Abstract
Burfi, an Indian traditional dairy dessert is highly popular, however, its low nutritive value and poor shelf life limits commercial viability. Kinnow juice was utilized to improve the phytonutritional profile and shelf stability of burfi. To further improve the quality, pectin was added at different concentrations (1–4%). Compared to control, inclusion of kinnow juice significantly (p < 0.05) improved minerals and phytochemical constituents as confirmed using FTIR analysis. Developed burfi presented a softer texture which was consistent with SEM results. Increasing the pectin levels, increased textural properties of kinnow burfi while decreased moisture content and water activity, without affecting color and nutritional properties. Based on desirable sensory and textural properties, kinnow burfi prepared with pectin (4% of added sugar) was selected for product development and evaluation of shelf life under room (25 ± 2 °C) and refrigerated (4 ± 2 °C) conditions. Irrespective of storage temperatures, moisture content and bioactive constituents decreased while titratable acidity, total and reducing sugars, free fatty acids, hardness of texture, and yeast and mould count increased significantly. Except moisture, the extent of quality changes was rapid at room temperature. The product showed high sensory acceptability as well as microbial safety up to 21 days at room temperature and 28 days under refrigeration.
Keywords: Kinnow, Bioactive constituents, Shelf life, Pectin, Limonin
Introduction
Citrus fruits (mandarins, grapefruit, lime/lemons, tangerines, etc.) are widely grown and consumed due to their rich nutritional profile, especially Vitamin C. Among the diverse citrus group, mandarins represent 22% of the world's citrus production and are positioned at the second most-produced citrus in the world (Mahawar et al. 2020). Kinnow, a high-yielding mandarin hybrid is cultivated extensively in Northern India. The fruit has its own functional significance due to presence of high level of vitamins, minerals, fibre, and phytochemicals (Mahawar et al. 2020). Despite its commercial prominence and nutritional benefits, the fruit suffers significant post-harvest losses (25–30%) due to shortage of cold storage and distribution chains in India (Purewal et al. 2021). Also, the availability of kinnow during off-season is limited due to its poor shelf life of 8–10 days. During peak production, kinnow is mostly processed into juice related beverages. However, delayed bitterness caused by development of limonin in the extracted juice, is the major problem faced by citrus juice processing industries. The intact fruit hardly contains limonin, however limonoate A- ring lactone, a non-bitter precursor (originally present in seeds) passes into the juice and is converted to limonin under acidic conditions of the juice, resulting in delayed bitterness after 3 to 4 h of juice extraction (Mahawar et al. 2020). This problem has significantly affected consumer acceptability and processing on commercial scale. Due to this reason, only 5% of the fruit is processed into juice and 95% is used for table purpose, leading to market gluts and price fall during peak fruiting season (Mahawar et al. 2020). Though considerable attempts have been explored in the past for modulating bitterness in citrus juices such as enzyme treatment, use of absorbents, and hydrocolloids (Purewal et al. 2021; Mahawar et al. 2020; Aggarwal and Sandhu 2004), still an effective and satisfactory method for removing or reducing bitterness without hampering taste, aroma and color of the juice has not been developed. Reduction of bitterness from kinnow juice extends the possibility of making value added products during off seasons.
The consumption of sweets is an integral part of the Indian dietary system. Around 50–55% of the milk produced in the country is converted into traditional sweet meats such as burfi, peda, kalakand, Gulab jamun, and milk cake (Rasane and Jha 2012). Burfi is Indian fudge made by boiling milk in open pan until desired semi solid mass called "khoa "is obtained. This mass is the base for manufacturing any kind of burfi. The obtained khoa is mixed with sugar till it thickens and reaches a solid consistency (Total solid content 70–75%) (Tiwari et al. 2014). Once confined to household productions, burfi is gaining an international market in recent years owning to its delicious flavor and texture. Burfi undergo several physical, biochemical, and microbiological changes during storage making it unfit for human consumption. The short shelf life (around 2–3 days under room and 4–6 days under refrigerated conditions) is one of the major constraints limiting their marketing in both domestic and export markets (Prasad et al. 2017).
In recent years, manufacturers are diversifying the production to include specialty items that cater to specific targeted populations (Arora et al. 2007). Nowadays many versions of burfi are available in the market such as khoa burfi (prepared with milk and sugar), chocolate burfi (cocoa powder), rava burfi (semolina), and doda burfi (germinated wheat flour). Some local manufacturers have also explored the addition of fruit pulps in burfi formulations due to their rich phytonutritional profile and potential health benefits. One such type of commercial available fruit based burfi is santra burfi which is highly popular in Nagpur district of Maharashtra, India. This type of burfi is prepared by the addition of orange pulp with sweetened khoa. Though it is marketed as orange burfi, the quantity of orange pulp used in its preparation is only 2%. Further the sensorial quality of the product is enhanced with the help of synthetic food colors and flavors. The shelf life of this burfi is also short about 8–9 days. The growing interests of health-conscious consumers now demand for more conscious, convenient, nutritious, and natural food options without any artificial or synthetic food ingredients. Looking at the current scenario, the potential of plant-based foods such as fruits can be exploited for developing novel traditional functional food products with enriched antioxidant properties and extended shelf-life. The concept of enhanced shelf life not only attracts consumers but also producers. Since Kinnow fruit is rich in bioactive metabolites so its utilization for development of nutritious burfi thus merits consideration. Also, very limited studies have been explored to study the microstructural properties of Indian traditional dairy products through scanning electron microscopy (SEM). Therefore, the present study was formulated to (i) develop phytonutrients rich kinnow burfi with extended shelf life and enhanced functionality and ii) evaluate the nutritional quality, microstructural properties and shelf stability of burfi during storage under room and refrigerated storage conditions.
Material and methods
Procurement of raw materials
Fully ripe Kinnow mandarins were procured from local market of Ludhiana, Punjab, India. Pectin was purchased from S.D. Fine Chemicals Ltd., Mumbai, India. Fresh Khoa (Moisture 29.50%, titarable acidity 0.40%, fat 18.50% and protein 18.45%) and coconut powder (Moisture 3.43%, fat 63.50%, and protein 6.70%) were purchased locally.
Extraction of kinnow juice
Kinnow was washed, manually peeled, and juice was extracted using screw type juice extractor (Kalsi Industries, Ludhiana, India). Immediately, after juice extraction, sugar was added as a sweetening agent to avoid limonin bitterness in the extracted juice and afterwards, the juice was kept under refrigerated conditions (4 ± 2 °C). Freshly extracted kinnow juice had 10.50% total solids, 0.64% acidity, 5.00% total sugars, 14.75 mg/100 g ascorbic acid and 28.0 ppm limonin content.
