Abstract
In this study, phosphorylation effects on the monosaccharide composition, structural attributes, morphology and radical-scavenging activities of Sanchi (Panax notoginseng) flower polysaccharides were investigated. Sanchi flower phosphorylated polysaccharides mainly comprised of Man, Rha, GluA, GalA, Glu, Gal and Xyl, but lacked GluN, Rib, Arab and Fuc in their compositions. FTIR analysis of phosphorylated polysaccharides showed an emergence of new absorption peak around spectral region of 1254 cm−1. NMR and FTIR analyses were indicative of the successful phosphorylation of the Sanchi flower polysaccharides. The introduction of phosphate groups into polysaccharides led to the induction of pore-like structures in polysaccharides configuration. Phosphorylation of polysaccharides led to concentration-dependent increasing tendencies in radical-scavenging activities. These findings demonstrated the positive impact of phosphorylation on Sanchi flower polysaccharides, which could potentially be used as a therapeutic agent.
Keywords: Sanchi polysaccharide, Phosphorylated, Antioxidant activity
Introduction
Sanchi (Panax notoginseng), which belongs to the genus Panax, is commonly termed as Chinese ginseng (Wu et al. 2021). The genus of Sanchi plant is same as that of Panax ginseng, commonly called Korean or Asian ginseng, which usually refers to the root that is widely known for its medicinal significance and health benefits (Peng et al. 2018; Zhang et al. 2018). Likewise, Sanchi has also been widely used for centuries as a traditional medicine in several Asian countries, particularly in China. Apart from root and leaves, flowers of this plant have also been utilized in medicinal formulations for disease prevention or treating cerebrovascular or cardiovascular ailments (Wu et al. 2021; Zhang et al. 2018). In recent years, Sanchi has received increased attention due to its pharmacological significance and more than 200 constituent compounds have been identified. Among Sanchi plant parts, root has been extensively studied, while other plant parts, such as flower buds and shoots have yet to be explored for their pharmacological activities and functional properties. Nonetheless, Sanchi flower has been reported to exhibit several therapeutic effects, such as anticancer, anti-inflammatory, hepato-protective, antimicrobial and antioxidant activities. It has also been reported to reduce tumor formation in special cancer types, such as colorectal cancer. The aforementioned effects of Sanchi flower have been attributed to the rich phytochemical profile comprising saponins contents (major constituent), volatile oils, flavonoids, and polysaccharides. As compared to Sanchi root, high content of saponins in Sanchi flower has been reported in published literature. Owing to these phytochemicals, Sanchi flower has been used to lower blood pressure, promote natural killer (NK) cell activity as well as to induce sleep (Wu et al. 2021; Zhang et al. 2018).
As biological macromolecules, the polysaccharides are composed of simple sugar units attached to each by means of glyosidic linkages. Natural polysaccharides are usually classified in terms of their origin, such as from plants (starch, cellulose, galactomannas, pectin, mucilage, and gums), animals (chitosan, chitin, hyaluronic acid and glycosaminoglycans), algae (galactans, alginates, carrageenan and agar), bacteria (dextran, xanthan, cellulose, gellan and levan) and fungi (chitin, chitosan, yeast glucans, pollulan and elsinan) (Xiong et al. 2019). Some of natural polysaccharides like starch, cellulose and chitin have commercial significance in several markets around the globe, ranging from paper production to industrial applications in food and nutraceutical manufacturing (Xie et al. 2020a; Yang et al. 2021; Torres et al. 2019). Moreover, natural polysaccharides have been reported to exhibit several multifaceted biological activities, such as antioxidant, anti-coagulant, antiviral, anti-tumor, immune-modulating, antihepatotoxic and antihyperlipidemic properties (Torres et al. 2019). Studies have shown that polysaccharides with good antioxidant properties help in scavenging of DPPH free radicals, hydroxyl ions and hydrogen peroxide, thereby protecting RAW 264.7 macrophages against oxidative stress (Costa et al. 2010; Tseng et al. 2008). Although, polysaccharides have been reported to exhibit numerous bioactivities, however, it is necessary for the polysaccharides to provide excellent immunomodulation with high therapeutic or preventive action, thereby necessitating further improvement of the polysaccharides (Xie et al. 2020b) as polysaccharides bioactivities are linked closely to the polymer’s molecular characteristics, such as aqueous solubility, chain conformation, molecular weight and monosaccharide composition (Xie et al. 2020a).
The molecular modification of polysaccharides is an effective way to alter the biological activities and molecular properties via the derivatization of functional groups, such as phosphate, sulfate, and acetyl groups (Xie et al. 2016). Among the different chemical modifications, phosphorylation has potential to improve the anti-tumor activity of polysaccharides (Deng et al. 2015). Nevertheless, the chemical modification of polysaccharides may cause the charged groups to exert significant influence on chain conformation and polysaccharides’ molecular weight, which in turn improve the biological activities (Xie et al. 2020a). Moreover, modification of the polysaccharides through phosphorylation might lead to the introduction of phosphate bonds into polysaccharides configuration. As phosphate radical has valency of three negative charges, it may enhance the electronegativity, which may consequently influence polysaccharides bioactivities (Xie et al. 2020a; Yang et al. 2021). To the best of our knowledge, there are no published reports available on the phosphorylated modification of Sanchi flower polysaccharides and influence on structural characteristics. In addition, the biological activities of phosphorylated derivatives of Sanchi flower were evaluated.
Materials and methods
Materials, chemicals and instruments
Sanchi flowers were obtained from a local superstore in Jilin City (coordinates: 43.8378° N, 126.5494° E), China. All the chemicals and reagents used for analytical purposes were assured to be of analytical grade. The instruments employed in this study comprised of electronic balance (SamHing Electronic Scale, SamHing Scales Factory Ltd., Hong Kong), water tank with digital display of electro-thermal constant temperature, rotatory evaporator, UV–Visible spectrophotometer, desktop refrigeration centrifuge, electro-thermal vacuum dryer and infrared spectrometer.
