Abstract
Spinal cord injury (SCI) is a devastating central nervous system disease, caused by physical traumas. With the characteristic of high disability rate, catastrophic dysfunction, and enormous burden on the patient’s family, SCI has become a tough neurological problem without efficient treatments. Contemporarily, the pathophysiology of SCI comprises complicated and underlying mechanisms, in which oxidative stress (OS) may play a critical role in contributing to a cascade of secondary injuries. OS substantively leads to ion imbalance, lipid peroxidation, inflammatory cell infiltration, mitochondrial disorder, and neuronal dysfunction. Hence, seeking the therapeutic intervention of alleviating OS and appropriate antioxidants is an essential clinical strategy. Previous studies have reported that traditional Chinese medicine (TCM) has antioxidant, anti-inflammatory, antiapoptotic and neuroprotective effects on alleviating SCI. Notably, the antioxidant effects of some metabolites and compounds of TCM have obtained numerous verifications, suggesting a potential therapeutic strategy for SCI. This review aims at investigating the mechanisms of OS in SCI and highlighting some TCM with antioxidant capacity used in the treatment of SCI.
Keywords: spinal cord injury, traditional Chinese medicine, oxidative strees, natural medicine, antioxidants
1 Introduction
Spinal cord injury (SCI) is a neurological injury disease caused by trauma or intraspinal lesions, which often leads to partial or complete loss of sensory and motor functions below the injury segment (Eckert and Martin, 2017). The damage to neuronal structure and function has the irreversible property, leaving SCI patients usually presented with a poor prognosis and a high rate of disability. The severe and long-term physical injury will not only reduce the patients’ quality of life but also result in consequences of serious economic burden on their families (Eli et al., 2021).
The spinal cord contains gray matter and white matter that include nerve cell bodies, along with the ascending tracts and descending tracts. According to the classification of the external physical impact and internal impacts, SCI can be divided into traumatic and non-traumatic spinal cord injury. The former is induced by trauma, such as acute stretch, acute distraction, compression, and axonal shearing (Eckert and Martin, 2017), which is mainly discussed in this review. The latter is often caused by compression of tumors and congenital diseases (Gedde et al., 2019). Degrees of disability can vary from slight sensory abnormality and motor dysfunction to severe paralysis, based on various locations and the traumatic extent of SCI.
The pathophysiological changes of SCI are very complex, so the mechanism of SCI is not well defined. But previous studies have found that oxidative stress (OS) caused by excessive reactive oxygen species (ROS) could aggravate the damage of neurons. Thus, our paper focused on the mechanisms of OS affecting SCI and discussed the etiology of OS from the perspective of traditional Chinese medicine (TCM) theory for SCI patients. Additionally, we highlighted some metabolites and compounds of TCM with antioxidant capacity used in the treatment of SCI, providing the potential treatment therapy of SCI via using the natural antioxidants derived from TCM.
2 Materials and methods
In this review, an electronic search of published articles published between 2000 and 2021 was conducted from Web of Science, PubMed, Science Direct, Google Scholar, and China National Knowledge Infrastructure (CNKI), with the use of the following keywords: “spinal cord injury, oxidative stress, and traditional Chinese medicine or herbal medicine or Chinese herbal medicine”.
Besides, to ensure the accurate scientific nomenclature for plants in our paper, all names and information on the source species were obtained and validated from Kewscience (http://mpns.kew.org/mpns-portal/) and pharmacopeia of the People’s Republic of China.
3 The pathophysiology of spinal cord injury
The pathophysiology of SCI is complicated, which contains acute phase, subacute phase and chronic phase (Supplementary Figure S1). The primary occurrence of SCI results from the initial trauma immediately, including acute stretch, acute distraction, compression, and axonal shearing (Katoh et al., 2019). The primary injury initiated by trauma will progressively cause the secondary injury, and further leads to the lesion of adjacent, uninjured tissue (Anjum et al., 2020). The pathophysiology of secondary injury mainly includes damage to neuronal fiber, blood-spinal cord barrier (BSCB) destruction, excessive free radical production (von Leden et al., 2017), ion imbalances (Garcia et al., 2018), inflammation (Zrzavy et al., 2021) neural cell necrosis and apoptosis (Wang et al., 2019), demyelination (Fan et al., 2018), Among the pathological processes of secondary injury, oxidative stress plays an indispensable and critical role in contributing to a poor microenvironment development, which is considered a hallmark of the secondary injury of SCI (Savikj et al., 2019).
3.1 The association between oxidative stress and spinal cord injury
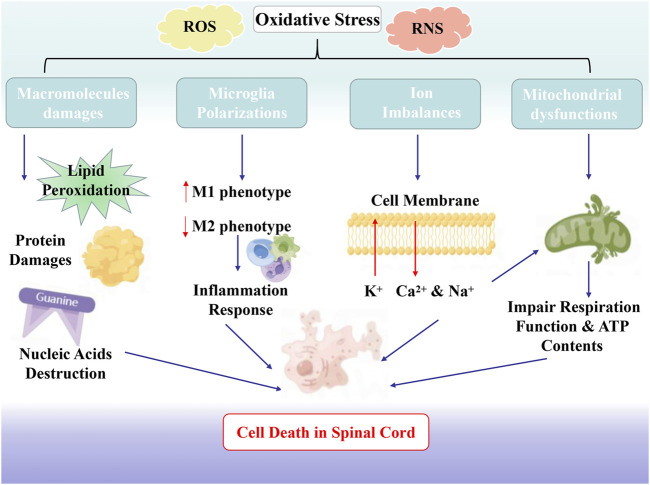
The essence of the OS reaction is the imbalance between oxidative free radicals and antioxidants. Oxidative free radicals include ROS and reactive nitrogen species (RNS) (Fatima et al., 2015). ROS contains superoxide, singlet oxygen, hydroxyl radical, and hydrogen peroxide, mainly generated by the mitochondria and microglia. RNS are also involved in the pathological mechanism of SCI, including nitric oxide (NO) and peroxynitrite (PON). Excessive generation of NO can induce neuronal apoptosis via cytotoxic effects (Cadenas, 2018) and PON are the key initiator of the lipids peroxidation (LP), as well protein nitration in the SCI (Hall et al., 2016) (Figure 1).
FIGURE 1.
The Association Between Oxidative Stress and SCI. Oxidative stress substantively leads to ion imbalance, lipid peroxidation, inflammatory cell infiltration, mitochondrial disorder, and neuronal dysfunction.
3.1.1 Oxidative stress causes the damage of biological macromolecules in spinal cord injury
Notably, the spinal cord is very vulnerable to oxidative damage because it contains overabundant polyunsaturated fatty acids that are particularly susceptible to ROS peroxidation (Anjum et al., 2020). Therefore, LP easily occurs after the trauma, which progressively includes three chemical phases, namely initiation, propagation, and termination (Hall et al., 2016). To begin with, highly reactive oxygen radical with the function of electron-snatching, such as -OH, -NO2, or -CO3, has a reaction with the polyunsaturated fatty acid composition of the membrane, such as arachidonic acid, eicosapentaenoic acid, and linoleic acid, causing the damage of membrane integrity. These types of radicals can be considered to have the characteristic called “electrophilic,” which snatches the electron from polyunsaturated fatty acid, leading to quenching of the original radicals whereas the polyunsaturated fatty acid (L), turns to a lipid radical (L•). As for the stage of propagation, L• sets off a chain reaction of constantly producing lipid peroxyl radicals (LOO•) and lipid hydroperoxides (LOOH). When the peroxidizable substrate is depleted or the reaction of lipid radical and another radical or radical scavenger produces other end products, including three carbon-containing malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE) (Hamann and Shi, 2009; Hall et al., 2010), LP greets its termination (Hall et al., 2016). As essential components of membranes, LP can lead to cellular dysfunction, eventually resulting in cell death.
In addition to lipids, proteins are also easy to be damaged by OS, and the damaging effects of OS on proteins are also fundamental factors of the continuous deterioration of SCI. Protein oxidation mainly includes breakage of the polypeptide chain, modification of amino-acid side chains, and protein degradation, as well as the conversed derivatives from protein, which are sensitive to proteolysis (Hawkins and Davies, 2019). The products of LP, MDA, and 4-HNE can compromise the structural and functional integrity of cellular proteins by undergoing a carbonyl ammonia cross-linking reaction to form covalent compounds (Wu et al., 2017). Besides, PON can trigger an exacerbated overload of intracellular Ca2+ overload, activating the cysteine protease calpain, which ultimately results in the damage of several cellular targets (Xiong et al., 2007).
Finally, the destructive effects of OS on nucleic acid substances are also not negligible factors. It is well documented that guanine is the most oxidation prone-nucleobase (Singh et al., 2019). When structured in the G-quadruplex entity, guanine can respond particularly towards OS (Singh et al., 2019), principally reflected in the generation of 8-oxoguanine via the initiation of 8-oxoguanine glycosylases (Every and Russu, 2013). Overproduced PON can also react with guanine to form 8-nitroguanine (8-NO(2)-G), resulting in the exchange of G and T bases to cause mutagenic damage (Piao et al., 2011). The consequences of base modification are mutations and genomic instability. Currently, the expression of certain microRNAs has been proven to be altered via OS. Excessive ROS generation can enhance the expression of miR-200c by negatively regulating the expression of Fas-associated phosphatase-1 (FAP-1), thereby inducing apoptosis (Yu et al., 2015).
3.1.2 Oxidative stress and microglia polarization in spinal cord injury
Microglia, polarized into two phenotypes: pro-inflammatory (M1) phenotype and anti-inflammatory (M2) phenotype, have great significance to the pathophysiological evolution of SCI (Fan et al., 2018). During acute trauma, microglia in the spinal cord tissue are primarily polarized to the M1 phenotype (Kroner et al., 2014). The polarized M1 has close correlations with the release of TNF-α, IL-6, IL-1β, ROS, NO, glutamate, and superoxide, which can trigger inflammation and OS, further initiating cascades of neurotoxic damage of SCI and contributing to apoptosis and necrosis (Anwar et al., 2016; Tang and Le, 2016). On the contrary, M2 phenotype plays critical role in antagonizing the pro-inflammatory responses and promoting neurodegeneration, accompanied by the release of several neurotrophic factors, anti-inflammatory cytokines (IL-4, IL-10, IL-13), and transforming growth factor beta (TGF-β) (Anwar et al., 2016; Tang and Le, 2016). Meanwhile, M2 phenotype can generate Arg1 that competes with iNOS for the arginine substrate and downregulate NO production, thereby reducing the collateral damage of OS to neuronal tissues (Yang et al., 2016). The homeostasis of the local microenvironment depends on the balance between M1 and M2. Therefore, inhibiting M1 or promoting the polarization of M2 may be an effective therapeutic strategy to promote functional recovery after SCI.
