Abstract
This study aimed to define the pharmacokinetics (PKs) of oral mannitol used as an osmotic laxative for bowel preparation for colonoscopy. The PKs of oral mannitol was evaluated in a substudy as part of a phase II dose‐finding, international, multicenter, randomized, parallel‐group, endoscopist‐blinded study. Patients were randomly assigned to take 50, 100, or 150 g mannitol. Venous blood samples were drawn at baseline (T 0), 1 h (T 1), 2 h (T 2), 4 h (T 4), and 8 h (T 8) after completion of mannitol self‐administration. The mean mannitol plasma concentrations (mg/ml) were dose‐dependent with a consistent difference among doses. The mean maximum concentration (C max) ± SD was 0.63 ± 0.15, 1.02 ± 0.28, and 1.36 ± 0.39 mg/ml, in the three dosage groups, respectively. The mean area under the curve from zero to infinity (AUC0−∞) was 2.667 ± 0.668, 4.992 ± 1.706, and 7.403 ± 3.472 mg/ml*h in the 50, 100, and 150 g mannitol dose groups, respectively. Bioavailability was similar in the three dose groups and was just over 20% (0.243 ± 0.073, 0.209 ± 0.081, and 0.228 ± 0.093 in the 50, 100, and 150 g mannitol dose groups, respectively). The present study showed that the bioavailability of oral mannitol is just over 20% and is similar for the three tested doses (50, 100, and 150 g). The linear increase in C max, AUC0−t8, and AUC0−∞ must be considered when choosing the oral mannitol dose for bowel preparation to avoid its systemic osmotic effects.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Oral mannitol has been used for bowel preparation for colonoscopy for many years. However, there is no adequate information about the pharmacokinetic (PK) characteristics.
WHAT QUESTION DID THIS STUDY ADDRESS?
The PK profile of oral mannitol is administered at three different doses.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
The mannitol plasma concentrations are consistently dose‐dependent. Bioavailability is similar for the three doses (just over 20%).
HOW MIGHT THIS CHANGE DRUG DISCOVERY, DEVELOPMENT, AND/OR THERAPEUTICS?
Mannitol 100 g is the ideal dose for bowel preparation without systemic osmotic effect.
INTRODUCTION
Colonoscopy is the gold‐standard method for thoroughly examining the colonic mucosa. 1 The main indications for colonoscopy are screening, diagnosis confirmation, and surveillance, 2 with millions of examinations performed annually. 3
Good quality colonoscopy is required to achieve favorable outcomes. 4 Unfortunately, up to one‐third of patients have unsatisfactory bowel cleansing, 5 , 6 with detrimental effects on colonoscopy completion, endoscopic accuracy, patient tolerance, and healthcare costs. 7 , 8 , 9 , 10 , 11
Many products for bowel preparation are available, with polyethylene glycol (PEG)‐based formulations the most commonly used in United States and Europe. However, PEG formulations have some disadvantages, including a large volume of solution, unpleasant taste, complicated timing, nocturnal problems (because of the split‐dose regimen), and high cost. 12 Despite the effectiveness of PEG preparations, these disadvantages significantly reduce patient adherence to colon cancer screening programs and to follow‐up of more serious chronic inflammatory colon diseases.
The availability of an easy‐to‐use bowel preparation requiring the consumption of a smaller volume of solution with a pleasant taste is an important unmet medical need. Consequently, the provision of such a product could favorably influence the diagnosis and follow‐up of colon diseases. 13
Mannitol is a sugar alcohol partly absorbed following oral administration. It acts as an osmotic laxative and was widely used in the late 1970s and early 1980s as a bowel cleansing agent for colonoscopy. Currently, mannitol is commonly used, although off‐label, in some Latin America countries, especially in Brazil where it is still the most popular agent for bowel cleansing thanks to its effectiveness, high patient acceptance, and lack of significant systemic side effects. 14 In Europe and the United States, mannitol is currently not used due to some cases of intestinal explosion that, despite the absence of controlled clinical trials, have been attributed to a mannitol‐induced increase of methane (CH4) and/or hydrogen (H2) intestinal concentration during the execution of diathermy‐electrocautery or argon plasma coagulation. 15 However, other extremely rare cases of intestinal explosion were also reported in patients prepared for colonoscopy using a standard enema, 15 PEG, and sorbitol, 16 suggesting that the causes of the explosion may be other than the drug used for bowel cleansing. Remarkably, these events have no longer been described in recent years, even in those countries where the use of mannitol is widespread, 17 probably because the routinary maneuvers of insufflation and washing to optimize bowel cleansing contribute to an effective gas exchange with the ambient air and equalize the distribution of combustible gases, overcoming the compartmentalization of the colon. 18 , 19 The above considerations lead us to believe that mannitol may represent an optimal choice for bowel cleansing as, with equal efficacy with PEG‐based preparations, it may be better accepted by patients because of its ease of use, small solution volume to be taken, pleasant taste, and rapidity of action, without a real increase of the intestinal explosion risk. These hypotheses were evaluated in the SATISFACTION study, a phase II/III, international, multicenter, randomized, parallel‐group, endoscopist‐blinded, dose‐finding/noninferiority study. In the phase II dose finding part of the study, three single doses of mannitol (50, 100, and 150 g), including the lowest and highest doses reported in the literature, were comparatively evaluated for bowel cleansing. The choice of doses used in the phase II part of the study was discussed and agreed with the European Regulatory Authorities. When used for bowel preparation, mannitol can be considered a topical drug, as its effectiveness is based on an osmotic effect with fluid retention in the intestinal lumen. The systemic osmotic effects associated with the absorption of mannitol are unwelcome in this context as they could cause adverse events. 20 , 21 Therefore, it is important to study the pharmacokinetics (PKs) of mannitol administered orally in order to identify the optimum dose for bowel preparation.