Preparation of Sanchi flower polysaccharides
Fresh flowers were washed and placed in a hot air oven (202-1, Taisite Instrument Co., Ltd, Tianjin, China) with the temperature set below 45 °C (40–43 °C ± 1). The oven-dried Sanchi flowers were crushed followed by sieving through a 50-mesh sieve to obtain powder of uniform particle size. Sanchi flower powder was mixed with the distilled water equivalent to mixing ratio of 1:30 g/mL. The reaction mixture was subjected to ultrasonic cleansing (sonication power, 100 W) using ultrasonic bath at an operational temperature of 70 °C for 60 min. Following sonication treatment, the solution was transferred to a 50 mL centrifuge tube and centrifuged (TD5A-WS, Xianglu Centrifuge Apparatus Co., Ltd., Changsha, China) at 3000 rpm for 10 min. Subsequently, the supernatant was filtered and then concentrated using the rotary evaporator (Heidolph, Germany) at 40 °C. Then, the freshly prepared reagent (prepared with dichloromethane and n-butanol in ratio of 5:1 was added to the solution concentrate for deproteinization. Then, the thoroughly-mixed solution was poured slowly into a separate funnel. The solution was allowed to stand for certain time interval (20 min) and then the upper layer that appeared on solution’s surface was taken out. Then, anhydrous ethanol was added in a proportionate volume ratio (1:3) to upper layer of solution and then the solution was allowed to stand overnight. Then, the solid residue was dissolved in the water, and then the solution was transferred into a dialysis bag, dialyzed against tap water (flowing water) for 3 d and again dialyzed against distilled water for 2 d. The precipitate was poured into a 50 mL centrifuge tube and allowed to centrifuge at 3000 rpm for 10 min. The supernatant was collected, separated and the obtained solid precipitate was subjected to oven drying (202-1, Taisite Instrument Co., Ltd., Tianjin, China) at a constant temperature of 40 °C. After oven-drying, the final Sanchi flower polysaccharide was obtained.
Preparation of phosphorylated polysaccharides of Sanchi flower
Sanchi flower polysaccharide (in 1:10 solid-to-liquid ratio) was weighed and solution was prepared by mixing with the distilled water by keeping in the water bath (HHS, Boxun Co., Ltd., Shanghai, China at constant temperature (80 °C) to achieve full dissolution of the solution. The above-mentioned Sanchi polysaccharide solution was taken into the 250 mL round bottom flask. Another solution mixture was prepared by adding with the 5% sodium polyphosphate and 1% sodium hexametaphosphate followed by thorough mixing to constant ratio of 5:1 by volume. After mixing, the solution’s reaction mixture was poured into the round bottom flask followed by stirring fully. The evenly mixed solution of 5% sodium polyphosphate and 1% sodium hexametaphosphate was then mixed with the Sanchi polysaccharide solution. Then, the round bottom flask was put into the water bath at 80 °C for 4 h. During incubation, constant monitoring of the pH was carried out (pH meter: (SYD-251, Jingsheng Instrument Co., Ltd., Shanghai, China) and maintained at pH of 7. Subsequently, the reaction mixture of solution was transferred to a beaker and sufficient amount of the absolute ethanol was added in the volume ratio of 1:1. Then, the reaction mixture was allowed to stand overnight. Supernatant was then collected and centrifuged (50 mL tube) at 3000 rpm. The solid matter was dissolved by adding distilled water, and the solution was transferred to a dialysis bag, dialyzed against tap water (flowing water) for 3 d, and dialyzed against distilled water for 2 d. Then, the solution was placed into an incubator (202-1, Taisite Instrument Co., Ltd., Tianjin, China) and dried at 30 °C and the final phosphorylated Sanchi flower polysaccharide was obtained.
Determination of monosaccharide compositions
The released monosaccharides were labeled with 1-phenyl-3- methyl-5- pyrazolone (PMP). Briefly, each sample (2 mg) was dissolved in 1 mL of 2 mol/L trifluoroacetic acid (TFA), and hydrolyzed under a sealed condition at 120 °C for 2 h in a hydrothermal reactor. Then, the residue was washed by co-evaporation using ethanol to remove the excessive TFA. 1-phenyl-3-methyl-5-pyrazolone (PMP) was prepared in the methanol solvent and 200 μL of methanolic PMP solution (0.5 mol/L) was mixed with the hydrolysate solution. Then, the mixing of 200 μL of NaOH solution (0.3 mol/L) was carried out. Then, the solution mixture was incubated for 60 min at 70 °C. The reaction was quenched by neutralizing with 200 μL of HCl (0.3 mol/L). Finally, the product was extracted (thrice) with chloroform (1 mL). The aqueous layer containing PMP-labeled derivative was filtered through a 0.45 μm membrane filter before further HPLC analysis. The Dionex Thermo Ultimate 3000 HPLC system (Dionex Co., CA, USA) equipped with an Ultimate 3000 diode array detector (DAD, Thermo Fisher Scientific, Waltham, MA, USA) was employed to detect PMP-labeled monosaccharides. The mobile phase was a mixture of acetonitrile (A) and 0.1 mol/mL phosphate buffer solution (PBS, pH 6.7) in a ratio of 82:18 (v/v). With 20 μL of injection volume, the samples were analyzed on a Supersil ODS2 column (5 μm, 4.6 × 250 mm2) at 30 °C with 0.8 mL/min of flow rate and 245 nm detection wavelength. Different types of monosaccharides were used as reference standards (mannose, ribose, rhamnose, glucuronic acid, galacturonic acid, glucose, galactose, xylose, arabinose and fucose).
Fourier transform infrared (FTIR) spectroscopy of phosphorylated polysaccharides
The samples of the Sanchi flower polysaccharides were dried (202-1, Taisite Instrument Co., Ltd., Tianjin, China). The dried samples of polysaccharides and their derivatives were ground in a mortar to form powders, which were pressed with the KBr pallets and subjected to spectrometric analysis through FTIR spectrometer (FTIR/NIR 400, Perkin–Elmer Inc., Waltham, MA, USA). The IR absorption spectrum of Sanchi flower polysaccharide powder was detected in the IR spectra ranging from 500 to 4000 cm−1.
Nuclear Magnetic Resonance (NMR) analysis of polysaccharides
As described by Chen et al. (2021), the Sanchi flower polysaccharide solution (50 mg/mL) was prepared using 99.99% deuterated water (D2O2) as a solvent. Bruker DPX NMR spectrometer (Bruker Instruments, Rheinstetten, Germany) was employed for recording of 31P NMR spectra of Sanchi flower polysaccharides.