3.1.3 Oxidative stress and ion imbalances in spinal cord injury
The imbalance of Na+, K+, and Ca2+ ions caused by OS is a vital mechanism for triggering necrotic cell death and apoptosis. OS significantly aggravates the influx of Ca2+ and Na+, as well as the decrease of intracellular K+ concentrations via destroying cell membrane structure, which could disturb ionic homeostasis and leads to necrotic cell death and apoptosis (Vanzulli and Butt, 2015). As an indispensable participator of synaptic transmission, Ca2+ plays a critical role in responding to the injuries of the CNS. Ca2+-pump ATPase in plasma and endoplasmic reticulum membrane is highly sensitive to OS. LP caused by OS can interfere with the transportation of Ca2+ and increase the intracellular Ca2+ concentrations via inhibiting the activation of Ca2+-pump ATPase. At the same time, the inhibition of Na+/K + -pump ATPase caused by OS can increase the accumulation of Na+and further promote the accumulation of Ca2+ in cells (Lowry et al., 2020). With the influx of Na+ and the alterations of change of osmotic pressure, cytotoxic cellular edema occurs, further promoting intracellular phospholipase activity and intracellular acidosis (Piao et al., 2011).
3.1.4 Oxidative stress and mitochondrial dysfunctions in spinal cord injury
Mitochondrial dysfunction is an important section of the cascade of traumatic cell death after SCI. Following the exacerbation of injury, there is a loss of homeostasis of mitochondria, together with the imbalance of synaptic homeostasis. As energy reservoirs, mitochondria can regulate cytosolic Ca2+ ion levels (Turtle et al., 2019). After trauma, the mitochondrial damage is mediated by the elevated Ca2+ levels in SCI. Elevated cytosolic Ca2+ levels can activate NADH dehydrogenase, increase ATP generation and promote ROS production. The surfeit of Ca2+ further disturbs the proton gradient and results in cell swelling or death by opening the mitochondrial permeability transition pores (mPTPs) (Cao et al., 2013). Besides, three PON forms (ONOO−, ONOOCO2, and ONOOH) can deplete stores of mitochondrial antioxidants, leading to protein nitration (Valdez et al., 2000). Excessive PON ulteriorly induced 4-HNE, 3-nitrotyrosine (3-NT), and protein carbonyl content in mitochondrial proteins, further aggravating mitochondrial dysfunction (Sullivan et al., 2007). An extremely low concentration of 4-HNE could significantly impair the respiration function of mitochondria (Xiao et al., 2017). Moreover, NO has been also shown to exist in mitochondria stemming from a NO synthase isoform. Because the neurons are particularly sensitive to energy, preventing the lack of energy prone to cause neurodegeneration is crucial. However, widespread damage to mitochondria can finally and easily cause neuronal death owing to the insufficient energy provided for cell survival (Golpich et al., 2017). Hence, mitochondria are highly regarded as the potential vital target for SCI pharmacological treatment. During the first 6 h after SCI, promoting mitochondrial fusion may even be used as a potential method of improving spinal cord function (Cao et al., 2013).
4 Mechanism of oxidative stress in the aetiology of traditional Chinese medicine for spinal cord injury
Therefore, alleviating OS is a critical step in therapeutic intervention in SCI. Seeking the appropriate antioxidant therapy to prevent OS after the injury has become a top priority. With its natural antioxidant capacity, TCM has recently become a novel and promising supplementary treatment. The TCM theory holds that the pathogenesis of SCI is characterized by blood stasis and deficiency of qi. In the theory of TCM, qi is regarded as a very delicate substance with strong vitality. Constituting the human body and maintaining human life activities, the concept of qi is consistent with the adenosine triphosphate (ATP) that is mainly produced in the mitochondrion (Wong and Ko, 2013; Yong et al., 2020). Moreover, Some TCM with the “Qi-invigorating” action has a close link to the safeguarding of mitochondrial function (Kang et al., 2020; Tian et al., 2021). Mitochondrial dysfunction induced by OS resembles the deficiency of qi. The function of qi lies in the power to promote blood flow. When the power of push is insufficient, flowing blood will generate the pathological product, blood stasis. The blockade of blood stasis obstructs the transportation and absorption of nutrients in channels and collaterals, leading to the body not being nurtured, which is consistent with the neurodegeneration caused by the lack of energy owing to the mitochondrial dysfunction induced by OS. The equivalence between qi deficiency and mitochondrial dysfunction provides a modern microbiology perspective for TCM to understand the pathological mechanism. Thus, TCM can treat SCI by supplementing qi, activating blood circulation, and removing blood stasis. Additionally, from the modern perspective, as natural antioxidants, TCM intervenes in SCI by attenuating OS and preventing mitochondrial dysfunction. The overlap of the two theories makes the feasibility of auxiliary treatment of SCI with TCM.
5 Therapeutic intervention with traditional Chinese medicine
Attracting a great quantity of attention, TCM has been considered a promising supplementary treatment in recent years. Active herbal extracts, metabolites (Tables 1, 2), traditional botanical drugs (Table 3), and formulas (Table 4) all have shown their effectiveness, playing vital roles in the prevention and treatment of SCI, especially as natural antioxidants.
TABLE 1.
Therapeutic intervention with active herbal extracts and metabolites.
| Active herbal extracts and metabolites | |||||
|---|---|---|---|---|---|
| Name | Source | Molecular formula | Molecular structure | Mechanisms | Effects on SCI |
| Quercetin | Bupleurum chinense DC [Apiaceae; Bupleuri Radix] and Morus alba L. [Moraceae; Mori Folium] | C15H10O7 |
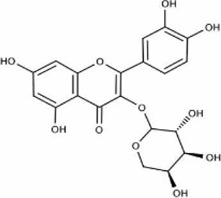
|
Decreases MDA and iNOS-positive cells, activates ATF2 pathway and suppresses NF-κB and STAT1 pathway | Decreases necroptosis, antioxidant properties |
| Gastrodin | Gastrodia elata Blume [Orchidaceae; Gastrodiae rhizome] | C13H18O7 |
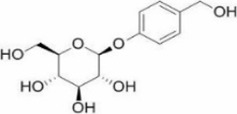
|
Enhances expressions of Nrf2 and modifies subunit of GCLm and GCLc, decreases ROS and MDA | Recovery of locomotor function and antioxidant properties |
| Asiatic acid | Centella asiatica (L.) Urb. [Apiaceae; Centeliae herba] | C30H48O5 |
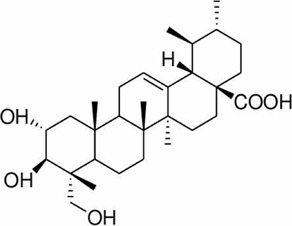
|
Suppresses myeloperoxidase activity, reduces ROS and MDA and blocks NF-kB/STAT3/ERK pathway | Increases BBB scores and inclined plane test scores, antioxidant effects |
| Tetramethylpyrazine | Ligusticum chuanxiong Hort. [Apiaceae; Chuanxiong Rhizoma] | C8H12N2 |
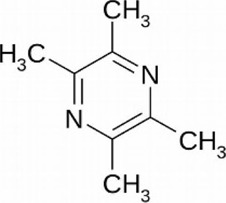
|
Reduces ROS production and inhibits p53/MAPK pathways | Prevents mitochondria dysfunction, antioxidant effects |
| Schisandrin B | Schisandra chinensis (Turcz) Baill. [Schisandraceae; Schisandrae Chinensis Fructus] | C23H28O7 |
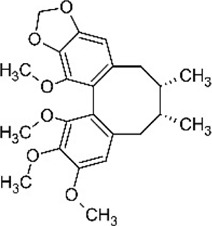
|
Increases SOD expression and decreases MDA expression | Improves the inclined plate test scores, antioxidant properties |
| Rosmarinic acid | Perilla frutescens (L.) Britton. [Lamiaceae; Perillae Fructus] | C18H16O8 |
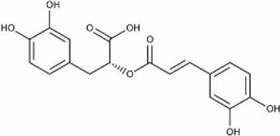
|
Decreased ROS and activates Nrf2 and HO-1 pathway | Mitigates cytotoxicity and inflammatory injury, antioxidant properties |
| Allicin | Allium sativum L. [Amaryllidaceae; Allii Sativi Bulbus] | C6H10OS2 |
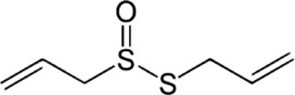
|
Increases Nrf2 nuclear translocation, inhibits ROS and LP. Inhibit lipid peroxidation by quenching free radicals | Accelerates recovery of motor functions, antioxidant properties |
| Resveratrol | Polygonum cuspidatum Sieb. et Zucc. [Polygonaceae; Polygoni Cuspidati Rhizoma et Radix] | C14H12O3 |
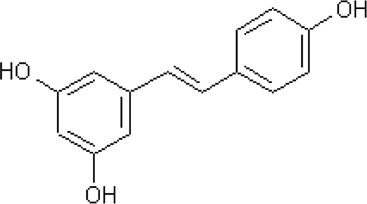
|
Inhibits the iNOS/p38MAPK pathway and activates Nrf2/HO-1 pathway | Strong antioxidant effects |
| Crocin | Crocus sativus L. [Iridaceae; Croci Stigma] | C44H64O24 |
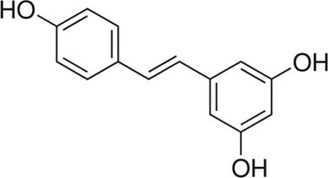
|
Enhances the expression level of neurotrophic factors in epidermal neural crest stem cells | Promotes spinal cord regeneration |
| Tetrandrine | Stephania tetrandra S.Moore [Menispermaceae; Stephaniae Tetrandrae Radix] | CHNO |
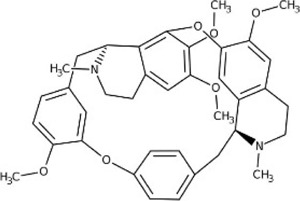
|
Reduces the production of pro-inflammatory factors and regulates PI3K/AKT/NF-κB pathway | Repairs the integrity of the blood-spinal cord barrier |
| Lycopene | Tomatoes, watermelons, and pink grapefruits | C40H56 |
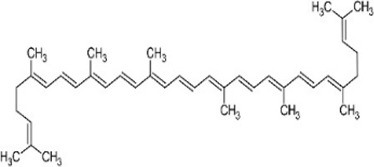
|
Inhibits MDA and LP | Strong antioxidant effects |
| Curcumin | Curcuma longa L. [Zingiberaceae; Curcumae Longae Rhizoma] | C21H20O6 |
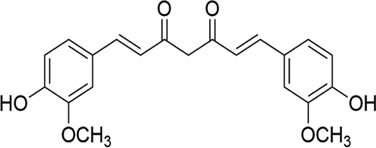
|
Enhances ability of lymphocytes to resist oxidative stress, increases the SOD content and protects the integrity of mitochondrial membranes | Improves mitochondrial dysfunction and strong antioxidant effects |
TABLE 2.