The current PK study was a substudy of the phase II dose‐finding part of the SATISFACTION study. The aim was to define the PK profile of the different doses of oral mannitol used in patients undergoing elective colonoscopy, and to provide data for choosing, also from a safety point of view, the dose to be used in the subsequent phase III comparison study with a PEG‐based preparation.
MATERIALS AND METHODS
Study design
The PKs of mannitol were evaluated in a substudy as part of a phase II dose‐finding, international, multicenter, randomized, parallel‐group, endoscopist‐blinded study. The PK substudy was conducted in accordance with the Declaration of Helsinki in four Italian centers and approved by the Research Ethics Committee of each center. All enrolled patients provided written informed consent. The inclusion criteria were: ability of the patient to consent and provide signed written informed consent, age greater than or equal to 18 years, men and women scheduled for elective (screening, surveillance, or diagnostic) colonoscopy to be prepared and performed according to European guidelines, and patients willing and able to complete the entire study and to comply with instructions. The main exclusion criteria were pregnancy or breast feeding, severe renal failure (estimated glomerular filtration rate [eGFR] < 30 ml/min/1.73 m2), severe heart failure (New York Heart Association [NYHA] Class III–IV), severe anemia (Hb < 8 g/dl), severe acute and chronically active inflammatory bowel disease, chronic liver disease (Child–Pugh class B or C), electrolyte imbalance, recent (<6 months) symptomatic acute ischemic heart disease, a history of significant gastrointestinal surgery, and use of laxatives or colon motility‐altering drugs.
All patients included in the phase II study were consecutively enrolled between June 2020 and November 2020 and randomized in a 1:1:1 ratio to three doses of oral mannitol: 50, 100, and 150 g dissolved, respectively, in 500, 750, and 1000 ml of room temperature water; the dilution volumes were determined according to the mannitol powder solubility, so that it was easy for the patient to obtain the solution to take. The osmolarity of the three doses was 0.605, 0.787, and 0.908 Osmol/Kg, respectively. Patients who agreed to be included in the PK substudy were enrolled sequentially until the required sample size (at least 10 evaluable patients in each dose group) was reached.
Patients had to drink the prepared mannitol solution on the day of the colonoscopy within 30 min for the 50 and 100 g doses and 60 min for the 150 g dose and complete the self‐administration at least 4 h before the endoscopic procedure.
Plasma mannitol assay
Mannitol plasma concentration was evaluated before mannitol self‐administration at baseline (T 0), and at 1 h ± 5 min (T 1), 2 h ± 5 min (T 2), 4 h ± 10 min (T 4), and 8 h ± 10 min (T 8) after completion of mannitol self‐administration.
As some foods contain mannitol, patients were required to restrict their diet (to avoid potential interference) for 24 h before drug self‐administration, and to fast overnight and for up to 8 h after mannitol intake.
After blood was collected, the tubes containing the blood samples (3 ml) were gently tilted 8–10 times for complete mixing with the anticoagulant (K2EDTA). The samples were then processed into plasma within 150–180 min after collection by centrifuging at 3000 revolutions per minute (rpm) for 20 min in a refrigerated centrifuge (Eppendorf 5702R with rotor A‐4‐38) at +4°C to achieve a clear plasma layer over the red cells. Two equal aliquots of plasma of at least 0.5 ml each were immediately transferred into two 1.8 ml NUNC storage tubes, which were frozen and maintained at −20°C or colder until shipment to a central bioanalytical laboratory (Aptuit, Verona, Italy).
Quantitative measurement of free mannitol in human plasma samples was performed using a validated bioanalytical liquid chromatography‐tandem mass spectrometry (LC‐MS/MS) method previously validated at Aptuit.
This study consisted of analytical runs containing a complete set of clinical study samples with an appropriate number of calibration standards (range 0.75–750 μg/ml) in a duplicate set. Quality control (QC) samples prepared from human mannitol‐free plasma, where “mannitol‐free” refers to plasma obtained from donors verbally screened as free of exogenous mannitol (i.e., from their diet) in the previous 36 h before blood collection, using D‐mannitol (also referred to as mannitol, supplied by Sigma‐Aldrich) as the reference standard. Calibration data were deemed acceptable if the back‐calculated concentration did not deviate from the nominal by more than 15% (20% at the lower limit of quantification [LLOQ]) and if no more than 25% of the calibration standards were rejected or lost for any other reason. A calibration standard was omitted from the regression if the back‐calculated concentration deviated from the nominal by more than 15% (20% at LLOQ). Individual QC results were deemed acceptable if the calculated concentration deviated by less than 15% from the nominal concentration. The analytical run was approved if no more than one‐third of the QC results exceeded the acceptable limit and at least 50% of the results at each concentration were within the acceptable limit. A control blank with and without the internal standard (i.e., D‐mannitol‐UL‐13C6 supplied by Santa Cruz Biotechnology, Inc.) was included in each run to evaluate the selectivity and the carryover (by injection of one or more blank samples after a high concentration standard).
