Abstract
Background & Aims
People with primary sclerosing cholangitis (PSC) have a variable and often progressive disease course that is associated with biliary and parenchymal changes. These changes are typically assessed by magnetic resonance imaging (MRI), including qualitative assessment of magnetic resonance cholangiopancreatography (MRCP). Our aim was to study the association of novel objective quantitative MRCP metrics with prognostic scores and patient outcomes.
Methods
We performed a retrospective study including 77 individuals with large-duct PSC with baseline MRCP images, which were postprocessed to obtain quantitative measures of bile ducts using MRCP+™. The participants’ ANALI scores, liver stiffness by vibration-controlled transient elastography, and biochemical indices were collected at baseline. Adverse outcome-free survival was measured as the absence of decompensated cirrhosis, liver transplantation (LT), or liver-related death over a 12-year period. The prognostic value of MRCP+-derived metrics was assessed by Cox regression modelling.
Results
During a total of 386 patients-years, 16 cases of decompensation, 2 LTs, and 5 liver-related deaths were recorded. At baseline, around 50% of the patients were classified as being at risk of developing disease complications. MRCP+ metrics, particularly those describing the severity of bile duct dilatations, were correlated with all prognostic factors. Univariate analysis showed that MRCP+ metrics representing duct diameter, dilatations, and the percentage of ducts with strictures and/or dilatations were associated with survival. In a multivariable-adjusted analysis, the median duct diameter was significantly associated with survival (hazard ratio 10.9, 95% CI 1.3–90.3).
Conclusions
MRCP+ metrics in people with PSC correlate with biochemical, elastographic, and radiological prognostic scores and are predictive of adverse outcome-free survival.
Lay summary
In this study, we assessed in people with primary sclerosing cholangitis (PSC) the association of novel objective quantitative MRCP metrics automatically provided by a software tool (MRCP+) with prognostic scores and patient outcomes. We observed that MRCP+ metrics in people with PSC correlate with biochemical, elastographic, and radiological prognostic scores and are predictive of adverse outcome-free survival.
Keywords: MRCP+, Prognosis, Magnetic resonance imaging, Liver stiffness, Cholestasis
Abbreviations: 3D, 3-dimensional; ERCP, endoscopic retrograde cholangiopancreatography; HR, hazard ratio; IBD, inflammatory bowel disease; IHBD, intrahepatic bile duct; LSM, liver stiffness measurement; LT, liver transplantation; MR, magnetic resonance; MRCP, magnetic resonance cholangiopancreatography; MRI, magnetic resonance imaging; PSC, primary sclerosing cholangitis; VCTE, vibration-controlled transient elastography
Graphical abstract
Highlights
-
•
The association of quantitative MRCP+ biliary metrics with PSC severity and prognosis is still unknown.
-
•
MRCP+ biliary metrics in PSC are correlated with prognostic factors (LSM, Mayo score, AOM, and MRI scores).
-
•
MRCP+ biliary metrics are independently associated with prognosis in PSC.
Introduction
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease of unknown aetiology characterised by inflammation and fibrosis of intrahepatic bile ducts (IHBDs) and/or extrahepatic bile ducts leading to the formation of multifocal strictures and bile duct dilatations.1 Although a rare condition, PSC’s progressive nature, which leads to eventual cirrhosis and associated complications, represents a significant cause of morbidity and mortality.2 With no available pharmacotherapeutic treatment to halt disease progression, liver transplantation (LT) currently represents the only effective therapy to extend survival in people with PSC. PSC is strongly linked (70–75% of patients) with inflammatory bowel disease (IBD) and is associated with an increased risk of hepatobiliary and colorectal cancers, particularly in people with concomitant IBD.2,3
Magnetic resonance cholangiopancreatography (MRCP) is currently the recommended imaging modality to diagnose PSC[4], [5], [6] and is also widely applied during follow-up of these patients for monitoring disease progression and the development of hepatobiliary cancers.4 Its regular use represents a unique opportunity for monitoring biliary and parenchymal changes during patient follow-up, which may predict the course of the disease. Within our group, we have observed that MRI changes in PSC are associated with radiological progression7 and prognosis.8,9 Furthermore, other groups reported evidence in favour of the use of MRI for prognostic purposes in PSC, focusing on the intrahepatic and extrahepatic biliary findings10 or parenchymal consequence of the disease.11 Previously, cholangiographic findings assessed by endoscopic retrograde cholangiopancreatography (ERCP) were found to be strongly associated with prognosis in these patients.12,13 However, because of its invasive nature, the use of ERCP is limited to therapeutic intervention and for ductal sampling of suspected malignant strictures.5
Currently, a limiting factor of MRCP is that interpreting the images relies upon qualitative assessment and is, as a result, highly subjective. Because of the heterogeneity of bile duct alterations observed in PSC, the adoption of quantitative rather than qualitative models for describing bile duct and parenchymal changes is crucial to improve diagnostic performance and to allow prospective assessment of disease evolution. Recently, imaging processing software capable of generating a 3-dimensional (3D) model of the biliary tree to simultaneously provide quantitative measures of biliary tree and gallbladder volume and assess the presence and severity of bile duct strictures and dilatations has been introduced.14 In a recent study involving 20 healthy volunteers, 10 individuals with biliary diseases (6 individuals with PSC), and 10 persons with non-biliary liver diseases, this novel software (quantitative MRCP, or MRCP+) provided accurate, repeatable, and reproducible measurement of the biliary tree. Certain metrics were also found to be sensitive to differences across duct width, highlighting quantitative metrics with particular relevance for capturing the features of strictures and dilatation associated with people with biliary disease.14 Very recently, 2 studies conducted in 65 and 76 adult persons with PSC found that MRCP+ metrics were significantly associated with biochemical prognostic scores and liver stiffness measurement (LSM) and suggested a role for refining risk stratification.15,16 MRCP+ metrics have also shown utility in characterising disease, stratification, and monitoring in paediatric patients17,18; however, at present, there are no studies that have investigated the correlation between MRCP+ and clinical outcomes in PSC. Therefore, here, we aimed to assess the role of MRCP+ for detecting biliary changes in a large retrospective cohort of people with PSC and to explore their association with disease severity and clinical outcome.