The specific data of references cited in Section 5.1
| Active herbal extracts and monomers | Study type | Experimental subjects | Dose | Duration | Controls |
|---|---|---|---|---|---|
| Quercetin | Preclinical trial | 40 male rats aged 18 months | In vivo: 20 mg/g/d, i.p | 10 days | 1 ml distilled water both intraperitoneally and subcutaneously |
| Preclinical trial | 240 adult male Sprague–Dawley rats | In vivo: 0.2 mg/kg/d, i.p | 14 days | Methylprednisolone 30 mg/kg/d, i.p | |
| Gastrodin | Preclinical trial | 60 male Sprang-Dawley rats | In vivo: 100 or 200 mg/kg/d, i.p | 5 days | Intraperitoneally treated with vehicle |
| Preclinical trial | 36 New Zealand white rabbits | In vivo: 100 mg/kg/d | 1 day | Perfuse normal saline 100 mg/kg | |
| Asiatic acid | Preclinical trial | 32 adult male Sprague-Dawley rats | In vivo: 75 mg/kg | 1 day | Vehicle |
| Preclinical trial | 150 Sprague-Dawley rats | In vivo: 30 mg/kg/d or 30 mg/kg/d intragastric injection | 3 days | Intragastric injection of vehicle 1 h after SCI | |
| Tetramethylpyrazine | Preclinical trial | Adrenal phaeochromocytoma PC12 cells | In vitro: 0.3, 0.6, and 1.2 μM | 10, 30, 60, and 120 min | Saline |
| Preclinical trial | 30 Sprague-Dawley rats | In vivo: 30 mg/kg, i.p | 30 min before occlusion | Normal saline | |
| Schisandrin B | Preclinical trial | 40 adult male Sprague-Dawley rats | In vivo: 50 mg/kg, orally | 5 days | Physiological saline (0.1 ml/100 g, i.p |
| Rosmarinic acid | Preclinical trial | 60 adult female Sprague-Dawley rats | In vivo: 10, 20, and 40 mg/kg, i.p | 28 days | Normal saline, i.p |
| Carnosic acid | Preclinical trial | Adult (8 weeks old) male CF-1 mice | In vivo: 0.3, 1.0, and 3.0 mg/kg, i.p | 2 days | Saline |
| Preclinical trial | Adult (8 weeks old) male CF-1 mice | In vivo: 1.0 mg/kg, i.p | 2 days | Sulforaphane 5.0 mg/kg | |
| Allicin | Preclinical trial | 90 female Sprague-Dawley rats | In vivo: 2, 10, and 50 mg/kg/d, i.p | 21 days | 0.9% NaCl daily |
| Preclinical trial | 40 adult BALB/c mice | In vivo: 1, 5, and 10 mg/kg/d, i.p | 7 days | 2 ml sterile saline | |
| Resveratrol | Preclinical trial | 35 Male Sprague–Dawley rats | In vivo: 10 mg/kg, i.p | 36 h | 1 ml of saline, i.p |
| Preclinical trial | 42 male Sprague-Dawley rats | In vivo:100 mg/kg | 48 h | Quercetin 200 mg/kg i.p | |
| Crocin | Preclinical trial | Epidermal neural crest stem cells | In vitro: 12.5, 50, 100, 200, 500, 1,000, 1,500, 2,000, and 2,500 µM | 72 h | 1 mM valproic acid |
| Preclinical trial | 25 Female Wistar rats | In vivo: 150 mg/kg/d, i.p | 14 days | Vehicle | |
| Tetrandrine | Preclinical trial | Spinal cord astrocytes | In vitro: 0.1, 1, 10, and 20 mM | 24 h | PI3K inhibitor LY294002 and NF-κB inhibitor PDTC |
| Preclinical trial | 48 healthy adult male or female New Zealand white rabbits | In vivo: 22.5 mg/kg, i.p | Before Ischemia reperfusion injury | Saline | |
| Lycopene | Preclinical trial | 30 adult male SD rats | In vivo: 5,10,20 mg/kg/d, i.p | 7 days | Saline |
| Curcumin | Preclinical trial | 40 male Wistar rats | In vivo: 200 mg/kg, i.p | Immediately after the trauma | 1 ml of rice bran oil and 30 mg/kg methylprednisolone sodium succinate |
| Preclinical trial | 39 male Sprague-Dawley rats | In vivo: 40 mg/kg/d, i.p | 6 days | Saline |
TABLE 3.
Therapeutic intervention with Chinese herbs.
| Chinese herbs | |||||
|---|---|---|---|---|---|
| Name | Species and source | Components | Picture | Effects in TCM theory | Antioxidant mechanisms |
| Salvia miltiorrhiza Bunge | Lamiaceae; Salviae Miltiorrhizae Radix et Rhizome | Hydrophilic depside derivatives (e.g., danishes, salvianolic acids A–C, E–G, caffeic acid, and ferulic acid) and lipophilic diterpenoids (e.g., tanshinones Ι, ΙΙA, andΙΙB, tanshinoneA, and tanshindiols A and B) |

|
Promotes blood circulation, removes blood stasis, and reduces pain | Improves SOD, decreases the MDA, inhibits MAPK pathways, and preserves CAT activities |
| Cistanche deserticola Ma | Orobanchaceae; Cistanches herba | Total glycosides (TGs, phenylethanoid glycosides, and other glycosides) and oligosaccharides |

|
Tonifies yang qi and blood, and moistens the intestines | Reduces p53, IL-6, and TNF-α, decreases MDA, increases SOD, GSH-Px, and CAT, facilitates Nrf-2 nuclear translocation |
TABLE 4.
Therapeutic intervention with traditional formulas.
| Name | Effects in TCM theory | Mechanisms |
|---|---|---|
| JisuiKang | Removes blood stasis and dredge meridians, reconciles qi and blood | (1) Protects the microstructure of neurons, such as mitochondria, dendritic spine, and endoplasmic reticulum |
| (2) Inhibits the expression of the Nogo receptor (NgR) in neurons and reduces the activation of the NgR/RhoA/ROCK signal pathway | ||
| (3) Inhibits the expression of NOS and the content of NO and MDA while improving the activity of SOD | ||
| Xuefuzhuyu Decoction | Promotes blood circulation and dissolves stasis | (1) Reduces the content of MDA in the spinal cord and promotes the relief of spinal cord edema |
| (2) Improve the content of SOD |
5.1 Therapeutic intervention with active herbal extracts and metabolites
5.1.1 Quercetin
Quercetin (C15H10O7), is abundant in a variety of plants, fruits, and vegetables, such as onion annd broccoli, as well as Bupleurum chinense DC [Apiaceae; Bupleuri Radix] and Morus alba L. [Moraceae; Mori Folium], which is one of the major flavonoids that are part of human diets. It plays a role in the prevention of various diseases such as cancer and cardiovascular diseases (Reyes-Farias and Carrasco-Pozo, 2019).
In recent years, it has been revealed that quercetin could protect the injured spinal cord by decreasing oxidative. Aged rats with quercetin (20 mg/kg/d intraperitoneally for 10 days) had a decreased level of MDA and reversed the major degenerative changes, restraining OS (Firgany and Sarhan, 2020). The underlying mechanism may be related to the regulation of p38 mitogen-activated protein kinase (MAPK) and activating transcription factor 2 (ATF2) pathway, thus quercetin antagonized OS. Even compared to the specific p38MAPK inhibitor SB203580, quercetin has stronger effects on enhancing SOD activity and inhibiting MDA in SCI rats. Immunohistochemistry consequences revealed that after quercetin administration (0.2 mg/kg/d intraperitoneally for 14 days), the postoperatively elevated rate of iNOS-positive cells was significantly decreased (Song et al., 2013).
5.1.2 Gastrodin
Gastrodin (C13H18O7) is a phenolic glycoside compound extracted from Gastrodia elata Blume [Orchidaceae; Gastrodiae rhizome]. Studies have indicated that gastrodin has various pharmacological effects, such as antihypertensive, lipid-lowering, and anticoagulant, which also beneficial functions in the protection of neurons, by inhibiting OS, regulating immune inflammation, and regulating ion channels (Liu et al., 2018).
Recently, gastrodin has emerged as a potential treatment for SCI. Administration of gastrodin (100 or 200 mg/kg/d intraperitoneally for 5 days) could enhance expressions of nuclear factor (erythroid-derived 2)-like 2 (Nrf2), modified subunit of γ-glutamylcysteine ligase (GCLm), and catalytic subunit of γ-glutamylcysteine ligase (GCLc), and subsequently improve the OS and recovery of locomotor function, as illustrated by the accumulation of Basso-Beattie-Bresnahan (BBB) scores (Du et al., 2016). Similarly, the spinal cord ischemia-reperfusion injury model by blocking the abdominal aorta under the renal artery and intervening with gastrodin showed an increase in SOD, glutathione peroxidase (GSH-Px), and total antioxidant capacity (T-AOC), as well as the decrease in ROS and MDA. Simultaneously, the reduction of mitochondrial swelling (MSD) also confirmed the effects of gastrodin (100 mg/kg/d intraperitoneally for 1 day) on protecting spinal cord ischemia-reperfusion injury by promoting the antioxidant capacity of spinal cord mitochondria and inhibiting the inflammatory response to the injury (Fang et al., 2016).
5.1.3 Asiatic acid
Asiatic acid (AA, C30H48O5) is a naturally occurring pentacyclic triterpenoid, which is found mainly in the Centella asiatica (L.) Urb. [Apiaceae; Centeliae herba]. It has been reported that AA has broad-spectrum anticancer abilities.