Preparation of kinnow burfi
Standardized recipe of kinnow burfi had the following ingredients: kinnow juice (125 g), sugar (100 g), khoa (50 g), coconut powder (14 g) and pectin (4% of added sugar). For preparation of burfi, extracted juice along with required amount of khoa and pectin was concentrated in a stainless steel pan by open pan boiling with continuous stirring until a paste like consistency was obtained (TSS 80˚B) (Tiwari et al. 2014). To this paste, coconut powder was added to further mask the bitter flavor and taste of Kinnow. This hot paste was then spread uniformly on greased trays and allowed to cool at refrigeration temperature (4 ± 2 °C) for 24 h. After setting, kinnow burfi was cut into rectangular pieces of 30 × 30 × 20 mm. Four different burfi formulations were prepared by varying the pectin amount (1 to 4% of added sugar) since it is the most important ingredient that significantly affects textural properties of a food product (Huang and Hsieh 2005). Samples prepared with 1% of pectin were not acceptable since these could not hold their structure well. Control sample (plain milk burfi; with 80% milk solids and 20% sugar) was prepared using the method of Wasnik et al. (2013) and used for quality and sensorial evaluation.
Physico-chemical parameters
Moisture, fat, ash, and protein contents of raw materials and formulated burfi samples were analyzed using standard AOAC (2019) methods of analysis. Titratable acidity, total and reducing sugars and free fatty acids (FFA) (as oleic acid) were determined using the methods of Kaur and Aggarwal (2016). Mineral analysis of burfi was performed by inductively coupled plasma atomic emission spectroscopy (ICP-AES) (Singh et al. 2021a). Color attributes L* (0–100, dark to light), a* (± green/red), and b* (± blue/yellow) were conducted using a Hunter Lab Calorimeter (Model CR 300 Konica Minolta Co, Osaka, Japan) (Singh et al. 2021b).
Textural characteristics (hardness, springiness, cohesiveness, gumminess, and chewiness) of burfi formulations were determined using texture analyzer (LLOYD texture instrument LR 5 K, England) equipped with a circular P-75 aluminium probe. For each reading, three replications were taken and test program was set as follows: samples were compressed to 50% (2 mm compression) of the original height (4 mm thickness); time between the compression: 5 s; post-test and testing speed: 1 mm/s.
Phytochemical parameters
Ascorbic acid content of the samples was determined using 2,4- Dichlorophenol-Indophenol dye method (Kaur et al. 2022a). Total phenolics was estimated using the Folin-Ciocalteu’s colorimetric method and the results were expressed as gallic acid equivalents (mg GAE/100 g) (Kaur et al. 2022b). Total flavonoids were estimated by the aluminum chloride assay method as mentioned by Kaur et al. (2022b) and expressed as quercetin equivalent (mg QE/100 g). Limonin content was estimated from the chloroform extract of the sample by colorimetric method as described by Aggarwal and Sandhu (2004). Antioxidant activity was determined using 1, 1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity method as explained by Kaur et al. (2022a).
Fourier transform-infrared (FTIR) analysis
Functional groups present in burfi samples were evaluated using FT-IR spectrometer Model: Nicolet-6700 (Thermo Electron Scientific Instruments Corporation, USA). All the samples were analyzed at ambient temperature and the spectra were recorded in the wave number range of 400–4000 cm−1. The spectra so obtained were interpreted as per Kaur et al. (2022b).
Microstructural characterization
Microstructure of burfi was examined using a scanning electron microscope (SEM) (Hitachi-S-3400 N; Tokyo, Japan). The gold coated burfi samples were inserted into the microscope and photographs were captured at different magnifications under an accelerating voltage of 25 kV as mentioned by Arora et al. (2007).
Microbiological analysis
Microbiological analysis of burfi (total plate count, yeast and mould count, coliform, and E. coli count) was conducted as per the procedure given by Farzana et al. (2017).
Sensory evaluation
Sensory attributes (color, flavor, texture, and overall acceptability) of burfi were evaluated using 9-point hedonic scale (1 = dislike extremely, 9 = like extremely) by a panel of 25 semi trained judges comprising of staff and students of Department of Food Science and Technology, Punjab Agricultural University, Ludhiana, India (Kaur and Aggarwal 2016). The panel contained members within the age group of 25–55 years having adequate knowledge of sensory characteristics of the product tested.
Shelf life determination
Burfi pieces (20 g each) were wrapped in butter paper and packed in polypropylene trays (150 mm × 125 mm × 25 mm). The samples were stored under room (25 ± 2 °C) and refrigerated (4 ± 2 °C) temperature conditions. Shelf stability of prepared product was assessed by analyzing the changes in physico-chemical (moisture, water activity, titratable acidity, total and reducing sugars and FFA), phytochemical (ascorbic acid, total phenols, total flavonoids, limonin, and DPPH radical scavenging activity), microbiological (total plate count, yeast and mould count and coliform count), and sensory characteristics at an interval of 7 days during storage.
Statistical analysis
All experiments were performed in triplicates and were statistically analyzed with One-way analysis of variance using the SPSS version 15.0 software. The difference in the means were analyzed using Tukey’s post hoc multiple range test at p < 0.05 (Statistical Package for Social Sciences, New York).
Results and discussion
Effect of addition of pectin on quality characteristics of different burfi formulations
In the absence of pectin, the moisture content in control burfi was 22.88% which was significantly (p < 0.05) different in comparison with kinnow burfi formulations (33.70 to 34.91%) (Table 1). The water activity of control was 0.71 (F0) which was significantly (p < 0.05) higher than kinnow burfi formulations (0.63 F3 to 0.66 F1) (Table 1) which might be due to higher initial water activity in khoa. The addition of pectin did not affect the moisture content of kinnow burfi formulations but it significantly (p < 0.05) affected the water activity (Table 1). Increasing the pectin concentration markedly reduced the water activity of kinnow burfi formulations which was probably due to increased pectin-water interaction that resulted in higher water binding capacity and fewer free water molecules (Salleh et al. 2017). All burfi formulations had moisture content in the range of 20–40% and water activity 0.6–0.9, thus can be considered as intermediate moisture dairy food (Prasad et al. 2017; Shrivas et al. 2018). Control burfi had significantly (p < 0.05) higher ash, protein, and fat content compared to kinnow burfi formulations (Table 1). These differences might be due to larger content of these constituents in khoa in comparison to kinnow juice. There was a significant (p < 0.05) difference in total and reducing sugars of control burfi and those formulated with addition of kinnow juice (Table 1). Titratable acidity also increased (0.68%) markedly (p < 0.05) with inclusion of kinnow juice compared to control (0.45%) which might be attributed to the higher acidic content of the juice. Non significant (p < 0.05) differences were observed in ash, protein, fat, total and reducing sugars, and titarable acidity for all formulated kinnow burfi samples, since the addition of pectin did not alter the content of these physico-chemical constituents in the samples. Also, no major changes were observed in phytochemical content and percent DPPH radical scavenging activity of formulated kinnow burfi samples indicating non-significant (p < 0.05) effect of addition of pectin on these parameters.
