Abstract
Background
Despite the improved medical and surgical managements, still there is a significant risk of developing acute cerebrovascular strokes after coronary artery bypass grafting (CABG). Our objectives were to study the immediate and long-term outcomes after CABG and to identify the possible predictors of post-CABG strokes.
Results
Between January 2016 and August 2020, 410 adult patients, mostly males (82.2%), were retrospectively enrolled after CABG. Acute postoperative strokes occurred in 31 (7.5%) patients; of them, 30 (96.8%) patients had ischemic stroke, while 1 (3.2%) had hemorrhagic stroke. Mechanical thrombectomy was done in two cases. The patients who developed acute cerebral stroke had significantly higher admission (p = 0.02) and follow-up (p < 0.001) SOFA scores, higher arterial blood lactate level (p < 0.001), longer hospitalization (p < 0.001) and more hospital mortality (p < 0.001) compared with the patients who did not develop stroke. Kaplan–Meier curves for 5-year mortality showed increased risk in those patients with postoperative stroke (HR: 23.03; 95% CI: 6.10–86.92, p < 0.001). After multivariate regression, the predictors of early postoperative stroke were carotid artery stenosis (CAS), postoperative atrial fibrillation, cardiopulmonary bypass time, prior cerebral stroke, admission SOFA score and chronic kidney disease (CKD). The predictors of late cerebrovascular stroke were CAS, combined CABG and valve surgery, CKD, atrial fibrillation, prior stroke and HbA1c.
Conclusions
The development of post-CABG acute cerebrovascular stroke is associated with longer hospitalization, multiple morbidities and increased mortality. Careful assessment and management of risk factors especially atrial fibrillation and carotid artery stenosis should be implemented to decrease this substantial complication after CABG.
Keywords: CABG, Coronary artery bypass grafting, SOFA score, Acute cerebrovascular stroke, Chronic kidney disease, Carotid artery stenosis, Mechanical thrombectomy, Postoperative atrial fibrillation, Cardiopulmonary bypass, Mortality
Background
The occurrence of cerebrovascular stroke after coronary revascularization is a substantial complication with a more frequency after CABG compared with percutaneous coronary interventions (PCI) [1, 2]. CABG is required in patients who are not suitable for PCI and still preferred in cases of significant coronary artery disease [3, 4]. Other indications of CABG include diabetes mellitus, left ventricular systolic dysfunction and rescue operation after failed trials of PCI [4, 5]. The development of postoperative cerebrovascular stroke is linked to prolonged hospitalization with high costs and increased hospital mortality [1, 2, 6]. Bucerius et al. [7] revealed the association between postoperative stroke and a sixfold increase in 30-day mortality. Due to advances in medications, percutaneous interventions and surgical techniques, the risk profiles of patients undergoing CABG change with increasing age and frequent comorbidities [8]. Also, atherosclerosis is a systemic vascular disease and patients subjected to CABG have high probabilities of significant atherosclerotic burden of different vessels including aorta, carotid and cranial arteries [9]. Our objectives were to study clinical outcomes of post-CABG acute cerebrovascular strokes and to identify the significant risk factors and potential predictors of postoperative stroke which will help to improve the patients' outcomes via adoption of new quality initiatives.
Methods
Study protocol
After getting our hospital ethical/research committee approval, we retrospectively enrolled all CABG patients (> 18 years) between 2016 and 2020 in this study. The data collected included demographic, perioperative clinical and laboratory variables, and the hospital outcomes. A follow-up was done to get the occurrence of a new stroke or mortality. The SOFA score was calculated after patient being admitted to ICU and 48 h later.
Definitions of the variables
Acute ischemic stroke means an acute neurological manifestations persisting ≥ 24 h due to brain focal infarction in a well-defined vascular territory with exclusion of other possible etiologies [10]. Post-CABG early stroke means occurrence of stroke during the first 7 days after CABG, while late stroke occurs beyond the first 7 postoperative days [1]. Carotid artery stenosis (CAS) means narrowing of carotid lumen ≥ 50% by a plaque [11, 12]. Chronic kidney disease (CKD) means glomerular filtration rate (GFR) ˂ 60 mL/min/1.73 m2 for ≥ 3 months, according to the guidelines of Kidney Disease Improving Global Outcomes (KDIGO) group [13]. Acute kidney injury (AKI) means acute reduction in GFR according to the RIFLE criteria [14].
Statistical analysis
The study results were presented using frequency with percentage for categorical data via the Chi-square (c2) test and using median with interquartile range (IQR) for quantitative data via the Mann–Whitney and Kruskal–Wallis tests. Data analysis was done using the statistical package for the Social Sciences (SPSS) version 26. Two-sided P-values < 0.05 were considered significant. After the univariate analysis, we used the significant variables for multivariate regression analysis to get the predictors of acute postoperative cerebrovascular stroke.
Results
Baseline characteristics
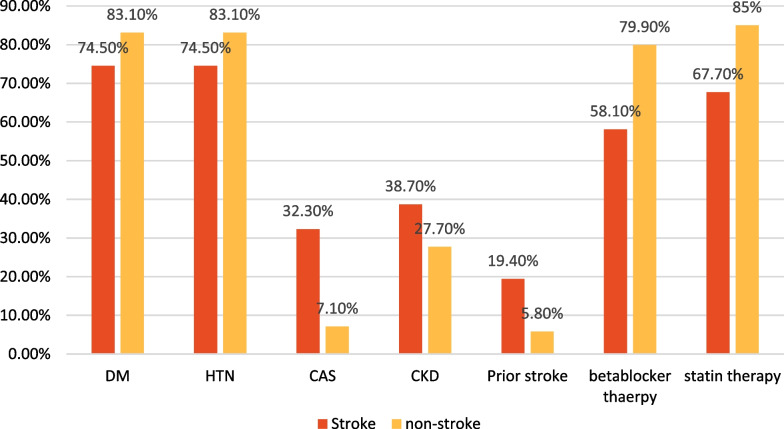
Our study enrolled 410 adult patients; of them, 31 (7.5%) patients developed acute cerebrovascular strokes after CABG. The patients with acute stroke had significantly more frequent prior strokes, carotid artery stenosis, CKD and prior cardiotomies while less frequent preoperative beta-blocker and statin therapies, and male gender compared to those who did not develop acute stroke (Table 1, Fig. 1).
Table 1.