Current research has shown that AA can be used to protect damaged spinal cord by reducing OS. AA treatment (75 mg/kg/d intraperitoneally for 1 day) significantly increased BBB scores and inclined plane test scores that were reduced by acute SCI. In addition, AA suppressed myeloperoxidase activity and reduced the levels of pro-inflammatory cytokines, ROS, and MDA, while increasing superoxide dismutase activity and glutathione production (Gurcan et al., 2017). Additionally, intragastric injection with AA (30 mg/kg or 75 mg/kg) was demonstrated that could lead to the downregulation of ROS, MDA, and tumor necrosis factor-alpha, while upregulating superoxide dismutase activity and glutathione production, which may be related to the inhibition of NLRP3 inflammasome pathway and the activation of Nrf2 and HO-1 (Jiang et al., 2016).
5.1.4 Tetramethylpyrazine
Tetramethylpyrazine (TMP, C8H12N2), a natural alkaloid which is isolated from the Chinese botanical drug Ligusticum chuanxiong Hort. [Apiaceae; Chuanxiong Rhizoma], has been found to have antioxidant and anti-inflammatory effects (AlKreathy et al., 2020).
Previous study reported the TMP treatment (30 mg/kg, intraperitoneally 30 min before occlusion) decreased the expression level of proinflammatory cytokines TNF-α and IL-1β, and inhibited the activation of NF-κB in spinal cord ischemia rats (Fan et al., 2011). Recently, TMP and monosialotetrahexosylganglioside (GM1) have been effectively used in the treatment of SCI. It is well documented that Selenium nanoparticles (SeNPs) have excellent antioxidant activity. As a novel multi-functionalized SeNPs, SeNPs@GM1/TMP, being loaded with TMP/GM1 and decorated with polysaccharide-protein complex (PTW)/PG-6 peptide, showed a strong protective effect against apoptosis after SCI. SeNPs@GM1/TMP (0.3, 0.6 and 1.2 μM) attenuated ROS overproduction via inhibiting p53 and MAPK in concentration dependent, thus preventing mitochondria dysfunction (Rao et al., 2019).
5.1.5 Schisandrin B
Schisandrin B (Sch B, C23H28O7), a dibenzocyclooctadiene lignan, is one of the most abundant active dibenzocyclooctadiene derivatives found in the Schisandra chinensis (Turcz.) Baill. [Schisandraceae; Schisandrae Chinensis Fructus]. Several studies had shown that Sch B has anti-fibrosis effects (Cuiqiong et al., 2020) and has a protective effect on myocardial injury (Zhang et al., 2017).
Currently, Xin et al. (2017) found that Sch B (50 mg/kg/day orally for 5 days) reduced the inflammatory response and inhibited OS in SCI model rats. ELISA kits showed that Sch B significantly increased SOD expression and decreased MDA expression levels compared with the untreated SCI group, which may be related to the inhibition of the P53 signaling pathway. Besides, Sch B improved the maximum angle of inclined plate test, behavioral examination scores, and inhibited spinal cord water content in rats with SCI.
5.1.6 Rosmarinic acid
Rosmarinic acid (RA, C18H16O8) exists in Perilla frutescens (L.) Britton. [Lamiaceae; Perillae Fructus]. RA was proved with multiple biological activities, including, anti-inflammatory, neuroprotective, and antiangiogenic abilities (Colica et al., 2018).
In the animal experiment conducted by Ma et al. (2020), they found that RA notably upregulated the activities of CAT, SOD and GSH-Px and downregulated the MDA levels, indicating the attenuation of SCI-induced oxidative damage. Among the three groups designed in their study, the highest content administration of RA (40 mg/kg) showed significantly highest levels of SOD, CAT and GSH-Px than groups with 20 mg/kg and 10 mg/kg RA (p < 0.05). Besides, Treatment with RA could remarkably increase Nrf2 and HO-1 levels to ameliorate the increase in oxidative injury and apoptosis induced by H2O2. Meanwhile, the activation of the Nrf2/HO-1 pathway further amplified the inhibition of the NF-κB pathway, mitigating LPS-induced cytotoxicity and inflammatory injury.
5.1.7 Allicin
Allicin (C6H10OS2), a thioester of sulfenic acid, is a natural antioxidant found in Allium sativum L. [Amaryllidaceae; Allii Sativi Bulbus]. Studies have shown that allicin has a good effect on a variety of diseases, such as bone diseases (Ding et al., 2016; Yang et al., 2020; Li et al., 2021) and neurodegenerative diseases (Zhang et al., 2018).
It is noteworthy that allicin played an important therapeutic role in SCI by inhibiting OS. SCI rats administrated with allicin showed better histological outcomes and accelerating recovery of motor functions, which may be related that allicin increased Nrf2 nuclear translocation in SCI rats (Lv et al., 2015). Additionally, the protective effects of allicin on antioxidation in SCI rats were dependent on the elevated production of NADH and inhibited levels of ROS (Wang and Ren, 2016). In the SCI model induced by glutamate, allicin was also shown to attenuate the release of lactate dehydrogenase (LDH), and inhibit OS by the mediation of heat shock protein 70 (HSP70)/inducible nitric oxide synthase (iNOS) pathway (Wang and Ren, 2016). Collectively, allicin may serve as a new therapeutic strategy for spinal cord injury (Liu et al., 2015).
5.1.8 Resveratrol
Resveratrol (C14H12O3) is a kind of natural non-flavonoid polyphenol found in many vegetables and fruits, mainly in grapes and Polygonum cuspidatum Sieb. et Zucc. [Polygonaceae; Polygoni Cuspidati Rhizoma et Radix]. Numerous studies have shown that resveratrol can serve as a good antioxidant, improving overall health by inhibiting OS (Wang et al., 2013; Chen et al., 2020; Recalde et al., 2020).
In the animal experiment conducted by Fu et al., resveratrol (10 mg/kg intraperitoneally) functioned as a strong antioxidant to protect the ischemia-damaged spinal cord by inhibiting the iNOS/p38MAPK signaling (Fu et al., 2018). Moreover, resveratrol (100 mg/kg intraperitoneally) was found that it had a good preventive effect on secondary injury caused by SCI (Çiftçi et al., 2016).
5.1.9 Crocin
Crocin (C44H64O24), belonging to apocarotenoid glycosides, is the main colorant and bioactive ingredient in Crocus sativus L. [Iridaceae; Croci Stigma]. Studies have shown that crocin has attracted much attention in the field of food and cosmetics. It can be used to produce functional products and as an anti-aging cosmetic agent for the skin (Fagot et al., 2018). Despite being used in dermatology, the results of Abdulkareem Aljumaily et al. (2021) verified that it can be used as a heart-protective agent for patients with cancers.
In recent years, crocin also has been found to have a significant effect on SCI. A recent investigation demonstrated that crocin may have therapeutic effects on SCI by enhancing the expression level of neurotrophic factors in epidermal neural crest stem cells (Baharvand et al., 2020). SCI rats treated with crocin (150 mg/kg/d intraperitoneally for 2 weeks) presented a notably reduced level of calcitonin gene-related peptide than the control group (Karami et al., 2013). In a word, crocin can greatly promote spinal cord regeneration and is an effective drug for treating SCI (Terraf et al., 2017).
5.1.10 Tetrandrine
Tetrandrine (CHNO) is an alkaloid, which mainly exists in the dry roots of Stephania tetrandra S. Moore [Menispermaceae; Stephaniae Tetrandrae Radix]. In recent years, studies have investigated that it can lessen lung damage, having a potential role in the treatment of asthma (Lin et al., 2019; Lin et al., 2020).
As for the aspect of tetrandrine treating SCI, scholars found tetrandrine was capable to protect damaged spinal cord astrocytes in rats and diminished the accumulation of IL-1β, IL-6, and TNF-α, which may be related to PI3K/AKT/NF-κB signaling pathway (Bao et al., 2016). After being intravenously injected with tetrandrine (22.5 mg/kg), New Zealand white rabbits with ischemia-reperfusion injury of the spinal cord showed a notable repair of the integrity of the blood-spinal cord barrier, related to that tetrandrine could change the BCL-2/Bax ratio and reduce the production of pro-inflammatory factors (Pu et al., 2020).
5.1.11 Lycopene
Lycopene (C40H56), an open-chain unsaturated structure, is the most important carotenoid in human plasma. Tomatoes, watermelons and pink grapefruits, are all rich sources of lycopene (Grabowska et al., 2019). As a functional pigment, lycopene has a variety of biological effects, among which the antioxidant effect has received extensive attention.
In recent years, numerous studies have found that lycopene could inhibit the production of MDA, and effectively resist ROS-mediated lipid peroxidation, thereby reducing the secondary damage of free radicals to spinal blood vessels and nerve cells. Animal experiments showed that lycopene also had an antioxidant effect on mitochondrial dysfunction in SCI rats. The experimental data showed that the mtDNA content and function-related genes-cytochrome b and mitochondrial organism’s function-Tfam of the SCI group significantly decreased. However, these changes were significantly hyperpolarized after intraperitoneally injecting lycopene (Hu et al., 2017). In addition, the outstanding effects of lycopene were directly proportional to the use time.
5.1.12 Curcumin
Curcumin (C21H20O6) is a natural polyphenol compound, which is extracted from traditional Chinese medicine Curcuma longa L. [Zingiberaceae; Curcumae Longae Rhizoma]. Studies have found that curcumin’s properties of anti-inflammatory, anti-glial cells, and antitumor activity (Vollono et al., 2019) (Barua and Buragohain, 2021).
In recent years, research results inferred that curcumin may play a therapeutic effect in SCI. In an animal experiment (Cemil et al., 2010), researchers applied curcumin (200 mg/kg/d intravenously for 1 day) to treat aneurysm clamp SCI and detected enzyme changes in the tissue. Their BBB score confirmed the recovery of nerve function after administrating with curcumin. Lin and his colleagues (Lin et al., 2011) established the spinal cord hemisection injury model and observed that curcumin (40 mg/kg/d intravenously for 6 days) could improve the motor function of SCI rats. The mechanism may be related to that curcumin increased the SOD content in spinal cord tissue, thereby promoting the production of GSH in astrocytes, improving mitochondrial dysfunction and lipid peroxidation, along with protecting the integrity of mitochondrial membranes.
5.2 Therapeutic intervention with Chinese botanical drugs
5.2.1 Salvia miltiorrhiza Bunge
As one of the most famous Chinese botanical drugs, Danshen, namely Salvia miltiorrhiza Bunge [Lamiaceae; Salviae Miltiorrhizae Radix et Rhizome] has the effects of promoting blood circulation, removing blood stasis, and reducing pain in TCM theory. Danshen has plenty of components, majorly including hydrophilic depside derivatives (e.g., danishes, salvianolic acids A–C, E–G, caffeic acid, and ferulic acid) and lipophilic diterpenoids (e.g., tanshinones Ι, ΙΙA, andΙΙB, tanshinoneA, and tanshindiols A and B).