Table 1.
Effect of addition of different pectin concentrations on quality characteristics of kinnow burfi formulations
| Formulations | F0 | F1 | F2 | F3 |
|---|---|---|---|---|
| Physicochemical characteristics | ||||
| Moisture (%) | 22.88 ± 0.73b | 34.91 ± 1.36a | 34.11 ± 0.99a | 33.70 ± 0.98a |
| Water activity | 0.71 ± 0.01a | 0.66 ± 0.00b | 0.65 ± 0.01c | 0.63 ± 0.00d |
| Ash (%) | 3.18 ± 0.070a | 1.47 ± 0.04b | 1.45 ± 0.04b | 1.48 ± 0.03b |
| Protein (%) | 14.25 ± 0.60a | 7.41 ± 0.25b | 7.40 ± 0.24b | 7.48 ± 0.30b |
| Total sugars (%) | 28.61 ± 1.00b | 54.89 ± 1.65a | 54.02 ± 1.89a | 55.20 ± 2.76a |
| Reducing sugars (%) | 25.09 ± 1.13b | 21.51 ± 0.93a | 20.06 ± 1.00a | 20.95 ± 0.70a |
| Fat (%) | 19.15 ± 0.73a | 2.77 ± 0.11b | 2.77 ± 0.08b | 2.79 ± 0.10b |
| Acidity (%) | 0.45 ± 0.01b | 0.68 ± 0.02a | 0.68 ± 0.01a | 0.68 ± 0.01a |
| Free fatty acids (%) | 0.10 ± 0.01a | 0.09 ± 0.00a | 0.08 ± 0.01a | 0.09 ± 0.00a |
| Color characteristics | ||||
| L* | 66.85 ± 1.47a | 43.75 ± 0.92b | 44.71 ± 0.94b | 44.79 ± 2.11b |
| a* | 6.71 ± 0.24a | 5.69 ± 0.18b | 5.34 ± 0.15b | 5.26 ± 0.20b |
| b* | 10.33 ± 0.22b | 20.12 ± 0.23b | 19.84 ± 0.54b | 19.95 ± 0.66a |
| Texture characteristics | ||||
| Hardness (N) | 51.52 ± 3.17a | 39.65 ± 1.89c | 44.66 ± 2.60bc | 47.31 ± 0.98ab |
| Springiness (mm) | 0.09 ± 0.00b | 0.17 ± 0.01a | 0.17 ± 0.02a | 0.17 ± 0.02a |
| Gumminess (N) | 4.83 ± 0.12a | 4.14 ± 0.09c | 4.59 ± 0.10b | 4.63 ± 0.79ab |
| Chewiness (N) | 1.02 ± 0.10a | 0.72 ± 0.05b | 0.75 ± 0.05b | 0.77 ± 0.08b |
| Cohesiveness (mm) | 0.17 ± 0.01a | 0.09 ± 0.00b | 0.10 ± 0.01b | 0.11 ± 0.01b |
| Phytochemical characteristics | ||||
| Ascorbic acid (mg/100 g) | ND | 4.38 ± 0.16a | 4.50 ± 0.13a | 4.52 ± 0.13a |
| Total phenolics (mg GAE/100 g) | ND | 670.09 ± 16.19a | 636.92 ± 24.09b | 624.19 ± 26.85c |
| Total flavonoids (mg QE/100 g) | ND | 142.48 ± 3.28a | 140.62 ± 3.23a | 138.24 ± 4.47a |
| Antioxidant activity (% DPPH inhibition) | ND | 33.19 ± 1.02a | 32.76 ± 0.88a | 30.27 ± 1.06a |
| Mineral matter (mg/100 g) | ||||
| Sodium | 10.12 ± 0.40b | 26.82 ± 1.18a | 26.52 ± 1.09a | 25.80 ± 0.98a |
| Magnesium | 9.52 ± 0.38b | 114.80 ± 4.79a | 113.02 ± 4.50a | 113.40 ± 5.01a |
| Phosphorus | 85.02 ± 3.25a | 11.23 ± 0.48b | 10.02 ± 0.40b | 10.68 ± 0.35b |
| Potassium | 95.12 ± 4.20a | 81.12 ± 3.38b | 81.00 ± 3.50b | 82.00 ± 4.00b |
| Calcium | 59.00 ± 3.01a | 25.92 ± 1.20b | 24.81 ± 1.00b | 29.12 ± 1.31b |
| Manganese | ND | 3.13 ± 0.10a | 3.01 ± 0.12a | 2.67 ± 0.10b |
| Iron | 0.20 ± 0.01c | 4.59 ± 0.20a | 3.02 ± 0.12b | 4.68 ± 0.19a |
| Copper | ND | 0.10 ± 0.01a | 0.11 ± 0.01a | 0.10 ± 0.00a |
| Sensory characteristics | ||||
| Appearance | 8.44 ± 0.31a | 8.40 ± 0.30a | 8.52 ± 0.25a | 8.50 ± 0.10a |
| Texture | 8.20 ± 0.37b | 7.52 ± 0.31ab | 7.80 ± 0.18ab | 8.21 ± 0.31a |
| Flavor | 7.16 ± 0.50a | 8.28 ± 0.25a | 8.40 ± 0.30a | 8.40 ± 0.36a |
| Overall acceptability | 7.60 ± 0.25b | 7.08 ± 0.30ab | 7.80 ± 0.30ab | 8.28 ± 0.31a |
ND Not detected; Results were expressed as mean ± standard deviation; n = 3; Mean in the same row with different alphabetical letters in superscripts is significantly different (p < 0.05); Formulation F0- Control (0% pectin); Formulation F1- 2% pectin; Formulation F2-3% pectin; F3-4% Pectin
Color characteristics (L*, a* and b*) of control and kinnow burfi formulations were significantly (p < 0.05) different from each other (Table 1). Addition of kinnow juice resulted in a decrease in L* value (66.85 to 43.75) (Table 1), or a darker appearance which could be attributed to the initial color of the added kinnow juice. All the burfi samples had the hunter a* (redness) and b* (yellowness) scale on the positive side, indicating the presence of an orange hue. As a whole, color of the formulated burfi samples was determined primarily by the original color of the kinnow juice which appeared to be orange-brown. Moreover, pectin addition did not result in a noticeable change in the color coordinates of formulated samples (Table 1).
