Abstract
This study aimed to evaluate the effect of Lactobacillus plantarum 200655 and fructooligosaccharides (FOS) on soymilk fermentation and the neuroprotective effects of fermented soymilk (FS). The addition of FOS did not affect the physicochemical properties during fermentation. It helped that L. plantarum 200655 survive for 21 days of storage at 4 °C. FOS increased the β-glucosidase activity of L. plantarum 200655, total phenolic content, and antioxidant activities, such as radical scavenging and reducing power of FS. In addition, FS with FOS exerted neuroprotective effects in SH-SY5Y cells against H2O2-induced oxidative stress. FS with 3% and 5% FOS (FS3 and FS5) significantly increased cell viability and gene expression of neuronal markers, such as brain-derived neurotrophic factor and tyrosine hydroxylase. Moreover, FS3 and FS5 significantly reduced lactate dehydrogenase release and the gene expression of Bax/Bcl-2 ratio, caspase-9, and caspase-3. These results indicated that FS3 and FS5, with enhanced antioxidant properties, could protect SH-SY5Y cells against H2O2-induced damage. Therefore, soymilk fermented with L. plantarum 200655 and FOS can be used as a prophylactic functional food with neuroprotective effects against oxidative stress.
Keywords: Lactobacillus plantarum, Fructooligosaccharide, Soymilk fermentation, Antioxidant activity, Neuroprotective effect
Introduction
Soybean is an important leguminous plant with high nutritional value and various health benefits. The soy products can alleviate the development of cancers, cardiovascular diseases, osteoporosis, and gastric disorders (Hubert et al. 2008). Chungkukjang, tempeh, and miso showed anti-cancer effects (Seo et al. 2009). Soybean protein showed antihypertensive effects (Mujtaba et al. 2021). Fermented soymilk with LAB could mitigate osteoporosis in both human and animal studies (Chiang and Pan 2011). Soymilk, the water extract of soybean, is a cholesterol and lactose-free product. Soymilk is the only vegetable food containing all the essential amino acids. Thus, soymilk has been consumed as an alternative to dairy products for lactose intolerance, allergies to milk proteins, or a vegan diet.
Fermentation with lactic acid bacteria (LAB) has been used to improve food quality and nutritional value. Soymilk has an undesirable beany flavor and indigestible oligosaccharides, triggering flatulence or stomach troubles (Hubert et al. 2008). LAB can reduce soy oligosaccharides during fermentation with α-galactosidase. Lactic fermentation can enhance the level of isoflavone aglycones, which are known to possess beneficial properties such as antioxidant activity. Isoflavone in soybean mainly exists in glucosides, which are more difficult to absorb than aglycones. The β-glycosidic bond of isoflavone glucosides could be hydrolyzed by β-glucosidase by LAB (Tsangalis et al. 2002). The β-glucosidase also influences the thermal stability of probiotics (Jang et al. 2018). Because the survival and metabolic activity of probiotics can be enhanced by the synergistic effects of synbiotics, the bio-functionality of soymilk can be improved through fermentation.
Oxidative stress is induced by an imbalance between reactive oxygen species (ROS) production and antioxidant defense systems. The brain is specifically susceptible to ROS because it requires a considerable oxygen supply and is rich in peroxide-sensitive fatty acids. Excessive ROS causes enzyme inactivation, protein oxidation, DNA breakage, and lipid peroxidation, resulting in neurodegenerative diseases such as Parkinson’s disease (PD), Alzheimer’s disease (AD), and Huntington’s disease (HD) (Tian et al. 2020). H2O2, the major ROS, quickly diffuses across the cell membrane and generates highly reactive radicals that induce neuronal apoptosis. Apoptosis is one of the most sensitive biomarkers for assessing ROS-induced oxidative stress. Apoptotic cells undergo morphological and biochemical changes, resulting in cell death (Nirmaladevi et al. 2014). Thus, inhibition of apoptosis and maintaining redox balance are important to protect neuronal cells against oxidative stress.
The probiotic Lactobacillus plantarum 200655 has been reported to protect neuroblastoma cells against H2O2 by increasing brain-derived neurotrophic factor (BDNF) and tyrosine hydroxylase (TH) and regulating apoptotic factors (Cheon et al. 2021). Therefore, this study aimed to apply L. plantarum 200655 and FOS to soymilk fermentation and investigate fermented soymilk's antioxidant activity and neuroprotective effects against H2O2-induced oxidative stress.
Material and methods
Used microorganisms and culture conditions
Lactobacillus plantarum 200655 (KCCM 12204P) isolated from kimchi was used as a starter. L. plantarum 200655 was cultured in Man, Rogosa, and Sharpe (MRS; Difco Laboratories, Detroit, MI, USA) broth at 37 °C for 24 h. The strain was stored at − 80 °C with 20% (v/v) glycerol until use.
Screening of prebiotics using growth assay
The availability of four prebiotics (fructooligosaccharides (FOS), galactooligosaccharides (GOS), inulin, and xylitol) was evaluated based on growth index (GI) (Kariyawasam et al. 2020). Briefly, MRS medium without glucose (negative control; MB cell, Seoul, Korea) was supplemented with 2% (w/v) prebiotics or glucose (positive control). Each medium was inoculated with 107 CFU/mL L. plantarum 200655 and incubated at 37 °C for 24 h. The optical density was measured at 600 nm, and the GI was calculated as follows:
where Asample and Acontrol represent the absorbance of each sample and positive control, respectively.
Fermentation of soymilk
Soymilk (Maeil Dairies Co., Seoul, Korea) was supplemented with filtered FOS (0%, 1%, 3%, and 5% (w/v)) with 0.45-μm pore size filter. And then, the prepared soymilk was sterilized at 121 °C for 15 min and cooled to 37 °C. Sterilized soymilk was inoculated with 2% (v/v) of L. plantarum 200655 and incubated at 37 °C until the pH reached 4.5. Fermented soymilk (FS) was stored for 21 days at 4 °C to evaluate cell viability and post-acidification.
