Abstract
Objectives
Alaska Native (AN) people experience twice the rate of colorectal cancer (CRC) as US Whites. There is a need for increased screening and early detection. We describe the development and implementation of a randomized controlled trial of the multi-target stool DNA test (mt-sDNA; Cologuard® Exact Sciences, Madison WI) to increase CRC screening among AN people.
Methods
A total of 32 rural/remote AN communities were randomized to a varied intensity intervention (patient navigation vs mailed health education) compared to 14 communities receiving usual opportunistic care. Outcome measures include screening completion and method used (mt-sDNA vs colonoscopy). Health care provider interviews and AN patient focus groups will be used to assess patient-, provider-, and system-level CRC screening promoters and barriers.
Results
The study began in April 2020 during the COVID-19 pandemic, resulting in a number of challenges and study adaptations. These included difficulty finding laboratory space, lack of timely mail service due to flight reductions across the state, and travel restrictions that led to postponement of in-person focus groups. Videoconferencing platforms for Tribal engagement replaced face-to-face interactions. After an extensive search, a laboratory with space available was identified and the preprocessing laboratory established. Study staff will work closely with patients to monitor mail service to get mt-sDNA kits sent on time. We are also exploring the use of videoconferencing platforms as alternatives to in-person focus groups.
Conclusions
Despite the challenges encountered during the COVID-19 pandemic, we successfully initiated the intervention and established the first mt-sDNA preprocessing laboratory in Alaska.
Keywords: Alaska Native, Colorectal neoplasms/prevention and control, Healthcare disparities, Community-based participatory research, Multi-target stool DNA
1. Introduction
Alaska Native (AN) people have the highest documented rate of colorectal cancer (CRC) in the world. Over the last four decades, CRC incidence and mortality have remained twofold higher compared to United States (US) White people, and is the most frequently diagnosed cancer in this population [1,2]. This health disparity has made CRC screening and prevention a priority among Tribal health leaders and AN communities.
Screening is an important method for detecting and diagnosing early stage CRC [3]. Early diagnosis increases the likelihood of successful treatment and decreases the potential for negative impacts on quality of life [[4], [5], [6]]. Approximately 76% of CRC related deaths occur among people who are non-adherent to CRC screening guidelines [7]. Although CRC screening rates have increased among the AN population, over 35% of the eligible population remain unscreened, which is higher than the national average [8]. Screening rates also vary considerably across the Tribal health regions of Alaska [9]. CRC screening is a supported service within the Alaska Tribal Health System, yet logistic hurdles and long-distance travel result in patients in remote and rural areas lacking access to CRC screening methods like colonoscopy. AN people also experience a high prevalence of Helicobacter pylori-associated gastric bleeding [10,11]. This can result in false positive guaiac-based fecal occult blood tests (FOBT) [12], which are consequently not recommended for AN people [13]. In some regions of Alaska the at-home fecal immunochemical test (FIT) is available, but colonoscopy has generally been the preferred screening test given the high incidence and mortality in this population [13].
The at-home multi-target stool DNA test (mt-sDNA; Cologuard®, Exact Sciences Laboratories, Madison, WI) has been established as an effective alternative to other CRC screening test options [14,15]. Mt-sDNA detects three DNA biomarkers for colorectal neoplasia and hemoglobin [15] and has a higher sensitivity for CRC compared to FIT [14,16]. A positive result has strong predictive value for CRC or advanced adenoma and requires a follow-up diagnostic colonoscopy to complete screening. Previous research has shown increases in screening adherence among patients who use mt-sDNA, even among those who have never been screened, as well as an improvement in the quality of follow-up colonoscopies [[17], [18], [19]]. In previous research, our team demonstrated high sensitivity of mt-sDNA for cancer and large polyps among AN adults [16], interest in using mt-sDNA among AN patients and their healthcare providers [20], and that mt-sDNA is a cost-effective screening strategy in this population [21]. However, it is unknown if mt-sDNA is feasible in rural/remote AN communities, and whether use of mt-sDNA will actually increase AN CRC screening adherence. This trial will evaluate the feasibility of mt-sDNA for increasing AN CRC screening rates using a multi-level, mixed methods community-based intervention. The purpose of this paper is to describe the Alaska study methods, formation of the mt-sDNA preprocessing laboratory in Alaska, and highlight challenges and adaptations to study procedures in response to the COVID-19 pandemic.