Textural properties of the formulated kinnow burfi samples were remarkably (p < 0.05) less than control burfi (Table 1). Hardness which is the most commonly evaluated parameter while determining the texture of milk products, varied significantly (p < 0.05) in control (51.52 N) and kinnow burfi samples (39.65 to 47.31 N) (Table 1). Control burfi was harder in texture which may be related to its lower moisture content (22.88%) compared to kinnow burfi (33.70–34.91%). Higher moisture content of kinnow juice gradually increased the inter-particle distance of the solids and weakened the strength of the solids, resulting in corresponding tender product (Wasnik et al. 2013). Likewise, control had higher gumminess, chewiness, and cohesiveness compared to kinnow burfi formulations (Table 1). With increase in the level of pectin, a drastic (p < 0.05) increase in the hardness of kinnow burfi samples was noticed (Table 1). Increasing the pectin concentration also increased the gumminess, chewiness and cohesiveness of the product which might be because of structural properties of pectin (Huang and Hsieh 2005).
Kinnow juice can be considered as a good source of essential minerals which are required for normal human body functioning (Mahawar et al. 2020; Purewal et al. 2021). Compared to control, mineral elements of burfi significantly increased with incorporation of kinnow juice, which was in the order of magnesium > potassium > sodium > calcium > phosphorus > iron > manganese > copper (Table 1). It is apparent that developed burfi contains key macro and micro minerals when compared with plain milk burfi and can provide appreciable amount of these elements as reported by Purewal et al. (2021).
For sensory attributes, kinnow burfi formulation (F3) received higher overall acceptability score (8.28 on a 9 point Hedonic scale) compared to control. F3 (4% pectin) was preferred on account of its proper setting with better texture and flavor. The panelists observed that formulation F1 and F2 did not hold their structure well and were very soft in texture. Hence, Formulation F3 was selected for product development and shelf life evaluation.
FTIR analysis
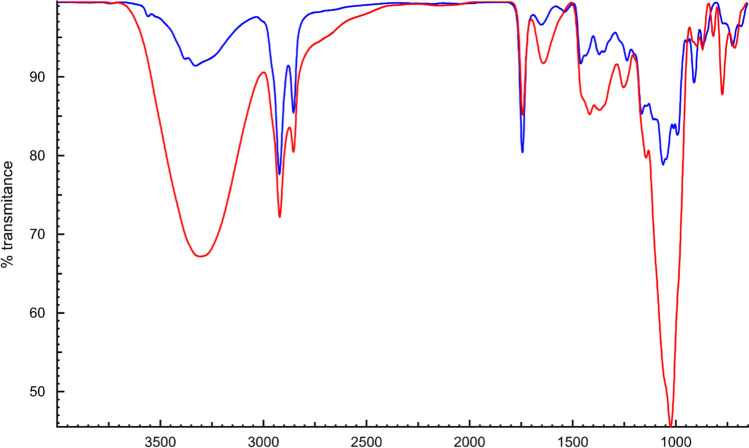
ATR-FTIR spectroscopy was used to identify the presence of functional compounds in control and kinnow burfi. (Fig. 1). FTIR spectra obtained for control and kinnow burfi exhibited different chemical structures in terms of form and intensity of major peaks; with some major peaks missing in control burfi. For Kinnow burfi, major peaks at 3273 and 3360 cm−1 represent the –OH stretching vibration which can be associated with the presence of phenols. The absorption bands between 2850–2927 cm−1 and 1631–1745 cm−1 shows aliphatic C-H stretch and C = O stretching vibration in a carbohydrate group (Singh et al. 2021a). Peaks at 1322–1365 cm−1 (CH3 asymmetrical/symmetrical stretch), 1206–1237 cm−1 (asymmetrical C–O–C stretch) and 1048–1065 cm−1 (stretching CH-CH, C–CH and C–OH) indicate the presence of phenolic compounds. Whereas C–CH stretching, C–OH observed in the absorption spectra range of 908–942, 848–867, and 723–754 confirm the presence of carbohydrates. In terms of wave number, highest peak was observed for Kinnow burfi (Fig. 1) which may explain the occurrence of bioactive components in it.
Fig. 1.
FTIR spectral (cm−1) of (A) control burfi; B kinnow burfi with 4% pectin
SEM analysis
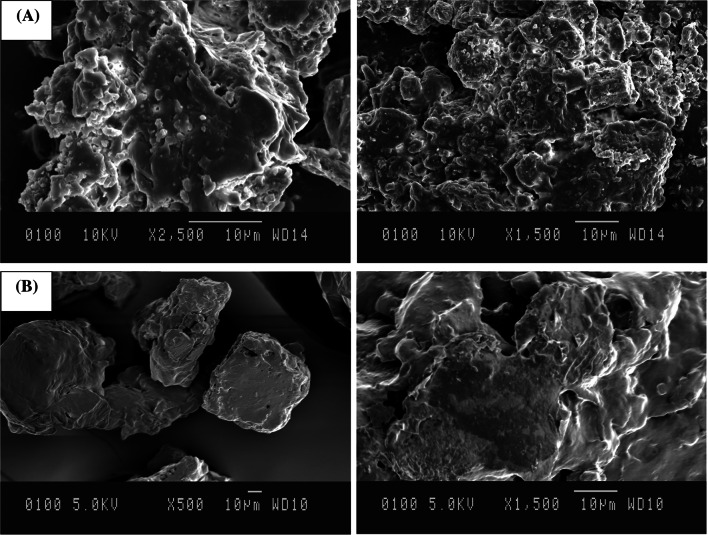
The morphology of control and kinnow burfi was analyzed by SEM (Fig. 2). Control burfi exhibited compact and well-defined globular structure (Fig. 2A), while the addition of kinnow juice resulted in the development of a loosely held or open microstructure (Fig. 2B). This difference can be ascribed to the presence of more milk solids in control sample resulting in a 3 D networking of casein micelles with very small pockets (Arora et al. 2007). The loose network in kinnow burfi formulations may explain their low hardness, cohesiveness, and accordingly gumminess and chewiness. With addition of kinnow juice, a reduction in denseness was noticed, indicating relatively soft texture compared to control (Fig. 2).
Fig. 2.