Viable cell counts, pH, and titratable acidity (TA)
Viable cell counts were measured by plate counting on MRS agar. An aliquot of each FS was serially diluted and plated on MRS agar. The plates were incubated for 24 h at 37 °C. The pH was measured using a pH meter (WTW, Weiheim, Germany). To determine titratable acidity (TA), 10 g of FS was suspended in 10 mL of distilled water and titrated with 0.1 N NaOH until the pH reached 8.3. The TA was expressed as follows:
Preparation of fermented soymilk supernatant
Antioxidant and neuroprotective effects were estimated using the supernatant of the FS. Briefly, 10 g of FS was homogenized with sterile distilled water (2.5 mL) and centrifuged at 14,240×g for 5 min at 4 °C. The separated supernatant was adjusted to pH 7.0, with 1 M NaOH, and filtered through a 0.45-μm pore size filter (Advantec, Tokyo, Japan). The acquired supernatant was stored at − 20 °C.
Determination of β-glucosidase activity
The β-glucosidase activity of L. plantarum 200655 in FS was measured by Jang et al. (2018). Two hundred microliters of FS were added to 400 μL of 5 mM 4-nitrophenyl β-D-glucopyranoside (ρNPG) in 0.2 M sodium phosphate buffer (pH 7.0) and incubated for 30 min at 37 ℃. The reaction was terminated by adding 800 μL of 1 M Na2CO3. The mixture was centrifuged at 14,240×g at 4 °C for 10 min. The supernatant was determined at 405 nm. One unit of β-glucosidase activity was defined as the release of 1 μM p-nitrophenol from the substrate per min.
Total phenolic content (TPC)
The total phenolic content (TPC) was measured using the modified method of Kim et al. (2020). Briefly, 50 μL of FS was mixed with 1 mL of 2% Na2CO3. After 5 min, 50 μL of 50% Folin-Ciocalteu reagent was added to the mixture. After 30 min, the absorbance was measured at 750 nm. TPC was expressed as mg gallic acid equivalent (GAE)/mL.
Determination of antioxidant activity
1,1-Diphenyl-2-picryl-hydrazyl radical (DPPH) radical scavenging activity was measured using a modified method by Bae et al. (2019). Briefly, 150 μL of the sample was mixed with 750 μL of 0.1 mM DPPH solution (Sigma-Aldrich (St. Louis, MO, USA)) in ethanol. After 30 min, the solution was centrifuged at 14,240 × g for 1 min. The absorbance of the supernatant was measured at 517 nm. DPPH radical scavenging activity was calculated as follows:
where Asample and Acontrol represent the absorbance of each sample and control, respectively.
2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) radical scavenging activity was measured using a modified method of Bae et al. (2019). ABTS solution was prepared by mixing 14 mM ABTS (Sigma-Aldrich) and 5 mM potassium persulfate in 0.1 M potassium phosphate buffer (pH 7.4) at a 1:1 ratio. ABTS solution was reacted for 16–18 h at room temperature and diluted to an absorbance of 0.7 ± 0.02 at 734 nm. Twenty microliters of sample were mixed with 980 μL of diluted ABTS solution and incubated for 5 min in the dark. The absorbance was measured at 734 nm. ABTS radical scavenging activity was calculated as follows:
where Asample and Acontrol represent the absorbance of each sample and control, respectively.
Superoxide anion scavenging activity was measured using a modified method by Bae et al. (2019). Briefly, 200 μL of the sample, 468 μM β-nicotinamide adenine dinucleotide (NADH) (in 20 mM potassium phosphate buffer, pH 7.4), and 300 μM nitrotetrazolium blue chloride (NBT) (Sigma-Aldrich) (in buffer) were mixed. Next, 200 μL of 60 μM phenazine methosulfate (PMS) (Sigma-Aldrich) (in buffer) was added to the mixture. The mixture was allowed to react for 5 min in the dark. Absorbance was measured at 560 nm. Superoxide anion scavenging activity was calculated as follows:
where Asample and Acontrol represent the absorbance of each sample and control, respectively.
FRAP assay was determined by Lee et al. (2021). FRAP solution was prepared by mixing 10 mM 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ) (in 40 mM HCl), 20 mM FeCl3, and 0.3 M sodium acetate buffer (pH 3.6) at a 1:1:10 ratio. Fifty microliters of sample were mixed with 950 μL of FRAP solution and incubated for 30 min. The absorbance was measured at 593 nm. FRAP value was expressed as FeSO4 equivalents using a standard curve.
The reducing power assay was performed using the method of Lee et al. (2021). Briefly, 50 μL of the sample was mixed with 250 μL of 1% potassium ferricyanide and 250 μL of 0.2 M sodium phosphate buffer (pH 6.6). The mixture was incubated for 20 min at 50 °C, and 250 μL of 10% trichloroacetic acid was added. Five hundred microliters of the mixture were reacted with 400 μL of distilled water and 100 μL of 0.1% FeCl3 for 30 min. The absorbance was measured at 700 nm. The reducing power was expressed as L-cysteine equivalents using a standard curve.
Cell culture
Human neuroblastoma SH-SY5Y cells were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA) and cultured in Dulbecco’s Modified Eagle Medium (DMEM; Hyclone™, Logan, UT, USA) supplemented with 10% (v/v) fetal bovine serum (FBS; Hyclone™) and 1% (v/v) penicillin–streptomycin (Sigma-Aldrich) in a humidified atmosphere containing 5% CO2 at 37 °C.
Cell viability assay using MTT assay and LDH assay
SH-SY5Y cells were seeded in 96-well plates (1 × 105 cells/well) and incubated for 24 h. Cells were pre-treated with twofold serially diluted FS for 4 h and exposed to 250 μM H2O2 for 20 h. Following treatment, the medium was replaced with thiazolyl blue tetrazolium bromide (MTT) solution (0.5 mg/mL). The MTT solution was removed after 4 h, and dimethyl sulfoxide was added to each well to dissolve the formazan crystals. Absorbance was measured at 570 nm, and the cell viability was expressed as follows:
where Acontrol and Asample are the absorbances of the control and a sample containing FS or H2O2, respectively.
Cytotoxicity was determined by measuring the amount of LDH released into the medium. SH-SY5Y cells were seeded in a 96-well plate and treated with FS and H2O2, as described above. Following treatment, cytotoxicity was measured using the EZ-LDH kit (DoGenBio, Seoul, Korea)) according to the manufacturer’s instructions.
