Abstract
There is increasing data implicating Chlamydia pneumoniae in the pathogenesis of atherosclerosis, and antibiotics may theoretically be useful to prevent secondary vascular complications. Three groups of New Zealand White specific-pathogen-free rabbits, fed cholesterol-free chow, were inoculated via the nasopharynx on three occasions, 2 weeks apart, with C. pneumoniae. Group I (n = 23) rabbits were untreated; group II (n = 24) rabbits were treated with azithromycin at 30 mg/kg of body weight daily for 3 days and then once every 6 days, starting 5 days after first inoculation and continuing until sacrifice (early treatment); and group III (n = 24) rabbits were treated with the same dose of azithromycin but initiated 2 weeks after the last inoculation. All animals were sacrificed at 10 to 11 weeks after initial inoculation and examined for signs of atherosclerosis of the aorta. Eight (34.8%) untreated rabbits developed early signs of atherosclerosis, whereas only one (4.2%) in the early-treatment group had such signs (P = 0.02). However, eight rabbits (33.3%) of the delayed-treatment group had atherosclerotic changes of the aorta and no significant reduction compared to untreated rabbits. Early treatment of C. pneumoniae-infected rabbits with azithromycin was highly effective (87%) in preventing atherosclerotic changes, but delayed treatment was ineffective. It is possible that longer or more aggressive antibiotic treatment may be needed to reverse preformed lesions or that antibiotics may not be of value once lesions have formed.
Chlamydia pneumoniae, a respiratory pathogen in humans, has been associated with coronary heart and carotid artery diseases in many seroepidemiological studies (6, 17, 20, 21, 24, 28, 29, 32, 33). Moreover, the organism can be detected in fatty streaks and atheromatous plaques from the aorta and coronary, carotid, and femoral-popliteal arteries but not in normal vessels (1, 2, 4, 13–15, 31). Viable organisms have also been recovered from atheromas of carotid and coronary arteries (12, 18, 26).
Further evidence of the role of C. pneumoniae in atherogenesis is supported by animal models with rabbits reported by us and others (7, 16, 23). Recently, two small pilot clinical trials have suggested that newer macrolides (which are effective in vitro against C. pneumoniae) may decrease secondary cardiovascular events after myocardial infarction or unstable angina (9, 10).
It is important, however, to determine whether antimicrobial agents are of value in preventing or reversing early aortic lesions that are inducible in the rabbit model with C. pneumoniae. These studies may provide insight into and guidance on the potential dosages and duration of therapy for future clinical trials in humans.
MATERIALS AND METHODS
This study was approved by the Animal Care Committee of our institution, and guidelines for care of the animals were strictly adhered to.
Animals.
One-month-old, male, New Zealand White, specific-pathogen-free rabbits, fed standard chow (no cholesterol supplementation), were used in the experiments. Three groups of animals (24 per group) were inoculated via the nasopharynx with a small catheter on three separate occasions, 2 weeks apart, with C. pneumoniae. Group I rabbits were untreated (controls); group II received azithromycin at 30 mg/kg of body weight daily for 3 days and then once every 6 days, starting after the 5th day post-initial inoculation and continuing until sacrifice; and group III received the same dosage schedule of azithromycin but start 2 weeks after the last inoculation. Animals were sacrificed and aortas were examined for signs of atherosclerosis 10 to 11 weeks after initial inoculation.
C. pneumoniae strains and inoculum.
TWAR strain AR-39 (Seattle, Wash.) and ATCC strain VR 1310 (originally respiratory isolates) were used in the studies. The organisms were grown in HEp-2 cells (27). Infected cells were harvested with sterile glass beads and ultrasonic disruption after 72 h. Cell culture-grown organisms were partially purified by one cycle each of low- and high-speed centrifugation, resuspended in sucrose-phosphate-glutamic acid buffer, and frozen in 1.0-ml aliquots at −70°C. Inoculum preparations were adjusted to contain 1.5 × 107 to 2.6 × 107 inclusion-forming units of C. pneumoniae. Contamination by Chlamydia trachomatis, Chlamydia psittaci, or Mycoplasma species was excluded by analysis with PCR genus- and species-specific primers (26) and monoclonal antibody staining.
