Abstract
Little is known about the impact of age on the processes governing human intestinal drug absorption. The Ussing chamber is a system to study drug transport across tissue barriers, but it has not been used to study drug absorption processes in children. This study aimed to explore the feasibility of the Ussing chamber methodology to assess pediatric intestinal drug absorption. Furthermore, differences between intestinal drug transport processes of children and adults were explored as well as the possible impact of age. Fresh terminal ileal leftover tissues from both children and adults were collected during surgery and prepared for Ussing chamber experiments. Paracellular (enalaprilat), transcellular (propranolol), and carrier‐mediated drug transport by MDR1 (talinolol) and BCRP (rosuvastatin) were determined with the Ussing chamber methodology. We calculated apparent permeability coefficients and efflux ratios and explored their relationship with postnatal age. The success rate for the Ussing chamber experiments, as determined by electrophysiological measurements, was similar between children (58%, N = 15, median age: 44 weeks; range 8 weeks to 17 years) and adults (67%, N = 13). Mean serosal to mucosal transport of talinolol by MDR1 and rosuvastatin by BCRP was higher in adult than in pediatric tissues (p = 0.0005 and p = 0.0091). In contrast, within our pediatric cohort, there was no clear correlation for efflux transport across different ages. In conclusion, the Ussing chamber is a suitable model to explore pediatric intestinal drug absorption and can be used to further elucidate ontogeny of individual intestinal pharmacokinetic processes like drug metabolism and transport.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
The intestine plays an important role in oral drug absorption. However, an information gap exists on age‐related differences in intestinal drug transport.
WHAT QUESTION DID THIS STUDY ADDRESS?
Can we study the ontogeny of intestinal drug transport with the Ussing chamber methodology?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
The Ussing chamber methodology is a feasible model to study pediatric intestinal drug transport of orally dosed drugs. Passive permeability as well as efflux transport can be studied with the use of this technique. Our exploratory data suggest lower intestinal MDR1 and BCRP transport in children, but sample size was too small to make definitive conclusions.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Characterization of age‐related absorption differences in the intestine could support pediatric intestinal physiologically‐based pharmacokinetic models that will help to develop advanced drug dosing guidelines and improve therapeutic efficacy and safety.
INTRODUCTION
Research in pediatric patients is challenging. Due to the low availability of pediatric intestinal tissue the ontogeny of intestinal drug absorption processes is not well known, making the prediction of oral drug doses for children challenging. 1 Many pediatric dosing regimens are often allometrically extrapolated from adult values based on bodyweight or body surface area. These methods assume that a linear or exponential relationship can be drawn from early development to adulthood. 2 , 3 However, development of a multiparameter system is not always a process that can be described by a single correlation. Therefore, setting the dose based on extrapolation of adults dosing regimens may result in inappropriate dose estimates for children. 4
Similar to adults, the most common route for drug administration in children is oral. Consequently, the physiological properties of the small intestine play an important role in dosing of oral drugs. 5 Drug absorption may be affected by maturational changes in intestinal permeability, which is determined by passive trans‐ and paracellular diffusion. 1 Intestinal drug transporters (DTs) and drug metabolizing enzymes (DMEs) play a key role in the absorption, distribution, and metabolism of a drug, influencing its pharmacokinetics. 6 Enterocytes express a wide variety of DTs, of which the efflux transporters multidrug resistance protein 1 (MDR1) and breast cancer resistance protein (BCRP) are highly relevant in clinical drug dosing. 6 , 7 Both transporters are members of the ATP binding cassette (ABC) superfamily. They are located on the apical membrane of the enterocytes and extrude xenobiotics by actively pumping them out of the cell back into the intestinal lumen, 8 , 9 thereby limiting oral bioavailability of substrate drugs. Contrary to their importance in drug absorption in adults, little is known about their ontogeny. Therefore, there is no clear understanding of the developmental impact of intestinal drug absorption processes on pharmacokinetics in children.
Organ specific maturation of DTs was established for the liver and kidneys by studying age‐dependent changes in their gene and protein expression. 10 , 11 , 12 However, maturation data on the intestine are scarce. Intestinal MDR1 is first detectable immunohistochemically during the 12th week of gestation, whereas mRNA levels reach adult values in neonates. 12 , 13 , 14 A recent study by Kiss et al., in 2021, showed lower protein expression of MDR1, BCRP, MRP2, and PEPT1 in different pediatric age groups compared to adults using targeted protein quantification. 15 A limitation of protein abundance measured by immunohistochemistry, Western blotting, and most recently with proteomics is that such data do not always provide an insight into the actual drug transport activity by DTs. 16
Hence, there is an unmet need for pediatric drug transporter data. Given the challenge of research in pediatric patients, an ex vivo model is required to generate data on pediatric intestinal drug absorption. The Ussing chamber is a well‐established ex vivo method that has been used to study drug absorption processes in human adult intestines. 17 , 18 , 19 , 20 However, data about drug absorption processes specifically in children is lacking. Therefore, the primary aim of this study was to assess the feasibility of the Ussing chamber to perform drug transport experiments with pediatric intestinal tissue. The secondary aim was to compare intestinal drug absorption processes between children and adults, and to explore the impact of age in the pediatric cohort on selected transport processes.
METHODS
Intestinal tissues
Human adult and pediatric small intestinal tissues were obtained as surgical leftover material during the years 2018–2021 at Radboud University Medical Center (Radboudumc), Nijmegen, The Netherlands. Adult terminal ileum tissues originated from (hemi)colectomies from patients with colonic cancer. Pediatric intestinal tissues were mainly obtained from ileal stoma closure surgeries (proximal to terminal ileum). Intestinal tissue distal from the stoma ending was used when possible, as this was exposed to nutrients in contrast to the distal tissue in most cases.
