Introduction
Members of the herpesvirus family have been implicated across nearly the spectrum of ocular disease. Vision loss due to these viral agents is possible, with the severity or extent depending on the site and degree of inflammation. Posterior involvement can take many forms such as uveitis, vitritis, infiltrative/necrotizing retinitis, retinal vasculitis, or optic neuritis.
A particularly devastating manifestation of herpesviral infection of the eye is the acute retinal necrosis (ARN) syndrome. This unfortunate condition is characterized by the triad of vitritis, retinitis and occlusive vasculitis. The majority of cases of ARN in particular which have identified a pathogen are attributed to varicella zoster virus (VZV) or herpes simplex virus (HSV).1 Atypical infectious causes can include toxoplasmosis, cytomegalovirus (CMV), Epstein-Barr virus (EBV) and human herpesvirus-6 (HHV6). However, reports of posterior HHV6 ocular disease are relatively rare, and the nature of this ubiquitous virus in states of ocular inflammation remains unclear. Additionally, guidelines for the treatment of this infrequently encountered clinical entity are very much in need.1, 2, 3, 4, 5, 6, 7, 8, 9
We present herein an interesting case of panuveitis complicated by fulminant retinal vasculitis and central retinal vascular occlusion in a patient who subsequently developed retrograde neuroinvasive disease. After undergoing extensive, multidisciplinary workup, we attribute this case, with some features of the ARN syndrome, to HHV6 as the causal pathogen. We hope to share a framework of treatment targeted towards HHV6 which successfully induced regression of multisystemic disease.
Case report
The patient's written consent was obtained. The design of the work has been approved by local ethical committees at the Naval Medical Center San Diego, CA. The procedures followed were in accordance with the ethical standards of the Helsinki Declaration of the World Medical Association.
A 19-year-old male with no medical or ophthalmic history presented to a local emergency department for progressive eye pain, red eye and vision loss of the right eye over the course of one day. During initial evaluation by emergency department providers, afferent function of the eye was reportedly intact, and he was referred to ophthalmic care the following morning.
The patient's symptoms rapidly progressed, and by the time of initial examination by a comprehensive ophthalmologist the following morning, vision had extinguished to the level of no light perception (NLP) OD. At this initial ophthalmic assessment, the intraocular pressure was reportedly elevated, and there was concern for acute central retinal artery occlusion as the cause of vision loss. This provider reportedly performed an attempted salvage anterior chamber paracentesis for rapid intraocular pressure reduction and subsequently referred the patient emergently to our institution for further care. No polymerase chain reaction (PCR) studies were completed on this aqueous sample.