2. Methods
This 5-year study began in April 2020 and was approved by the Alaska Area Institutional Review Board (IRB). It received Tribal research review approval from participating sites: the Alaska Native Tribal Health Consortium, Southcentral Foundation, and the Yukon-Kuskokwim Health Corporation. A Research Consultation Committee comprised of Alaska Native/American Indian people provided guidance on study instruments and materials during study development. As individuals will be selected for each intervention trial arm based on their randomized community, we obtained a waiver of informed consent for eligibility and group assignment to avoid potential selection/volunteer bias. This was approved by the privacy officers of the participating Tribal health organizations and the protocol approved by the Alaska Area IRB. The study is registered with the Clinical Trials Registry (NCT04336397) and was designed in accordance with the Consolidated Standards of Reporting Trials (CONSORT) statement. The risks posed by this intervention do not exceed the threshold of minimal risk, therefore an external data safety monitoring board/committee is not required. However, in keeping with the principles of community-based participatory research and respect for tribal data, we established a data safety monitoring committee with four experts independent of the study protocol to provide study oversight and consultation.
2.1. Study aims and design
The study has two primary aims: 1) To identify patient-, provider-, and system-level factors associated with CRC screening preferences, uptake, and follow-up, and 2) To test the hypothesis that multi-level high and medium intensity community-based interventions using mt-sDNA, as compared with usual opportunistic care, will increase CRC screening rates among AN people in rural/remote communities. This study will use a community-based, cluster randomized controlled trial design to compare varied intensity intervention to usual care, as well as CRC screening method used (colonoscopy vs mt-sDNA). The study design was informed by the Reach Effectiveness Adoption Implementation Maintenance Framework (RE-AIM) [[22], [23], [24]]. The intervention trial will be complemented by Health Belief Model-informed [22,25,26] health care provider key informant interviews and patient focus groups to assess multi-level promoters and barriers to CRC screening, including beliefs and preferences for screening with colonoscopy vs mt-sDNA.
2.2. Intervention trial
2.2.1. Study population
The study will be conducted in the 75,000 square mile Yukon-Kuskokwim Delta region, which is home to approximately 23,000 people who are primarily Alaska Native [27]. Community size ranges from 25 to 6500 people. All communities in the region are located off the road system and are accessible only by airplane, with seasonal access by snowmobile or boat.
2.2.2. Eligibility and recruitment
Eligibility criteria include: AN adults ages 45–75 living in communities served by the participating Tribal health organization in southwest Alaska, contact information available in the electronic health record (EHR) system, and have had at least one clinic visit in the Alaska Tribal Health System in the past three years. Ineligibility criteria include: history of familial adenomatous polyposis, hereditary non-polyposis CRC, previous colonoscopic evidence of inflammatory bowel disease, Crohn's disease, colorectal adenomas, CRC, 1st degree relative with prior CRC diagnosed at age 60 or younger, or positive FOBT in the last 6 months. Age range and risk criteria are based on Cologuard® testing requirements. Patients already adherent to screening guidelines (colonoscopy within 10 years, sigmoidoscopy within five years, or FOBT within preceding 12 months) are excluded from the intervention trial. Approximately 1540 eligible patients (n = 770 per intervention arm) living in 32 communities (n = 16 per intervention arm) will be offered the intervention; this target sample size accounts for a 25% drop-out rate. During and following the intervention a sub-set of patients (10%) who have completed CRC screening will be randomly selected to evaluate their awareness and response to the CRC screening intervention. This brief (3–5 min) phone survey will include questions to assess perceived severity, perceived susceptibility, perceived benefits, perceived barriers, and self-efficacy relevant to CRC screening.
