q. A review of isolation process, structural characteristics, and bioactivities of water-soluble polysaccharides from Dendrobium plants. Bioact. Carbohydrates Diet. Fibre. 2013;1:131–147. doi: 10.1016/j.bcdf.2013.04.001. [DOI] [Google Scholar]
- Xing Y., Li C., Luan Y., Xu M., Wang H., Li Y., Jia L. Anti-aging effect of Codnopsis Bulleyana forrest ex Diels on D-galactose-induced aging mouse model. For. Chem. Rev. 2022:2348–2364. [Google Scholar]
- Xing Y.M., Chen J., Cui J.L., Chen X.M., Guo S.X. Antimicrobial activity and biodiversity of endophytic fungi in Dendrobium devonianum and Dendrobium thyrsiflorum from Vietman. Curr. Microbiol. 2011;62:1218–1224. doi: 10.1007/s00284-010-9848-2. [DOI] [PubMed] [Google Scholar]
- Xu Z. Epoxycytochalasin H: an endophytic Phomopsis compound induces apoptosis in A2780 cells through mitochondrial damage and endoplasmic reticulum stress. OncoTargets Ther. 2020;13:4987–4997. doi: 10.2147/OTT.S253716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Zhang X., Ma J., Yang Y., Zhou J., Xu J. Secondary metabolites produced by mangrove endophytic fungus Aspergillus fumigatus HQD24 with immunosuppressive activity. Biochem. Systemat. Ecol. 2020;93:1–4. doi: 10.1016/j.bse.2020.104166. [DOI] [Google Scholar]
- Xue R., Meng Q., Li J., Feng J., Shi H. Worse or better?—cirrhosis with hepatocellular carcinoma. Med. Hypotheses. 2016;97:85–87. doi: 10.1016/j.mehy.2016.10.019. [DOI] [PubMed] [Google Scholar]
- Yang J., Tong Y., Zhu K., Jiang Y., Yan Y., Chen S., Wang P. Optimization of mechanochemical-assisted extraction and decoloration by resins of polysaccharides from petals of Crocus sativus L. J. Food Process. Preserv. 2018;42:1–11. doi: 10.1111/jfpp.13369. [DOI] [Google Scholar]
- Yashin A., Yashin Y., Xia X., Nemzer B. Antioxidant activity of spices and their impact on human health: a review. Antioxidants. 2017;6:1–18. doi: 10.3390/antiox6030070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehia R.S., Osman G.H., Assaggaf H., Salem R., Mohamed M.S.M. Isolation of potential antimicrobial metabolites from endophytic fungus Cladosporium cladosporioides from endemic plant Zygophyllum mandavillei. South Afr. J. Bot. 2020:313–319. doi: 10.1016/j.sajb.2020.02.033. [DOI] [Google Scholar]
- Yu Y., Hou S., Sun Z., Zhang M., Zhang T., Zhang Y. Drechmeria panacis sp. nov., an endophyte isolated from Panax notoginseng. Int. J. Syst. Evol. Microbiol. 2018;68:3255–3259. doi: 10.1099/ijsem.0.002971. [DOI] [PubMed] [Google Scholar]
- Zabalgogeazcoa I. Review. Fungal endophytes and their interaction with plant pathogens. Spanish J. Agric. Res. 2008;6:138–146. doi: 10.5424/sjar/200806s1-382. [DOI] [Google Scholar]
- Zaher A.M., Makboul M.A., Moharram A.M., Tekwani B.L., Calderón A.I. A new enniatin antibiotic from the endophyte Fusarium tricinctum Corda. J. Antibiot. 2015;68:197–200. doi: 10.1038/ja.2014.129. [DOI] [PubMed] [Google Scholar]
- Zeng Y.J., Yang H.R., Ou X.Y., Su H.H., Zong M.H., Yang J.G., Lou W.Y. Fungal polysaccharide similar with host Dendrobium officinale polysaccharide: preparation, structure characteristics and biological activities. Int. J. Biol. Macromol. 2019;141:460–475. doi: 10.1016/j.ijbiomac.2019.08.238. [DOI] [PubMed] [Google Scholar]
- Zeng Y.J., Yang H.R., Wu X.L., Peng F., Huang Z., Pu L., Zong M.H., Yang J.G., Lou W.Y. Structure and immunomodulatory activity of polysaccharides from Fusarium solani DO7 by solid-state fermentation. Int. J. Biol. Macromol. 2019;137:568–575. doi: 10.1016/j.ijbiomac.2019.07.019. [DOI] [PubMed] [Google Scholar]
- Zeng Y., Yang H., Zong M., Yang J., Lou W. Novel antibacterial polysaccharides produced by endophyte Fusarium solani. Bioresour. Technol. 2019;288:1–5. doi: 10.1016/j.biortech.2019.121596. [DOI] [PubMed] [Google Scholar]
- Zhang S., Yang Y., Li J., Qin J., Zhang W., Huang W., Hu H. Physiological diversity of orchids. Plant Divers. 2018;40:196–208. doi: 10.1016/j.pld.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T.T., Xu X.L., Jiang M.H., Jiang J.G. Hepatoprotective function of Penthorum chinense pursh. Food Funct. 2013;4:1581–1585. doi: 10.1039/c3fo60245a. [DOI] [PubMed] [Google Scholar]
- Zhao J.C., Wang Y.L., Zhang T.Y., Chen Z.J., Yang T.M., Wu Y.Y., Sun C.P., Ma X.C., Zhang Y.X. Indole diterpenoids from the endophytic fungus Drechmeria sp. as natural antimicrobial agents. Phytochemistry. 2018;148:21–28. doi: 10.1016/j.phytochem.2018.01.010. [DOI] [PubMed] [Google Scholar]
- Zhao J., Ge L.Y., Xiong W., Leong F., Huang L.Q., Li S.P. Advanced development in phytochemicals analysis of medicine and food dual purposes plants used in China (2011-2014) J. Chromatogr. A. 2016;1428:39–54. doi: 10.1016/j.chroma.2015.09.006. [DOI] [PubMed] [Google Scholar]
- Zhao J., Luan Z., Liang J., Cheng Z., Sun C., Wang Y., Zhang M., Zhang T., Wang Y., Yang T. Drechmerin H, a novel 1(2), 2(18)-diseco indole diterpenoid from the fungus Drechmeria sp. as a natural agonist of human pregnane X receptor. Bioorg. Chem. 2018;79:250–256. doi: 10.1016/j.bioorg.2018.05.001. [DOI] [PubMed] [Google Scholar]
- Zhao J., Lv G.P., Chen Y.W., Li S.P. Advanced development in analysis of phytochemicals from medicine and food dual purposes plants used in China. J. Chromatogr. A. 2011;1218:7453–7475. doi: 10.1016/j.chroma.2011.06.041. [DOI] [PubMed] [Google Scholar]
- Zhao J., Zhou L., Wang J., Shan T. Endophytic fungi for producing bioactive compounds originally from their host plants. Curr. Res. 2010:567–576. [Google Scholar]
- Zhu H., Xu W., Wang N., Jiang W., Cheng Y., Guo Y., Yao W., Hu B., Du P., Qian H. Anti-fatigue effect of Lepidium meyenii Walp. (Maca) on preventing mitochondria-mediated muscle damage and oxidative stress in vivo and vitro. Food Funct. 2021;12:3132–3141. doi: 10.1039/d1fo00383f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.