According to the Dutch Code of Conduct for Responsible Use, no informed consent was needed for the anonymous left‐over tissue collection from adults. Informed consent for the use of pediatric left‐over tissue and clinical data was obtained from the parents/legal guardians and/or the children. Radboudumc Medical Ethics Board waived the need for formal ethics approval according to the Dutch Law on Human Research for pediatric tissues.
Preparation of the Ussing chamber setup
EM‐LVSYS‐6 Ussing Chamber Systems were used, manufactured by Physiologic Instruments (Cat. No. P2400; Alpine, CA). The chambers were filled with 3 ml freshly made Krebs bicarbonate buffer and were continuously oxygenated with carbogen gas (95% O2, 5% CO2). Four electrodes (Cat. No. P2020‐IS and P2020‐VS; Physiologic Instruments) were used to continuously measure the viability of the tissue: the potential difference (PD), and the short circuit current (SCC; Figure 1). The narrow end of the electrode tips was filled with 6% agarose to create an agarose‐salt bridge and the remainder was completely filled with 3 M potassium chloride. The electrodes were firmly attached to the tips after which they were connected to the chambers. The quality of the electrodes was verified by measuring the PD and the SCC between the electrode pairs, setting a background reference.
FIGURE 1.
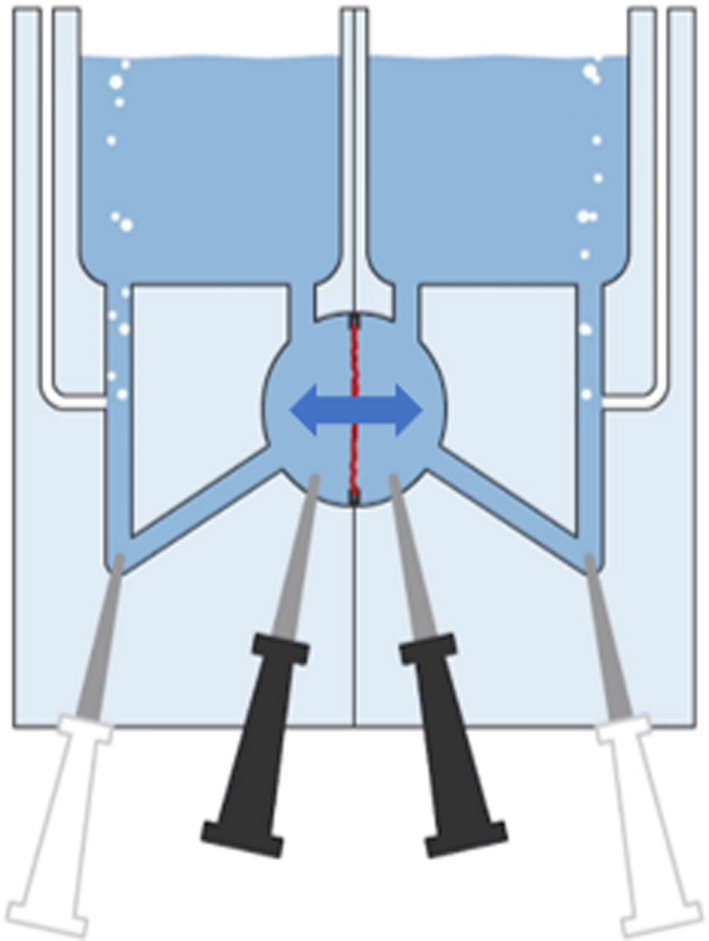
Schematic representation of the Ussing chamber. The tissue, shown in red, separates the mucosal and serosal compartments. Arrow indicates bidirectional transport. Black electrodes measure potential difference (PD). White electrodes measure short circuit current (SCC).
Tissue preparation
Intestinal specimens were collected directly at the time of resection and kept in ice‐cold carbogenated Krebs buffer. Transport time to the laboratory was less than 15 min. The tissues were opened longitudinally, washed thoroughly, and stored in continuously carbogenated ice‐cold Krebs buffer to avoid ischemia. Without overstretching, the tissue was pinned down on a silicon bed (DC Sylgard 184; Dow Inc., Midland, MI) in a temperature‐controlled preparation basin (made by the custom solutions team at UCB Biopharma SRL), filled with continuously carbogenated Krebs bicarbonate buffer (10°C) and the serosa was carefully removed, as described by Sjoberg et al. 17
A maximum of six tissue pieces per patient were mounted in inserts (P2410, exposed area 0.2 cm2 or P2413, exposed area 0.71 cm2) and were placed into the Ussing chambers with prewarmed (37°C), carbogenated Krebs buffer. The preparation of the pediatric tissues (maximum n = 6) was similar, but 0.2 cm2 and 0.031 cm2 (exposed area, P2407) inserts were used to accommodate the small tissue sizes. Once the tissue was placed inside the Ussing chamber, electrophysiology measurements were started. Digital signals were recorded by the Clamp software (Scientific Instruments, Aachen, Germany). Criteria for a viable tissue were set to PD greater than or equal to 4 (mV) and SCC greater than or equal to 100 (μA/cm2), as determined by Sjoberg et al. 17 Tissues not meeting electrophysiology threshold criteria were excluded from further analysis.
Transport experiments
Marker compounds were selected to probe the individual transport routes. Propranolol (10 μM; Sigma‐Aldrich, St. Louis, MO) and enalaprilat (10 μM; Cayman Chemicals, Ann Arbor, MI) were chosen to evaluate passive transcellular and paracellular transport respectively. 17 , 21 To study transport by BCRP and MDR1, the drug substrates rosuvastatin (4 μM; Carbosynth Ltd., Berkshire, UK) and talinolol (2 μM; Cayman Chemicals) were chosen. 22 , 23 , 24 , 25 , 26 , 27 Samples of 50 μl were taken from both sides of the segment and replaced with fresh buffer (intervals of 15 min on the acceptor side and 15 or 60 min on the donor side).