Characteristics and profiles of patients
| Variables | All patients (410) | Stroke group (31,7.5%) | Non-stroke group (379,92.5%) | P value | |
|---|---|---|---|---|---|
| Age (years) | 60 (55–68) | 66 (54–75) | 60 (55–68) | 0.06 | |
| Gender, male (n, %) | 337 (82.2) | 19 (61.3) | 318 (83.9) | 0.002 | |
| BMI (kg/m2) | 28.05 (25.8–31.8) | 28 (25–31.8) | 28.1 (25.6–31.8) | 0.88 | |
| Smoking (n, %) | 159 (38.8) | 11 (35.5) | 148 (39.1) | 0.69 | |
| Diabetes mellitus (n, %) | 338 (82.4) | 23 (74.5) | 315 (83.1) | 0.209 | |
| Hyperlipidemia (n, %) | 245 (59.8) | 19 (61.3) | 226 (59.6) | 0.85 | |
| Chronic Kidney disease (n, %) | 117 (28.5) | 12 (38.7) | 105 (27.7) | 0.041 | |
| Hypertension (n, %) | 351 (85.6) | 25 (80.6) | 326 (86) | 0.42 | |
| Bronchial asthma (n, %) | 44 (10.7) | 5 (16.1) | 39 (10.3) | 0.359 | |
| Hypothyroidism (n, %) | 42 (10.2) | 3 (9.7) | 39 (10.3) | 1 | |
| Hyperthyroidism (n, %) | 3 (0.7) | 0 (0) | 3 (0.8) | 1 | |
| Preoperative AF (n, %) | 26 (6.3) | 2 (6.5) | 24 (6.3) | 1 | |
| Malignancy (n, %) | 26 (6.3) | 2 (6.5) | 24 (6.3) | 1 | |
| Renal/liver transplant (n, %) | 17 (4.1) | 1 (3.2) | 16 (4.2) | 1 | |
| Prior stroke (n, %) | 28 (6.8) | 6 (19.4) | 22 (5.8) | 0.013 | |
| Prior TIAs (n, %) | 24 (5.9) | 3 (9.7) | 21 (5.5) | 0.41 | |
| Carotid artery stenosis (n, %) | 37 (9) | 10 (32.3) | 27 (7.1) | < 0.001 | |
| Peripheral vascular disease (n, %) | 44 (10.7) | 3 (9.7) | 41 (10.8) | 1 | |
| Prior myocardial infarction (n, %) | 257 (62.7) | 24 (77.4) | 233 (61.5) | 0.07 | |
| Prior PCI (n, %) | 137 (33.4) | 10 (32.3) | 127 (33.5) | 0.88 | |
| Prior cardiac surgeries | 27 (6.6) | 7 (22.6) | 20 (5.3) | 0.002 | |
| Preoperative LVEF% (n, %) | More than 55% | 141 (34.4) | 10 (32.3) | 131 (34.6) | 0.052 |
| 45–55% | 106 (25.9) | 4 (12.9) | 102 (26.9) | ||
| 35–45 | 88 (21.5) | 6(19.4) | 82 (21.6) | ||
| Less than 35% | 75 (18.3) | 11 (35.5) | 64 (16.9) | ||
| Preoperative drugs (n, %) | Beta-blockers | 321 (78.3) | 18 (58.1) | 303 (79.9) | 0.004 |
| Statins | 343 (83.7) | 21 (67.7) | 322 (85) | 0.013 | |
| Fibrates | 45 (11) | 2 (6.5) | 43 (11.3) | 0.55 | |
| ACEI/ARBs | 187 (45.6) | 10 (32.3) | 177 (46.7) | 0.12 | |
| Steroids | 18 (4.4) | 1 (3.2) | 17 (4.5) | 1 | |
| Immune suppression | 11 (2.7) | 1 (3.2) | 10 (2.6) | 0.58 | |
| Preoperative IABP (n, %) | 15 (3.7) | 3 (9.7) | 12 (3.2) | 0.09 | |
| Preoperative ECMO (n, %) | 2 (0.5) | 2 (6.5) | 0 (0) | 0.006 | |
BMI body mass index, AF atrial fibrillation, CAS carotid artery stenosis, PCI percutaneous coronary intervention, ACEI angiotensin-converting enzyme inhibitors, IABP intra-aortic balloon pump, LVEF left ventricle ejection fraction, TIAs transient ischemic attacks, ARBs angiotensin receptor blockers, ECMO extracorporeal membrane oxygenation
Fig. 1.
Preoperative variables of the enrolled patients
Laboratory variables
Preoperatively, the glycated hemoglobin (HbA1c) was 8.1% [7.4–9.1] vs. 7.1% [6.4–8.1], p = 0.005. The peak blood lactate level was 12.5 [7.6–14.7] vs. 5.1[3.7–8.5], p < 0.001, and the peak troponin level was 980 [474–1560] vs. 608 [387–1149], p = 0.004 in those with and without strokes, respectively. The peri-operative laboratory variables were summarized in Table 2.
Table 2.
Laboratory variables
| Variables | All patients | Stroke group | Non-stroke group | P value |
|---|---|---|---|---|
| Preoperative platelets (10ˆ9/L) | 246 (202–308) | 304 (229–352) | 243 (201–304) | 0.08 |
| Preoperative hemoglobin (g/L) | 131 (115–142) | 118 (108–136) | 131 (116–143) | 0.052 |
| Preoperative serum creatinine (umol/L) | 90 (74–111) | 85 (64–107) | 90 (74–113) | 0.1 |
| Preoperative serum bilirubin (umol/L) | 6.6 (4.6–11.1) | 10.4 (6.6–17.4) | 6.4 (4.6–10.4) | 0.002 |
| Preoperative INR | 1 (1–1.1) | 1.2 (1.1–1.2) | 1 (1–1.1) | 0.06 |
| Preoperative fibrinogen (g/L) | 3.2 (2.85–3.65) | 3.43 (2.88–3.62) | 3.19 (2.85–3.7) | 0.72 |