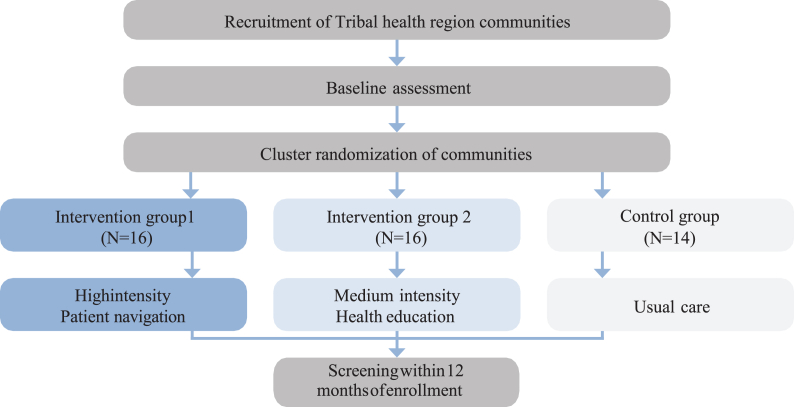
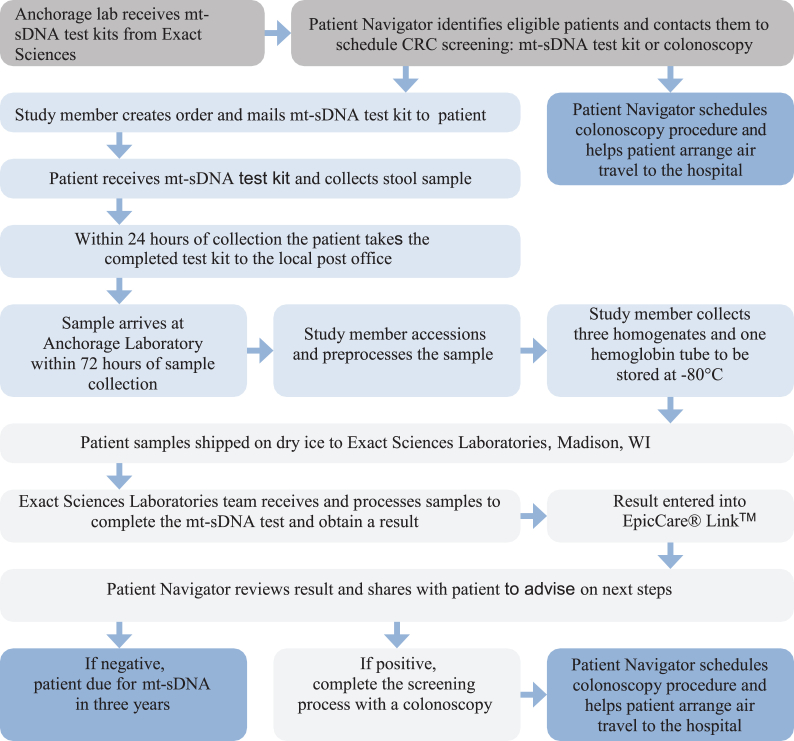
Communities served by the participating regional Tribal health organization will be randomly assigned to either intervention or control to get to the total number of participants needed for statistical comparison between intervention arms. Intervention communities will then be randomized into high (patient navigation) or medium (mailed health education) intensity intervention arms matched by population size (Fig. 1). Eligible patients in communities assigned to the medium intensity intervention will be mailed a letter from a patient navigator informing the patient that they are due for CRC screening along with a culturally tailored pamphlet containing information on the two CRC screening test options (i.e., colonoscopy and mt-sDNA). If the patient has not responded within two weeks, the patient navigator will follow up with one phone call contact attempt. Patients in communities assigned to the high intensity intervention will be contacted by the patient navigator using telephone and mail for up to six contact attempts. The remaining 14 communities in the region will serve as the control group and will continue receiving opportunistic screening recommendations at clinic visits per usual care. Colonoscopies will be done at the regional Tribal hospital. Patients who choose mt-sDNA will be sent the mt-sDNA kit via postal mail for at-home sample collection. Patients with positive mt-sDNA results will be notified and scheduled for a follow-up colonoscopy at the regional Tribal hospital or the Alaska Native Medical Center (Fig. 2).
Fig. 1.
Cluster randomization design of rural and remote communities in Alaska into intervention arms of varied intensity for colorectal cancer screening outreach and uptake.
Fig. 2.
Study intervention flow/process for colorectal cancer screening with either mt-sDNA or colonoscopy among Alaska Native people living in rural and remote communities in Alaska.
2.2.3. Data collection
Data for patients in the trial will be obtained from the Tribal health organization EHR and entered and stored in Research Electronic Data Capture (REDCap), a Health Insurance Portability and Accountability Act compliant data collection software. Demographic information such as age, sex, community of residence at enrollment, as well as family history of CRC and screening test preference will be collected at baseline. Information from the endoscopic procedure (e.g., test adherence, date performed, test outcome, biopsy collection and description) will be obtained for patients who choose colonoscopy. Mt-sDNA data collection will include preprocessing variables (e.g., date test received, weight of stool sample, validity of sample, date and time of preprocessing procedures) and results (positive or negative).