Recently, studies found that many components of Danshen could play a critical antioxidant role in improving SCI. Dihydrotanshinone I (DI) could alleviate the pathological damage to the spinal cord and promote neuronal functional recovery by suppressing iNOS, and total oxidant status levels, while improving the (total antioxidant status) TAS level. Moreover, the experiment revealed that the HMGB1/TLR4/NOX4 pathway may participate in the effects of DI on SCI (Yu and Qian, 2020). Intraperitoneal administration with tanshinone IIA (TIIA) inhibited OS by significantly rescuing the activity of SOD and decreasing the MDA. Notably, TIIA had strong analgesic actions via inhibiting MAPKs pathways to depress the activation of microglial and immune responses (Cao et al., 2015). Salvianolic acid B(SalB) In the SCI rat models, groups administered with SalB markedly preserved the activities of SOD and CAT, playing an antioxidant role. (Fu et al., 2014).
5.2.2 Cistanche deserticola ma
Cistanche deserticola Ma [Orobanchaceae; Cistanches herba] is a commonly used drug for tonifying yang qi and blood, and moistening the intestines in TCM theory. Modern medicine has proved that Cistanche deserticola is effective for cardiovascular and cerebrovascular diseases (Wang et al., 2020). A considerable number of components of Cistanche deserticola were shown to be antioxidants, including total glycosides (TGs, phenylethanoid glycosides, and other glycosides) and oligosaccharides.
It was reported that oligosaccharides from Cistanche deserticola significantly altered LP, GSH, superoxide dismutase, acetylcholine esterase, catalase, nitric acid, acetylcholine esterase, and ROS in the SCI rats. Extract supplementation of oligosaccharides reduced mRNA expression levels of iNOS, p53, IL-6, TNF-α and cyclooxygenase-2 more than 20%, showing effective effects against inflammation, apoptosis, and OS (Zhang et al., 2019). Moreover, according to the experimental results of Wang et al. (2020) TGs could significantly decrease MDA levels, while increasing antioxidant activities, such as SOD, GSH-Px, and CAT, via remarkably facilitating Nrf-2 nuclear translocation. Additionally, phenylethanoid glycosides, an extract of Cistanche deserticola also had the ability to enhance SOD activity and decreasing MDA content (Xuan and Liu, 2008).
5.3 Therapeutic intervention with traditional formulas.
5.3.1 JisuiKang
JSK, a TCM formula derived from classic prescriptions Buyang Huanwu Decoction, is composed of Plantago asiatica L. [Plantaginaceae; Plantaginis Semen] 15 g, Paeonia lactiflora Pall. [Paeoniaceae; Paeoniae Radix Rubra] 12 g, Ligusticum chuanxiong Hort. [Apiaceae; Chuanxiong Rhizoma] 10 g, Rheum palmatum L. [Polygonaceae; Rhei Radix et Rhizoma] 10 g, Angelica sinensis (Oliv.) Diels [Apiaceae; Angelicae Sinensis Radix] 12 g, Salvia miltiorrhiza Bunge [Lamiaceae; Salviae Miltiorrhizae Radix et Rhizome] 20 g, Poria cocos (Schw.) Wolf [Polyporaceae; Poria] 10 g, Magnolia officinalis Rehd. et Wils. [Magnoliaceae; Magnoliae Officinalis Cortex] 10 g; Astragalus mongholicus Bunge [Fabaceae; Astragali Radix] 30 g, Cistanche deserticola Ma [Orobanchaceae; Cistanches herba] 10 g, Eupolyphaga sinensis Walker [Corydidae; Eupolyphaga Steleophaga] 10 g, Scolopendra subspinipes mutilans L. Koch [Scolopendridae; Scolopendra] 10 g, Epimedium brevicornu Maxim. [Berberidaceae; Epimedii Folium] 10 g, Alpinia oxyphylla Miq. [Zingiberaceae; Alipiniae Oxyphyllae Fructus] 10 g, Alisma orientale (Sam.) Juzep. [Alismataceae; Alismatis Rhizoma] 10 g, Citrus aurantium L. [Rutaceae; Aurantii Fructus Immaturus] 10 g. Many clinical tests on SCI patients have demonstrated that JSK has satisfactory clinical efficacy (Guo et al., 2017). JSK could improve the motor function of SCI rats by protecting the microstructure of neurons, such as mitochondria, dendritic spine, and endoplasmic reticulum. Moreover, JSK inhibited the expression of the Nogo receptor (NgR) in neurons and reduced the activation of the NgR/RhoA/ROCK signal pathway to improve the motor function of SCI rats. These effects indicated the clinical values of JSK as a potential nerve regeneration agent (Wu et al., 2020).
As for the aspects of antioxidation, JSK could inhibit the expression of NOS and decrease the content of NO and MDA, while improving the activity of SOD. Thus, JSK directly inhibited the process of LP after SCI, as well as weakened the secondary degeneration and necrosis of the spinal cord caused by free radicals. On the other hand, JSK could gradually enhance the activity of endogenous antioxidants and enhance the scavenging effects of free radicals (Zhou et al., 2009). Moreover, in the clinical trial including 84 SCI patients, Wang et al. (2008) demonstrated that JSK treatment had significantly beneficial effects in improving kinetic score, grades of spinal injury and effectiveness of the treatment (p < 0.05). Clinical trial including 68 SCI patients demonstrated that the effective rate of JSK treatment was 94.3%, which may be related to the inhibited expressions of GFAP and CSPG proteins (Shao, 2020).
5.3.2 Xuefuzhuyu decoction
Originated in Qing Dynasty, XFZYD is considered one of the most significant decoctions for promoting blood circulation and dissolving stasis to treat cardiovascular and cerebrovascular diseases. The abundant meta-analysis, clinical trials, and animal experiments showed that XFZYD had effects on treating hyperlipidemia (Liao et al., 2014), coronary heart disease (Liao et al., 2014), liver fibrosis (Zhou et al., 2014), and so on. Composed of 11 kinds of traditional botanical drugs, including Angelica sinensis (Oliv.) Diels [Apiaceae; Angelicae Sinensis Radix] 9 g, Rehmannia glutinosa Libosch. [Orobanchaceae; Rehmanniae Radix] 9 g, Prunus persica (L.) Batsch [Rosaceae; Persicae Semen] 12 g, Citrus aurantium L. [Rutaceae; Aurantii Fructus] 6 g, Paeonia lactiflora Pall. [Paeoniaceae; Paeoniae Radix Rubra] 6 g, Carthamus tinctorius L. [Asteraceae; Carthami Flos] 9 g, Ligusticum chuanxiong Hort. [Apiaceae; Chuanxiong Rhizoma] 5 g, Bupleurum chinense DC. [Apiaceae; Bupleuri Radix] 3 g, Glycyrrhiza uralensis Fisch. [Fabaceae; Glycyrrhizae Radix et Rhizoma] 6 g, Platycodon grandiflorum (Jacq.) A. DC. [Campanulaceae; Platycodonis Radix] 5 g, Achyranthes bidentata Blume [Amaranthaceae; Achyranthis Bidentatae Radix] 9 g, XFZYD was found to be beneficial for the treatment of SCI by attenuating OS.
XFZUD could promote the microcirculation reperfusion of spinal cord stasis, reduce the content of MDA in the spinal cord and promote the relief of spinal cord edema, so as to protect the injured spinal cord (Liu et al., 2006). Meanwhile, a current study showed that the content of SOD in XFZYD containing serum group was significantly higher than that in the injury control group and blank serum group (Liu et al., 2017). Besides, the level of SOD in the XFZYD group was similar to that in the neurotrophic factor protection (NFP) group, and this similitude also existed in the cell survival rate of these two groups (XFZYD group = 0.529 ± 0.010, NFP group = 0.548 ± 0.056) (Liu et al., 2017).
6 Conclusion
The complicated pathophysiologic mechanisms contained in SCI lead to the existing circumstances that fully restorative treatments of SCI do not exist. Among the various pathophysiologic mechanisms, OS plays a vital role in secondary injury, triggering a series of free-radical-mediated damages, including damages to biological macromolecules, ion imbalances, and mitochondrial dysfunctions. Thus, lessening OS may become an effective therapeutic strategy for SCI.
From classical TCM theory, the mentioned active herbal extracts, metabolites, traditional botanical drugs, and formulas can treat SCI via invigorating Qi, activating blood circulation, and removing blood stasis, which is in line with our above-mentioned TCM treatment principles for SCI. On the other hand, from the modern perspective, the mentioned TCM intervene in SCI by suppressing M1, enhancing SOD activity, decreasing MDA levels, promoting mitochondrial functions, and other pathways to attenuate the impacts of OS on SCI. In the treatment of SCI, traditional botanical drugs and formulas used for thousands of years, have the advantages of synergistic effect and multitarget action, which are more in line with the medication law of TCM theory. However, “Drug-Drug Interaction” (DDI) occurs in the traditional botanical drugs and formulas owing to their numerous compounds, which may cause the complication of predicting and preventing adverse reactions. On the contrary, active herbal extracts and metabolites, new concepts proposed in modern medicine compared to traditional botanical drugs and formulas, have the superiority of simple composition, direct targeting, high safety, and clear effects, avoiding the problems of adverse reactions. Therefore, active herbal extracts and metabolites with scavenging capacity may be more suitable to be the supplementary treatment to improve SCI, particularly resveratrol and quercetin.
However, the investigations on the application of TCM in SCI are generally published in low-impact journals, suggesting that this field of study is still in the initial stages. Additionally, promising results have been widely verified in animal models, instead of the implementation in human clinical applications, which makes us more aware of the obstacles and limitations of TCM in SCI. Therefore, in-depth studies in this study are extremely necessary. Accordingly, we make the following three recommendations and perspectives for subsequent research in this area: 1) to comprehensively understand traditional botanical drugs and formulas, more clinical investigations need to be done, including underlying possible therapeutic mechanisms, the best route of administration, dosage, and timing. 2) combine the active herbal extracts and metabolites with emerging nanotechnology or modern tissue scaffold therapies, improve the effect of nerve function repair and reconstruction after SCI. 3) in-depth research on the traditional theory of TCM and the mechanism of antioxidants in TCM is needed to reveal the modern mechanism of the traditional efficacy of benefiting qi in TCM theory, thereby identifying appropriate treatments for SCI within the TCM treatment protocol.
