Abstract
Calendula officinalis Linn contains the highest quantity of lutein in the orange variety of Calendula flowers. It has several benefits like; it protects the eyes from free radicals associated with UV rays on the eye retina. In the present work, we checked the enhancement of lutein content in C. officinalis flowers by solid-state fermentation examined by using four different species of Lactobacillus (L. rhamnosus, L. casei spp. casei, and L. plantrum). These microorganisms were isolated and allowed to ferment over the fresh (FC) and dried (DC) petals of C. officinalis for 10 days in the incubator. The fermented and non-fermented petals were extracted by the hexane extraction method and the presence of lutein was confirmed by HPLC technique, using a reversed-phase C18 column and gradient elution with a mobile phase composed of acetonitrile and methanol (40:60), flow rate of 1.0 ml/min and the UV detection at 446 nm. The highest amount of lutein (9.92 mg/g) was found in dried orange variety Calendula petals fermented by Lactobacillus rhamnosus. However, the orange variety of FC petals showed the highest concentration (40.66 mg/g) in Lactobacillus plantarum. Experiment results concluded that 1 kg of FC petals contains 4.0% of lutein fermented by Lactobacillus plantarum and 1 kg of DC petals contains 0.99% of lutein fermented by Lactobacillus rhamnosus compared with non-fermented and commercial lutein. The non-fermented FC and DC orange flowers contain 1.1% and 0.4% of lutein and commercial lutein contains 0.2%. The process mentioned above was also carried out using ethanol as the solvent for extraction, which showed satisfactory yield, 1 kg of fresh flowers of Calendula contains 0.43% of lutein fermented by Lactobacillus plantarum and 1 kg of DC contain 0.52% of lutein fermented by Lactobacillus rhamnosus.
Keywords: Calendula officinalis, Lutein, Lactobacillus species, HPLC, Sold-state fermentation
Introduction
Calendula officinalis Linn. commonly known as pot Calendula or marigold, is one of the traditionally used medicinal plants in India, China, Europe and the US (Muley et al. 2009) and has been of therapeutic importance since the beginning of 12th century. It belongs to the Asteraceae (Compositae) family. The colour of the petals of yellow and orange verities (Fig. 1) originated from carotenoids pigments. It has been evidenced to portray several promising benefits, for instance, it has a role in cancer prevention, it enhances the function of immune system (Chew et al. 1996), inhibits the auto-oxidation of cellular lipids (Zhang et al. 1991) and also age-related macular degeneration (Fullmer and Shao 2001; Seddon et al. 1994). Recent studies have been focused on the use of Calendula petals extract as a human nutritional supplement, therefore, research interest has developed in processing new methods for the production of xanthophylls (Lutein) such as chemical synthesis (Kreienbuhl et al. 2000) and fermentation technology (Gierhart 1994; Hirschberg and Harker 1999; Jacobson et al. 2000). These methods do ensnare innovative efforts, but involve the use of toxic solvents in the chemical synthesis method, which render them inadequate. An alternative method i.e., fermentation technology, has been proposed to improve both xanthophylls extraction and purification efficiency (Ausich and Sanders 1997; Khachik 1995; Philip 1997). As a suitable method of extraction that could enhance extractive yield, solid-state fermentation (SSF) appears to be superior to submerged fermentation technology (SmF) in several aspects. The function of physiological and genetic characteristics of the microorganism applied during growth on solid substrates examined with aqueous solution has so far been all but neglected even though it may be the microbiology that makes SSF advantageous over SmF biotechnology (Hölker et al. 2004; Balakrishnan, 1996).