After the initial analysis was completed and results were reviewed, additional runs were included to evaluate incurred sample reproducibility (incurred sample reanalysis [ISR] of 24 samples, which provided representation across patients and period, with selected timepoints near the time to maximum observed concentration [T max] and elimination phase). In order to demonstrate acceptable ISR, at least 66.7% of the repeat results had to be within 20% of the mean (of the original and repeat result). Concentrations of mannitol in human plasma samples were determined from the appropriate calibration line using the software application BioLims.
The laboratory technician who performed the analysis was in a blind condition.
Statistical analysis
The phase II dose‐finding study sample size (50 patients in each dose group) was based on the precision of the estimate within each treatment group, that is, the 95% confidence interval (95% CI) half‐width of the proportion of patients in each treatment group with adequate bowel cleansing (Boston Bowel Preparation Scale [BBPS] total score ≥6, with BBPS ≥2 for each segment).
For example, a sample of 50 patients gives a 95% CI width of 12%, 11%, and 10% in the case of adequate bowel cleansing percentages of 75%, 80%, and 85%, respectively.
Based on the obtained concentrations of mannitol in plasma samples, the PK parameters (i.e., the mannitol maximum observed concentration [C max], the T max, the terminal elimination half‐life [t 1/2], the area under the concentration‐time curve from T 0 to the last blood sampling timepoint with a measurable concentration [AUC0−t8] and from T 0 to infinity [AUC0−∞], and the bioavailability of each mannitol dose) were calculated as described below:
C max (ng/ml) was calculated as the maximum concentration at the four timepoints (T 1, T 2, T 4, and T 8).
T max (min) was calculated as minutes (T i) at the earliest occurrence of C max.
T 1/2 (i.e., the time in minutes required for C max to reach half of its original value) was estimated as ln(2)/λ Z, where λ Z is the terminal phase elimination rate constant, estimated by linear regression analysis of logarithmically transformed concentration versus time data.
AUC0−t8, measured in ng/ml*h, was calculated using a linear trapezoidal rule for increasing concentrations and the logarithmic trapezoidal rule for decreasing concentrations (linear‐up/log‐down).
AUC0−∞, measured in ng/ml*h, was calculated according to the trapezoidal rule until T 4; then, the area after the last available concentration (T 8) was calculated under the mono‐exponential model, generally accepted as suitable for the elimination phase of a drug, by taking into account the mannitol t 1/2 (as the mean of the values obtained for each dose group).
Bioavailability was calculated by dividing the AUC0−∞ corrected for the patient’s body weight by the AUC0−∞ after i.v. administration of mannitol 1 g/kg body weight, as reported by Rudehill et al. 22
Descriptive statistics of the above quantitative variables (mean, SD, minimum, first quartile, median, third quartile, and maximum) have been reported for each treatment group; 95% CIs have also been calculated. Statistical analysis was performed using SAS release 9.4 or later (SAS Institute, Inc., Cary, NC).
RESULTS
Patient characteristics
The PK population, defined as all randomized patients who completed the mannitol treatment and had at least one PK assessment, included 42 patients: 15 in the 50 g dose group, 11 in the 100 g dose group, and 16 in the 150 g dose group.
Demographic data for the PK population are summarized in Table 1. Overall, patients who participated in the PK substudy were ~56 years old, all but two (i.e., 40 out of 42) were White, and 19 (45.24%) were women, of whom the majority (68.42%) were menopausal. After mannitol self‐administration, all punctual sampling for the calculation of mannitol t ½ were satisfied in only 31 of the 42 patients in the PK population (i.e., 13, 7, and 11 patients in the 50, 100, and 150 g dose groups, respectively).
TABLE 1.
Demographic characteristics of the study population
| 50 g mannitol (N = 15) | 100 g mannitol (N = 11) | 150 g mannitol (N = 16) | Total (N = 42) | |
|---|---|---|---|---|
| Age at study entry (years) | ||||
| n | 15 | 11 | 16 | 42 |
| Mean (SD) | 59.8 (11.47) | 52.4 (11.17) | 56.1 (11.35) | 56.4 (11.45) |
| Median | 58.0 | 55.0 | 54.5 | 55.0 |
| Q1; Q3 | 51.0; 69.0 | 42.0; 61.0 | 47.5; 65.0 | 49.0; 66.0 |
| Min; max | 40; 81 | 33; 68 | 37; 78 | 33; 81 |
| Sex, n (%) | ||||
| Male | 9 (60.00) | 5 (45.45) | 9 (56.25) | 23 (54.76) |
| Female | 6 (40.00) | 6 (54.55) | 7 (43.75) | 19 (45.24) |
| Ethnicity, n (%) | ||||
| Hispanic or Latino | 0 | 0 | 2 (12.50) | 2 (4.76) |
| Not Hispanic or Latino | 15 (100.00) | 11 (100.00) | 14 (87.50) | 40 (95.24) |
| Female reproductive status, a n (%) | 6 | 6 | 7 | 19 |
| Childbearing potential | 1 (16.67) | 2 (33.33) | 3 (42.86) | 6 (31.58) |
| Menopause | 5 (83.33) | 4 (66.67) | 4 (57.14) | 13 (68.42) |
Abbreviations: Q1, first quartile; Q3, third quartile; SD, standard deviation.