Scanning electron micrographs of (A) control burfi; B kinnow burfi with 4% pectin
Shelf life studies
Physicochemical parameters
Moisture content and water activity play a very critical role in acceptability of khoa based sweets during storage as far as microbial growth, rates of deteriorative reactions and chemical/ physical properties are concerned. Moisture content of kinnow burfi decreased significantly (p < 0.05) during 21 days of storage at both storage temperatures (Fig. 3A). However, the rate of decrease was faster (28.75%) in samples stored at low temperature than those stored at ambient conditions (10.30%) (Fig. 3A). The decrease in moisture content during refrigerated storage might be due to drying at low temperature and surface evaporation (Shrivas et al. 2018). At the end of 28 days, almost 30% moisture was lost in burfi samples stored under refrigerated conditions. With further extension of storage, burfi became very brittle and unacceptable. Shrivas et al. (2018) and Jha et al. (2014) reported decrease in moisture content in rava burfi and lal peda (milk based confection) during storage at room (30 ± 2 °C) and refrigerated (4–7 °C) conditions.
Fig. 3.
Effect of storage on physicochemical properties i.e. A moisture, B water activity, C titratable acidity, D total and reducing sugars, D Free fatty acids, F color, and phytochemical properties of kinnow burfi stored at room (G) and refrigerated (H) storage conditions; Different alphabets indicate significant (p<0.05) difference during storage
Prior storage, the water activity in kinnow burfi was 0.63. After 21 days, non-significant (p > 0.05) decreasing trend in water activity was observed at both temperatures (Fig. 3B) which might be attributed to the loss in moisture during storage. Relationship between moisture and water activity has been widely investigated (Huang and Hsieh 2005; Prasad et al. 2017).
Titratable acidity is an important physico-chemical factor which affects product quality in terms of flavor and shelf-stability. During storage, titratable acidity of kinnow burfi increased significantly (p < 0.05) irrespective of storage temperatures (Fig. 3C). However, the rate of increase was higher (1.7 times) for samples stored under room temperature conditions compared to refrigeration (1.5 times increase). The increase in acidity might be due to formation of various organic acids by degradation of reducing sugars (Bajwa et al. 2005; Londhe et al. 2012).
Sugars are the most important constituent of milk based sweets and are essential factor for color, flavor, and preservation. An upward (p < 0.05) trend was observed in reducing sugar contents of kinnow burfi during storage at both temperatures (Fig. 3D). After 21 days of storage, the average reducing sugars content was almost doubled (41.64%) compared to initial value (20.95%) which might be due to acid hydrolysis of sucrose and conversion of polysaccharides to mono-saccharides (Sharma et al. 2013). More changes in reducing sugars were observed at room temperature storage compared to low temperature (Fig. 3D). Similar pattern was reported in carrot milk cake by Bajwa et al. (2005). Likewise, an increase in total sugars content in kinnow burfi was also witnessed during the entire storage period, irrespective of storage temperatures (Fig. 3D). The rate of increase was higher (14.45%) at room temperature conditions which might be due to rapid hydrolysis of polysaccharides and inversion of non- reducing sugars at elevated conditions (Deepika et al. 2016).
Free fatty acids (FFA) plays a major role in shelf life determination of burfi and production of the free fatty acids is the best predictors of fat deterioration which could be used to monitor the extent of fat spoilage (Shrivas et al. 2018). As evident from Fig. 3E, FFA of kinnow burfi increased significantly (p < 0.05) with extension in the storage days; with higher values recorded for samples stored at room temperature. FFA increased from 0.09% to 0.17 and 0.38% after storage of 21 days at refrigerated and room temperature, respectively (Fig. 3E). As per codex standards, the maximum limit for FFA in milk fat products is 0.3 to 0.4%, above which it is rendered unfit for consumption (Codex Alimentarius Commission 2011). In this study up to 21 days, none of the samples showed FFA above the prescribed limit. However, further storage of burfi under ambient conditions exceeded the prescribed limit and thereafter the product became unacceptable due to development of rancid flavor and visible mould growth. The increase in FFA during room temperature storage may be related to the hydrolysis of fat which is primarily affected by the higher microbial growth (yeast and moulds). Secondly, the increased acidity during storage might also have made conditions conductive for fat hydrolysis (Shrivas et al. 2018). The results of increasing FFA contents during storage are well supported by previous studies on various milk based products (Gupta et al. 2010; Jha et al. 2014; Prasad et al. 2017).
Color characteristics
Color is one of the most important sensory parameter in determining the acceptability of a food product as it considerably affects consumer acceptability and its market value. As depicted in Table 2 at both the storage temperatures, all the color parameters (L*, a* and b*) changed significantly (p < 0.05) with the progression in storage period. L* value which is an indicator of lightness reduced to about 9 and 13% from the initial value in burfi samples stored at refrigerated and room temperature storage, respectively (Table 2). It was observed that burfi stored at room temperature was darker in color (Fig. 3F) which might be due to degradation of color pigments due to higher exposure to light at ambient storage temperature (Patil et al. 2015). A similar decrease in a* (redness) and b* (yellowness) values was also observed during storage (Table 2).
Table 2.
Effect of storage on the instrumental color and textural properties of kinnow burfi
| Test parameters | Storage conditions | Storage period (days) | ||||
|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | ||
| Color properties | ||||||
| L* | RF | 44.79 ± 2.11a | 42.81 ± 1.63ab | 41.10 ± 1.56ab | 40.51 ± 0.93b | 40.02 ± 0.80b |
| RT | 44.79 ± 2.11a | 41.39 ± 2.03ab | 40.03 ± 0.92b | 38.91 ± 1.28b | ||
| a* | RF | 5.26 ± 0.20a | 5.16 ± 0.23a | 5.00 ± 0.14a | 4.91 ± 0.19a | 4.83 ± 0.12a |
| RT | 5.26 ± 0.20a | 5.02 ± 019a | 4.95 ± 0.13a | 4.38 ± 0.14b | ||
| b* | RF | 19.95 ± 0.66a | 19.82 ± 0.95ab | 19.01 ± 0.44ab | 18.57 ± 0.54ab | 18.07 ± 0.48b |
| RT | 19.95 ± 0.66a | 18.86 ± 0.74ab | 18.39 ± 0.63ab | 18.27 ± 0.51ab | ||