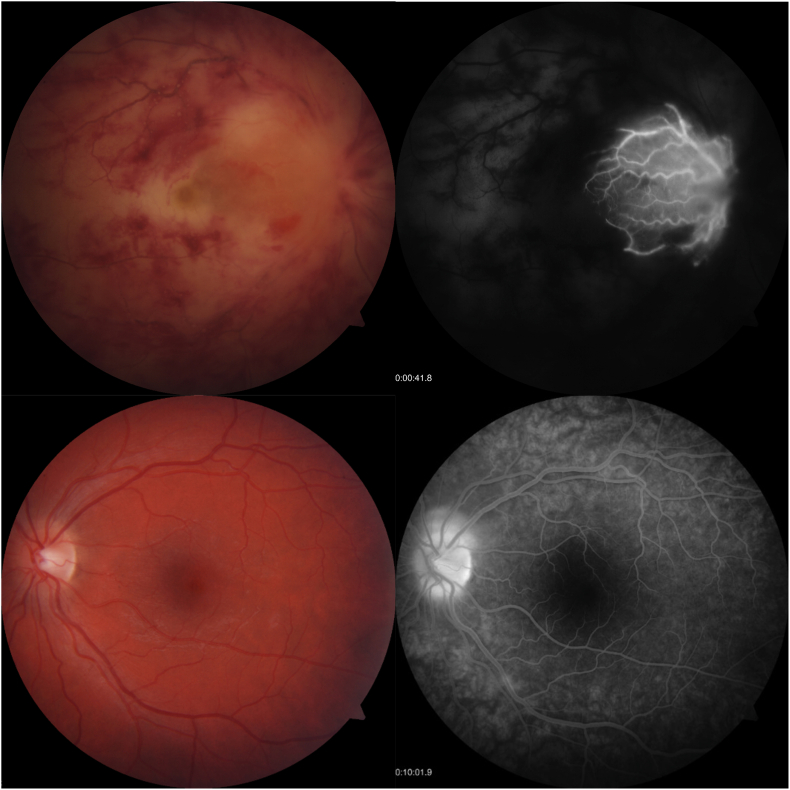
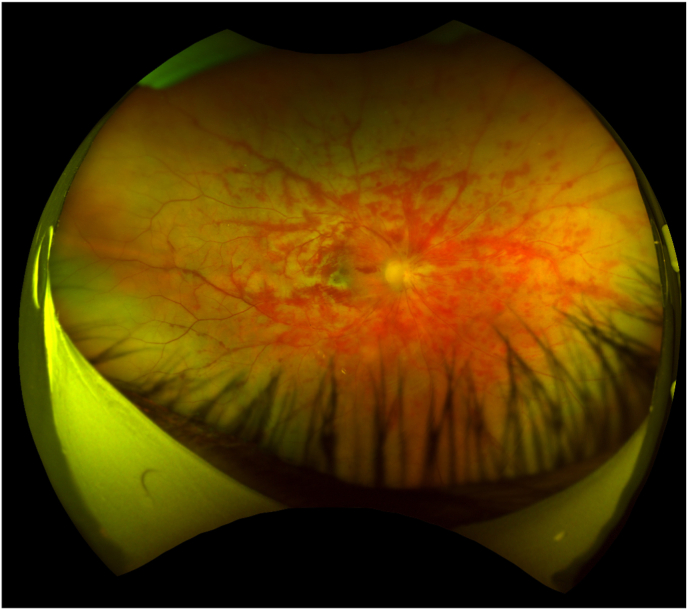
On arrival at our institution, examination revealed a hyperemic eye with elevated intraocular pressure of 45 mmHg and panuevitis. The posterior pole was remarkable for vitreous haze, optic nerve edema, exudative macular detachment and diffuse retinal hemorrhages along the major vascular arcades with a blood and thunder appearance (Fig. 1). The periphery revealed large, discrete inferior patches of retinal whitening concerning for infiltrative retinitis. Optical coherence tomography (OCT) of the macula revealed inner retinal hyper-reflectivity and large subretinal fluid. Collected later in the disease course, widefield imaging at 2 weeks after disease onset demonstrated evolving retinal hemorrhages, submacular exudate, and peripheral retinal whitening concerning for infiltrative retinitis (Fig. 2).
Fig. 1.
Fundus photographs and fluorescein angiography performed early in disease course. The right eye demonstrated extinguished central retinal artery and vein flow evident in early frames, with late inflammatory leakage around a cilioretinal artery. There were areas of blocked choroidal fluorescence underlying retinal hemorrhages and exudate. In the left eye, late frames demonstrated papillitis with minimal inferior arcade vasculitis, prompting treatment with intravitreal foscarnet.
Fig. 2.
Widefield imaging taken at 2 weeks after disease onset demonstrates evolving retinal hemorrhages with occlusive vasculitis, submacular exudate, and peripheral retinal whitening concerning for retinitis.
On our initial assessment, the pattern of ocular involvement and rapid progression was felt to be most concerning for a viral etiology, such as the ARN syndrome, and the patient was admitted to our institution for further evaluation and treatment. Initial treatment consisted of intravenous acyclovir (10 mg/kg three times daily) and topical ocular antihypertensives, atropine and prednisolone. Emphasis was placed on early management of presumed viral uveitis due to VZV or HSV. Our initial differential diagnosis included panuveitis, ARN, toxoplasma chorioretinitis, CMV retinitis, syphilis, sarcoidosis, tuberculosis, lymphoma, Behcet's disease and acute multifocal hemorrhagic retinal vasculitis. Intravenous fluorescein angiogram (IVFA) demonstrated extinguished central retinal artery and vein flow OD in a pattern of combined central retinal artery and vein occlusion, with inflammatory leakage around the cilioretinal artery (Fig. 1). There were areas of blocked choroidal fluorescence underlying retinal hemorrhages and exudate. In the left eye, there were features of early inflammatory optic neuritis and vasculitis. In light of finding early involvement of the fellow eye, intravitreal foscarnet was administered (2.4mg/0.1mL).