During incubation and the actual transport experiment, the mucolytic agent N‐acetylcysteine (NAC; Sigma‐Aldrich, St. Louis, MO, final concentration 70 or 700 μg/ml) was added to reduce mucus adhesion to the tissue and sustain the redox balance of the tissue. Buffered NAC was added to the serosal side of the tissue, whereas unbuffered NAC was added to the mucosal side, resulting in a rather physiological pH of 7.4 on the serosal side and 6.5 on the mucosal side, respectively. After 30 min of incubation, the buffer was removed from both half‐chambers and replaced with 37°C warm, fresh buffer including the test compounds (final DMSO concentration 0.18%) and NAC.
Liquid chromatography‐tandem mass spectrometry
Compound concentrations of each sample were determined by liquid chromatography‐tandem mass spectrometry (LC‐MS/MS). Sample preparation started with protein precipitation with three volumes of methanol containing both d5‐enalaprilat and labetalol as internal standards. The samples were mixed well, and centrifuged. 1 μl of the supernatant was directly injected into the LC‐MS/MS system for analysis. Propranolol, enalaprilat, talinolol, and rosuvastatin were quantified with LC‐MS/MS, using an Acquity ultraperformance liquid chromatography (UPLC; Waters, Milford, MA) coupled to a Xevo TQ‐S (Waters) triple quadrupole mass spectrometer. The compounds were separated on a HSS T3 analytical column (1.8 μm; 100 × 2.1 mm, Acquity UPLC, Waters, Ireland). The elution gradient was as follows: 0–1 min, from 20% B to 80% B; 1–2 min, 100% B; and 2–3 min, 20% B. Solvent A consisted of 0.1% formic acid in H2O and solvent B consisted of 0.1% formic acid in acetonitrile. The column temperature was set to 40°C, and the flow rate was 300 μl/min. The effluent from the UPLC was passed directly into the electrospray ion source. Positive electrospray ionization was achieved using nitrogen as a desolvation gas with ionization voltage of 600 V. The source temperature was set to 500°C with argon used as collision gas. Detection of all compounds was based on isolation of the protonated molecular ion, [M + H]+ and subsequent MS/MS fragmentations and a multi‐reaction monitoring (MRM) were carried out. The following MRM transitions were used: for propanol m/z 260.2 (parent ion) to m/z 116.0 and 183.0 (both product ions), for enalaprilat m/z 348.9 (parent ion) to m/z 206.0 and 90.9 (both product ions), for talinolol m/z 364.1 (parent ion) to m/z 99.9 and 308.1 (both product ions), for rosuvastatin m/z 482.0 (parent ion) to m/z 258.1 and 300.1 (both product ions), for d5‐enalaprilate m/z 354.2 (parent ion) to m/z 211.1 and 95.9 (both product ions), and for labetalol m/z 329.2 (parent ion) to m/z 91.0 (product ion).
Transport activity
The linear part of the acquired time‐concentration curve on the acceptor side, obtained during the time of the above defined tissue viability criteria, was used to calculate the apparent permeability coefficient (P app) for each compound according to Equation 1.
| (1) |
where dQ/dt is the rate of drug transport from one half chamber to the other (either mucosal to serosal or serosal to mucosal), A the exposed area of the tissue, and C 0 the initial concentration of the compound investigated in the donor compartment.
Efflux ratios (ERs) were determined according to Equation 2 for each tissue.
| (2) |
Statistics
All data are presented with descriptive statistics. Data are summarized using mean P app value per patient. In the graphs mean P app per age group are shown. Summarized pediatric and adult data, as well as data from patients younger and older than 1 year of postnatal age were compared using mixed models testing. Therefore, statistical p values are based on estimated marginal means ± SE. Values discussed in the paper are depicted as mean individual values ± SD. Table 2 summarizes means and estimated marginal means. The impact of age was explored using linear regression, with a Spearman test. Data analysis was performed using IBM SPSS Statistics software (SPSS Statistics for Windows, version 25.0; IBM, Armonk, NY) and GraphPad Prism software (version 8.0.1; La Jolla, CA).
TABLE 2.
Estimated marginal mean ± SE calculated using mixed model statistics and means ± SD per age group and transport direction of the different substrates
| Drug | Age group | Direction | Estimated marginal mean ± SE (×10–6 cm/s) | Mean ± SD (×10–6 cm/s) | N, n |
|---|---|---|---|---|---|
| Propranolol | Pediatric | M > S | 7.98 ± 1.45 | 7.49 ± 4.58 | 11, 19 |
| S > M | 7.74 ± 1.47 | 8.27 ± 4.70 | 10, 16 | ||
| Adult | M > S | 11.98 ± 1.28 | 12.38 ± 6.15 | 11, 26 | |
| S > M | 10.34 ± 1.27 | 10.65 ± 5.02 | 12, 26 | ||
| Enalaprilat | Pediatric | M > S | 2.91 ± 0.37 | 2.76 ± 1.36 | 11, 21 |
| S > M | 2.93 ± 0.39 | 3.02 ± 0.9 | 10, 16 | ||
| Adult | M > S | 2.95 ± 0.34 | 2.94 ± 1.69 | 11, 26 | |
| S > M | 2.65 ± 0.34 | 2.67 ± 1.48 | 12, 25 | ||
| Talinolol | Pediatric | M > S | 4.94 ± 1.33 | 4.80 ± 3.11 | 11, 19 |
| S > M | 11.06 ± 1.39 | 11.32 ± 4.26 | 10, 16 | ||
| Adult | M > S | 6.60 ± 1.16 | 7.00 ± 4.52 | 11, 15 | |
| S > M | 17.87 ± 1.15 | 18.27 ± 6.87 | 12, 26 | ||
| Rosuvastatin | Pediatric | M > S | 3.80 ± 0.88 | 4.73 ± 2.04 | 11, 18 |
| S > M | 7.53 ± 1.00 | 7.53 ± 3.66 | 10, 14 | ||
| Adult | M > S | 6.32 ± 0.97 | 6.32 ± 2.75 | 11, 26 | |
| S > M | 11.16 ± 0.91 | 11.16 ± 5.49 | 12, 17 |
Note: N = amount of patients, n = amount of chambers.