Relative gene expression by RT-PCR
RT-PCR was conducted to evaluate the expression of neuronal biomarkers and apoptotic genes in SH-SY5Y cells. SH-SY5Y cells were seeded in 6-well plates at 2 × 106 cells/well and incubated for 24 h. Cells were pre-treated with FS for 4 h and treated with 250 μM H2O2 for 3 h. According to the manufacturer's instructions, total RNA was isolated using the RNeasy Mini Kit (Qiagen, Hilden, Germany). The extracted RNA was converted to cDNA using a cDNA synthesis kit (Thermo-Fisher Scientific, Waltham, MA, USA). Gene expression was determined using the SYBR Green PCR Master mix (Thermo-Fisher Scientific) with real-time PCR (Thermo-Fisher Scientific). RT-PCR was performed as follows: initial denaturation at 95 °C for 10 min, followed by 40 cycles of 95 °C for 20 s, 60 °C for 20 s, and 72 °C for 30 s, followed by a final extension at 72 °C for 5 min. The relative gene expression levels were normalized to GAPDH expression and analyzed using the 2−ΔΔCt method. Specific primer sequences were used BDNF, TH, Bax, Bcl-2, caspase-3, caspase-9, and GAPDH (Cheon et al. 2021; Nirmaladevi et al. 2014; Tu et al. 2013).
Statistical analysis
All experiments were repeated in triplicate and are presented as the mean ± standard deviation. One-way analysis of variance, Duncan’s multiple range test, and Student’s t-test were used to determine significant differences. Values were considered significant at p < 0.05, and all analyses were performed using SPSS (IBM, Chicago, IL, USA).
Results and discussion
Screening of prebiotics using growth index
Specific probiotics selectively ferment prebiotics based on glycosidic linkages, chain length, monomer constituents, and the overall structure. L. plantarum 200655 selectively utilized prebiotics for growth since there were significant differences after 24 h (p < 0.05, data not shown). The negative control and xylitol showed the poorest GI values of 11.11% and 9.96%, respectively. A GI value below 25% indicates a complete inhibition of bacterial growth, and a range between 25 and 75% implies partial inhibition (Bevilacqua et al. 2016). Although the GI was slightly increased in inulin (29.31%) compared to the negative control, FOS and GOS had higher effects on growth (94.67% and 88.70%, respectively). Since FOS showed the highest GI value, FOS was considered the most potential candidate for fermentation. One of the essential criteria for prebiotic availability is chain length. FOS and inulin are included in fructans, but FOS has less than 10 and inulin has up to 60 DP. Since the chain length of FOS is shorter than that of inulin, FOS could significantly support the growth of probiotics than inulin (Davani-Davari et al. 2019; Sharma and Kanwar 2018). Kariyawasam et al. (2020) reported that FOS stimulated the growth of Lactobacillus brevis strains as much as glucose, whereas other prebiotics showed weak growth.
Bacterial growth and acidity changes during soymilk fermentation
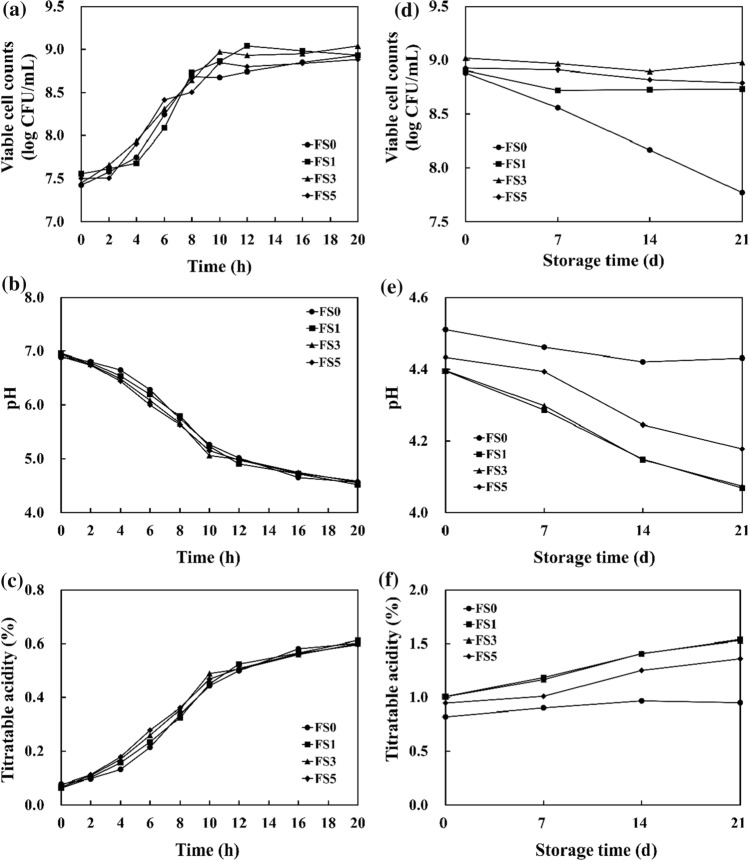
The growth of L. plantarum 200655, pH, and TA of soymilk during fermentation was investigated. L. plantarum 200655 showed similar growth patterns regardless of whether soymilk contained FOS (Fig. 1a). Viable cells ranged from 8.88 to 9.04 log CFU/mL at the end of fermentation. Like these results, five Lactobacillus strains reached the highest viable cell numbers (8.17 to 8.93 log CFU/mL) after 24 h in soymilk, the reason is sugar usability (Rekha and Vijayalakshmi 2011).
Fig. 1.
Changes of viable cell counts, pH, and titratable acidity of FS during fermentation (a–c) and refrigerated storage (d–f). FS0, fermented soymilk without FOS; FS1, fermented soymilk with 1% FOS; FS3, fermented soymilk with 3% FOS; FS5, fermented soymilk with 5% FOS
Gradual decreases in pH and increases in TA were observed during fermentation (Fig. 1b, c). The decline in pH caused soymilk coagulation, which indicated sufficient acid production for curd formation. Solidification of soy proteins begins at pH 6.4, and yogurt-like consistency of soymilk was observed between 6 and 12 h of fermentation (Rekha and Vijayalakshmi 2011). The initial pH was 6.89 to 6.90, and it took the same time (20 h) to reach pH 4.5 in all soymilks. Meanwhile, TA increased from 0.06–0.08% to 0.60–0.61%. FOS supplementation did not influence acid production or viability regardless of FOS concentration. This result implies that the presence of FOS did not inhibit the growth of L. plantarum 200655 during soymilk fermentation.