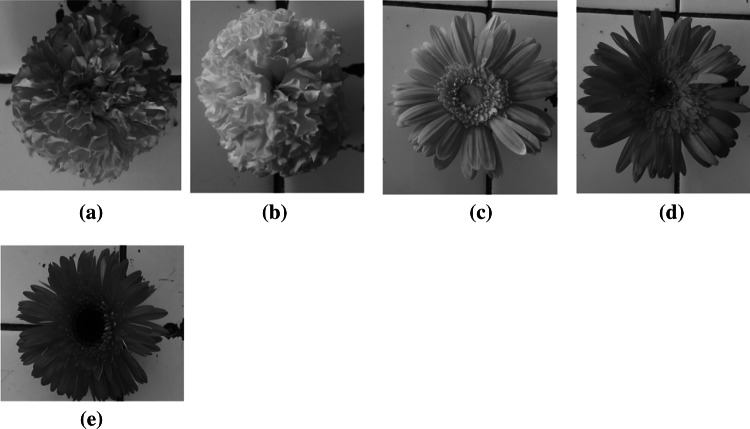
Fig. 1.
The various kind of Calendula flowers: a Orange colour closed calendula (OCC), b yellow colour closed calendula (YCC), c Yellow colour Open calendula (YOC), d Orange colour open calendula (OOC), and e Pink colour open calendula (POC) (color figure online)
SSF possess various benefits, such as higher fermentation potency, higher end-concentration of outcomes, higher outcome stability, lower catabolic repression, cultivation of microorganisms specialized for water-insoluble substrates or mixed cultivation of various fungi, and last but not the least, lower demand on sterility due to the low water activity used in SSF (Orzua et al. 2009; Rodriguez-Duran et al. 2011).
Though this method has been used to extract lutein and enhance its yield using n-hexane as a solvent and also used alternative solvent like ethanol which was necessary since the former cannot be used in nutraceuticals. Thus, keeping in mind the drawbacks of the existing methods like SmF, the present study was designed to enhance the lutein yield by Solid-state fermentation with Lactobacillus species.
Materials and methods
Chemicals and reagents
MRS and Potato dextrose broth was purchased from Central drug house (P) Ltd. Delhi. Freeze-dried Cultures ampoules of lactic acid bacteria (LAB) (Lactobacillus casei spp. casei − 017, Lactobacillus fermentum − 141, Lactobacillus plantarum − 020) were obtained from National Collection of Dairy Cultures, NDRI, Karnal, India, which were incubated at 35 °C for 48 h in an anaerobic condition. The Brand (Setu Eye Max, lutemax 2020), it contained lutein 10 mg and zeaxanthin 2 mg use as marker compound purchased from Omni wellness and nutraceutical private limited. Furthermore, skimmed milk powder and curd order from Mother Dairy Fruit & Vegetable Pvt Ltd, A-3, Sector-1, Noida, Uttar Pradesh-201 301, India.
Collection and processing of Petals
The different verities of C. officinalis flowers were procured from Chattarpur, Delhi in the month of September. The samples were identified and authenticated by Dr. Vidhu Aeri, Professor of Pharmacognosy, Department of Pharmacognosy & Phytochemistry, SPER, Jamia Hamdard, and New Delhi.
The petals were removed from the head of flowers and were spread on a filter paper for two days at room temperature. The semi-dried petals were then dried in a hot air oven at a temperature not more than 70 °C to prevent the degradation of carotenoids. The moisture content of petals was calculated on dry and wet basis. The dried material was powdered and stored in airtight containers at 4 °C for further use.
Moisture content of different varieties of Calendula petals
The various kind of Calendula flowers i.e. Orange colour open calendula (OOC), yellow colour open calendula (YOC), and pink colour open calendula (POC) with Orange colour closed calendula (OCC) and Yellow colour closed calendula (YCC) were dried in the hot air oven at a temperature below 70 °C till the moisture content became constant. The flowers were dried to avoid any type of hindrance during the extraction process by virtue of the moisture present in raw materials. The moisture content was estimated on both wet and dry basis.
Lutein extraction from tablets
Setu Eye Max (lutemax 2020), it contained lutein 10 mg and zeaxanthin 2 mg (100% Pure). We took one tablet and gently ground it in a mortar. An aliquot was dissolved in 10 mL of solvent (hexane or ethanol) in a flask. It was vortexes for 5–10 min and centrifuged for 10 min at 3000 rpm. Two layers were formed; upper layer was collected and filtered for further analysis. This extract of lutein was used as standard.