Author contributions
ZH and JW conceived the presented idea. JT was responsible for the planning and administration of the topic of this article. ZH took the lead in drafting the manuscript with input from all authors. CL, JW, and ZW collected the literatures and analyzed them. HZ and JH interpreted results from a clinical point of view. All authors read and approved the final manuscript.
Funding
This work was funded by The Second Batch of Scientific Research Projects for the Business Construction of the National Traditional Chinese Medicine Clinical Research Base (NO. JDZX2015268). Relevant picture materials are from Figdraw. The free ID is 533420148.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.976757/full#supplementary-material
Glossary
- 3- NT
3-nitrotyrosine
- 4- HNE
4-hydroxynonenal
- AA
asiatic acid
- ATF2
activating transcription factor 2
- ATP
adenosine triphosphate
- ATRA
all-trans retinoic acid
- BBB
Basso-Beattie-Bresnahan
- BSCB
blood-spinal cord barrier
- CA
Carnosic acid
- DDI
Drug-Drug Interaction
- EGb
Ginkgo biloba extract
- EGCG
Epigallocatechin gallate
- FAP-1
Fas-associated phosphatase-1
- GSH-Px
glutathione peroxidase
- IRI
ischemia-reperfusion injury
- iROS
intracellular reactive oxygen species
- JSK
JisuiKang
- LP
lipid peroxidation
- MAPK
mitogen-activated protein kinase
- MDA
malondialdehyde
- NO
nitric oxide
- OS
oxidative stress
- PON
peroxynitrite
- Q- PCR
Quantitative real-time polymerase chain reaction
- RA
Rosmarinic acid
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- Sch B
Schisandrin B
- SCI
Spinal cord injury
- SeNPs
Selenium nanoparticles
- T- AOC
total antioxidant capacity
- TCM
traditional Chinese medicine
- TMP
Tetramethylpyrazine
- XFZYD
Xuefuzhuyu Decoction
References
- Abdulkareem Aljumaily S. A., Demir M., Elbe H., Yigitturk G., Bicer Y., Altinoz E. (2021). Antioxidant, anti-inflammatory, and anti-apoptotic effects of crocin against doxorubicin-induced myocardial toxicity in rats. Environ. Sci. Pollut. Res. Int. 28, 65802–65813. [DOI] [PubMed] [Google Scholar]
- Alkreathy H. M., Alghamdi M. K., Esmat A. (2020). Tetramethylpyrazine ameliorates indomethacin-induced gastric ulcer in rats: Impact on oxidative, inflammatory, and angiogenic machineries. Saudi Pharm. J. 28, 916–926. 10.1016/j.jsps.2020.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjum A., Yazid M. D., Fauzi Daud M., Idris J., Ng A. M. H., Selvi Naicker A., et al. (2020). Spinal cord injury: Pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int. J. Mol. Sci. 21, E7533. 10.3390/ijms21207533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar M. A., Al Shehabi T. S., Eid A. H. (2016). Inflammogenesis of secondary spinal cord injury. Front. Cell. Neurosci. 10, 98. 10.3389/fncel.2016.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baharvand Z., Nabiuni M., Tahmaseb M., Amini E., Pandamooz S. (2020). Investigating the synergic effects of valproic acid and crocin on bdnf and gdnf expression in epidermal neural crest stem cells. Acta Neurobiol. Exp. (Wars). 80, 38–46. 10.21307/ane-2020-004 [DOI] [PubMed] [Google Scholar]
- Bao G., Li C., Qi L., Wang N., He B. (2016). Tetrandrine protects against oxygen-glucose-serum deprivation/reoxygenation-induced injury via pi3k/akt/nf-?b signaling pathway in rat spinal cord astrocytes. Biomed. Pharmacother. 84, 925–930. 10.1016/j.biopha.2016.10.007 [DOI] [PubMed] [Google Scholar]
- Barua N., Buragohain A. K. (2021). Therapeutic potential of curcumin as an antimycobacterial agent. Biomolecules 11, 1278. 10.3390/biom11091278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenas S. (2018). Mitochondrial uncoupling, ros generation and cardioprotection. Biochim. Biophys. Acta. Bioenerg. 1859, 940–950. 10.1016/j.bbabio.2018.05.019 [DOI] [PubMed] [Google Scholar]
- Cao F. L., Xu M., Wang Y., Gong K. R., Zhang J. T. (2015). Tanshinone iia attenuates neuropathic pain via inhibiting glial activation and immune response. Pharmacol. Biochem. Behav. 128, 1–7. 10.1016/j.pbb.2014.11.004 [DOI] [PubMed] [Google Scholar]
- Cao Y., Lv G., Wang Y. S., Fan Z. K., Bi Y. L., Zhao L., et al. (2013). Mitochondrial fusion and fission after spinal sacord injury in rats. Brain Res. 1522, 59–66. 10.1016/j.brainres.2013.05.033 [DOI] [PubMed] [Google Scholar]
- Cemil B., Topuz K., Demircan M. N., Kurt G., Tun K., Kutlay M., et al. (2010). Curcumin improves early functional results after experimental spinal cord injury. Acta Neurochir. 152, 1583–1590. 10.1007/s00701-010-0702-x [DOI] [PubMed] [Google Scholar]
- Chen W., Zhao Z., Zhao S., Zhang L., Song Q. (2020). Resveratrol and puerarin loaded polymeric nanoparticles to enhance the chemotherapeutic efficacy in spinal cord injury. Biomed. Microdevices 22, 69. 10.1007/s10544-020-00521-4 [DOI] [PubMed] [Google Scholar]
- Çiftçi U., Delen E., Vural M., Uysal O., Turgut Coşan D., Baydemir C., et al. (2016). Efficiacy of resveratrol and quercetin after experimental spinal cord injury. Ulus. Travma Acil Cerrahi Derg. 22, 423–431. 10.5505/tjtes.2016.44575 [DOI] [PubMed] [Google Scholar]
- Colica C., Di Renzo L., Aiello V., De Lorenzo A., Abenavoli L. (2018). Rosmarinic acid as potential anti-inflammatory agent. Rev. Recent Clin. Trials 13, 240–242. 10.2174/157488711304180911095818 [DOI] [PubMed] [Google Scholar]
- Cuiqiong W., Chao X., Xinling F., Yinyan J. (2020). Schisandrin B suppresses liver fibrosis in rats by targeting mir-101-5p through the tgf-Β signaling pathway. Artif. Cells Nanomed. Biotechnol. 48, 473–478. 10.1080/21691401.2020.1717507 [DOI] [PubMed] [Google Scholar]
- Ding G., Zhao J., Jiang D. (2016). Allicin inhibits oxidative stress-induced mitochondrial dysfunction and apoptosis by promoting pi3k/akt and creb/erk signaling in osteoblast cells. Exp. Ther. Med. 11, 2553–2560. 10.3892/etm.2016.3179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F., Wang X., Shang B., Fang J., Xi Y., Li A., et al. (2016). Gastrodin ameliorates spinal cord injury via antioxidant and anti-inflammatory effects. Acta Biochim. Pol. 63, 589–593. 10.18388/abp.2016_1272 [DOI] [PubMed] [Google Scholar]
- Eckert M. J., Martin M. J. (2017). Trauma: Spinal cord injury. Surg. Clin. North Am. 97, 1031–1045. 10.1016/j.suc.2017.06.008 [DOI] [PubMed] [Google Scholar]
- Eli I., Lerner D. P., Ghogawala Z. (2021). Acute traumatic spinal cord injury. Neurol. Clin. 39, 471–488. 10.1016/j.ncl.2021.02.004 [DOI] [PubMed] [Google Scholar]
- Every A. E., Russu I. M. (2013). Opening dynamics of 8-oxoguanine in dna. J. Mol. Recognit. 26, 175–180. 10.1002/jmr.2262 [DOI] [PubMed] [Google Scholar]
- Fagot D., Pham D. M., Laboureau J., Planel E., Guerin L., Nègre C., et al. (2018). Crocin, A natural molecule with potentially beneficial effects against skin ageing. Int. J. Cosmet. Sci. 40, 388–400. 10.1111/ics.12472 [DOI] [PubMed] [Google Scholar]
- Fan B., Wei Z., Yao X., Shi G., Cheng X., Zhou X., et al. (2018). Microenvironment imbalance of spinal cord injury. Cell Transpl. 27, 853–866. 10.1177/0963689718755778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L., Wang K., Shi Z., Die J., Wang C., Dang X. (2011). Tetramethylpyrazine protects spinal cord and reduces inflammation in A rat model of spinal cord ischemia-reperfusion injury. J. Vasc. Surg. 54, 192–200. 10.1016/j.jvs.2010.12.030 [DOI] [PubMed] [Google Scholar]
- Fang H., Zhang J. C., Yang M., Li H. F., Zhang J. P., Zhang F. X., et al. (2016). Perfusion of gastrodin in abdominal aorta for alleviating spinal cord ischemia reperfusion injury. Asian pac. J. Trop. Med. 9, 688–693. 10.1016/j.apjtm.2016.05.007 [DOI] [PubMed] [Google Scholar]
- Fatima G., Sharma V. P., Das S. K., Mahdi A. A. (2015). Oxidative stress and antioxidative parameters in patients with spinal cord injury: Implications in the pathogenesis of disease. Spinal Cord. 53, 3–6. 10.1038/sc.2014.178 [DOI] [PubMed] [Google Scholar]
- Firgany A. E. L., Sarhan N. R. (2020). Quercetin mitigates monosodium glutamate-induced excitotoxicity of the spinal cord motoneurons in aged rats via P38 mapk inhibition. Acta Histochem. 122, 151554. 10.1016/j.acthis.2020.151554 [DOI] [PubMed] [Google Scholar]
- Fu J., Fan H. B., Guo Z., Wang Z., Li X. D., Li J., et al. (2014). Salvianolic acid B attenuates spinal cord ischemia-reperfusion-induced neuronal injury and oxidative stress by activating the extracellular signal-regulated kinase pathway in rats. J. Surg. Res. 188, 222–230. 10.1016/j.jss.2013.11.1118 [DOI] [PubMed] [Google Scholar]