| Textural properties | ||||||
| Hardness (N) | RF | 47.31 ± 0.98b | 51.53 ± 2.19 cd | 66.10 ± 2.00b | 79.95 ± 3.80a | 81.52 ± 3.39a |
| RT | 47.31 ± 0.98b | 48.34 ± 1.72d | 49.62 ± 2.12 cd | 55.63 ± 2.20c | ||
| Springiness (mm) | RF | 0.17 ± 0.02a | 0.09 ± 0.01bcd | 0.08 ± 0.01 cd | 0.08 ± 0.01 cd | 0.07 ± 0.00d |
| RT | 0.17 ± 0.02a | 0.15 ± 0.02a | 0.12 ± 0.01b | 0.11 ± 0.00bc | ||
| Gumminess (N) | RF | 4.63 ± 0.79a | 5.03 ± 0.50a | 5.28 ± 0.46a | 5.49 ± 0.28a | 5.88 ± 0.83a |
| RT | 4.63 ± 0.79a | 4.96 ± 0.73a | 5.01 ± 0.39a | 5.22 ± 0.70a | ||
| Chewiness (N) | RF | 0.77 ± 0.18c | 0.99 ± 0.23bc | 1.14 ± 0.18bc | 2.79 ± 0.10a | 3.01 ± 0.08a |
| RT | 0.77 ± 0.18c | 0.83 ± 0.12c | 1.06 ± 0.10bc | 1.22 ± 0.12b | ||
| Cohesiveness (mm) | RF | 0.11 ± 0.01a | 0.08 ± 0.00bc | 0.07 ± 0.00 cd | 0.06 ± 0.00d | 0.06 ± 0.00d |
| RT | 0.11 ± 0.01a | 0.09 ± 0.01b | 0.08 ± 0.01bc | 0.07 ± 0.01 cd | ||
RF Refrigeration temperature; RT Room temperature; Results were expressed as mean ± standard deviation; n = 3, Mean in the same row with different alphabetical letters in superscript is significantly different (p < 0.05)
Textural characteristics
Texture is an important parameter in burfi which affects the sensorial properties and consumer acceptability of the product. Hardness is an indicator of the most direct response to taste, directly affecting chewiness, gumminess and cohesiveness in texture profile analysis (Jha et al 2014). In the present study, an increase (p < 0.05) in hardness in burfi, irrespective of the storage temperature was observed (Table 2). The rate of increase was comparatively (p < 0.05) rapid at refrigerated conditions which could be related to continuous reduction in moisture and subsequent increase in total solids. Jha et al. (2014) and Londhe et al. (2012) established an inverse relationship between the moisture content of peda samples and their hardness values and reported that hardness increased with decrease in moisture content. Likewise, Tiwari et al. (2014) and Shrivas et al. (2018) observed continuous increase in hardness in burfi samples with the progress of storage period due to moisture loss. A significant (p < 0.05) reduction in springiness and cohesiveness was noticed in burfi, irrespective of storage temperatures (Table 2) which could be related to the loss of moisture during storage resulting in less cohesion of the matrix of kinnow burfi. The other two parameters i.e. gumminess and chewiness increased significantly during storage at both temperatures; rate of increase being faster at refrigerated temperature which could be due to increased hardness resulting from higher moisture loss at low temperature conditions. These findings are in agreement with the results of Jha et al. (2014) who observed an increase in chewiness and gumminess of brown peda during storage at 4 and 37ºC.
Phytochemical parameters and antioxidant activity
Plant foods are known for their bioactive phytochemicals which possess good antioxidant potential, therefore effect of processing and storage on these constituents is tremendously gaining ground in the field of food technology (Kaur and Aggarwal 2016).
During storage, a significant (p < 0.05) loss in ascorbic acid content in kinnow burfi was noticed at both temperatures (Fig. 3G and H). Maximum retention (88.0%) was found in burfi stored at low temperature. After 21 days of storage, ascorbic acid content showed gradual degradation of about 12.16% and 17.70% at refrigerated and room temperatures, respectively (Fig. 3G and H) which might be due to oxidation of ascorbic acid to dehydro ascorbic acid during storage (Kaur and Aggarwal 2016). Further, 84.0% ascorbic acid was retained in burfi samples during extended storage of 28 days at low temperature (Fig. 3H). Refrigerated storage reduced the rate of ascorbic acid oxidation. Djaoudene and Louaileche (2017) reported almost 76.5% retention of ascorbic acid in orange jam after 30 days of storage at 25ºC compared to jam stored at accelerated (35ºC) conditions with 70% retention.
Storage period was found to impart a profound (p < 0.05) effect on total phenolics and flavonoids content of burfi samples, the average loss reaching almost 25.0% and 33.0%, respectively of the initial content after 21 days of storage at both temperatures (Fig. 3G and H). The decrease was more prominent during room temperature storage compared to refrigeration (Fig. 3G). The decrease might be due to oxidation and hydrolysis. Al-Weshahy et al. (2013) suggested degradation of bioactive compounds to be temperature dependent with maximum decrease associated with high temperature storage.
Irrespective of storage temperature, a gradual increase in limonin content in burfi with advancing storage period was noticed (Fig. 3G and H) which might be due to conversion of limonoate A- ring lactone (non-bitter precursor) to limonin (bitter) as explained earlier. Burfi kept at refrigeration conditions recorded less increase (8.82%) in limonin compared to its counterpart with 17.8% increase. Similar findings were reported by Bhardwaj and Nandal (2014) during storage of kinnow juice.
At both storage temperatures, a significant (p < 0.05) decrease in DPPH radical scavenging activity of kinnow burfi was observed, with higher loss (24.40%) recorded at room temperature storage (Fig. 3G) compared to refrigerated (9.10%) after 21 days of storage (Fig. 3H). The decrease might be attributed to decrease in bioactive concentration during storage. Prasad et al. (2017) reported higher loss in radical scavenging activity of burfi samples to the extent of about 29.2% and 14.40% at elevated (37˚C) and low (4˚C) temperature conditions, respectively.
Microbial and sensory parameters
In the present study, microbiological analysis of the stored kinnow burfi was carried out up to 35 days at room and refrigerated temperatures (Table 3). During storage, burfi samples showed an increasing trend in total plate count at both temperatures (Table 3), however the values were within the acceptable range (FSSAI 2011). For most of the intermediate dairy desserts, such as burfi, mould growth is reported to be the prominent factor in limiting their shelf life. The yeast and mould count was not detected in fresh burfi samples and up to 14th day of refrigerated storage but appeared under room temperature storage (Table 3). After 21 days, the fungal colonies increased rapidly at ambient conditions and exceeded the limit compared to low temperature which may be related to higher acidity and FFA content observed during ambient temperature storage (Prasad et al. 2017).
Table 3.