Cell viability and post-acidification during storage
FS was stored for 21 days at 4 °C, and samples were evaluated at intervals of 7 days (Fig. 1d–f). A significant reduction in cell viability was observed in FS0, which decreased from 8.88 to 7.77 log CFU/mL after 21 days (p < 0.05). On the other hand, the viable cell numbers in FS with FOS marginally decreased during this period. FS with FOS showed significantly higher viable cell numbers than FS0, ranging from 8.73 to 8.98 log CFU/mL at 21 days (p < 0.05). This implies that the presence of FOS helped L. plantarum 200655 survive during refrigerated storage. In contrast, the dramatically decreased viability in FS0 may be due to diminishing nutrients.
One of the most convenient and reliable methods to evaluate the acceptability of products and metabolic activity of probiotics is to assess post-acidification and lactic acid production (Mishra and Mishra 2013). The pH of all FS ranged from 4.40 to 4.51 at day 1 and showed a sustained reduction from 4.07 to 4.43 at 21 days (p < 0.05). The pH significantly decreased in FS with FOS than in FS0 during storage. In contrast, TA increased more in the presence of FOS during storage (p < 0.05). The initial TA was 0.82–1.01% and raised to 0.95–1.54% after 21 days. FS0 showed a significantly lower TA value (p < 0.05). Active probiotics produce acidic metabolites, such as lactic acid, which lowers pH and increases acidity. If yogurt with prebiotics showed a higher pH reduction than plain yogurt, it indicated that probiotic strains had better metabolic activity.
β-Glucosidase activity and total phenolic contents (TPC) in FS
The β-glucosidase activity of L. plantarum 200655 and total phenolic content (TPC) in FS was determined after fermentation (Table 1). Soymilk is known to be a suitable medium for lactobacilli to produce enough β-glucosidase because β-glucosidase or isoflavone glucoside-hydrolyzing enzymes could be induced in soymilk. As shown in Table 1, L. plantarum 200655 in FS0 possess 6.66 mU/mL of β-glucosidase activity, whereas activities significantly increased in FS with FOS compared to FS0 (p < 0.05). Supplementation with FOS seems to influence β-glucosidase production positively. The addition of 3% FOS resulted in the highest levels compared with the other concentrations. The production of β-glucosidase depends on starter microorganisms, fermentation periods, and the sugar content in the media. When the glucose concentration in the MRS medium increases, the number of Bifidobacterium strains capable of β-glucosidase increases (Tsangalis et al. 2002). Lim (2012) reported that Lactobacillus paracasei GK74 showed a significant increase in β-glucosidase activity when fermented in soymilk containing FOS.
Table 1.
β-Glucosidase activity of L. plantarum 200655 and total phenolic contents in FS
| Sample | β-Glucosidase activity (mU/mL) | Total phenolic contents (mg GAE/mL) |
|---|---|---|
| FS0 | 6.66 ± 0.12d | 11.81 ± 0.10c |
| FS1 | 6.95 ± 0.18c | 18.07 ± 0.15b |
| FS3 | 8.04 ± 0.31a | 18.42 ± 0.13a |
| FS5 | 7.72 ± 0.18b | 18.21 ± 0.16b |
Different letters (a–d) indicate statistical differences in the same column (p < 0.05)
FS0, fermented soymilk without FOS; FS1, fermented soymilk with 1% FOS; FS3, fermented soymilk with 3% FOS; FS5, fermented soymilk with 5% FOS
The highest TPC was measured at FS3, followed by FS5, FS1, and FS0. Similar to β-glucosidase activity, TPC in FS with FOS was significantly higher than in FS0 (p < 0.05). FS3 had the highest beta-glucosidase activity and TPC (8.04 mU/mL; 18.42 mg GAE/mL), followed by FS5 (7.72 mU/mL; 18.21 mg GAE/mL), FS1(6.95 mU/mL, 18.07 mg GAE/mL), and FS0 (6.66 mU/mL; 11.81 mg GAE/mL). The reason is enzyme activity have limit in substrate increase. When cranberry phenolics and chitosan oligosaccharides were added to soymilk, the total soluble phenolic content significantly increased after fermentation (Apostolidis et al. 2011). Some studies have been reported that the increase of phenolic content is positively correlated with enhanced β-glucosidase activity and isoflavone aglycones by lactic fermentation (Rekha and Vijayalakshmi 2011).
Antioxidant activities of FS
ROS and free radical production play key roles in regulating redox reactions and oxidative stress, significant causes of neurodegeneration. Antioxidants are involved in neutralizing ROS and other free radicals (Nirmaladevi et al. 2014). A single assay could not demonstrate the total antioxidant activity of the compounds because the total antioxidant activity is related to multiple reaction mechanisms. Thus, the relevance between antioxidant potential and compounds should be assessed using various methods.
The DPPH assay is an easy and rapid method based on single electron transfer (SET) and hydrogen atom transfer (HAT) reactions. As shown in Table 2, the DPPH radical scavenging activity of FS0 was 47.26%, whereas that of FS with FOS significantly increased by 57.16%, 60.53%, and 56.54%, respectively (p < 0.05). FS3 showed the highest activity compared to other FOS concentrations, whereas FS1 and FS5 had similar activities. Contrary to these results, Lim (2012) reported that yogurt supplemented with FOS did not enhance DPPH radical scavenging activity. Li et al. (2018) reported that soymilk fermented with L. plantarum YS-1 showed better DPPH radical scavenging activity than fermented with yogurt starter strain.
Table 2.
Antioxidant activities of FS
| Antioxidant assay | Sample | |||
|---|---|---|---|---|
| FS0 | FS1 | FS3 | FS5 | |
| DPPH radical scavenging activity (%) | 47.26 ± 1.74c | 57.16 ± 1.44b | 60.53 ± 1.38a | 56.54 ± 0.79b |
| ABTS radical scavenging activity (%) | 70.96 ± 1.79c | 75.02 ± 1.31b | 76.82 ± 1.45a | 77.81 ± 0.95a |
| Superoxide anion scavenging activity (%) | 47.84 ± 1.88b | 50.69 ± 4.08ab | 54.72 ± 6.71a | 50.12 ± 5.97ab |
| FRAP (μM FeSO4 equivalent) | 365.88 ± 12.55c | 416.22 ± 14.35b | 440.19 ± 26.57a | 408.97 ± 6.79b |
| Reducing power (mM L-Cysteine equivalent) | 1.25 ± 0.04c | 1.43 ± 0.02b | 1.59 ± 0.06a | 1.59 ± 0.03a |
Different letters (a–d) indicate statistical differences in the same row (p < 0.05)
FS0, fermented soymilk without FOS; FS1, fermented soymilk with 1% FOS; FS3, fermented soymilk with 3% FOS; FS5, fermented soymilk with 5% FOS. The data are presented as the mean ± standard deviation of three independent experiments
The ABTS assay is based on the HAT reaction, where one hydrogen atom transfers from free radicals to an antioxidant. FS5 showed the highest ABTS scavenging activity (77.81%), followed by FS3, FS1, and FS0 (Table 2). FOS supplementation significantly enhanced the activities (p < 0.05). All FS samples exhibited higher radical scavenging activity than the DPPH assay. Since the ABTS assay can measure the antioxidant activity of compounds, whether hydrophilic or lipophilic, scavenging activity from ABTS assay is generally higher than DPPH assay (Liang and Kitts 2014).