RESULTS
In total, we included data obtained on tissues from 15 pediatric and 13 adult patients. After mounting, each chamber was checked individually for electrophysiology values. The median postnatal age of pediatric patients was 44 weeks (range: 8 weeks–17 years) and the pediatric intestinal material originated predominantly from stoma closure at the level of the ileum (Table 1). Figure 2 shows a flowchart of included and excluded patients as well as reasons for tissue or measurement exclusion.
TABLE 1.
List of pediatric samples, including postnatal age of the patient, the medical condition, and surgical procedure
| Postnatal age (weeks) | Tissue region | Type of surgery | (Previous) disease state |
|---|---|---|---|
| 8 | Proximal ileum | Stoma closure surgery | Proximal ileum atresia |
| 13 | Terminal ileum | Stoma closure surgery | Colon Hirschsprung |
| 14 | Terminal ileum | Stoma closure surgery | Ileal atresia |
| 19 | Terminal ileum | Stoma closure surgery | Ileal atresia |
| 26 | Terminal ileum | Stoma closure surgery | Ileal atresia |
| 39 | Terminal ileum | Stoma closure surgery | Ileal atresia |
| 44 | Ileum | Ileostomy revision | Colon ischemia |
| 48 | Terminal ileum | Stoma closure surgery | Ileal atresia |
| 48 | Terminal ileum | Stoma closure surgery | Ileal atresia |
| 57 | Terminal ileum | Stoma closure surgery | Previous necrotic enterocolitis |
| 95 | Terminal ileum | Stoma closure surgery | Ileal atresia |
| 154 | Terminal ileum | Stoma closure surgery | Ileal atresia |
| 177 | Terminal ileum | Revision of ileostomy | Chronic intestinal pseudo‐obstruction caused by underlying genetic disease |
| 637 | Terminal ileum | Trauma surgery | Emergency closure |
| 906 | Terminal ileum | Stoma closure surgery | Colitis Ulcerosa |
FIGURE 2.
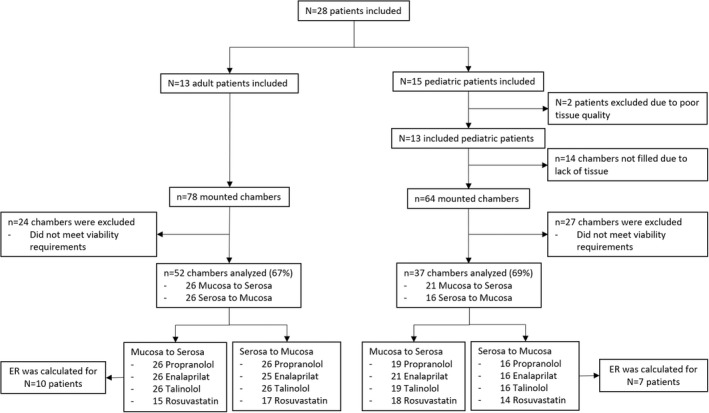
Flowchart of adult and pediatric included patients and Ussing measurements by substrate and direction. N = number of patients, n = number of Ussing chambers. ER, efflux ratio.
For pediatric tissue, the experiments were considered successful when electrophysiology values were similar to adult values (data not shown). Based on tissue availability and viability values 37 out of 64 mounted pediatric chambers were analyzed; a success rate of 58%. From adult tissue, 52 out of 78 mounted chambers were analyzed; a success rate of 67%. In these tissues, we successfully studied bidirectional passive and active intestinal transport using the selected compounds. The tissues excluded showed electrophysiology values below our pre‐set viability threshold values. A lower success rate for pediatric intestinal tissues was expected as tissues health and viability might be influenced by the origin of the tissues (stoma endings) or might be damaged by underlying illnesses.
Mean P app values per age group can be found in Table 2 and Figure 3. Individual mean P app values per patient of propranolol, enalaprilat, talinolol, and rosuvastatin versus age are shown in Figure 4. In general, comparable mucosal to serosal and serosal to mucosal P app values were obtained for drug transport between the pediatric and adult age groups, with the exception of (efflux) transport from the serosal to mucosal direction. The transport of talinolol by MDR1 was significantly lower in pediatric patients compared to adults (p = 0.0005; Table 2). A similar outcome was observed for rosuvastatin transport by BCRP, a lower P app of 7.5 ± 3.7 × 10−6 cm/s in pediatric patients compared to 11.2 ± 5.5 × 10−6 cm/s in adult patients was found (p = 0.0091). Interestingly, when analyzing the relationship between the talinolol P app,(s−m) across all age groups using Spearman's correlation, a moderate correlation was observed with an R value of 0.49 (p = 0.0283; Figure 4). However, within the pediatric subpopulation no correlation was observed with age.
FIGURE 3.
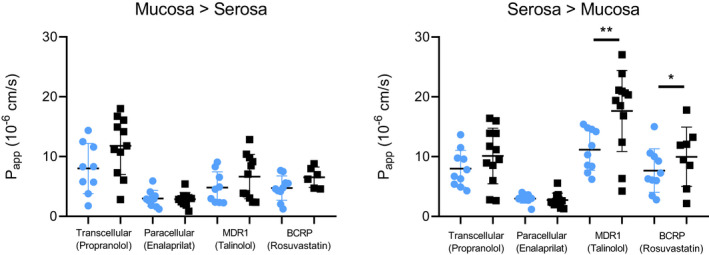
Mean P app values per patient for each drug in both age groups. Mean value per age group ± SD are shown. Blue circles represent the pediatric age group (0–18 years old), black squares represent the adult age group. Statistics performed following mixed models based on estimated marginal means. *p = 0.0091, **p = 0.0005. P app, apparent permeability coefficient.