Culture of microorganism
The freeze-dried culture ampoules were activated by spreading into the MRS agar (Fischer) plates, incubated at 35 °C for 48 h in an anaerobic condition. A colony was picked from each plate, grown in MRS agar, streaked into MRS agar slants and incubated at 35 °C. These cultures were used for fermentation process. The remaining LAB cultures plates were stored in a refrigerator for further use (Karami et al. 2017).
Solid state fermentation
MRS broth of 50 ml was added into slants of LAB (Lactobacillus casei species casei, Lactobacillus plantarum) in a laminar flow cabinet. LAB (10%) was poured over the dried (DC) and fresh Calendula (FC) petals (27.1 and 65.6 g, respectively). Lactobacillus casei 6.5 ml was added over 65.6 g of fresh Calendula petals. The glass container with Calendula petals was sealed with transparent film and allowed to ferment in an incubator at 35 °C for ten days. The same process was repeated for other Lactobacillus species.
Hexane extraction method
Accurately weighed one gram of fermented and non-fermented petals powder was transferred in a beaker, followed by addition of 20 ml of hexane (95% Purity). The mixture was agitated on a shaker for 2 h at 35 °C at 131 rpm in dark. After 2 h, the samples were taken out. The sample colour was changed either to yellow or orange. The sample was filtered and transferred into glass vials and stored in a refrigerator for further analysis.
Ethanol extraction method
Accurately weighed one gram of fermented and non-fermented powder was transferred in a beaker, followed by addition of 20 ml of ethanol (99.9% Purity). The mixture was agitated on shaker for 2 h at 35 °C at 131 rpm in dark. After 2 h the sample was withdrawn. The sample colour changed either to yellow or orange. The sample was filtered and transferred into glass vials and stored in a refrigerator for further analysis.
UV-Vis spectroscopic method
Different concentrations of the standard were prepared in hexane by a serial dilution method and absorbance was checked at the wavelength, 446–470 nm by comparing with blank reference solvent. The standard plot was drawn between the concentration and absorbance and R2 was calculated. Similarly, the absorbance of the samples was measured at wavelength ranging from 446 to 470 nm and the concentration of each sample was calculated by the equation obtained from standard plot. The highest peak of Calendula petals extract was observed at wavelength of 446 nm (Hajare et al. 2013).
High-performance liquid chromatography (HPLC) method
The HPLC was performed using Shimadzu HPLC SPINLAB ID S0001/1707 (Japan) equipped with Rheodyne-7725i injection valve (20 µl) (Rheodyne, USA), LC-10 AT VP pumps and SPD-10 A VP UV-V as detector along with integrated CLASS-VP 6.14 SP1 software. Analysis was performed on reverse phase column, steel packed, 250 × 4.60 mm diameter of 5 µ. The elute solution of acetonitrile: methanol (40:60) was used as a mobile phase with a flow rate 1.0 ml/min and the detector were set at 446 nm. The injection volumes were 20 µl throughout the experiment. All the solvents and samples were sonicated and filtered through 0.45 μm filter (Millipore) before use in HPLC. The identification of lutein was done by retention time, UV spectra and by comparing the peak area of sample with that of the standard (Tinoi et al. 2006).
Statistical analysis of data
All data were reported as mean (standard error of mean for at least four replicates in each treatment. Simple regression analysis was performed using graphpad prism 6.01 (San Diego, CA, United states).
Results and discussion
Moisture content of different varieties of Calendula petals
Calendula flowers (OOC, YOC, POC, OCC and YCC) indicated that the OOC showed highest moisture content 83.33% on wet basis and the YOC showed the highest moisture content 31.81% on dry basis.