| Preoperative serum albumin (g/L) | 39.8 (36.7–42.4) | 39.1 (34.4–40.2) | 40 (37–42.5) | 0.052 |
| HbA1c (%) | 7.2 (6.4–8.2) | 8.1 (7.4–9.1) | 7.1 (6.4–8.1) | 0.005 |
| Peak lactate (mmol/L) | 5.5 (3.8–8.9) | 12.5 (7.6–14.7) | 5.1 (3.7–8.5) | < 0.001 |
| Peak troponin (ng/L) | 634 (392–1180) | 980 (474–1560) | 608 (387–1149) | 0.004 |
| Hemoglobin day 1 (g/L) | 98 (91–107) | 90 (86–102) | 98 (92–107) | 0.001 |
| Platelets day 1 (10ˆ9/L) | 189 (139–232) | 204 (106–275) | 189 (140–231) | 0.97 |
| Serum albumin day 1(g/L) | 36 (32.8–38.6) | 34.2 (30–36.5) | 36.3 (33–38.7) | 0.006 |
| Serum bilirubin day 1 (umol/L) | 9.75 (7.2–15.6) | 16.8 (10.3–28) | 9.5 (7–14) | < 0.001 |
| Serum creatinine day 1 (umol/L) | 86.5 (74–116) | 89 (76–108) | 86 (74–119) | 0.78 |
| Hemoglobin day 3 (g/L) | 91 (88–97) | 89 (85–94) | 92 (88–97) | 0.027 |
| Platelets day 3 (10ˆ9/L) | 167 (127–211) | 157 (83–223) | 169 (127–211) | 0.12 |
| Serum albumin day 3(g/L) | 36 (33–37.8) | 34.1 (30.6–36.7) | 36.3 (33.4–37.8) | 0.042 |
| Serum bilirubin day 3 (umol/L) | 12.85 (9–19.9) | 19.6 (13.8–36.3) | 12.4 (8.8–19.6) | < 0.001 |
Perioperative variables
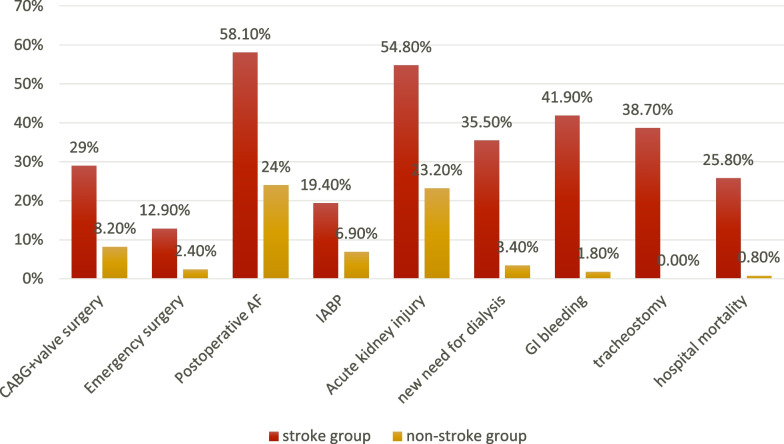
The patients with acute postoperative stroke had significantly longer cardiopulmonary bypass time, more frequent emergency surgeries, higher SOFA scores, higher frequencies of postoperative atrial fibrillation, ventricular arrhythmias, AKI, new need for dialysis, more inotropes days, more ventilator days, frequent tracheostomies, frequent ECMO support, frequent IABP support, frequent gastrointestinal bleeding, longer ICU stay, postoperative ward stay and increased hospital mortality compared to those who did not develop stroke, respectively (Fig. 2, Table 3).
Fig. 2.
Postoperative variables of the studied patients
Table 3.
Perioperative variables and outcomes
| Variables | All patients | Stroke group | Non-stroke group | P value | |
|---|---|---|---|---|---|
| Type of surgery | Isolated CABG (n, %) | 370 (90.2) | 22 (71) | 348 (91.8) | 0.001 |
| CABG plus valve surgery (n, %) | 40 (9.8) | 9 (29) | 31 (8.2) | ||
| Cardiopulmonary bypass (minutes) | 100 (77–126) | 102 (90.5–157.5) | 99 (76–125) | 0.034 | |
| Aortic clamping time (minutes) | 58 (43–83) | 69 (46–84.5) | 57 (43–83) | 0.351 | |
| Approach | Sternotomy (n, %) | 398 (97.1) | 31 (100) | 367 (96.8) | 0.612 |
| Minimally invasive (n, %) | 12(2.9) | 0 | 12 (3.2) | ||
| Urgency of surgery | Elective (n, %) | 397 (96.8) | 27 (87.1) | 370 (97.6) | 0.012 |
| Emergent (n, %) | 13 (3.2) | 4 (12.9) | 9 (2.4) | ||
| Initial SOFA score | 2 (1–3) | 3 (2–4) | 2 (1–3) | 0.02 | |
| SOFA at 48 h | 1 (0–2) | 5 (1–7) | 1 (0–2) | < 0.001 | |
| Postoperative atrial fibrillation (n, %) | 109 (26.6) | 18 (58.1) | 91 (24) | < 0.001 | |
| Ventricular arrhythmias (n, %) | 24 (5.9) | 6 (19.4) | 18 (4.7) | 0.006 | |
| Intra-cardiac thrombi (n, %) | 7 (1.7) | 2 (6.5) | 5 (1.3) | 0.09 | |
| Acute kidney injury (n, %) | 105 (25.6) | 17 (54.8) | 88 (23.2) | < 0.001 | |
| New need for hemodialysis (n, %) | 24 (5.9) | 11 (35.5) | 13 (3.4) | < 0.001 | |
| Exploration for bleeding (n, %) | 27 (6.6) | 4 (12.9) | 23 (6.1) | 0.137 | |
| Gastrointestinal bleeding (n, %) | 20 (4.9) | 13 (41.9) | 7 (1.8) | < 0.001 | |
| Sternotomy wound infection (n, %) | 53 (12.9) | 7 (22.6) | 46 (12.1) | 0.1 | |
| Wound debridement (n, %) | 37 (9) | 7 (22.6) | 30 (7.9) | 0.014 | |
| ICU days | 4 (3–6) | 11 (6–42) | 4 (3–5) | < 0.001 | |
| Inotropes days | 2 (1–2) | 6 (2–10) | 1 (1–2) | < 0.001 | |
| Ventilator days | 1 (1–2) | 10 (2–32) | 1 (1–2) | < 0.001 | |
| Post-ICU ward days | 5 (3–8) | 11 (2–57) | 5 (3–7) | 0.015 | |
| Postoperative ECMO | 8 (2) | 4 (12.9) | 4 (1.1) | 0.002 | |
| ECMO days | 9 (6–13) | 9 (6–13) | 9.5 (7–13) | 1 | |
| Postoperative IABP (n, %) | 32 (7.8) | 6 (19.4) | 26 (6.9) | 0.025 | |