Superoxide anions are the first ROS produced in the body. Superoxide anions can be converted into other free radicals, such as H2O2, hydroxyl radicals, and singlet oxygen if they are overproduced than enzymes involved in the antioxidant system (Sharma and Singh 2012). FS0 displayed a superoxide anion scavenging activity of 47.84% (Table 2). FS3 showed a significant increase of 54.72%, whereas the addition of 1% and 5% FOS showed insignificant increase compared to FS0. Unfermented soybean broth showed no activity on superoxide anion scavenging activity. The concentration of isoflavone aglycones in fermented soy germ extracts showed a good linear correlation with superoxide anion scavenging activity (Hubert et al. 2008).
The FRAP assay is a SET-based colorimetric method that estimates the ferric reducing capacity to reduce Fe3+-TPTZ complex to Fe2+-TPTZ (Liang and Kitts 2014). The FRAP value of FS0 was 365.88 μM FeSO4, while FOS supplementation significantly increased antioxidant capacity by 13.76%, 20.31%, and 11.78% (p < 0.05).
As shown in Table 2, FS0 presented 1.25 mM L-cysteine equivalent of reducing power and significantly increased by 14.60%, 27.47%, and 27.73%, respectively, following FOS concentration (p < 0.05). The reducing power of soymilk and soy whey increased after fermentation compared to that of unfermented ones (Liu et al. 2005). The higher reducing power may be attributed to metabolites produced during fermentation, which could stabilize and terminate the radical chain reaction (Yang et al. 2000).
Biological antioxidants can prevent the uncontrolled formation of active oxygen species and free radicals and inhibit related reactions. Antioxidants exert an effect through multiple mechanisms, such as inhibiting the chain reaction, decomposition of peroxides, and chelating transition metal ions. The intake of natural antioxidants can decrease the cumulative effects of oxidatively damaged molecules (Sharma and Singh 2012). Based on these results, it could be deduced that FS supplemented with FOS had enhanced antioxidant potential and may protect against oxidative stress.
Effect of FS on H2O2-induced cytotoxicity in SH-SY5Y cells
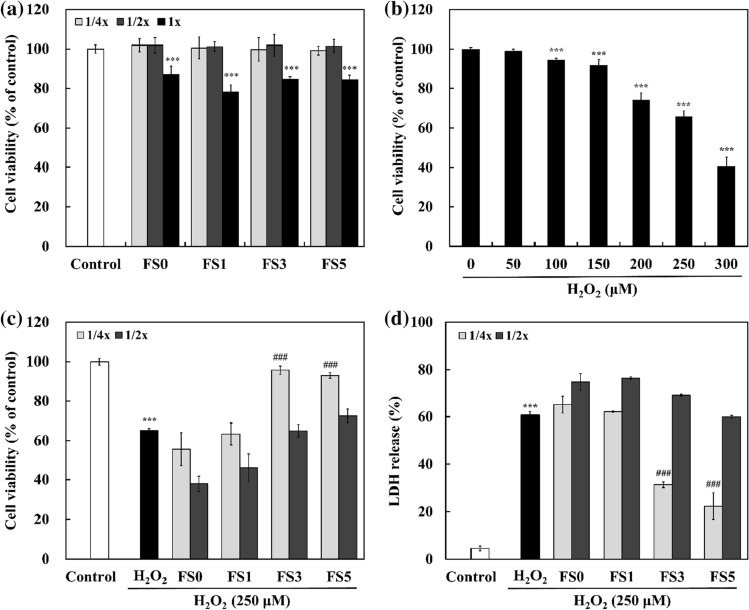
FS exerted neuroprotective effects on H2O2-treated SH-SY5Y cells by increasing cell viability and preventing LDH release. The cytotoxicity of FS on SH-SY5Y cells was determined after 24 h of treatment (Fig. 2a). Whereas 1 × FS significantly decreased the cell viability to below 90% compared to the control group, the other concentrations had no significant cytotoxicity on cell viability. Exposure to various concentrations of H2O2 (50–300 μM) for 20 h decreased cell viability in a dose-dependent manner (Fig. 2b). Cell viability was significantly decreased by 65.84% after exposure to 250 μM H2O2 (p < 0.05). Thus, 250 μM H2O2 was used in subsequent experiments. As shown in Fig. 2c, pretreatment with all 1/2 × FS did not protect SH-SY5Y cells against H2O2-induced cytotoxicity. In 1/4 × FS, no notable changes were observed in FS0 and FS1 compared to the H2O2-treated group. However, FS3 and FS5 significantly increased cell viability by 95.74% and 93.00%, respectively (p < 0.05). The neuroprotective effects of FS were further estimated using the LDH assay. LDH is a cytosolic enzyme that is released into the medium when the plasma membrane is damaged. The released LDH corresponds to damaged or dead cells (Huang et al. 2020). According to Ko et al. (2019), the metabolized product of daidzein (7,8,4′-trihydroxyisoflavone, THIF) significantly inhibited cell death and LDH leakage in SH-SY5Y cells against 6-hydroxydopamine (6-OHDA). As shown in Fig. 2d, LDH release was significantly increased in the H2O2-treated group (60.77%) compared to the control group (4.36%), indicating that H2O2 decreased the plasma membrane integrity. Similar to the MTT assay, 1/2 × FS did not prevent LDH release. However, 1/4 × FS3 and FS5 showed significantly lower LDH release by 31.27% and 22.36% than the H2O2-treated group, while FS0 and FS1 showed similar LDH release. These results indicated that FS3 and FS5 could prevent SH-SY5Y cells from H2O2-induced oxidative stress.
Fig. 2.
