Abstract
Cancer is among the major cause of demise worldwide. Though the array of anticancer chemical medications is available but unfortunately, they are also associated with negative health effects. The invaluable therapeutic potential of spices makes them an integral part of our daily diet. Therefore, the present work focuses on the traditional uses of 46 spices and the phytochemical analysis of 31 spices. Out of them, only 29 spices are explored for their cytotoxicity against different cancer cell lines. The pre-clinical and clinical anticancer studies of spices along with their toxicity, mechanism of actions like Wnt/β-catenin, phosphatidylinositol-3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR), JAK/STAT, mitogen-activated protein kinase (MAPK), Notch-mediated pathways and Quantitative structure-activity relationship (QSAR) studies were also focused. Curcumin was found as one of the most explored bioactive in every aspect such as in-vitro, in-vivo, clinical as well as SAR anticancer studies while some other bioactive such as 1,8-Cineole, trans-Anethole, Diosgenin, Trigonelline are either unexplored or least explored for their clinical and SAR studies. In fact, traditional medicinal uses of spices also provide solid shreds of evidence for the new leads towards the invention of novel anticancer agents. Therefore, further research can be designed for the anticancer marketed formulation from spices after having their placebo and related toxicological data.
Keywords: Anticancer, Cytotoxicity, Inflammation, Natural products, QSAR, Traditional uses
Chemical compounds studied in this article: Curcumin, Pubchem CID: 839564, Cardamonin, Pubchem CID: 557026, Eugenol, Pubchem CID: 13876103, Piperine, Pubchem CID: 553590, 6-Gingerol, Pubchem CID: 391126, Capsaicin, Pubchem CID: 1265957, Cinnamaldehyde, Pubchem CID: 637511, Linalool, Pubchem CID: 391430, Rosmarinic acid, Pubchem CID: 4445104, Thymol, Pubchem CID: 21105998
Graphical abstract
Highlights
-
•
Herbs and spices possess potent anticancer therapeutic potential.
-
•
Spices inhibit the excessive synthesis of reactive oxygen species.
-
•
Curcumin, 6-gingerol, thymoquinone, eugenol, capsaicin are major anticancer agents.
-
•
Wnt/β-catenin, JAK/STAT, MAPK and Notch are anticancer signaling pathways.
-
•
Further clinical studies are necessary to corroborate anticancer potential of spices.
Abbreviations
- PCD
Programmed cell death
- NF-κB
Nuclear factor kappa B
- NEDD
Neural precursor cell expressed developmentally down-regulated protein
- CSCs
Cancer stem cells
- IL
Interleukin
- MMP
Matrix metallopeptidases
- VEGF
Vascular endothelial growth factor
- IC
Inhibitory concentration
- YAP
Yes-associated protein
- TAZ
Transcriptional coactivator with PDZ-binding motif
- TNF-α
Tumor necrosis factor-α
- EGFR
Epidermal growth factor receptor
- TRPV1
Transient receptor potential vanilloid type-1
- Bcl-2
B-cell lymphoma 2
- CRC
Colorectal cancer
- OXA
Oxaliplatin
- MNU
N-nitroso N-methyl Urea
- PTEN
Phosphatase and tensin homolog
- PARP
Poly ADP-ribose polymerase
- ER-α
Estrogen receptor alpha
- HDAC
Histone deacetylase
- DMH
Dimethylhydrazine
- DSS
Dextran sulphate sodium
- GSK3β
Glycogen synthase kinase 3β
- CK1α
Casein kinase 1α
- DVL
Disheveled
- TCF
T cell-specific factor
- LEF
Lymphoid enhancer-binding factor
- CBP
CREB binding protein
- APC
Adenomatous polyposis coli gene
- AXIN
Anti-Neurexin
- PI3K
Phosphatidylinositol-3-kinase
- mTOR
Mammalian target of rapamycin
- PH
Pleckstrin-homology
- MAPK
Mitogen-activated protein kinase
- RTKs
Receptor tyrosine kinases
- NICD
Notch intracellular domain
- NEC
Notch extracellular subunit
- NTM
Notch transmembrane fragment
- NEXT
Notch extracellular truncated
- CSL
C-protein binding factor 1/Suppressor of Hairless/Lag-1
- GRAS
Generally recognized as safe
- HNPMI
N-(2-hydroxy-5-nitrophenyl (4′-methylphenyl) methyl) indoline
- NQO1, NAD(P)H
quinone oxidoreductase 1
- Keap1
Kelch-like erythroid cell derived protein with CNC homology[ECH]-associated protein 1
- Nrf2
[NF-E2]-related factor 2
- QSAR
Quantitative structure-activity relationship
- ISO
International Organization for Standardization
1. Introduction
Cancer is a dreadful disease and is one of the major deleterious causes of death all over the globe (Bhagat and Chaturvedi, 2016). According to World Health Organization (WHO)-Cancer report (2018), about 9.6 million deaths were reported to be caused by cancer. The disease is featured by unlimited cell division, growth, and distant migration. It is mainly caused by carcinogenic agents which can be categorized into two groups i.e., genotoxic agents and non-genotoxic agents. The genotoxic agents are those which directly interfere with the genetic material and induce the alteration in the cell cycle whereas the non-genotoxic agents indirectly induce cancer (Anwar et al., 2020). Though numerous conventional anticancer modalities such as surgery, radiation therapy, chemotherapy, immunotherapy, photodynamic therapy, cancer vaccinations, stem cell transformation, and combination thereof are often available but they are effectively used only for compromising the small-sized tumors and are also associated with several negative effects. Chemotherapy and radiotherapy lead to the damages of normal cells along with the tumor's cells. For instance; radiotherapy retards the bone and muscle growth in children suffering from cancer. Moreover, due to the continuous use of conventional anticancer therapies, many patients have also reported adverse effects like anemia, immunosuppression, diarrhea, cough, nausea, constipation, bleeding, hair loss, infection, vomiting, appetite loss, pain, drowsiness, irritability and sadness. These factors necessitate the requirement of natural and effective treatment of cancer with fewer adverse effects (Zheng et al., 2016; Mora et al., 2022).
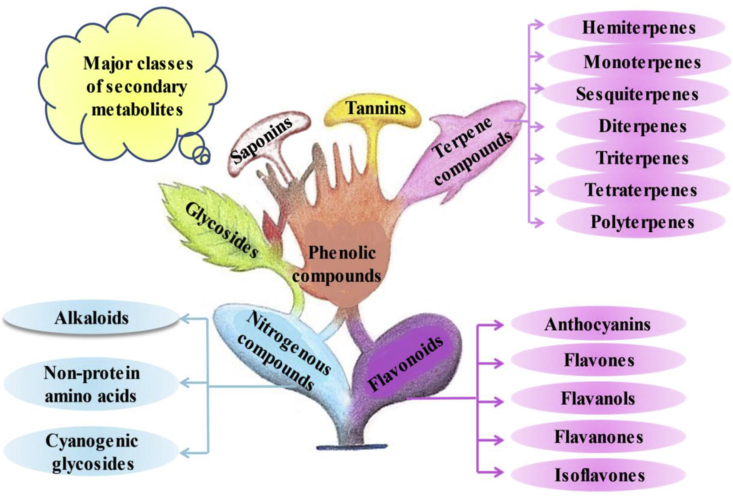
Natural products are considered palliative over chemotherapy. There are numerous natural products with highly diversified structures and functions. They are also termed “secondary metabolites”. The classification of these secondary metabolites is depicted in Fig. 1 (Das and Gezici, 2018). These products play a key role in drug development against cancer as well as other diseases too like cardiovascular disorders, pathogenic infections, digestive problems, neurological issues, and so on. They also regulate the endogenous defense system and interaction (in terms of the competition) with other organisms. They cover a wide pool of chemical space instead of synthetic compound libraries, thus, providing a great source of compounds that can be isolated and developed as a novel drug. Approximately, more than 70% of anticancer medications are developed from natural products. For instance, vincristine, vinblastine, paclitaxel, docetaxel, ellipticine, berberine, combretastatins, cephalotaxus, campothecin, curcumin and capsaicin are some of the most established examples of plant-derived natural products utilized for cancer prevention (Iqbal et al., 2017; Dallavalle et al., 2020; Atanasov et al., 2021). Besides, there are some factors that influence the frequent use of anticancer natural products. The major disadvantage is the scarcity of international standardization and documentation on the composition, quality, efficacy, safety, manufacturing practices, regulation, and approval processes of natural products. One another limitation that greatly affects the anticancer use of natural products is their poor solubility and bioavailability (Fridlender et al., 2015). Despite, these limitations, natural products are always preferable over chemical drugs due to their eco-friendly, less toxicity, and omnipresence properties.
Fig. 1.
Classification of natural products/secondary metabolites.
Spices are also considered as one of the most promising anticancer agents due to their inverse relation with oncological incidences such as abnormal cell growth, irregular cell cycle, cell cycle arrest, abnormal apoptosis, damaging of healthy tissues, tumor inducing signaling pathways (Perez-Ortiz et al., 2020; Kammath et al., 2021). These are the naturally-fragmented dried plant parts including seeds, bark, roots, flowers, and fruits, especially used for flavoring, seasoning, coloring, and preserving food products (Singh et al., 2021a). Globally, thousands of spices are used for coloring, flavoring, and preserving food items. The use and preferences of these spices vary from region to region. Therefore, it seems difficult to enlist all the spices in a single shot. In India, under the act of parliament, a total of 52 spices are considered under the purview of Spices Board, however, 109 spices are mentioned in the International Organization for Standardization (ISO) list. Many spices are native to India; hence the country is aptly recognized as the land of spices. Generally, spices are used in minor quantities and especially used for preserving and imparting colour and taste to the food items but apart from this, they also possess tremendous health benefits (Kunnumakkara et al., 2018; Sachan et al., 2018). From millennia, several studies have ascertained the effectiveness of spices in the preparation of folkloric medicines for managing routine maladies and rejuvenating overall health. The pharmacological properties of spices are rendered by their active compounds (Sachan et al., 2018). They consist of alkaloids, flavonoids, terpenoids, phenols, phenylpropanoids, anthocyanins, fibers, sugar, fat, protein, ash, calcium, iron, vitamin B, vitamin C, carotene, gum, essential oils, and many more. They are rich source of sodium and fat and able to meet their dietary requirement. Thus, they exert beneficial health effects on salt and sugar reduction (Balasubramanian et al., 2016).
Spices are a rich reservoir of useful dietary bioactive and are popularly considered as the primary source of nutraceuticals (Srinivasan, 2017a). Many dietary spices have been well reported with numerous pharmacological effects such as antioxidant, antimicrobial, antidiabetic, wound healing, anti-inflammatory as well as immunomodulatory effect. All these pharmacological potentials are primarily being responsible for the anticancer efficacy of spices. Many spices such as Curcuma longa (Turmeric), Cinnamomum verum (Cinnamon), Nigella sativa (Black cumin), Cuminum cyminum (Cumin), Zingiber officinale (Ginger), Trigonella foenum-graecum (Fenugreek), Allium sativum (Garlic), Crocus sativus (Saffron), Piper nigrum (Black pepper) and Capsicum annum (Chilli powder) are well documented for their anticancer property (Zheng et al., 2016). Numerous bioactives from spices such as curcumin, sulfur compounds, 6-gingerol, thymoquinone, eugenol, and capsaicin are also well reported for their anticancer potential (Srinivasan, 2017a). Therefore, the present study summarizes the up-to-date information on the anticancer role of spices. The various ethnomedicinal uses of spices were described to indicate that a single plant can be used in treating other diseases too apart from cancer. Spices’ bioactives were discussed to signify the role of a single phytocompound against several cancer types. The possible mechanism of action is depicted in figures to ease the understanding of signaling pathways. Structure-activity relationship (SAR) studies, pre-clinical and clinical studies are also well illustrated to strengthen the research going on cancer. Ultimately, the study provides solid pieces of evidences on the anticancer role of spices and baseline for the younger researchers particularly those working on the anticancer drug development from natural products.
2. Methodology
A comprehensive literature survey was conducted from 2015 to 2022 on the anticancer potential of species by referencing the major scientific databases such as Google Scholar, Science Direct, PubMed, Scopus, Web of Science and WHO. Spices listed under purview of Spices Board of India were only included for the current study. The reference articles were obtained via using the keywords like spices, anticancer potential, active compounds, anticancer potential of spice's bioactives, ethnomedicinal uses of spices and the SAR studies of spices bioactives to gather available information. Spices used for traditional uses were of primary concern to collect the valuable information, by adopting the comprehensive selection criteria. The articles dealing with the anticancer compound isolation were of primary focus. The articles related to spices but on different aspects like different kind of stress mitigation, stability issues, physiochemical and biochemical profiling, germination indices, genomic variations, packaging and adulteration were excluded. Chemical structures were drawn via ChemDraw ultra-8.0. Primarily, the current review focuses on the anticancer efficacy of ethnomedicinally used spices in India.
3. Ethnomedicinal uses of Indian spices
All across the globe, over 80 spices are grown and out of them near 50 spices are cultivated in India. It is the biggest producer of spices and that's why it is popular all over the world for its spices and traditional medicines (Balasubramanian et al., 2016). The history of the use of spices is as old as the history of mankind. The uses of spices are also well reported in “Epic of Gilgamesh” and the “Bhagavad Gita”. In Ayurveda, spices are reported to be used in improving the state of mood and mind. They are a rich source of flavoring, coloring, and preserving agents. They not only improve the quality of food but also enhance the secretion of saliva as well as improve the digestive system. On the traditional medicinal scale, they are used to treat cold, influenza, nausea, stomach ache, and vomiting. Plenty of spices like cinnamon, black pepper, turmeric, and ginger have been used on a large scale during covid-19. These spices can also be used to cure other disorders. For instance; the long pepper can be traditionally used in curing respiratory disorders, gastric issues, tongue paralysis, diarrhea, fever, hepatitis, stomachache, cholera, cough, and tumors (Prasad and K Tyagi, 2016). Besides, they are also used for embalming mummies because of their flavoring properties (Gottardi et al., 2016). They also possess purgative, laxative, expectorant, carminative, and diuretic agents (Sachan et al., 2018). All these aforementioned studies provide scientific evidence to some extent for the effective use of spices in our daily diet, to improve human well-being. The various ethnomedicinal uses of some commonly used spices are summarized in Table 1.
Table 1.