2.2.4. Preprocessing lab
Nationally, mt-sDNA use is limited to states where patient samples can be mailed to Madison, WI for processing within 72 h of sample collection. The remote nature of many AN communities renders this impractical if not impossible. Therefore, this study also includes the creation of a mt-sDNA preprocessing laboratory in Anchorage, AK to extend the time between sample collection and laboratory analysis (Fig. 2). Samples will be preprocessed and preserved at −80 °C, packaged with dry ice, and sent weekly to Exact Sciences Laboratories in Madison, WI. Test results will be obtained via the Exact Sciences Laboratories’ clinical provider portal EpicCare® Link™. Exact Sciences Laboratories provided in-person support to set up lab and IT equipment as well as virtual trainings for ordering kits, printing and scanning barcodes, and preprocessing samples. Study team members were certified on competencies for preprocessing samples prior to study implementation to ensure compliance with federal regulations for Cologuard® use.
2.3. Patient-, provider-, and system-level screening factors
2.3.1. Eligibility and recruitment
Eligibility criteria for the health care provider key informant interviews will include employment as a physician, physician assistant, nurse practitioner, or community health aide/practitioner at the participating Tribal health organization. Providers will be recruited via organization email listservs, internal organization newsletters, and presentations at medical team meetings. A total of 25–30 provider interviews will be conducted using a snowball sampling technique until data saturation has been met [28]. Patients will be eligible to participate in the focus groups if they meet the randomized controlled trial intervention criteria. Recruitment will occur via direct outreach from the patient navigator using medical record data. Each focus group will include 6–8 participants, stratified by sex, with four groups total.
2.3.2. Procedures and outcome measures
Prior to conducting interviews and focus groups, informed consent will be obtained using the e-Consent Framework in REDCap. A short electronic questionnaire will be administered to collect general demographic information about the participants (age, sex, community). Provider interviews will take about 30–60 min and patient focus groups will last approximately 2 h. Phone interviews and focus group sessions will be audio taped and all recordings will be transcribed verbatim using Temi software (temi.com). Qualitative computer software (ATLAS.ti) will be used for analysis, as needed. All interview and focus group participants will receive a gift card in consideration for their time. Measures from the qualitative component (e.g., patient satisfaction with mt-sDNA vs colonoscopy, provider attitudes and beliefs) will be incorporated into secondary analyses to help explain the magnitude and direction of intervention trial results.
2.4. Sample size estimates and statistical analysis
The study sample size allocation is based on a pre-specified ability to detect from baseline a difference of 10% or more in screening participation rates for usual care vs any intervention and a difference of 10% or more in screening for high intensity vs medium intensity outreach. Given these expected improvements in screening rates, and the expected non-intervention screen rate of 59% based on current rates among AN people, we anticipate more than 80% power to detect a statistically significant difference between screening rates in the medium and high intensity interventions (α = 0.05; n = 616 in each of the three study arms, accounting for an anticipated 25% drop-out rate). Intention-to-treat analysis will be used such that all randomized participants will be included in the arm to which they were originally assigned [29].
The primary intervention outcome measure will be completion of an incident CRC screening episode recorded in the EHR within one year of follow-up after randomization and intervention. This will be defined as having a colonoscopy; mt-sDNA with a negative result; or mt-sDNA with a positive result followed by diagnostic colonoscopy within 90 days of a positive mt-sDNA result. A secondary outcome measure will include the rate of positive mt-sDNA follow-up to diagnostic colonoscopy due to the patient navigation system. Incident CRC and advanced adenoma [adenomatous polyp ≥1 cm or containing >25% villous component or high-grade dysplasia, or sessile serrated adenoma/polyp ≥1 cm] detection will be compared between the three arms.