Neuroprotective effects of FS on H2O2-induced cytotoxicity in SH-SY5Y cells. a Effects of FS on SH-SY5Y cell viability; b effects of different concentrations of H2O2 on SH-SY5Y cell viability; c effects of FS on H2O2-treated SH-SY5Y cell viability; d LDH release of SH-SY5Y cells treated with FS and H2O2. SH-SY5Y cells were pre-treated with FS for 4 h and exposed to H2O2 (250 μM) for 20 h. The data are presented as the mean ± standard deviation of three independent experiments. FS0, fermented soymilk without FOS; FS1, fermented soymilk with 1% FOS; FS3, fermented soymilk with 3% FOS; FS5, fermented soymilk with 5% FOS. ***p < 0.001, compared to control group; ###p < 0.001, compared to H2O2-treated group
Effects of FS on gene expression in SH-SY5Y cells
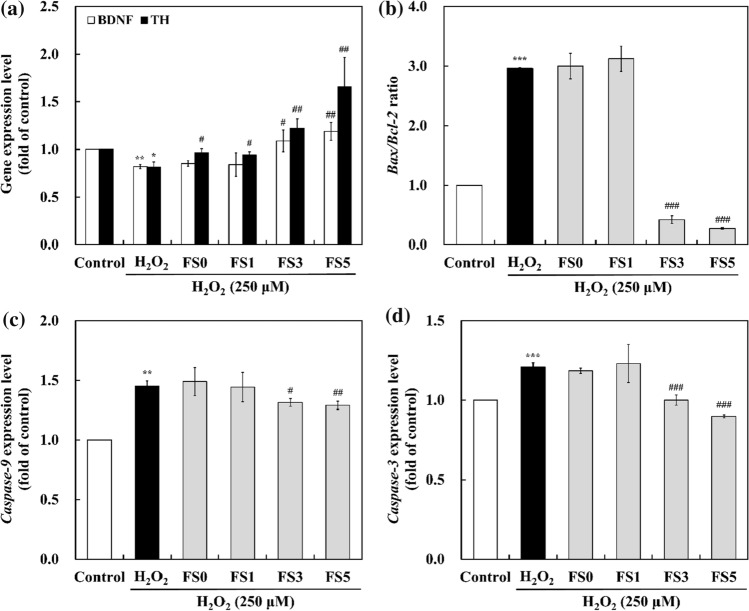
The gene expression of neuronal biomarkers and apoptosis-related factors in SH-SY5Y cells was measured by RT-PCR (Fig. 3). BDNF and TH play essential roles in neuronal development and neurotransmitter synthesis. BDNF is a neurotrophin that regulates the function, survival, growth, and differentiation of neuronal cells. TH is the rate-limiting enzyme involved in dopamine synthesis, which converts tyrosine to a dopamine precursor. Decreased BDNF and TH expression, which leads to dopamine deficiency, were observed in patients with PD. Although the mechanisms underlying the suppression of TH gene expression have not been established, the downregulated TH gene may be associated with transcriptional inhibition (Ko et al. 2019). As shown in Fig. 3a, exposure to H2O2 alone significantly decreased BDNF and TH gene expression by 0.82 and 0.81-fold, respectively (p < 0.05). Pretreatment with 1/4 × FS3 and FS5 significantly increased BDNF by 1.09-and 1.19-fold and TH expression levels by 1.23-and 1.66-fold, respectively (p < 0.05). However, 1/4 × FS0 and FS1 showed similar BDNF expression and slightly higher TH expression than the H2O2-treated group. The development of PD is attributed to oxidative stress associated with mitochondrial dysfunction (Huang et al. 2020). Increased BDNF and TH expression could inhibit neuronal apoptosis induced by different stimuli in SH-SY5Y cells (Cheon et al. 2021; Nirmaladevi et al. 2014; Tian et al. 2020). Thus, the elevated BDNF and TH expression in FS3- and FS5-treated cells may increase the survival of SH-SY5Y cells.
Fig. 3.
Relative mRNA expression of neuronal biomarkers and apoptosis-related genes using RT-PCR. a BDNF and TH expression level; b Bax/Bcl-2 ratio; c caspase-9 expression level; d caspase-3 expression level. SH-SY5Y cells were pre-treated with 1/4 × FS for 4 h and exposed to H2O2 (250 μM) for 3 h. The data are presented as the mean ± standard deviation of three independent experiments. FS0, fermented soymilk without FOS; FS1, fermented soymilk with 1% FOS; FS3, fermented soymilk with 3% FOS; FS5, fermented soymilk with 5% FOS. *p < 0.05, **p < 0.01, ***p < 0.001, compared to control group; #p < 0.05, ##p < 0.01, ###p < 0.001, compared to H2O2-treated group
Bax and Bcl-2 proteins are members of the Bcl-2 family that regulate the permeabilization of the mitochondrial membrane and the release of cytochrome C from the intermembrane mitochondrial space to the cytoplasm. Bax and Bcl-2 perform opposite actions. Thus, the Bax/Bcl-2 ratio is a better indicator of apoptotic cell death than the expression of each factor alone. H2O2 significantly increased the Bax/Bcl-2 ratio by 2.96-fold compared to the control group (Fig. 3b). Pretreatment with 1/4 × FS0 and FS1 did not significantly increase the ratio; FS3 and FS5 downregulated the ratio by 0.42- and 0.27-fold, respectively. This result suggested that FS3 and FS5 may suppress mitochondrial dysfunction by balancing Bax and Bcl-2. Xu et al. (2009) reported that genistein and daidzein could protect vascular endothelial cells against H2O2-induced oxidative stress by decreasing the Bax/Bcl-2 ratio.
Caspases are involved in regulating the two different apoptotic pathways induced by various stimuli. Released cytochrome C in the cytosol activates caspase-9, which is the initiator of caspase-induced apoptosis. Activated caspase-9 induces caspase-3 activation, triggering apoptosis or cell death (Hu et al. 2015). Because caspase-9 and caspase-3 are primarily related to neuronal apoptosis, the suppression of caspase may be necessary to protect against oxidative stress. H2O2 treatment increased the gene expression of caspase-9 and caspase-3 by 1.45- and 1.21-fold, respectively (Fig. 3c, d). While the expression of two caspases in FS0 and FS1 was similar to that in the H2O2-treated group, FS3 and FS5 significantly decreased the gene expression, indicating that FS3 and FS5 could modulate the caspase cascades.