Percentages were computed on female patients belonging to the pharmacokinetics (PKs) population.
Plasma mannitol concentrations
Mannitol concentrations (mg/ml) in plasma samples for each blood collection timepoint are summarized by treatment group in Table 2.
TABLE 2.
Summary of mannitol concentrations by timepoint
| 50 g mannitol (N = 15) | 100 g mannitol (N = 11) | 150 g mannitol (N = 16) | |
|---|---|---|---|
| Mannitol plasma concentrations (mg/ml) | |||
| T 0 | |||
| n | 15 | 11 | 16 |
| Mean (SD) | 0.0006 (0.00193) | 0.0002 (0.00062) | 0.0001 (0.00033) |
| Median | 0.0000 | 0.0000 | 0.0000 |
| Q1; Q3 | 0.0000; 0.0000 | 0.0000; 0.0000 | 0.0000; 0.0000 |
| Min; max | 0.000; 0.007 | 0.000; 0.002 | 0.000; 0.001 |
| T 1 | |||
| n | 15 | 11 | 16 |
| Mean (SD) | 0.6218 (0.14869) | 0.9777 (0.28581) | 1.2996 (0.38100) |
| Median | 0.6387 | 0.9201 | 1.2922 |
| Q1; Q3 | 0.4951; 0.7018 | 0.8375; 1.2190 | 0.9655; 1.6511 |
| Min; max | 0.402; 0.951 | 0.532; 1.617 | 0.715; 1.798 |
| T 2 | |||
| n | 15 | 11 | 16 |
| Mean (SD) | 0.4689 (0.12169) | 0.8647 (0.26881) | 1.1747 (0.40002) |
| Median | 0.4408 | 0.8498 | 1.2887 |
| Q1; Q3 | 0.3881; 0.5886 | 0.6217; 1.0242 | 0.7649; 1.4889 |
| Min; max | 0.274; 0.673 | 0.499; 1.392 | 0.520; 1.700 |
| T 4 | |||
| n | 15 | 11 | 16 |
| Mean (SD) | 0.2734 (0.08228) | 0.5606 (0.22477) | 0.7339 (0.35330) |
| Median | 0.2661 | 0.6241 | 0.7427 |
| Q1; Q3 | 0.2123; 0.3086 | 0.3269; 0.8144 | 0.4220; 0.9672 |
| Min; max | 0.160; 0.472 | 0.238; 0.845 | 0.241; 1.281 |
| T 8 | |||
| n | 14 a | 11 | 16 |
| Mean (SD) | 0.0931 (0.05362) | 0.1889 (0.09435) | 0.2754 (0.16519) |
| Median | 0.0761 | 0.2374 | 0.2605 |
| Q1; Q3 | 0.0640; 0.0818 | 0.0987; 0.2619 | 0.1266; 0.4063 |
| Min; max | 0.032; 0.215 | 0.050; 0.316 | 0.070; 0.629 |
Note: T 0 = baseline before mannitol self‐administration; T 1 = 1 h, T 2 = 2 h, T 4 = 4 h, T 8 = 8 h after completion of mannitol self‐administration.
Abbreviations: Q1, first quartile; Q3, third quartile; SD, standard deviation.
The plasma sample for one patient was collected outside of the protocol‐defined time window, and therefore the sample result was excluded from the calculation of summary statistics.
At T 0, before mannitol self‐administration, mannitol was basically absent in plasma samples in all treatment groups. However, the average ± SD mannitol concentration at T 0 was slightly above zero in all groups, possibly due to the dietary intake of a small amount of mannitol the day before by a few patients, despite the required dietary restrictions.
Of note, at T 8, the plasma sample for one patient in the 50 g mannitol dose group was collected outside the protocol‐defined time window, and therefore the sample result was excluded from the calculation of summary statistics for the respective nominal timepoint.
The time/concentration curves for each treatment group are shown in Figure 1. Mannitol reached its C max in plasma at the first sampling point after drug self‐administration, that is, at T 1 (1 h ± 5 min after completion of mannitol self‐administration), followed by a substantially linear decrease at the following timepoints in all treatment groups.
FIGURE 1.