Demographic characteristics will be compared (sex, age) between those screened using MT-sDNA and colonoscopy, as well as by intervention status (high intensity, medium intensity, usual care) in simple frequency tables. Multivariable models will be used to examine the role of intervention status in determining screening rates, adjusting for potential confounders such as age, sex, and other demographic characteristics. We will assess the proportion of persons screened (or the screening rate) using logistic and/or Poisson regression in the multivariable linear models. The effectiveness of the interventions may vary by sex, age, and rurality; this will be explored using frequency tables and crossing outcome proportions by levels of sex, age, and rurality. Examination of interaction terms (sex by intervention, age by intervention, etc.) in multivariable models, as well as subset analysis as appropriate to illuminate such differences, will allow us to evaluate differences across these covariates in effectiveness of the intervention arms. Lastly, we will compare the mt-sDNA sample viability rate from the Alaska study with national sample quality estimates (96%). These study results will help determine whether adding mt-sDNA helps increase screening rates; which test AN patients prefer (colonoscopy vs mt-sDNA); and whether the mt-sDNA test is feasible in rural/remote Alaska given the challenging geography and limitations to mail service and delivery.
Of note, the qualitative findings will be used to explain and triangulate the quantitative results; however, the quantitative and qualitative data will be kept analytically distinct. Statistical techniques will be used to analyze screening adherence data while thematic analysis will be used to analyze interview data. The integrity of each method will be preserved while capitalizing on the potential for enhanced understanding from combining the two sets of findings [22].
3. Study challenges and adaptations
The COVID-19 pandemic has presented challenges to all sectors of society, including government, healthcare systems, businesses, and individuals, as well as to the conduct of research. This study began during the height of pandemic-related closures. Multiple unanticipated issues during the study development phase included challenges related to identifying laboratory space for preprocessing samples, delays in mail service, and restrictions on travel.
3.1. Laboratory space
One critical element impacted by the COVID-19 pandemic was obtaining mt-sDNA preprocessing laboratory space. Initially, the preprocessing laboratory was going to be at the Alaska Native Medical Center, a tertiary care center for all AN people statewide. However, the initiation of the study coincided with a massive effort to conduct COVID-19 testing, which meant that there was no longer space available given the tremendous burden of COVID-19 test volumes. Several university laboratories were available, but not useable because they lacked Clinical Laboratory Improvement Amendments (CLIA) certification. After contacting almost 30 laboratories that met criteria, the State of Alaska Public Health Laboratory agreed to the use of their facility and bench space for the duration of study sample collection and processing.
3.2. Mail service
The United States Postal Service (USPS) contracts mail carriers (personnel and aircraft) to provide mail services to rural communities that are located off the road-system and only accessible by small aircraft. Circumstances such as weather and carrier availability affect the frequency and schedule of mail services in these communities and the pandemic created additional challenges. At the beginning of the pandemic, the main interstate airline in Alaska experienced substantial economic hardship. To offset costs, they reduced flights and canceled routes to some of the remote AN communities. This resulted in less frequent, or elimination of, mail service for some communities. While flights and mail delivery have now resumed, they continue to be less frequent and unpredictable. This presents a sizeable problem for use of mt-sDNA since it must be mailed to the patient for their use, and then received within 72 h of sample collection.
We are also exploring another method in which patients return their completed mt-sDNA kits to the local clinic. The clinic staff will then give the sample to the mail carrier airline pilot, who delivers the sample to Bethel, AK. Staff from the hub hospital in Bethel pick up the sample and then send via air cargo plane from Bethel to our Anchorage pre-processing lab. This “hand carry” method for clinical biospecimens is already in use in this region and may prove more efficient than the USPS mail system, despite the greater coordination and steps needed to complete the process.
3.3. Travel
This study uses the principles of community-based participatory research as a culturally-appropriate framework for doing research with Indigenous communities [30]. One aspect of community-based participatory research is the deep involvement of Tribal organizations and community in the research process [22,23,[31], [32], [33]]. In previous work by the study investigators, numerous in-person meetings in Tribal communities and with Tribal health leadership were held to review study design, methods, instruments, and implementation. Due to the COVID-19 pandemic, travel restrictions and community mandates precluded in-person study meetings. Virtual meeting platforms like Zoom were deployed to gather feedback and input into the study, develop methods, and share study progress. The original study design included conducting in-person focus groups, two with patients residing in remote communities and two with patients living in the regional hub community. The COVID-19 pandemic led to the postponement of planned in-person focus groups. The study team has explored the possibility of conducting virtual focus groups in addition to in-person focus groups. Studies suggest data richness is similar among face-to-face focus groups and online focus groups [34,35]. A potential benefit of conducting virtual focus groups is the ability to invite patients from multiple geographical locations to increase the diversity of perspectives shared [36]. However, this might be more challenging due to issues with Internet connectivity and access in rural and remote communities [37] and comfort with technology among this population [38].