Patients and methods
This was a retrospective analysis using data collected from a longitudinal study that conformed to the ethical guidelines of the 1975 Declaration of Helsinki and had local ethical approval from the committee of Saint-Antoine Hospital, Paris. Informed consent was waived for retrospective analysis of imaging characteristics.
Inclusion criteria were as follows: diagnosis of large-duct PSC and 1 baseline liver MRI with MRCP (including T1- and T2-weighted magnetic resonance [MR] images and 3D-MRCP) available for MRCP+ postprocessing analysis. Diagnosis of large-duct PSC was defined by the association of chronic cholestasis, typical features of MRCP, and no cause of secondary sclerosing cholangitis.19 The liver MRI acquired closest in time to the diagnosis of PSC was considered as the baseline MRI, and the date of this MRI was defined as the date of inclusion. A hundred patients were planned to be included in this study, selected among 124 patients of a previous study8 on the basis of their disease severity assessed using ANALI score without gadolinium ≤2 or >2 in a 1:1 ratio. Exclusion criteria included the following: age at inclusion <18 years, absence of typical features of PSC on MRCP, small-duct PSC, PSC-autoimmune hepatitis variant, previous LT, previous choledocojejunostomy, or hepatic comorbidities, secondary sclerosing cholangitis, cholangiocarcinoma, hepatocellular carcinoma, and decompensated cirrhosis or extrahepatic cholestasis caused by a dominant stenosis of extrahepatic bile ducts at the time of inclusion.
MRI
MRI was performed according to the protocol for 3D-MRCP previously described9 and in line with the indications provided in the position statement of the International PSC Study Group.4 Two expert radiologists, blinded to clinical information, reviewed all MR images in consensus at Saint-Antoine Hospital in Paris and calculated the 2 ANALI scores.9
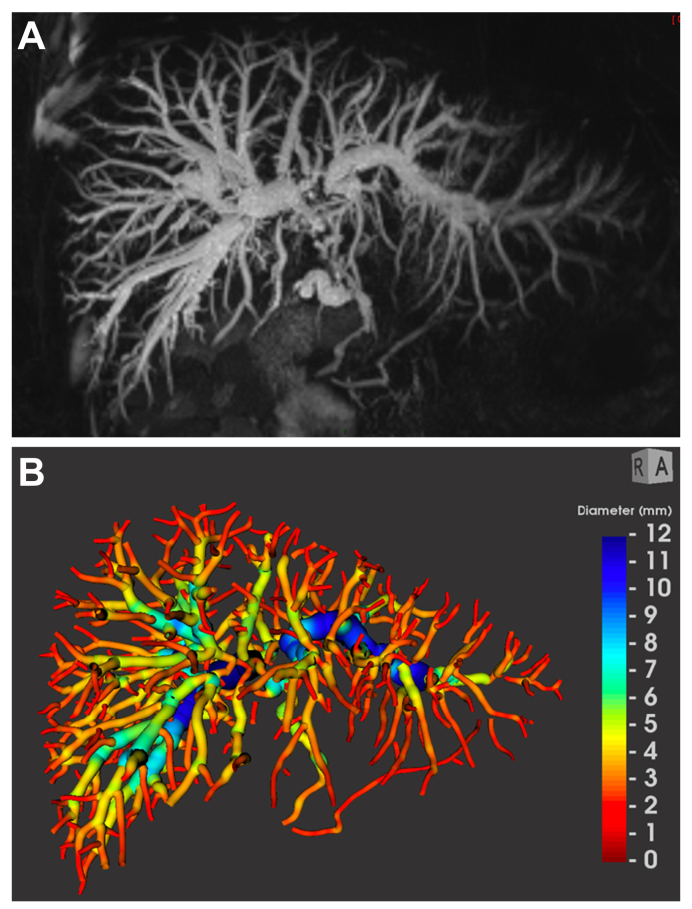
Using MRCP+, a proprietary MRI postprocessing software tool (Perspectum Ltd., Oxford, UK), 3D T2-weighted MRCP images from the research MRI examinations were processed to generate a quantitative model of the biliary ducts and cross-sectional diameters identifying candidate strictures and dilatations, as extensively reported by Goldfinger et al.14 (Fig. 1). The gallbladder has been automatically segmented and its volume provided separately. The cystic and pancreatic ducts were excluded when calculating the biliary tree volume. All the metrics assessed automatically by the software, for the purpose of this study, were derived from the whole biliary tree, and their definitions are available in the Supplementary information (Table S1).