Ethnomedicinal uses of Indian spices used for cancer treatment.
| S. No. | Botanical Name | Family | Common Name | Plant part used | Prominent Ethnomedicinal use | Ref. (s) |
|---|---|---|---|---|---|---|
| 1. |
 Piper nigrum L. |
Piperaceae | Black pepper | Fruit |
|
de Souza Grinevicius et al. (2016); Kunnumakkara et al. (2018); Perez-Ortiz et al. (2020) |
| 2. |
 Syzygium aromaticum (L.) Merr. & L.M. Perry |
Myrtaceae | Cloves | Flower buds and essential oil |
|
Sachan et al. (2018); Kello et al. (2020) |
| 3. |
 Zingiber officinale Roscoe Zingiber officinale Roscoe |
Zingiberaceae | Ginger | Rhizome |
|
Gottardi et al. (2016); Mansingh et al. (2020); Perez-Ortiz et al. (2020) |
| 4. |
 Cinnamomum verum J. Presl Cinnamomum verum J. Presl |
Lauraceae | Cinnamon | Bark |
|
Gottardi et al. (2016); Srinivasan (2017b); Singh et al. (2021a) |
| 5. |
 Curcuma caesia Roxb. Curcuma caesia Roxb. |
Zingiberaceae | Black turmeric | Rhizome |
|
Mukunthan et al. (2017) |
| 6. |
 Curcuma longa L. |
Zingiberaceae | Turmeric | Rhizome |
|
Perez-Ortiz et al. (2020) |
| 7. |
 Myristica fragrans Houtt. |
Myristicaceae | Nutmeg/Jaiphal | Seeds |
|
Gottardi et al. (2016) |
| 8. |
 Origanum majorana L. |
Lamiaceae | Marjoram | Leaves |
|
Khaleel et al., 2016 |
| 9. |
 Capsicum annuum L. |
Solanaceae | Chili pepper | Fruit |
|
Chamikara et al. (2016) |
| 10. |
 Nigella sativa L. |
Ranunculaceae | Black seed | Seeds |
|
Majeed et al. (2021) |
| 11. |
 Elettaria cardamomum (L.) Maton |
Zingiberaceae | Small cardamom | Fruits |
|
Ashokkumar et al. (2020) |
| 12. |
 Apium graveolens Apium graveolensL. |
Apiaceae | Celery | Leaves |
|
Perez-Ortiz et al. (2020) |
| 13. |
 Foeniculum vulgare Mill. |
Apiaceae | Fennel | Seeds and fruits |
|
Farid et al. (2020) |
| 14. |
 Cuminum cyminum L. |
Apiaceae | Cumin | Seeds and fruits |
|
Goodarzi et al. (2020); Perez-Ortiz et al. (2020); Singh et al. (2021b) |
| 15. |
 Trigonella foenum-graecum L. |
Fabaceae | Fenugreek | Seeds and leaves |
|
Mohamadi et al. (2018); Sanlier and Gencer (2020); Singh et al. (2022) |
| 16. |
 Brassica juncea L. Czern |
Brassicaceae | Mustard | Seeds |
|
Kunnumakkara et al. (2018); Sanlier and Gencer (2020) |
| 17. |
 Murraya koenigii (L.) Spreng. |
Rutaceae | Curry leaf | Leaf |
|
Sanlier and Gencer (2020) |
| 18. |
 Coriandrum sativum L. |
Apiaceae | Coriander | Whole plant |
|
Sari et al. (2021) |
| 19. |
 Pimpinella anisum L. Pimpinella anisum L. |
Apiaceae | Aniseed | Seeds and fruits |
|
Sun et al. (2019) |
| 20. |
 Trachyspermum ammi (L.) Sprague |
Apiaceae | Ajwain | Seeds and fruits |
|
Chahal et al. (2017a) |
| 21. |
 Carum carvi L. Carum carvi L. |
Apiaceae | Caraway | Fruits |
|
Miraj and Kiani (2016) |
| 22. |
 Anethum graveolens L. |
Apiaceae | Dill | Seeds |
|
Chahal et al. (2017b) |
| 23. |
 Allium sativum L. |
Alliaceae | Garlic | Bulb |
|
Gudalwar et al. (2021) |
| 24. |
 Garcinia indica (Thouars) Choisy Garcinia indica (Thouars) Choisy |
Clusiaceae | Kokam | Fruit rind |
|
Chate et al. (2019) |
| 25. |
 Mentha piperita L. Mentha piperita L. |
Lamiaceae | Mint | Leaf |
|
Mahendran and Rahman, 2020 |
| 26. |
 Petroselinum crispum (Mill.) Fuss |
Apiaceae | Parsley | Seeds and leaf |
|
Agyare et al. (2017) |
| 27. |
 Punica granatum L. Punica granatum L. |
Punicaceae | Pomegranate | Seeds |
|
Ge et al. (2021) |
| 28. |
 Crocus sativus L. Crocus sativus L. |
Iridaceae | Saffron | Dried stigmas |
|
Cardone et al. (2020) |
| 29. |
 Vanilla planifolia Jacks. Ex Andrews Vanilla planifolia Jacks. Ex Andrews |
Orchidaceae | Vanilla | Pod |
|
Ahmad et al. (2020) |
| 30. |
 Illicium verum Hook.f. |
Schisandraceae | Star anise | Fruit |
|
Rocha and Tietbohl (2016) |
| 31. |
 Acorus calamus L. |
Araceae | Sweet flag | Rhizome |
|
Khwairakpam et al. (2018) |
| 32. |
 Alpinia galangal (L.) Willd. Alpinia galangal (L.) Willd. |
Zingiberaceae | Greater galanga | Rhizome |
|
Khairullah et al. (2020) |
| 33. |
 Armoracia rusticana P. Gaertn., B. Mey. & Scherb. Armoracia rusticana P. Gaertn., B. Mey. & Scherb. |
Brassicaceae | Horse Radish | Root |
|
Yasmeen et al. (2020) |
| 34. |
 Capparis spinosa L. Capparis spinosa L. |
Capparidaceae | Caper | Flower buds |
|
Zhang and Ma (2018) |
| 35. |
 Ferula asafoetida H. Karst. Ferula asafoetida H. Karst. |
Apiaceae | Asafoetida | Resins |
|
Upadhyay (2017) |
| 36. |
 Hyssopus officinalis L. Hyssopus officinalis L. |
Lamiaceae | Hyssop | Leaf |
|
Tahir et al. (2018) |
| 37. |
 Juniperus communis L. Juniperus communis L. |
Cupressaceae | Juniper berry | Berry |
|
Raina et al. (2019) |
| 38. |
 Laurus nobilis L. Laurus nobilis L. |
Lauraceae | Bay leaf | Leaf |
|
Elkiran et al. (2018) |
| 39. |
 Ocimum basilicum L. Ocimum basilicum L. |
Lamiaceae | Basil | Leaf |
|
Shahrajabian et al. (2020) |
| 40. |
 Papaver somniferum L. Papaver somniferum L. |
Papaveraceae | Poppy seeds | Seeds |
|
Raut and Ghotankar (2019) |
| 41. |
 Rosmarinus officinalis L. |
Lamiaceae | Rosemary | Leaf |
|
Kompelly et al. (2019) |
| 42. |
 Salvia officinalis L. Salvia officinalis L. |
Lamiaceae | Sage | Leaf |
|
Sharma et al. (2019) |
| 43. |
 Thymus vulgaris L. Thymus vulgaris L. |
Lamiaceae | Thyme | Leaf |
|
Dauqan, and Abdullah (2017) |
| 44. |
 Origanum vulgare L. Origanum vulgare L. |
Lamiaceae | Oregano | Leaf |
|
Oniga et al. (2018) |
| 45. |
 Artemisia dracunculus L. Artemisia dracunculus L. |
Asteraceae | Tarragon | Leaf |
|
Ekiert et al. (2021) |
| 46. |
 Tamarindus indica L. Tamarindus indica L. |
Caesalpiniaceae | Tamarind | Fruit |
|
Menezes et al. (2016) |
Nowadays, there is the concept of repurposing the drug i.e., the drug that is already established against a particular type of disease is also explored for its therapeutics against other diseases, so that a single drug can be recruited against several disorders. Therefore, from Table 1, it can be inferred that the spices which possess immense pharmacology against several disorders can be further explored in novel drug development.
4. Phytochemistry of Indian anticancer dietary spices
Spices are the rich reservoir of many therapeutically active compounds viz. alkaloids, phenolic compounds, flavonoids, quinines, amino acids, polypeptides, terpenoids, vitamins etc. Approximately 180 chemical compounds from spices have been authenticated to be used against different degenerative diseases. Out of them, numerous bioactive are also being evidenced for cancer risk management. Cinnamaldehyde, allyl isothiocyanate, gingerol, Ar-tumerone, shagol, sulfur-containing compounds, zingiberene, anethole, estragole, ferulic acid, caryophyllene, 1,8-cineole, thymoquinone, eugenyl acetate, eugenol, limonene, sabinenecamphene, curcuminoids, curcumin, myrceneajoene, linalool, phenethyl isothiocynate, myristicin, allicin, alliin, methiin, trigonelline, cuminaldehyde, citral, safrole, quercetin, rutin, leutin, rosmarinic acid, capsaicin and many other compounds derived from spices play a pivotal role in cancer prevention (Mughal, 2019).
In a study by Vutakuri and Somara (2018), two bioactives viz. Diindolylmethane and indole-3-carbinol from Elettaria cardamomum (Cardamom) were reported for breast cancer treatment. But still many bioactives of spices are either unexplored or least explored for their anticancer potential hence herein we listed different bioactives derived from spices which are specifically used for cancer treatment. The list of anticancer bioactives from spices along with their structures and their anticancer specificity towards a particular type of cancer is summarized in Table 2.
Table 2.
Structure of novel anticancer compounds isolated from Indian spices.
| S. No. | Spices used | Class of compound | Name of the compound | Structure of the compound | Cancer type | Ref (s) |
|---|---|---|---|---|---|---|
| 1. | Crocus sativus L. | Carotenoid | Crocin (Pubchem CID: 17339399) |  |
Breast, lung, leukemia, reproductive system and digestive system cancer | Zheng et al. (2016); Hire et al. (2017); Mir et al. (2020) |
| Crocetin (Pubchem CID: 4444644) | ||||||
| Organic oxides | Safranal (Pubchem CID: 55000) | 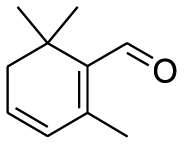 |
||||
| 2. | Curcuma longa L. | Polyphenol | Curcumin (Pubchem CID: 839564) |  |
Pancreatic, lung, bladder, colon, breast, gastric, nasopharyngeal, hepatobiliary and prostate cancer | Basha et al. (2016); Zheng et al. (2016) |
| 3. | Curcuma aromatica Salisb. | Sesquiterpenoid | Ar-tumerone (Pubchem CID: 485257) |  |
breast cancer | Parida et al. (2020) |
| 4. | Syzygium aromaticum (L.) Merr. & L.M. Perry | Flavonoid | Kumatakenin (Pubchem CID: 4477326) |  |
Ovarian, cervical, oral squamous cell carcinoma, digestive system and breast cancer | Zheng et al. (2016); Woo et al. (2017); Das et al. (2018); Choudhury et al. (2020) |
| Phenylpropanoid | Eugenol (Pubchem CID: 13876103) |  |
||||
| 5. | Elettaria cardamomum (L.) Maton | Indoles | Diindolylmethane (Pubchem CID: 8928276) |  |
Breast, lung, ovarian, prostate, colon, gastric and leukemia | Bhagat and Chaturvedi (2016); Vutakuri and Somara (2018); Nawaz et al. (2020); Yue et al. (2020) |
| 3-Alkylindoles | Indole-3-carbinol (Pubchem CID: 3581) | 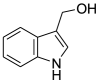 |
||||
| Chalconoid | Cardamonin (Pubchem CID: 557026) | 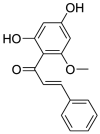 |
||||
| Monoterpene | Limonene (Pubchem CID: 388386) |  |
||||
| 6. | Piper nigrum L. | Piperamide | Piperine (Pubchem CID: 553590) |  |
Breast, prostate colon cancer and osteosarcoma | de Souza Grinevicius et al. (2016) |
| Alkaloid | Piperlongumine (Pubchem CID: 553441) |  |
Lung, breast, ovarian, gastric and colon cancer | Prasad and K Tyagi, 2016; Tripathi and Biswal (2020) | ||
| 7. | Trigonella foenum-graecum L. | Alkaloid | Trigonelline (Pubchem CID: 5369) | 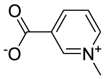 |
Blood and breast cancer | Kunnumakkara et al. (2018); Mohamadi et al. (2018) |
| Diosgenin (Pubchem CID: 89870) |  |
|||||
| 8. | Zingiber zerumbet (L.) Roscoe ex Sm. | Sesquiterpene | Zerumbone (Pubchem CID: 4580581) |  |
Colorectal cancer | Sithara et al. (2018) |
| 9. | Zingiber officinale Roscoe | Phenolics | 6-Gingerol (Pubchem CID: 391126) |  |
Breast, colorectal, prostate, lung cancer, melanoma and glioblastoma | Geng et al. (2016); Zheng et al. (2016); Kammath et al. (2021) |
| Shogaols | Shogaol (Pubchem CID: 4445106) |  |
||||
| Paradols | Paradol (Pubchem CID: 85173) |  |
||||
| 10. | Capsicum annuum L. | Alkaloids | Capsaicin (Pubchem CID: 1265957) |  |
Lung, colon, breast, cervical, prostate and tongue carcinoma | Geng et al. (2016); Kunnumakkara et al. (2018) |
| 11. | Nigella sativa L. | Monoterpene | Thymoquinone (Pubchem CID: 9861) |  |
Colon, bladder, lung, ovarian and gastric cancer | Zhang et al. (2016); Majeed et al. (2021) |
| 12. | Cinnamomum verum J. Presl | Aldehyde | Cinnamaldehyde (Pubchem CID: 637511) |  |
Leukemia, oral, liver, lung, prostate, breast and colon cancer | Singh et al. (2021a) |
| 13. | Cinnamomum tamala (Buch.-Ham.) T. Nees & Eberm. | Monocyclic sesquiterpene | α-Caryophyllene (Pubchem CID: 4444853) |  |
Lung, breast and brain cancer | Thanekar et al. (2016) |
| 14. | Allium sativum L. | Organosulfur compound | Allicin (Pubchem CID: 58548) |  |
Colon cancer | Perez-Ortiz et al. (2020) |
| Diallyl sulphide (Pubchem CID: 11128) | Colon, skin, breast prostate and upper digestive tract cancer | Zheng et al. (2016); Kunnumakkara et al. (2018) | ||||
| S-Allyl-L-cysteine (Pubchem CID: 7969672) |  |
Prostate cancer | Guldiken et al. (2018) | |||
| 15. | Rosmarinus officinalis L. | Diterpenoids | Carnosic acid (Pubchem CID: 58635) |  |
Lung, colorectal, breast, kidney, liver, prostate cancer and leukemia | Zheng et al. (2016); Kunnumakkara et al. (2018); Corveloni et al. (2020); Kammath et al. (2021) |
| Carnosol (Pubchem CID: 390568) |  |
|||||
| Rosmarinic acid (Pubchem CID: 4445104) | 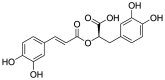 |
|||||
| 16. | Myristica fragrans Houtt. | Benzofurans | Licarin B (Pubchem CID: 4945284) |  |
Skin carcinoma | Kunnumakkara et al. (2018); Perez-Ortiz et al. (2020) |
| Phenylpropene | Myristicin (Pubchem CID: 4125) |  |
||||
| 17. | Illicium verum Hook.f. | Phenylpropene | Estragole (Pubchem CID: 13850247) | 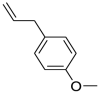 |
Hepatocellular carcinoma | Kunnumakkara et al. (2018) |
| trans-Anethole (Pubchem CID: 553166) |  |
Osteosarcoma | Kunnumakkara et al. (2018) | |||
| 18. | Salvia officinalis L. | Monoterpene cyclic ether | 1,8-Cineole (Pubchem CID: 2656) |  |
Hepatocellular and lung cancer | Kunnumakkara et al. (2018) |
| 19. | Garcinia indica (Thouars) Choisy | Benzophenone | Garcinol (Pubchem CID: 10199485) |  |
Breast, colon and squamous cell carcinoma | Duan et al. (2018); Kunnumakkara et al. (2018) |
| 20. | Brassica juncea (L.) Czern. | Isothiocyanate | Sulforaphane (Pubchem CID: 7851806) |  |
Lung, colorectal and bladder cancer | Kunnumakkara et al. (2018) |
| 21. | Coriandrum sativum L. | Terpene alcohol | Linalool (Pubchem CID: 391430) |  |
Breast cancer | Zheng et al. (2016) |
| 22. | Ocimum basilicum L. | Triterpenoids | Ursolic acid (Pubchem CID: 191497) |  |
Breast and colorectal cancer | Kunnumakkara et al. (2018) |
| 23. | Mentha piperita L. | Triterpenoids | Carvone (Pubchem CID: 21106424) |  |
Leukemia and skin cancer | Kunnumakkara et al. (2018) |
| 24. | Origanum vulgare | Monoterpenoids | Carvacrol (Pubchem CID: 21105867) |  |
Breast cancer | Baranauskaite et al. (2017) |
| 25. | Thymus vulgaris L. | Monoterpenoids | Thymol (Pubchem CID: 21105998) | 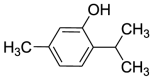 |
Colorectal cancer | Zeng et al. (2020) |
| 26. | Ferula asafoetida H. Karst. | Sesquiterpene coumarins | Gummosin (Pubchem CID: 5442126) |  |
Breast and prostate cancer | Iranshahy et al. (2019) |
| 27. | Capparis spinosa L. | Glucopyranosides | 3-methyl-2-buten-1-yl β-D-glucopyranoside (Pubchem CID: NR) |  |
Breast cancer | Salih et al. (2020) |
| 28. | Vanilla planifolia Jacks. Ex Andrews | Phenolic aldehyde | Vanillin (Pubchem CID: 1183) |  |
Colorectal cancer | Xie et al. (2020) |
| 29. | Murraya koenigii (L.) Spreng. | Carbazole alkaloid | Girinimbine (Pubchem: 87534) |  |
Colon cancer | Iman et al. (2017) |
| 30. | Cuminum cyminum L. | Flavonoids | Luteolin-7-O-glucoside (Pubchem CID: 4444241) |  |
Breast cancer | Goodarzi et al. (2020) |
| 31. | Origanum majorana L. | Flavonoids | Hesperetin (Pubchem CID: 65234) |  |
Brain and cervical cancer | Erenler et al. (2016) |
| Benzenediol | Hydroquinone (Pubchem CID: 764) | 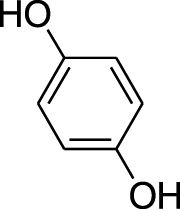 |
NR* = Not reported.
Table 2 shows that some of the compounds were evaluated for their anticancer potential against more than one cancer while some of the compounds were reported only against one cancer type. Therefore, these compounds can be evaluated against other cancer types too. The anticancer efficacy of these compounds can be evaluated after the structural modification of these compounds. It is hypothesized that the structural modification is featured by enhanced functionalization. The same hypothesis can be applied on these bioactives too.