Effect of storage on microbial and sensory quality of kinnow burfi
| Test parameters | *Permissible limits | Temperature | Storage period (days) | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | 35 | |||
| Microbiological evaluation | ||||||||
| Total plate count (cfu/g) | < 2.5 × | RF | 1.8 × 102 | 3.9 × 102 | 1.7 × 103 | 5.4 × 103 | 8.6 × 103 | 1.3 × 104 |
| RT | 1.8 × 102 | 6.1 × 102 | 2.9 × 103 | 8.2 × 103 | 1.8 × 104 | - | ||
| Yeast and mould count (cfu/g) | < 1.0 × | RF | Absent | Absent | Absent | Absent | 2.0 × 101 | 5.0 × 101 |
| RT | Absent | Absent | 3.7 × 101 | 4.8 × 101 | 1.2 × 102 | – | ||
| Coliform (cfu/g) | < | RF | < 101 | < 101 | < 101 | < 101 | < 101 | < 101 |
| RT | < 101 | < 101 | < 101 | < 101 | < 101 | - | ||
| E. coli (cfu/g) | Absent | RF | Absent | Absent | Absent | Absent | Absent | Absent |
| RT | Absent | Absent | Absent | Absent | Absent | – | ||
| Sensory evaluation | ||||||||
| Color | – | RF | 8.50 ± 0.37a | 8.40 ± 0.25a | 8.32 ± 0.30a | 7.50 ± 0.36ab | 7.10 ± 0.39b | – |
| RT | 8.50 ± 0.37a | 8.20 ± 0.40ab | 7.92 ± 0.70ab | 7.44 ± 0.59ab | ||||
| Texture | – | RF | 8.21 ± 0.62a | 8.20 ± 0.38a | 7.84 ± 0.50ab | 6.88 ± 0.38bc | 6.10 ± 0.19c | – |
| RT | 8.21 ± 0.62a | 8.20 ± 0.35a | 7.16 ± 0.48abc | 6.92 ± 0.26bc | ||||
| Flavour | – | RF | 8.40 ± 0.31a | 8.32 ± 0.50a | 7.80 ± 0.62ab | 7.00 ± 0.46bc | 6.88 ± 0.50bc | – |
| RT | 8.40 ± 0.31a | 8.32 ± 0.43a | 7.60 ± 0.50ab | 6.10 ± 0.38c | ||||
| Overall acceptability | – | RF | 8.28 ± 0.50a | 8.20 ± 0.28a | 7.84 ± 0.33ab | 7.40 ± 0.44abc | 6.28 ± 0.47c | – |
| RT | 8.28 ± 0.50a | 8.24 ± 0.62a | 7.20 ± 0.60ab | 6.84 ± 0.36bc | ||||
*As per FSSAI, (2011); < 10 indicate absence of test organisms in 1 g of sample’ RF = Refrigeration temperature; RT = Room temperature; results were expressed as mean ± standard deviation; n = 3, Mean in the same row with different alphabetical letters in superscript are significantly different (p < 0.05)
Coliform, an indicator organism used to identify hygienic conditions maintained during food processing and packaging, was absent in both fresh as well as stored burfi samples thus the developed product was considered microbiologically safe and shelf stable for at least 21 days under ambient temperature conditions. Control burfi was also assessed for microbiological safety during storage which showed very short life of about 3 days at room temperature. With incorporation of kinnow juice, shelf life of burfi was enhanced due to its high antioxidant potential and lower water activity which lowers the growth of micro-organisms.
Though micro-organisms are accountable to the food spoilage, yet food quality and consumer acceptability largely depend on sensory evaluation (Singh et al., 2021a). A decreasing (p < 0.05) trend in all sensory attributes was observed during storage, irrespective of storage temperature; changes being more rapid during room temperature storage (Table 3). Color scores decreased significantly (p < 0.05) during storage which is also supported by the hunter color values (Table 2). Higher flavor changes were observed by the panelists in kinnow burfi stored at room temperature which may be related to oxidative and physico-chemical changes, in addition to increased microbial load during room temperature storage, as mentioned in the previous sections. Regardless of the storage conditions, burfi samples showed prominent (p < 0.05) decrease in texture scores. However, the panelist found that burfi stored at low temperature was comparatively harder which is well supported by the instrumental textural analysis (Table 2). Texture of burfi stored at low temperature was acceptable (6.10 on a 9 point Hedonic scale) upto 28 days and with extended storage, the samples became very brittle and showed an increase in hardness to an unacceptable level. Based on microbial and sensory acceptability, it can be concluded that the shelf life of kinnow burfi was 21 days at room temperature which may be additionally extended upto 28 days, if the samples are stored at lower temperature conditions. Similar results were found for rava burfi, where faster deteriorative sensorial changes occurred at 30 ± 2 °C while the same product had a shelf life of 35 days without any sign of fungal spoilage at lower temperature (7 ± 2 °C) (Shrivas et al. 2018).
Conclusion
In this study, phytonutrients rich kinnow burfi with enhanced functionality and extended shelf life was developed. Developed burfi was found rich in minerals and phytochemical constituents compared to traditional milk burfi. The shelf life of burfi increased manifolds (upto 21 day) at room temperature compared to control which was shelf stable for only 4 days. Developed burfi also remained organoleptically acceptable at refrigerated storage for up to 28 days; however the texture was unacceptable due to increased hardness during extended storage. The enhanced shelf life of burfi may increase its commercial viability for export in national and international markets. Moreover, valorisation of kinnow may be helpful in coping with seasonal availability and minimizing the market glut.
Acknowledgements
Authors acknowledge the support and facilities provided by the authorities of Punjab Agricultural University, Ludhiana, Punjab, India.
Abbreviations
- TSS
Total soluble solids
- FFA
Free fatty acids
- SEM
Scanning electron microscopy
- FTIR
Fourier transform-infrared spectroscopy
- DPPH
1, 1-Diphenyl-2-picrylhydrazyl
Author contributions
SK: Conceptualization, Methodology, Investigation, Validation, Writing-original draft; PA: Conceptualization, Resources, Supervision, Writing-review, and editing; NK: Methodology, Investigation, Software, Validation, Writing-original draft, review and editing.
Funding
This research did not receive any specific grant from funding agencies.
Data Availability
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
There is no conflict of interest pertaining to this manuscript.
Ethical approval
Not applicable
Consent to participate
All of three authors are aware of the submission and have approved it.
A statement about originality of work
This manuscript describes original work and is not under consideration by any other journal.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sukhpreet Kaur, Email: sukhpreetnagra1@gmail.com.
Navjot Kaur, Email: navjotkkingra@gmail.com.