Fig. 1.
MRCP and MRCP+ of a 36-year-old man with intrahepatic and extrahepatic PSC.
(A) Maximum intensity projection image and (B) the corresponding parametric biliary 3D-tree model generated from MRCP+, colour coded according to duct diameter. The gallbladder has been automatically segmented and its volume provided separately. The cystic and pancreatic ducts were excluded. MRCP, magnetic resonance cholangiopancreatography; PSC, primary sclerosing cholangitis.
Clinical data collection
Clinical and biological data were retrospectively collected from patients’ records and included the following: date of PSC diagnosis, association with IBD, the closest biochemical analysis to the date of inclusion (±3 months), LSM by vibration-controlled transient elastography (VCTE) at inclusion (±6 months), and clinical events that occurred after inclusion (LT, death and cause of death, and cirrhosis decompensation defined as the occurrence of variceal bleeding, ascites, hepatic encephalopathy, or hepatorenal syndrome). The revised Mayo risk and Amsterdam–Oxford risk scores were also calculated for each included patient with available data.20,21
Statistical analysis
Continuous variables were expressed as median and IQR, whereas categorical variables were reported as number and percentage. Correlations between clinical markers and radiological markers were examined using exploratory analysis. The Spearman's rank correlation coefficient was calculated. Groupwise mean differences in MRCP+ metrics were examined using the independent-samples t test or 1-way ANOVA where appropriate, presented using uncorrected p values. To assess the prognostic value of MRCP+-derived metrics, we considered as endpoints adverse outcome-free survival, which was defined as the time elapsed from the date of inclusion to the last visit or occurrence of an adverse event defined as liver-related death, LT, or cirrhosis decompensation. A binary logistic c-statistic analysis including Youden’s index calculation was applied to determine the optimal threshold of MRCP+-derived metrics that best predicted the adverse outcomes in a univariate analysis. The prognostic variables and their respective weight on the rates of survival without adverse outcomes were determined using univariable- and multivariable-adjusted Cox backward stepwise regression analysis. Survival rates were calculated based on the Kaplan–Meier estimates. Statistical analysis was performed using IBM SPSS Statistics v.26.R (IBM; Armonk, NY, USA).
Results
General characteristics
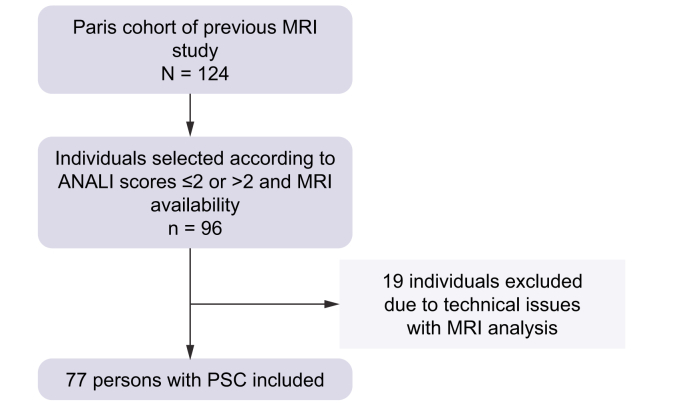
Of the 124 patients followed up at the Reference Centre for Inflammatory Biliary Diseases and Autoimmune Hepatitis of Saint-Antoine Hospital in Paris and included in a previous study,8 100 patients were selected according to the above-mentioned criteria, and 96 patients were considered eligible for this study; 4 cases were excluded owing to the lack of high-quality digitalised MRI images to be used with MRCP+ at the time of inclusion. After excluding further 19 cases owing to technical issues (4 owing to low data quality [e.g. motion artefact and gastrointestinal contamination] and 15 owing to either missing 3D-MRCP series, inconsistent slice orientation, or inappropriate native resolution [1 voxel dimension more than double that of the others]), which made the images unquantifiable using the MRCP+ software, 77 patients were included in the final analyses as shown in Fig. 2.
Fig. 2.
Flowchart of the study.
MRI, magnetic resonance imaging; PSC, primary sclerosing cholangitis.
Of the 77 persons with PSC included in the final analysis, 52 (67.5%) were male, and median age at PSC diagnosis was 34 (21–43) years. The association with IBD was documented in 58 (75.3%) patients, of whom 31 (53.4%) had associated ulcerative colitis, 23 (39.7%) Crohn disease, and 4 (6.9%) indeterminate colitis. Biochemical indices, LSM, and distributions according to the ANALI scores at the time of inclusion are reported in Table 1. At inclusion, approximately 50% of patients were classified as being at risk of developing disease complications according to biochemical, radiological, and elastographic prognostic factors, as shown in Table 2.
Table 1.