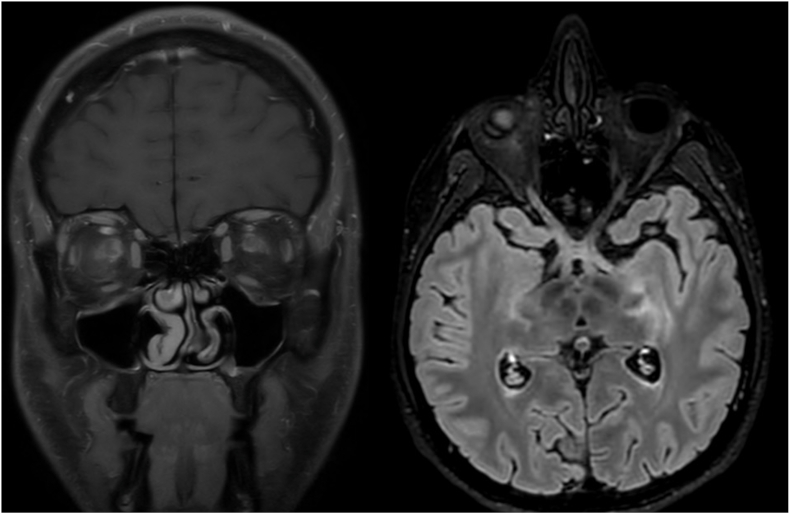
Early during the hospital course, the patient complained of headache and malaise concurrent with his ocular symptoms. Magnetic resonance imaging (MRI) with angiography of the head was performed, which revealed robust right-sided optic neuritis and perineuritis involving the entire optic nerve with retrograde post-chiasmatic extension, meningoencephalitis, and left sided optic neuritis (Fig. 3). Consideration was given to a broad spectrum of potential causes of the patient's evolving neuroinvasive disease, and a multidisciplinary approach was pursued. Multiple services including Ophthalmology, Infectious Disease, Neurology, Rheumatology, Hematology/Oncology were involved to evaluate for multisystemic disease with possible rheumatological or neoplastic process/lymphoma. In light of these radiographic findings, lumbar puncture was completed.
Fig. 3.
MRI of the brain taken early in disease course, demonstrating bilateral optic neuritis and perineuritis with post-chiasmatic extension and involvement of the left temporal stem, left calcarine fissure and occipital horn, indicating evolving meningoencephalitis. Examination of the left eye revealed early papillitis. Aggressive immunosuppression with intravenous methylprednisolone 1g/day was administered for a period of 3 days, followed by transition to oral high dose prednisone.
Results of the cerebrospinal fluid samples demonstrated lymphocytic pleocytosis with atypical lymphocytes and elevated protein (71.4 mg/dL) - a pattern felt to be most consistent with viral process. Samples were also sent for multiplex PCR paneling, which revealed active HHV6 replication. Subsequent serum studies further subtyped this to HHV6B. This PCR panel explicitly found no evidence of VZV, HSV, toxoplasmosis, CMV, or EBV.
Our extensive, multidisciplinary workup found no frank evidence to favor an alternative pathology. There were no pertinent findings for alternative, more typical infectious agents, specifically including no cerebrospinal fluid (CSF) evidence of acute VZV, HSV, toxoplasmosis, CMV, or EBV. Comprehensive systemic evaluation for underlying rheumatologic vasculitides, immunosuppression/HIV, neoplasia/lymphoma or primary CNS inflammation/vasculitis similarly failed to reveal any causal pathology. The only positive finding extensive workup reveal was the presence of robust HHV6 replication. We elected to transition therapy from acyclovir to a regimen with better activity against HHV6. The patient was treated with intravenous ganciclovir (5 mg/kg every 12 hours) and foscarnet (90 mg/kg every 12 hours). This regimen was selected based on literature mostly from transplant patients experiencing severe HHV6 CNS disease, but also would continue to cover any potential herpes virus.