UV-Vis spectroscopic method
The OC Calendula petals showed the highest amount of lutein (53.51 mg/g) at 445 nm. Hence, further analysis was carried out on this particular variety.
The UV-Vis spectrum of fermented fresh petals of Calendula petals indicated a peak in two different solvents. In hexane, the peak was observed at 472.20 nm, 443.60 nm, 421.70 nm, 415.70 nm and in ethanol; peak was also indicated at 472.70 nm, 444.50 nm, and 423.50 nm. The peaks were identified in the range of 400–500 nm.
High-performance liquid chromatography (HPLC) method
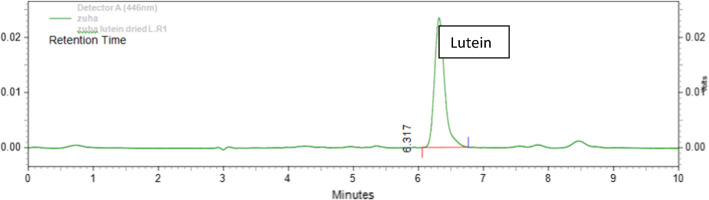
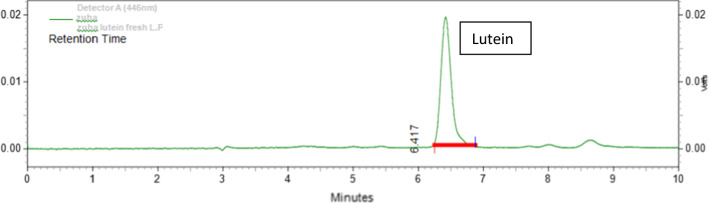
The lutein present in the extract was confirmed by the separation in HPLC method. The eluents were continuously monitored in a UV detector at 446 nm. Peak identification was based on the retention time in comparison with standard plot and the peak appearance between 5.10 and 6.45 min confirmed the presence of lutein. The peak area and concentration of respective samples are shown in Tables 1 and 2. Comparative analysis of these samples in both the solvents (hexane and ethanol) is shown in Figs. 2 and 3. The concentration was calculated from the equation of the standard plot graph. A significantly higher concentration of lutein was extracted through hexane method in comparison to alcohol method in both fresh and dried petals. Increased yield of lutein was obtained using fermentation process as compared non-fermentation process. Fermented experiments produced significantly higher concentration (p < 0.001) of lutein from fresh petal with hexane as the solvent while in dried petal with ethanol as the solvent. The highest amount of lutein was found in orange DC petals fermented by Lactobacillus rhamnosus in the concentration of 9.92 mg/g. However, the orange FC petals showed the highest concentration of lutein in Lactobacillus plantarum, the concentration being about 40.66 mg/g.
Table 1.
Fresh and Dried petals (fermented and non-fermented) extracted by hexane method
| Sample | Peak area (446 nm) FC |
Conc. (mg/g) FC |
Peak area (446 nm) DC |
Conc. (mg/g) DC |
|---|---|---|---|---|
| Non-fermented | ||||
| Fresh | 16,450 | 11.14 ± 1.1 | ||
| Dried | 4594 | 4.12 ± 0.06 | ||
| Commercial | 1100 | 2.06 ± 0.01 | ||
| fermenteda | ||||
| Lactobacillus rhamnosus | 10,735 | 7.76 ± 0.10 | 14,384 | 9.92 ± 0.1 |
| Lactobacillus casei spp. casei | 6930 | 5.5 ± 0.01 | 639 | 7.78 ± 0.01 |
| Lactobacillus plantrum | 66,359 | 40.66 ± 0.02 | 5057 | 4.4 ± 0.04 |
Mean ± SEM, n > 4
ap<0.001 versus non-fermented process in Fresh Calendula (FC) petals
Table 2.