| IABP days | 3 (2–4) | 3 (2–3) | 3 (2–4) | 0.644 | |
| Tracheostomy (n, %) | 12 (2.9) | 12 (38.7) | 0 (0) | < 0.001 | |
| Hospital mortality (n, %) | 11 (2.7) | 8 (25.8) | 3 (0.8) | < 0.001 | |
| Discharge with tube feeding (n, %) | 13 (3.2) | 12 (38.7) | 1 (0.3) | < 0.001 | |
| Discharge with tracheostomy breathing (n, %) | 10 (2.4) | 10 (32.3) | 0 (0) | < 0.001 | |
| Stroke during follow-up (n, %) | 15 (3.7) | 0 (0) | 15 (4) | 0.61 | |
| Mortality during follow-up (n, %) | 28 (6.8) | 2 (6.5) | 26 (6.9) | 1 | |
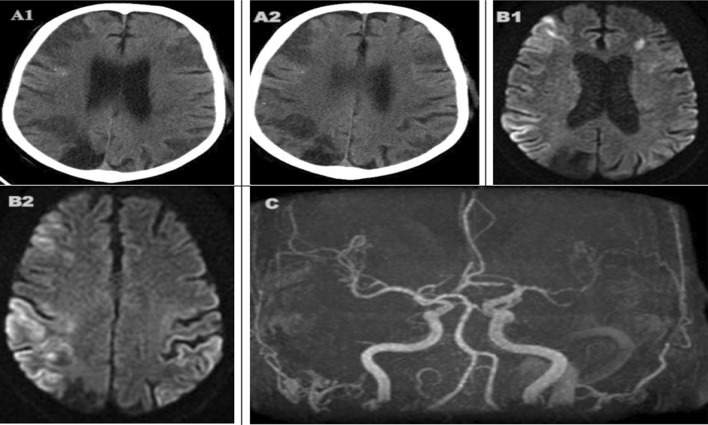
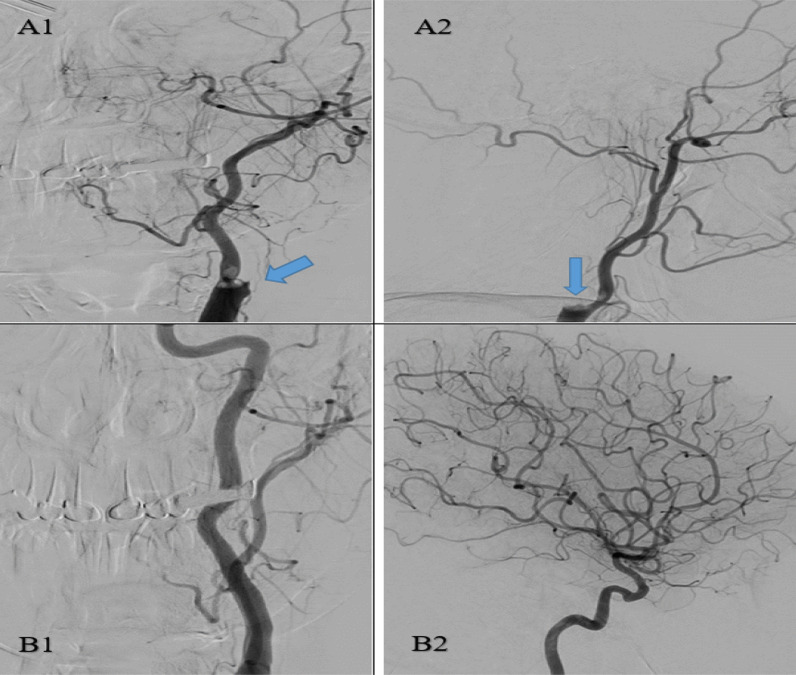
According to brain imaging studies, there were territorial infarcts and watershed infarcts. Mechanical thrombectomy was done in two cases with large vessels occlusion (Figs. 3 and 4).
Fig. 3.
A 64-years-old man with old right occipital old stroke and multiple scattered calcific emboli, presented with delayed neurological recovery after CABG. CT brain (A1, A2) showed acute hypo-densities in bilateral fronto-parietal and right occipito-temporal regions representing watershed infarctions. MRI (B1, B2) was done after 6 days and showed external and internal watershed infarctions involving bilateral cortical and subcortical areas. MR angiography (C) demonstrates multifocal moderate-to-severe bilateral middle cerebral arteries stenosis associated with paucity of distal vessels with few collaterals' formation
Fig. 4.
A 53 years-old-woman with atrial fibrillation that developed postoperative acute right-sided hemiparesis. Left carotid angiogram showed a filling defect (consistent with an embolic clot) completely occluding the internal carotid artery and partially occluding the origin of external carotid artery (A1 & A2). Complete recanalization of both internal and external carotid arteries was achieved after mechanical thrombectomy (B1 & B2)
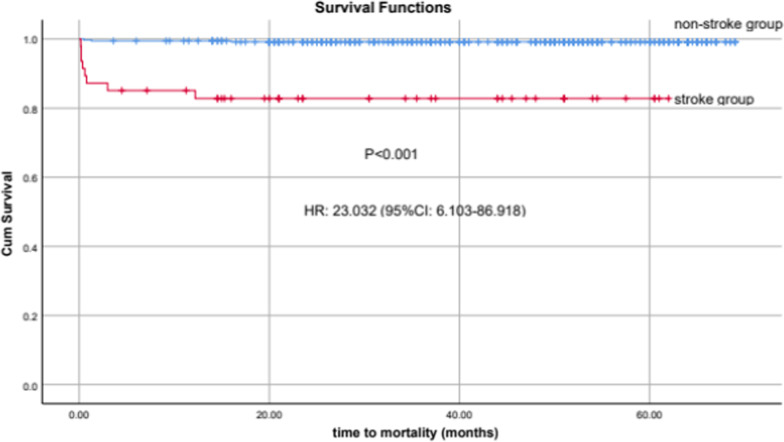
Kaplan–Meier curves of mortality during 5-years follow-up showed increased risk in those patients with postoperative stroke (HR: 23.032; 95% CI: 6.103–86.918, p < 0.001) (Fig. 5).
Fig. 5.
Kaplan–Meier curves of survival for 5-years follow-up
Predictors of post-CABG strokes
Regression analysis was done to detect the predictors of post-CABG cerebrovascular stroke. Early stroke was independently predicted with CAS, CPB time, initial SOFA score, prior stroke, postoperative AF and CKD. Late cerebrovascular stroke was independently predicted with CAS, AF, prior stroke, CABG plus valve surgery, HbA1c and CKD (Table 4).
Table 4.
Multivariate regression analysis
| Studied variables | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Early stroke | |||
| Carotid artery stenosis | 8.223 | 2.92–23.1 | < 0.001 |
| Cardiopulmonary bypass time | 5.64 | 1.97–16.11 | 0.001 |
| Postoperative AF | 3.014 | 1.158–7.84 | 0.024 |
| Initial SOFA score | 1.643 | 1.194–2.26 | 0.002 |
| Prior stroke | 4.412 | 1.286–15.14 | 0.018 |
| Chronic kidney disease | 1.28 | 1.05–3.75 | 0.017 |
| Late stroke | |||
| Carotid artery stenosis | 9.38 | 3.72–23.66 | < 0.001 |
| CABG plus valve surgery | 7.44 | 2.75–20.145 | < 0.001 |
| Chronic kidney disease | 2.33 | 1.056–5.165 | 0.036 |
| HbA1c | 1.431 | 1.071–1.914 | 0.016 |
| Prior stroke | 3.725 | 1.179–11.768 | 0.025 |
| Atrial fibrillation | 2.35 | 1.025–5.41 | 0.016 |
Discussion
Our study reported that 7.5% of CABG patients developed acute postoperative cerebrovascular strokes, and the occurrence of stroke was associated with longer hospitalization, many morbidities and increased mortality. Moreover, Kaplan–Meier curves of 5-year survival showed increased mortality in those patients with postoperative stroke (HR: 23.03; 95% CI: 6.10–86.92, p < 0.001). Many studies reported different incidences and mortality after CABG due to differences with the study designs, studied populations and surgical techniques [7, 9, 15, 16].