5. In-vitro cytotoxicity of Indian spices
Being a multifactorial disease, it is difficult to tackle carcinogenesis with monodrug therapy. Sometimes monodrug therapy also exerts some negative effects which can be countered with adjuvant drugs (Geng et al., 2016). Due to such safety concerns, the plant bioactives are preferred to cure this deadly disease sophisticatedly as compared to the chemotherapy. Spices are the rich reservoir of many bioactives viz. alkaloids, terpenes, flavonoids, phenylpropanoids and anthocyanins which possess anticancer therapeutic potential. These bioactives have the potential to halt the excessive synthesis of reactive oxygen species (ROS) and nitrogen radicals which ultimately prevents the many metabolic disorders associated with them (Singh and Yadav, 2022). These bioactives also augments the endogenous antioxidant system. The suppression of oxidative stress ultimately reduces the risk of cancer initiation and progression (Bhagat and Chaturvedi, 2016).
Therefore, spices’ derived bioactives can be used frequently for prevention of different kind of malignancies and for combating more than one cancer type such as breast, lung, fore stomach, liver, pancreas, colorectal and oral cancer (Srinivasan, 2017a). For instance, in a study by Ramaswamy et al. (2017), the antitumor potential of ginger, dalchini and ajwain decoctions was examined against lung cancer cell lines. It was observed that a concentration ranges from 25 to 50 μg/ml was effective in combating cancer cell proliferation while the concentration above 50 μg/ml was insignificant due to its intolerability. The significant anticancer effect of various spices and herbs was also screened against cancerous cells and their cyclooxygenase-2 (COX-2) inhibitory activity in HCA-7 cell lines of colorectal cancer (CRC) (Jaksevicius et al., 2017).
Numerous spices in the following order: turmeric, bay leaf, ginger, sage and rosemary have significantly suppressed the cell growth along with the down-regulation of COX-2 expression and activity. In another study by Saeed (2017), the synergistic anticarcinogenic activity of clove/dalchini water decoction was screened on HepG2 cell lines of liver carcinoma and it was found that the extract has exerted significant cytotoxicity as compared to the control. The anticancer efficacy of turmeric, ginger and garlic mixture on MCF-7, ZR-75 and MDA-MB 231 cell lines of breast cancer was carried out and it was found that the combined extract has induced apoptosis more profoundly in MCF-7 and ZR-75 cell lines followed by MDA-MB 231 cell lines (Vemuri et al., 2017). Thymoquinone from Nigella sativa can be used for the prevention of metastasis of tumor cells to the other body parts and phytoestrogen from red clove to stop breast cancer. Crocin, from saffron was also reported to downregulates the progression of cancer cell (Kammath et al., 2021). The in-vitro cytotoxicity studies of spice’ derived extracts or a specific compound are summarized in Table 3.
Table 3.
Cytotoxicity of extracts derived from Indian spices (in-vitro).
| S. No. | Botanical Name | Family | Common Name | Plant part | Active compound/extract | Cancer type | Cell line | Mechanism of action | Ref (s). |
|---|---|---|---|---|---|---|---|---|---|
| 1. | Zingiber zerumbet (L.) Roscoe ex Sm. | Zingiberaceae | Ginger | Rhizome | Zerumbone | Colorectal cancer | SW480 cell line | Induced programmed cell death (PCD), cell cycle arrest and inhibited cell migration | Sithara et al. (2018) |
| 2. | Curcuma caesia Roxb. | Zingiberaceae | Black turmeric | Rhizome | Hexane | liver Adenocarcinoma | HepG2 cell lines | Blockage of cell cycle at G2/M phase and finally induced PCD | Mukunthan et al. (2017) |
| 3. | Curcuma aromatica Salisb. | Zingiberaceae | Wild turmeric | Rhizome EO | Ar-tumerone | Breast cancer | MCF7 cells | Inhibition of cancerous cell | Parida et al. (2020) |
| 4. | Curcuma longa L. | Zingiberaceae | Turmeric | Rhizome | Curcumin | Pancreatic cancer | L3.6 pl and MIA PaCa-2 cells | DNA synthesis phase arrest, inhibit surviving expression, Increased PCD and halted nuclear factor kappa B (NF-κB) translocation | Basha et al. (2016) |
| Patu8988 and Panc-1 | Down regulation of neural precursor cell expressed developmentally downregulated protein-4 (NEDD-4) (oncoprotein) | Su et al. (2017) | |||||||
| Patu8988 and Panc-1 cells | Inhibition of cell growth, invasion, migration and induction of PCD | Zhou et al. (2016) | |||||||
| Bladder cancer stem cells | UM-UC-3 and EJ cells | Down regulation of cancer stem cell (CSC) markers, inhibited sonic hedgehog pathway, cell proliferation and induced PCD | Wang et al. (2017) | ||||||
| Colon cancer | SW480 and SW620 cells | Decreased p53 level, cell proliferation and alteration in phosphoproteome | Sato et al. (2017) | ||||||
| Colon carcinoma | CRC cells | Inhibition of Wnt/β-catenin & sonic hedgehog signalings | Luers (2018) | ||||||
| Colorectal cancer | HT29 cells | Inhibit the tumor growth due to their anti-angiogenic effect | Yue et al. (2016) | ||||||
| Colon cancer | HCT 116 cell line | Suppression of cellular protein synthesis, induced mitochondrial dysfunction, Inactivation of lysosomal activity and apoptosis | Wang et al. (2016) | ||||||
| Gastric cancer | MGC-803 cell line | Decreased cell viability, colony formation, cell migration and caspases activation to induce PCD | He et al. (2017) | ||||||
| Colon cancer | DLD-1, HCT116 and LoVo cells | High expression of Caspase-3 and -7, inhibited cell growth and ultimately lead to PCD | Montgomery et al. (2016) | ||||||
| Breast canc Er |
MCF-10F, MDA-MB-231 and Tumor-2 cells | Suppression of the expression of different proteins, cadherins (E, N) and catenins, vimentins, fibronectins, epithelial mesenchymal transition and cell proliferation | Gallardo and Calaf (2016) | ||||||
| MDA-MB-231 cells | Inhibitory concentration (IC)50 = 49 ± 2.08 μg/ml | Ahmad et al. (2016) | |||||||
| MDA-MB-231 cells | Alteration of cellular cytoskeleton, morphology and cellular migration | Li et al. (2016) | |||||||
| MCF-7 cells | Inhibition of LPA induced RhoA/ROCK/matrix metallopeptidases (MMP) signaling, cell invasion | Sun et al. (2016) | |||||||
| Regulation and inhibition of breast cancer promoting genes and tumour progression | Wang et al. (2018) | ||||||||
| Suppression the expression of phosphorylated protein p44/42 in MAP Kinase pathway, activation of p53, caspase-3, 9, B-cell lymphoma-2 (Bcl-2) and Bax activity | Bhuiyan (2020) | ||||||||
| Prostate cancer | PC3 cells | Halts the cell growth and finally lead to PCD | Irshad et al. (2017) | ||||||
| Hepatocellular cancer | Hepa 1–6 cells | Reactivation of estrogen receptor alpha (ER-α) gene expression, Inhibition of cell growth and induced PCD | Sanaei et al. (2020) | ||||||
| Curcumin along with silibinin B | Colorectal cancer | CRC cell line | Induced cell death by both PCD and autophagy | Horita et al. (2019) | |||||
| Curcumin analogues | Prostate cancer, colon cancer, lung cancer and pancreatic cancer | PC-3, HT-29, H1299 and BxPC-3 cells | Inhibition of cell growth, suppression of NF-κB and induced apoptosis | Zhang et al. (2016) | |||||
| WZ35 analogue | Gastric cancer | BGC-823 and SGC-7901 cells | Downregulation of yes-associated protein (YAP), activation of JNK and inhibition of glycolysis | Chen et al. (2020) | |||||
| 5. | Piper nigrum L. | Piperaceae | Black pepper | Fruit | Piperine | Breast and colon cancer | MCF-7 and HT-29 cells | DNA damage, decreased cell viability, cell proliferation, cell cycle arrest and induced apoptosis | de Souza Grinevicius et al. (2016) |
| Prostate cancer | PC3 cells | Inhibition of cell growth and induction of apoptosis | Irshad et al. (2017) | ||||||
| 6. | Crocus sativus L. | Iridaceae | Saffron | Dried red stigma | Crocin | Breast cancer | HCC70, HCC1806, HeLa and CCD1059sk cells | Depolymerization of microtubules, inhibit mitosis, tubulin assembly and cell proliferation | Hire et al. (2017) |
| Leaf biowastes | Crocetin | Breast carcinoma | MCF-7 cells | Inhibition of ER-α and histone deacetylase 2 (HDAC2) | Mir et al. (2020) | ||||
| 7. | Nigella sativa L. | Ranunculaceae | Black cumin | Seed oil | Thymoquinone | Colon cancer | COLO205 and HCT116 cells | Inhibition of NF-κB | Zhang et al. (2016) |
| 8. | Allium sativum L. | Amaryllidaceae | Garlic | Bulb | Thiosulfinate-enriched extract | Colon cancer | Caco-2 and HT-29 cells | Decreasing cell viability | Perez-Ortiz et al. (2020) |
| 9. | Syzygium aromaticum (L.) Merr. & L.M. Perry | Myrtaceae | Clove | Flower bud | Kumatakenin | Ovarian cancer | SKOV3 and A2780 cells | Induced cytotoxicity, PCD, increased activity of caspase 3, 8, 9 and inhibition of interleukin (IL)-10, MMP-2/9 and vascular endothelial growth factor (VEGF)- Cancer promoting factors | Woo et al. (2017) |
| Eugenol | Cervical cancer | HeLa cells | Modification of cellular morphology and ultimately induced PCD | Das et al. (2018) | |||||
| Esophageal cancer | TE-13 cells | Inhibition of cellular growth | Zheng et al. (2016) | ||||||
| Breast cancer | MCF-7 cells | Downregulation of β-catenin and CSC markers | Choudhury et al. (2020) | ||||||
| Induction of oxidative stress, DNA damage, alteration of Akt, p38 MAPK, JNK and Erk 1/2 signaling pathways and induced cell death | Kello et al. (2020) | ||||||||
| 10. | Elettaria cardamomum (L.) Maton | Zingiberaceae | Small cardamom | Fruits | Cardamonin | Human melanoma | M14 and A375 cell lines | Inhibited cell viability, reduced cell density, upregulation of BAX protein and induced apoptosis | Yue et al. (2020) |
| 11. | Cinnamomum tamala (Buch.-Ham.) T. Nees & Eberm. | Lauraceae | Tejapatta | Leaves | Eugenol and α-caryophyllene | Lung, breast and brain cancer | A549, MCF-7 and U-87MG cells | Cell-specific cytotoxicity | Thanekar et al. (2016) |
| 12. | Cinnamomum zeylanicum Blume | Lauraceae | Dalchini | Bark | Ethanolic extract | Breast carcinoma | MDA-MB-231 cells | IC50 = 25 μg/ml and DNA fragmentation | Husain et al. (2018) |
| 13. | Myristica fragrans Houtt. | Myristicaceae | Mace | Outer aril | Dichloromethane | Oral cancer | Oral cancer cell lines | DNA fragmentation and genotoxicity | Cinthura et al. (2017) |
| 14. | Allium wallichii Kunth | Alliaceae | Himalayan Onion | Whole plant | Aqueous ethanol | Prostate cancer, Breast Cancer and cervical cancer, Burkitt's lymphoma | PC3, MCF-7, HeLa and B- Lymphoma cell line | IC50 = 69.69 μg/ml (PC3), IC50 = 55.29 μg/ml (MCF-7), IC50 = 46.51 μg/ml (HeLa) and IC50 = 3.817 ± 1.99 mg/ml (B-Lymphoma) |
Bhandari et al. (2017) |
| 15. | Origanum marjoran L. | Lamiaceae | Marjoram | Aerial parts | Aqueous | Mammary adencarcinoma | AMN-3 cell line | Inhibition of cell growth | Khaleel et al. (2016) |
| Hesperetin and hydroquinone | Rat brain tumor and cervical cancer | C6 and HeLa cells | Hesperetin IC50 = 64.38 μg/ml (C6 cells) Hydroquinone IC50 = NR | ||||||
| 16. | Rosmarinus officinalis L. | Lamiaceae | Rosemary | Leaves | Dimethyl sulfoxide | Breast cancer | MDA-MB-231 cell line | Inhibition of cell growth, cell survival and lead to PCD | Jaglanian and Tsiani (2020) |
| Carnosic acid | Lung cancer | IMR-90 and NCI–H460 cell line | Blockage of cell cycle, inhibition of cellular proliferation and apoptosis | Corveloni et al. (2020) | |||||
| 17. | Pimpinella anisum L. | Apiaceae | Anise | Seeds | Aqueous | Oral cancer | KB cell line | Induced Cytotoxicity and Changes in cellular morphology | Mukunda et al. (2020) |
| 18. | Foeniculum vulgare Mill. | Apiaceae | Fennel | Seeds | Protein fraction | Breast and pancreatic cancer | MCF-7 and AsPC-1 cells | Induced cytotoxicity in MCF-7 cells but no significant inhibition of AsPC-1 cell lines | Megeressa et al. (2020) |
| 19. | Coriandrum sativum L. | Apiaceae | Coriander | Roots | Ethyl acetate extract | Breast carcinoma | MCF-7 cells | Halts the process of tumor cells migration | Zheng et al. (2016) |
| 20. | Origanum vulgare | Lamiaceae | Oregano | Whole | Carvacrol | Breast carcinoma | MDA-MB-231 cells | IC50 = 199 μM | Baranauskaite et al. (2017) |
| 21. | Thymus vulgaris L. | Lamiaceae | Thyme | Whole | Thymol | Colorectal cancer | HCT-116 and Lovo cells | Inhibition of cell growth and metastasis via downregulation of Wnt/β-catenin and activation of BAX/Bcl-2 signaling pathway | Zeng et al. (2020) |
| 22. | Ferula asafoetida H. Karst. | Apiaceae | Asafoetida | Resin | Gummosin | Breast and prostate cancer | MCF-7 and PC-3 cells | IC50 = 32.1 μg/ml (MCF-7) and IC50 = 30 μg/ml (PC-3) | Iranshahy et al. (2019) |
| 23. | Capparis spinosa L. | Capparidaceae | Caper | Fruit | 3-Methyl-2-buten-1-yl β-D-glucopyranoside | Breast cancer | NR | Significantly declined the efficacy of the enzyme coenzyme colin estersase and killed the cancer cells | Salih et al. (2020) |
| 24. | Armoracia rusticana P. Gaertn., B. Mey. & Scherb. | Brassicaceae | Horseradish | Above and underground parts | 5-Phenylpentylisothiocyanate | Colon and cervical cancer | Caco-2 and HeLa cells | IC50 = 3.4 μg/ml (Caco-2) IC50 = 5.9 μg/ml (HeLa) |
Dekić et al. (2017) |
| 25. | Alpinia galangal (L.) Willd. | Zingiberaceae | Greater galanga | Rhizomes | Acetoxy Chavicol Acetate | Cervical, and breast cancer | HeLa, MCF-7 and T47D cells | Induction of apoptosis via the activation of Caspase-3 cascade | Suhendi et al. (2017) |
| 26. | Vanilla planifolia Jacks. Ex Andrews | Orchidaceae | Vanilla | Beans | Vanillin | Colorectal cancer | HT-29 cells | Suppression of cell proliferation and Caspases induced apoptosis | Xie et al. (2020) |
| 27. | Garcinia indica (Thouars) Choisy | Clusiaceae | Kokam | Fruit rind | Garcinol | Gallbladder cancer | GBC cells | Downregulation of mRNA levels to suppress the activity of MMP2 and MMP9 | Duan et al. (2018) |
| 28. | Murraya koenigii (L.) Spreng. | Rutaceae | Curry leaf | Leaf | Girinimbine | Colon cancer | HT-29 cells | Inhibition of inflammation and cell viability and finally induced apoptosis | Iman et al. (2017) |
| 29. | Cuminum cyminum L. | Apiaceae | Cumin | Seeds and fruits | Luteolin-7 -O-glucoside | Breast cancer | MCF-7 cells | Selective cytotoxicity | Goodarzi et al. (2020) |
NR* = Not reported.