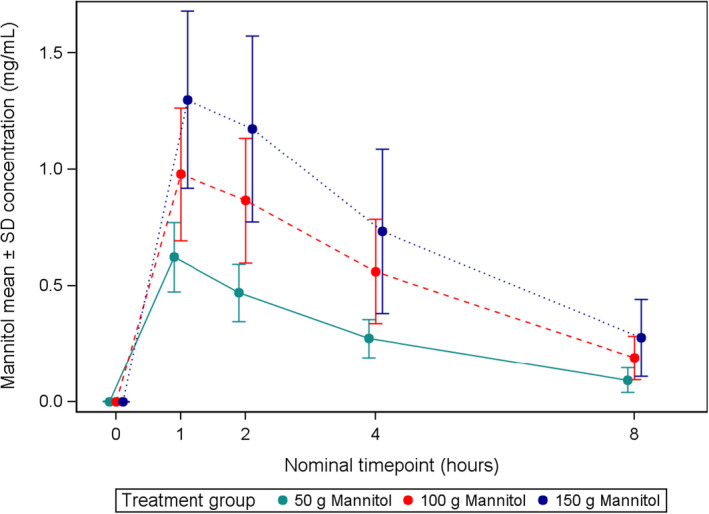
Mean mannitol concentrations ± SD over time by dose level.
PK parameters
A summary description of mannitol PK parameters is provided in Table 3 by treatment group.
TABLE 3.
Summary of PK parameters
| 50 g mannitol (N = 15) | 100 g mannitol (N = 11) | 150 g mannitol (N = 16) | |
|---|---|---|---|
| C max (mg/ml) | |||
| n | 15 | 11 | 16 |
| Mean (SD) | 0.630 (0.145) | 1.024 (0.279) | 1.359 (0.391) |
| 95% CI | 0.550; 0.711 | 0.836; 1.211 | 1.150; 1.567 |
| Median | 0.644 | 0.944 | 1.510 |
| Q1; Q3 | 0.505; 0.702 | 0.850; 1.219 | 1.031; 1.695 |
| Min; max | 0.402; 0.951 | 0.532; 1.617 | 0.716; 1.798 |
| T max (h) | |||
| n | 15 | 11 | 16 |
| Median | 1.000 | 1.000 | 1.000 |
| Q1; Q3 | 0.920; 1.000 | 1.000; 2.000 | 1.000; 1.920 |
| Min; max | 0.920; 2.000 | 0.920; 2.080 | 0.920; 2.030 |
| AUC0−t8 (mg/ml*h) | |||
| n | 15 | 11 | 16 |
| Mean (SD) | 2.231 (0.521) | 4.159 (1.348) | 5.590 (2.181) |
| 95% CI | 1.943; 2.520 | 3.253; 5.065 | 4.428; 6.752 |
| Median | 2.130 | 4.390 | 6.065 |
| Q1; Q3 | 1.830; 2.690 | 2.890; 5.300 | 3.515; 7.420 |
| Min; max | 1.300; 3.160 | 2.010; 6.360 | 2.270; 8.570 |
| AUC 0−∞ (mg/ml*h) | |||
| n | 15 | 11 | 16 |
| Mean (SD) | 2.667 (0.668) | 4.992 (1.706) | 7.403 (3.472) |
| 95% CI | 2.297; 3.037 | 3.846; 6.139 | 5.618; 9.189 |
| Median | 2.515 | 5.421 | 7.928 |
| Q1; Q3 | 2.161; 3.241 | 3.384; 6.560 | 4.434; 9.715 |
| Min; max | 1.523; 4.020 | 2.270; 7.560 | 2.650; 15.751 |
| t 1/2 (h) | |||
| n a | 13 | 7 | 11 |
| Mean (SD) | 2.584 (0.840) | 2.419 (0.579) | 2.678 (0.489) |
| 95% CI | 2.076; 3.091 | 1.883; 2.954 | 2.350; 3.007 |
| Median | 2.470 | 2.320 | 2.600 |
| Q1; Q3 | 2.070; 2.530 | 1.990; 2.850 | 2.260; 3.240 |
| t 1/2 (h) | |||
| Min; max | 1.840; 4.690 | 1.800; 3.480 | 2.010; 3.370 |
| Bioavailability b | |||
| n | 15 | 11 | 16 |
| Mean (SD) | 0.243 (0.073) | 0.209 (0.081) | 0.228 (0.093) |
| 95% CI | 0.202; 0.283 | 0.154; 0.264 | 0.180; 0.276 |
| Median | 0.230 | 0.197 | 0.229 |
| Q1; Q3 | 0.209; 0.254 | 0.129; 0.286 | 0.169; 0.294 |
| Min; max | 0.144; 0.435 | 0.103; 0.326 | 0.095; 0.391 |
Abbreviations: AUC 0−∞ , area under concentration–time curve T 0−∞ ; AUC0−t , area under concentration–time curve T 0–T 8; CI, confidence interval; C max, maximum concentration; PK, pharmacokinetic; Q1, first quartile; Q3, third quartile; SD, standard deviation; t 1/2, terminal elimination half‐life; T max, time to reach maximum concentration.
Only 31 patients of the 42 patients in the PK population (13, 7 and 11 patients in the 50, 100, and 150 g dose groups, respectively) satisfied all punctual sampling for the calculation of mannitol t ½.