- Fu S., Lv R., Wang L., Hou H., Liu H., Shao S. (2018). Resveratrol, an antioxidant, protects spinal cord injury in rats by suppressing mapk pathway. Saudi J. Biol. Sci. 25, 259–266. 10.1016/j.sjbs.2016.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia V. B., Abbinanti M. D., Harris-Warrick R. M., Schulz D. J. (2018). Effects of chronic spinal cord injury on relationships among ion channel and receptor mrnas in mouse lumbar spinal cord. Neuroscience 393, 42–60. 10.1016/j.neuroscience.2018.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gedde M. H., Lilleberg H. S., Assmus J., Gilhus N. E., Rekand T. (2019). Traumatic vs non-traumatic spinal cord injury: A comparison of primary rehabilitation outcomes and complications during hospitalization. J. Spinal Cord. Med. 42, 695–701. 10.1080/10790268.2019.1598698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golpich M., Amini E., Mohamed Z., Azman Ali R., Mohamed Ibrahim N., Ahmadiani A. (2017). Mitochondrial dysfunction and biogenesis in neurodegenerative diseases: Pathogenesis and treatment. CNS Neurosci. Ther. 23, 5–22. 10.1111/cns.12655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowska M., Wawrzyniak D., Rolle K., Chomczynski P., Oziewicz S., Jurga S., et al. (2019). Let food Be your medicine: Nutraceutical properties of lycopene. Food Funct. 10, 3090–3102. 10.1039/c9fo00580c [DOI] [PubMed] [Google Scholar]
- Guo Y., Ma Y., Pan Y. L., Zheng S. Y., Wang J. W., Huang G. C. (2017). Jisuikang, A Chinese herbal formula, increases neurotrophic factor expression and promotes the recovery of neurological function after spinal cord injury. Neural Regen. Res. 12, 1519–1528. 10.4103/1673-5374.215264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurcan O., Gurcat A. G., Kazanci A., Senturk S., Bodur E., Karaca E. U., et al. (2017). Effect of asiatic acid on the treatment of spinal cord injury: An experimental study in rats. Turk. Neurosurg. 27, 259–264. 10.5137/1019-5149.JTN.15747-15.2 [DOI] [PubMed] [Google Scholar]
- Hall E. D., Vaishnav R. A., Mustafa A. G. (2010). Antioxidant therapies for traumatic brain injury. Neurotherapeutics 7, 51–61. 10.1016/j.nurt.2009.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall E. D., Wang J. A., Bosken J. M., Singh I. N. (2016). Lipid peroxidation in brain or spinal cord mitochondria after injury. J. Bioenerg. Biomembr. 48, 169–174. 10.1007/s10863-015-9600-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann K., Shi R. (2009). Acrolein scavenging: A potential novel mechanism of attenuating oxidative stress following spinal cord injury. J. Neurochem. 111, 1348–1356. 10.1111/j.1471-4159.2009.06395.x [DOI] [PubMed] [Google Scholar]
- Hawkins C. L., Davies M. J. (2019). Detection, identification, and quantification of oxidative protein modifications. J. Biol. Chem. 294, 19683–19708. 10.1074/jbc.REV119.006217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W., Wang H. B., Liu Z. F., Liu Y. L., Wang R., Luo X., et al. (2017). Neuroprotective effects of lycopene in spinal cord injury in rats via antioxidative and anti-apoptotic pathway. Neurosci. Lett. 642, 107–112. 10.1016/j.neulet.2017.02.004 [DOI] [PubMed] [Google Scholar]
- Jiang W., Li M., He F., Bian Z., He Q., Wang X., et al. (2016). Neuroprotective effect of asiatic acid against spinal cord injury in rats. Life Sci. 157, 45–51. 10.1016/j.lfs.2016.05.004 [DOI] [PubMed] [Google Scholar]
- Kang S., Piao Y., Kang Y. C., Lim S., Pak Y. K. (2020). Qi-activating quercetin alleviates mitochondrial dysfunction and neuroinflammation in vivo and in vitro . Arch. Pharm. Res. 43, 553–566. 10.1007/s12272-020-01238-x [DOI] [PubMed] [Google Scholar]
- Karami M., Bathaie S. Z., Tiraihi T., Habibi-Rezaei M., Arabkheradmand J., Faghihzadeh S. (2013). Crocin improved locomotor function and mechanical behavior in the rat model of contused spinal cord injury through decreasing calcitonin gene related peptide (cgrp). Phytomedicine 21, 62–67. 10.1016/j.phymed.2013.07.013 [DOI] [PubMed] [Google Scholar]
- Katoh H., Yokota K., Fehlings M. G. (2019). Regeneration of spinal cord connectivity through stem cell transplantation and biomaterial scaffolds. Front. Cell. Neurosci. 13, 248. 10.3389/fncel.2019.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroner A., Greenhalgh A. D., Zarruk J. G., Passos Dos Santos R., Gaestel M., David S. (2014). Tnf and increased intracellular iron alter macrophage polarization to A detrimental M1 phenotype in the injured spinal cord. Neuron 83, 1098–1116. 10.1016/j.neuron.2014.07.027 [DOI] [PubMed] [Google Scholar]
- Li D., Liang H., Li Y., Zhang J., Qiao L., Luo H. (2021). Allicin alleviates lead-induced bone loss by preventing oxidative stress and osteoclastogenesis via sirt1/foxo1 pathway in mice. Biol. Trace Elem. Res. 199, 237–243. 10.1007/s12011-020-02136-5 [DOI] [PubMed] [Google Scholar]
- Liao J., Tian J., Li T., Song W., Zhao W., Du J. (2014). Xuefuzhuyu decoction for hyperlipidemia: A systematic review and meta-analysis of randomized clinical trails. J. Tradit. Chin. Med. 34, 411–418. 10.1016/s0254-6272(15)30040-6 [DOI] [PubMed] [Google Scholar]
- Lin M. S., Lee Y. H., Chiu W. T., Hung K. S. (2011). Curcumin provides neuroprotection after spinal cord injury. J. Surg. Res. 166, 280–289. 10.1016/j.jss.2009.07.001 [DOI] [PubMed] [Google Scholar]
- Lin Y., Yao J., Wu M., Ying X., Ding M., Wei Y., et al. (2020). Erratum to "tetrandrine ameliorates airway remodeling of chronic asthma by interfering tgf-ß1/nrf-2/Ho-1 signaling pathway-mediated oxidative stress. Can. Respir. J. 2020, 6958283. 10.1155/2020/6958283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Yao J., Wu M., Ying X., Ding M., Wei Y., et al. (2019). Tetrandrine ameliorates airway remodeling of chronic asthma by interfering tgf-ß1/nrf-2/Ho-1 signaling pathway-mediated oxidative stress. Can. Respir. J. 2019, 7930396. 10.1155/2019/7930396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D. K., Wang G., Zhao G., Chi X. F. (2017). Protective effect of xuefu zhuyu serum on anoxic injury of dorsal root neurons in rats, 189–191. [Google Scholar]
- Liu R. Z., Pan H. S., Tang X. J., Peng J., Bao J. (2006). Effect of xuefu zhuyu decoction on neurons and vascular perfusion in rabbits after decompression of acute cervical spine cord damage in an early time. Chin. J. Of Clin. Rehabilitation 10. [Google Scholar]
- Liu S. G., Ren P. Y., Wang G. Y., Yao S. X., He X. J. (2015). Allicin protects spinal cord neurons from glutamate-induced oxidative stress through regulating the heat shock protein 70/inducible nitric oxide synthase pathway. Food Funct. 6, 321–330. 10.1039/c4fo00761a [DOI] [PubMed] [Google Scholar]
- Liu Y., Gao J. L., Peng M., Meng H. Y., Ma H. B., Cai P. P., et al. (2018). A review on central nervous system effects of gastrodin. Front. Pharmacol. 9, 24. 10.3389/fphar.2018.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry C. A., Golod M. E., Andrew R. D., Bennett B. M. (2020). Expression of neuronal Na(+)/K(+)-Atpase alpha subunit isoforms in the mouse brain following genetically programmed or behaviourally-induced oxidative stress. Neuroscience 442, 202–215. 10.1016/j.neuroscience.2020.07.009 [DOI] [PubMed] [Google Scholar]
- Lv R., Mao N., Wu J., Lu C., Ding M., Gu X., et al. (2015). Neuroprotective effect of allicin in A rat model of acute spinal cord injury. Life Sci. 143, 114–123. 10.1016/j.lfs.2015.11.001 [DOI] [PubMed] [Google Scholar]
- Ma Z., Lu Y., Yang F., Li S., He X., Gao Y., et al. (2020). Rosmarinic acid exerts a neuroprotective effect on spinal cord injury by suppressing oxidative stress and inflammation via modulating the Nrf2/HO-1 and TLR4/NF-κB pathways. Toxicol. Appl. Pharmacol. 397, 115014. 10.1016/j.taap.2020.115014 [DOI] [PubMed] [Google Scholar]
- Piao F., Li S., Li Q., Ye J., Liu S. (2011). Abnormal expression of 8-nitroguanine in the brain of mice exposed to arsenic subchronically. Ind. Health 49, 151–157. 10.2486/indhealth.ms1058 [DOI] [PubMed] [Google Scholar]
- Pu X. W., Wang L. H., Lu T. S., Yao S. D., Yang J. W., Luo C. S. (2020). Effects of tetrandrine pretreatment on spinal cord ischemia-reperfusion injury in rabbits. Int. J. Of Clin. Exp. Med. 13, 7499–7508. [Google Scholar]
- Rao S., Lin Y., Du Y., He L., Huang G., Chen B., et al. (2019). Designing multifunctionalized Selenium nanoparticles to reverse oxidative stress-induced spinal cord injury by attenuating ros overproduction and mitochondria dysfunction. J. Mat. Chem. B 7, 2648–2656. 10.1039/c8tb02520g [DOI] [PubMed] [Google Scholar]
- Recalde M. D., Miguel C. A., Noya-Riobó M. V., González S. L., Villar M. J., Coronel M. F. (2020). Resveratrol exerts anti-oxidant and anti-inflammatory actions and prevents oxaliplatin-induced mechanical and thermal allodynia. Brain Res. 1748, 147079. 10.1016/j.brainres.2020.147079 [DOI] [PubMed] [Google Scholar]
- Reyes-Farias M., Carrasco-Pozo C. (2019). The anti-cancer effect of quercetin: Molecular implications in cancer metabolism. Int. J. Mol. Sci. 20, E3177. 10.3390/ijms20133177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savikj M., Kostovski E., Lundell L. S., Iversen P. O., Massart J., Widegren U. (2019). Altered oxidative stress and antioxidant defence in skeletal muscle during the first year following spinal cord injury. Physiol. Rep. 7, E14218. 10.14814/phy2.14218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y. (2020). “Clinical observation of "jisuikang,” in Treating spinal cord injury and experimental study on promoting nerve regeneration after spinal cord injury (Doctor: Nanjing University Of Traditional Chinese Medicine; ). [Google Scholar]