Fresh and Dried petals (fermented and non-fermented) extracted by ethanol method
| Sample | Peak area (446 nm) FC |
Conc. (mg/g) FC |
Peak area (446 nm) DC |
Conc. (mg/g) DC |
|---|---|---|---|---|
| Non-fermented | ||||
| Fresh | 6648 | 0.6 ± 0.01 | ||
| Dried | 642 | 0.48 ± 0.01 | ||
| Commercial | 1342 | 0.5 ± 0.02 | ||
| fermenteda | ||||
| Lactobacillus rhamnosus | 5084 | 0.58 ± 0.05 | 256,381 | 5.26 ± 0.02 |
| Lactobacillus casei spp. casei | 4051 | 0.56 ± 0.02 | 4067 | 0.57 ± 0.02 |
| Lactobacillus plantrum | 206,633 | 4.32 ± 0.05 | 6372 | 0.6 ± 0.01 |
Mean ± SEM, n > 4
ap<0.001 versus non-fermented process in Dried Calendula (DC) petals
Fig. 2.
Comparison of concentration of lutein in fresh petals of fermented and non- fermented Calendula petals extracted from hexane and ethanol. *p < 0.05; **p < 0.01; ***p < 0.001- hexane vsersus ethanol
Fig. 3.
Comparison of concentration of lutein in dried petals of fermented and non- fermented Calendula petals extracted from hexane and ethanol. ***p < 0.001- hexane vsersus ethanol
From the above results it was found that the yield of lutein increased after fermentation with Lactobacillus species. The extracted oleoresin from the Calendula petals flower fermented in solid-state was analyzed by HPLC. It showed that the highest amount of lutein was found in orange DC petals fermented by Lactobacillus plantarum, the concentration being about 40.66 mg/gin hexane and 4.32 mg/g in ethanol. This yield was compared to the yield of commercial Calendula petals flower derived from industrial ensilage processes, which gave an average output of 11.54 ± 2 g/kg Calendula petals flower (dry weight) from Rhodotorula glutinis YB-252 using the solid-state fermentation process (Hernández-Almanza et al. 2014). The yield was also compared with previous reports (Luis et al. 2004; Delgado-Vargas and Paredes-López 1997).
The experiments discussed here indicate that the process of solid-state fermentation is a suitable alternative to enhance the yield of lutein (4%) extracted from Calendula petals when compared with non-fermented fresh (1%) and dried (0.4%) petals. While the yield percentage is better than the results of previous studies (Delgado-Vargas and Paredes-López 1997). The variance in concentration can be due to differences in both the Calendula variety, treatment and the method used in particular. However, in our solid-state fermentation technique, we used Calendula petals and also Lactobacillus species, which was safe to use. The process is longer than usual. However, it can be used for short scale purposes. Though, enzymatic treatments reduce the processing time (Delgado-Vargas and Paredes-López 1997) and can be used on a large scale (Figs. 4 and 5).
Fig. 4.
HPLC chromatogram of dried orange variety Calendula petals fermented by Lactobacillus rhamnosus
Fig. 5.
HPLC chromatogram of fresh orange variety Calendula petals fermented by Lactobacillus plantarum
Additionally, there is insufficient ability to store large amounts of raw material. The modular of the solid-state fermentation system employed in this work allows for the monitoring and quantification of critical variables. Once the device operates under optimum or near-optimum conditions, the recovery of lutein from Calendula flowers is increased significantly without loss, with additional improvement in the profiles of main components.
Conclusion
The OCC were found to contain the highest amount i.e., 51.51 mg/g, 53.51 mg/g and 48 mg/g at different wavelengths i.e., 420 nm, 445 and 475 nm, respectively of lutein in hexane by UV-Vis spectrophotometry. The HPLC method was developed to quantify lutein in OCC. The peak eluted at 5.317 min with a peak area of 3,717,093 nm, thus was identified as lutein. HPLC conditions: C18 column, gradient solvent of acetonitrile: methanol (40:60, v/v), linear gradient over 15 min, flow rate 0.1 ml/min, and detection at 446 nm. A Solid-state fermentation enhanced the extraction of lutein by using different Lactobacillus species i.e., Lactobacillus rhamnosus, Lactobacillus plantarum, Lactobacillus casei spp. casei. The UV-Vis spectrophotometry indicated the presence of lutein in both extracted solvents. HPLC analysis shows that lutein was found in different concentrations in fermented and non-fermented varieties of Calendula petals. HPLC conditions: C18 column, gradient solvent of acetonitrile: methanol (40:60, v/v), linear gradient over 15 min, flow rate 0.1 ml/min and detection at 446 nm. The highest amount of lutein was found in orange color DC petals fermented by Lactobacillus rhamnosus in the concentration of about 9.92 mg/g in hexane and 5.26 mg/g in ethanol.