Scanning electron microscopic analysis of polysaccharides
Polysaccharides derivatives as well as phosphorylated polysaccharides of dried Sanchi flower were prepared with a thin gold film to carry out using environment scanning electron microscopic (ESEM, Quanta 250 FEG, FEI Company, USA), at the magnification of 500 and 1000x.
In vitro radical scavenging activities analyses
DPPH radical-scavenging activity (RSA) assay
The radical-scavenging activities of polysaccharides and phosphorylated polysaccharides of Sanchi flower were determined according to the reported method of Huang et al. (2018) with some modifications. The anhydrous ethanol was mixed with the DPPH solution (1 mM) to prepare the fresh solution prior to carrying measurements and incubated under darkness. Aqueous polysaccharides samples were taken in an amount of 0.5 mL and thorough mixed with DPPH solution (2 mL) and ultrapure water (1.5 mL). Then, the solution was incubated under darkness for 30 min. The samples were analyzed for the DPPH-RSA spectrophotometrically (UV-1800, Shimadzu Instruments Mfg. Co., Ltd., Kyoto, Japan) at a detection wavelength of 517 nm against ascorbic acid as a positive control.
Hydroxyl-RSA assay
The hydroxyl-RSA of the polysaccharides and phosphorylated polysaccharides of Sanchi flower was determined as per the reported method of Xie et al. (2020a) with slight modifications. Briefly, reaction mixture containing Sanchi flower polysaccharides was prepared. This reaction mixture was further mixed with 0.1% H2O2 (1 mL) and 1.5 mM FeSO4 (1 mL). Then, after thorough mixing, the solution mixture was allowed to stand at 37 °C temperature of 10 min. Afterwards, 6 mM salicylic acid–ethanol solution (1 mL) was added to the reaction mixture and then allowed to stand for a time interval of 30 min at 37 °C. The samples were analyzed for the hydroxyl-RSA spectrophotometrically (UV-1800, Shimadzu Instruments Mfg. Co., Ltd., Kyoto, Japan) at a detection wavelength of 510 nm, whereas the ascorbic acid (vitamin C) was employed as a positive control. The hydroxyl-RSA was calculated as per the equation given below:
where A0 is denoted for the absorbance of the standard control group while A1 for the absorbance of the samples.
ABTS-RSA assay
The polysaccharides and phosphorylated polysaccharides of the Sanchi flower were analyzed for ABTS-RSA based on a method by Li et al. (2018a, b). In brief, 2.6 mM potassium persulfate solution was subjected to through mixing with 7.4 mM ABTS solution. The thoroughly mixed solution was allowed to stand for a time ranging from 12 to 16 h at room temperature under darkness and then subjected to dilutions 20–40 times followed by measurement of the absorbance using a spectrophotometer (UV-1800, Shimadzu Instruments Mfg. Co., Ltd., Kyoto, Japan) at an absorbance wavelength of 734 nm, whereas the ascorbic acid (vitamin C) was employed as a positive control.
Statistical analysis
All the experiments were carried out in triplicate and results were documented in terms of mean ± S.D. The difference between the means were calculated using the Duncan multiple range test and statistical analysis was performed using Microsoft Excel and SPSS 20.0 (IB, USA) at a significant level of p < 0.05.
Results and discussion
Monosaccharide composition analysis of polysaccharides
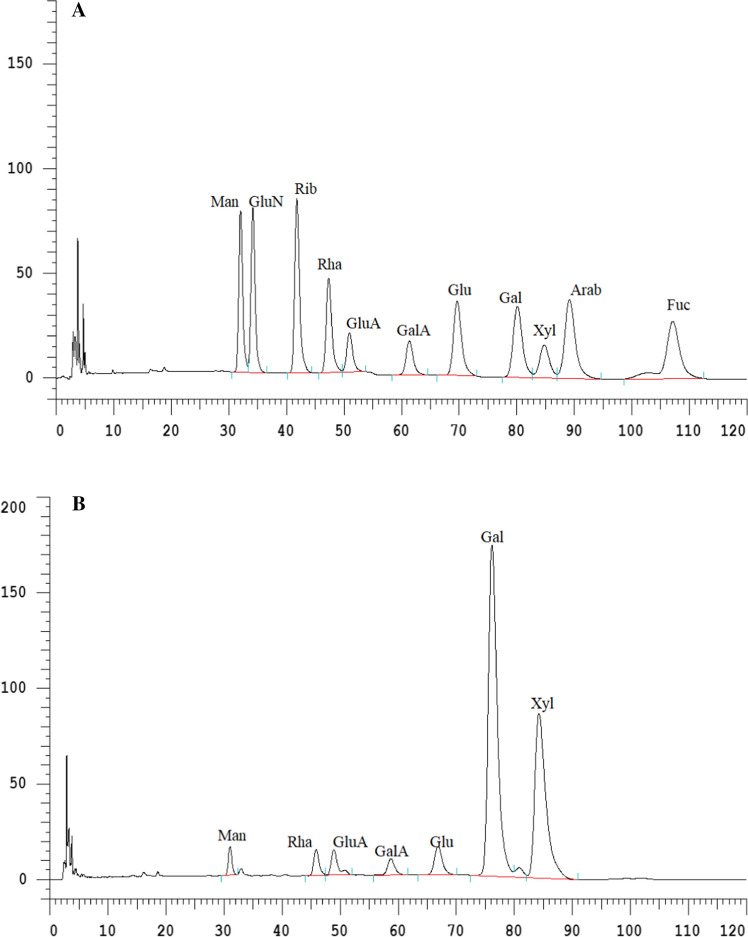
The results of HPLC-based analysis of monosaccharide composition of the Sanchi flower polysaccharides as well as phosphorylated polysaccharides are shown in Fig. 1A, B, respectively. Molar ratios of monosaccharide components of Sanchi flower are shown in Table 1. The monosaccharides, mannose (Man), Rhamnose (Rha), galacturonic acid (GluA), gluconic acid (GalA), glucose (Glu), galactose (Gal), xylose (Xyl) exhibited molar ratios of 1.0, 2.11, 1.7, 1.28, 2.52, 21.57 and 17.68, respectively. The phosphorylated polysaccharides of Sanchi flower exhibited heteropolysacchrides, which mainly comprised of Man, Rha, GluA, GalA, Glu, Gal and Xyl with retention times of 32.05, 47.35, 50.93, 61.37, 69.63, 80.10 and 84.77 min, respectively. However, the phosphorylated polysaccharides did not show the presence of GluN, Rib, Arab and Fuc, while these monosaccharides were present in non-phosphorylated polysaccharides. As shown in Table 1, in phosphorylated Sanchi flower polysaccharides, Gal (21.57 mg/g) and Xyl (17.68 mg/g) showed the highest concentrations, whereas these two monosaccharides were found to be considerably lower in non-phosphorylated polysaccharides. Conversely, Man, Rha, GluA and GalA showed significantly (p < 0.05) higher quantities in the case of polysaccharide samples as compared to phosphorylated polysaccharide samples. This could be due to the effect of phosphorylation, which causes the introduction of phosphate linkages into the polysaccharide’s configuration. Owing to the three negative charges of valency of phosphate radical, the increased degree of electronegativity could possibly exert influence on the physicochemical properties and biological activities. Similar results were reported by Mei et al. (2020), who reported that β-D-glucan and its phosphorylated derivative were of high purity and exhibited successful substitution. These results were in agreement with the findings reported by the Xie et al. (2020a), wherein authors concluded that phosphorylated modification of Cyclocarya paliurus polysaccharides caused significant changes in the monosaccharide composition of polysaccharides.