References
- Aggarwal P, Sandhu KS. Effect of hydrocolloids on the limonin content of Kinnow juice. J Food, Agric Environ. 2004;2:44–48. [Google Scholar]
- Al-Weshahy A, El-Nokety M, Bakhete M, Rao V. Effect of storage on antioxidant activity of freeze-dried potato peels. Food Res Int. 2013;50:507–512. doi: 10.1016/j.foodres.2010.12.014. [DOI] [Google Scholar]
- AOAC (2019) Official Methods of Analysis of AOAC International. AOAC International
- Arora S, Singh VP, Yarrakula S, et al. Textural and microstructural properties of burfi made with various sweetners. J Texture Stud. 2007;38:684–697. doi: 10.1111/j.1745-4603.2007.00120.x. [DOI] [Google Scholar]
- Bajwa U, Gupta M, Sandhu KS, et al. Changes in physico-chemical, sensory and microbiological characteristics during storage of carrot-milk cake in various packages. J Food Sci Technol. 2005;42:119–126. [Google Scholar]
- Bhardwaj R, Nanda U. Effect of storage temperature on physico-chemical and sensory evaluation of Kinnow Mandarin juice blends. J Food Process Technol. 2014 doi: 10.4172/2157-7110.1000361. [DOI] [Google Scholar]
- Codex Alimentarius Commission (2011) Milk and Milk Products Second edition Milk and Milk Products
- Deepika PP, Marakand DS, Thakur PK. Effect of packaging on quality of enriched fruit bars from aonla (Emblicaofficinalis G.) during storage. Int J Agric Environ Biotechnol. 2016;9:411. doi: 10.5958/2230-732x.2016.00053.x. [DOI] [Google Scholar]
- Djaoudene O, Louaileche H. Effect of storage time and temperature on the nutritional quality of commercial orange jam. SDRP J Food Sci Technol. 2017 doi: 10.25177/jfst.1.2.5. [DOI] [Google Scholar]
- Farzana T, Mohajan S, Saha T, et al. Formulation and nutritional evaluation of a healthy vegetable soup powder supplemented with soy flour, mushroom, and moringa leaf. Food Sci Nutr. 2017;5:911–920. doi: 10.1002/fsn3.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FSSAI (2011) Food Safety and Standards (Food products standards and food additives Regulations, 2011). https://www.fssai.gov.in/upload/uploa dfiles/files/Food_Additives_Regulations.Pdf. Accessed 10 Sept 2020 Publisher’s
- Gupta V, Vijayalakshmi NS, Ashwini B, et al. Shelf life enhancement of coconut burfi - an Indian traditional sweet. J Food Qual. 2010;33:329–349. doi: 10.1111/j.1745-4557.2010.00312.x. [DOI] [Google Scholar]
- Huang X, Hsieh FH. Physical properties, sensory attributes, and consumer preference of pear fruit leather. J Food Sci. 2005 doi: 10.1111/j.1365-2621.2005.tb07133.x. [DOI] [Google Scholar]
- Jha A, Kumar A, Jain P, et al. Physico-chemical and sensory changes during the storage of lal peda. J Food Sci Technol. 2014;51:1173–1178. doi: 10.1007/s13197-012-0613-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Aggarwal P. Development and quality characteristics of nutritionally enhanced potato legume based wari- an Indian traditional savoury. J Food Sci Technol. 2016;53:1899–1908. doi: 10.1007/s13197-015-2123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur N, Aggarwal P, Kaur N, Kaur S. Nutritional improvement of bhujia by incorporating colored bell peppers. J Food Process Preserv. 2022 doi: 10.1111/jfpp.16569. [DOI] [Google Scholar]
- Kaur N, Aggarwal P, Kumar V, Kaur S. Influence of different extraction techniques on the extraction of phytochemicals and antioxidant activities from Syzygium cumini (jamun) pomace using Taguchi orthogonal array design: a qualitative and quantitative approach. Biomass Convers Biorefinery. 2022 doi: 10.1007/s13399-022-02826-1. [DOI] [Google Scholar]
- Londhe G, Pal D, Raju PN. Effect of packaging techniques on shelf life of brown peda, a milk-based confection. LWT - Food Sci Technol. 2012;47:117–125. doi: 10.1016/J.LWT.2011.12.025. [DOI] [Google Scholar]
- Mahawar MK, Jalgaonkar K, Bibwe B, et al. Post-harvest processing and valorization of Kinnow mandarin (Citrus reticulate L.): a review. J Food Sci Technol. 2020;57:799–815. doi: 10.1007/s13197-019-04083-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil RV, Sawant PJ, Sawant DN, Todkar SR. Physicochemical analysis and sensory evaluation of burfi enriched with dried date. J Anim Res. 2015;5:131–134. doi: 10.5958/2277-940X.2015.00022.4. [DOI] [Google Scholar]
- Prasad W, Khamrui K, Sheshgiri S. Effect of packaging materials and essential oils on the storage stability of Burfi, a dairy dessert. J Packag Technol Res. 2017;1:181–192. doi: 10.1007/s41783-017-0018-x. [DOI] [Google Scholar]
- Purewal SS, Punia S, Kaur P, et al. Unraveling the efficacy of different treatments towards suppressing limonin and naringin content of Kinnow juice: an innovative report. Lwt. 2021;152:112341. doi: 10.1016/j.lwt.2021.112341. [DOI] [Google Scholar]
- Rasane P, Jha A. Sensory and texture profile analysis of Burfi samples manufactured in Varanasi. J Dairy Foods Home Sci. 2012;31:168–172. [Google Scholar]
- Salleh RM, Ying TL, Mousavi L. Development of fruit bar using Sapodilla (Manilkara zapota L.) J Food Process Preserv. 2017;41:1–7. doi: 10.1111/jfpp.12806. [DOI] [Google Scholar]
- Sharma SK, Chaudhary SP, Rao VK, et al. Standardization of technology for preparation and storage of wild apricot fruit bar. J Food Sci Technol. 2013;50:784–790. doi: 10.1007/s13197-011-0396-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivas AA, Pinto SV, Patel SM, Balakrishnan S. Effect of storage on composition, physico-chemical, rheology, sensory and microbiological quality of Indian cookie Rava Burfi. J Appl Nat Sci. 2018;10:88–97. doi: 10.31018/jans.v10i1.1585. [DOI] [Google Scholar]
- Singh R, Kaur S, Aggarwal P. Exploration of potato starches from non-commercial cultivars in ready to cook instant non cereal, non glutinous pudding mix. LWT - Food Sci Technol. 2021;150:111966. doi: 10.1016/j.lwt.2021.111966. [DOI] [Google Scholar]
- Singh R, Kaur S, Sachdev PA. A cost effective technology for isolation of potato starch and its utilization in formulation of ready to cook, non cereal, and non glutinous soup mix. J Food Meas Charact. 2021;15:3168–3181. doi: 10.1007/s11694-021-00887-w. [DOI] [Google Scholar]
- Tiwari S, Chetana R, Puttaraju S, Khatoon S. Physico-chemical characteristics of burfi prepared by using medium chain triglyceride rich margarines. J Food Sci Technol. 2014;51:136–141. doi: 10.1007/s13197-011-0478-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasnik PG, Nikam PB, Dhotre AV, et al. (2013) Physico-chemical and textural properties of Santra burfi as influenced by orange pulp content. J Food Sci Technol. 2013;522(52):1158–1163. doi: 10.1007/S13197-013-1044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.