After 48 hours of systemic coverage with this new treatment regimen, high dose intravenous methylprednisolone was administered, followed by a transition to oral prednisone on a slow taper. No deterioration in visual function of the left eye was noted. Repeat IVFA after 1 week of treatment demonstrated decreased inflammation of the left optic nerve and no further evidence of retinal vasculitis. Repeat MRI after 2 weeks of treatment demonstrated reduced central inflammation.
Oral corticosteroids were tapered gradually over several weeks as the patient continued to demonstrate good response to antiviral treatment. Intravenous ganciclovir was transitioned to oral valganciclovir at dosing of 900 mg every 12 hours after 2 weeks of sustained therapy, and foscarnet was discontinued after 6 weeks. Serial serum HHV6B viral load testing revealed down trending titers at all timepoints. Valganciclovir was continued at dosing 900mg daily for 6 months of therapy. At final follow up, no deterioration in afferent function of the left eye was noted, and the patient's visual outcome was NLP OD, 20/20 OS.
Discussion
Human herpesvirus-6 is a lymphotropic virus belonging to the beta-herpesvirus subfamily, and is genetically quite similar to CMV.10 It was discovered in 1986 and initially named Human B-Lymphotrophic Virus due to its speculated association with lymphomas in patients with HIV.11 There are two major variants of the virus (HHV-6A and HHV-6B) with distinctive traits.12 HHV-6 readily infects T-lymphocytes and is nearly ubiquitous in human populations with >95% seroprevalence in adults.13 Primary infection typically occurs early in life, causing the febrile diseases exanthem subitum and roseola infantum. It is estimated that approximately 1% of humans have inherited the virus genome from the genome of parental germ cells.14 Like the other herpesviruses, it is an enveloped DNA virus which can establish latent infection in human hosts, with reactivation having been linked to hepatitis, pneumonitis and severe infections of the central nervous system in both immunosuppressed and immunocompetent patients.
In the present case, a young patient presented with acute panuveitis and retinal vasculitis complicated by central retinal artery and vein occlusion. There were several features about his presentation that were suggestive of the ARN syndrome. Workup revealed concurrent neuroinvasive disease and early involvement of the fellow eye. The only pertinent positive finding despite extensive multidisciplinary evaluation was the presence of active HHV6 replication in the CSF and serum.
Our arrival at the diagnosis of HHV6-associated ocular and neuroinvasive disease presented several challenges. The pattern and nature of ocular involvement in this case exhibited features suggestive of a viral etiology, with concern for possible ARN syndrome. Namely, the primarily affected eye demonstrated acute and robust hypertensive panuevitis with areas of peripheral retinal whitening concerning for necrotizing retinitis. Atypical for ARN and unfortunate for our patient, there were diffuse retinal hemorrhages and vasculitis, with vision loss in this case being acutely driven by retinal ischemia as a result of combined central retinal artery and vein occlusion. Despite this, the patient's initial presentation and subsequent evaluation were very suggestive of VZV or HSV as the proximate cause, and we elected not to proceed with initial diagnostic analysis of ocular fluids given our high suspicion for VZV or HSV disease.
When we discovered the presence of concurrent neuroinvasive disease, we pursued an extensive, multidisciplinary approach to ensure other infectious and non-infectious explanations for the patient's syndrome were thoroughly evaluated. He had lymphocytic pleocytosis with numerous atypical lymphocytes and elevated protein on lumbar puncture, supporting an active viral infection. However, no genetic material for HSV, VZV, toxoplasmosis, CMV or EBV could be found in the CSF or the blood despite active CNS disease.
The presence of HHV6 activity in the CSF and blood was felt to be the likely cause of both the ocular disease and the neuroinvasive disease. This conclusion, however, has several important limitations. At no point did we collect aqueous or vitreous fluid samples for PCR. It is entirely possible the patient had a primary ocular uveitis/vasculitis that responded very well to our aggressive immunosuppressive regimen. Results of ocular fluid sampling also may have elicited intraocular VZV or HSV. However, in the presence of active and concurrent central nervous system disease with CSF studies demonstrating HHV6 replication to the exclusion of alternative infectious agents, we conclude that the ocular disease was attributable to HHV6.