- Singh A., Kukreti R., Saso L., Kukreti S. (2019). Oxidative stress: Role and response of short guanine tracts at genomic locations. Int. J. Mol. Sci. 20, E4258. 10.3390/ijms20174258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Liu J., Zhang F., Zhang J., Shi T., Zeng Z. (2013). Antioxidant effect of quercetin against acute spinal cord injury in rats and its correlation with the P38mapk/inos signaling pathway. Life Sci. 92, 1215–1221. 10.1016/j.lfs.2013.05.007 [DOI] [PubMed] [Google Scholar]
- Sullivan P. G., Krishnamurthy S., Patel S. P., Pandya J. D., Rabchevsky A. G. (2007). Temporal characterization of mitochondrial bioenergetics after spinal cord injury. J. Neurotrauma 24, 991–999. 10.1089/neu.2006.0242 [DOI] [PubMed] [Google Scholar]
- Tang Y., Le W. (2016). Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol. Neurobiol. 53, 1181–1194. 10.1007/s12035-014-9070-5 [DOI] [PubMed] [Google Scholar]
- Terraf P., Kouhsari S. M., Ai J., Babaloo H. (2017). Tissue-engineered regeneration of hemisected spinal cord using human endometrial stem cells, poly Ε-caprolactone scaffolds, and crocin as A neuroprotective agent. Mol. Neurobiol. 54, 5657–5667. 10.1007/s12035-016-0089-7 [DOI] [PubMed] [Google Scholar]
- Tian J., Huang Y., Wu T., Huang H. D., Ko K. M., Zhu B. T., et al. (2021). The use of Chinese yang/qi-invigorating tonic botanical drugs/herbal formulations in ameliorating chronic kidney disease by enhancing mitochondrial function. Front. Pharmacol. 12, 622498. 10.3389/fphar.2021.622498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turtle J. D., Henwood M. K., Strain M. M., Huang Y. J., Miranda R. C., Grau J. W. (2019). Engaging pain fibers after A spinal cord injury fosters hemorrhage and expands the area of secondary injury. Exp. Neurol. 311, 115–124. 10.1016/j.expneurol.2018.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez L. B., Alvarez S., Arnaiz S. L., Schopfer F., Carreras M. C., Poderoso J. J., et al. (2000). Reactions of peroxynitrite in the mitochondrial matrix. Free Radic. Biol. Med. 29, 349–356. 10.1016/s0891-5849(00)00301-4 [DOI] [PubMed] [Google Scholar]
- Vanzulli I., Butt A. M. (2015). Mglur5 protect astrocytes from ischemic damage in postnatal cns white matter. Cell Calcium 58, 423–430. 10.1016/j.ceca.2015.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollono L., Falconi M., Gaziano R., Iacovelli F., Dika E., Terracciano C., et al. (2019). Potential of curcumin in skin disorders. Nutrients 11, E2169. 10.3390/nu11092169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Leden R. E., Yauger Y. J., Khayrullina G., Byrnes K. R. (2017). Central nervous system injury and nicotinamide adenine dinucleotide phosphate oxidase: Oxidative stress and therapeutic targets. J. Neurotrauma 34, 755–764. 10.1089/neu.2016.4486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Zhang L., Ndong J. C., Hettinghouse A., Sun G., Chen C., et al. (2019). Progranulin deficiency exacerbates spinal cord injury by promoting neuroinflammation and cell apoptosis in mice. J. Neuroinflammation 16, 238. 10.1186/s12974-019-1630-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Li R., Tu P., Chen J., Zeng K., Jiang Y. (2020). Total glycosides of Cistanche deserticola promote neurological function recovery by inducing neurovascular regeneration via nrf-2/keap-1 pathway in mcao/R rats. Front. Pharmacol. 11, 236. 10.3389/fphar.2020.00236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. D., Shi Y. M., Li L., Guo J. D., Zhang Y. P., Hou S. X. (2013). Treatment with resveratrol attenuates sublesional bone loss in spinal cord-injured rats. Br. J. Pharmacol. 170, 796–806. 10.1111/bph.12301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. W., Wu M., Huang G. C. (2008). Effect of jisuikang on kinetic dysfunction in patients after spinal injury. Chin. J. Integr. Med. 14, 190–193. 10.1007/s11655-008-9006-x [DOI] [PubMed] [Google Scholar]
- Wang S., Ren D. (2016). Allicin protects traumatic spinal cord injury through regulating the hsp70/akt/inos pathway in mice. Mol. Med. Rep. 14, 3086–3092. 10.3892/mmr.2016.5651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H. S., Ko K. M. (2013). Herba Cistanches stimulates cellular glutathione redox cycling by reactive oxygen species generated from mitochondrial respiration in H9c2 cardiomyocytes. Pharm. Biol. 51, 64–73. 10.3109/13880209.2012.710242 [DOI] [PubMed] [Google Scholar]
- Wu C., Zhou Y., Tu P., Yang G., Zheng S., Pan Y., et al. (2020). Jisuikang promotes the repair of spinal cord injury in rats by regulating ngr/rhoa/rock signal pathway. Evid. Based. Complement. Altern. Med. 2020, 9542359. 10.1155/2020/9542359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Zheng C., Wu J., Xue J., Huang R., Wu D., et al. (2017). The pathologic mechanisms underlying lumbar distraction spinal cord injury in rabbits. Spine J. 17, 1665–1673. 10.1016/j.spinee.2017.05.024 [DOI] [PubMed] [Google Scholar]
- Xiao M., Zhong H., Xia L., Tao Y., Yin H. (2017). Pathophysiology of mitochondrial lipid oxidation: Role of 4-hydroxynonenal (4-hne) and other bioactive lipids in mitochondria. Free Radic. Biol. Med. 111, 316–327. 10.1016/j.freeradbiomed.2017.04.363 [DOI] [PubMed] [Google Scholar]
- Xin D. Q., Hu Z. M., Huo H. J., Yang X. J., Han D., Xing W. H., et al. (2017). Schisandrin B attenuates the inflammatory response, oxidative stress and apoptosis induced by traumatic spinal cord injury via inhibition of P53 signaling in adult rats. Mol. Med. Rep. 16, 533–538. 10.3892/mmr.2017.6622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Rabchevsky A. G., Hall E. D. (2007). Role of peroxynitrite in secondary oxidative damage after spinal cord injury. J. Neurochem. 100, 639–649. 10.1111/j.1471-4159.2006.04312.x [DOI] [PubMed] [Google Scholar]
- Xuan G. D., Liu C. Q. (2008). Research on the effect of phenylethanoid glycosides (peg) of the Cistanche deserticola on anti-aging in aged mice induced by D-galactose. Zhong Yao Cai 31, 1385–1388. [PubMed] [Google Scholar]
- Yang J., Song X., Feng Y., Liu N., Fu Z., Wu J., et al. (2020). Natural ingredients-derived antioxidants attenuate H(2)O(2)-induced oxidative stress and have chondroprotective effects on human osteoarthritic chondrocytes via keap1/nrf2 pathway. Free Radic. Biol. Med. 152, 854–864. 10.1016/j.freeradbiomed.2020.01.185 [DOI] [PubMed] [Google Scholar]
- Yang Y., Liu F., Tang M., Yuan M., Hu A., Zhan Z., et al. (2016). Macrophage polarization in experimental and clinical choroidal neovascularization. Sci. Rep. 6, 30933. 10.1038/srep30933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong R. L., Dong J. Z., Zhang L., Wu L. (2020). Effects of electroacupuncture at "Zusanli"(St36) on ultrastructure and mitochondrial dynamics of skeletal muscle in rats with spleen qi deficiency syndrome. Zhen Ci Yan Jiu 45, 15–20. 10.13702/j.1000-0607.1903376 [DOI] [PubMed] [Google Scholar]
- Yu D. S., Lv G., Mei X. F., Cao Y., Wang Y. F., Wang Y. S., et al. (2015). Mir-200c regulates ros-induced apoptosis in murine bv-2 cells by targeting fap-1. Spinal Cord. 53, 182–189. 10.1038/sc.2014.185 [DOI] [PubMed] [Google Scholar]
- Yu L., Qian J. (2020). Dihydrotanshinone I alleviates spinal cord injury via suppressing inflammatory response, oxidative stress and apoptosis in rats. Med. Sci. Monit. 26, E920738. 10.12659/MSM.920738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Wang P., Xue Y., Liu L., Li Z., Liu Y. (2018). Allicin ameliorates cognitive impairment in app/ps1 mice via suppressing oxidative stress by blocking jnk signaling pathways. Tissue Cell 50, 89–95. 10.1016/j.tice.2017.11.002 [DOI] [PubMed] [Google Scholar]
- Zhang H., Xiang Z., Duan X., Jiang J. L., Xing Y. M., Zhu C., et al. (2019). Antitumor and anti-inflammatory effects of oligosaccharides from Cistanche deserticola extract on spinal cord injury. Int. J. Biol. Macromol. 124, 360–367. 10.1016/j.ijbiomac.2018.11.132 [DOI] [PubMed] [Google Scholar]
- Zhang W., Sun Z., Meng F. (2017). Schisandrin B ameliorates myocardial ischemia/reperfusion injury through attenuation of endoplasmic reticulum stress-induced apoptosis. Inflammation 40, 1903–1911. 10.1007/s10753-017-0631-4 [DOI] [PubMed] [Google Scholar]
- Zhou J. Z., Ma Y., Yin S. J., Sun S. J. (2009). Experiment study of jisuikang on treatment of rat after spinal cord injury. Nanchang: Journal Of Jiangxi University Of Traditional Chinese Medicine, 21. 10.3969/j.issn.1005-9431.2009.02.030 [DOI] [Google Scholar]
- Zhou Y. N., Sun M. Y., Mu Y. P., Yang T., Ning B. B., Ren S., et al. (2014). Xuefuzhuyu decoction inhibition of angiogenesis attenuates liver fibrosis induced by CCl₄ in mice. J. Ethnopharmacol. 153, 659–666. 10.1016/j.jep.2014.03.019 [DOI] [PubMed] [Google Scholar]
- Zrzavy T., Schwaiger C., Wimmer I., Berger T., Bauer J., Butovsky O., et al. (2021). Acute and non-resolving inflammation associate with oxidative injury after human spinal cord injury. Brain. 144, 144–161. 10.1093/brain/awaa360 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.