4. Discussion
This randomized controlled trial is designed to investigate which outreach practices increase CRC screening in rural/remote AN populations, and whether the addition of mt-sDNA as a new screening option substantially increases screening rates. At the beginning of the COVID-19 pandemic, elective procedures such as cancer screenings were put on hold to manage the spread of the virus in hospitals. The result was a substantial decline in cancer prevention and early screening detection, as well as increased patient reluctance to obtain screening [3,39,40]. The circumstances of the COVID-19 pandemic have also created special challenges for clinical trial and translational health research [41]. This has been true for the development of this CRC screening intervention trial. COVID-19 vaccination efforts continue in Alaska, but many AN communities are still contending with COVID-19 outbreaks and related community closures and travel restrictions. Challenges including delayed mail delivery of mt-sDNA, colonoscopy availability and patient willingness to be screened will likely continue to impact the study going forward. The COVID-19 pandemic has required us to think creatively and implement effective solutions that may not have been considered prior to the pandemic while still ensuring the validity of the study methodology.
Estimated trajectories for cancer incidence have increased since pre-COVID-19, especially in minority populations [40], but effective, feasible, at-home screening tests could help ameliorate this disparity. This research addresses a health disparity of established community concern. It will generate data that will have broad-reaching clinical and policy outcomes for the Alaska Tribal Health System and potentially help alleviate some of the burden usual CRC screening (i.e., colonoscopy) can have on patients and the health care system. If the results demonstrate an increase in CRC screening rates with the use of mt-sDNA the preprocessing laboratory could become a permanent service after study completion. This study will advance scientific knowledge and clinical practice by revealing strategies that work to increase screening among the AN population and help curb the extreme morbidity and mortality due to CRC in this population. If this study demonstrates an improvement in CRC screening rates in Alaska, it may also be relevant to rural and remote communities in other parts of the US and worldwide.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Rutten provides scientific input to research studies through a contracted services agreement between Mayo Clinic and Exact Sciences. Dr. Kisiel is listed as an inventor of Mayo Clinic intellectual property, licensed to Exact Sciences (Madison, Wisconsin), for which he may receive royalties, paid to Mayo Clinic. He consults and receives research support under a sponsored research agreement between Mayo Clinic and Exact Sciences. All other authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgements
This research was supported by the National Institutes of Health National Cancer Institute under award number R01CA247642. We acknowledge the contributions and support of the Alaska Native Tribal Health Consortium and the Yukon-Kuskokwim Health Corporation Boards of Directors; Exact Sciences, who provided Cologuard® test kits and preprocessing materials and implementation support at no cost; and the Alaska State Laboratory, who provided laboratory space at no cost. The content presented is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Zimpelman G.I., Miller K.N., Carlo D.D., Schade T.L., Provost E.M., Britton C.I., Nash S.H. 2021. Cancer in Alaska Native People: 1969-2018, the 50-year Report. [Google Scholar]
- 2.Kelly J.J., Alberts S.R., Sacco F., Lanier A.P. Colorectal cancer in Alaska Native people, 2005-2009. Gastrointest Cancer Res. Sep 2012;5(5):149–154. [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer Screening during the COVID-19 Pandemic. Accessed April 23, 2021. Atlanta, GA.