FIGURE 4.
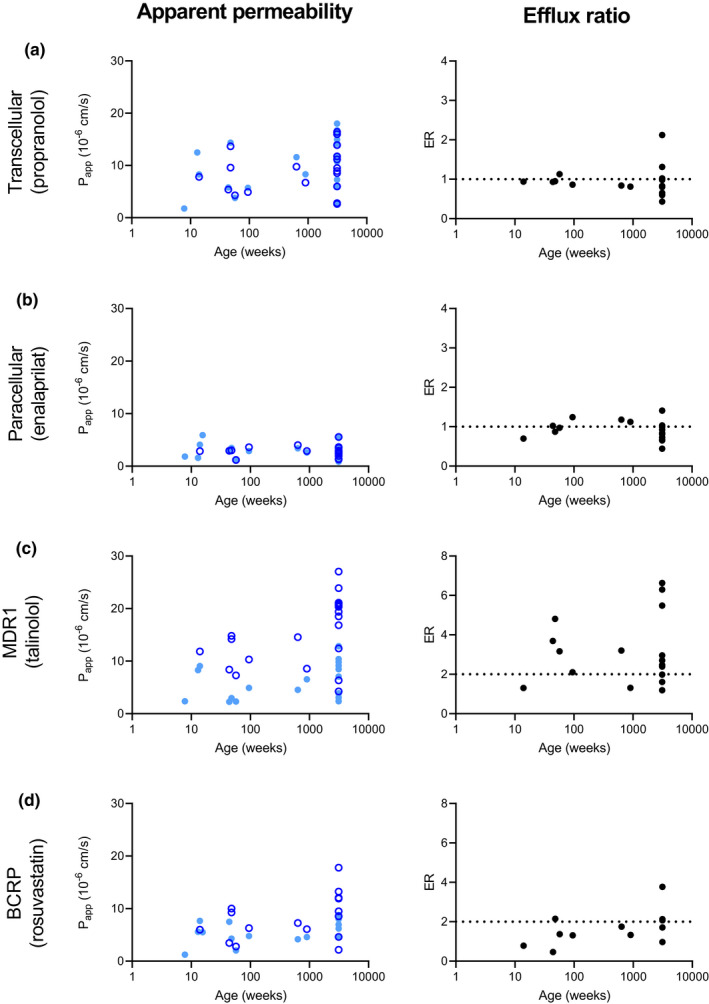
Apparent permeability coefficient (P app) and efflux ratio (ER) values versus age for probe compounds determined in this study. Each point represents a mean value per patient. Light blue closed circles: mucosal to serosal transport of the substrate. Dark blue open circles: serosal to mucosal transport of the substrate. Black circles: mean ER per patient. (a) transcellular transport determined with substrate propranolol. (b) Paracellular transport determined with substrate enalaprilat. (c) Transport by MDR1 determined with substrate talinolol. Spearman correlation showed a correlation for MDR1 with age Rs value of 0.49 (p = 0.0283). (d) Transport by BCRP determined with substrate rosuvastatin. No correlation between ER of talinolol and rosuvastatin was found, Rs value of 0.57 (p > 0.05).
Average ER for propranolol transport in pediatric patients was 0.9 ± 0.1 and adult patients was 1.0 ± 0.5. For enalaprilat ERs of 1.0 ± 0.2 and 0.9 ± 0.3 were calculated for pediatric and adult patients, respectively (Figure 4). The mean ER per tissue for passive transport fluctuates around one for propranolol and enalaprilat across the whole age range, confirming the lack of active transporter contribution to the absorption of propranolol and enalaprilat in this model. Average ERs for talinolol were 2.8 ± 1.3 for pediatric and 3.4 ± 2.0 for adult patients. Rosuvastatin ERs found were 1.3 ± 0.6 for pediatric and 2.1 ± 1.0 for adult patients. No correlation between ER of talinolol and rosuvastatin was observed with an R s value of 0.57 (p > 0.05; Figure 4). ERs for both actively transported drugs are similar across the age range and demonstrate comparable active transport measurements in pediatric and adult intestinal tissue with use of the Ussing chamber (Figure 4).
DISCUSSION
The Ussing chamber methodology is a feasible method to study pediatric intestinal drug transport processes, enabling quantification of paracellular, transcellular, MDR1‐ and BCRP‐mediated transport across pediatric small intestinal samples. The experiments with pediatric small intestinal tissues were considered successful when electrophysiological recordings were similar to adult values (data not shown), passive permeability and active transport were evaluated, and ERs were in line with the transport directions. Similar to the adult Ussing methodology, a high ratio of collected pediatric samples were suitable for measurements, and those that were excluded were too small in size, showed low viability, or had serious underlying disease. Besides that, similar passive transport values were found for pediatric and adult age groups and a possibly lower serosal to mucosal transport by the efflux transporters MDR1 and BCRP.