Fig. 1.
HPLC chromatograms of Sanchi flower monosaccharide of polysaccharides (A) and (B) monosaccharide of phosphorylated polysaccharides
Table 1.
Molar ratio of monosaccharide components of Sanchi (Panax notoginseng) flower
| Man | GluN | Rib | Rha | GluA | GalA | Glu | Gal | Xyl | Arab | Fuc |
|---|---|---|---|---|---|---|---|---|---|---|
| 1.00 | – | – | 2.11 | 1.7 | 1.28 | 2.52 | 21.57 | 17.68 | – | – |
FTIR analysis of polysaccharides from Sanchi flower
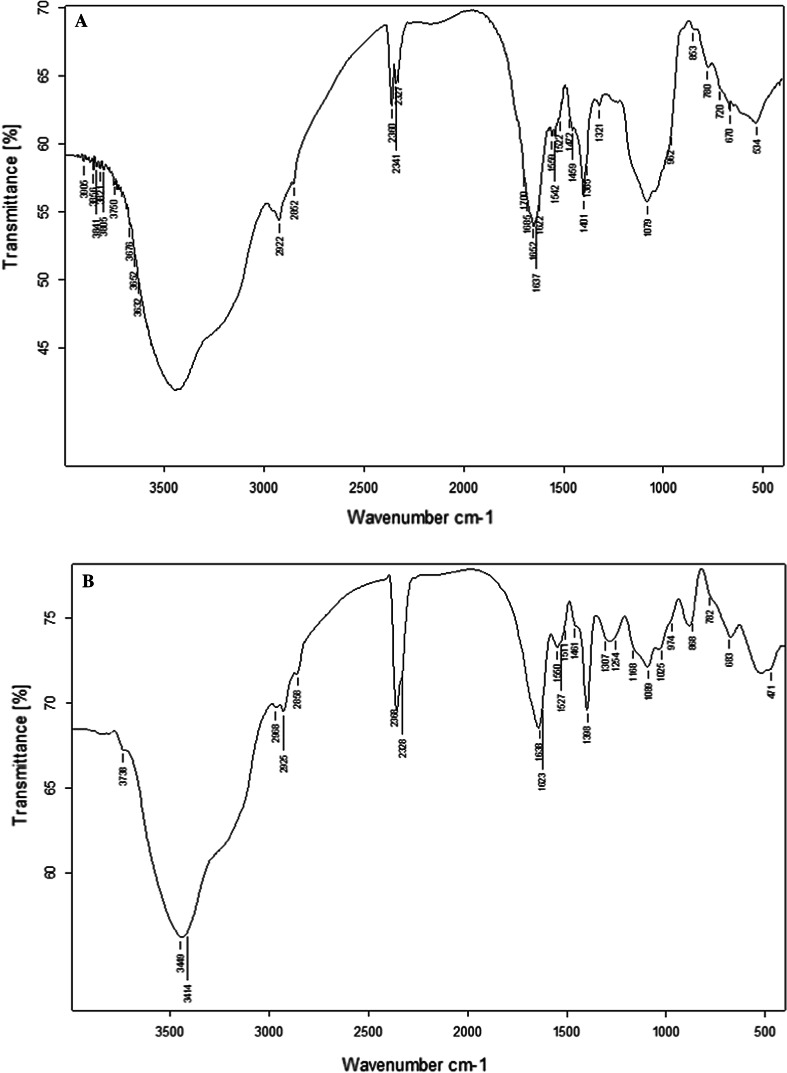
The results of FTIR spectroscopic analysis of Sanchi flower polysaccharides and phosphorylated polysaccharides are illustrated in Fig. 2A, B, respectively. It is evident from the Fig. 2 that the characteristic wider peak existing in the IR regions of 3414 and 3449 cm−1 correspond to the absorption peak of −OH stretching vibration (Ramachandraiah and Chin 2017). Polysaccharide molecules has been reported to exhibit several hydroxyl groups in their configuration and the IR hydroxyl (O–H) peak widening might be ascribed to the intermolecular and intramolecular hydrogen linkages. The characteristic peak in the IR region at 889 cm−1 was evident of the particular absorption peak of angular vibration as characteristic of C–H stretching of β-D-pyranose. This implied that that the component of the Sanchi flower polysaccharides comprising of β-D-pyranose ring and the characteristic molecule was linked through the β-glycosidic bond. Furthermore, the IR absorption peaks in the spectral regions of 1254 cm−1, 1373 cm−1 and 2923 cm−1 were evident of the presence of β-(1 → 3) linkage. IR spectral regions at 2925 and 2968 cm−1 corresponded to the absorption peak of vibrational stretches of C–H group, whereas, the absorption peaks at 1527 and 1638 cm−1 indicated the bending vibrations of hydroxyl (O–H) vibrations. The IR absorption peaks at IR spectral regions of 1398 and 1461 cm−1 were ascribed to carboxyl (C–O) stretching vibration. The IR peak at 974 cm−1 was the characteristic absorption peak of β-glycosidic linkage. The three consecutive absorption peaks at IR regions of 1168, 1089 and 1025 cm−1 was indicative of the probable presence of pyran rings in the phosphorylated Sanchi flower polysaccharides. Moreover, the IR vibration pattern at spectral region of 782 cm−1 was because of C–O–C vibrational stretching owing to the presence of D-pyranose ring (Yang et al. 2021). It was evident from the results of IR spectroscopy that polysaccharides prior to and after exposure to phosphorylation exhibited quite similar patterns in terms of absorption peak intensities, wave number, peak width and absorption waveform. The IR absorption peak specific to the β-chain was not found at the IR region of 890 cm−1. Furthermore, the absorption peaks at IR regions of 1254 and 2919 cm−1 corresponded to the CH2OH and C–H absorption peaks which showed weaker peaks, which might be possible because of the substitution during phosphorylation of phosphoric acidic group. Moreover, the absorption peaks occurring in range of 1403 and 1254 cm−1 showed great variability because of substitution reaction and absorption peak developed owing to P=O substitution linkage (Mei et al. 2020). However, the emergence of new absorption peak around 1254 cm−1 in the FTIR spectra of phosphorylated polysaccharides could be attributed to the stretching vibration caused by P=O existing in the phosphate group (Yang et al. 2021). This implied the successful introduction of phosphate linkages into the polysaccharide’s configuration of Sanchi polysaccharides. Similar results were observed in a previous by Chen et al. (2021) on phosphorylated polysaccharides.