Bioavailability has been calculated by dividing the AUC0−∞ corrected for the patients’ average body weight by the AUC0−∞ after i.v. administration of mannitol 1 g/kg body weight, as reported by Rudehill et al. 18
C max after mannitol self‐administration increases linearly with dose (0.630 ± 0.145, 1.024 ± 0.278, and 1.359 ± 0.391 mg/ml in the 50, 100, and 150 g dose groups, respectively).
Like C max, mean AUC0−t8 also increases linearly with dose (2.231 ± 0.521, 4.159 ± 1.348, and 5.590 ± 2.181 mg/ml*h in the 50, 100, and 150 g dose groups, respectively). A similar increase was also observed for AUC0−∞ (2.667 ± 0.668, 4.992 ± 1.706, and 7.403 ± 3.472 mg/ml*h in the 50, 100, and 150 g dose groups, respectively).
To test the linearity, we carried out three regression analyses with dosage as the independent variable and AUC0−∞, AUC0−t8, and C max as the respective dependent variables. The statistical significance is p < 0.0001 for all the analyses with a slope of 2.106 for AUC0−∞, 1.741 for AUC0−t8, and 0.0364 for C max.
Conversely, mean T max is similar in the three dose groups and is just over an hour with median value (range) of 1.000 h (0.920–2.000 h), 1.000 (0.920–2.080 h), and 1.000 h (0.920–2.030 h) in the 50, 100, and 150 g dose groups, respectively.
Mean t 1/2 is similar in the three dose groups and is ~2.5 h (2.584 ± 0.840 h, 2.419 ± 0.579 h, and 2.678 ± 0.489 h in the 50, 100, and 150 g groups, respectively).
Bioavailability is similar in the three dose groups and is just over 20% (0.243 ± 0.073, 0.209 ± 0.081, and 0.228 ± 0.093 in the 50 g, 100 g, and 150 g dose groups, respectively).
Tolerability
At least one treatment‐emergent adverse event (TEAE) related to the study drug was reported for two out of 65 patients (3.1%) in the 50 g dose group, six out of 57 (10.5%) in the 100 g group, and 12 out of 57 (21.1%) in the 150 g group. The most frequent TEAE related to the study drug were vomiting (one patient in the 50 g dose group, 2 in the 100 g group, and 8 in the 150 g group) and nausea (4 patients in the 100 g group and 3 in the 150 g group).
No deaths were reported, and only one patient in the highest dose group experienced a treatment‐emergent serious adverse event (TESAE; i.e., a syncope not related to the study drug).
DISCUSSION
Mannitol is a sugar alcohol with an osmotic effect due to fluid retention in the intestinal lumen and is commonly used in Latin America (mainly in Brazil) as an off‐label formulation for bowel cleansing before colonoscopy. 17 , 23 , 24 Oral mannitol for bowel preparation is cheap and has a pleasant taste, low volume (<1 L), and very early onset of action, which allows same‐day bowel preparation even for a morning colonoscopy. 12 As a result, mannitol is an easy‐to‐use bowel preparation with high patient acceptability.
Mannitol used for bowel preparation can be considered a topical drug, as its effectiveness is based on an osmotic effect with fluid retention in the intestinal lumen. The systemic osmotic effects resulting from mannitol absorption, potentially useful for other indications, are undesirable in this context as they could lead to adverse effects. Furthermore, the absorbed mannitol fraction is obviously not useful for bowel preparation. Consequently, it is important to determine the PKs of mannitol administered orally at doses that significantly modify intestinal transit times so that the optimal dose for bowel preparation can be chosen.
Therefore, we conducted a phase II dose‐finding, international, multicenter, randomized, parallel‐group, endoscopist‐blinded study with a substudy to define the PK profile of different doses of oral mannitol in patients undergoing colonoscopy in a real‐life clinical setting.
The results of the present study showed that the bioavailability of mannitol is just over 20% and is similar for the three doses used (50, 100, and 150 g). We know that over 50 g of oral mannitol induces osmotic diarrhea and reduces intestinal transit time. In contrast, lower doses of oral mannitol (0.5–2 g) do not have any significant osmotic effects and have a higher absorption rate (up to 60%). 25
The similar bioavailability for the three doses tested explains the linear increase in C max, AUC0−t8, and AUC0−∞ observed as the administered dose increased. This linear increase should be considered when choosing the dose to be used for bowel preparation. The effects of the 100 g dose (equal to 1.5 g/kg of body weight for a weight of 65 kg) are the closest to those produced by the venous route (i.v.) to obtain systemic osmotic effects (1 g/kg of body weight in a 20% solution). The difference between the mean AUC0−∞ obtained (4.992 mg/ml*h) with the 100 g dose and that reported by Rudehill et al. 22 after i.v. administration of 1 g/kg of body weight (16.076 mg/ml*h) suggests that clinically relevant systemic osmotic effects are unlikely. With the highest dose of 150 g, even though bioavailability is substantially the same, the absolute amount of mannitol reaching the systemic circulation is greater, with a mean AUC0−∞ (7.403 mg/ml*h) that is closest to that reported after i.v. administration of the therapeutic dose of 1 g/kg body weight, as reported by Rudehill et al. In this case, the quantitative relationship between AUC0−∞ after oral and i.v. administration does not allow osmotic systemic effects, even if of reduced intensity, to be ruled out. Therefore, the 50 and 100 g doses of oral mannitol have the optimal relationship between the absolute quantity of drug that remains in the intestinal lumen, useful for bowel preparation, and the absolute quantity of drug that reaches the systemic circulation and is potentially capable of causing unwanted systemic osmotic effects.