Characteristics of people with PSC at the time of inclusion.
| Characteristic | Value, n (%) |
|---|---|
| Male sex | 52 (67.5) 0 (0%) |
| Age at inclusion, years | 35 (25–49) 0 (0%) |
| Type of PSC | |
| Intrahepatic only | 24 (31.2) |
| Intrahepatic and extrahepatic | 53 (68.8) 0 (0%) |
| IBD | 58 (75.3) |
| Ulcerative colitis | 31 (53.4) |
| Crohn disease | 23 (39.7) |
| Indeterminate | 4 (6.9) 0 (0%) |
| Total bilirubin (μmol/L) | 15.0 (10.9–20.0) 0 (0%) |
| AST (×ULN) | 1.1 (0.7–2.2) 2 (2.7%) |
| ALP (×ULN) | 1.4 (0.7–2.7) 2 (2.7%) |
| GGT (×ULN) | 2.3 (0.7–6.6) 2 (2.7%) |
| Serum albumin (g/L) | 41.5(38.2–44.2) 6 (8.5%) |
| PLT (×109/L) | 275 (209–339) 2 (2.6%) |
| Liver stiffness (kPa) | 9.5 (6.7–12.2) 0 (0%) |
| ANALI score without gadolinium | |
| 0 | 17 (22.1) |
| 1 | 12 (15.6) |
| 2 | 6 (7.8) |
| 3 | 14 (18.2) |
| 4 | 17 (22.1) |
| 5 | 11 (14.3) 0 (0%) |
| ANALI score with gadolinium | |
| 0 | 22 (34.4) |
| 1 | 5 (7.8) |
| 2 | 37 (57.8) 13 (17%) |
Quantitative variables are expressed as medians (IQR). Nominal variables are expressed as absolute number (percentage). Italic indicates missing data within the cohort and are expressed as absolute number (percentage).
ALP, alkaline phosphatase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transpeptidase; IBD, inflammatory bowel disease; PLT, platelet count; PSC, primary sclerosing cholangitis; ULN, upper limit of normal.
Table 2.
Distribution of people with PSC according to prognostic factors.
| Prognostic factor | n (%) |
|---|---|
| ANALI score without gadolinium >2 | 42 (54.5) |
| ANALI score with gadolinium >1 | 37 (48.1) |
| Liver stiffness >9.6 kPa | 38 (49.4) |
| Amsterdam–Oxford risk score | |
| Low risk | 7 (22.6) |
| Intermediate risk | 7 (22.6) |
| Intermediate–moderate risk | 10 (32.3) |
| Severe risk | 7 (22.6) |
| Revised Mayo risk score | −0.39 (−1.0 to 0.5) |
ANALI score with gadolinium was available in 64 (83%) patients, Amsterdam–Oxford risk score in 31 (40.3%) patients, and the revised Mayo score in 66 (85.7%) patients. Values are expressed as number (percentage) except for Revised Mayo risk score which is expressed as median (interquartile range).
PSC, primary sclerosing cholangitis.
Follow-up and clinical events
A total of 386 patient-years were available in the entire cohort. Among the 77 patients, 24 (31%) experienced at least 1 adverse outcome in an average of 4.9 ± 2.9 years (range 1–12 between 2006 and 2018) after inclusion. The adverse outcomes included the following: 16 cirrhosis decompensations, 3 LTs (for end-stage liver disease), and 5 liver-related deaths (4 from cholangiocarcinoma and 1 from acute cholangitis complicated by septic shock).
Correlations between MRCP+-derived metrics and prognostic indices at baseline
The distribution of patients according to MRCP+-derived metrics is detailed in Table S2. Exploratory analysis assessing the correlations between different MRCP+ metrics are reported in Table S3 and showed, as expected, several correlations between MRCP+ metrics describing strictures and dilatations. Exploratory analysis examining the correlations among biochemical indices, LSM, ANALI scores, and composite scores with all MRCP+-derived metrics are shown in Table S4. The percentages of normal bile ducts, defined as ducts with median diameters 1–3 mm, was inversely correlated with cholestatic indices, LSM, ANALI scores, and Mayo risk score (R between −0.38 and −0.58, all p <0.01), while positively correlating with albumin (R = 0.27, p = 0.02). Conversely, the percentages of ducts with median diameters between 7–9 and >9 mm, indicating severe IHBD dilatations, were positively correlated with cholestatic indices, Mayo risk score, the Amsterdam–Oxford model, and LSM (0.25 ≤ R ≤ 0.46, all p <0.003) and negatively correlated with serum albumin (R = −0.34, p <0.01). Accordingly, we observed that MRCP+ metrics quantifying absolute, sum, and relative dilatation severity (i.e. maximum dilatation severity, mean absolute dilatation severity, and maximum absolute dilatation severity) were positively correlated with cholestatic indices, ANALI scores, LSM, Mayo risk score, and the Amsterdam–Oxford model (0.23 ≤ R ≤ 0.57, all p <0.01). Finally, the percentage of ducts with strictures or dilatations and the percentage of tree centreline that was abnormal were positively correlated with cholestatic indices, LSM, ANALI scores, and Mayo risk score (0.24 ≤ R ≤ 0.48, all p <0.01). No significant correlations were observed between biliary volume or gallbladder volume and biochemical indices or LSM.
Distribution of people with MRCP-derived metrics according ANALI score without gadolinium
Exploratory analysis of the distribution of MRCP+-derived metrics according to the prognostic categories as defined by the ANALI score without gadolinium >2 are shown in Table S5. Findings of note revealed that the number of modelled ducts detected by the MRCP+ software was higher in persons with ANALI score without gadolinium >2. These patients (without gadolinium >2) were characterised by the following: a higher number of strictures (p <0.01), which were more severe (p = 0.03) and longer (p <0.01); a higher number of dilatations (p <0.01), which were also more severe (p <0.01); and a higher number of ducts with strictures and/or dilatations (p <0.01). Additionally, the sum of ducts with an abnormal length was higher in persons with ANALI score without gadolinium >2 (p <0.01).