Soymilk fermented by L. plantarum YS-1 protected H2O2-damaged Caco-2 cells by reducing intracellular ROS and MDA levels and increasing the expression of antioxidant enzymes (Li et al. 2018). Ko et al. (2019) reported that the high antioxidant activity of 7,8,4′-THIF may protect SH-SY5Y cells exposed to the neurotoxin 6-OHDA. 7,8,4′-THIF could inhibit lipid peroxidation and increase antioxidant enzyme levels. In our study, FS3 and FS5 increased cell viability and protected the plasma membrane of SH-SY5Y cells against H2O2. FS3 and FS5 attenuated the neuronal biomarkers and mitochondria-dependent apoptotic pathways. These results may be attributed to the enhanced antioxidant properties of FS3 and FS5.
Conclusion
These results showed that L. plantarum 200655 could be used as a starter microorganism for soymilk fermentation with FOS. FOS supplementation protected L. plantarum 200655 from surviving during refrigerated storage and induced higher β-glucosidase and phenol production, leading to higher antioxidant activities. Moreover, FS with 3% and 5% FOS could inhibit H2O2-induced oxidative stress in SH-SY5Y cells by increasing cell viability and expression of neuronal markers and lowering LDH release and expression of apoptosis-related genes. Thus, soymilk fermented with L. plantarum 200655 and FOS can be used as a functional food to prevent neurodegenerative diseases.
Abbreviations
- BDNF
Brain-derived neurotrophic factor
- FOS
Fructooligosaccharides
- FS
Fermented soymilk
- GI
Growth index
- GOS
Galactooligosaccharides
- ROS
Reactive oxygen species
- TH
Tyrosine hydroxylase
- LAB
Lactic acid bacteria
- PD
Parkinson’s disease
- AD
Alzheimer’s disease
- HD
Huntington’s disease
Authors' contributions
GHC: investigation, methodology, writing—original draft, validation. HJB: investigation, methodology. NKL: conceptualization, validation, writing—review and editing. HDP: conceptualization, supervision, writing—review and editing, validation.
Funding
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, and Forestry (IPET) through the Innovational Food Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (#119009-3).
Availability of data and materials
All data and material generated or analyzed during this study are included in this article.
Declarations
Conflict of interest
All authors did not have conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
All authors consent to participate.
Consent for publication
All authors consent to publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Apostolidis E, Kwon YI, Shinde R, Ghaedian R, Shetty K. Inhibition of Helicobacter pylori by fermented milk and soymilk using select lactic acid bacteria and link to enrichment of lactic acid and phenolic content. Food Biotechnol. 2011;25:58–76. doi: 10.1080/08905436.2011.547118. [DOI] [Google Scholar]
- Bae WY, Kim HY, Park EH, Kim KT, Paik HD. Improved in vitro antioxidant properties and hepatoprotective effects of a fermented Inula britannica extract on ethanol-damaged HepG2 cells. Mol Biol Rep. 2019;46:6053–6063. doi: 10.1007/s11033-019-05040-x. [DOI] [PubMed] [Google Scholar]
- Bevilacqua A, Sinigaglia M, Speranza B, Altieri C. Effect of prebiotic compounds on the growth and survival of Bifidobacteria in a laboratory medium. Adv J Food Sci Technol. 2016;11:770–774. doi: 10.19026/ajfst.11.2790. [DOI] [Google Scholar]
- Cheon MJ, Lee NK, Paik HD. Neuroprotective effects of heat-killed Lactobacillus plantarum 200655 isolated from kimchi against oxidative stress. Probiotics Antimicrob Proteins. 2021;13:788–795. doi: 10.1007/s12602-020-09740-w. [DOI] [PubMed] [Google Scholar]
- Chiang SS, Pan TM. Antiosteoporotic effects of Lactobacillus-fermented soy skim milk on bone mineral density and the microstructure of femoral bone in ovariectomized mice. J Agric Food Chem. 2011;59:7734–7742. doi: 10.1021/jf2013716. [DOI] [PubMed] [Google Scholar]
- Davani-Davari Dorna, Negahdaripour Manica, Karimzadeh Iman, Seifan Mostafa, Mohkam Milad, Masoumi Seyed, Berenjian Aydin, Ghasemi Younes. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods. 2019;8:92. doi: 10.3390/foods8030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakri EFM, Lim SM, Musa NH, Hasan MH, Adam A, Ramasamy K. Lactobacillus fermentum LAB 9-fermented soymilk with enriched isoflavones and antioxidants improved memory in vivo. Sains Malays. 2016;45:1289–1297. [Google Scholar]
- Hu XL, Niu YX, Zhang Q, Tian X, Gao LY, Guo LP, Meng WH, Zhao QC. Neuroprotective effects of kukoamine B against hydrogen peroxide-induced apoptosis and potential mechanisms in SH-SY5Y cells. Environ Toxicol Pharmacol. 2015;40:230–240. doi: 10.1016/j.etap.2015.06.017. [DOI] [PubMed] [Google Scholar]
- Huang B, Liu J, Fu S, Zhang Y, Li Y, He D, Ran X, Yan X, Du J, Meng T, Gao X, Liu D. α-Cyperone attenuates H2O2-induced oxidative stress and apoptosis in SH-SY5Y cells via activation of Nrf2. Front Pharmacol. 2020;11:281. doi: 10.3389/fphar.2020.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert J, Berger M, Nepveu F, Paul F, Daydé J. Effects of fermentation on the phytochemical composition and antioxidant properties of soy germ. Food Chem. 2008;109:709–721. doi: 10.1016/j.foodchem.2007.12.081. [DOI] [PubMed] [Google Scholar]
- Jang HJ, Song MW, Lee NK, Paik HD. Antioxidant effects of live and heat-killed probiotic Lactobacillus plantarum Ln1 isolated from kimchi. J Food Sci Technol. 2018;55:3174–3180. doi: 10.1007/s13197-018-3245-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariyawasam KMGMM, Yang SJ, Lee NK, Paik HD. Probiotic properties of Lactobacillus brevis KU200019 and synergistic activity with fructooligosaccharides in antagonistic activity against foodborne pathogens. Food Sci Anim Resour. 2020;40:297–310. doi: 10.5851/kosfa.2020.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariyawasam KMGMM, Lee NK, Paik HD. Synbiotic yoghurt supplemented with novel probiotic Lactobacillus brevis KU2000019 and fructooligosaccharides. Food Biosci. 2021;39:100835. doi: 10.1016/j.fbio.2020.100835. [DOI] [Google Scholar]