Fig. 2.
FTIR spectrogram of Sanchi flower polysaccharide (A) and phosphorylated polysaccharide (B)
31P NMR of phosphorylated polysaccharide from Sanchi flower
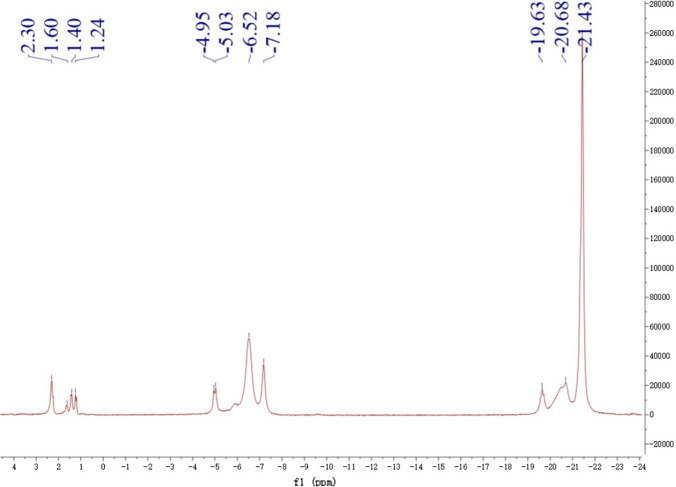
Phosphorylated polysaccharides of Sanchi flower were studied and 31P NMR spectrum are demonstrated in Fig. 3. As the phosphate group belongs to the category of electron absorber group, the NMR spectrum showed a clear shift of C1–C6 owing to the possible introduction of phosphate group, whereas the number of split peaks of each C exhibited a reducing tendency. NMR analysis in conjunction with that of FTIR spectra was indicative of the successful phosphorylation of the Sanchi flower polysaccharides. It was evident from the results of NMR spectra of phosphorylated polysaccharides that the three groups of peaks were mainly observed in the NMR spectra, which implied that phosphorylation caused introduction of phosphate groups into the structural configurations of Sanchi flower polysaccharides by replacing three hydroxyl (O–H) groups. Furthermore, the intensity of the peak belonging to first groups was the highest at − 21.43 ppm indicating that this position was the easiest to be replaced. The first group exhibiting triple peaks showed chemical shifts of − 21.43, − 20.68, and − 19.63 ppm whereas the second group of triple peaks exhibited peaks at − 7.18, − 6.52 and − 0.4.95. Conversely, third group of quadruple peaks showed intensity peaks at 1.24, 1.40, 1.60 and 2.30 ppm. Similar results were reported by Mei et al. (2020), who reported that peaks presence at 61.38 ppm, 68.83 ppm, 73.28 ppm, 76.92 ppm, and 86.98 ppm might be possibly evident of the carbon positions of C6, C4, C2, C5 and C3. This was also consistent with the structural configuration of (1 → 3)-β-D-glucan which comprises of 6 carbon positions. Moreover, in case of phosphorylated (1 → 3)-β-D-glucan, several peaks at 3.8 ppm, 2.9 ppm and 1.8 ppm, respectively were observed. It could be inferred that phosphorylated derivatives of Sanchi flower polysaccharide exhibited several substitution sites of phosphorus and mainly the phosphates were positioned at glucan position of C2, C4 and C6 carbons. Furthermore, the it has also been reported in published literature that phosphate acts as the weak electron absorber and in this case the chemical shifts showed shifting of C1–C6 and obviously due to the substitution of phosphate groups, the peak splitting of carbon showed reducing tendency. Similar to findings of this study, the hydroxyl groups showed the replacement by the phosphate groups in case of phosphorylation of polysaccharide derived from the purple sweet potato. Moreover, authors reported that position of 21.82 ppm was the easiest in structural configuration to replace as compared to other positions (Yang et al. 2021). These results were in agreement with the findings of Xiong et al. (2019) wherein authors reported successful phosphorylation of native ginseng polysaccharides.
Fig. 3.