When lutein extracted with hexane, 1 kg of FC petals contains 4.0% of lutein fermented by Lactobacillus plantarum and 1 kg of DC petals contains 0.99% of lutein fermented by Lactobacillus rhamnosus compared with non-fermented and commercial lutein. Likewise, in ethanol solvent, 1 kg of FC petals contains 0.43% of lutein fermented by Lactobacillus plantarum, and 1 kg of DC petals contains 0.52% of lutein fermented by Lactobacillus rhamnosus. Furthermore, the non-fermented FC and DC orange flowers contain 1.1% and 0.4% of lutein and commercial lutein contains 0.2%. Ethanol, which is already in common use in the food industry, can further be used as a solvent of choice in newer researches contributing to this field.
Abbreviations
- L
Lactobacillus
- FC
Fresh Petals
- DC
Dried Petals
- C.
Calendula
- SSF
Solid State Fermentation
- SmF
Submerged Fermentation Technology
- OOC
Orange colour open calendula
- YOC
Yellow colour open calendula
- POC
Pink colour open calendula
- OCC
Orange colour closed calendula
- YCC
Yellow colour closed calendula
- °C
Degree Celsius
- Mg
Milligrams
- Ml
Millilitres
- Min
Minutes
- Rpm
Revolutions per minute
- G
Grams
- Kg
Kilograms
- nm
Nanometre
- µl
Micro litre
- LAB
Lactic acid Bacteria
- MRS
De Man, Rogosa and Sharpe agar
- HPLC
High-performance liquid chromatography
- UV-Vis
Ultraviolet-visible
Authors' contributions
VA conceived, supervised the work and edited the manuscript, ZR carried out the experiments and wrote the MS.
Funding
NA.
Data Availability
The authors declare that all data supporting the findings of this study are available within the article.
Code availability
NA.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
NA.
Consent to participate
NA.
Consent for publication
NA.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ausich RL, Sanders DJ (1997) U.S. Patent No. 5,648,564. Washington, DC: U.S. Patent and Trademark Office
- Balakrishnan K. Production of biologically active secondary metabolites in solid state fermentation. J Sci Ind Res. 1996;55:365–372. [Google Scholar]
- Chew BP, Wong MW, Wong TS. Effects of lutein from marigold extract on immunity and growth of mammary tumors in mice. Anticancer Res. 1996;16(6B):3689–3694. [PubMed] [Google Scholar]
- Delgado-Vargas F, Paredes-López O. Effects of enzymatic treatments of marigold flowers on lutein isomeric profiles. J Agric Food Chem. 1997;45(4):1097–1102. doi: 10.1021/jf960474a. [DOI] [Google Scholar]
- Fullmer LA, Shao A. The role of lutein in eye health and nutrition. Cereal Foods World. 2001;46(9):408–413. [Google Scholar]
- Gierhart DL. Production of zeaxanthin and zeaxanthin containing compositions. US Patent. 1994;5:308759. [Google Scholar]
- Hajare R, Ray A, Shreya TC, Avadhani MN, Selvaraj IC. Extraction and quantification of antioxidant lutein from various plant sources. Int J Pharm Sci Rev. 2013;22(1):152–157. [Google Scholar]
- Hernández-Almanza A, Montañez-Sáenz J, Martínez-Ávila C, Rodríguez-Herrera R, Aguilar CN. Carotenoid production by Rhodotorula glutinis YB-252 in solid-state fermentation. Food Bioscience. 2014;7:31–36. doi: 10.1016/j.fbio.2014.04.001. [DOI] [Google Scholar]