From Table 3, it is observed that there is no specific bioactive that persist in all the spices for their anticancer effect. The anticancer therapeutic effects of these spices are mainly attributed to the presence of their unique bioactives that vary from spice to spice. For examples, curcumin from Curcuma longa, cinnamaldehyde from Cinnamomum verum, cuminaldehyde from Cuminum cyminum, crocin from Crocus sativus, thymoquinone from Nigella sativa, eugenol from Syzygium aromaticum, cardamonin from Elettaria cardamomum, thymol from Thymus vulgaris and so on are specific to a particular spice for their anticancer effect. Sometimes, more than one bioactive from a single spice can also exhibit anticancer potential such as kumatakenin and eugenol are two different anticancer bioactive isolated from Syzygium aromaticum. Likewise, Crocus sativus possess two different anticancer bioactive including crocin and crocetin. On the contrary, a single bioactive can be reported from more than one spice but their therapeutic effects depend upon the yield of that particular bioactive. Hence, many bioactive compounds are responsible for the anticancerous activity of different spices.
Inflammatory tissues are one of the origins of cancer cells that increases the risk of carcinogenesis. Inefficient clearance of infected tissues during chronic inflammation leads to the tissue deterioration, ROS generation and ultimately DNA damage and mutation. Under these inflammatory conditions, the cells remain continuously proliferating in order to maintain homeostasis. This process becomes the major driving force for the development of initial tumor cells. Chronic inflammation is also the cause of overproduction of cytokines like TNF-α and IL-6. These cytokines trigger the malignancy and metastasis of cancerous cells. Therefore, the compound which exhibits anti-inflammatory potential can be precisely used to eliminate inflammation induced cancer pathology (Dupré and Malik, 2018).
Curcumin is a natural anti-inflammatory agent. It attenuates the production of pro-inflammatory and profibrotic cytokines by targeting the various inflammatory mediators like COX-2, inducible nitric oxide synthase and NF-κB, thus halts the excessive generation of free radicals. The whole mechanism will eventually aid to the amelioration of tissue toxicity. Curcumin anti-inflammatory efficacy induced NF-κB modulation and downregulation of it signaling cascade also help in reducing the angiogenesis, tumor progression and metastasis (Farhood et al., 2019). From Table 3, it can be inferred that curcumin is highly explored and a representative anticancer spice’ bioactive. The compound alone or in combination with other drugs possesses significant anticancer efficacy against several types of cancers such as colon, pancreatic, breast, prostate, bladder, gastric and hepatocellular cancer. The compound exerts its anticancer effect via affecting the microtubule assembly that ultimately lead to the mitotic arrest. However, no treatment has yet been approved by the FDA. The reason behind this might be the non-specific action of curcumin which not only impacts the tumor cells but also imply its possible impact on normal cells. In addition, the poor bioavailability and insolubility of curcumin in water make it least absorbed in liver and intestinal walls (Fridlender et al., 2015). Therefore, further studies can be planned on the improvement of different physicochemical properties of curcumin and its disease-specific action. Inspire from curcumin and its anticancer efficacy, other spices and their bioactives can also be evaluated for their physicochemical properties, anticancer efficacy, toxicological evaluation and safety concern.
6. Structure-activity relationship (SAR)
Bioactives of spices (because of their active functional entities) gives a lead for designing the novel chemical compounds that consists of more than one compound that are linked via different bonding in a single structure, featured by improved therapeutics (Singh et al., 2021a). Various curcumin analogues were synthesized on the basis of its SAR studies to overcome its different limitations such as bioavailability, fastest metabolism, less solubility and absorption, which hinder its clinical efficacy (Gupta et al., 2017). They reported that introduction of a strong electron withdrawing group (acetone or cyclo-hexanone spacer, 4′-weak electrons donating and 2′-electron withdrawing groups) in curcumin will ultimately lead to its increased cytotoxicity due to its more electronegative character.
The SAR studies of cinnamaldehyde and eugenol was conducted to evaluate the compound's toxicity on human adipose-derived mesenchymal stem cells (HASCs). It was observed that both the compounds were non-toxic at their lower concentrations, easily biodegradable and can be metabolized through cytochrome-P450 (Absalan et al., 2017). The QSAR study of various derivatives of indole namely “N-(2-hydroxy-5-nitrophenyl (4′-methylphenyl) methyl) indoline (HNPMI)” has shown good anti breast cancer results, even better than cyclophosphamide (positive control). QSAR model revealed the presence of indole ring, aromaticity, nitrogen and oxygen like constituents, attributed to the biological efficacy of HNPMI (Palanivel et al., 2020). Various SAR studies of various spice's bioactives are clubbed in Table 4.
Table 4.
Summary of QSAR studies and associated pharmacological effect of spice's bioactives.
| S. No. | Active compound | Analogues | Conc./IC50 obtained | Pharmacological effects | Molecular targets | References |
|---|---|---|---|---|---|---|
| 1. | Curcumin | Bisdemethoxycurcumin | 10–40 μM | Cytotoxicity against ovarian cancer, CCL4-induced hepatotoxicity in rats, Colon cancer | COX-2, matrix metalloproteinase, STAT3 and Notch signaling, PI3K/Akt and Wnt/β-catenin pathway | Gupta et al. (2017) |
| Demethoxycurcumin | 20 μM | Combating lead-induced neurotoxicity in rats, proliferation of breast cancer cells | ||||
| Tetrahydrocurcumin | 34–112.5 μM | Suppression of COX-dependent arachidonic acid metabolism, intrinsic apoptosis pathway in breast cancer | ||||
| Hydrazinocurcumin | NR | Inhibition of colon cancer via suppression the cell cycle | ||||
| 2. | Cinnamaldehyde | End products obtained after cinnamaldehyde metabolism by cytochrome-P450 | 2.5 μM/ml | Non-genotoxic and low toxicity towards hASCs | May bind to DNA and proteins | Absalan et al. (2017) |
| 3. | Eugenol | End products obtained after eugenol metabolism by cytochrome-P450 | 0.1 μg/ml | Non-genotoxic but carcinogenic and mutagenic and low-toxic in nature towards hASCs | May bind to DNA and proteins | Absalan et al. (2017) |
| 4-[(2S)-2,3-dihydroxypropyl]-2-methoxyphenyl 2-hydroxybenzoate | 26.56 μmol/ml - 286.81 μmol/ml | Significantly inhibited Bcl-2 expression in HT29 colon cancer | Hydrophobic nature plays important role | Fadilah et al. (2020) | ||
| 4. | Orientin | Fenofibryl glucuronide | 100 μg/ml (202.389 μM) | Results showed 41% cell mortality of HepG2 cells after an exposure of 96 h | Due to its anti proliferative properties | Sharma et al. (2016) |
| 5. | Indole | HNPMI | 10–100 μM | Inducing anti-proliferative and PCD of breast cancer cells | Downregulation of PI3K/S6K1 genes via the upregulation of epidermal growth factor receptor (EGFR) | Palanivel et al. (2020) |
| 6. | Ursolic acid | 6r and 6q compounds | NR | Significant anticancer properties against T24 cell lines of bladder cancer | Down regulation of NF-κB signalings | Yadav et al. (2019) |
| 7. | Sulforaphane | NR | NR | Upregulation of NAD(P)H:quinone oxidoreductase 1 (NQO1) (carcinogen detoxification enzyme) | Kelch-like erythroid cell derived protein with CNC homology [ECH]-associated protein-1 (Keap-1)-[NF-E2]-related factor-2 (Nrf-2) pathway | Vaghefinezhad et al. (2021) |
| 8. | Different phytochemicals from Brassicaceae | Glucoraphanin | pIC50 = 4.28 μm |
Prevent tumor inflammation | NF-κB inducing kinase | Devi et al. (2017) |
| 9. | Myristicin | 6-allyl-4-phenoxybenzo[d][1,3]dioxole (M1) | Log P = 3.97 | Antioxidant effect (Due to large number of electrons attributed to higher polarity) | More solubility in water as antioxidant drug due to its highest log P value | Muliadi et al. (2021) |
| 10. | Piperic acid | 2- (3,4-dihydroxyphenyl)ethyl ester | 17 μM | Tumor specificity and cytotoxicity | Induced PCD and breakdown of caspase-3 | Sakagami et al. (2017) |
| 11. | Rosmarinic acid | Derivatives formed via the substitution of metal ions sodium with silver and amines with imidazole | IC50 > 200 μg/ml | Anti-glioblastoma | Upregulation of caspases 3,7,8 & 9, to induce PCD and modification of IL-17A downstream angiogenesis signalings | Khan et al. (2019) |
| 12. | Capsazepine | N-[2-(4-chlorophenyl)ethyl]-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline-2-carbothioamide and N-benzyl-6,7-dihydroxy-1-phenyl-1,2,3,4-tetrahydroisoquinoline-2-carbothioamide | IC50 < 15 μM | HeLa cervical cancer | Target transient receptor potential vanilloid subtype 1 (TRPV1) | De et al. (2019) |
| 13. | Cardamonin | Cu(II) complex of cardamonin: [Cu(C16H13O4)2(H2O)2]·2H2O | IC50 = 13.2 μM (A549 cell lines) IC50 = 13.2 μM (HK1 cells) |
Anticancer activity against lung cancer and NPC cells | Caspases mediated DNA damage, cell cycle arrest and resulted PCD as well as suppression of mTOR signalings | Break et al. (2018) |
NR* = Not reported.
From Table 4, it can be inferred that curcumin is highly explored for SAR studies as compared to the other bioactives. Curcumin analogues were reported to possess significant anticancer activity after introducing the strong electron withdrawing group. The strong electron withdrawing group can also be introduced in other bioactives too, in order to enhance their anticancer efficacy.
7. Anticancer mechanism of actions of Indian dietary spices and their principal bioactives
Spices can induce the PCD and halt the process of migration, invasion and proliferation of the tumour cells to combat cancer (Zheng et al., 2016). Different phytoconstituents of spices exerts different actions to suppress cancer cell growth. In a study by Mughal (2019), it was postulated that the phytoconstituents from spices have the ability to suppress the expression of cyt p450, isozymes like CYP-1A1, COX-2, signal transducer and activator of transcription-3 (STAT-3), which are crucial to tumourogenesis. The bioactives decelerates the process of carcinogenesis via the suppression of IL-6 receptors, epidermal growth factors (EGF), the protein involved in cell cycle and the suppression of Kappa-B. Spices active constituents have the potential to suppress ROS production, cell division and stimulate apoptosis. They have the potential to regulate inflammation and immunocompetence which ultimately contribute to their cancer treatment efficacy (Srinivasan, 2017a).
The anticancer mechanism of curcumin was performed in mice bearing colon cancer. The tumor proliferation was mediated via the dysregulation of Wnt/β-catenin pathways. Western blotting data revealed that curcumin treatment has downregulated the expression of β-catenin along with miR-130a and ultimately suppressed the Wnt/β-catenin signaling pathway. MiR-130 expression was supposed to antagonize the anticancer effect of curcumin. Curcumin treated cells were noticed with decelerated cell viability and cell proliferation (Dou et al., 2017). In another study by Zhou et al. (2016), the anticancer mechanism of action of curcumin was assessed in treating pancreas cancer and it was suggested that curcumin treatment has significantly abolished the over expression of YAP and transcriptional co-activator with PDZ-binding motif (TAZ) and associated Notch-1 expression, which play critical role in pancreatic cancer cell proliferation and ultimately halted the cell proliferation and invasion.
The cancer treating mechanism of curcumin was evaluated in colon cancer bearing male Balb/c nude mice and it was reported that Sirtuin (silent mating type information regulation 2 homolog) 1 (SIRT1) (an NAD+ -dependent histone/protein deacetylase) was overexpressed in colon cancer proliferation. The SIRT1 protein helps in viability and migration of human colon cancer cells. The tumor bearing mice were administrated with curcumin. Curcumin possesses the ability to suppress the expression of SIRT1 protein. It was noticed that curcumin treatment has significantly suppressed the SIRT1 protein expression without influencing its mRNA expression. Furthermore, post translational modification and proteasomal degradation of SIRT1 protein was also noticed. Moreover, it was also reported that curcumin bind to cysteine 67 domain of SIRT1 oncoprotein and replaced it with alanine (Lee et al., 2018). The anticancer mechanism of 6-gingerol and capsaicin (isolated from ginger and red chili, respectively) was investigated on lung carcinoma. Capsaicin exerted cancer promoting effect due to the enhanced level of EGFR level whereas reduced level of TRPV1. On contrary, 6-gingerol individually or synergistically with capsaicin, has significantly increased the TRPV1 content and ultimately reduced the EGFR, NF-κB and cyclin D1 level and finally decreased the pulmonary carcinoma metastasis (Geng et al., 2016). The anticancer mechanism of thymoquinone (TQ) was tested on COLO205 and HCT116 cell lines of colon cancer. TQ treatment resulted into the significant reduction of phosphorylated p65 level in the nucleus, VEGF, c-Myc and Bcl-2 factors which ultimately mediated the suppression of NF-κB and cell death (Zhang et al., 2016, Zhang et al., 2016).
The antitumor mechanism of curcumin was studied in oxaliplatin (OXA) acquired resistant CRC cell lines. The OXA resistant CRC cells overexpressed the NF-κB transcription factor and associated CXC-chemokines (CXCL8, CXCL1 and CXCL2). It was observed that NF-κB signaling cascade can be inhibited through the curcumin alone or in combination with OXA. The compounds also made the CXCL8 and CXCL1 gene silencing via the suppression of the Akt/NF-κB pathway (Ruiz de Porras et al., 2016). Curcumin ability was examined for its cancer treating potential in pancreatic cancer. The compound was well demonstrated for its anticancer ability via deactivation the NEDD4 (oncoprotein) and stimulating the phosphatase and tensin homolog (PTEN) and p73 expression (Su et al., 2017). Cardamonin was also reported to exhibit the cell cycle blockage and PCD inducing potential in malignant cells (Nawaz et al., 2020). In a study by Manayi et al. (2018), the preclinical study was carried out on piperine alongwith its self-renewal, proliferation and survival inhibiting potential. It was also found that the compound possesses significant antimutagenic activity and can suppress the expression of multidrug resistance transporters such as P-gp and MRP-1 and exerts cancer cell specific cytotoxicity.
Eugenol was evaluated for its antitumor potency on CSC markers and its main regulator β-catenin in MCF-7 cell lines. It was observed that eugenol facilitated the N-terminal phosphorylation of Ser37 residue which mediated the suppression of β-catenin and CSC markers, these series of events play crucial role for exerting their anticancer potential (Choudhury et al., 2020). The mechanism of action of curcumin was examined in Hepa 1–6 cell lines of hepatocellular cancer. The compound treatment has exerted dose dependent cell growth inhibition, anti-proliferative and apoptotic effects mediated via the reactivation of estrogen receptor alpha (ERα) gene expression (Sanaei et al., 2020). Rosemary extract has significantly inhibited the phosphorylation/activation of Akt and mTOR signaling and increased the breakdown of poly ADP-ribose polymerase (PARP) apoptotic marker and ultimately induced PCD in triple-negative MDA-MB-231 cells of mammary cancer (Jaglanian and Tsiani, 2020). Curcumin can modulate the multiple pathways such as NF-κB, PI3K/AKT/mTOR, JAK/STAT, MAPK and notch signaling, as associated with cell proliferation, cell cycle regulation, senescence and PCD of breast cancer cells, therefore, the compound can be targeted for drug discovery against breast cancer (Banik et al., 2017). In a study by Kunnumakkara et al. (2018), the various signaling pathways including COX-2, TNF-α, NF-κB, PI3K/Akt/mTOR, MAPK and JAK/STAT mediated by spices bioactives to exert their anti-tumorigenic effect was reported therefore it can be inferred that these spice's bioactives can be effectively used to prevent various types of cancer. In this regard, the different anticancer mechanisms of actions mediated by spice's bioactives are described as follows:
7.1. Wnt/β-catenin signaling pathway
Upon activation of Wnt/β-catenin signalings, Wnt binds to their receptor (composed of frizzled proteins and LPR5/6) which in turn activates the cytoplasmic protein disheveled (DVL). The activated DVL protein will lead to the suppression of degradation complex as composed of adenomatous polyposis coli gene (APC), anti-neurexin (AXIN), glycogen synthase kinase 3β (GSK3β) and casein kinase 1α (CK1α). Subsequently, the phosphorylation and inhibition of GSK3β will increase the concentration of cytosolic β-catenin. The non-phosphorylated cytosolic β-catenin will move to the nucleus. In nucleus, it communicates with T cell-specific factor (TCF)/lymphoid enhancer-binding factor (LEF) and co-activators like CREB binding protein (CBP), in order to induce gene transcription. In case of Wnt off state or in the absence of Wnt, the destruction complex induces the phosphorylation of cytosolic β-catenin and subsequently its ubiquitin-mediated proteosomal degradation with no gene transcription (Zhang and Wang, 2020). The overall Wnt/β-catenin signaling pathway is depicted in Fig. 2.
Fig. 2.
Overview of Wnt/β-catenin signaling pathway.