31P NMR spectrum of phosphorylated polysaccharide from Sanchi flower
Microstructural analysis of polysaccharide from Sanchi flower
Polysaccharides and phosphorylated polysaccharides samples of Sanchi flower were analyzed through SEM to elucidate information pertaining to surface appearance and topography. The micrographs of non-phosphorylated polysaccharides of Sanchi flower are depicted in Figs. 4A, A1 at 500 and 1000×, respectively. The micrographs of phosphorylated polysaccharides of Sanchi flower are provided in Fig. 4B, B1 obtained at 500 and 1000×, respectively. It was evident from the results that non-phosphorylated polysaccharides had smooth surface attributes with intact molecular configuration comprising of bumps and sags. Although, phosphorylated polysaccharides exhibited slight rough surface morphology with fissures and cracks, the overall structural configurations of phosphorylated and non-phosphorylated polysaccharides were almost similar in terms of intactness. Moreover, it was observed that the introduction of phosphate groups into polysaccharides resulted in more pore-like structures in the polysaccharides configuration. The differences in surface morphology and microstructural attributes were indicative of the fact that phosphorylation caused modification changes in surface features of Sanchi flower polysaccharides. These results were consistent with findings of Xie et al. (2020a) who also reported changes due to phosphorylation in polysaccharides of Cyclocarya (C. paliurus). Nonetheless, studies have reported that phosphorylation-induced changes in plant-derived polysaccharides may exert significant influence on technological properties and biological activities of the polysaccharides (Xie et al. 2020a).
Fig. 4.
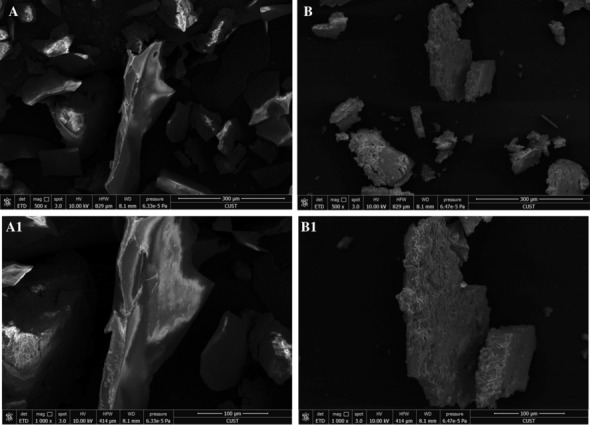
Environmental Scanning electron microscopy (ESEM) images of Sanchi flower polysaccharide (A, A1) and its phosphorylated polysaccharide (B, B1). A (×500); A1 (×1000); B (×500); B1 (×1000)
In vitro radical scavenging activities analyses of Sanchi flower polysaccharide
DPPH-RSA
Non-phosphorylated polysaccharides and phosphorylated polysaccharides samples of Sanchi flower were analyzed for their DPPH-RSA. DPPH radical has been exploited to evaluate the antioxidant potential of natural compounds as the DPPH radial may exhibit molecular stability after acceptance of hydrogen radical or an electron. The results of DPPH-RSA of non-phosphorylated, phosphorylated polysaccharides and ascorbic acid (positive control) are shown in Table 2. The non-phosphorylated polysaccharides showed concentration-dependent increasing tendency of DPPH-RSA ranging from 4.35 to 34.71% at concentration range of 0.2–1.0 mg/mL. On the other hand, the DPPH-RSA of phosphorylated polysaccharides ranged from 9.92 to 39.22% and concentration-dependent increases in DPPH-RSA were evident from the results (Table 2). Ascorbic acid was employed as a positive control, which exhibited DPPH-RSA in the range of 90.86–97.69% at concentrations ranging from 0.2 to 1.0 mL. Overall, phosphorylation caused noticeable effect on DPPH-RSA with phosphorylated polysaccharides showing higher DPPH-RSA than that of non-phosphorylated polysaccharides. Hence, it may be implied the introduction of phosphate groups into polysaccharides configuration elevated its ability to scavenge DPPH free radicals, particularly at high concentration levels of phosphorylated polysaccharides. It is important to note that antioxidant activity of polysaccharides is contingent upon monosaccharide type, glyosidic bonds, uronic acid and molecular weight (Xie et al. 2020a). Therefore, chemical modifications are undertaken to cause structural changes in polysaccharides, which consequently lead to modifications in molecular weight, monosaccharide composition, uronic acid and other polysaccharide features (Li et al. 2018a, b). It has also been reported by other researchers that DPPH is very stable free radical which comprises of nitrogen in its configuration at the central position. The stability of the DPPH radical usually is because of the space barrier of three benzene rings occurring in its structural configuration and their resonance stabilization which causes lone pair electrons being trapped in central nitrogen atom to not play their role in pairing. It also has been reported by Mei et al. (2020) that phosphorylation of (1 → 3)-β-D-glucan exhibited linear relationship with scavenging ability. It has also been concluded in previous researchers that reducing/scavenging ability might be attributable to the polysaccharides sugar content. The scavenging ability would be higher in case of higher sugar content in the polysaccharides molecules (Yang et al. 2021).
Table 2.