- Hirschberg J, Harker M (1999) U.S. Patent No. 5,935,808. Washington, DC: U.S. Patent and Trademark Office
- Hölker U, Höfer M, Lenz J. Biotechnological advantages of laboratory-scale solid-state fermentation with fungi. Appl Microbiol Biotechnol. 2004;64(2):175–186. doi: 10.1007/s00253-003-1504-3. [DOI] [PubMed] [Google Scholar]
- Jacobson GK, Jolly SO, Sedmak JJ, Skatrud TJ, Wasileski JM. Astaxanthin over-producing strains of Phaffiarhodozyma, method for their cultivation and their use in animal feeds. US Patent. 2000;6:015684. [Google Scholar]
- Karami S, Roayaei M, Hamzavi H, Bahmani M, Hassanzad-Azar H, Leila M, Rafieian-Kopaei M. Isolation and identification of probiotic Lactobacillus from local dairy and evaluating their antagonistic effect on pathogens. Int J Pharm Invest. 2017;7(3):137. doi: 10.4103/jphi.JPHI_8_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachik F (1995) Process for isolation, purification, and recrystallization of lutein from saponified marigold oleoresin and uses thereof. US Patent 5,382,714.
- Kreienbuhl P, Rudin P, Rudolph W. Method of making carotenoids. US Patent. 2000;6:150561. [Google Scholar]
- Luis N-BJ, Hugo J-I, Enrique B-A, Ramiro R-M, Octavio P-L. An optimization study of solid-state fermentation: xanthophylls extraction from marigold flowers. Appl Microbiol Biotechnol. 2004;65(4):383–390. doi: 10.1007/s00253-004-1615-5. [DOI] [PubMed] [Google Scholar]
- Muley BP, Khadabadi SS, Banarase NB (2009) Phytochemical constituents and pharmacological activities of Calendula officinalis Linn (Asteraceae): a review.Tropical journal of pharmaceutical research, 8(5)
- Orzua MC, Mussatto SI, Contreras-Esquivel JC, Rodriguez R, De la Garza H, Teixeira JA, Aguilar CN. Exploitation of agro industrial wastes as immobilization carrier for solid-state fermentation. Ind Crops Prod. 2009;30:24–27. doi: 10.1016/j.indcrop.2009.02.001. [DOI] [Google Scholar]
- Philip T. Purification of lutein-fatty acid esters from plant materials. US Patent. 1997;4:048203. [Google Scholar]
- Rodriguez-Duran LV, Contreras-Esquivel JC, Rodriguez R, Prado-Barragan LA, Aguilar CN. Optimization of tannase production by Aspergillus niger in solid-state packedbedbiorreactor. J Microbiol Biotechnol. 2011;21:960–967. doi: 10.4014/jmb.1103.03025. [DOI] [PubMed] [Google Scholar]
- Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, Farber MD, Gragoudas ES, Haller J, Miller DT, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA. 1994;272(18):1413–1420. doi: 10.1001/jama.1994.03520180037032. [DOI] [PubMed] [Google Scholar]
- Tinoi J, Rakariyatham N, Deming RL. Determination of major carotenoid constituents in petal extracts of eight selected flowering plants in the north of Thailand. Chiang Mai J Sci. 2006;33(2):327–334. [Google Scholar]
- Zhang LX, Cooney RV, Bertram JS. Carotenoids enhance gap junctional communication and inhibit lipid peroxidation in C3H/10T1/2 cells: relationship to their cancer chemopreventive action. Carcinogenesis. 1991;12(11):2109–2114. doi: 10.1093/carcin/12.11.2109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that all data supporting the findings of this study are available within the article.
NA.