There is an additional potential explanation of the degree of HHV6 activity that further confounds our favored diagnosis, which is the ubiquitous nature of HHV6 and its documented presence in ocular inflammatory states. The advent of polymerase chain reaction (PCR) testing has identified HHV6 in aqueous and vitreous samples in a variety of ocular inflammatory conditions.15 A virological analysis of 350 patients with uveitis or endophthalmitis found HHV6 DNA in 7 patients (2%), suggesting that HHV6 viral replication occurs during states of ocular inflammation, but the authors could not conclude the role of HHV6 as the cause of uveitis.15 Previous work has demonstrated the ability of HHV-6 to directly infect retina pigment epithelium.16 Causality is challenging to establish, given the ubiquitous nature of the virus and potential for genomic integration.17
Of available reports documenting HHV6 as a cause of ocular disease, treatment regimens have been varied. In one report, a 63-year-old male without pre-existing medical illness developed unilateral optic disc edema and retinal vasculitis and was found to have HHV6 DNA in his aqueous humor.3 His condition resolved with oral prednisone alone, with vision reported as 10/20 in the involved eye. In another report, a young patient with infectious mononucleosis and elevated IgM titers to EBV subsequently developed inflammatory encephaloradiculitis, fourth cranial nerve palsy, bilateral ARN and was found to have HHV6 on CSF analysis.5 His condition improved with intravenous acyclovir and corticosteroids, with visual acuity of 20/20 OU at last follow up. Other notable cases in English-language literature are described in Table 1.
Table 1.
Described cases of HHV6 associated retinitis/uveitis/optic neuritis.3, 4, 5, 6, 7, 8 Abbreviations: PCR – Polymerase chain reaction; CSF – Cerebrospinal fluid; RT – Reverse transcriptase; IV – Intravenous; VEP – Visual evoked potentials.
| Author and journal | Date | Age | Immune status | Site of isolation | Laterality | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| Ogata et al. Jpn J Ophthalmol10 | 2011 | 63 | Competent | Aqueous/multiplex PCR | Left | Steroids + ganciclovir | Recovered full vision |
| Moschettini et al. J Clin Virol11 | 2006 | 23 | Competent | CSF/Serum PCR; RT-nested PCR for HHV6 mRNA | Bilateral | Ganciclovir | Relapses, ultimately successful treatment |
| Papageorgiou et al. Ocul Immunol Inflamm12 | 2014 | 22 | Competent | CSF | Bilateral | Acyclovir + steroids | Recovered full vision |
| Maslin et al. J Infect13 | 2007 | 81 | DM, AML (10 months remission) | CSF PCR, Aqueous humor PCR | Bilateral | Intravitreal foscarnet → ganciclovir | Recovered previous vision |
| Mechai et al. J Med Virol14 | 2007 | 59 | HIV, CD4 > 200 | CSF, blood | Bilateral | IV foscarnet + IV ganciclovir→ cidofovir + valganciclovir | Recovered vision, VEP remained abnormal |
| Hino et al. Br J Haematol15 | 2015 | 39 | MDS s/p HLA matched BMT | CSF PCR | Bilateral | Foscarnet IV | Stabilized but persistent memory deficit |
| Oberacher-Velten et al. Graefes Arch Clin Exp Ophthalmol16 | 2005 | 31 | Diabetes | Blood serology | Bilateral | Foscarnet IV | Recovered vision |
Our present case presented with several features suggestive of the ARN syndrome, despite what we favor to be an atypical cause. A subset of herpetic ocular disease, the acute retinal necrosis (ARN) syndrome is a visually devastating entity characterized by the triad of vitritis, retinitis and occlusive vasculitis. Affected eyes will typically demonstrate discrete or initially isolated peripheral areas of retinal inflammation and necrosis that, if untreated, may coalesce and progress posteriorly to the macula. Involvement of the fellow eye can occur in up to 70% of untreated patients, usually within a few months.18 Of affected eyes, 48% will have vision worse than 20/200 six months after onset, as a result of retinal detachment (20–73% of affected eyes), chronic uveitis, epiretinal membrane formation, ischemia, edema or optic neuropathy.19 Central retinal vascular occlusions are rare but devastating complications of the ARN syndrome.20, 21, 22 Immunodeficient patients may present with minimal intraocular inflammation and early involvement of the posterior pole as a result of unimpeded spread of virus, a condition referred to as progressive outer retinal necrosis (PORN). Viral retinitis is fortunately rare, with an estimated incidence of approximately 1 in 2 million per year.23,24 Classically, ARN is attributed to varicella zoster virus (VZV) or herpes simplex virus (HSV).1 Atypical causes can include toxoplasmosis, CMV, EBV, and more recently, there are reports of associations with HHV6.1, 2, 3, 4, 5, 6, 7, 8, 9