Antioxidant activities of Sanchi flower polysaccharide and its phosphorylated derivative (Unit: %)
| Antioxidant activity | Concentration (mg/mL) | Polysaccharide | Derivative | Ascorbic acid |
|---|---|---|---|---|
|
DPPH RSA |
0.2 | 4.35 ± 0.12eC | 9.92 ± 0.53eB | 90.86 ± 0.30bA |
| 0.4 | 15.06 ± 0.12dC | 16.51 ± 0.77dB | 97.10 ± 0.07aA | |
| 0.6 | 27.88 ± 0.12cC | 26.24 ± 0.01cB | 97.65 ± 0.01aA | |
| 0.8 | 33.76 ± 0.07bC | 35.57 ± 0.18bB | 97.77 ± 0.01aA | |
| 1.0 | 34.71 ± 0.07aC | 39.22 ± 0.77aB | 97.69 ± 0.07aA | |
|
ABTS RSA |
0.2 | 11.73 ± 0.25eC | 12.54 ± 0.06eB | 98.12 ± 0.30aA |
| 0.4 | 14.66 ± 0.15dC | 20.43 ± 0.20dB | 98.05 ± 0.17aA | |
| 0.6 | 22.15 ± 0.08cC | 24.92 ± 0.05cB | 98.01 ± 0.09aA | |
| 0.8 | 23.78 ± 0.07bC | 28.02 ± 0.8bB | 97.97 ± 0.11aA | |
| 1.0 | 25.73 ± 0.09aC | 32.04 ± 0.13aB | 98.16 ± 0.07aA | |
|
Hydroxyl RSA |
0.2 | 67.78 ± 0.20eC | 69.53 ± 0.08eB | 85.64 ± 0.25eA |
| 0.4 | 68.91 ± 0.10dC | 71.37 ± 0.15dB | 88.36 ± 0.19dA | |
| 0.6 | 70.45 ± 0.08cC | 74.78 ± 0.11cB | 89.48 ± 0.15cA | |
| 0.8 | 72.20 ± 0.17bC | 77.01 ± 0.09bB | 92.64 ± 0.13bA | |
| 1.0 | 75.35 ± 0.19aC | 80.91 ± 0.15aB | 99.56 ± 0.10aA |
A–CMeans followed by different letters in each row are significantly different (p < 0.05)
a–eMeans followed by different letters in each column are significantly different (p < 0.05)
ABTS-RSA
ABTS-RSA of non-phosphorylated polysaccharides and phosphorylated polysaccharides samples of Sanchi flower are shown in Table 2. It was evident from the results that the ABTS-RSA of the non-phosphorylated polysaccharides exhibited concentration-dependent increasing tendency ranging from 11.73 to 25.73% at concentrations ranging from 0.2 to 1.0 mg/mL. On the other hand, phosphorylated polysaccharides showed concentration-dependent increases in ABTS-RSA ranging from 12.54 to 32.04%, whereas ascorbic acid (positive control) showed ABTS-RSA from 98.12 to 98.16% at concentration range of 0.2–1.0 mL. Nevertheless, phosphorylation caused considerable impact on ABTS-RSA with phosphorylated polysaccharides showing significantly (p < 0.05) higher ABTS-RSA as compared to those of non-phosphorylated polysaccharides. These results indicate that introduction of phosphate groups into polysaccharides led to increased ability to scavenge ABTS free radicals, especially at high concentration levels of phosphorylated polysaccharides. While both ascorbic acid and phosphorylated polysaccharides exhibited different degrees of ABTS scavenging effect, ascorbic acid showed stronger scavenging effect (p < 0.05) as compared to that of phosphorylated polysaccharides.
Hydroxyl-RSA
Hydroxyl radical is a highly reactive chemical agent, which can cause oxidation of sugar molecules. Hydroxyl-RSA evaluates the capacity of polysaccharides to scavenge hydroxyl radicals (Xie et al. 2020a). The hydroxyl-RSA of non-phosphorylated, phosphorylated polysaccharides and ascorbic acid (positive control) are shown in Table 2. Although, the best scavenging effect was observed with ascorbic acid, the polysaccharides modified through phosphorylation also showed superior hydroxyl-RSA, which was significantly higher (p < 0.05) than that of non-phosphorylated polysaccharides. It was evident from the results that the hydroxyl-RSA of the non-phosphorylated polysaccharides from Sanchi flower exhibited concentration-dependent increasing tendency ranging from 67.78 to 75.35%. On the other hand, the hydroxyl-RSA of phosphorylated polysaccharides ranged from 69.53 to 80.91% with concentration-dependent rises in hydroxyl-RSA (Table 2). Thus, it can be suggested that the introduction of phosphate radical groups into polysaccharides structural configuration led to effective improvement in the hydroxyl-RSA of phosphorylated polysaccharides. These results were consistent with the findings reported by Yang et al. (2021), whereby phosphorylated polysaccharide from sweet purple potato exhibited good scavenging effects on the hydroxyl radicals. Moreover, the scavenging ability showed concentration-dependent increases with corresponding rises in the sugar content of polysaccharides. After the introduction of the phosphate groups in the structural configuration of polysaccharides, the density of electrons on the polysaccharides carbon chain showed rising trend which possibly results in increased radical-scavenging ability of the hydroxyl groups (Yang et al. 2021).
Conclusion
In this study, phosphorylated modification of Sanchi flower polysaccharides was carried out. In addition, the influence on structural characteristics and biological activities of phosphorylated derivatives of Sanchi flower were evaluated. The phosphorylated polysaccharides of Sanchi flower mainly comprised of Man, Rha, GluA, GalA, Glu, Gal and Xyl, while GluN, Rib, Arab and Fuc were absent in their compositions. The emergence of new absorption peak in FTIR spectra indicated the successful introduction of phosphate linkages into the polysaccharides configuration of Sanchi polysaccharides. As the phosphate group belongs to the category of electron absorber group, the NMR spectrum showed a clear shift of C1-C6. NMR analysis in conjunction with that of FTIR spectra findings was indicative of the successful phosphorylation of the polysaccharides. This phosphorylation-induced changes could exert considerable influence on the technological properties (e.g. water solubility) and biological activities of Sanchi flower polysaccharides. Phosphorylation caused concentration-dependent increases in radical-scavenging effects of phosphorylated polysaccharides on DPPH, ABTS, and hydroxyl radicals. Hence, phosphorylation of Sanchi polysaccharides resulted in modified polysaccharides with superior antioxidant activities, which could be potentially be used as a therapeutic agent in the treatment of some chronic diseases. It was also evident from this study results that polysaccharide derivitization is one of the significant technological modality for improving biological activities of polysaccharides. However, in this regard, further investigation is needed pertaining to structure–activity relationship of polysaccharides.
Acknowledgements
The authors declare that (1) the work described has not been published before (except in the form of an abstract, a published lecture or academic thesis), (2) this study is not under consideration for publication elsewhere, (3) the submission to JFST publication has been approved by all authors as well as the responsible authorities (4) if accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright holder and (5) JFST will not be held legally responsible should there be any claims for compensation or dispute on authorship.
Abbreviations
- NK
Natural killer
- DPPH
2,2-Diphenyl-1-picrylhydrazyl
- PMP
1-Phenyl-3- methyl-5- pyrazolone
- TFA
Trifluoroacetic acid
- FTIR
Fourier transform infrared
- NMR
Nuclear Magnetic Resonance
- ESEM
Environment scanning electron microscopic
- RSA
Radical-scavenging activity
- ABTS
Azinobis (3-ethyl-benzothiazoline-6-sulfonic acid)
- Man
Mannose
- Rha
Rhamnose
- GluA
Galacturonic acid
- GalA
Gluconic acid
- Glu
Glucose
- Gal
Galactose
- Xyl
Xylose
Author contributions
NH: Methodology, Formal analysis, Writing-Original Draft. KA: Formal analysis, Writing-Original Draft, Revision, Writing-Review & Editing. ZW: Resources, Data curation, Visualization. SY: Resources, Data curation, Visualization. GJ: Conceptualization, Supervision, Data curation, Validation. KR: Conceptualization, Writing-Review & Editing, Investigation.