Serology.
Antibodies (immunoglobulin G [IgG]) to C. pneumoniae were measured by the microimmunofluorescence test (MRL Diagnostics, Cypress, Calif.). The IgG serum antibody fractions were measured by using fluorescein-isothiocyanate-conjugated goat anti-rabbit IgG (Jackson Laboratories, West Grove, Pa.). Blood was obtained from the earlobe marginal veins on the day of sacrifice. Our previous studies of 36 rabbits showed no detectable C. pneumoniae antibodies at baseline before inoculation. IgM and IgA antibodies were not tested because anti-rabbit IgM and IgA conjugates were not available commercially. The C. pneumoniae antibodies were tested in all three groups by screening at a 1:16 dilution against purified elementary bodies of C. pneumoniae (AR-39). All serum samples positive at 1:16 had their titers determined in twofold dilutions to end point.
Pathology.
At sacrifice, the entire aorta from the ascending aorta to the iliac bifurcation was removed and cleansed of any fat. The aorta was split longitudinally, and sections were taken from the arch and descending and abdominal aorta.
All specimens obtained were fixed in 10% buffered formalin, processed, and paraffin embedded. Staining with hematoxylin and eosin stain and an elastic stain (Movat’s pentachrome) was performed on sections for histological examination. Aortic lesions were graded histologically, modified from the work of Daley et al. (5), as follows: grade I, early fatty streaks, defined as consisting of foamy cells in the intima; grade II, advanced fatty streaks, defined as consisting of approximately equal numbers of foamy cells and spindle-shaped cells; grade III, spindle cell lesion, defined as consisting of spindle-shaped (smooth muscle) cells and other products; grade IV, advanced atheromatous lesion, defined as the presence of a core containing pools of extracellular lipid and/or necrotic debris and fibrous cap. Calcification of a fibromuscular lesion without a lipid core was also classified as grade IV. None of the infected rabbits developed the classic grade IV lesion with a lipid core, but rather they had fibromuscular lesions with calcification.
Myxoid lesions were defined as isolated thickening of the intima-media with ground substance or myxoid-like material which stained green with Movat’s stain but had no other features of atherosclerosis as defined. These lesions were usually associated with fragmentation and separation of the elastic fibers in the media. Periaortitis represented focal areas of accumulation of inflammatory mononuclear cells between the outer wall of the aorta and the adventitia. These cells consisted of macrophages and T and B lymphocytes, shown by immunohistochemical staining with specific monoclonal antibodies.
Immunohistochemical study.
Immunohistochemical staining (14) for C. pneumoniae antigen was performed on paraffin-embedded sections by the labelled strept avidin-biotin-peroxidase method (3) with the Histo-Stain kit of Zymed. The antibodies used included C. pneumoniae-specific monoclonal antibody RR-402 and Chlamydia genus-specific antibody CF-2 (Washington Research Foundation, Seattle), and a second C. pneumoniae-specific monoclonal antibody, Chlamydia Cel Pn (Cell Lab). In selected cases, assays with factor VIII and smooth muscle actin (DAKO, Carpinteria, Calif.) and T cells, B cells, and macrophage markers specific for rabbits (Serotec) were performed on adjacent sections to identify cells of endothelium, smooth muscle, T and B cells, and macrophages, respectively.
A negative control consisting of phosphate-buffered saline instead of primary antibodies was used in each run. Positive controls for C. pneumoniae included positive lung and spleen cells from our previous study (7), and cell pellets of the C. pneumoniae inoculum prepared in HEp-2 cells were fixed in formalin and embedded in paraffin.
Data analysis.
The prevalence of atherosclerotic lesions in the treated groups was compared to that in the untreated group by chi-square analysis or Fisher’s exact test.