Individual prognostic performance of MRCP-derived metrics
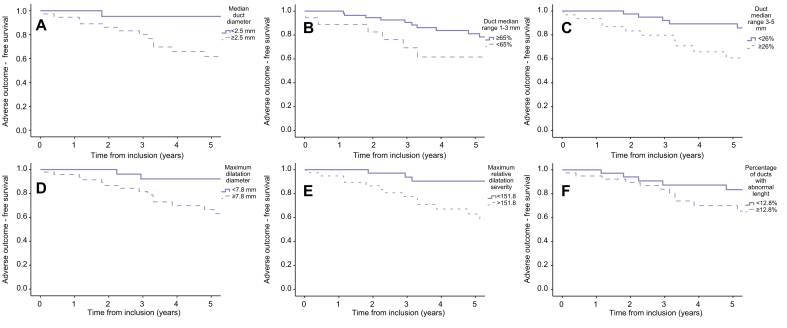
In a univariate analysis, median duct diameter (hazard ratio [HR] 17.79, 95% CI 2.37–133.80), the percentage of ducts with median diameter 3–5 mm (HR 5.23, 95% CI 1.03–13.42), the maximum dilatation diameter (HR 5.23, 95% CI 2.03–13.43), the maximum absolute (HR 3.19, 95% CI 1.23–8.23) and relative dilatation severity (HR 4.70, 95% CI 1.74–12.70), the percentage of ducts with strictures or dilatations (HR 3.14, 95% CI 1.07–9.24), and the percentage of ducts with abnormal duct lengths (HR 3.39, 95% CI 1.33–8.65) were significantly associated with the occurrence of adverse outcomes (Fig. 3). However, the percentage of ducts with median diameter 1–3 mm (HR 2.53, 95% CI 1.09–5.86) was inversely associated with the occurrence of adverse outcomes. The cut-off values with the highest total sensitivity and specificity of these MRCP-derived metrics are summarised in Table 3.
Fig. 3.
Adverse outcome-free survival according to different MRCP+-derived biliary metrics.
(A) Kaplan–Meier curves for adverse outcome-free survival according to median duct diameter, (B) percentage of duct with median range between 1 and 3 mm, (C) percentage of duct with median range between 3 and 5 mm, (D) maximum dilatation diameter, (E) maximum relative dilatation severity, and (F) percentage of ducts with abnormal length.
Table 3.
Performance of MRCP-derived metrics for the prediction of adverse outcomes.
| AUROC (95% CI) | Cut-off | SE (%) | SP (%) | |
|---|---|---|---|---|
| Median diameter | 0.787 (0.684–0.890) p <0.001 | 2.5 mm | 79.2 | 68.9 |
| Duct median range 1–3 mm | 0.219 (0.112–0.325) p <0.001 | 65% | 62.5 | 17.0 |
| Duct median range 3–5 mm | 0.783 (0.679–0.886) p <0.001 | 26% | 75.0 | 72.7 |
| Max dilatation diameter | 0.652 (0.528–0.776) p = 0.034 | 7.8 mm | 83.3 | 47.2 |
| Max absolute dilatation severity | 0.649 (0.524–0.774) p = 0.037 | 4.1 mm | 70.8 | 62.3 |
| Max relative dilatation severity | 0.688 (0.564–0.812) p = 0.009 | 151.8 | 79.2 | 62.3 |
| Percentage of ducts with strictures or dilatations | 0.666 (0.540–0.792) p = 0.020 | 20.9 | 83.3 | 49.1 |
| Percentage of abnormal duct length | 0.684 (0.557–0.811) p = 0.01 | 12.8 | 75.0 | 66.5 |
ROC analysis was conducted. AUROC, area under the receiver operating curve; MRCP, magnetic resonance cholangiopancreatography; SE, sensitivity; SP, specificity.
In a multivariate Cox regression model, including all the significant variables at univariate analysis except the percentage of ducts with median diameter 1–3 mm and the maximum absolute dilatation severity caused by collinearity, the median duct diameter was independently associated with adverse outcome-free survival (HR 10.93, 95% CI 1.32–90.32, p = 0.027), and a trend toward a significant association was observed between maximum relative dilatation severity and survival (p = 0.053).
Discussion
In this study of 77 persons with PSC, we evaluated the association of radiological findings assessed using an MRI postprocessing software tool that gives an objective, quantitative, and continuous model of the biliary ducts and cross-sectional diameters with clinical severity and prognosis. Results revealed that several metrics, and in particular the severity of bile duct dilatations, are directly and significantly correlated with the cholestatic indices of the disease. Moreover, the median diameter of ducts, measured using MRCP+, were negatively correlated with albumin, indicating a direct relationship between the severity of bile duct disease and the decrease of liver synthetic function. These observations confirm the clinical meaning of dilatations in identifying patients with more severe disease and the importance of objective quantification.