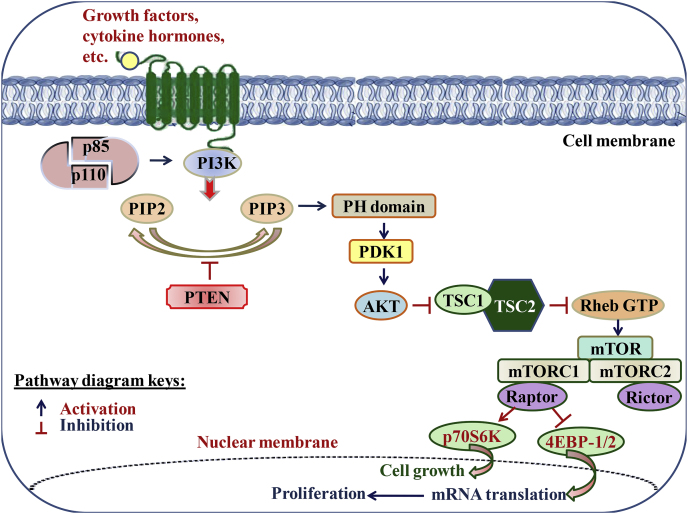
7.2. PI3K/Akt/mTOR signaling pathway
The extracellular stimulus, such as growth factors, cytokines and hormones stimulate the dimerization of the p110 catalytic subunit with regulatory p85 subunit, to activate PI3K. Upon activation, PI3K stimulates the phosphorylation of PIP2 into PIP3 that recruits a subset of pleckstrin-homology (PH) or other lipid-binding domains of downstream targets which in response stimulates other signaling proteins, such as PDK1 and kinases AKT. The activated Akt impacts various downstream effectors, such as mTOR and finally activates the cell growth and cell survival pathways. PTEN deleted on chromosome 10 negatively regulates the PI3K signaling by dephosphorylating PIP3 to PIP2 and thus prevents the activation of downstream kinases (Kawade et al., 2018). The PI3K/Akt/mTOR signaling pathway is well presented in Fig. 3.
Fig. 3.
Overview of PI3K/Akt/mTOR signaling pathway.
7.3. JAK/STAT signaling pathway
In noncanonical mode of signaling, JAK2 phosphorylates the histone 3 which ultimately transfers CBX5 from chromatin and associate with STAT5A to inhibit gene transcription. In canonical context, cytokines bind to receptor that induces the phosphorylation of JAK kinases. The activated JAK led to STAT phosphorylation and subsequently dimerises and translocates it to the nucleus. In nucleus, dimeric STAT associates with the sequence in the target gene promoters and resultantly activates gene transcription (Qureshy et al., 2020). The JAK/STAT signaling pathway is elucidated in Fig. 4.
Fig. 4.
Overview of JAK/STAT signaling pathway.
7.4. MAPK signaling pathway
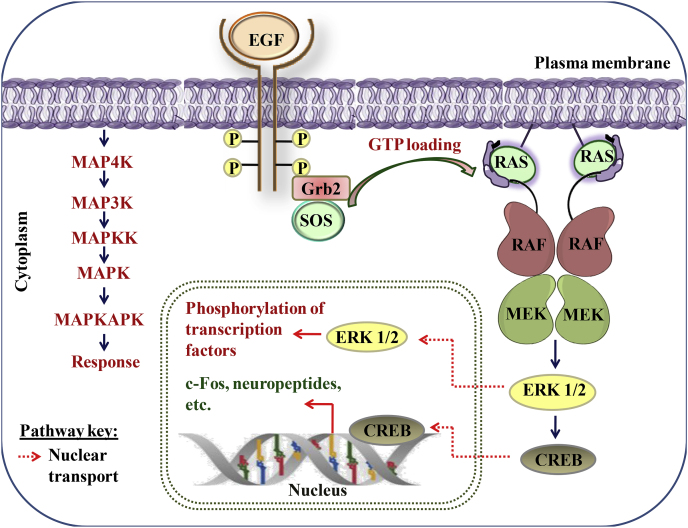
MAPKs activation plays a critical role in inflammation-associated cancer proliferation. Neoplastic transformation is facilitated by the active mutations of Ras-Raf-MEK-ERK. ERK regulates the inflammatory cytokines that promotes the inflammation-associated cancer. The phosphorylated ERK was also detected predominantly in pancreatic cancer. The Ras activation induces the local inflammation by enhancing the level of CXCL-8 and IL-8 that finally promotes neo-vascularization and cancer progression, therefore, MAPKs pathway can be a suitable target for treating cancer. The signaling cascade of MAPK kinase is described as follows:
Upon ligand binding, receptor tyrosine kinases (RTKs) stimulate guanine exchange factors, Sos proteins, which transfers GTP to Ras GTPases. Downstream activation of RAS/RAF and MEK subsequently activates the ERK1/2 transcription factor activator which further phosphorylates a number of downstream effectors. The activation of ERK signaling play significant role in cell death, proliferation and cytoskeletal remodeling (Yuan et al., 2020). The MAPK signaling pathway is shown in Fig. 5.
Fig. 5.
Overview of MAPK signaling pathway.
7.5. Notch signaling pathway
Upon ligand interaction with notch receptor, a two step proteolytic cleavage is stimulated by ADAM family proteases and γ-secretase, to release notch intracellular domain (NICD), which subsequently moves to nucleus and bind with C-protein binding factor 1/Suppressor of Hairless/Lag-1 (CSL) and finally lead to the conversion of repressor complex of notch target genes to activator complex (Venkatesh et al., 2018). The Notch signaling pathway is represented in Fig. 6.
Fig. 6.
Overview of Notch signaling pathway.
8. Supremacy of natural products over synthetic anticancer medications
Despite the availability of enormous conventional anticancer medications, the researchers have always remained engaged in searching new therapeutic agents from natural resources. The main reason behind this is the efficacy and safety of natural products over synthetic drugs. The synthetic drugs work in action orientation manner for a particular disease. Moreover, the efficacy of synthetic medicines is time-bounded and non-curative. On contrary, natural entities play their action synergistically and offer a holistic treatment approach. Hence natural compounds can be used safely to cure more than one ailment at a single time at optimum concentration (Singh et al., 2021a). Some of the natural products derived from spices like capsaicin (red pepper); curcumin (turmeric); cyanidin glycosides (red onion); gingerol (ginger); crocin, crocetin and safranal (saffron) are most renowned anticancer agents that also possess other therapeutic effects too. Therefore, these phytocompounds can be used to treat other body ailments along with cancer at minimum risk. These bioactives can target heterogenous populations of cancer cells at a single time. They also have the ability to regulate several key pathways involved in carcinogenesis whereas the synthetic medicines lack this efficacy (Turrini et al., 2020). Along with, the synthetic medicines are associated with several adverse effects. For example; irinotecan (adverse effects: diarrhea, sensory neuropathy, neutropenia), melphalan, cisplatin, cyclophosphamide, oxaliplatin, carboplatin (adverse effects: toxicity of pulmonary, cardiovascular, hematological, gastrointestinal and renal system), doxorubicin (adverse effects: cardiotoxicity) (Iqbal et al., 2017). Hence, it is always preferable to cure several health issues with natural products instead of synthetic medicines.
9. Synergistic anticancer effect of spices
As we discussed in section 8 that spices and their bioactives follow a synergistic approach for their therapeutic action. These bioactives can stimulate more than one signaling pathway involved in different types of cancer, so a single spice's bioactive can be used to treat more than one cancer type. They induce their anticancer effect by the activation of tumor suppressing genes, apoptosis, deacceleration of tumor angiogenesis, cell cycle arrest and so on. From Table 3, it can be inferred that curcumin can be used to treat multiple cancer types such as pancreatic, breast, bladder, gastric and colon cancer. Likewise, piperine was also reported to be used for curing breast, colon, and prostate cancer (Turrini et al., 2020). The multiple cancer treating effect of a single bioactive can also be supported by Table 2. However, the clinical evidences on their multiple cancer treating effect are lacking. Therefore, future clinical studies can be planned to explore the bioavailability, bioefficacy, biosafety, mechanism of action and optimum dose of spice’ bioactives needed for combating various cancer types (Almatroodi et al., 2021).
10. Bioavailability and bioefficacy of phytocompounds
Bioavailability is defined as the quantity of micronutrients and phytocompounds i.e., absorbed, distributed to its target organs/tissues where it metabolized and finally excreted. Though, it is not feasible to compute the précised amount of biologically available phytochemicals in humans because it needed in-vivo experimentations. This is the reason why several phytocompounds which show excellent potential in in-vitro experimentation fail to display the same potential during in-vivo evaluation. There are some factors that influence the bioavailability of a compound. These factors are categorized under two categories i.e., exogenous and endogenous. The exogenous factors include the complex nature of food matrix, chemical nature, amount and structure of the compounds co-ingested whereas the endogenous factors include the mucosal mass, duration to transit in intestine, metabolism, binding of proteins in blood/tissues, etc. However, some of the phytocompounds can be metabolized to make them biologically active whereas other phenolic extracts show a low bioavailability after ingestion. The polyphenolic compounds also interfere with the bioavailability and potential of the food that we eat. For instance; the ingestion of blueberries along with milk led to a deficit in-vivo antioxidant activity of blueberries. The association also decreased the absorption of caffeic acid. Moreover, bioaccessibility is essential for the bioavailability of a compound. It can be described as the amount released from the food matrix into the lumen and absorbed in the intestine. Hence, bioavailability is directly proportional to the bioaccessibility of a compound. Bioaccessibility is affected by several factors including pH, temperature, texture, gut microbiota, plant cell wall composition, and so on. However, data were lacking on these two terms but a key was provided to understand the bioefficacy of phytocompounds being bioactive. Hence, phytocompounds are required to be biologically available in order to achieve their full potential (Anwar et al., 2021).
11. Bio-toxicity and bio-safety aspects of spices
Bio-toxicity of natural products can be defined as the toxic effects exerted by natural product on living organism. Spices are also considered as to exert toxic effects on living beings due to the presence of heavy metals (Pb, As, Cd), mycotoxins and especially aflatoxins. Some of the spices like red chillies, black pepper, nutmeg exert high aflatoxin risk whereas turmeric and ginger possess medium aflatoxin risk. Other spices including mace, cloves, cardamom, cinnamon, cumin, coriander and fennel are reported to possess low aflatoxin risk. The aflatoxin is carcinogenic (categorized as group 1 carcinogen by the International Agency for Research on Cancer), teratogenic, mutagenic, hepatotoxic, growth retardant and immunosuppressant in nature (Akhtar et al., 2020). Keeping in mind, the above-mentioned bio-toxicity of these spices, the bio-safety of these spices should be evaluated. Bio-safety of spices refers to the protection of living entities from the toxins released from the spices. As spices and their bioactives are basically considered safe, therefore, they are widely utilized in preparation of various cuisines at appropriate doses. They work in dose-dependent manner. Dose dependency refers to the change in effect on changing the dose of the drug. It helps in quantifying the drug effect at the level of molecule, cell, tissue, organ, organ system, and organism. It also helps in calculating the optimum dose at which the drug shows its maximal efficacy. Their isolated compounds also work in dose-dependent manner and are well documented for their therapeutic potentials especially for cancer preventing action without significant toxicity. For instance, Curcuminoids is categorized as “generally recognized as safe” (GRAS) by national cancer institute. Besides their anticancer approach, they are also noticed with some occasional toxic issues under some specific conditions. Curcumin is generally safe up to 12 gm daily doses, with minor adverse effects (Gupta et al., 2017). Curcumin was reported to possess DNA damaging effect in the presence of Cu2+ (both in-vitro and in-vivo). Safrole, a natural constituent of spices extracted essential oil was also noticed with some oncogenic effects but the content of safrole is reduced up to its safe level by high temperature during cooking. Apart from these toxicities, some anticancer compounds which show anti-platelet activities might induce over bleeding in patients suffering from bone marrow suppression (Zheng et al., 2016). In another study by Guldiken et al. (2018), it was opined that spices and herbs’ bioactives safety is dose dependent. They are generally safe and health promoting at lower concentration whereas at high doses they cause health deteriorating symptoms. Some of the spices mediated toxicity is summarized in Table 5. Therefore, further studies are needed for the in-depth exploration of the bio-toxicity and bio-safety of spices and the derived anticancer compounds, so that their anticancer safe dose can be recommended.
Table 5.
Toxicological effects of some spices.
| Name of spice/bioactives | Study type | Body part affected | Resulted adverse effects |
|---|---|---|---|
| Curcumin | In-vitro & in-vivo | Any | DNA damage |
| Chilli | Clinical study | Human stomach | Gastric cancer |
| Gall bladder | Carcinogenic | ||
| Safrole from Areca nut | Human esophagus | ||
| Star anise | CNS | Neutotoxic | |
| Thyme oil | In-vitro | Human lymphocytes | Genotoxic |
| Carvacrol | Lung fibroblast cells | DNA damage, clastogenic | |
| Allium sativum | Adipocytes cells | Cytotoxic | |
| Saffron | In-vivo | Mice liver | Hepatotoxic |
| Chilli, coriander, fennel, ajwain, cardamom, cumin and dark pepper | Gastrointestinal part of albino rats | Gastrointestinal toxicity | |
| Ginger | Mice | ||
| Clinical study | Human stomach | Gastric problems |
Source: Guldiken et al. (2018).
12. In-vivo anticancer activity of Indian spices
The in-vivo anticancer efficacy of Indian spices can be evaluated by different studies like pre-clinical studies and clinical studies. The pre-clinical studies are those which are performed to evaluate the efficacy of a drug/medical treatment in animals. These studies provide preliminary data regarding the safety of new drug. On the basis of pre-clinical data, clinical studies can be performed. Clinical studies are those which are performed in people. The various in-vivo anticancer studies of Indian spices are discussed below:
12.1. Pre-clinical studies
A pre-clinical trial on colon cancer bearing male Balb/c nude mice was performed to evaluate the antitumor efficacy of turmeric ethanolic extract and curcumin. After 30 days of dose (75 mg/kg) administration, the mice were found with increased level of WBC, neutrophils and lymphocytes counts. The extract has also inhibited the tumor growth significantly (Yue et al., 2016). The potency of curcumin was tested for treating the colon carcinoma. The SW480 cells of colon cancer were introduced in to the female nude mice to generate the xenograft tumor model to study the curcumin effect. The mice were intraperitoneal injected with a dose of 200 mg/kg for 5 days and were observed daily. Curcumin treated mice were noticed with prolonged life span with suppressed proliferation of tumor cells (Dou et al., 2017). In another study by Lee et al. (2018), the colon cancer xenograft tumors were established by injecting HCT-116 colon cancer cells into the male Balb/c nude mice and were treated with curcumin dissolved in corn oil at a dose of 50 mg/kg and 100 mg/kg for 8 times/three weeks. The treatment has showed the significant silencing of SIRT1 protein and resultantly reduced the tumor volume.
The aqueous extract of ginger was examined on N-nitroso N-methyl Urea (MNU) caused gastric carcinoma in albino Wistar rats. The extract supplementation has significantly reduced the pathological changes associated with inflammation and oxidative stress and finally suppressed the tumor progression (Mansingh et al., 2020). The compound 6-gingerol and capsaicin, the main constituent of ginger and red chili, respectively were screened for their anticancer efficacy. The study was performed on urethane-induced lung adenocarcinoma in female ICR mice-model. The animals were supplemented with both ginger and red chili at a dose of 50 mg/kg, once a day for 20 weeks. Results revealed that both the constituents were antagonistic in nature and 6-gingerol significantly suppressed the lung carcinoma and also reversed the cancer-promoting effect of capsaicin (Geng et al., 2016).
The different extracts of Cinnamomum tamala was tested for their anticancer efficacy in fibrosarcoma-induced mouse tumor model. Among different extracts the petroleum ether fraction of methanol extract was the most potent anticancer agent. The fraction has significantly arrested the tumor growth and proliferation through the inhibition of topoisomerase-I enzyme activity (Thanekar et al., 2016). Eugenol, as one of the most common bioactive of many spices was examined in the breast tumor bearing Female Swiss Albino mice and it was demonstrated that eugenol treatment has noticeably delayed the translocation of β-catenin to nucleus and promoted its cytoplasmic degradation which has ultimately reduced the mRNA expression and formation of enriched stemness of secondary mammosphere facilitated via the low level of CD44+/CD24-/low population. All these alterations significantly reduced the volume of solid tumors and induced apoptosis of malignant cells (Choudhury et al., 2020).
A study was carried out on 5–6 weeks old male Balb/c mice to examine the piperlongumine (an alkaloid found in Piper longum) effect on 1,2-dimethylhydrazine (DMH)/dextran sulphate sodium (DSS) induced colon cancer. The compound treatment has noticeably suppressed the expression of Ras/PI3K/Akt/mTOR signaling that perform critical function during colon carcinogenesis and proliferation. The compound has also arrested the cell cycle growth at G2/M phase and restored the cell death (inhibited via the Ras proteins and PI3K/Akt signaling cascade) by down-regulating the expression of Bcl-2. The report of hepatic and renal toxicity declared that piperlongumine was safe, without any significant negative symptoms (Kumar and Agnihotri, 2019).