Interestingly, the three doses of oral mannitol tested in the present study were also found to have a comparable mean plasma t ½ of about 2.5 h. As expected, this result is in line with the conclusions reported by Rudehill et al. after administration of i.v. mannitol (2.44 ± 0.85 h), as this parameter is independent of the route of administration. This finding related to the elimination of mannitol has two clinically relevant consequences. First, any systemic osmotic side effects from 50, 100, or 150 g oral mannitol are unlikely, and, if they do occur, are of limited extent and of short duration. Second, oral mannitol is completely eliminated in less than 24 h. Therefore, patient surveillance for any adverse events related to systemic osmotic effects can be limited to the day of the colonoscopy.
Study limitations
PK sampling was limited as the study was pragmatically conducted in patients who were undergoing bowel preparation for a colonoscopy; under these conditions, and particularly in the first 2 h after taking mannitol, the scheduling of repeated sampling at fixed times while the patient has repeated bowel discharges is not possible; a sampling schedule compatible with the patient’s clinical situation was therefore adopted.
The study was carried out in patients with an indication to colonoscopy, for whom there was no ethical justification for the i.v. administration of mannitol in relation to the intense pharmacological effects and the not infrequent adverse events associated with this treatment, which is only justified in patients with a specific critical indication (endocranial hypertension, renal insufficiency in the anuric phase, and acute glaucoma). For this reason, the bioavailability assessment was not performed in the same patients with a cross‐over study, but with reference to PK data reported in the literature for i.v. administration of mannitol in patients who were candidates for neurosurgery.
In conclusion, the present PK study showed that 50, 100, and 150 g doses of oral mannitol have 20% bioavailability and a mean plasma t ½ of 2.5 h with unlikely systemic osmotic effects. This evidence suggests that either 50 or 100 g of oral mannitol could be safely adopted for bowel cleansing solutions for colonoscopy preparation.
AUTHOR CONTRIBUTIONS
M.C. wrote the manuscript. M.C., M.V., G.E.T., A.O., B.M.C., and A.P. designed the research. G.F., C.S., P.S., M.V., G.E.T., G.M., M.S., I.B., P.C., A.Q., L.S., and C.T. performed the research. M.C., B.M.C., and A.O. analyzed the data.
FUNDING INFORMATION
This study was funded by NTC, Milan, Italy.
CONFLICT OF INTEREST
A.O. is an employee of NTC. M.C. and B.M.C. are consultants with NTC. N.T.C. is developing a bowel cleansing preparation based on mannitol. All other authors declared no competing interests for this work.
ACKNOWLEDGEMENTS
SATISFACTION Study Group: Flaminia Cavallaro, Manuela Codazzi, Germana De Nucci, Giuseppe De Roberto, Massimo Devani, Dhanai Di Paolo, Luca Elli, Carsten Hinkel, Ralf Jakobs, Daniel Janke, Vincenza Lombardo, Mauro Lovera, Franco Radaelli, Davide Ravizza, Peter Uebel, Jean Christoph Valats, Johanna Vollmar, Tim Zimmermann, and Giorgio Ciprandi.
The authors would like to thank the contract research organization OPIS (Desio, MB, Italy) for skillful management of the trial.
Fiori G, Spada C, Soru P, et al. Pharmacokinetics of oral mannitol for bowel preparation for colonoscopy. Clin Transl Sci. 2022;15:2448‐2457. doi: 10.1111/cts.13373
Giancarla Fiori and Cristiano Spada should be considered joint first authors and Marino Carnovali and Maurizio Vecchi should be considered joint senior authors.
The study was registered on EudraCT (2019–002856‐18) and ClinicalTrials.gov (NCT04759885).