When assessing the prognostic values of MRCP+-derived metrics, we observed several metrics (including median ducts diameter, dilatations, the percentage of ducts with strictures or dilatations, and the percentage of ducts with abnormal length) to be associated with event-free survival by univariate analysis. In particular, median duct diameter survived multivariate analysis, thus demonstrating it to be independently associated with survival. This is in some way expected, as previous observations by Ruiz et al.7 showed that the severity of IHBD dilatations was one of the radiological parameters predictive of radiological progression over time among individuals along with dysmorphy and the presence of portal hypertension, and their combination in the ANALI score without gadolinium was independently associated with survival in these patients.9 Differently from the study by Ruiz et al.,7 the MRCP+ metrics provide a description only of bile duct changes and are automatically calculated here for the whole biliary tree, as for the nature of the PSC that affects the whole biliary tree in most cases. However, we did not consider that this may have affected the results of our reported association with survival, as our and other groups’ results showed that the severity of IHBD changes, but not extrahepatic bile duct changes, in PSC is associated with radiological progression and survival.7,13
Taking advantage of previous observations, we here explored the relationship between MRCP+ metrics and the ANALI score without gadolinium, and as expected, we observed positive linear associations. Indeed, we observed that more severe stenosis and dilatations were present in the group of persons with ANALI score without gadolinium >2. This observation is at least in part explained by the presence of IHBD dilatation in the ANALI score without gadolinium. However, the number of modelled ducts did not correlate with biochemical severity, LSM, or prognosis, but we observed that the number of modelled ducts is higher in persons with ANALI without gadolinium >2 together with a higher number and severity of strictures and dilatations observed in this group. We also observed a direct correlation between the number of modelled ducts and most of the metrics describing the severity and the extent of strictures and dilatations, which clearly depict more severe diseases. Biliary tree volume was higher in persons with ANALI without gadolinium >2 and also in ANALI with gadolinium >1, but no associations between biliary tree volume and cholestatic indices or adverse outcome-free survival were observed. Similarly, Selvaraj et al.16 recently reported that the volume of the whole biliary tree was not correlated with cholestatic indices, LSM, the Enhanced Liver Fibrosis (ELF®) test, and prognostic scores except for a moderate correlation with the Amsterdam–Oxford score. However, when the analysis was limited to the intrahepatic biliary tree volume, several correlations with cholestatic indices and prognostic scores were reported.16 We can argue about the lack of association between the biliary tree volume and the adverse outcome and we may speculate that this apparently conflicting observation is related to the fact that in PSC biliary tree volume may increase in the intermediate stage of the disease when marked dilatations develop as a response of downstream inflammatory strictures. Then, during the disease course, the development of peribiliary and parenchymal fibrosis leads to a progressive aggravation of the severity and extent of the strictures and a reduction of dilatations with the appearance of the so-called severe pruning of the biliary tree.12 In addition, there is a significant loss of volume of certain hepatic segments that results in hepatic dysmorphy and also in a loss of volume of the bile ducts,22 and consequently, the biliary tree volume may decrease from the previous stage with an associated aggravation of cholestasis. This is concordant with previous observations by Olsson and Asztély,13 which demonstrated the association between the severity of intrahepatic strictures and the early onset of jaundice and shorter survival. However, this hypothesis should be validated in larger multicentric studies with repeated measurement of biliary tree volume and the other metrics during the disease course in the same patient.
Thinking about other possible applications of MRCP+ in people with PSC, we may suggest that a prospective use of MRCP+ in individual patients could represent a useful tool for monitoring biliary changes and biliary tree volume after endoscopic treatment of severe strictures of the extrahepatic bile duct. Indeed, because of its repeatability and reproducibility,14 MRCP+ could provide useful supporting clinically relevant information to radiologists and clinicians concerning the need of further treatments and monitoring response to therapy.
MRCP+ could also overcome the poor to moderate inter-reader agreement of the ANALI scores that was recently suggested.16,23 Regarding the feasibility of MRCP+, in our cohort, we observed that 4.2% of cases were excluded because of low data quality (e.g. motion artefacts), a value below the one reported by Gilligan et al.,18 where 30% of their paediatric cases were excluded because of motion artefact, and similar to that by Selvaraj et al.,16 where they excluded 5% because of low data quality. Additionally, 15 patients (15.6%) were excluded because of protocol-related reasons (e.g. missing 3D-MRCP dataset available for software application or nonisotropic resolution). Given the retrospective nature of our study, these constrains were not possible to overcome, but the recent results published by Selvaraj et al.,16 where they do not exclude patients because of protocol-related issues, suggests that the prospective use of MRCP+ could improve its applicability.
Recent results from Ismail et al.15 confirmed that MRCP+ metrics are associated with biochemical prognostic scores and ANALI scores, similar to that found in this study, suggesting that MRCP+ has the potential as a risk stratifier in PSC together with liver stiffness by magnetic resonance elastography and ANALI scores. In contrast to our results, they reported the most relevant association among stricture severity, instead of dilatations, and predicted prognosis.15 This is reasonably because they evaluated the association between MRCP+ and biochemical prognostic scores instead of actual survival as we performed. In the paper by Selvaraj et al.,16 intrahepatic dilatation severity assessed using MRCP+ demonstrated excellent performance to classify patients into the high-risk and low-risk groups according to different prognostic scores. We here confirm that the severity of bile duct dilatation is associated with a more severe disease, and we also provide data in favour of its role in the prediction of clinical outcomes.