Bucerius et al. [7] studied a total of 16,184 patients and reported that 1.9% of isolated CABG patients and 7.4% of combined valve and CABG surgery patients developed acute postoperative stroke and confirmed that postoperative stroke was linked to a sixfold increase in 30-day mortality. Cao et al. [9] studied 430 CABG patients and reported an incidence of acute stroke of 7.4%. Dacey et al. [15] studied 35,733 CABG patients and reported occurrence of acute stroke in 1.61% with increased mortality and decreased 5-year survival among patients with physical limitations. Tarakji et al. [16] retrospectively studied 45,432 CABG patients with a 1.6% incidence of acute stroke and reported the association of stroke with worse many outcomes and longer hospitalization compared with the non-stroke group.
According to our results, the inotropic support, arrhythmias, postoperative IABP and ECMO support and multi-organ dysfunctions were significantly more frequent in the stroke group compared with the non-stroke group. Gaudino et al. [1] reported that thromboembolism and hypoperfusion were the causes of intra-operative stroke, while early postoperative stroke occurs due to unstable hemodynamics and arrhythmias. Post-cardiotomy low cardiac output syndrome (LCOS) represents a life-threatening state with severe reduction in cardiac systolic function and cardiac index resulting in impaired global tissue perfusion, multi-organ dysfunction and increased mortality [17, 18]. Algarni et al. [18] studied 25,176 CABG patients and reported that LCOS was predicted with emergency surgery, re-operative CABG, female gender and severe left ventricular systolic dysfunction. Our results showed significantly increased frequencies of AKI and dialysis in the stroke group which may be related to the postoperative renal hypoperfusion and increased inotropic support ± mechanical circulatory support. We used postoperative arterial blood lactate level to detect tissue hypoperfusion, and the patients with acute cerebral stroke showed progressive hyperlactatemia. Post-cardiotomy hyperlactatemia has been linked to increased mortality and different morbidities [19–21]. We used the SOFA scoring of the studied patients and for follow-up of organ functions after 48 h. The SOFA score was used in many studies and proved a high performance in different patients' groups including post-cardiotomies and patients on ECMO support [22, 23]. The SOFA score was associated with the early postoperative stroke in our regression analysis.
Perioperative cerebral embolism may occur due to cardiac, aortic, carotid, or cardiopulmonary bypass circuit sources. In this study, CPB time was significantly prolonged in the acute cerebral stroke group and was a predictor of early stroke. J. Bucerius et al. [7] reported that CPB time ≥ 120 min was a predictor of stroke and also reported the small stroke incidence with beating heart surgery. Aortic cannulation, application or removal of clamping and proximal graft anastomoses are important embolic sources during CABG [24, 25]. Ascending aorta atherosclerosis was documented in ≥ 50% of CABG patients and dislodgment of atheromatous debris or calcium can occur during application or removal of cross-clamping [25, 26]. It was reported that postoperative stroke occurred in 8.7% and 1.8% in patients with and without ascending aorta atherosclerosis, respectively [27]. There is a synergistic relation between cerebral embolism and hypoperfusion during CABG especially with the presence of carotid atherosclerosis resulting in watershed infarcts [28, 29]. Watershed infarcts may be cortical/external between the cortical branches of cerebral arteries or internal in the white matter between cortical and perforator branches of cerebral arteries [29]. Gottesman et al. [28] showed the association between watershed infarcts and intraoperative hypotension and reported the increased mortality with these infarcts. Also that study reported that MRI (48%) was better than CT scans (22%) for detection of watershed infarcts.
Carotid artery stenosis (CAS) was associated with both early and late postoperative strokes in our study. Raffa et al. [30] recently reported the increased risk of post-cardiotomy stroke with presence of CAS regardless of the degree of stenosis. Naylor et al. [31] reported that CAS was a predictor of postoperative stroke and the risk positively correlated with increased severity of CAS. Stamou et al. [32] retrospectively studied 16,528 CABG patients and reported that the stroke predictors included CAS, CKD, DM, prior cerebrovascular stroke, left ventricular systolic dysfunction, atrial fibrillation and age more than 75 years.
Our regression analysis showed that presence of previous cerebrovascular stroke was a predictor of both early and late new postoperative stroke and this finding agreed with other previous studies [7, 32]. So proper preoperative assessment for recurrent strokes should be done including carotids and ascending aorta evaluation.
Postoperative atrial fibrillation (POAF) occurred in 26.6% of our studied patients and was a predictor of early post-CABG stroke in the multivariate analysis. Moreover, atrial fibrillation was a predictor of late post-CABG stroke. POAF was reported in about 25% of isolated CABG patients and 50% after combined CABG and valve cardiotomies [33, 34]. POAF was previously considered as transient post-cardiotomy arrhythmia, but it was linked to prolonged hospitalization, readmissions and increased mortality [35, 36].
Lahtinen et al. [34] studied 2630 CABG patients and reported that 36.5% of the patients with postoperative strokes had experienced attacks of POAF. Despite POAF was related to local inflammation with atriotomy incisions and pericardial disruption or systemic inflammation with cardiopulmonary bypass and catecholamine infusions, there are patients’ factors that make POAF persistent like underling cardiac dysfunction. Recently, POAF was reported as a predisposing factor to late stroke during follow-up after CABG [35, 37]. Compared with the non-stroke group, our results showed that preoperative use of beta-blockers and statin therapies was significantly less frequent, while postoperative inotropes therapy was more frequent in the acute cerebral stroke group. Peri-operative use of beta-blockers was recommended as a class I according to European Associations of Cardiothoracic Surgeons (EACTS) and the European Society of Cardiology (ESC) guidelines [38]. Perioperative β-blockers therapy, unless contraindicated, was recommended as a class I, while amiodarone was given a class IIa recommendation according to the European Association of Cardiothoracic Anesthetists (EACTA) and the Society of Cardiovascular Anesthesiologists (SCA) guidelines [39].