RESULTS
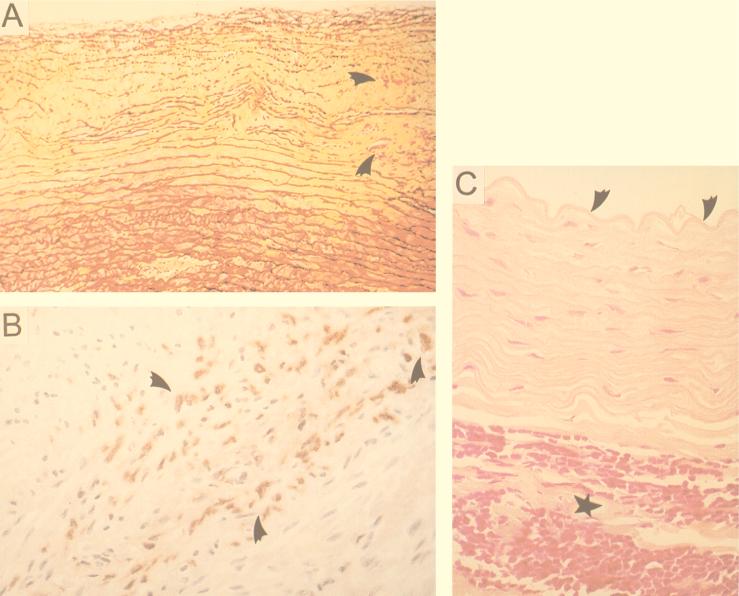
One of the control untreated animals was sacrificed early because of an accident and was not included in the analysis. Eight (34.8%) of the 23 untreated rabbits had early signs of patchy atherosclerosis (predominantly grade III lesions). Another five (21.7%) rabbits had myxoid-like material accumulating between the intima-media but no other changes suggestive of atherosclerosis. Eight (34.8%) rabbits demonstrated focal mononuclear cell infiltration in the adventitia of the abdominal aorta classified as periaortitis (Fig. 1).
FIG. 1.
(A) Myxoid ground substance accumulation and elastic fragmentation in intima-media (arrowheads indicate adjacent grade III lesion). Movat’s stain was used. (B) Grade III lesion with C. pneumoniae demonstrated by immunohistochemistry (area indicated by arrowheads). (C) Periaortitis, infiltration of aortic adventitia by lymphocytes and macrophages (indicated by star) and unremarkable intima (arrowheads indicate internal elastic lamina).
The effects of the azithromycin treatment on the various pathological entities are summarized in Table 1. Early treatment with azithromycin was highly effective in preventing early atherosclerotic lesions (87%) and periaortitis (100%) but had no effect on the myxoid-like changes. Delayed treatment was ineffective in reducing or reversing atherosclerotic lesions and myxoid-like changes but was highly effective in reducing periaortitis (100%). The results of the immunostaining for C. pneumoniae antigen are summarized in Table 2. Azithromycin did not decrease the prevalence of detectable antigen between the untreated controls and early-treated rabbits, but C. pneumoniae was detected more frequently in the delayed-treatment group than in the early-treatment group.
TABLE 1.
Effects of early and delayed treatment with azithromycin on aortic lesions
| Condition | No treatmenta(n = 23) | Early treatmenta(n = 24) | P | Delayed treatmenta(n = 24) | P |
|---|---|---|---|---|---|
| Atherosclerosis | 8 (34.8) | 1 (4.2) | 0.02 | 8 (33.3) | NSb |
| Myxoid-like change | 5 (21.7) | 8 (33.3) | NS | 4 (16.7) | NS |
| Periaortitis | 8 (34.8) | 0 | <0.02 | 0 | <0.02 |
Number of rabbits (percent) showing condition.
NS, not statistically significant (P > 0.05).
TABLE 2.
C. pneumoniae antigen detection after treatment with azithromycin
| Treatment group (n) | No. of aortic segments with C. pneumoniae antigen/total no. (%) | No. of segments (%) showing
degreea:
|
No. of animals (%) with C. pneumoniae antigen | |
|---|---|---|---|---|
| 1+ | 2+ | |||
| Control (23) | 11/69 (15.9) | 10 | 1 (9.1) | 8 (34.8) |
| Early azithromycin (24) | 14/72 (19.4) | 12 | 2 (14.3) | 9 (37.5) |
| Delayed azithromycin (24) | 45/72 (62.5) | 39 | 6 (13.3) | 21 (87.5) |
1+, 5 to 10 cells positive in one focus. 2+, two or more foci positive.