However, MRCP+ does not take into account liver parenchymal consequences of this biliary disease, which have been strongly associated with radiological and clinical outcomes in people with PSC,7,9 and thus, further studies in this field are needed to integrate MRI hepatobiliary changes with clinical and biochemical disease severity. Nevertheless, in this study, MRCP+ showed good correlation with LSM, and thus, their combination can possibly be used to integrate the observed changes in the biliary tree with LSM and disease progression. Moreover, possible combination with other MRI markers such as corrected T1 could also provide better interpretation of MRI hepatobiliary changes with disease severity. This exploratory study has shown that MRCP+ is a promising tool to assess bile duct disease severity in PSC and its correlation with clinical outcome but is underpowered to select the most indicative MRCP + metrics of severe disease and radiological and clinical progression. For this reason, a multicentric analysis in a large cohort of well-characterised people with PSC is highly warranted. Indeed, it is necessary to understand what candidate MRCP+ metrics describing strictures and dilatations are strongly associated with different disease stages, what metrics are predictive of a stable vs. progressive disease course, and the interval time for changing between different metrics, which then can be used in the setting of clinical trials. Moreover, by using the MRCP+ metrics, automatically derived using the whole biliary tree, we here missed the opportunity to compare the prognostic value of the IHBD metrics with the ANALI score without gadolinium and eventually to incorporate the intrahepatic MRCP+ metrics in the ANALI score to possibly overcome its low inter-reader agreement.23 Finally, in a further study including a multicentric cohort of people with PSC, we could also test the predictive performance of MRCP+ metrics against other non-invasive prognostic tools such biochemical scores, ANALI scores, and LSM, either on their own or in combination with each other. To date, despite data showing the prognostic value of MRI findings in PSC and its use in routine care, MRI has not been applied in clinical trials for risk stratification or as surrogate endpoints, as prospective studies are not yet available, likely in part owing to the long time courses needed for data collection in this chronic condition. This study has used retrospective data to attempt to bridge this knowledge gap and directly predict outcome-free survival using quantitative MRCP.
This study has several limitations including the small size, the limited number of major clinical events observed in this cohort, and the retrospective design. All these limitations may have reduced the significance of the association between MRCP+ metrics and prognosis and did not allow us to compare the prognostic value of MRCP+ metrics with other prognostic scores. Despite this, we have shown predictive value in measures of duct diameter, highlighting the usefulness of quantitative MRCP+.
In conclusion, we here propose further supporting arguments in favour of the use of MRI for prognostic purposes in PSC.
Financial support
This study was supported by Imagerie Saint Antoine, ISA, Assistance Publique – Hôpitaux de Paris, Sorbonne University, Department of Radiology, Saint-Antoine Hospital, 184 rue du Fauborg Saint-Antoine, 75012 Paris, France.
Authors’ contributions
Study concept and design: NC, LA, CF, SF.
Acquisition of data: NC, LA, SEM, QV, SL, CF, SF.
Analysis and interpretation of data: NC, LA, SF, CF.
Drafting of the manuscript: NC, LA, SF.
Critical revision of the manuscript for important intellectual content: All the authors.
Statistical analysis: NC.
Study supervision: LA, OC, CC, SL.
Data availability statement
The data that support the findings of this study are available on request from the corresponding authors (LA and NC). The data are not publicly available owing to privacy restrictions.
Conflicts of interest
NC, SEM, QC, SL, CC, OC, and LA declare no conflict of interest related to this paper. CF and SF are employed by Perspectum Ltd.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
Perspectum provided in-kind research support in the form of access to MRCP+ software to the study team.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2022.100577.
Contributor Information
Nora Cazzagon, Email: nora.cazzagon@unipd.it.
Lionel Arrivé, Email: lionel.arrive@aphp.fr.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Karlsen T.H., Folseraas T., Thorburn D., Vesterhus M. Primary sclerosing cholangitis – a comprehensive review. J Hepatol. 2017;67:1298–1323. doi: 10.1016/j.jhep.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 2.Weismüller T.J., Trivedi P.J., Bergquist A., Imam M., Lenzen H., Ponsioen C.Y., et al. Patient age, sex, and inflammatory bowel disease phenotype associate with course of primary sclerosing cholangitis. Gastroenterology. 2017;152:1975–1984.e8. doi: 10.1053/j.gastro.2017.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trivedi P.J., Crothers H., Mytton J., Bosch S., Iqbal T., Ferguson J., et al. Effects of primary sclerosing cholangitis on risks of cancer and death in people with inflammatory bowel disease, based on sex, race, and age. Gastroenterology. 2020;159:915–928. doi: 10.1053/j.gastro.2020.05.049. [DOI] [PubMed] [Google Scholar]
- 4.Schramm C., Eaton J., Ringe K.I., Venkatesh S., Yamamura J. MRI working group of the IPSCSG. Recommendations on the use of magnetic resonance imaging in PSC – a position statement from the International PSC Study Group. Hepatology. 2017;66:1675–1688. doi: 10.1002/hep.29293. [DOI] [PubMed] [Google Scholar]