Adult P app values found in this study reflect the scarce results reported in literature. Michiba et al., in 2021, showed comparable propranolol and lower rosuvastatin transport in jejunum in mucosal to serosal direction. For propranolol 7.8 ± 4.6 × 10−6 cm/s (N = 6, n = 29) versus 12.4 ± 6.2 × 10−6 cm/s (N = 13, n = 27) in our study and for rosuvastatin 1.6 ± 0.6 × 10−6 cm/s (N = 6, n = 21) versus 6.3 ± 2.8 × 10−6 cm/s (N = 11, n = 15) in our study. 19 Differences might be explained by regional alterations between the jejunum and ileum. Sjoberg et al., in 2013, showed a comparable propranolol permeability of 22.4 ± 14.6 × 10−6 cm/s (N = ?, n = 5) across the ileum in mucosal to serosal direction. Their higher P app value might be explained by their different setup with intensive stirring and the lack of a pH gradient. 28 Furthermore, they determined the rosuvastatin transport from mucosal to serosal direction in the jejunum, showing a similar P app value to our ileum transport, 7.0 ± 1.0 × 10−6 cm/s (n = 3) versus 6.3 ± 2.8 × 10−6 cm/s (N = 11, n = 15), respectively. 17 Sjoberg et al., in 2013, discussed that Ussing chamber experiments show a better correlation between laboratories and with human in vivo fraction absorbed, compared to in vitro models, such as Caco‐2 cells. They composed a correlation curve of jejunal P app measurements from different laboratories using the human Ussing chamber compared to in vivo fraction absorbed. 17 Our ileal P app,m−s values of propranolol and rosuvastatin combined with fraction absorbed (propranolol 95% and rosuvastatin 50%) fit in their compiled jejunal correlation curve, suggesting the suitability of our model. 17
Paracellular and transcellular transport P app values and variability were comparable between the pediatric and adult age groups in both directions, demonstrating the feasibility of applying pediatric intestinal tissue in the Ussing chamber. Interestingly, mean talinolol and rosuvastatin transport by MDR1 and BCRP was lower in pediatric patients compared to adults for the serosal to mucosal direction only, which could indicate age‐related differences in transporter expression and/or functionality. However, as neither a relationship was found with age evaluated on a continuous scale nor with age groups and ER, it is too early to conclude that intestinal MDR1 and BRCP activity is lower in children. Should efflux transport be lower in pediatric patients compared to the adult age group, the in vivo bioavailability of substrate drugs might be altered in pediatric patients. More pediatric data, from across the whole age range and more detailed data analysis into underlying disease, medication, and food intake, among many other factors influencing DTs are needed to be more conclusive. Furthermore, studies with the use of selective transporter inhibitors may be needed to further explore the role of age on individual transporter activities.
The US Food and Drug Administration (FDA) considers a substrate for efflux transporter MDR1 sufficiently demonstrated when ER in Caco‐2 transport assays is greater than or equal to 2. 29 In our study, we observed pediatric ER for talinolol and rosuvastatin of 2.8 ± 1.3 and 1.3 ± 0.6, respectively. The ER for talinolol found in this study was significantly higher than the passive transported compounds. The ER of rosuvastatin does not meet the FDA in vitro requirement, neither does the mean ER differ significantly from the passive transported drug. Nevertheless, a Caco‐2 methodology is hard to compare to the use of fresh intestinal tissue due to their lack of in vivo representation. 18 , 30
Earlier studies found an age‐related variation in mRNA expression and protein abundance for several drug transporters during early development, which could influence their transport capacity. 12 , 15 , 31 For example, Kiss et al. reported lower protein expression of MDR1 in ileum tissue from infants (0–2 years) compared to adults and lower BCRP in infants (0–2 years) and adolescents (12–18 years) compared to adults. However, they did not find a positive correlation with postnatal age for MDR1 or BCRP in ileal tissues. 15 In contrast, gene expression analysis by Mooij et al. did not show a difference between MDR1 expression in neonates (0–1 month) compared to adult pooled intestinal regions. Of note, mRNA expression levels and protein abundances are not always proportional to the activity of the proteins. Therefore, determining gene expression combined with functional data from the Ussing chamber could be a valuable follow‐up study. Post‐translational modifications and subcellular localization, for example, can influence the transporter's function and efficiency, as reviewed by Czuba et al. 32 Thus, a larger study cohort is needed to confirm these results.
Our study has several limitations. First, tissue access was always the main challenge in conducting pediatric studies. The number of planned surgical procedures affecting the small intestine is low, whereas emergency surgeries pose a challenge in obtaining informed consent from the parents of the patients. Most patients included in our study were previously diagnosed with small intestinal atresia, a developmental malformation that results in an obstruction or disconnection of the proximal and distal ends. These tissues are macroscopically normal, however, their level of physiological similarity to normal tissue is unknown. In addition, the adult terminal ileum was obtained from patients suffering from colon cancer. Tissues surrounding cancer areas might have an altered drug transporter expression. For instance, colorectal cancer tissue shows increased levels of MDR1. 33 Gene expression adjacent to the tumor could have been changed. 34 Hence, in all cases, underlying disease, pretreatment, drug history, or lifestyle factors might have influenced intestinal drug transport measurements. Follow‐up studies could be improved by comparing the youngest age groups to adolescents. However, adolescent intestinal patients are often diagnosed with inflammatory bowel disease, which makes it difficult to obtain (close to) healthy tissue. 35
Besides that, only nine patients with an age less than or equal to 1 year were included in this study (ER could be calculated for just 3 patients). As reviewed by Van Groen et al., in 2018, most hepatic DTs and DMEs are found to substantially mature during this first period of life, either increasing or decreasing in expression or activity levels. For instance, the hepatic expression of the efflux transporter MRP2 increased gradually from neonatal to adult level during infancy. 36 Hence, a correlation with age might be seen when including more patients below the postnatal age of 1 year. Besides that, ontogeny of DTs can be affected by other factors, such as ethnicity, sex, genotype, medications, and food type, however, due to the scarcity of samples this was not examined during this study.
An advantage of the Ussing chamber is that ex vivo primary tissue is used, which closely represents the intestinal barrier. Transporter functionality can be more easily related to in vivo functionality and corresponding age/characteristics of the patient than in vitro models as Caco‐2 cell lines. 17 , 37 Apparent permeability values obtained can be incorporated in physiologically‐based pharmacokinetic (PBPK) models to improve dosing guidelines for the studied population. Alternative techniques to study pediatric intestinal drug absorption could include patient‐derived organoids or organ‐specific exosomes. 38 , 39 However, for both models, it is still unknown if they retain their age‐specific properties. Currently, we are working on the possibility of comparing drug transport and metabolism in intestinal organoids from pediatric and adult patients with results obtained in the Ussing chamber. If these organoids retain their age‐specific characteristics, this could be the next step in pediatric pharmacokinetic studies.