REFERENCES
- 1. Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Gastroitest Endosc. 2015;81:31‐53. [DOI] [PubMed] [Google Scholar]
- 2. Fassil H, Adams KF, Weinmann S, et al. Approaches for classifying the indications for colonoscopy using detailed clinical data. BMC Cancer. 2014;14:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Joseph DA, Meester RGS, Zauber AG, et al. Colorectal cancer screening. Estimated future colonoscopy need and current volume and capacity. Cancer. 2016;122:2479‐2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shenbagaraj L, Thomas‐Gibson S, Stebbing J, et al. Endoscopy in 2017: a national survey of practice in the UK. Frontline Gastroenterol. 2019;10(1):7‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chapman W. The importance of adequate bowel cleansing for effective colonoscopy. Brit J Nursing. 2020;29:S3‐S8. [Google Scholar]
- 6. Gkolfakis P, Tziatzios G, Papanicolau IS, Triantafyllou K. Strategies to improve inpatients' quality of bowel preparation for colonoscopy: a systematic review and meta‐analysis. Gastroenterol Res Pract. 2019;2019:5147208‐5147215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. John J, Al‐Douri A, Candelaria B, et al. Colonoscopy quality and adherence to post polypectomy surveillance guidelines in an underinsured clinic system. Gastroenterol Res Pract. 2020;6240687:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Siegel RL, Miller KD, Jemal AL. Cancer statistics, 2019. CA Cancer J Clin. 2018;69:7‐34. [DOI] [PubMed] [Google Scholar]
- 9. Doubeni CA, Laiyemo AO, Major JM, et al. Socioeconomic status and the risk of colorectal cancer. Cancer. 2012;118(14):3636‐3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Millien VO, Mansour NM. Bowel preparation for colonoscopy in 2020: a look at the past, present, and future. Curr Gastroenterol Rep. 2020;22:28. [DOI] [PubMed] [Google Scholar]
- 11. Bechtold ML, Mir F, Puli SR, Nguyen DL. Optimizing bowel preparation for colonoscopy. A guide to enhance quality of visualization. Ann Gastroenterol. 2016;29:137‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tontini GE, Prada A, Sferrazza S, Ciprandi G, Vecchi M. The unmet needs for identifying the ideal bowel preparation. JGH Open. 2021;5(10):1135‐1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hassan C, East J, Radaelli F, et al. Bowel preparation for colonoscopy: European society of gastrointestinal endoscopy (ESGE) guideline. Endoscopy. 2019;45:142‐150. [DOI] [PubMed] [Google Scholar]
- 14. Paulo GA d, Martins FPB, Macedo EP d, Gonçalves MEP, Ferrari AP. Safety of mannitol use in bowel preparation: a prospective assessment of intestinal methane (CH4) levels during colonoscopy after mannitol and sodium phosphate (NaP) bowel cleansing. Arq Gastroenterol. 2016;53(3):196‐202. [DOI] [PubMed] [Google Scholar]
- 15. Ladas SD, Karamanolis G, Ben‐Soussan E. Colonic gas explosion during therapeutic colonoscopy with electrocautery. World J Gastroenterol. 2007;13(40):5295‐5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Josemanders DFGM, Spillenaar Bilgen EJ, van Sorge AA, Wahab P, de Vries RA. Colonic explosion during endoscopic polypectomy: avoidable complication or bad luck? Endoscopy. 2006;38(9):943‐944. [DOI] [PubMed] [Google Scholar]
- 17. Sousa JB d, Silva SME, Fernandes MB d L. Colonoscopies performed by resident physicians in a university teaching hospital: a consecutive analysis of 1000 cases. ABCD Brazil Arch Dig Surg. 2012;25(1):9‐12. [DOI] [PubMed] [Google Scholar]
- 18. Monahan DW, Peluso FE, Goldner F. Combustible colonic gas levels during flexible sigmoidoscopy and colonoscopy. Gastrointest Endosc. 1992;38:40‐43. [DOI] [PubMed] [Google Scholar]
- 19. Bond JH, Levitt MD. Factors affecting the concentration of combustible gases in the colon during colonoscopy. Gastroenterology. 1975;68:1445‐1448. [PubMed] [Google Scholar]
- 20. Cloyd JC, Snyder BD, Cleeremans B, Bundlie SR, Blomquist CH, Lakatua DJ. Mannitol pharmacokinetics and serum osmolality in dogs and humans. J Pharmacol Exp Ther. 1986;236(2):301‐306. [PubMed] [Google Scholar]
- 21. Kaneda K, Baker MT, Han TH, Weeks JB, Todd MM. Pharmacokinetic characteristics of bolus‐administered mannitol in patients undergoing elective craniotomy. J Clin Pharmacol. 2010;50(5):536‐543. [DOI] [PubMed] [Google Scholar]
- 22. Rudehill A, Gordon E, Ohman G, Lindqvist C, Andersson P. Pharmacokinetics and effects of mannitol on hemodynamics, blood, and cerebrospinal fluid electrolytes, and osmolality during intracranial surgery. J Neurosurg Anesthesiol. 1993;5(1):4‐12. [DOI] [PubMed] [Google Scholar]
- 23. Müller S, Francesconi CFDM, Maguilnik I. Randomized clinical trial comparing sodium picosulfate with mannitol in the preparation for colonoscopy in hospitalized patients. Arq Gastroenterol. 2007;44(3):244‐249. [DOI] [PubMed] [Google Scholar]
- 24. Vieira MC, Hashimoto CL, Carrilho FJ. Bowel preparation for performing a colonoscopy: prospective randomized comparison study between a low‐volume solution of polyethylene glycol and bisacodyl versus bisacodyl and a mannitol solution. Arq Gastroenterol. 2012;49(2):162‐168. [DOI] [PubMed] [Google Scholar]
- 25. Cobden I, Hamilton I, Rothwell J, Axon AT. Cellobiose/mannitol test: physiological properties of probe molecules and influence of extraneous factors. Clin Chem Acta. 1985;148(1):53‐62. [DOI] [PubMed] [Google Scholar]