- Kim HY, Bae WY, Yu HS, Chang KH, Hong YH, Lee NK, Paik HD. Inula britannica fermented with probiotic Weissella cibaria D30 exhibited anti-inflammatory effect and increased viability in RAW 264.7 cells. Food Sci Biotechnol. 2020;29:569–578. doi: 10.1007/s10068-019-00690-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko YH, Kim SK, Kwon SH, Seo JY, Lee BR, Kim YJ, Hur KH, Kim SY, Lee SY, Jang CG. 7,8,4′-Trihydroxyisoflavone, a metabolized product of daidzein, attenuates 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells. Biomol Ther. 2019;27:363–372. doi: 10.4062/biomolther.2018.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Song MW, Kim KT, Hong WS, Paik HD. Antioxidant effect and sensory evaluation of yogurt supplemented with hydroponic ginseng rood extract. Foods. 2021;10:639. doi: 10.3390/foods10030639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Long X, Pan Y, Zhao X, Song JL. Study on soybean milk fermented by Lactobacillus plantarum YS-1 reduced the H2O2-induced oxidative damage in Caco-2 cells. Biomed Res. 2018;29:357–364. doi: 10.4066/biomedicalresearch.29-17-3132. [DOI] [Google Scholar]
- Liang N, Kitts DD. Antioxidant property of coffee components: assessment of methods that define mechanisms of action. Molecules. 2014;19:19180–19208. doi: 10.3390/molecules191119180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SM. Synbiotic potential of yoghurt manufactured with probiotic lactic acid bacteria isolated from mustard leaf kimchi and prebiotic furctooligosaccharide. Microbiol Biotechnol Lett. 2012;40:226–236. doi: 10.4014/kjmb.1204.04002. [DOI] [Google Scholar]
- Liu JR, Chen MJ, Lin CW. Antimutagenic and antioxidant properties of milk-kefir and soymilk-kefir. J Agric Food Chem. 2005;53:2467–2474. doi: 10.1021/jf048934k. [DOI] [PubMed] [Google Scholar]
- Mishra S, Mishra HN. Effect of synbiotic interaction of fructooligosaccharide and probiotics on the acidification profile, textural and rheological characteristics of fermented soy milk. Food Bioprocess Technol. 2013;6:3166–3176. doi: 10.1007/s11947-012-1021-4. [DOI] [Google Scholar]
- Mujtaba N, Jahan N, Sultana B, Zia MA. Isolation and characterization of antihypertensive peptides from soy bean protein. Braz J Pharm Sci. 2021;57:e19061. doi: 10.1590/s2175-97902020000419061. [DOI] [Google Scholar]
- Nirmaladevi D, Venkataramana M, Chandranayaka S, Ramesha A, Jameel NM, Srinivas C. Neuroprotective effects of bikaverin on H2O2-induced oxidative stress mediated neuronal damage in SH-SY5Y cell line. Cell Mol Neurobiol. 2014;34:973–985. doi: 10.1007/s10571-014-0073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekha CR, Vijayalakshmi G. Isoflavone phytoestrogens in soymilk fermented with β-glucosidase producing probiotic lactic acid bacteria. Int J Food Sci Nutr. 2011;62:111–120. doi: 10.3109/09637486.2010.513680. [DOI] [PubMed] [Google Scholar]
- Santos WLD, Silva EFTD, Silva MEBD, Silva EGD, Bomfim AGJ, Moreira KA. Effect of in vitro gastrointestinal digestion on the antioxidant potential of yogurt added with probiotic culture containing Bacillus subtilis. Diversitas J. 2020;5:1750–1763. doi: 10.17648/diversitas-journal-v5i3-1070. [DOI] [Google Scholar]
- Seo HR, Kim JY, Kim JH, Park KY. Identification of Bacillus cereus in a chungkukjang that showed high anticancer effects against AGS human gastric adenocarcinoma cells. J Med Food. 2009;12:1274–1280. doi: 10.1089/jmf.2009.0081. [DOI] [PubMed] [Google Scholar]
- Sharma Sakshi, Kanwar Sarbjit Singh. Effect of prebiotics on growth behavior of Lactobacillus plantarum and their impact on adherence on strict anaerobic pathogens to intestinal cell lines. Journal of Food Safety. 2018;38:e12384. doi: 10.1111/jfs.12384. [DOI] [Google Scholar]
- Sharma SK, Singh AP. In vitro antioxidant and free radical scavenging activity of Nardostachys jatamansi DC. J Acupunct Meridian Stud. 2012;5:112–118. doi: 10.1016/j.jams.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Tian W, Zhao J, Lee JH, Akanda MR, Cho JH, Kim SK, Choi YJ, Park BY. Neuroprotective effects of Cornus officianlis on stress-induced hippocampal deficits in rats and H2O2-induced neurotoxicity in SH-SY5Y neuroblastoma cells. Antioxidants. 2020;9:27. doi: 10.3390/antiox9010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsangalis D, Ashton JF, Mcgill AEJ, Shan NP. Enzymatic transformation of isoflavone phytoestrogens in soymilk by β-glucosidase-producing Bifidobacteria. J Food Sci. 2002;67:3104–3133. doi: 10.1111/j.1365-2621.2002.tb08866.x. [DOI] [Google Scholar]
- Tu Y, Cheng S, Zhang S, Sun H, Xu Z. Vincristine induces cell cycle arrest and apoptosis in SH-SY5Y neuroblastoma cells. Int J Mol Med. 2013;31:113–119. doi: 10.3892/ijmm.2012.1167. [DOI] [PubMed] [Google Scholar]
- Xu SZ, Zhong W, Ghavideldarestani M, Saurabh R, Lindow SW, Atkin SL. Multiple mechanisms of soy isoflavones against oxidative stress-induced endothelium injury. Free Radic Biol Med. 2009;47:167–175. doi: 10.1016/j.freeradbiomed.2009.04.021. [DOI] [PubMed] [Google Scholar]
- Yang JH, Mau JL, Ko PT, Huang LC. Antioxidant properties of fermented soybean broth. Food Chem. 2000;71:249–254. doi: 10.1016/S0308-8146(00)00165-5. [DOI] [Google Scholar]
- Zhang H, Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr Opin Food Sci. 2016;8:33–42. doi: 10.1016/j.cofs.2016.02.002. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and material generated or analyzed during this study are included in this article.