In summary, we successfully used pediatric small intestinal tissues in the Ussing chamber setup to measure transepithelial drug transport and explored the impact of age with a success rate of 58%. In general, passive permeability, as well as efflux by MDR1 and BCRP, appear in a similar range in adults and children older than 14 weeks of postnatal age. Efflux transport may be lower in this pediatric population, but this needs to be confirmed in a larger study cohort, including neonates. Ultimately, intestinal Ussing data may support pediatric intestinal PBPK models that will help to develop advanced drug dosing guidelines and improve therapeutic efficacy and safety.
AUTHOR CONTRIBUTIONS
E.J.S., M.K., P.v.d.B., F.G.M.R., E.v.d.S., and S.N.d.W. wrote the manuscript. J.N., J.v.d.H., P.v.d.B., S.M.B.I.B., and S.d.W. designed the research. E.J.S., M.K., J.v.d.H., and S.N.d.W. performed the research. E.J.S., M.K., P.v.d.B., L.v.R., F.G.M.R., and S.N.d.W. analyzed the data. J.N., P.v.d.B., J.v.d.H., S.M.B.I.B., M.W.J.S., and A.L.U. contributed new reagents/methodology/analytical tools.
FUNDING INFORMATION
This study was funded by UCB BioPharma SRL, Braine‐l'Alleud, Belgium, and the Dutch Ministry of Economic Affairs by means of the PPP Allowance made available by the Top Sector Life Sciences & Health to stimulate public‐private partnerships and the Research Institute of Health Science of Radboudumc, Nijmegen, The Netherlands.
CONFLICT OF INTEREST
J.N. and A.‐L.U. were employees of UCB biopharma during the course of this study. All other authors declared no competing interests for this work.
Streekstra EJ, Kiss M, van den Heuvel J, et al. A proof of concept using the Ussing chamber methodology to study pediatric intestinal drug transport and age‐dependent differences in absorption. Clin Transl Sci. 2022;15:2392‐2402. doi: 10.1111/cts.13368
Eva J. Streekstra and Márton Kiss contributed equally to this work.
REFERENCES
- 1. Stillhart C, Vučićević K, Augustijns P, et al. Impact of gastrointestinal physiology on drug absorption in special populations – An UNGAP review. Eur J Pharm Sci. 2020;147:105280. [DOI] [PubMed] [Google Scholar]
- 2. Sawrey EL, Subramanian MW, Ramirez KA, Snyder BS, Logston BB, Russell GB. Use of body surface area for dosing of vancomycin. J Pediatr Pharmacol Ther. 2019;24:296‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ritschel WA. Handbook of Basic Pharmacokinetics. Drug Intelligence Publications; 1980. [Google Scholar]
- 4. van den Anker J, Reed MD, Allegaert K, Kearns GL. Developmental changes in pharmacokinetics and pharmacodynamics. J Clin Pharmacol. 2018;58(Suppl 10):S10‐S25. [DOI] [PubMed] [Google Scholar]
- 5. Stewart KD, Johnston JA, Matza LS, et al. Preference for pharmaceutical formulation and treatment process attributes. Patient Prefer Adherence. 2016;10:1385‐1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zamek‐Gliszczynski MJ, Taub ME, Chothe PP, et al. Transporters in drug development: 2018 ITC recommendations for transporters of emerging clinical importance. Clin Pharmacol Ther. 2018;104:890‐899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. International Transporter, C . Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fardel O, Lecureur V, Guillouzo A. The P‐glycoprotein multidrug transporter. Gen Pharmacol Vasc S. 1996;27:1283‐1291. [DOI] [PubMed] [Google Scholar]
- 9. Wang X, Wu X, Wang C, et al. Transcriptional suppression of breast cancer resistance protein (BCRP) by wild‐type p53 through the NF‐κB pathway in MCF‐7 cells. FEBS Lett. 2010;584:3392‐3397. [DOI] [PubMed] [Google Scholar]
- 10. Cheung KWK, Groen BD, Spaans E, et al. A comprehensive analysis of ontogeny of renal drug transporters: mRNA analyses, quantitative proteomics, and localization. Clin Pharmacol Ther. 2019;106:1083‐1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Groen BD, van de Steeg E, Mooij MG, et al. Proteomics of human liver membrane transporters: a focus on fetuses and newborn infants. Eur J Pharm Sci. 2018;124:217‐227. [DOI] [PubMed] [Google Scholar]
- 12. Mooij MG, Schwarz UI, de Koning BAE, et al. Ontogeny of human hepatic and intestinal transporter gene expression during childhood: age matters. Drug Metab Dispos. 2014;42:1268‐1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Konieczna A, Erdosova B, Lichnovska R, Jandl M, Cizkova K, Ehrmann J. Differential expression of ABC transporters (MDR1, MRP1, BCRP) in developing human embryos. J Mol Histol. 2011;42:567‐574. [DOI] [PubMed] [Google Scholar]
- 14. Mizuno T, Fukuda T, Masuda S, et al. Developmental trajectory of intestinal MDR1/ABCB1 mRNA expression in children. Br J Clin Pharmacol. 2014;77:910‐912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kiss M, Mbasu R, Nicolaï J, et al. Ontogeny of small intestinal drug transporters and metabolizing enzymes based on targeted quantitative proteomics. Drug Metab Dispos. 2021;49:1038‐1046. [DOI] [PubMed] [Google Scholar]
- 16. Streekstra EJ, Russel FGM, van de Steeg E, de Wildt SN. Application of proteomics to understand maturation of drug metabolizing enzymes and transporters for the optimization of pediatric drug therapy. Drug Discov Today Technol. 2021;39:31‐48. [DOI] [PubMed] [Google Scholar]