In a study by Jwaid et al. (2021), the different doses (500 mg/kg of body weight and 1000 mg/kg of body weight) of A. dracunculus L. extraction (extracted with 80% of ethanol) was tested against the proliferation of bone marrow and spleen cells of mice. Results revealed that the dose 500 mg/kg of body weight has significantly increased the mitotic index (i.e., 12.02 ± 1.16 and 10.48 ± 1.28 for bone marrow and spleen cells, respectively). On the contrary, the dose 1000 mg/kg of body weight has significantly decreased the mitotic index (i.e., 4.18 ± 0.73 and 3.76 ± 0.45 for bone marrow and spleen cells, respectively).
12.2. Clinical studies
A study by Farid et al. (2020), was carried out to evaluate the anti-mutagenic effect of Foeniculum vulgare seeds extract on human blood sample collected from 20 healthy persons (10 males and 10 females), who are not used to smoke. The samples were irradiated to induce elevated levels of cytokinines such as IL-1β, IL-6, IL-8, and Tumor necrosis factor-α (TNF-α). The fennel decoction has noticeably led to the reduction of cytokines levels and ameliorated negative effect caused by elevated cytokines.
The effect of ginger was tested on the complications associated with platinum-based adjuvant chemotherapy for ovarian cancer. The study was conducted on 49 patients, administrated with 2 gm of ginger capsules. Results revealed that ginger treatment has significantly prevented the nausea, vomiting, metabolic disorders, digestive problems, reproductive system problems, reduced the level of prostaglandins, stimulated appetite and alleviated the toxic effects of chemotherapy. Gingerol and shogaol from ginger was found to be responsible for such activities, so these compounds can be investigated further for their cancer preventive efficacy (Shokri et al., 2017). The above clinical data however, supports the clinical anticancer role of spice’ bioactives but the data is not enough to substantiate their efficacy with commercially available anticancer drugs, therefore more clinically studies can be planned on anticancer efficacy of spice's bioactives.
13. Conclusion and future recommendations
Through this treatise, it is evident that the temptation towards spices is not only because of their aromatic and seasonings power but more importantly credited to their diverse medicinal attributes too. Within this context, the present study provides a clear understanding regarding the role of spices in cancer prevention, which has become the emperor of all maladies. More importantly, the review provides the ethnomedicinal basis for the reliable anticancer effectiveness of spices and their active constituents which otherwise always questioned due to the lack of enough scientific evidences. Overall, spices’ bioactives have the ability to interact with multiple check points and also target various mediators to dysregulate or halt the various signaling pathways involved in cancer proliferation. Henceforth, in order to avoid inflating cost of advanced medication alongwith their associated fatal side issues, spices and their bioactives can be introduced as fresh lead for the development of cost-effective novel drugs. However, from the current study the anticancer role of spices is clear on traditional, pre-clinical and clinical scale to SAR studies to some extent but in-spite of these studies, sufficient placebo studies are also required to establish the anticancer role of spices on cancer patients. Future investigations are also needed on in-depth molecular mechanism of anticancer spices which can open new avenues for the better management of cancer patients.
Ethical approval
Not required.
CRediT authorship contribution statement
Neetu Singh: Writing – original draft, Conceptualization, Investigation, Data curation. Surender Singh Yadav: Conceptualization, Supervision, Validation, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Financial assistance from the Council for Scientific and Industrial Research (CSIR), New Delhi, Science and Engineering Research Board, Department of Science and Technology, Government of India, New Delhi and Haryana State Council for Science and Technology (HSCST), Panchkula and DST-FIST, New Delhi is thankfully acknowledged.
References
- Absalan A., Mesbah-Namin S.A., Tiraihi T., Taheri T. Cinnamaldehyde and eugenol change the expression folds of AKT1 and DKC1 genes and decrease the telomere length of human adipose-derived stem cells (hASCs): an experimental and in silico study. Iran. J. Basic Med. Sci. 2017;20:316. doi: 10.22038/ijbms.2017.8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agyare C., Appiah T., Boakye Y.D., Apenteng J.A. 2017. Petroselinum crispum: a Review. Medicinal Spices and Vegetables from Africa; pp. 527–547. [DOI] [Google Scholar]
- Ahmad H., Khera R.A., Hanif M.A., Ayub M.A., Jilani M.I. Medicinal Plants of South Asia. Elsevier; 2020. Vanilla; pp. 657–669. [DOI] [Google Scholar]
- Ahmad R., Srivastava A.N., Khan M.A. Evaluation of in vitro anticancer activity of rhizome of Curcuma longa against human breast cancer and Vero cell lines. Evaluation. 2016;1:1–6. [Google Scholar]
- Akhtar S., Riaz M., Naeem I., Gong Y.Y., Ismail A., Hussain M., Akram K. Risk assessment of aflatoxins and selected heavy metals through intake of branded and non-branded spices collected from the markets of Multan city of Pakistan. Food Control. 2020;112 doi: 10.1016/j.foodcont.2020.107132. [DOI] [Google Scholar]
- Almatroodi S.A., Syed M.A., Rahmani A.H. Potential therapeutic targets of curcumin, most abundant active compound of turmeric spice: role in the management of various types of cancer. Recent Pat. Anti-Cancer Drug Discov. 2021;16:3–29. doi: 10.2174/1574892815999201102214602. [DOI] [PubMed] [Google Scholar]
- Anwar S., Almatroudi A., Alsahli M.A., Khan M.A., Khan A.A., Rahmani A.H. Natural products: implication in cancer prevention and treatment through modulating various biological activities. Anti Cancer Agents Med. Chem. 2020;20:2025–2040. doi: 10.2174/1871520620666200705220307. [DOI] [PubMed] [Google Scholar]
- Anwar S., Khan S., Almatroudi A., Khan A.A., Alsahli M.A., Almatroodi S.A., Rahmani A.H. A review on mechanism of inhibition of advanced glycation end products formation by plant derived polyphenolic compounds. Mol. Biol. Rep. 2021;48:787–805. doi: 10.1007/s11033-020-06084-0. [DOI] [PubMed] [Google Scholar]
- Ashokkumar K., Murugan M., Dhanya M.K., Warkentin T.D. Botany, traditional uses, phytochemistry and biological activities of cardamom [Elettaria cardamomum (L.) Maton]–A critical review. J. Ethnopharmacol. 2020;246 doi: 10.1016/j.jep.2019.112244. [DOI] [PubMed] [Google Scholar]
- Atanasov A.G., Zotchev S.B., Dirsch V.M., Supuran C.T. Natural products in drug discovery: advances and opportunities. Nat. Rev. Drug Discov. 2021;20:200–216. doi: 10.1038/s41573-020-00114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian S., Roselin P., Singh K.K., Zachariah J., Saxena S.N. Postharvest processing and benefits of black pepper, coriander, cinnamon, fenugreek, and turmeric spices. Crit. Rev. Food Sci. Nutr. 2016;56:1585–1607. doi: 10.1080/10408398.2012.759901. [DOI] [PubMed] [Google Scholar]
- Banik U., Parasuraman S., Adhikary A.K., Othman N.H. Curcumin: the spicy modulator of breast carcinogenesis. J. Exp. Clin. Cancer Res. 2017;36:1–16. doi: 10.1186/s13046-017-0566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranauskaite J., Kubiliene A., Marksa M., Petrikaite V., Vitkevičius K., Baranauskas A., Bernatoniene J. The influence of different oregano species on the antioxidant activity determined using HPLC postcolumn DPPH method and anticancer activity of carvacrol and rosmarinic acid. BioMed Res. Int. 2017;2017:1–7. doi: 10.1155/2017/1681392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basha R., Connelly S.F., Sankpal U.T., Nagaraju G.P., Patel H., Vishwanatha J.K., El-Rayes B. Small molecule tolfenamic acid and dietary spice curcumin treatment enhances antiproliferative effect in pancreatic cancer cells via suppressing Sp1, disrupting NF-kB translocation to nucleus and cell cycle phase distribution. J. Nutr. Biochem. 2016;31:77–87. doi: 10.1016/j.jnutbio.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagat N., Chaturvedi A. Spices as an alternative therapy for cancer treatment. SRP. 2016;7:46–56. doi: 10.5530/srp.2016.7.7. [DOI] [Google Scholar]
- Bhandari J., Muhammad B., Thapa P., Shrestha B.G. Study of phytochemical, anti-microbial, anti-oxidant, and anti-cancer properties of Allium wallichii. BMC Compl. Alternative Med. 2017;17:1–9. doi: 10.1186/s12906-017-1622-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuiyan S. Curcumin downregulates the expression of p44/42 MAPK and causes caspase-mediated cell inhibition in MCF-7 breast cancer cells. Bioresearch Communications. 2020;6:801–805. [Google Scholar]
- Break M.K.B., Hossan M.S., Khoo Y., Qazzaz M.E., Al-Hayali M.Z., Chow S.C., Khoo T.J. Discovery of a highly active anticancer analogue of cardamonin that acts as an inducer of caspase-dependent apoptosis and modulator of the mTOR pathway. Fitoterapia. 2018;125:161–173. doi: 10.1016/j.fitote.2018.01.006. [DOI] [PubMed] [Google Scholar]
- Cardone L., Castronuovo D., Perniola M., Cicco N., Candido V. Saffron (Crocus sativus L.), the king of spices: an overview. Sci. Hortic. 2020;272 doi: 10.1016/j.scienta.2020.109560. [DOI] [Google Scholar]
- Chahal K.K., Dhaiwal K., Kumar A., Kataria D., Singla N. Chemical composition of Trachyspermum ammi L. and its biological properties: a review. J. Pharmacogn. Phytochem. 2017;6:131–140. [Google Scholar]
- Chahal K.K., Monika A.K., Bhardwaj U., Kaur R. Chemistry and biological activities of Anethum graveolens L.(dill) essential oil: a review. J. Pharmacogn. Phytochem. 2017;6:295–306. [Google Scholar]
- Chamikara M.D.M., Dissanayake D.R.R.P., Ishan M., Sooriyapathirana S.D.S.S. Dietary, anticancer and medicinal properties of the phytochemicals in chili pepper (Capsicum spp.) Ceylon J. Sci. 2016;45:5–20. doi: 10.4038/cjs.v45i3.7396. [DOI] [Google Scholar]
- Chate M.R., Kakade S.B., Neeha V.S. Kokum (Garcinia indica) fruit: a review. Asian J. Dairy Food Res. 2019;38:329–332. doi: 10.18805/ajdfr.DR-1493. [DOI] [Google Scholar]
- Chen T., Zhao L., Chen S., Zheng B., Chen H., Zeng T., Wang L. The curcumin analogue WZ35 affects glycolysis inhibition of gastric cancer cells through ROS-YAP-JNK pathway. Food Chem. Toxicol. 2020;137 doi: 10.1016/j.fct.2020.111131. [DOI] [PubMed] [Google Scholar]
- Choudhury P., Barua A., Roy A., Pattanayak R., Bhattacharyya M., Saha P. Eugenol restricts Cancer Stem Cell population by degradation of β-catenin via N-terminal Ser37 phosphorylation-an in vivo and in vitro experimental evaluation. Chem. Biol. Interact. 2020;316 doi: 10.1016/j.cbi.2020.108938. [DOI] [PubMed] [Google Scholar]
- Cinthura C., Gayathri R., Priya V. Genotoxicity analysis of mace (Myristica Fragrans) on oral cancer cell line by DNA fragmentation. J. Pharmaceut. Sci. Res. 2017;9:205. [Google Scholar]
- Corveloni A.C., Semprebon S.C., Baranoski A., Biazi B.I., Zanetti T.A., Mantovani M.S. Carnosic acid exhibits antiproliferative and proapoptotic effects in tumoral NCI-H460 and nontumoral IMR-90 lung cells. J. Toxicol. Environ. Health, Part A. 2020;83:412–421. doi: 10.1080/15287394.2020.1767741. [DOI] [PubMed] [Google Scholar]
- Dallavalle S., Dobričić V., Lazzarato L., Gazzano E., Machuqueiro M., Pajeva I., Fruttero R. Improvement of conventional anti-cancer drugs as new tools against multidrug resistant tumors. Drug Resist. Updates. 2020;50 doi: 10.1016/j.drup.2020.100682. [DOI] [PubMed] [Google Scholar]
- Das A., Harshadha K., Sk D.K., Jayaprakash B. Evaluation of therapeutic potential of eugenol-a natural derivative of Syzygium aromaticum on cervical cancer. Asian Pac. J. Cancer Prev. APJCP. 2018;19:1977. doi: 10.22034/APJCP.2018.19.7.1977. /10.22034%2FAPJCP.2018.19.7.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das K., Gezici S. Secondary plant metabolites, their separation and identification, and role in human disease prevention. Ann. Phytomed. 2018;7:13–24. doi: 10.21276/ap.2018.7.2.3. [DOI] [Google Scholar]
- Dauqan E.M., Abdullah A. Medicinal and functional values of thyme (Thymus vulgaris L.) herb. J. appl. biol. 2017;5(2) doi: 10.7324/JABB.2017.50203. 0-2. [DOI] [Google Scholar]
- De L.C.J., Valdez M., Ruiz F., III, Gonzales K., Mitchell W., McHardy S.F., Gonzales C.B. Synthesis and SAR of novel capsazepine analogs with significant anti-cancer effects in multiple cancer types. Bioorg. Med. Chem. 2019;27:208–215. doi: 10.1016/j.bmc.2018.11.040. [DOI] [PubMed] [Google Scholar]
- de Souza Grinevicius V.M.A., Kviecinski M.R., Mota N.S.R.S., Ourique F., Castro L.S.E.P.W., Andreguetti R.R., Pedrosa R.C. Piper nigrum ethanolic extract rich in piperamides causes ROS overproduction, oxidative damage in DNA leading to cell cycle arrest and apoptosis in cancer cells. J. Ethnopharmacol. 2016;189:139–147. doi: 10.1016/j.jep.2016.05.020. [DOI] [PubMed] [Google Scholar]
- Dekić M.S., Radulović N.S., Stojanović N.M., Randjelović P.J., Stojanović-Radić Z.Z., Najman S., Stojanović S. Spasmolytic, antimicrobial and cytotoxic activities of 5-phenylpentyl isothiocyanate, a new glucosinolate autolysis product from horseradish (Armoracia rusticana P. Gaertn., B. Mey. & Scherb., Brassicaceae) Food Chem. 2017;232:329–339. doi: 10.1016/j.foodchem.2017.03.150. [DOI] [PubMed] [Google Scholar]
- Devi R.J., Devi N.G., Bavanilatha M., Kavitha B.T., Jameela B.M., Kanimozhi S. Three-dimensional quantitative structural activity relationships modeling studies of phytochemicals from brassicaceae as potent inhibitors against tumor inflammation. Asian J. Pharmaceut. Clin. Res. 2017;10:321–323. doi: 10.22159/ajpcr.2017.v10i1.15361. [DOI] [Google Scholar]
- Dou H., Shen R., Tao J., Huang L., Shi H., Chen H., Wang T. Curcumin suppresses the colon cancer proliferation by inhibiting Wnt/β-catenin pathways via miR-130a. Front. Pharmacol. 2017;8:877. doi: 10.3389/fphar.2017.00877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y.T., Yang X.A., Fang L.Y., Wang J.H., Liu Q. Anti-proliferative and anti-invasive effects of garcinol from Garcinia indica on gallbladder carcinoma cells. Die Pharmazie-An Int. J. Pharm. Sci. Res. 2018;73:413–417. doi: 10.1691/ph.2018.8366. [DOI] [PubMed] [Google Scholar]
- Dupré A., Malik H.Z. Inflammation and cancer: what a surgical oncologist should know. Eur. J. Surg. Oncol. 2018;44:566–570. doi: 10.1016/j.ejso.2018.02.209. [DOI] [PubMed] [Google Scholar]