Funding
This work was supported by Foundation of Ph.D. Research Project, Jilin Medical University (No. JYBS2019009), Jilin Province, China.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Declarations
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Ethics approval
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nailin Huo and Kashif Ameer have contributed equally to this work.
Contributor Information
Guihun Jiang, Email: jiangguihun1@hotmail.com.
Karna Ramachandraiah, Email: karna@sejong.ac.kr.
References
- Chen F, Huang G, Huang H. Preparation, analysis, antioxidant activities in vivo of phosphorylated polysaccharide from Momordica charantia. Carbohyd Polym. 2021;252:117179. doi: 10.1016/j.carbpol.2020.117179. [DOI] [PubMed] [Google Scholar]
- Costa LS, Fidelis GP, Cordeiro SL, Oliveira RM, Sabry DDA, Câmara RBG, Nobre LTDB, Costa MSSP, Almeida-Lima J, Farias EHC, Leite EL. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed Pharmacother. 2010;64(1):21–28. doi: 10.1016/j.biopha.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Deng C, Fu H, Xu J, Shang J, Cheng Y. Physiochemical and biological properties of phosphorylated polysaccharides from Dictyophora indusiata. Int J Biol Macromol. 2015;72:894–899. doi: 10.1016/j.ijbiomac.2014.09.053. [DOI] [PubMed] [Google Scholar]
- Huang L, Shen M, Zhang X, Jiang L, Song Q, Xie J. Effect of high-pressure microfluidization treatment on the physicochemical properties and antioxidant activities of polysaccharide from Mesona chinensis Benth. Carbohyd Polym. 2018;200:191–199. doi: 10.1016/j.carbpol.2018.07.087. [DOI] [PubMed] [Google Scholar]
- Li S, Song Z, Liu T, Liang J, Yuan J, Xu Z, Zhang D. Polysaccharide from Ostrea rivularis attenuates reproductive oxidative stress damage via activating Keap1-Nrf2/ARE pathway. Carbohyd Polym. 2018;186:321–331. doi: 10.1016/j.carbpol.2018.01.075. [DOI] [PubMed] [Google Scholar]
- Li XL, Tu XF, Thakur K, Zhang YS, Zhu DY, Zhang JG, Wei ZJ. Effects of different chemical modifications on the antioxidant activities of polysaccharides sequentially extracted from peony seed dreg. Int J Biol Macromol. 2018;112:675–685. doi: 10.1016/j.ijbiomac.2018.01.216. [DOI] [PubMed] [Google Scholar]
- Mei X, Tang Q, Huang G, Long R, Huang H. Preparation, structural analysis and antioxidant activities of phosphorylated (1 → 3)-β-d-glucan. Food Chem. 2020;309:125791. doi: 10.1016/j.foodchem.2019.125791. [DOI] [PubMed] [Google Scholar]
- Peng M, Yi YX, Zhang T, Ding Y, Le J. Stereoisomers of saponins in Panax notoginseng (Sanqi): a review. Front Pharmacol. 2018;9:188. doi: 10.3389/fphar.2018.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandraiah K, Chin KB. Impact of drying and micronization on the physicochemical properties and antioxidant activities of celery stalk. J Sci Food Agric. 2017;97:4539–4547. doi: 10.1002/jsfa.8321. [DOI] [PubMed] [Google Scholar]
- Torres FG, Troncoso OP, Pisani A, Gatto F, Bardi G. Natural polysaccharide nanomaterials: an overview of their immunological properties. Int J Mol Sci. 2019;20(20):5092. doi: 10.3390/ijms20205092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng YH, Yang JH, Mau JL. Antioxidant properties of polysaccharides from Ganoderma tsugae. Food Chem. 2008;107(2):732–738. doi: 10.1016/j.foodchem.2007.08.073. [DOI] [Google Scholar]
- Wu Z, Ameer K, Jiang G. Effects of superfine grinding on the physicochemical properties and antioxidant activities of Sanchi (Panax notoginseng) flower powders. J Food Sci Technol. 2021;58(1):62–73. doi: 10.1007/s13197-020-04514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie JH, Tang W, Jin ML, Li JE, Xie MY. Recent advances in bioactive polysaccharides from Lycium barbarum L., Zizyphus jujuba Mill, Plantago spp., and Morus spp.: structures and functionalities. Food Hydrocolloids. 2016;60:148–160. doi: 10.1016/j.foodhyd.2016.03.030. [DOI] [Google Scholar]
- Xie L, Shen M, Wen P, Hong Y, Liu X, Xie J. Preparation, characterization, antioxidant activity and protective effect against cellular oxidative stress of phosphorylated polysaccharide from Cyclocarya paliurus. Food Chem Toxicol. 2020;145:111754. doi: 10.1016/j.fct.2020.111754. [DOI] [PubMed] [Google Scholar]
- Xie L, Shen M, Hong Y, Ye H, Huang L, Xie J. Chemical modifications of polysaccharides and their anti-tumor activities. Carbohyd Polym. 2020;229:115436. doi: 10.1016/j.carbpol.2019.115436. [DOI] [PubMed] [Google Scholar]
- Xiong X, Huang G, Huang H. The antioxidant activities of phosphorylated polysaccharide from native ginseng. Int J Biol Macromol. 2019;126:842–845. doi: 10.1016/j.ijbiomac.2018.12.266. [DOI] [PubMed] [Google Scholar]
- Yang W, Zhang Y, Tang A, Ruan Q, Huang G. Preparation and antioxidant activity of phosphorylated polysaccharide from purple sweet potato. Chem Biol Drug Des. 2021;98(5):828–834. doi: 10.1111/cbdd.13936. [DOI] [PubMed] [Google Scholar]
- Zhang S, Chen C, Lu W, Wei L. Phytochemistry, pharmacology, and clinical use of Panax notoginseng flowers buds. Phytother Res. 2018;32(11):2155–2163. doi: 10.1002/ptr.6167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Not applicable.