- 17. Sjoberg A, Lutz M, Tannergren C, Wingolf C, Borde A, Ungell AL. Comprehensive study on regional human intestinal permeability and prediction of fraction absorbed of drugs using the Ussing chamber technique. Eur J Pharm Sci. 2013;48:166‐180. [DOI] [PubMed] [Google Scholar]
- 18. Haslam IS, O'Reilly DA, Sherlock DJ, Kauser A, Womack C, Coleman T. Pancreatoduodenectomy as a source of human small intestine for Ussing chamber investigations and comparative studies with rat tissue. Biopharm Drug Dispos. 2011;32:210‐221. [DOI] [PubMed] [Google Scholar]
- 19. Michiba K, Maeda K, Kurimori K, et al. Characterization of the human intestinal drug transport with ussing chamber system incorporating freshly isolated human jejunum. Drug Metab Dispos. 2021;49:84‐93. [DOI] [PubMed] [Google Scholar]
- 20. Kisser B, Mangelsen E, Wingolf C, et al. The ussing chamber assay to study drug metabolism and transport in the human intestine. Curr Protoc Pharmacol. 2017;77:7.17.1‐7.17.9. [DOI] [PubMed] [Google Scholar]
- 21. Roos C, Dahlgren D, Sjogren E, Tannergren C, Abrahamsson B, Lennernas H. Regional intestinal permeability in rats: a comparison of methods. Mol Pharm. 2017;14:4252‐4261. [DOI] [PubMed] [Google Scholar]
- 22. Morrison RA, Chong S, Marino AM, et al. Suitability of enalapril as a probe of the dipeptide transporter system: in vitro and in vivo studies. Pharm Res. 1996;13:1078‐1082. [DOI] [PubMed] [Google Scholar]
- 23. Pretorius E, Bouic PJ. Permeation of four oral drugs through human intestinal mucosa. AAPS PharmSciTech. 2009;10:270‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gramatté T, Oertel R, Terhaag B, Kirch W. Direct demonstration of small intestinal secretion and site‐dependent absorption of the beta‐blocker talinolol in humans. Clin Pharmacol Ther. 1996;59:541‐549. [DOI] [PubMed] [Google Scholar]
- 25. Huang L, Wang Y, Grimm S. ATP‐dependent transport of rosuvastatin in membrane vesicles expressing breast cancer resistance protein. Drug Metab Dispos. 2006;34:738‐742. [DOI] [PubMed] [Google Scholar]
- 26. Oswald S, Terhaag B, Siegmund W. In vivo probes of drug transport: commonly used probe drugs to assess function of intestinal P‐glycoprotein (ABCB1) in humans. In: Fromm MF, Kim RB, eds. Drug Transporters. Springer Berlin Heidelberg; 2011:403‐447. [DOI] [PubMed] [Google Scholar]
- 27. Lee CA, O'Connor MA, Ritchie TK, et al. Breast cancer resistance protein (ABCG2) in clinical pharmacokinetics and drug interactions: practical recommendations for clinical victim and perpetrator drug‐drug interaction study design. Drug Metab Dispos. 2015;43:490‐509. [DOI] [PubMed] [Google Scholar]
- 28. Neuhoff S, Ungell AL, Zamora I, Artursson P. pH‐dependent passive and active transport of acidic drugs across Caco‐2 cell monolayers. Eur J Pharm Sci. 2005;25:211‐220. [DOI] [PubMed] [Google Scholar]
- 29. FDA . In vitro drug interaction studies—cytochrome p450 enzyme‐ and transporter‐mediated drug interactions; 2020. Accessed February 16, 2022. https://www.fda.gov/media/134582/download
- 30. Sun H, Chow ECY, Liu S, Du Y, Pang KS. The Caco‐2 cell monolayer: usefulness and limitations. Expert Opin Drug Metab Toxicol. 2008;4:395‐411. [DOI] [PubMed] [Google Scholar]
- 31. Mooij MG, de Koning BEA, Lindenbergh‐Kortleve DJ, et al. Human intestinal PEPT1 transporter expression and localization in preterm and term infants. Drug Metab Dispos. 2016;44:1014‐1019. [DOI] [PubMed] [Google Scholar]
- 32. Czuba LC, Hillgren KM, Swaan PW. Post‐translational modifications of transporters. Pharmacol Ther. 2018;192:88‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Beklen H, Gulfidan G, Arga KY, Mardinoglu A, Turanli B. Drug repositioning for P‐glycoprotein mediated co‐expression networks in colorectal cancer. Front Oncol. 2020;10:1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aran D, Camarda R, Odegaard J, et al. Comprehensive analysis of normal adjacent to tumor transcriptomes. Nat Commun. 2017;8:1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rosen MJ, Dhawan A, Saeed SA. Inflammatory bowel disease in children and adolescents. JAMA Pediatr. 2015;169:1053‐1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van Groen BD, Nicolaï J, Kuik AC, et al. Ontogeny of hepatic transporters and drug‐metabolizing enzymes in humans and in nonclinical species. Pharmacol Rev. 2021;73:597‐678. [DOI] [PubMed] [Google Scholar]
- 37. Gotoh Y, Kamada N, Momose D. The advantages of the ussing chamber in drug absorption studies. J Biomol Screen. 2005;10:517‐523. [DOI] [PubMed] [Google Scholar]
- 38. van Groen BD, Allegaert K, Tibboel D, de Wildt SN. Innovative approaches and recent advances in the study of ontogeny of drug metabolism and transport. Br J Clin Pharmacol. 2020;1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nantasanti S, de Bruin A, Rothuizen J, Penning LC, Schotanus BA. Concise review: organoids are a powerful tool for the study of liver disease and personalized treatment design in humans and animals. Stem Cells Transl Med. 2016;5:325‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]