- 5.Aabakken L., Karlsen T.H., Albert J., Arvanitakis M., Chazouilleres O., Dumonceau J.-M., et al. Role of endoscopy in primary sclerosing cholangitis: European Society of Gastrointestinal Endoscopy (ESGE) and European Association for the Study of the Liver (EASL) Clinical Guideline. Endoscopy. 2017;49:588–608. doi: 10.1055/s-0043-107029. [DOI] [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver EASL Clinical Practice Guidelines on sclerosing cholangitis. J Hepatol. 2022;77:761–806. doi: 10.1016/j.jhep.2022.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Ruiz A., Lemoinne S., Carrat F., Corpechot C., Chazouillères O., Arrivé L. Radiologic course of primary sclerosing cholangitis: assessment by three-dimensional magnetic resonance cholangiography and predictive features of progression. Hepatology. 2014;59:242–250. doi: 10.1002/hep.26620. [DOI] [PubMed] [Google Scholar]
- 8.Cazzagon N., Lemoinne S., El Mouhadi S., Trivedi P.J., Gaouar F., Kemgang A., et al. The complementary value of magnetic resonance imaging and vibration-controlled transient elastography for risk stratification in primary sclerosing cholangitis. Am J Gastroenterol. 2019;114:1878–1885. doi: 10.14309/ajg.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 9.Lemoinne S., Cazzagon N., El Mouhadi S., Trivedi P.J., Dohan A., Kemgang A., et al. Simple magnetic resonance scores associate with outcomes of patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2019;17:2785–2792.e3. doi: 10.1016/j.cgh.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Tenca A., Mustonen H., Lind K., Lantto E., Kolho K.-L., Boyd S., et al. The role of magnetic resonance imaging and endoscopic retrograde cholangiography in the evaluation of disease activity and severity in primary sclerosing cholangitis. Liver Int. 2018;38:2329–2339. doi: 10.1111/liv.13899. [DOI] [PubMed] [Google Scholar]
- 11.Schulze J., Lenzen H., Hinrichs J.B., Ringe B., Manns M.P., Wacker F., et al. An imaging biomarker for assessing hepatic function in patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2019;17:192–199.e3. doi: 10.1016/j.cgh.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Ponsioen C.Y., Vrouenraets S.M.E., Prawirodirdjo W., Rajaram R., Rauws E.A.J., Mulder C.J.J., et al. Natural history of primary sclerosing cholangitis and prognostic value of cholangiography in a Dutch population. Gut. 2002;51:562–566. doi: 10.1136/gut.51.4.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsson R.G., Asztély M.S. Prognostic value of cholangiography in primary sclerosing cholangitis. Eur J Gastroenterol Hepatol. 1995;7:251–254. [PubMed] [Google Scholar]
- 14.Goldfinger M.H., Ridgway G.R., Ferreira C., Langford C.R., Cheng L., Kazimianec A., et al. Quantitative MRCP imaging: accuracy, repeatability, reproducibility, and cohort-derived normative ranges. J Magn Reson Imaging. 2020;52:807–820. doi: 10.1002/jmri.27113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ismail M.F., Hirschfield G.M., Hansen B., Tafur M., Elbanna K.Y., Goldfinger M.H., et al. Evaluation of quantitative MRCP (MRCP+) for risk stratification of primary sclerosing cholangitis: comparison with morphological MRCP, MR elastography, and biochemical risk scores. Eur Radiol. 2021;32:67–77. doi: 10.1007/s00330-021-08142-y. [DOI] [PubMed] [Google Scholar]
- 16.Selvaraj E.A., Ba-Ssalamah A., Poetter-Lang S., Ridgway G.R., Brady J.M., Collier J., et al. A quantitative magnetic resonance cholangiopancreatography metric of intrahepatic biliary dilatation severity detects high-risk primary sclerosing cholangitis. Hepatol Commun. 2022;6:795–808. doi: 10.1002/hep4.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janowski K., Shumbayawonda E., Cheng L., Langford C., Dennis A., Kelly M., et al. Quantitative multiparametric MRI as a non-invasive stratification tool in children and adolescents with autoimmune liver disease. Sci Rep. 2021;11 doi: 10.1038/s41598-021-94754-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilligan L.A., Trout A.T., Lam S., Singh R., Tkach J.A., Serai S.D., et al. Differentiating pediatric autoimmune liver diseases by quantitative magnetic resonance cholangiopancreatography. Abdom Radiol. 2020;45:168–176. doi: 10.1007/s00261-019-02184-z. [DOI] [PubMed] [Google Scholar]
- 19.European Association for the Study of the Liver EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237–267. doi: 10.1016/j.jhep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Kim W.R., Therneau T.M., Wiesner R.H., Poterucha J.J., Benson J.T., Malinchoc M., et al. A revised natural history model for primary sclerosing cholangitis. Mayo Clin Proc. 2000;75:688–694. doi: 10.4065/75.7.688. [DOI] [PubMed] [Google Scholar]
- 21.de Vries E.M., Wang J., Williamson K.D., Leeflang M.M., Boonstra K., Weersma R.K., et al. A novel prognostic model for transplant-free survival in primary sclerosing cholangitis. Gut. 2018;67:1864–1869. doi: 10.1136/gutjnl-2016-313681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arrivé L., Hodoul M., Arbache A., Slavikova-Boucher L., Menu Y., El Mouhadi S. Magnetic resonance cholangiography: current and future perspectives. Clin Res Hepatol Gastroenterol. 2015;39:659–664. doi: 10.1016/j.clinre.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Grigoriadis A., Ringe K.I., Andersson M., Kartalis N., Bergquist A. Assessment of prognostic value and interreader agreement of ANALI scores in patients with primary sclerosing cholangitis. Eur J Radiol. 2021;142 doi: 10.1016/j.ejrad.2021.109884. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding authors (LA and NC). The data are not publicly available owing to privacy restrictions.