Chronic kidney disease was a significant variable associated with both early and late cerebrovascular strokes after CABG in our regression analysis. Safaie et al. [40] reported the increased mortality and frequent morbidities after CABG in patients with CKD. Li et al. [41] reported the association between CKD and 30 days’ mortality and multiple outcomes including acute postoperative stroke.
Despite our results showed insignificant frequencies of diabetes mellitus (DM) between patients with and without acute postoperative strokes, the glycated hemoglobin (HbA1c) levels were significantly different between both groups reflecting a difference in preoperative blood sugar control. The HbA1c was a predictor of late cerebrovascular stroke after CABG in our regression analysis. Nyström et al. [42] studied 53,820 CABG patients and reported the higher risk of cerebrovascular strokes in patients with DM during follow-up.
Chronic hyperglycemia was associated with increased cardiovascular and cerebrovascular risks due to endothelial dysfunction, oxidative stress and enhanced atherosclerosis. The atherosclerotic burden with DM was proved in different studies, and the risks associated with DM were related to the control of blood glucose level [43–45]. Recently, Gao et al. [46] conducted the CARE- II study and reported the extensive carotid atheromas with significant calcifications and lipid-rich necrotic cores in patients with DM.
Since the use of thrombolysis during peri-operative period is contraindicated and strokes were linked to increased mortality, mechanical cerebral thrombectomy has been an emergent procedure to improve the neurological outcomes [47, 48]. The experience of postoperative cerebral thrombectomy is limited to few case reports [49, 50]. In our heart center, we decreased the use of narcotic analgesia and deep sedation immediately after cardiac surgery for close neurological assessment and early detection of any focal sign of neurological impairment. In case of presumed stroke, stroke code is activated and brain imaging with perfusion study will be done for possibility of mechanical thrombectomy in case of large vessel occlusion. Cerebral thrombectomy is considered in case of ischemic stroke with proximal large artery occlusion within 6 h of manifestations and it may be extended to 24 h according to CT perfusion data [48, 51]. Early detection of acute stroke is particularly a crucial step in proper management and the presence of established easy referral pathway to the neurosciences department allowed us to use the stroke code after cardiotomy and providing the mechanical thrombectomy procedure for the proper candidates.
Conclusions
The development of post-CABG acute cerebrovascular stroke is associated with longer hospitalization, multiple morbidities and increased mortality. Careful assessment and management of risk factors especially atrial fibrillation and carotid artery stenosis should be implemented to decrease this substantial complication after CABG.
Study limitations
The study was a single-center retrospective analysis. We could not assess the extent of preoperative aortic atherosclerosis and the blood sugar control data during hospital perioperative stay.
Acknowledgements
The abstract was presented in Acute Cardiovascular Care association congress (ACVC 2022) of the European Society of Cardiology, and it was published in the European Heart Journal supplement. https://academic.oup.com/ehjacc/article/11/Supplement_1/zuac041.048/6576944.
Abbreviations
- SOFA
Sequential organ failure assessment
- POAF
Postoperative atrial fibrillation
- CABG
Coronary artery bypass grafting
- DM
Diabetes mellitus
- CKD
Chronic kidney disease
- CT
Computed tomography
- AF
Atrial fibrillation
- CAS
Carotid artery stenosis
- OR
Odds ratio
- CPB
Cardiopulmonary bypass
- AKI
Acute kidney injury
- CI
Confidence interval
- TIAs
Transient ischemic attacks
- MRI
Magnetic resonance imaging
- LVEF
Left ventricular ejection fraction
Author contributions
ML participated in study design, data collection, statistical analysis, drafting of manuscript. MA participated in study design and data analysis. ZA participated in study design, analysis of data and manuscript revision. MM, SMA, MA, SAA and BA participated in study design, data collection and interpretation of data. All authors have read and approved the manuscript.
Funding
The authors did not receive any fund for the study nor publication.
Availability of data and materials
The data of the study are available with the corresponding author.
Declarations
Ethics approval and consent to participate
The study was approved by the ethical committee of King Faisal Specialist Hospital and Research Center (KFSHRC), given a reference number (2225181) and waived from a specific consent as there are no personal identifiable data or photographs.
Consent for publication
For the 2 patients mentioned in figures 3 and 4, we got informed consents.
Competing interests
The authors declare that there is no competing interest.
Footnotes
The original version of this article was revised: The “consent for publication” and “acknowledgement” sections have been update.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/8/2022
A Correction to this paper has been published: 10.1186/s43044-022-00318-1
References
- 1.Gaudino M, Angiolillo DJ, Di Franco A, Capodanno D, Bakaeen F, Farkouh ME, Fremes SE, Holmes D, Girardi LN, Nakamura S, Head SJ. Stroke after coronary artery bypass grafting and percutaneous coronary intervention: incidence, pathogenesis, and outcomes. J Am Heart Assoc. 2019;8(13):e013032. doi: 10.1161/JAHA.119.013032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Head SJ, Milojevic M, Daemen J, Ahn JM, Boersma E, Christiansen EH, Domanski MJ, Farkouh ME, Flather M, Fuster V, Hlatky MA. Mortality after coronary artery bypass grafting versus percutaneous coronary intervention with stenting for coronary artery disease: a pooled analysis of individual patient data. Lancet. 2018;391(10124):939–948. doi: 10.1016/S0140-6736(18)30423-9. [DOI] [PubMed] [Google Scholar]