Serology.
All animals in the control untreated group had antibodies to C. pneumoniae at a >1:16 dilution (range, 1:32 to 1,024) and one (4.2%) of the animals in the early-treated group had positive serology of 1:16, but antibodies in the rest were undetectable (<1:16). Six (25%) of the animals in the delayed-treatment group had undetectable antibodies, but the remaining 18 (75%) had antibodies ranging from a 1:16 to a 1:256 dilution. None of the rabbits with undetectable antibodies had evidence of aortic lesions.
DISCUSSION
We have previously shown that C. pneumoniae infection in the rabbit can induce early lesions of atherosclerosis de novo, whereas these changes are not seen with sham-infected controls or Mycoplasma pneumoniae-infected rabbits (20, 29). This study clearly demonstrates that early treatment with azithromycin, 5 days after initial infection, was highly effective (87%) in preventing atherosclerosis-like lesions or periaortitis. The significance of the periaortitis and myxoid-like changes are not clear, and we cannot explain the reason why the myxoid lesions were not decreased with treatment but periaortitis was prevented. Myxoid or ground substance (glycosaminoglycan) accumulation is commonly seen in human atherosclerotic lesions (preatheroma and more advanced lesions) but may also be present in Marfan’s syndrome and vascular inflammation (30, 34). This is believed to be a response of smooth muscle cells to inflammatory stimuli, with increased production of glycosaminoglycan. Since we did not analyze the biochemical nature of our lesions, we can only assume that they are similar based on the histochemical staining (Movat’s stain). Delayed treatment (6 weeks after initial infection) was ineffective in reducing or reversing early atherosclerotic lesions but still effective (100%) in preventing periaortitis. Treatment with the macrolide did not significantly decrease the prevalence of C. pneumoniae antigen in the aorta. Detectable antigen in the aorta was more frequently localized to atherosclerosis-like lesions or areas of periaortitis but could be seen in areas without histological changes. Although not all lesions with atherosclerotic changes demonstrated C. pneumoniae antigen, this could be related to sampling at a specific microscopy level. However, we could not determine whether there were viable organisms, as C. pneumoniae is extremely difficult to recover by culture from rabbits and can be recovered only from the respiratory tract within 2 to 3 days of inoculation (22).
The results of the C. pneumoniae serology assay showed that early treatment was largely effective in abrogating the antibody response (and was correlated with lack of aortic lesions), compared to delayed treatment. This would suggest that the antigens detectable in the early-treated group represent nonviable bacteria or existence in a latent stage without stimulation of the immune system.
In a study of different design, Muhlestein et al. (23) were able to show that azithromycin at 30 mg/kg intramuscularly daily for 1 week and then twice weekly for another 6 weeks (starting 3 days after final triple inoculation of C. pneumoniae) was able to reverse the enhanced intimal thickening of the aortas in the rabbits fed a 0.25% cholesterol-enriched chow. Our study did not use a cholesterol-enriched diet and therefore, it looked at the de novo effect of C. pneumoniae infection on induction of early atherosclerosis. Moreover, in our study the animals were treated by oral gavage with less frequent dosing.