- 4.Atkin W.S., Edwards R., Kralj-Hans I., et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. May 8 2010;375(9726):1624–1633. doi: 10.1016/S0140-6736(10)60551-X. S0140-6736(10)60551-X [pii] [DOI] [PubMed] [Google Scholar]
- 5.Mandel J.S., Bond J.H., Church T.R., et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N. Engl. J. Med. May 13 1993;328(19):1365–1371. doi: 10.1056/nejm199305133281901. [DOI] [PubMed] [Google Scholar]
- 6.Selby J.V., Friedman G.D., Quesenberry C.P., Weiss N.S. A case–control study of screening sigmoidoscopy and mortality from colorectal cancer. N. Engl. J. Med. 1992;326(10):653–657. doi: 10.1056/nejm199203053261001. [DOI] [PubMed] [Google Scholar]
- 7.Doubeni C.A., Fedewa S.A., Levin T.R., et al. Modifiable failures in the colorectal cancer screening process and their association with risk of death. Gastroenterology. Jan 2019;156(1):63–74 e6. doi: 10.1053/j.gastro.2018.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alaska Department of Health and Social Services . 2019. Alaska Behavioral Risk Factor Surveillance System. [Google Scholar]
- 9.Indian Health Service . 2017. Alaska Area Aggregate GPRA [Government Performance and Results Act of 1993] Clinical Performance Report, CRS [Clinical Reporting System] Version 18.0. [Google Scholar]
- 10.Yip R., Limburg P.J., Ahlquist D.A., et al. Pervasive occult gastrointestinal bleeding in an Alaska native population with prevalent iron deficiency. Role of Helicobacter pylori gastritis. JAMA. Apr 9 1997;277(14):1135–1139. doi: 10.1001/jama.1997.03540380049030. [DOI] [PubMed] [Google Scholar]
- 11.Parkinson A.J., Gold B.D., Bulkow L., et al. High prevalence of Helicobacter pylori in the Alaska native population and association with low serum ferritin levels in young adults. Clin. Diagn. Lab. Immunol. Nov 2000;7(6):885–888. doi: 10.1128/cdli.7.6.885-888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Redwood D., Provost E., Asay E., et al. Comparison of fecal occult blood tests for colorectal cancer screening in an Alaska Native population with high prevalence of Helicobacter pylori infection, 2008-2012. Prev. Chronic Dis. 2014;11:E56. doi: 10.5888/pcd11.130281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alaska Native Medical Center . 2013. Colorectal Cancer Screening Guidelines. [Google Scholar]
- 14.Imperiale T.F., Ransohoff D.F., Itzkowitz S.H., et al. Multitarget stool DNA testing for colorectal-cancer screening. N. Engl. J. Med. Apr 3 2014;370(14):1287–1297. doi: 10.1056/NEJMoa1311194. [DOI] [PubMed] [Google Scholar]
- 15.Heigh R.I., Yab T.C., Taylor W.R., et al. Detection of colorectal serrated polyps by stool DNA testing: comparison with fecal immunochemical testing for occult blood (FIT) PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0085659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Redwood D.G., Asay E.D., Blake I.D., et al. Stool DNA testing for screening detection of colorectal neoplasia in Alaska native people. Mayo Clin. Proc. Jan 2016;91(1):61–70. doi: 10.1016/j.mayocp.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Prince M., Lester L., Chiniwala R., Berger B. Multitarget stool DNA tests increases colorectal cancer screening among previously noncompliant Medicare patients. World J. Gastroenterol. Jan 21 2017;23(3):464–471. doi: 10.3748/wjg.v23.i3.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berger B.M., Schroy P.C., 3rd, Rosenberg J.L., et al. Colorectal cancer screening using stool DNA analysis in clinical practice: early clinical experience with respect to patient acceptance and colonoscopic follow-up of abnormal tests. Clin. Colorectal Cancer. Jan 2006;5(5):338–343. doi: 10.3816/CCC.2006.n.003. [DOI] [PubMed] [Google Scholar]
- 19.Johnson D.H., Kisiel J.B., Burger K.N., et al. Multitarget stool DNA test: clinical performance and impact on yield and quality of colonoscopy for colorectal cancer screening. Gastrointest. Endosc. Mar 2017;85(3):657–665. doi: 10.1016/j.gie.2016.11.012. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redwood D.G., Blake I.D., Provost E.M., Kisiel J.B., Sacco F.D., Ahlquist D.A. Alaska native patient and provider perspectives on the multitarget stool DNA test compared with colonoscopy for colorectal cancer screening. J. Prim. Care Community Health. Jan-Dec 2019;10 doi: 10.1177/2150132719884295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redwood D.G., Dinh T.A., Kisiel J.B., et al. Cost-effectiveness of multitarget stool DNA testing vs colonoscopy or fecal immunochemical testing for colorectal cancer screening in Alaska native people. Mayo Clin. Proc. May 2021;96(5):1203–1217. doi: 10.1016/j.mayocp.2020.07.035. [DOI] [PubMed] [Google Scholar]
- 22.Tariq S., Woodman J. Using mixed methods in health research. JRSM short Rep. Jun 2013;4(6) doi: 10.1177/2042533313479197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ivankova N.V., Creswell J.W., Stick S.L. Using mixed-methods sequential explanatory design: from theory to practice. Field Methods. 2006;18:3–20. [Google Scholar]
- 24.Weiner B.J., Lewis C.C., Stanick C., et al. Psychometric assessment of three newly developed implementation outcome measures. Implement. Sci. Aug 29 2017;12(1):108. doi: 10.1186/s13012-017-0635-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glanz K., Rimer B.K., Lewis F.M. fourth ed. Jossey-Bass; 2008. Health Behavior and Health Education: Theory, Research, and Practice. [Google Scholar]
- 26.Weinstein N.D. Testing four competing theories of health-protective behavior. Health Psychol. : Off. J. Div. Health Psychol. Am. Psychol. Assoc. Jul 1993;12(4):324–333. doi: 10.1037//0278-6133.12.4.324. [DOI] [PubMed] [Google Scholar]
- 27.Yukon Kuskokwim Health Corporation. About the YK Delta: The Yukon-kuskokwim Delta region, a lowland river delta, is our traditional homeland. Accessed November 15, 2021. https://www.ykhc.org/story/about-yk/.