The management of our patient in this report presented several challenges. At the outset, the nature of HHV6B as the causal pathogen was unclear. Furthermore, the severity of the patient's presentation dictated a very aggressive initial regimen. After initial treatment with intravenous acyclovir presumptively for coverage of HSV/VZV, we elected to transition to foscarnet and ganciclovir for better coverage of HHV6. We selected these agents based on evidence of in vitro of activity against the HHV6 virus, the possibility of ganciclovir resistance via UL69 mutation, as well as limited reports, mainly from transplant literature, of successful control of neuroinvasive HHV6 infection with these agents.8,25, 26, 27 Likewise, given HHV6 similarity to CMV, we adopted a framework of CMV retinitis in the immunocompromised host, noting that high quality randomized control trials for this condition are not available.
After transition from acyclovir, an agent with no appreciable activity against the betaherpesviruses but robust activity against HSV and VZV, to foscarnet/ganciclovir, he had notable improvement. Certainly, the immunosuppressive dosing of corticosteroids contributed to this improvement, however the sustained improvement in the face of multisystemic viral disease added confidence that our therapeutic plan was appropriate. Given the course of the patient's disease and response to this targeted treatment, we believe the clinical and laboratory findings in the present case are attributable to active HHV6 disease and not indicative of reactivated inherited viral chromosomal integration, a phenomenon which has been previously described.17
With regard to monitoring of progression and length of treatment, work by Zerr and colleagues in hematopoeietic stem cell patients showed correlation of serum HHV6 DNA with response to treatment.28 We elected to follow this paradigm with our patient after we showed that, at 2 weeks of treatment, he had improved symptoms, improved ophthalmic exam, and improvement on MRI, and this correlated with a significant reduction in serum HHV6 viral load. In absence of true precedent, we aimed for a treatment duration based on CMV retinitis in HIV regimen as based on the Opportunistic Infections Guidelines.29 The patient was monitored closely for toxicities, especially myelosuppression and renal injury, but required only intermittent electrolyte repletion.
HHV 6 has been identified with increasing frequency in states of intraocular and central nervous system inflammation. Given a paucity of available data, great uncertainty exists in appropriate treatment of this pathogen in relation to ocular disease. We present herein a case of visually devastating viral retinitis in a previously healthy patient attributable to HHV6 and the novel treatment regimen which induced regression of disease. We hope that our experience with this case may offer a framework for potential therapeutic regimens for future cases of multisystemic neuroinvasive disease attributable to HHV6.
Disclaimer
The views expressed are solely those of the authors and do not reflect the official policy or position of the US Navy, the Department of Defense, or the US Government.
All authors attest that they meet the current ICMJE criteria for Authorship.
Consent to publish this case report has been obtained from the patient in writing.
No funding or grant support.
The following authors have no financial disclosures: CS, PW, AGH, RA, DT, BP.
Conflict of interest
None.
Acknowledgements
None.