- Ekiert H., Świątkowska J., Knut E., Klin P., Rzepiela A., Tomczyk M., Szopa A. Artemisia dracunculus (Tarragon): a review of its traditional uses, phytochemistry and pharmacology. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.653993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkiran O., Akbaba E., Bagci E. Constituents of essential oils from leaves and seeds of Laurus nobilis L.: a chemotaxonomic approach. Bangladesh J. Bot. 2018;47:893–901. doi: 10.3329/bjb.v47i4.47379. [DOI] [Google Scholar]
- Erenler R., Sen O., Aksit H., Demirtas I., Yaglioglu A.S., Elmastas M., Telci I. Isolation and identification of chemical constituents from Origanum majorana and investigation of antiproliferative and antioxidant activities. J. Sci. Food Agric. 2016;96:822–836. doi: 10.1002/jsfa.7155. [DOI] [PubMed] [Google Scholar]
- Fadilah F., Andrajati R., Arsianti A., Paramita R.I., Erlina L., Yanuar A. Synthesis and in vitro activity of eugenyl benzoate derivatives as BCL-2 inhibitor in colorectal cancer with QSAR and molecular docking approach. bioRxiv. 2020;2020:1–20. doi: 10.1101/2020.08.17.253591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhood B., Mortezaee K., Goradel N.H., Khanlarkhani N., Salehi E., Nashtaei M.S., Sahebkar A. Curcumin as an anti‐inflammatory agent: implications to radiotherapy and chemotherapy. J. Cell. Physiol. 2019;234:5728–5740. doi: 10.1002/jcp.27442. [DOI] [PubMed] [Google Scholar]
- Farid A., Kamel D., Montaser S.A., Ahmed M.M., El Amir M., El Amir A. Assessment of antioxidant, immune enhancement, and antimutagenic efficacy of fennel seed extracts in irradiated human blood cultures. J. Radiat. Res. Appl. Sci. 2020;13:260–266. doi: 10.1080/16878507.2020.1728963. [DOI] [Google Scholar]
- Fridlender M., Kapulnik Y., Koltai H. Plant derived substances with anti-cancer activity: from folklore to practice. Front. Plant Sci. 2015;6:799. doi: 10.3389/fpls.2015.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo M., Calaf G.M. Curcumin inhibits invasive capabilities through epithelial mesenchymal transition in breast cancer cell lines. Int. J. Oncol. 2016;49:1019–1027. doi: 10.3892/ijo.2016.3598. [DOI] [PubMed] [Google Scholar]
- Ge S., Duo L., Wang J., Yang J., Li Z., Tu Y. A unique understanding of traditional medicine of pomegranate, Punica granatum L. and its current research status. J. Ethnopharmacol. 2021;271 doi: 10.1016/j.jep.2021.113877. [DOI] [PubMed] [Google Scholar]
- Geng S., Zheng Y., Meng M., Guo Z., Cao N., Ma X., Du G. Gingerol reverses the cancer-promoting effect of capsaicin by increased TRPV1 level in a urethane-induced lung carcinogenic model. J. Agric. Food Chem. 2016;64:6203–6211. doi: 10.1021/acs.jafc.6b02480. [DOI] [PubMed] [Google Scholar]
- Goodarzi S., Tabatabaei M.J., Mohammad Jafari R., Shemirani F., Tavakoli S., Mofasseri M., Tofighi Z. Cuminum cyminum fruits as source of luteolin-7-O-glucoside, potent cytotoxic flavonoid against breast cancer cell lines. Nat. Prod. Res. 2020;34:1602–1606. doi: 10.1080/14786419.2018.1519824. [DOI] [PubMed] [Google Scholar]
- Gottardi D., Bukvicki D., Prasad S., Tyagi A.K. Beneficial effects of spices in food preservation and safety. Front. Microbiol. 2016;7:1394. doi: 10.3389/fmicb.2016.01394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudalwar B.R., Nimbalwar M.G., Panchale W.A., Wadekar A.B., Manwar J.V., Bakal R.L. Allium sativum, a potential phytopharmacological source of natural medicine for better health. GSC Adv. Res. Rev. 2021;6:220–232. doi: 10.30574/gscarr.2021.6.3.0049. [DOI] [Google Scholar]
- Guldiken B., Ozkan G., Catalkaya G., Ceylan F.D., Yalcinkaya I.E., Capanoglu E. Phytochemicals of herbs and spices: health versus toxicological effects. Food Chem. Toxicol. 2018;119:37–49. doi: 10.1016/j.fct.2018.05.050. [DOI] [PubMed] [Google Scholar]
- Gupta A.P., Khan S., Manzoor M.M., Yadav A.K., Sharma G., Anand R., Gupta S. Anticancer curcumin: natural analogues and structure-activity relationship. Stud. Nat. Prod. Chem. 2017;54:355–401. doi: 10.1016/B978-0-444-63929-5.00010-3. [DOI] [Google Scholar]
- He B., Wei W., Liu J., Xu Y., Zhao G. Synergistic anticancer effect of curcumin and chemotherapy regimen FP in human gastric cancer MGC-803 cells. Oncol. Lett. 2017;14:3387–3394. doi: 10.3892/ol.2017.6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hire R.R., Srivastava S., Davis M.B., Kumar Konreddy A., Panda D. Antiproliferative activity of crocin involves targeting of microtubules in breast cancer cells. Sci. Rep. 2017;7:1–11. doi: 10.1038/srep44984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horita M.A., Reed M., Ganapathy A., Ezekiel U. Anticancer effect of curcumin‐silibinin B combination in colorectal cancer cells is mediated by apoptosis and autophagy. Faseb. J. 2019;33:471–477. doi: 10.1096/fasebj.2019.33.1_supplement.471.7. [DOI] [Google Scholar]
- Husain I., Ahmad R., Chandra A., Raza S.T., Shukla Y., Mahdi F. Phytochemical characterization and biological activity evaluation of ethanolic extract of Cinnamomum zeylanicum. J. Ethnopharmacol. 2018;219:110–116. doi: 10.1016/j.jep.2018.02.001. [DOI] [PubMed] [Google Scholar]
- Iman V., Mohan S., Abdelwahab S.I., Karimian H., Nordin N., Fadaeinasab M., Noor S.M. Anticancer and anti-inflammatory activities of girinimbine isolated from Murraya koenigii. Drug Des. Dev. Ther. 2017;11:103. doi: 10.2147/DDDT.S115135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal J., Abbasi B.A., Mahmood T., Kanwal S., Ali B., Shah S.A., Khalil A.T. Plant-derived anticancer agents: a green anticancer approach. Asian Pac. J. Trop. Biomed. 2017;7:1129–1150. doi: 10.1016/j.apjtb.2017.10.016. [DOI] [Google Scholar]
- Iranshahy M., Farhadi F., Paknejad B., Zareian P., Iranshahi M., Karami M., Abtahi S.R. Gummosin, a sesquiterpene coumarin from Ferula assafoetida is preferentially cytotoxic to human breast and prostate cancer cell lines. Avicenna J. Phytomedicine. 2019;9:446. [PMC free article] [PubMed] [Google Scholar]
- Irshad S., Ashfaq A., Muazzam A., Yasmeen A. Antimicrobial and anti-prostate cancer activity of turmeric (Curcuma longa L.) and black pepper (Piper nigrum L.) used in typical Pakistani cuisine. Pakistan J. Zool. 2017;49:1665–1669. doi: 10.17582/journal.pjz/2017.49.5.1665.1669. [DOI] [Google Scholar]
- Jaglanian A., Tsiani E. Rosemary extract inhibits proliferation, survival, Akt, and mTOR signaling in triple-negative breast cancer cells. Int. J. Mol. Sci. 2020;21:810. doi: 10.3390/ijms21030810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaksevicius A., Carew M., Mistry C., Modjtahedi H., Opara E.I. Inhibitory effects of culinary herbs and spices on the growth of HCA-7 colorectal cancer cells and their COX-2 expression. Nutrients. 2017;9:1051. doi: 10.3390/nu9101051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jwaid A.H., Hassan A.F., Kasim A.A. The effect of artemisia dracunculus L. On mitotic index in bone marrow and spleen cells of mice: in vivo study. Indian J. Forensic Med. Toxicol. 2021;15:1879. [Google Scholar]
- Kammath A.J., Nair B., Nath L.R. Curry versus cancer: potential of some selected culinary spices against cancer with in vitro, in vivo, and human trials evidences. J. Food Biochem. 2021;45 doi: 10.1111/jfbc.13285. [DOI] [PubMed] [Google Scholar]
- Kawade V.S., Satpute P.S., Dhulap S.A., Gurjar S. Therapeutic potential of PI3K/Akt/mTOR signalling pathway: effective combination therapy for cancer. Indian J. Pharmaceut. Sci. 2018;80:702–708. doi: 10.4172/pharmaceutical-sciences.1000410. [DOI] [Google Scholar]
- Kello M., Takac P., Kubatka P., Kuruc T., Petrova K., Mojzis J. Oxidative stress-induced DNA damage and apoptosis in clove buds-treated MCF-7 cells. Biomolecules. 2020;10:139. doi: 10.3390/biom10010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khairullah A.R., Solikhah T.I., Ansori A.N.M., Fadholly A., Ram S.C., Ansharieta R., Anshori A. A review of an important medicinal plant: Alpinia galanga (L.) willd. Sys. Rev. Pharm. 2020;11:387–395. doi: 10.31838/srp.2020.10.62. [DOI] [Google Scholar]
- Khaleel F.M., Hameed A.S., Taghreed U.M. In vitro cytotoxic effect of aqueous extract of Origanum marjoram on AMN-3 cell line. J. Nat. Sci. Res. 2016;6(16) [Google Scholar]
- Khan A.U., Khan M., Khan M.M. Antifungal and antibacterial assay by silver nanoparticles synthesized from aqueous leaf extract of Trigonella foenum-graecum. BioNanoScience. 2019;9:597–602. doi: 10.1007/s12668-019-00643-x. [DOI] [Google Scholar]
- Khwairakpam A.D., Damayenti Y.D., Deka A., Monisha J., Roy N.K., Padmavathi G., Kunnumakkara A.B. Acorus calamus: a bio-reserve of medicinal values. J. Basic Clin. Physiol. Pharmacol. 2018;29:107–122. doi: 10.1515/jbcpp-2016-0132. [DOI] [PubMed] [Google Scholar]
- Kompelly A., Kompelly S., Vasudha B., Narender B. Rosmarinus officinalis L.: an update review of its phytochemistry and biological activity. J. drug deliv. ther. 2019;9:323–330. http://jddtonline.info/index.php/jddt/article/view/2218 [Google Scholar]
- Kumar S., Agnihotri N. Piperlongumine, a piper alkaloid targets Ras/PI3K/Akt/mTOR signaling axis to inhibit tumor cell growth and proliferation in DMH/DSS induced experimental colon cancer. Biomed. Pharmacother. 2019;109:1462–1477. doi: 10.1016/j.biopha.2018.10.182. [DOI] [PubMed] [Google Scholar]
- Kunnumakkara A.B., Sailo B.L., Banik K., Harsha C., Prasad S., Gupta S.C., Aggarwal B.B. Chronic diseases, inflammation, and spices: how are they linked? J. Transl. Med. 2018;16:1–25. doi: 10.1186/s12967-018-1381-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.H., Song N.Y., Suh J., Kim D.H., Kim W., Ann J., Surh Y.J. Curcumin suppresses oncogenicity of human colon cancer cells by covalently modifying the cysteine 67 residue of SIRT1. Cancer Lett. 2018;431:219–229. doi: 10.1016/j.canlet.2018.05.036. [DOI] [PubMed] [Google Scholar]
- Li Y., Wang P., Chen X., Hu J., Liu Y., Wang X., Liu Q. Activation of microbubbles by low-intensity pulsed ultrasound enhances the cytotoxicity of curcumin involving apoptosis induction and cell motility inhibition in human breast cancer MDA-MB-231 cells. Ultrason. Sonochem. 2016;33:26–36. doi: 10.1016/j.ultsonch.2016.04.012. [DOI] [PubMed] [Google Scholar]
- Luers E.C. vol. 2018. ProQuest.; 2018. Prevention and Treatment of Colorectal Cancer with Turmeric and its Main Active Constituent, Curcumin; pp. 1–15. [Google Scholar]
- Mahendran G., Rahman L.U. Ethnomedicinal, phytochemical and pharmacological updates on Peppermint (Mentha× piperita L.)—a review. Phytother Res. 2020;34:2088–2139. doi: 10.1002/ptr.6664. [DOI] [PubMed] [Google Scholar]
- Majeed A., Muhammad Z., Ahmad H., Hayat S.S.S., Inayat N., Siyyar S. Nigella sativa L.: uses in traditional and contemporary medicines–An overview. Acta Ecol. Sin. 2021;41:253–258. doi: 10.1016/j.chnaes.2020.02.001. [DOI] [Google Scholar]
- Manayi A., Nabavi S.M., Setzer W.N., Jafari S. Piperine as a potential anti-cancer agent: a review on preclinical studies. Curr. Med. Chem. 2018;25:4918–4928. doi: 10.2174/0929867324666170523120656. [DOI] [PubMed] [Google Scholar]
- Mansingh D.P., Pradhan S., Biswas D., Barathidasan R., Vasanthi H.R. Palliative role of aqueous ginger extract on N-nitroso-N-methylurea-induced gastric cancer. Nutr. Cancer. 2020;72:157–169. doi: 10.1080/01635581.2019.1619784. [DOI] [PubMed] [Google Scholar]
- Megeressa M., Do V., Ahmed A. Cytotoxic activity of proteins extracted from fennel (Foeniculum vulgare) seeds against human cancer cell lines. Faseb. J. 2020;34 doi: 10.1096/fasebj.2020.34.s1.06422. 1-1. [DOI] [Google Scholar]
- Menezes A.P.P., Trevisan S.C.C., Barbalho S.M., Guiguer E.L. Tamarindus indica L. A plant with multiple medicinal purposes. J. Pharmacogn. Phytochem. 2016;5:50. [Google Scholar]
- Mir M.A., Ganai S.A., Mansoor S., Jan S., Mani P., Masoodi K.Z., Ahmad P. Isolation, purification and characterization of naturally derived Crocetin beta-d-glucosyl ester from Crocus sativus L. against breast cancer and its binding chemistry with ER-alpha/HDAC2. Saudi J. Biol. Sci. 2020;27:975–984. doi: 10.1016/j.sjbs.2020.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miraj S., Kiani S. Pharmacological activities of Carum carvi L. Der Pharm. Lett. 2016;8:135–138. https://www.scopus.com/inward/record.uri?eid=2-s2.0-84973652564&partnerID=40&md5=27c4404b1927b9d2593d548fcd0e6dd0 [Google Scholar]
- Mohamadi N., Sharififar F., Pournamdari M., Ansari M. A review on biosynthesis, analytical techniques, and pharmacological activities of trigonelline as a plant alkaloid. J. Diet. Suppl. 2018;15:207–222. doi: 10.1080/19390211.2017.1329244. [DOI] [PubMed] [Google Scholar]
- Montgomery A., Adeyeni T., San K., Heuertz R.M., Ezekiel U.R. Curcumin sensitizes silymarin to exert synergistic anticancer activity in colon cancer cells. J. Cancer. 2016;7:1250. doi: 10.7150/jca.15690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora D.C., Kristoffersen A.E., Overvåg G., Jong M.C., Mentink M., Liu J., Stub T. Safety of Complementary and Alternative Medicine (CAM) treatment among children and young adults who suffer from adverse effects of conventional cancer treatment: a systematic review. Integr. Cancer Ther. 2022;21 doi: 10.1177/15347354221105563. 10.1177%2F15347354221105563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mughal M.H. Spices; a mechanistic anticancer treatise. J. Nutr. Food Res. Technol. 2019;2:14–19. doi: 10.30881/jnfrt.00016. [DOI] [Google Scholar]
- Mukunda A., Pynadath M.K., Kadar N., Mohan A. Cytotoxic effect of anise seed (Pimpinella anisum) extract on KB cell line-a comparative study with CISPLATIN. J. Oral Maxillofac. Pathol. 2020;11:1–5. [Google Scholar]
- Mukunthan K.S., Satyan R.S., Patel T.N. Pharmacological evaluation of phytochemicals from South Indian Black Turmeric (Curcuma caesia Roxb.) to target cancer apoptosis. J. Ethnopharmacol. 2017;209:82–90. doi: 10.1016/j.jep.2017.07.021. [DOI] [PubMed] [Google Scholar]
- Muliadi M., Basimin M.Q., Jayali A.M. Density functional theory for QSAR antioxidant compound myristicin derivatives. Indones. J. Chem. 2021;9:49–56. doi: 10.30598//ijcr.2021.9-mul. [DOI] [Google Scholar]
- Nawaz J., Rasul A., Shah M.A., Hussain G., Riaz A., Sarfraz I., Selamoglu Z. Cardamonin: a new player to fight cancer via multiple cancer signaling pathways. Life Sci. 2020;250 doi: 10.1016/j.lfs.2020.117591. [DOI] [PubMed] [Google Scholar]
- Oniga I., Pușcaș C., Silaghi-Dumitrescu R., Olah N.K., Sevastre B., Marica R., Hanganu D. Origanum vulgare ssp. vulgare: chemical composition and biological studies. Molecules. 2018;23:2077. doi: 10.3390/molecules23082077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanivel S., Murugesan A., Subramanian K., Yli-Harja O., Kandhavelu M. Antiproliferative and apoptotic effects of indole derivative, N-(2-hydroxy-5-nitrophenyl (4′-methylphenyl) methyl) indoline in breast cancer cells. Eur. J. Pharmacol. 2020;881 doi: 10.1016/j.ejphar.2020.173195. [DOI] [PubMed] [Google Scholar]