- 28.Goodman L. Snowball sampling. Annu. Maths. Statis. 1961;32(1):148–170. [Google Scholar]
- 29.McCoy C.E. Understanding the intention-to-treat principle in randomized controlled trials. West. J. Emerg. Med. Oct 2017;18(6):1075–1078. doi: 10.5811/westjem.2017.8.35985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solomon T.G.A., Randall L.L., editors. Conducting Health Research with Native American Communities. American Public Health Association; 2014. [Google Scholar]
- 31.Minkler M., Wallerstein N., editors. Community-based Participatory Research for Health. Jossey-Bass; 2003. [Google Scholar]
- 32.Israel B.A., Eng E., Schulz A.J., Parker E.A., editors. Methods in Community-Based Participatory Research for Health. Jossey-Bass; 2005. [Google Scholar]
- 33.Dickerson D., Baldwin J.A., Belcourt A., et al. Encompassing cultural contexts within scientific research methodologies in the development of health promotion interventions. Prev. Sci. Jun 29 2018 doi: 10.1007/s11121-018-0926-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eigege C.Y., Daundasekara S.S., Gomez M.L., Walton Q.L., Hernandez D.C. Six feet apart: the feasibility of transitioning qualitative research to meet the emerging research needs during a pandemic. Int. J. Qual. Methods. 2022 doi: 10.1177/16094069211069442. [DOI] [Google Scholar]
- 35.Abrams K.M., Wang Z., Song Y.J., Galindo-Gonzalez S. Data richness trade-offs between face-to-face, online audiovisual, and online text-only focus groups. Soc. Sci. Comput. Rev. 2015;33(1):80–96. doi: 10.1177/0894439313519733. [DOI] [Google Scholar]
- 36.Matthews K.L., Baird M., Duchesne G. Using online meeting software to facilitate geographically dispersed focus groups for health workforce research. Qual. Health Res. 2018;28(10):1621–1628. doi: 10.1177/1049732318782167. [DOI] [PubMed] [Google Scholar]
- 37.Power J.M., Braun K.L., Bersamin A. Exploring the potential for technology-based nutrition education among WIC recipients in remote Alaska native communities. J. Nutr. Educ. Behav. 2017;49(7):S186–S191. doi: 10.1016/j.jneb.2016.11.003. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robinson R.F., Dillard D.A., Hiratsuka V.Y., et al. Formative evaluation to assess communication technology access and health communication preferences of Alaska native people. Int. J. Indig. Health. 2015;10(2):88–101. doi: 10.18357/ijih.102201515042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen R.C.H.K., Du S., Barron J., Katz A.J. Association of cancer screening deficit in the United States with the COVID-19 pandemic. JAMA Oncol. 2021 doi: 10.1001/jamaoncol.2021.0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.IQVIA . 2021. Shifts in Healthcare Demand, Delivery and Care during the COVID-19 Era.https://wwwiqviacom/insights/the-iqvia-institute/covid-19/shifts-in-healthcare-demand-delivery-and-care-during-the-covid-19-era [Google Scholar]
- 41.Hashem H., Abufaraj M., Tbakhi A., Sultan I. Obstacles and considerations related to clinical trial research during the COVID-19 pandemic. Front. Med. 2020;7 doi: 10.3389/fmed.2020.598038. [DOI] [PMC free article] [PubMed] [Google Scholar]