Azithromycin, a newer macrolide antibiotic of the azalide subclass, has in vitro activity against C. pneumoniae similar to that of erythromycin with a MIC against 90% of strains of 0.125 to 0.25 μg/ml (11). Although the absolute bioavailability is only 37%, this drug penetrates intracellularly in a wide variety of tissues (including macrophages where C. pneumoniae may reside), with an intracellular concentration of 200 to 500 times the levels in plasma or extracellular levels (25). The dosage used in our study was designed to mimic the human dose of 500 mg once a week being used in an ongoing clinical trial (WIZARD study). Azithromycin has a long terminal half-life of 63 h in humans, allowing once-a-week dosing, and a slightly shorter half-life in rabbits (24a). It is possible that with more frequent dosing and longer therapy we may have greater efficacy with the delayed treatment. But it is also possible that we may not be able to reverse preformed atherosclerotic lesions, as we previously showed that grade I to III lesions can be formed within a month of C. pneumoniae infection in rabbits (7). Since in the delayed-treatment group the rabbits received at least 4 to 5 weeks of therapy without benefit, and the normal lifespan of a rabbit is 7 to 8 years, 10-fold less than that of humans, this would suggest that short-term therapy in humans may not be effective and that clinical trials should use a longer duration, of more than 40 to 50 weeks of treatment, to assess the clinical benefit of azithromycin in vascular complications of atherosclerosis.
ACKNOWLEDGMENTS
This study was funded by a grant from Pfizer Inc.
We are grateful to D. Bajhan for her assistance in preparing the manuscript.
REFERENCES
- 1.Blasi F, Derti F, Erba M, Cosentini R, Raccarelli R, Rinaldi A, Fagetti L, Exposito G, Ruberti U, Allegra L. Detection of Chlamydia pneumoniae but not Helicobacter pylori in atherosclerotic plaques of aortic aneurysms. J Clin Microbiol. 1996;34:2766–2769. doi: 10.1128/jcm.34.11.2766-2769.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell L A, O’Brien E R, Cappuccio A L, Kuo C C, Wang S P, Stewart D, Grayston J T. Detection of Chlamydia pneumoniae TWAR in human coronary atherectomy tissues. J Infect Dis. 1995;172:585–588. doi: 10.1093/infdis/172.2.585. [DOI] [PubMed] [Google Scholar]
- 3.Cartun R W, Pedersen C A. An immunocytochemical technique offering increased sensitivity and lowered costs with a streptavidin horseradish peroxidase conjugate. J Histotechnol. 1989;12:P273–P277. [Google Scholar]
- 4.Chiu B, Viira E, Tucker W, Fong I W. Chlamydia pneumoniae, cytomegalovirus and herpes simplex virus in atherosclerosis of the carotid artery. Circulation. 1997;96:2144–2148. doi: 10.1161/01.cir.96.7.2144. [DOI] [PubMed] [Google Scholar]
- 5.Daley S J, Klemp K F, Guyton J R, Rogers K A. Cholesterol-fed and casein-fed rabbit models of atherosclerosis (part 2) Arterioscler Thromb. 1994;14:105–114. doi: 10.1161/01.atv.14.1.105. [DOI] [PubMed] [Google Scholar]
- 6.Danesh J, Collins R, Petro R. Chronic infections and coronary heart disease: is there a link? Lancet. 1997;350:430–436. doi: 10.1016/S0140-6736(97)03079-1. [DOI] [PubMed] [Google Scholar]
- 7.Fong I W, Chiu B, Viira E, Fong M W, Jang D, Mahony J. Rabbit model for Chlamydia pneumoniae infection. J Clin Microbiol. 1997;35:58–62. doi: 10.1128/jcm.35.1.48-52.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fong I W, Chiu B, Viira E, Jang D, Mahony J B. Abstracts of the 98th General Meeting of the American Society for Microbiology. Washington, D.C.: American Society for Microbiology; 1998. Atherosclerosis can be induced by Chlamydia pneumoniae in rabbits, abstr. B-439; p. 129. [Google Scholar]
- 9.Gupta S, Leatham E W, Carrington D, Mendall M A, Kaski J C, Camm A J. Elevated Chlamydia pneumoniae antibodies, cardiovascular events and azithromycin in male survivors of myocardial infarction. Circulation. 1997;96:404–407. doi: 10.1161/01.cir.96.2.404. [DOI] [PubMed] [Google Scholar]
- 10.Gurfinkel E, Bozovich G, Daroca A, Beck E, Mautner B for the ROXIS Study Group. Randomised trial of roxithromycin in non-Q- wave coronary syndromes: ROXIS Pilot Study. Lancet. 1997;350:404–407. doi: 10.1016/s0140-6736(97)07201-2. [DOI] [PubMed] [Google Scholar]