- 3.Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Ståhle E, Feldman TE, Van Den Brand M, Bass EJ, Van Dyck N. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360(10):961–972. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 4.Capodanno D, Capranzano P, Di Salvo ME, Caggegi A, Tomasello D, Cincotta G, Miano M, Patané M, Tamburino C, Tolaro S, Patané L. Usefulness of SYNTAX score to select patients with left main coronary artery disease to be treated with coronary artery bypass graft. JACC Cardiovasc Interv. 2009;2(8):731–738. doi: 10.1016/j.jcin.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Writing Committee Members. Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, Bittl JA, Cohen MG, DiMaio JM, Don CW. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: executive summary: a report of the american college of cardiology/american heart association joint committee on clinical practice guidelines. J Am Coll Cardiol. 2022;79(2):197–215. doi: 10.1016/j.jacc.2021.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Sun LY, Tu JV, Lee DS, Beanlands RS, Ruel M, Austin PC, Eddeen AB, Liu PP. Disability–free survival after coronary artery bypass grafting in women and men with heart failure. Open Heart. 2018;5(2):e000911. doi: 10.1136/openhrt-2018-000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bucerius J, Gummert JF, Borger MA, Walther T, Doll N, Onnasch JF, Metz S, Falk V, Mohr FW. Stroke after cardiac surgery: a risk factor analysis of 16,184 consecutive adult patients. Ann Thorac Surg. 2003;75(2):472–478. doi: 10.1016/S0003-4975(02)04370-9. [DOI] [PubMed] [Google Scholar]
- 8.Cornwell LD, Omer S, Rosengart T, Holman WL, Bakaeen FG. Changes over time in risk profiles of patients who undergo coronary artery bypass graft surgery: the veterans affairs surgical quality improvement program (VASQIP) JAMA Surg. 2015;150(4):308–315. doi: 10.1001/jamasurg.2014.1700. [DOI] [PubMed] [Google Scholar]
- 9.Cao L, Li Q, Bi Q, Yu Q-J. Risk factors for recurrent stroke after coronary artery bypass grafting. J Cardiothorac Surg. 2011;6:157. doi: 10.1186/1749-8090-6-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, Elkind MS, George MG, Hamdan AD, Higashida RT, Hoh BL. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart association/American stroke association. Stroke. 2013;44(7):2064–2089. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21(2):93–111. doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Grant EG, Benson CB, Moneta GL, Alexandrov AV, Baker JD, Bluth EI, Carroll BA, Eliasziw M, Gocke J, Hertzberg BS, Katanick S. Carotid artery stenosis: gray-scale and Doppler US diagnosis—society of radiologists in ultrasound consensus conference. Radiology. 2003;229(2):340–346. doi: 10.1148/radiol.2292030516. [DOI] [PubMed] [Google Scholar]
- 13.Levin A, Stevens PE, Bilous RW, Coresh J, De Francisco AL, De Jong PE, Griffith KE, Hemmelgarn BR, Iseki K, Lamb EJ, Levey AS, Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1–50. [Google Scholar]
- 14.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure–definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):1–9. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dacey LJ, Likosky DS, Leavitt BJ, Lahey SJ, Quinn RD, Hernandez F, Jr, Quinton HB, Desimone JP, Ross CS, O'Connor GT, Northern New England Cardiovascular Disease Study Group Perioperative stroke and long-term survival after coronary bypass graft surgery. Ann Thorac Surg. 2005;79(2):532–536. doi: 10.1016/j.athoracsur.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 16.Tarakji KG, Sabik JF, Bhudia SK, Batizy LH, Blackstone EH. Temporal onset, risk factors, and outcomes associated with stroke after coronary artery bypass grafting. JAMA. 2011;305(4):381–390. doi: 10.1001/jama.2011.37. [DOI] [PubMed] [Google Scholar]
- 17.Lomivorotov VV, Efremov SM, Kirov MY, Fominskiy EV, Karaskov AM. Low-cardiac-output syndrome after cardiac surgery. J Cardiothorac Vasc Anesth. 2017;31(1):291–308. doi: 10.1053/j.jvca.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 18.Algarni KD, Maganti M, Yau TM. Predictors of low cardiac output syndrome after isolated coronary artery bypass surgery: trends over 20 years. Ann Thorac Surg. 2011;92(5):1678–1684. doi: 10.1016/j.athoracsur.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 19.Lindsay AJ, Xu M, Sessler DI, Blackstone EH, Bashour CA. Lactate clearance time and concentration linked to morbidity and death in cardiac surgical patients. Ann Thorac Surg. 2013;95(2):486–492. doi: 10.1016/j.athoracsur.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 20.Laimoud M, Alanazi M. The clinical significance of blood lactate levels in evaluation of adult patients with veno-arterial extracorporeal membrane oxygenation. Egypt Heart J. 2020;72:74. doi: 10.1186/s43044-020-00108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mak NT, Iqbal S, de Varennes B, Khwaja K. Outcomes of post-cardiac surgery patients with persistent hyperlactatemia in the intensive care unit: a matched cohort study. J Cardiothorac Surg. 2016;24(11):33. doi: 10.1186/s13019-016-0411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambden S, Laterre PF, Levy MM, Francois B. The SOFA score—development, utility and challenges of accurate assessment in clinical trials. Crit Care. 2019;23(1):1–9. doi: 10.1186/s13054-019-2663-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laimoud M, Alanazi M. The validity of SOFA score to predict mortality in adult patients with cardiogenic shock on venoarterial extracorporeal membrane oxygenation. Crit Care Res Prac. 2020;2020:1–9. doi: 10.1155/2020/3129864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergman P, Van Der Linden J. Atherosclerosis of the ascending aorta as a major determinant of the outcome of cardiac surgery. Nat Clin Pract Cardiovasc Med. 2005;2(5):246–251. doi: 10.1038/ncpcardio0192. [DOI] [PubMed] [Google Scholar]
- 25.Head SJ, Börgermann J, Osnabrugge RL, Kieser TM, Falk V, Taggart DP, Puskas JD, Gummert JF, Kappetein AP. Coronary artery bypass grafting: part 2—optimizing outcomes and future prospects. Eur Heart J. 2013;34(37):2873–2886. doi: 10.1093/eurheartj/eht284. [DOI] [PubMed] [Google Scholar]
- 26.Gerriets T, Schwarz N, Sammer G, Baehr J, Stolz E, Kaps M, Kloevekorn WP, Bachmann G, Schönburg M. Protecting the brain from gaseous and solid micro-emboli during coronary artery bypass grafting: a randomized controlled trial. Eur Heart J. 2010;31(3):360–368. doi: 10.1093/eurheartj/ehp178. [DOI] [PubMed] [Google Scholar]
- 27.van der Linden J, Hadjinikolaou L, Bergman P, Lindblom D. Postoperative stroke in cardiac surgery is related to the location and extent of atherosclerotic disease in the ascending aorta. J Am Coll Cardiol. 2001;38(1):131–135. doi: 10.1016/s0735-1097(01)01328-6. [DOI] [PubMed] [Google Scholar]
- 28.Gottesman RF, Sherman PM, Grega MA, Yousem DM, Borowicz LM, Jr, Selnes OA, Baumgartner WA, McKhann GM. Watershed strokes after cardiac surgery: diagnosis, etiology, and outcome. Stroke. 2006;37(9):2306–2311. doi: 10.1161/01.STR.0000236024.68020.3a. [DOI] [PubMed] [Google Scholar]