References
- 1.Ganatra J.B., Chandler D., Santos C., Kuppermann B., Margolis T.P. Viral causes of the acute retinal necrosis syndrome. Am J Ophthalmol. 2000;129(2):166–172. doi: 10.1016/s0002-9394(99)00316-5. [DOI] [PubMed] [Google Scholar]
- 2.Tran T.H., Rozenberg F., Cassoux N., Rao N.A., LeHoang P., Bodaghi B. Polymerase chain reaction analysis of aqueous humour samples in necrotising retinitis. Br J Ophthalmol. 2003;87(1):79–83. doi: 10.1136/bjo.87.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogata N., Koike N., Yoshikawa T., Takahashi K. Human herpesvirus 6-associated uveitis with optic neuritis diagnosed by multiplex PCR. Jpn J Ophthalmol. 2011;55(5):502–505. doi: 10.1007/s10384-011-0069-4. [DOI] [PubMed] [Google Scholar]
- 4.Moschettini D., Franceschini R., Vaccaro N.M., et al. Human herpesvirus-6B active infection associated with relapsing bilateral anterior optic neuritis. J Clin Virol. 2006;37(4):244–247. doi: 10.1016/j.jcv.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Papageorgiou E., Ch'ng S., Kulkarni A., Anwar S., Empeslidis T. Fourth cranial nerve palsy and bilateral acute retinal necrosis following human herpesvirus 6 infection of the central nervous system. Ocul Immunol Inflamm. 2014;22(3):228–232. doi: 10.3109/09273948.2013.856533. [DOI] [PubMed] [Google Scholar]
- 6.Maslin J., Bigaillon C., Froussard F., Enouf V., Nicand E. Acute bilateral uveitis associated with an active human herpesvirus-6 infection. J Infect. 2007;54(4):e237–e240. doi: 10.1016/j.jinf.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Mechai F., Boutolleau D., Manceron V., et al. Human herpesvirus 6-associated retrobulbar optic neuritis in an HIV-infected patient: response to anti-herpesvirus therapy and long-term outcome. J Med Virol. 2007;79(7):931–934. doi: 10.1002/jmv.20833. [DOI] [PubMed] [Google Scholar]
- 8.Hino Y., Doki N., Sekiya N., Takaki Y., Ohashi K. Optic neuritis as an initial manifestation of human herpesvirus 6 reactivation after unrelated bone marrow transplantation. Br J Haematol. 2016;172(5):654. doi: 10.1111/bjh.13826. [DOI] [PubMed] [Google Scholar]
- 9.Oberacher-Velten I.M., Jonas J.B., Junemann A., Schmidt B. Bilateral optic neuropathy and unilateral tonic pupil associated with acute human herpesvirus 6 infection: a case report. Graefes Arch Clin Exp Ophthalmol. 2005;243(2):175–177. doi: 10.1007/s00417-004-0986-8. [DOI] [PubMed] [Google Scholar]
- 10.Lawrence G.L., Chee M., Craxton M.A., Gompels U.A., Honess R.W., Barrell B.G. Human herpesvirus 6 is closely related to human cytomegalovirus. J Virol. 1990;64(1):287–299. doi: 10.1128/jvi.64.1.287-299.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salahuddin S.Z., Ablashi D.V., Markham P.D., et al. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science. 1986;234(4776):596–601. doi: 10.1126/science.2876520. [DOI] [PubMed] [Google Scholar]
- 12.Schirmer E.C., Wyatt L.S., Yamanishi K., Rodriguez W.J., Frenkel N. Differentiation between two distinct classes of viruses now classified as human herpesvirus 6. Proc Natl Acad Sci U S A. 1991;88(13):5922–5926. doi: 10.1073/pnas.88.13.5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braun D.K., Dominguez G., Pellett P.E. Human herpesvirus 6. Clin Microbiol Rev. 1997;10(3):521–567. doi: 10.1128/cmr.10.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Komaroff A.L., Zerr D.M., Flamand L. Summary of the 11th international conference on human herpesviruses-6A, -6B, and -7. J Med Virol. 2020;92(1):4–10. doi: 10.1002/jmv.25576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugita S., Shimizu N., Watanabe K., et al. Virological analysis in patients with human herpes virus 6-associated ocular inflammatory disorders. Invest Ophthalmol Vis Sci. 2012;53(8):4692–4698. doi: 10.1167/iovs.12-10095. [DOI] [PubMed] [Google Scholar]