- 11.Hammerschlag M R, Qumei K K, Roblin P M. In vitro activities of azithromycin, clarithromycin, l-ofloxacin, and other antibiotics against Chlamydia pneumoniae. Antimicrob Agents Chemother. 1992;36:1573–1574. doi: 10.1128/aac.36.7.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson L A, Lee A, Campbell L A, Kuo C C, Rodriguez D I, Grayston J T. Isolation of Chlamydia pneumoniae (TWAR) from carotid atherosclerotic plaque specimen obtained by endarterectomy. J Infect Dis. 1997;176:292–295. doi: 10.1086/517270. [DOI] [PubMed] [Google Scholar]
- 13.Kuo C C, Coulson A S, Campbell L A, Cappuccio A L, Lawrence R D, Wang S P, Grayston J T. Detection of Chlamydia pneumoniae in atherosclerotic plaques in the walls of arteries of lower extremities from patients undergoing bypass operation for arterial obstruction. J Vasc Surg. 1997;26:29–31. doi: 10.1016/s0741-5214(97)70143-5. [DOI] [PubMed] [Google Scholar]
- 14.Kuo C C, Gown A M, Benditt E P, Grayston J T. Detection of Chlamydia pneumoniae in aortic lesions of atherosclerosis by immunocytochemical stain. Arterioscler Thromb. 1993;13:1500–1504. doi: 10.1161/01.atv.13.10.1501. [DOI] [PubMed] [Google Scholar]
- 15.Kuo C C, Shor A, Campbell L A, Fukushi H, Patton D L, Grayston J T. Demonstration of Chlamydia pneumoniae in atherosclerotic lesions of coronary arteries. J Infect Dis. 1993;167:841–849. doi: 10.1093/infdis/167.4.841. [DOI] [PubMed] [Google Scholar]
- 16.Laitinen K, Laurila A, Pyhala L, Leinonen M, Saikku P. Chlamydia pneumoniae infection induces inflammatory changes in the aortas of rabbits. Infect Immun. 1997;65:4832–4835. doi: 10.1128/iai.65.11.4832-4835.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linnanmaki E, Leinonen M, Matilla K, Nieminen M S, Valtonen V, Saikku P. Chlamydia pneumoniae specific circulating immune complexes in patients with chronic coronary heart disease. Circulation. 1993;87:1130–1134. doi: 10.1161/01.cir.87.4.1130. [DOI] [PubMed] [Google Scholar]
- 18.Maas M, Bartels C, Engel P M, Momat U, Sievers H H. Endovascular presence of viable Chlamydia pneumoniae is a common phenomenon in coronary artery disease. J Am Coll Cardiol. 1998;31:827–832. doi: 10.1016/s0735-1097(98)00016-3. [DOI] [PubMed] [Google Scholar]
- 19.Mahony J B, Luinstra K E, Chernesky M A. Diagnosis of Chlamydia trachomatis and Chlamydia pneumoniae respiratory tract infections by multiplex PCR. In: Ofila J, et al., editors. Chlamydial infections. Bologna, Italy: Societa Editrice Esculapio; 1994. pp. 370–373. [Google Scholar]
- 20.Melnick S, Shakar E, Folsom A, Grayston J T, Sonlie P, Wang S P, Szklo M. Past infection by Chlamydia pneumoniae strain TWAR and asymptomatic carotid atherosclerosis. Am J Med. 1993;95:499–504. doi: 10.1016/0002-9343(93)90332-j. [DOI] [PubMed] [Google Scholar]
- 21.Mendall M A, Carrington D, Strachan D, Patel P, Molineaux N, Levy J, Toosey T, Camm A J, Northfield T C. Chlamydia pneumoniae: risk factor for seropositivity and association with coronary artery disease. J Infect. 1995;30:121–128. doi: 10.1016/s0163-4453(95)80006-9. [DOI] [PubMed] [Google Scholar]
- 22.Moazed T C, Kuo C-C, Patton D L, Grayston J T, Campbell L A. Experimental rabbit models of Chlamydia pneumoniae infection. Am J Pathol. 1996;148:667–676. [PMC free article] [PubMed] [Google Scholar]
- 23.Muhlestein J B, Anderson J L, Hammond E H, Zhao L, Trehan S, Schwobe E P, Carlquist J F. Infection with Chlamydia pneumoniae accelerates the development of atherosclerosis and treatment with azithromycin prevents it in a rabbit model. Circulation. 1998;97:633–636. doi: 10.1161/01.cir.97.7.633. [DOI] [PubMed] [Google Scholar]
- 24.Patel P, Mendall M A, Carrington D, Strachan D P, Leatham E, Molineaux N, Levy J, Blakeston C, Seymour C A, Camm A J, Northfield T C. Association of Helicobacter pylori and Chlamydia pneumoniae infections with coronary heart disease and cardiovascular risk factor. BMJ. 1995;311:711–714. doi: 10.1136/bmj.311.7007.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24a.Pfizer Inc. In-house data. Personal communication.