- 29.Naylor AR, Mehta Z, Rothwell PM, Bell PR. Carotid artery disease and stroke during coronary artery bypass: a critical review of the literature. Eur J Vasc Endovasc Surg. 2002;23(4):283–294. doi: 10.1053/ejvs.2002.1609. [DOI] [PubMed] [Google Scholar]
- 30.Raffa GM, Agnello F, Occhipinti G, Miraglia R, Lo Re V, Marrone G, Tuzzolino F, Arcadipane A, Pilato M, Luca A. Neurological complications after cardiac surgery: a retrospective case-control study of risk factors and outcome. J Cardiothorac Surg. 2019;14(1):1–9. doi: 10.1186/s13019-019-0844-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.NaylorAR M, Rothwell PM, et al. Carotid artery disease and stroke during coronary artery bypass: a critical review of the litera·· 23· ture. Eur J Vasc Endovase Surg. 2002;23:283–294. doi: 10.1053/ejvs.2002.1609. [DOI] [PubMed] [Google Scholar]
- 32.Stamou SC, Hill PC, Dangas G, Pfister AJ, Boyce SW, Dullum MK, Bafi AS, Corso PJ. Stroke after coronary artery bypass: incidence, predictors, and clinical outcome. Stroke. 2001;32(7):1508–1513. doi: 10.1161/01.str.32.7.1508. [DOI] [PubMed] [Google Scholar]
- 33.D'Agostino RS, Jacobs JP, Badhwar V, Fernandez FG, Paone G, Wormuth DW, Shahian DM. The society of thoracic surgeons adult cardiac surgery database: 2018 update on outcomes and quality. Ann Thorac Surg. 2018;105(1):15–23. doi: 10.1016/j.athoracsur.2017.10.035. [DOI] [PubMed] [Google Scholar]
- 34.Lahtinen J, Biancari F, Salmela E, Mosorin M, Satta J, Rainio P, Rimpiläinen J, Lepojärvi M, Juvonen T. Postoperative atrial fibrillation is a major cause of stroke after on-pump coronary artery bypass surgery. Ann Thorac Surg. 2004;77(4):1241–1244. doi: 10.1016/j.athoracsur.2003.09.077. [DOI] [PubMed] [Google Scholar]
- 35.Burrage PS, Low YH, Campbell NG, O'Brien B. New-onset atrial fibrillation in adult patients after cardiac surgery. Curr Anesthesiol Rep. 2019;9(2):174–193. doi: 10.1007/s40140-019-00321-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinberg BA, Zhao Y, He X, Hernandez AF, Fullerton DA, Thomas KL, Mills R, Klaskala W, Peterson ED, Piccini JP. Management of postoperative atrial fibrillation and subsequent outcomes in contemporary patients undergoing cardiac surgery: insights from the society of thoracic surgeons CAPS-Care atrial fibrillation registry. Clin Cardiol. 2014;37(1):7–13. doi: 10.1002/clc.22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Megens MR, Churilov L, Thijs V. New-onset atrial fibrillation after coronary artery bypass graft and long-term risk of stroke: a meta-analysis. J Am Heart Assoc. 2017;6(12):e007558. doi: 10.1161/JAHA.117.007558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 39.O'Brien B, Burrage PS, Ngai JY, Prutkin JM, Huang CC, Xu X, Chae SH, Bollen BA, Piccini JP, Schwann NM, Mahajan A. Society of Cardiovascular Anesthesiologists/European Association of Cardiothoracic Anaesthetists practice advisory for the management of perioperative atrial fibrillation in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2019;33(1):12–26. doi: 10.1053/j.jvca.2018.09.039. [DOI] [PubMed] [Google Scholar]
- 40.Safaie N, Chaichi P, Habibzadeh A, Nasiri B. Postoperative outcomes in patients with chronic renal failure undergoing coronary artery bypass grafting in madani heart center: 2000–2010. J Cardiovasc Thorac Res. 2011;3(2):53–56. doi: 10.5681/jcvtr.2011.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X, Zhang S, Xiao F. Influence of chronic kidney disease on early clinical outcomes after off-pump coronary artery bypass grafting. J Cardiothorac Surg. 2020;15(1):199. doi: 10.1186/s13019-020-01245-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nyström T, Holzmann MJ, Sartipy U. Long-term risk of stroke in patients with type 1 and type 2 diabetes following coronary artery bypass grafting. J Am Heart Assoc. 2015;4(11):e002411. doi: 10.1161/JAHA.115.002411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Natali A, Vichi S, Landi P, Severi S, L'Abbate A, Ferrannini E. Coronary atherosclerosis in Type II diabetes: angiographic findings and clinical outcome. Diabetologia. 2000;43(5):632–641. doi: 10.1007/s001250051352. [DOI] [PubMed] [Google Scholar]
- 44.Laimoud M, Faris F, Elghawaby H. Intravascular evaluation of coronary atherosclerotic lesions among Egyptian diabetic patients with acute coronary syndromes. Egypt Heart J. 2018;70(4):237–241. doi: 10.1016/j.ehj.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wagenknecht LE, Zaccaro D, Espeland MA, Karter AJ, O'Leary DH, Haffner SM. Diabetes and progression of carotid atherosclerosis: the insulin resistance atherosclerosis study. Arterioscler Thromb Vasc Biol. 2003;23(6):1035–1041. doi: 10.1161/01.ATV.0000072273.67342.6D. [DOI] [PubMed] [Google Scholar]
- 46.Gao X, Song J, Watase H, Hippe DS, Zhao X, Canton G, Tian F, Du R, Ji S, Yuan C, CARE-II Investigators Differences in carotid plaques between symptomatic patients with and without diabetes mellitus: a care-II study. Arterioscler Thromb Vasc Biol. 2019;39(6):1234–1239. doi: 10.1161/ATVBAHA.118.312092. [DOI] [PubMed] [Google Scholar]
- 47.Mistry EA, Mistry AM, Nakawah MO, Chitale RV, James RF, Volpi JJ, Fusco MR. Mechanical thrombectomy outcomes with and without intravenous thrombolysis in stroke patients: a meta-analysis. Stroke. 2017;48(9):2450–2456. doi: 10.1161/STROKEAHA.117.017320. [DOI] [PubMed] [Google Scholar]
- 48.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Dávalos A, Majoie CB, van der Lugt A, De Miquel MA, Donnan GA. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–1731. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 49.Madeira M, Martins C, Koukoulis G, Marques M, Reis J, Abecassis M. Mechanical thrombectomy for stroke after cardiac surgery. J Card Surg. 2016;31(8):517–520. doi: 10.1111/jocs.12776. [DOI] [PubMed] [Google Scholar]
- 50.Yun JW, Ahn SW, Kim YH, Min J, Choi YS, Chae YK, Lee ES, Kang Y. Intraarterial mechanical thrombectomy for the treatment of postoperative cerebral infarction: a case report. Korean J Anesthesiol. 2014;66(5):402–406. doi: 10.4097/kjae.2014.66.5.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Evans MR, White P, Cowley P, Werring DJ. Revolution in acute ischaemic stroke care: a practical guide to mechanical thrombectomy. Pract Neurol. 2017;17(4):252–265. doi: 10.1136/practneurol-2017-001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of the study are available with the corresponding author.