- 16.Arao Y., Soushi S., Sato Y., et al. Infection of a human retinal pigment epithelial cell line with human herpesvirus 6 variant A. J Med Virol. 1997;53(2):105–110. [PubMed] [Google Scholar]
- 17.Pantry S.N., Medveczky P.G. Latency, integration, and reactivation of human herpesvirus-6. Viruses. 2017;9(7) doi: 10.3390/v9070194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palay D.A., Sternberg P., Jr., Davis J., et al. Decrease in the risk of bilateral acute retinal necrosis by acyclovir therapy. Am J Ophthalmol. 1991;112(3):250–255. doi: 10.1016/s0002-9394(14)76725-x. [DOI] [PubMed] [Google Scholar]
- 19.Meghpara B., Sulkowski G., Kesen M.R., Tessler H.H., Goldstein D.A. Long-term follow-up of acute retinal necrosis. Retina. 2010;30(5):795–800. doi: 10.1097/IAE.0b013e3181c7013c. [DOI] [PubMed] [Google Scholar]
- 20.Yeh S., Fahle G., Flaxel C.J., Francis P.J. Central retinal vascular occlusion associated with acute retinal necrosis. Arch Ophthalmol. 2012;130(4):514–517. doi: 10.1001/archophthalmol.2011.1735. [DOI] [PubMed] [Google Scholar]
- 21.Shah S.P., Hadid O.H., Graham E.M., Stanford M.R. Acute retinal necrosis presenting as central retinal artery occlusion with cilioretinal sparing. Eur J Ophthalmol. 2005;15(2):287–288. doi: 10.1177/112067210501500220. [DOI] [PubMed] [Google Scholar]
- 22.Kang S.W., Kim S.K. Optic neuropathy and central retinal vascular obstruction as initial manifestations of acute retinal necrosis. Jpn J Ophthalmol. 2001;45(4):425–428. doi: 10.1016/s0021-5155(01)00336-7. [DOI] [PubMed] [Google Scholar]
- 23.Muthiah M.N., Michaelides M., Child C.S., Mitchell S.M. Acute retinal necrosis: a national population-based study to assess the incidence, methods of diagnosis, treatment strategies and outcomes in the UK. Br J Ophthalmol. 2007;91(11):1452–1455. doi: 10.1136/bjo.2007.114884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cochrane T.F., Silvestri G., McDowell C., Foot B., McAvoy C.E. Acute retinal necrosis in the United Kingdom: results of a prospective surveillance study. Eye. 2012;26(3):370–377. doi: 10.1038/eye.2011.338. quiz 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nash P.J., Avery R.K., Tang W.H., Starling R.C., Taege A.J., Yamani M.H. Encephalitis owing to human herpesvirus-6 after cardiac transplant. Am J Transplant. 2004;4(7):1200–1203. doi: 10.1111/j.1600-6143.2004.00459.x. [DOI] [PubMed] [Google Scholar]
- 26.Yoshikawa T., Yoshida J., Hamaguchi M., et al. Human herpesvirus 7-associated meningitis and optic neuritis in a patient after allogeneic stem cell transplantation. J Med Virol. 2003;70(3):440–443. doi: 10.1002/jmv.10414. [DOI] [PubMed] [Google Scholar]
- 27.Birnbaum T., Padovan C.S., Sporer B., et al. Severe meningoencephalitis caused by human herpesvirus 6 type B in an immunocompetent woman treated with ganciclovir. Clin Infect Dis. 2005;40(6):887–889. doi: 10.1086/427943. [DOI] [PubMed] [Google Scholar]
- 28.Zerr D.M., Gupta D., Huang M.L., Carter R., Corey L. Effect of antivirals on human herpesvirus 6 replication in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34(3):309–317. doi: 10.1086/338044. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan J.E., Benson C., Holmes K.K., et al. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the national institutes of health, and the HIV medicine association of the infectious diseases society of America. MMWR Recomm Rep (Morb Mortal Wkly Rep) 2009;58(RR-4):1–207. quiz CE201-204. [PubMed] [Google Scholar]