- Parida R., Mohanty S., Nayak S. Chemical composition and anti-proliferative activity of essential oil from rhizomes of micropropagated Curcuma aromatica in eastern India. J. Biol. Act. Prod. Nat. 2020;10:1–7. doi: 10.1080/22311866.2020.1737230. [DOI] [Google Scholar]
- Perez-Ortiz J.M., Galan-Moya E.M., de la Cruz-Morcillo M.A., Rodriguez J.F., Gracia I., Garcia M.T., Redondo-Calvo F.J. Cost effective use of a thiosulfinate-enriched Allium sativum extract in combination with chemotherapy in colon cancer. Int. J. Math. Stat. 2020;21:2766. doi: 10.3390/ijms21082766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S., K Tyagi A. Historical spice as a future drug: therapeutic potential of piperlongumine. Curr. Pharmaceut. Des. 2016;22:4151–4159. doi: 10.2174/1381612822666160601103027. [DOI] [PubMed] [Google Scholar]
- Qureshy Z., Johnson D.E., Grandis J.R. Targeting the JAK/STAT pathway in solid tumors. J. cancer metastatis treat. 2020;2020:27. doi: 10.20517/2394-4722.2020.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina R., Verma P.K., Peshin R., Kour H. Potential of Juniperus communis L. as a nutraceutical in human and veterinary medicine. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e02376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy L., Shankar M., Thilagavathi G. Analysis of consortium of spices: ginger, cinnamon and ajwain on their phytochemical content and anticancerous effect on lung Cancer cell lines. J. Pharmacogn. Phytochem. 2017;6:83–88. [Google Scholar]
- Raut V.R.A., Ghotankar A.M. Review of Khuskhus (khaskhas) (seeds of Papaver somniferum linn.) with special reference of ayurveda medicine. J. Ayurveda Integr. Med. 2019;4:228–231. http://jaims.in/jaims/article/view/793 [Google Scholar]
- Rocha L., Tietbohl L.A.C. Essential Oils in Food Preservation, Flavor and Safety. Academic Press; 2016. Staranise (Illicium verum hook) oils; pp. 751–756. [DOI] [Google Scholar]
- Ruiz de Porras V., Bystrup S., Martínez-Cardús A., Pluvinet R., Sumoy L., Howells L., Martínez-Balibrea E. Curcumin mediates oxaliplatin-acquired resistance reversion in colorectal cancer cell lines through modulation of CXC-Chemokine/NF-κB signalling pathway. Sci. Rep. 2016;6:1–17. doi: 10.1038/srep24675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachan A.K., Kumar S., Kumari K., Singh D. Medicinal uses of spices used in our traditional culture: Worldwide. JMPS. 2018;6:116–122. [Google Scholar]
- Saeed N.M. Combination of spices aqueous extracts as antioxidant and novel anticancer agents in human liver cancer cell line. Int. J. Biol. Chem. 2017;11:1–8. [Google Scholar]
- Sakagami H., Uesawa Y., Masuda Y., Tomomura M., Yokose S., Miyashiro T., Sugita Y. Quantitative structure–cytotoxicity relationship of newly synthesized piperic acid esters. Anticancer Res. 2017;37:6161–6168. doi: 10.21873/anticanres.12065. [DOI] [PubMed] [Google Scholar]
- Salih A.A., Idham H.A., Ismaeel N.K. Extraction of (3-Methyl-2-buten-1-yl β-D-glucopyranoside) of the fruit of plant Capparis spinosa and study effect on breast cancer cells. EurAsia J. BioSci. 2020;14:4359–4362. [Google Scholar]
- Sanaei M., Kavoosi F., Arabloo M. Effect of curcumin in comparison with trichostatin A on the reactivation of estrogen receptor alpha gene expression, cell growth inhibition and apoptosis induction in hepatocellular carcinoma Hepa 1-6 cell lline. Asian Pac. J. Cancer Prev. APJCP. 2020;21:1045. doi: 10.31557/APJCP.2020.21.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanlier N., Gencer F. Role of spices in the treatment of diabetes mellitus: a minireview. Trends Food Sci. Technol. 2020;99:441–449. doi: 10.1016/j.tifs.2020.03.018. [DOI] [Google Scholar]
- Sari D.P., Bellatasie R., Ifora I. Anti-inflammatory properties of coriandrum sativum L.: a review. Int. Res. J. Pharm. 2021;4:34–38. [Google Scholar]
- Sato T., Higuchi Y., Shibagaki Y., Hattori S. Phosphoproteomic analysis identifies signaling pathways regulated by curcumin in human colon cancer cells. Anticancer Res. 2017;37:4789–4798. doi: 10.21873/anticanres.11885. [DOI] [PubMed] [Google Scholar]
- Shahrajabian M.H., Sun W., Cheng Q. Chemical components and pharmacological benefits of Basil (Ocimum basilicum): a review. Int. J. Food Prop. 2020;23:1961–1970. doi: 10.1080/10942912.2020.1828456. [DOI] [Google Scholar]
- Sharma U.K., Sharma A.K., Pandey A.K. Medicinal attributes of major phenylpropanoids present in cinnamon. BMC Compl. Alternative Med. 2016;16:1–11. doi: 10.1186/s12906-016-1147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma Y., Fagan J., Schaefer J., Yashaswini Sharma C. Ethnobotany, phytochemistry, cultivation and medicinal properties of Garden sage (Salvia officinalis L.) J. Pharmacogn. Phytochem. 2019;8:3139–3148. [Google Scholar]
- Shokri F., Gharebaghi P.M., Esfahani A., Sayyah-Melli M., Shobeiri M.J., Ouladsahebmadarek E., Ghojazadeh M. Comparison of the complications of platinum-based adjuvant chemotherapy with and without ginger in a pilot study on ovarian cancer patients. Int J Womens Health Reprod Sci. 2017;5:324–331. doi: 10.15296/ijwhr.2017.55. [DOI] [Google Scholar]
- Singh N., Rao A.S., Nandal A., Kumar S., Yadav S.S., Ganaie S.A., Narasimhan B. Phytochemical and pharmacological review of Cinnamomum verum J. Presl-a versatile spice used in food and nutrition. Food Chem. 2021;338 doi: 10.1016/j.foodchem.2020.127773. [DOI] [PubMed] [Google Scholar]
- Singh N., Yadav S.S. A review on health benefits of phenolics derived from dietary spices. Curr. Res. Food Sci. 2022;5:1508–1523. doi: 10.1016/j.crfs.2022.09.009.s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N., Yadav S.S., Kumar S., Narashiman B. A review on traditional uses, phytochemistry, pharmacology, and clinical research of dietary spice Cuminum cyminum L. Phytother Res. 2021;35:5007–5030. doi: 10.1002/ptr.7133. [DOI] [PubMed] [Google Scholar]
- Singh N., Yadav S.S., Kumar S., Narashiman B. Ethnopharmacological, phytochemical and clinical studies on Fenugreek (Trigonella foenum-graecum L.) Food Biosci. 2022;46 [Google Scholar]
- Sithara T., Dhanya B.P., Arun K.B., Sini S., Dan M., Kokkuvayil Vasu R., Nisha P. Zerumbone, a cyclic sesquiterpene from Zingiber zerumbet induces apoptosis, cell cycle arrest, and antimigratory effects in SW480 colorectal cancer cells. J. Agric. Food Chem. 2018;66:602–612. doi: 10.1021/acs.jafc.7b04472. [DOI] [PubMed] [Google Scholar]
- Srinivasan K. Antimutagenic and cancer preventive potential of culinary spices and their bioactive compounds. PharmaNutrition. 2017;5:89–102. doi: 10.1016/j.phanu.2017.06.001. [DOI] [Google Scholar]
- Srinivasan K. Ginger rhizomes (Zingiber officinale): a spice with multiple health beneficial potentials. PharmaNutrition. 2017;5:18–28. doi: 10.1016/j.phanu.2017.01.001. [DOI] [Google Scholar]
- Su J., Zhou X., Yin X., Wang L., Zhao Z., Hou Y., Wang Z. The effects of curcumin on proliferation, apoptosis, invasion, and NEDD4 expression in pancreatic cancer. Biochem. Pharmacol. 2017;140:28–40. doi: 10.1016/j.bcp.2017.05.014. [DOI] [PubMed] [Google Scholar]
- Suhendi A., Wikantyasning E.R., Setyadi G., Wahyuni A.S., Da'i M. Acetoxy chavicol acetate (ACA) concentration and cytotoxic activity of Alpinia galanga extract on HeLa, MCF7 and T47D cancer cell lines. Indones. J. Cancer. Chemoprevention. 2017;8:81–84. [Google Scholar]
- Sun K., Duan X., Cai H., Liu X., Yang Y., Li M., Wang J. Curcumin inhibits LPA-induced invasion by attenuating RhoA/ROCK/MMPs pathway in MCF7 breast cancer cells. Clin. Exp. Med. 2016;16:37–47. doi: 10.1007/s10238-015-0336-7. [DOI] [PubMed] [Google Scholar]
- Sun W., Shahrajabian M.H., Cheng Q. Anise (Pimpinella anisum L.), a dominant spice and traditional medicinal herb for both food and medicinal purposes. Cogent Biol. 2019;5 doi: 10.1080/23312025.2019.1673688. [DOI] [Google Scholar]
- Tahir M., Khushtar M., Fahad M., Rahman M.A. Phytochemistry and pharmacological profile of traditionally used medicinal plant Hyssop (Hyssopus officinalis L.) J. Appl. Pharmaceut. Sci. 2018;8:132–140. doi: 10.7324/JAPS.2018.8721. [DOI] [Google Scholar]
- Thanekar D., Dhodi J., Gawali N., Raju A., Deshpande P., Degani M., Juvekar A. Evaluation of antitumor and anti-angiogenic activity of bioactive compounds from Cinnamomum tamala: In vitro, in vivo and in silico approach. South Afr. J. Bot. 2016;104:6–14. doi: 10.1016/j.sajb.2015.09.014. [DOI] [Google Scholar]
- Tripathi S.K., Biswal B.K. Piperlongumine, a potent anticancer phytotherapeutic: perspectives on contemporary status and future possibilities as an anticancer agent. Pharmacol. Res. 2020;156 doi: 10.1016/j.phrs.2020.104772. [DOI] [PubMed] [Google Scholar]
- Turrini E., Sestili P., Fimognari C. Overview of the anticancer potential of the “king of spices” Piper nigrum and its main constituent piperine. Toxins. 2020;12:747. doi: 10.3390/toxins12120747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay P.K. Pharmacological activities and therapeutic uses of resins obtained from Ferula asafoetida Linn.: a Review. Int. J. Green Pharm. 2017;11:240–247. doi: 10.22377/ijgp.v11i02.1033. [DOI] [Google Scholar]
- Vaghefinezhad N., Farsani S.F., Gharaghani S. In silico drug-designing studies on sulforaphane analogues: pharmacophore mapping, molecular docking and QSAR modeling. Curr. Drug Discov. Technol. 2021;18:139–157. doi: 10.2174/1570163816666191112122047. [DOI] [PubMed] [Google Scholar]
- Vemuri S.K., Banala R.R., Subbaiah G.P.V., Srivastava S.K., Reddy A.G., Malarvili T. Anti-cancer potential of a mix of natural extracts of turmeric, ginger and garlic: a cell-based study. Egypt. j. basic appl. sci. 2017;4:332–344. doi: 10.1016/j.ejbas.2017.07.005. [DOI] [Google Scholar]
- Venkatesh V., Nataraj R., Thangaraj G.S., Karthikeyan M., Gnanasekaran A., Kaginelli S.B., Basalingappa K.M. Targeting Notch signalling pathway of cancer stem cells. Stem Cell Invest. 2018;5:1–12. doi: 10.21037/sci.2018.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vutakuri N., Somara S. Natural and herbal medicine for breast cancer using Elettaria cardamomum (L.) Maton. Int J Herbal Med. 2018;6:91–96. [Google Scholar]
- Wang D., Kong X., Li Y., Qian W., Ma J., Wang D., Zhong C. Curcumin inhibits bladder cancer stem cells by suppressing Sonic Hedgehog pathway. Biochem. Biophys. Res. Commun. 2017;493:521–527. doi: 10.1016/j.bbrc.2017.08.158. [DOI] [PubMed] [Google Scholar]
- Wang J., Zhang J., Zhang C.J., Wong Y.K., Lim T.K., Hua Z.C., Lin Q. In situ proteomic profiling of curcumin targets in HCT116 colon cancer cell line. Sci. Rep. 2016;6:1–8. doi: 10.1038/srep22146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Li J., Zhao Y., Li Y., Yin L. Investigating the therapeutic potential and mechanism of curcumin in breast cancer based on RNA sequencing and bioinformatics analysis. Breast Cancer. 2018;25:206–212. doi: 10.1007/s12282-017-0816-6. [DOI] [PubMed] [Google Scholar]
- Woo J.H., Ahn J.H., Jang D.S., Lee K.T., Choi J.H. Effect of kumatakenin isolated from cloves on the apoptosis of cancer cells and the alternative activation of tumor-associated macrophages. J. Agric. Food Chem. 2017;65(36):7893–7899. doi: 10.1021/acs.jafc.7b01543. [DOI] [PubMed] [Google Scholar]
- Xie Y., Zhang J., Wang C., Fan Q., Zhang Y. Vanillin an active constituent from Vanilla bean induces apoptosis and inhibits proliferation in human colorectal adenocarcinoma cell line. Phcog. Mag. 2020;16:197. doi: 10.4103/pm.pm_235_19. [DOI] [Google Scholar]
- Yadav D., Nath Mishra B., Khan F. 3D-QSAR and docking studies on ursolic acid derivatives for anticancer activity based on bladder cell line T24 targeting NF-kB pathway inhibition. J. Biomol. Struct. Dyn. 2019;37:3822–3837. doi: 10.1080/07391102.2018.1528888. [DOI] [PubMed] [Google Scholar]
- Yasmeen A., Qayyum F., Mughal A.A., Amjad O. Phytochemical and biological investigation of armoracia rusticana. Int. J. Phar. Integr. Health Sci. 2020;1:58–68. [Google Scholar]
- Yuan J., Dong X., Yap J., Hu J. The MAPK and AMPK signalings: interplay and implication in targeted cancer therapy. J. Hematol. Oncol. 2020;13:1–19. doi: 10.1186/s13045-020-00949-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue G.G.L., Kwok H.F., Lee J.K.M., Jiang L., Wong E.C.W., Gao S., Bik-San Lau C. Combined therapy using bevacizumab and turmeric ethanolic extract (with absorbable curcumin) exhibited beneficial efficacy in colon cancer mice. Pharmacol. Res. 2016;111:43–57. doi: 10.1016/j.phrs.2016.05.025. [DOI] [PubMed] [Google Scholar]
- Yue Y., Liu L., Liu P., Li Y., Lu H., Li Y., Duan X. Cardamonin as a potential treatment for melanoma induces human melanoma cell apoptosis. Oncol. Lett. 2020;19:1393–1399. doi: 10.3892/ol.2019.11242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q., Che Y., Zhang Y., Chen M., Guo Q., Zhang W. Thymol Isolated from Thymus vulgaris L. inhibits colorectal cancer cell growth and metastasis by suppressing the Wnt/β-catenin pathway. Drug Des. Dev. Ther. 2020;14:2535. doi: 10.2147/DDDT.S254218. 10.2147%2FDDDT.S254218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Ma Z.F. Phytochemical and pharmacological properties of Capparis spinosa as a medicinal plant. Nutrients. 2018;10:116. doi: 10.3390/nu10020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Bai Y., Yang Y. Thymoquinone chemosensitizes colon cancer cells through inhibition of NF-κB. Oncol. Lett. 2016;12:2840–2845. doi: 10.3892/ol.2016.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Li D., Liu Y., Wang H., Zhang C., Huang H., Zheng X. Potential anticancer activity of curcumin analogs containing sulfone on human cancer cells. Arch. Biol. Sci. 2016;68:125–133. doi: 10.2298/ABS150323134Z. [DOI] [Google Scholar]
- Zhang Y., Wang X. Targeting the Wnt/β-catenin signaling pathway in cancer. J. Hematol. Oncol. 2020;13:1–16. doi: 10.1186/s13045-020-00990-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., Zhou Y., Li Y., Xu D.P., Li S., Li H.B. Spices for prevention and treatment of cancers. Nutrients. 2016;8:495. doi: 10.3390/nu8080495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Su J., Feng S., Wang L., Yin X., Yan J., Wang Z. Antitumor activity of curcumin is involved in down-regulation of YAP/TAZ expression in pancreatic cancer cells. Oncotarget. 2016;7 doi: 10.18632/oncotarget.12596. 10.18632%2Foncotarget.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]