- 25.Rakita R M. Intracellular activity, potential clinical uses of antibiotic. ASM News. 1998;64:570–575. [Google Scholar]
- 26.Ramirez J the Chlamydia pneumoniae/Atherosclerotic Study Group. Isolation of Chlamydia pneumoniae from the coronary artery of a patient with atherosclerosis. Ann Intern Med. 1996;125:979–982. doi: 10.7326/0003-4819-125-12-199612150-00008. [DOI] [PubMed] [Google Scholar]
- 27.Roblin P M, Dumornay W, Hammerschlag M R. Use of HEp-2 cells for improved isolation and passage of Chlamydia pneumoniae. J Clin Microbiol. 1992;30:1968–1971. doi: 10.1128/jcm.30.8.1968-1971.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saikku P, Leinonen M, Tenkanen L, Linnanmaki E, Ekman M R, Manninen V, Manttari M, Frick M H, Huttunen J K. Chronic Chlamydia pneumoniae infection as a risk factor for coronary heart disease in the Helsinki heart study. Ann Intern Med. 1992;116:273–278. doi: 10.7326/0003-4819-116-4-273. [DOI] [PubMed] [Google Scholar]
- 29.Saikku P, Matilla K, Nieminen M S, Makela P H, Huttunen J K, Valtonen V. Serological evidence of an association of a novel chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet. 1988;ii:983–986. doi: 10.1016/s0140-6736(88)90741-6. [DOI] [PubMed] [Google Scholar]
- 30.Salisbury B G J, Hajjar D P, Minick C R. Altered glycosaminoglycan metabolism in injured arterial wall. Exp Mol Pathol. 1985;42:306–319. doi: 10.1016/0014-4800(85)90081-4. [DOI] [PubMed] [Google Scholar]
- 31.Shor A, Kuo C C, Patton D L. Detection of Chlamydia pneumoniae in coronary artery fatty streaks and atheromatous plaques. S Afr Med J. 1992;82:158–161. [PubMed] [Google Scholar]
- 32.Thom D H, Grayston J T, Siscovick D S, Wang S P, Weiss N S, Daling J R. Association of prior infection with Chlamydia pneumoniae and angiographically demonstrated coronary artery disease. JAMA. 1992;268:68–72. [PubMed] [Google Scholar]
- 33.Thom D H, Wang S-P, Grayston J T, Siscovick D S, Stewart D K, Kronmal R A, Weiss N S. Chlamydia pneumoniae strain TWAR antibody and angiographically demonstrated coronary artery disease. Arterioscler Thromb. 1991;11:547–551. doi: 10.1161/01.atv.11.3.547. [DOI] [PubMed] [Google Scholar]
- 34.Wasty F, Alavi M Z, Moore S. Distribution of glycosaminoglycans in the intima of human aortas: changes in atherosclerosis and diabetes mellitus. Diabetologia. 1993;36:316–322. doi: 10.1007/BF00400234. [DOI] [PubMed] [Google Scholar]